Abstract
The essentiality of zinc in humans was established in 1963. During the past 50 y, tremendous advances in both clinical and basic sciences of zinc metabolism in humans have been observed. The major factor contributing to zinc deficiency is high phytate-containing cereal protein intake in the developing world, and nearly 2 billion subjects may be zinc deficient. Conditioned deficiency of zinc has been observed in patients with malabsorption syndrome, liver disease, chronic renal disease, sickle cell disease, and other chronic illnesses. Major clinical problems resulting from zinc deficiency in humans include growth retardation; cell-mediated immune dysfunction, and cognitive impairment. In the Middle East, zinc-deficient dwarfs did not live beyond the age of 25 y, and they died because of intercurrent infections. In 1963, we knew of only 3 enzymes that required zinc for their activities, but now we know of >300 enzymes and >1000 transcription factors that are known to require zinc for their activities. Zinc is a second messenger of immune cells, and intracellular free zinc in these cells participate in signaling events. Zinc has been very successfully used as a therapeutic modality for the management of acute diarrhea in children, Wilson’s disease, the common cold and for the prevention of blindness in patients with age-related dry type of macular degeneration and is very effective in decreasing the incidence of infection in the elderly. Zinc not only modulates cell-mediated immunity but is also an antioxidant and anti-inflammatory agent.
Introduction
In 1869, the essentiality of zinc for the growth of Aspergillus niger was observed (1). In 1933, zinc was shown to be essential for the growth of the rats (2). Although by 1960, the essentiality of zinc for growth in various animal species was reported, it was considered improbable that zinc deficiency in humans could lead to significant clinical problems.
I was trained as a clinical scientist at the University of Minnesota, Minneapolis, MN, and after my training, a strange set of circumstances brought me to Shiraz, Iran. The story of zinc began when an Iranian physician presented to me at the medical center grand rounds, a 21-y-old man who looked like a 10-y-old boy and who was severely anemic. His genitalia were infantile. He had rough and dry skin, mental lethargy, hepatosplenomegaly, and geophagia. He ate only bread (whole wheat flour) and had no intake of animal protein. He consumed 0.5 kg of clay daily. He was severely iron deficient but had no blood loss. Later, I discovered that this syndrome was common in the villages of Shiraz, Iran (3).
Iron deficiency alone could not account for all the features that we observed in this case because growth retardation and testicular atrophy are not seen in iron-deficient experimental animals. An examination of the periodic table suggested to me that deficiency of another transitional element, perhaps zinc, may have also been present, which may account for growth retardation and hypogonadism. We hypothesized that a high phosphate content in the diet and geophagia may have decreased the availability of both iron and zinc, which resulted in deficiency of both elements (3).
Our later studies in Egypt documented conclusively that zinc deficiency occurred in humans and that zinc supplementation resulted in 12.7–15.2 cm of growth in 1 y and that genitalia became normal within 3–6 mo of zinc supplementation (4–6).
For nearly a decade, the idea that zinc deficiency occurred in humans remained very controversial. Several reports, however, confirmed our observation, and in 1974, the National Research Council of the National Academy of Sciences declared zinc as an essential element for humans and established a recommended dietary allowance (RDA)4 (7). In 1978, the FDA made it mandatory to include zinc in the total parenteral nutrition fluids (8).
The details of the circumstances leading to the discovery of human zinc deficiency in the Middle East have been presented in another paper.
In this paper, the nutritional deficiency of zinc, which is present throughout the developing world, and a conditioned deficiency of zinc, which may complicate many diseased states, are discussed. Zinc has also been recognized to have a therapeutic role in the management of certain diseases. This is also presented.
Chronology of zinc-related observations in humans
Eggleton (9) in China demonstrated in 1939 that the zinc contents of the toenails, fingernails, and skin were significantly decreased in poor Chinese individuals with beriberi compared with healthy subjects, and he suggested that zinc deficiency may be a factor in beriberi. In another publication, Eggleton (10) analyzed zinc and copper in 13 different organs and tissues from 26 Chinese subjects of both sexes ranging in age from 156 d to 60 y who had died of miscellaneous causes. He showed that both elements were present in all the tissues examined. He also observed that whereas zinc was concentrated in all organs and tissues, copper was concentrated only in the liver, brain, kidney, and hair, in descending order. The cerebellum contained more zinc and copper than the cerebrum.
By using the dithizone technique, Lutz (11) analyzed zinc in various human tissues and concluded that the total zinc content of a 70-kg man was ~2.2 g. This figure is remarkably close to what is accepted today (12). Absorption and excretion of zinc were first investigated by McCance and Widdowson (13). They showed that the principal route of zinc excretion was in the feces, and only a small quantity of zinc was excreted in the urine.
Vikbladh (14) analyzed serum zinc by the dithizone technique and reported that the normal level was 19.7 ± 0.24 μmol/L, a value similar to what has been reported by modern techniques. In another paper, Vikbladh (15) reported that serum zinc was decreased in many chronic diseases, including liver disease. In 1956, Vallee et al. (12) reported that the serum zinc concentration was decreased in patients with cirrhosis of the liver and suggested that this represented a conditioned deficiency of zinc due to hyperzincuria.
Nutritional deficiency of zinc
The first case of zinc deficiency in the United States was reported by Caggiano et al. (16) in 1969 in a Puerto Rican subject with dwarfism, hypogonadism, hypogammaglobulinemia, giardiasis, strongyloidosis, and schistosomiasis. Zinc supplementation resulted in improved growth and development. In 1972, Hambidge et al. (17) reported the occurrence of nutritional zinc deficiency in Mexican-American children from Denver, CO. They responded well to zinc supplementation.
In 1972, Halsted et al. (18) published the results of their study involving a group of 15 men who were rejected at the Iranian Army Induction Center because of “malnutrition.” Two women, 19 and 20 y old, were also included in their study. The unique feature was that all their subjects were 19 and 20 y old. Their clinical features were similar to those reported by Prasad et al. in 1961 (3) and 1963 (4). They were studied for 6–12 mo. One group received a well-balanced diet containing adequate animal protein plus a placebo capsule. A second group was given the same diet plus a capsule of zinc sulfate containing 27 mg of elemental zinc. A third group received the diet without additional supplement for 6 mo. The 2 women lived in the house of Dr. Ronaghy and received the same treatment and observation program. The zinc-supplemented group gained considerably in height and showed evidence of early onset of sexual function as defined by nocturnal emission in males and menarche in females compared with those receiving only a well-balanced diet.
Severe deficiency of zinc
Acrodermatitis enteropathica.
In 1973, Barnes and Moynahan (19) reported a 2-y-old girl with severe acrodermatitis enteropathica who was being treated with diiodohydroxyquinolone and a lactose-deficient synthetic diet but was not showing any satisfactory response to this therapy. The serum zinc concentration was significantly decreased. They, therefore, administered oral zinc sulfate to correct this deficiency. Surprisingly, the skin lesions and gastrointestinal symptoms cleared after zinc supplementation. When zinc was inadvertently omitted from the child’s regimen, the child suffered a relapse; however, she again completely responded to oral zinc therapy. The authors attributed zinc deficiency to the synthetic diet that the patient received. The authors, however, soon realized that zinc might have been fundamental to the pathogenesis of this rare inherited disorder and that the clinical improvement reflected correction of the zinc status in the patient. This original observation was quickly confirmed in other patients with acrodermatitis enteropathica (AE) throughout the world. The underlying pathogenesis of the zinc deficiency in these patients is due to malabsorption of zinc caused by a mutation in ZIP4, an intestinal zinc transporter (20).
AE is a lethal, autosomal, recessive trait that usually occurs in infants of Italian, Armenian, or Iranian lineage (21). The disease develops in the early months of life soon after weaning from breastfeeding. The dermatologic manifestations of severe zinc deficiency in patients with AE include bullous pustular dermatitis of the extremities and oral, anal, and genital areas around the orifices, paronychia, and alopecia. Ophthalmic signs include blepharitis, conjunctivitis, photophobia, and corneal opacities. Neuropsychiatric signs include irritability, emotional instability, tremors, and occasional cerebellar ataxia. Weight loss, growth retardation, and male hypogonadism are also prominent clinical features. Congenital malformation of fetuses and infants born of pregnant women with AE has been commonly observed (22).
AE patients have an increased susceptibility to infections. Thymic hypoplasia, absence of germinal centers in lymph nodes, and plasmacytosis in the spleen are seen consistently. All T cell–mediated functional abnormalities are completely corrected with zinc supplementation. Abnormal chemotoxis is also corrected with zinc therapy in AE patients. The clinical course is downhill with failure to thrive and complicated by intercurrent bacterial, fungal, viral, and other opportunistic infections. Gastrointestinal disturbances are severe and include diarrhea, malabsorption, steatorrhea, and lactose intolerance. The disease, if unrecognized and untreated, is fatal. Zinc supplementation, however, results in complete recovery.
The AE gene has been localized to a ∼3.5-cm region on the 8q24 chromosome. The gene encodes a histidine-rich protein, now referred to as ZIP-4, which is a member of a large family of transmembrane proteins known as zinc transporters. In patients with AE, mutations in this gene have been documented (20).
Total parenteral nutrition.
In 1975, Kay and Tasman-Jones (23) reported the occurrence of severe zinc deficiency in subjects receiving total parenteral nutrition for prolonged periods without zinc. Okada et al. (24) and Arakawa et al. (25) reported similar findings without zinc. These observations have been documented by several investigators, and, indeed, in the United States, zinc is now being routinely included in total parenteral fluids for subjects who are likely to receive such therapy for extended period.
Patients on total parenteral nutrition with diarrhea may lose up to 6 to 12 mg/d of zinc. The excessive loss of zinc results in severe deficiency of zinc. Manifestations such as dermatologic, alopecia, neuropsychiatric, weight loss, and intercurrent infections are commonly seen. There are a negative nitrogen balance and impaired carbohydrate utilization. If zinc deficiency is not recognized and treated, the condition may become fatal (8). Zinc deficiency may also be common in very low birth weight infants because fortified human milk and preterm formula may not contain zinc; as such, physicians must be aware of this problem (26).
Penicillamine therapy.
A severe deficiency of zinc has also been observed in patients with Wilson’s disease who received penicillamine therapy as decoppering agent. This treatment may induce excessive zinc loss and cause severe deficiency of zinc (27).
In summary, the manifestations of severe zinc deficiency in humans include bullous pustular dermatitis, alopecia, diarrhea, emotional disorders, weight loss, intercurrent infections due to cell-mediated immune dysfunctions, hypogonadism in males, neurosensory disorders, and problems with healing of ulcers. Severe deficiency of zinc, if untreated, may become fatal.
Moderate deficiency of zinc
The manifestations of a moderate deficiency of zinc include growth retardation, male hypogonadism in adolescents, rough skin, poor appetite, mental lethargy, delayed wound healing, cell-mediated immune dysfunctions, and abnormal neurosensory changes. These manifestations have been reported in subjects with nutritional deficiency of zinc as observed originally in Iran and Egypt (3, 4) and many subjects with conditioned deficiency of zinc.
It is now apparent that a nutritional deficiency of zinc in humans is fairly prevalent throughout the world, particularly in areas where cereal proteins are primary in local diets. In Turkey, geophagia is also a common problem, and the majority of the adolescents in the villages in Turkey with geophagia exhibit both iron and zinc deficiencies (22).
Cavdar et al. (22) observed a decreased zinc level in almost 30% of low socioeconomic status pregnant women in Turkey. Their diet consisted of mainly cereals. Maternal zinc deficiency was associated with severe congenital malformation of the central nervous system in the fetuses, and maternal morbidity was increased.
Zinc and growth.
Growth is the first limiting effect of zinc deficiency in experimental animals (28). Zinc deficiency decreases circulating insulin-like growth factor 1 (IGF-1) concentration independent of total energy intake (29).
In humans, zinc deficiency decreases circulating IGF-1 concentration (30, 31). IGF-1 receptor possesses tyrosine kinase activity (28). On activation of the receptor by IGF, a cascade of phosphorylation occurs within the cell leading to regulation of cell cycle and cell division. Tyrosine phosphorylation of the receptor is essential for its activation, and I hypothesize that because zinc has been shown to inhibit various protein tyrosine phosphatases (32), phosphorylation of the tyrosine kinase receptor by zinc is perhaps the most important critical step of zinc action on human growth.
IGF-1 activation leads to stimulation of thymidine uptake in cells (33). In our earlier studies, we showed that in zinc-deficient rats, the activity of deoxythymidine kinase (TK), an enzyme required for conversion of deoxythymidine to deoxythymidine 5′-monophosphate, a precursor of thymidine triphosphate, is considerably decreased in the implanted sponge connective tissue, and this reduced activity of TK decreased DNA, protein, and collagen synthesis in rats (34).
Thus, it appears that zinc has multiple roles in growth. It is required for IGF-1 generation, phosphorylation of IGF-1 receptor, and upregulation of the activity of TK, all of which are involved in cell division and growth.
Mild deficiency of zinc
Although the clinical, biochemical, and diagnostic aspects of severe and moderate levels of zinc deficiency in humans are well defined, the recognition of mild deficiency of zinc remains very difficult. We therefore developed an experimental model of zinc deficiency in humans to define mild zinc deficiency.
In a group of human volunteers, we induced a mild state of zinc deficiency by dietary means. Adult male volunteers were hospitalized at the Clinical Research Center of the University of Michigan Medical School Hospital, Ann Arbor, MI. A semipurified diet that supplied ~3.0–5.0 mg/d of zinc was used to induce zinc deficiency (35).
The volunteers were given a hospital diet containing animal protein daily for 4 wk. This diet averaged 12 mg/d of zinc, consistent with the RDA. After this, they received 3.0–5.0 mg/d of zinc while consuming a soy protein–based experimental diet. This regimen was continued for 28 wk. After this, the volunteers received 2 cookies containing 27 mg of zinc supplement. This supplementation was continued for 12 wk.
Throughout this study, the level of all nutrients including protein, amino acids, vitamins, and minerals (both micro and macro elements) were kept constant, meeting the RDA except for zinc. By this technique, we were able to induce a specific mild deficiency of zinc in human volunteers (35–38).
As a result of mild deficiency of zinc, we observed a decreased serum testosterone level, oligospermia, decreased natural killer cell lytic activity, decreased IL-2 activity of T helper cells, decreased serum thymulin activity, hyperammonemia, hypogeusia, decreased dark adaptation, and decreased lean body mass (36–38). This study clearly established that even a mild deficiency of zinc in humans affects clinical, biochemical, and immunological functions adversely.
Zinc and immune cells.
Zinc is a second messenger for immune cells, and its intracellular status is directly altered by an extracellular stimulus and then intracellular zinc participates in signaling events (39). Hirano et al. (40) showed that a decrease in intracellular free zinc is critical for LPS-mediated CD4+ T-cell activation by dendritic cells (DCs). LPS binds to Toll-like receptor 4 on DCs and initiates Myd88 and TRIF [a domain containing adapter–inducing interferon (IFN)-β]-mediated signaling (39). TRIF-mediated signaling increases the ZnT (a solute carrier )-5 mRNA and decreases ZIP-6 mRNA, thus resulting in a decrease in the intracellular free zinc in DCs. Reduction in intracellular free zinc increases surface expression of major histocompatibility complex class II molecules, which is important for the activation of CD4+ T cells (39, 40).
Zinc affects the activity of monocytes/macrophages in several ways. Zinc is involved in monocyte/macrophage development (41–43) and regulates its functions such as phagocytosis and proinflammatory cytokine production. LPS stimulation of zinc-sufficient monocytes results in downregulation of inflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-8 (37–39). Zinc inhibits the membrane phosphodiesterase, leading to elevated levels of the second messenger guanosine 3′,5′ cyclic monophosphate, which is followed by a subsequent suppression of the nuclear factor κB (NF-κB)–dependent mRNA of TNF-α, IL-1β, and other inflammatory cytokines (40–42). Additionally, zinc induces A-20, which inhibits NF-κB signaling via TNF receptor–associated factor pathways, resulting in downregulation of the mRNA of inflammatory cytokines (44–46). Based on this, we propose that zinc is an important anti-inflammatory agent.
Zinc deficiency affects T helper subset 1 cell function adversely in humans (36–38, 43). Serum thymulin activity and generation of T helper subset 1 cell cytokine, IL-2, and IFN-γ were affected within 8–12 wk of institution of a zinc-restricted diet (3–5 mg/d) in human volunteers, whereas plasma zinc decreased after 20 to 24 wk of the institution of the experimental diet. This suggests that T helper subset 1 cells are very sensitive to zinc restriction. T helper subset 2 cell cytokines were not affected as a result of the institution of zinc-deficient diet in humans.
In the human malignant lymphoblastoid cell line of Th0 phenotype cells, a human malignant lymphoblastoid cell line of the Th0 phenotype, we showed that in zinc-sufficient cells, mRNA levels of IFN-γ, IL-12 receptor β2, and T-bet (a transcription factor involved in T-cell differentiation) in phorbol-12 myristate 13 acetate (PMA)/phytohemagglutinin-P (PHA)–stimulated cells were increased compared with zinc-deficient cells (47). Although intracellular free (noncovalently bound zinc) zinc increased only slightly in PMA/PHA-stimulated cells, in concanavalin A–stimulated cells in zinc-sufficient medium, there was an increased sustained level of intracellular free zinc compared with the zinc-deficient cells (47). We concluded that stimulation of cells by concanvalin A via T-cell receptor, there was a release of intracellular free zinc that functioned as a signal transduction molecule for the generation of IFN-γ, T-bet, and IL-12 receptor β2 mRNA required for T helper subset 1 cell differentiation (47).
Conditioned deficiency of zinc
GI disorders.
A moderate level of zinc deficiency has been observed in many gastrointestinal disorders. These include malabsorption syndrome, Crohn’s disease, regional ileitis, and steatorrhea.
In 1968, MacMahon et al. (48) were the first to report zinc deficiency in a patient who had steatorrhea. Zinc deficiency in patients with malabsorption syndrome is now well recognized, and most physicians are aware of this problem.
Liver disease.
Low serum and hepatic zinc and, paradoxically, hyperzincuria were reported in patients with cirrhosis of the liver many years ago (12). Some patients with cirrhosis of the liver who had night blindness did not respond to vitamin A supplementation; however, they responded to zinc administration (21).
Elevated blood ammonia levels are known to be a factor in the development of hepatic coma. It is known that zinc-deficient rats have a defect in the metabolism of sulfur-containing amino acids. Zinc deficiency also affects urea synthesis and thus an abnormality related to metabolism of amino acids and ammonia may act in concert to produce hepatic coma. We reported earlier that dietary zinc restriction may lead to hyperammonemia (49). Rabbani and Prasad (49) observed a decrease in hepatic ornithine transcarbamoylase activity and an increase in plasma ammonia levels in zinc-deficient rats. Additionally, increased activity of the purine nucleotide enzyme adenosine monophosphate deaminase as a result of zinc deficiency has also been observed, which may also contribute to increased ammonia levels (50).
Zinc therapy has been reported to be beneficial in subjects with hepatic encephalopathy (51). More studies are needed in this important area. It is likely that some of the clinical features of cirrhosis of the liver, such as loss of body hair, testicular hypofunction, poor appetite, mental lethargy, difficulty in healing, abnormal cell–mediated immunity, and night blindness, may indeed be related to the secondary zinc-deficient state induced by hyperzincuria.
Renal diseases.
Mahajan et al. (52, 53) were the first to document that patients with chronic renal failure showed decreased concentration of zinc in plasma, leukocytes, and hair; increased plasma ammonia levels; and increased activity of plasma ribonuclease. Uremic hypogeusia improved after zinc supplementation. Impotence is common in uremic males and is not improved by hemodialysis. A double-blind clinical trial of zinc supplementation showed that zinc deficiency was a reversible cause of sexual dysfunction in uremia (53).
Zinc deficiency in sickle cell disease.
Our studies have documented the occurrence of zinc deficiency in adult sickle cell disease (SCD) patients (54, 55). Growth retardation, hypogonadism in males, hyperammonemia, abnormal dark adaptation, and cell-mediated immune dysfunction in SCD patients have been related to a deficiency of zinc. The biochemical evidence of zinc deficiency in SCD patients was decreased levels of zinc in the plasma, erythrocytes, and hair; hyperzincuria; decreased activity of certain zinc-dependent enzymes such as carbonic anhydrase in erythrocytes, alkaline phosphatase in the neutrophils, deoxythymidine kinase activity in newly synthesizing skin connective tissue and collagen; and hyperammonemia (54, 55). Inasmuch as zinc is known to be an inhibitor of RNA, increased activity of this enzyme in plasma was considered to also be evidence of zinc deficiency. Zinc supplementation in SCD patients resulted in significant improvement in secondary sexual characteristics, normalization of plasma ammonia level, and correction of dark adaptation abnormality. Zinc supplementation also increased zinc levels in plasma, erythrocytes, and neutrophils. The expected response to zinc supplementation in enzyme activities was also observed. Increased longitudinal growth and body weight in 14- to 18-y-old SCD patients were observed. Zinc supplementation also corrected impaired delayed-type hypersensitivity reaction and decreased natural killer cell lytic activity in SCD patients (21, 54, 55).
A 3-mo placebo-controlled zinc supplementation trial (25 mg zinc as zinc acetate 3 times a day) in 36 SCD patients showed that zinc-supplemented subjects had a decreased incidence of infections, increased hemoglobin and hematocrit levels, and increased plasma zinc and antioxidant power compared with the placebo group (55). Plasma nitrite and nitrate, lipid peroxidation products, DNA oxidation products, and soluble vascular cell adhesion molecule 1 (VCAM-1) decreased in the zinc-supplemented group compared with the placebo group. Zinc-supplemented subjects showed significant decreases in LPS-induced TNF-α and IL-1β mRNA and TNF-induced NF-κB DNA binding in mononuclear cells (MNCs) compared with the placebo group (55). Zinc supplementation also increased relative levels of IL-2 and IL-2 receptor α mRNA in PHA-stimulated MNCs (55).
Therapeutic impact of zinc
Zinc and infectious diseases.
Acute diarrhea in children.
Supplementation with zinc has been shown to prevent and treat diarrhea in children younger than 5 y of age, decreasing both diarrhea morbidity and mortality (56, 57). Zinc deficiency is also correlated with the risk of respiratory tract infections, but the benefit of supplementation appears to be limited to more severe episodes and in populations with a high incidence of zinc deficiency (57).
Diarrhea causes the breakdown of absorptive mucosa, resulting in poor absorption of nutrients, including zinc. Studies conducted earlier linked diarrheal illness to the loss of endogenous zinc (57). Children with low plasma zinc were observed to be more susceptible to diarrhea pathogens, propagating a cycle of deficiency and infection.
There is extensive evidence supporting the efficacy of zinc supplementation for the prevention of childhood diarrhea (57). In 2004, WHO issued a global recommendation for the daily supplementation with 20 mg zinc in children 6 mo of age and older and 10 mg of zinc in infants younger than 6 mo for 10–14 d on diarrheal onset.
Meta-analysis of routine supplementation for as long as 3 mo in 7 studies providing 1–2 times the RDA of elemental zinc 5–7 times per week found an 18% reduction in diarrheal incidence, a 25% decrease in diarrhea prevalence, and a 33% reduction in persistent diarrhea episodes among supplemented children compared with children who received placebo (57).
A meta-analysis of 3 randomized, controlled trials providing short-course zinc supplementation with 2–4 times the daily RDA for 2 wk after the onset of an episode of acute or persistent diarrhea was reported. The pooled analysis showed an 11% decrease in diarrhea incidence and a 34% decrease in diarrhea prevalence during 3-mo observation period (57).
Severe infection and zinc in children.
Serious bacterial infections are a major cause of death in early infancy in developing countries. In 1 study, the effect of zinc as an adjunct to antibiotics in infants with probable serious bacterial infection was assessed. A randomized, double-blind, placebo-controlled zinc supplementation trial in infants 7–120 d of age with probable serious bacterial infections at 3 hospitals in New Delhi, India, was conducted (58). The patients were stratified according to whether they were underweight or had diarrhea at enrollment, and they received either 10 mg zinc or placebo orally every day in addition to standard antibiotic treatment. The primary outcome was treatment failure, which was defined as the need to change antibiotics within 7 d of randomization, the need for intensive care management, or death at any time within 21 d. Significantly fewer treatment failures occurred in the zinc group (10%) compared with the placebo group (17%). Ten infants receiving zinc died compared with 17 receiving placebo (58).
Zinc for the treatment of the common cold.
The common cold is one of the most frequently occurring diseases in the world (59, 60). More than 20 viruses cause the common cold, and these include rhinovirus, coronavirus, adenovirus, respiratory syncytial virus, and parainfluenza virus. Annually adults in the United States may develop a common cold 2–4 times in a year, and children may develop colds 6–8 times in a year. The morbidity and subsequent financial loss resulting from absenteeism from work are substantial. Previously prescribed treatments have not provided a consistent relief of symptoms.
Eby et al. (61) in 1984 were the first to show in a double-blind, placebo-controlled trial that zinc gluconate lozenges administered every 2 h were effective in decreasing the severity and duration of common cold. We tested the efficacy of zinc acetate lozenges in the common cold in 50 volunteers who were recruited within 24 h of symptoms of the common cold developing and conducted a randomized, double-blind, placebo-controlled trial (59). Participants took 1 lozenge containing 12.8 mg zinc (as acetate) or placebo every 2 to 3 h while awake as soon as common cold symptoms developed. Subjective symptom scores for sore throat, nasal discharge, nasal congestion, sneezing, cough, scratchy throat, hoarseness, muscle ache, fever, and headache were recorded daily for 12 d. Plasma zinc and proinflammatory cytokines were assayed on day 1 and after participants were well.
Twenty-five in the zinc group and 23 in the placebo group completed the study. Compared with the placebo group, the zinc group had shorter overall duration of cold symptoms (4.5 vs. 8.1 d, P < 0.01), cough (3.1 vs. 6.3 d, P = 0.01), and nasal discharge (4.1 vs. 5.8 d, P = 0.02), and decreased total severity scores for all symptoms (P < 0.002).
In another study, we recruited 50 ambulatory volunteers within 24 h of common cold symptoms developing for randomized, double-blind, placebo-controlled trial of zinc (60). Participants took 1 lozenge containing 13.3 mg of zinc (as zinc acetate) or placebo every 2–3 h while awake. The subjective scores of clinical symptoms were recorded daily. Plasma zinc, soluble IL-1 receptor antagonist (ra), soluble TNF receptor 1, soluble ICAM-1 were assayed on days 1 and 5 (60).
Compared with the placebo group, the zinc group had a shorter mean overall duration of the cold (4.0 vs 7.1 d, P = 0.001), cough (2.1 vs 5.0 d, P < 0.001), and nasal discharge (3.0 vs 4.5 d, P = 0.02). Blinding of subjects was adequate, and adverse effects were comparable in the 2 groups. Symptom severity scores were significantly decreased in the zinc group (P = 0.002). The mean changes between zinc and placebo groups (before vs after therapy) showed significant differences in soluble IL-1-ra (P = 0.033) and soluble ICAM-1 level (P = 0.04). Both decreased in the zinc group, and the mean changes in zinc and placebo group (before vs after therapy) showed a significant difference (P < 0.001)
Our results suggest that common cold viruses increase oxidative stress, which activates macrophages and monocytes and results in increased production of both the inflammatory cytokines and the anti-inflammatory product soluble IL-1ra; thus, a decrease in soluble IL-1ra in the zinc group suggests that zinc decreased the activation of monocytes and macrophages by decreasing oxidative stress. We previously showed that zinc functions as an antioxidant (44).
Human rhinovirus type 24 “docks” with ICAM-1 on the surface of the somatic cells (59, 60). Thus, zinc may act as an antiviral agent by reducing ICAM-1 levels. We previously showed that zinc functions as a downregulator of NF-κB activity involved in the gene expression of adhesion molecules such as ICAM-1 (46).
We conclude that zinc acetate lozenges given within 24 h of the onset of common cold in proper doses are very effective in decreasing the duration and severity of the common cold. We propose that the beneficial effects seen in the zinc group were due to the antioxidant and anti-inflammatory effects of zinc. We also suggest that a decrease in plasma ICAM-1 levels due to zinc therapy may have decreased the docking of the cold viruses on the surface of somatic cells.
A meta-analysis selected randomized, double-blind, placebo-controlled trials using zinc for at least 5 consecutive days to treat or at least 5 mo to prevent the common cold were included for analysis (62). Thirteen therapeutic trials (966 participants) and 2 preventive trials (394 participants) were included for analysis. Studies reporting the duration and severity of cold symptoms suggest that zinc significantly reduced the overall duration and severity of common cold symptoms if the therapy was started within 24 h of the onset of the cold. Zinc supplementation for prevention of the common cold showed that the incidence of the common cold, school absenteeism, and use of antibiotics were decreased (62).
In another meta-analysis, 13 placebo-controlled studies examined the therapeutic effect of zinc lozenges on common cold symptoms (63). Five of the trials used a total daily zinc dose of <75 mg and uniformly found no effect. Three trials used zinc acetate in daily doses of >75 mg; the pooled results showed a 42% reduction in the duration of colds. Five trials used zinc salts rather than acetate in daily doses of >75 mg; the pooled results showed a 20% reduction in the duration of common colds.
In another report, the investigators included 17 randomized, controlled trials to analyze the effect of zinc on the common cold (64). Although the authors observed an increased heterogeneity due to age, zinc dose, and chemical formulation, they concluded that zinc may shorten the duration of the common cold (64).
To optimize the therapeutic effect of zinc lozenges on the common cold, one must pay attention to several issues. First, zinc therapy must begin within 24 h of the onset of cold symptoms. Second, the total daily dose of elemental zinc should be >75 mg. Third, the chemical formulation should be optimal so that zinc is ionized in the oral cavity at pH 7.4. Zinc acetate and zinc gluconate are good salts to use; however, if citric acid, glycine, tartarate, and other binders are used, zinc is prevented from ionization. Thus, it is critical that the solution chemistry of the preparation is proper. Physicians and health practitioners must realize that one cannot treat common cold symptoms by swallowing zinc tablets, zinc syrup, or zinc lozenges. Zinc lozenges must be used orally and allowed to dissolve slowly in the mouth, which will then allow ionic zinc to be released, absorbed, and transported to the virally infected nose.
Zinc therapy for Wilson’s disease
Wilson’s disease is an inherited autosomal disorder of copper accumulation. The excretion of liver copper in the bile is decreased. This leads to failure of proper copper excretion in the stool and to the accumulation of copper in the liver. Eventually not only the liver but also brain and other organs are damaged due to excess copper accumulation. Patients typically present with liver disease, neurological disease (movement disorder), or psychiatric disturbances in the second to fourth decades of life. In many cases, the diagnosis is either missed or delayed (65, 66).
The gene for Wilson’s disease has been now identified. The genetic mutation leads to a defective production of a protein called ATP7B (membrane-bound copper-binding ATP), which is responsible for key step in biliary excretion of copper (65, 66). The disease is recessive; thus, both copies of the ATP7B gene have to be mutated to cause a failure in biliary excretion of copper and produce the disease. The gene for Wilson’s disease codes for a membrane-bound copper-binding ATP-type protein that probably acts as a copper pump, in either the plasma membrane or the intracellular membrane. A large number of mutations in this gene causing Wilson’s disease have been identified. This complicates the development of an easy DNA test for the diagnosis of this disease.
It is important to establish an early diagnosis of Wilson’s disease because effective therapeutic measures may prevent accumulation of copper and serious damage to organs such as the liver and brain. Ninety percent of the Wilson’s disease patients have low levels of ceruloplasmin, and ceruloplasmin-bound copper and nonceruloplasmin-bound copper are elevated in the plasma. Measurement of the 24-h urinary copper is a good diagnostic test because this is consistently elevated in these patients (65, 66). Urinary copper, however, may be elevated in patients with obstructive liver disease also who do not have Wilson’s disease.
A slit-lamp examination for corneal copper deposits (Kayser-Fleischer rings) is a very useful noninvasive diagnostic test for Wilson’s disease. They are positive in only 50% of the hepatic cases and are invariably present in neurologic cases of Wilson’s disease.
The initial treatment objective is to decrease copper levels or otherwise affect copper such that new copper toxicity is avoided. It is also desirable to prevent copper from shifting from one pool to the other while decoppering is being done. Initial copper control treatment may take 2–4 mo (65, 66).
Several years ago, we were using 150 mg elemental zinc in 6 divided doses for the treatment of sickle cell disease (SCD) patients (67, 68). We had observed that zinc was an effective antisickling drug. We observed that at this level of zinc therapy, our treatment resulted in inducing copper deficiency in these patients (69). This led Brewer et al. (65, 66) to develop zinc as an effective anticopper drug for Wilson’s disease.
Zinc competes with copper for similar binding sites, and oral zinc decreases uptake of copper very efficiently (70). Zinc may also act by induction of intestinal cell metallothionein. Metallothionein T, once induced, has a high affinity for binding copper and prevents the serosal transfer of copper into the blood. The intestinal cells turnover rapidly and take the complexed copper into the stool for final excretion. Zinc not only blocks food copper but also the copper that is endogenously excreted via salivary, gastric, and other gastrointestinal juices. Thus, zinc is effective in producing a chronic negative copper balance. Fifty milligrams of elemental zinc (as acetate) is given orally 3 times a day for the management of Wilson’s disease patients. Zinc is given in a fasting or postabsorptive state. The only side effect is that 10% of the subjects may have gastric discomfort. This is usually observed after the first morning dose and can be avoided if zinc is administered between breakfast and lunch or after dinner before going to bed.
For maintenance therapy, zinc is the drug of choice (65, 66). Relatively speaking, zinc has no toxicity and is nonteratogenic; thus, it can be given to subjects of all ages and even the pregnant women. Zinc has been approved by FDA for the treatment of Wilson’s disease patients.
Zinc and age-related macular degeneration
Age-related macular degeneration (AMD) affects nearly 25% of individuals older than 65 y of age, and late-stage disease accounts for nearly 50% of legal blindness in Europe and North America (71). Newsome et al. (72) demonstrated that concentrations of zinc are reduced in human eyes with signs of AMD and suggested that zinc deficiency may lead to oxidative stress and retinal damage.
The Age-Related Eye Disease Study Group, supported by National Eye Institute/NIH, conducted an 11-center double-blind clinical trial in patients with dry-type AMD (73). A total of 3640 participants were enrolled. Their ages ranged from 55 to 80 y, and the average follow-up period was 6.3 y. Participants were randomly assigned to receive daily orally one of the following: 1) antioxidants (vitamin C 500 mg, vitamin E 400 iu, and β-carotene 15 mg); 2) zinc 80 mg as zinc oxide and copper 2 mg as copper oxide to prevent copper deficiency induced by zinc; 3) antioxidants plus zinc; or 4) placebo.
In the group taking the antioxidant plus zinc supplements, the risk of advanced AMD developing was reduced by ∼25% and vision loss by ∼19%. In the group taking zinc alone, the risk of advanced AMD developing was reduced by ∼21% and vision loss by 11%. In the group taking the vitamins alone, the risk of advanced AMD developing was decreased by 17%, and vision loss was decreased by 10%. No significant side effects were noted in subjects who received high levels of therapeutic zinc (73). This study confirmed the antioxidant effect of zinc in humans. Interestingly, only the zinc-supplemented group showed increased longevity (74). The risk of mortality was reduced by 27% in the Age-Related Eye Disease Study Group studies in subjects 55–81 y of age who received only therapeutic zinc daily. At present, most ophthalmologists throughout the world are using zinc and vitamins as supplement for the treatment of the dry type of AMD.
Zinc supplementation in the elderly decreases the incidence of infection
The daily intake of zinc in elderly subjects in the Western world including the United States is only ∼8–10 mg, whereas the RDA is 15 mg. The elderly frequently do not eat the usual 3 meals a day and skip either breakfast or lunch. Many live alone and do not cook a proper meal for themselves. Our study in the Detroit area showed that 35% of the well-off ambulatory elderly subjects may have a deficiency of zinc based on their plasma zinc levels. Zinc deficiency and susceptibility to infections due to cell-mediated immune dysfunction have been reported to occur in the elderly (75, 76). The third NHANES (1988–1914) also found that elderly persons (older than 71 y) were at the greatest risk of inadequate zinc intake (77).
Oxidative stress and increased inflammatory cytokines have been recognized as important contributing factors for several chronic diseases attributed to aging, such as atherosclerosis and related cardiovascular disorders, mutagenesis and cancer, neurodegenerative disorders, type 2 diabetes mellitus, and Alzheimer’s disease (AD). Together, O·2-, H2O2, and OH radicals are reactive oxygen species (ROS), and excessive generation of ROS causes oxidative stress. Inflammatory cytokines such as TNF-α and IL-1β, generated by activated monocytes, are also known to generate greater levels of ROS. Chronic inflammatory processes have been implicated in high cardiovascular mortality in elderly subjects (78).
Our previous studies showed that zinc supplementation in individuals 20 to 50 y of age decreased oxidative stress markers, such as malondialdehyde (MDA), 4-hydroxyalkenals (HAEs), and 8-hydroxydeoxyguanine in the plasma; downregulated the ex vivo induction of TNF-α and IL-1β mRNA in MNCs; and provided protection against TNF-α–induced NF-κβ activation in isolated MNCs (44). We also showed previously that in the human promyelocytic leukemia cell line (HL-60), which differentiates to the monocyte and macrophage phenotype in response to PMA, zinc upregulates the expression of A20 and the binding of A20 transactivating factor to DNA, which results in the inhibition of NF-κB activation (44, 45).
Inasmuch as zinc deficiency and susceptibility to infections due to cell-mediated immune dysfunctions have been observed in the elderly, we conducted a randomized, placebo-controlled trial of zinc supplementation in 50 healthy elderly subjects (55–87 y) of both sexes and all ethnic groups from St. Patrick’s senior citizen center, Detroit, MI. One subject in zinc group dropped out on the second day; thus, we had complete data for 49 subjects (24 in the zinc group and 25 in the placebo group). Exclusion criteria were as follows: life expectancy of <8 mo, progressive neoplastic disease, severe cardiac dysfunction, significant kidney disease, significant liver disease, and subjects who were not competent mentally. Zinc supplementation consisted of 45 mg elemental zinc (as gluconate) daily for 12 mo.
A comparison of the baseline data between the younger subjects (ages 18–54, n = 31) and the elderly subjects showed that the plasma zinc was lower and the percentage of cells producing IL-1β and TNF-α and the generated cytokines were significantly higher in the elderly subjects (76). VCAMs, vascular endothelial cell adhesion molecules, and E-selectin in the plasma also were significantly higher in the elderly. IL-10 generated by T helper subset 2 cells, which is known to regulate negatively IL-2 generation from T helper subset 1 cells, was significantly higher in the elderly. A similar observation with respect to IL-10 generation by Th2 cells in the elderly has been also reported by Cakman et al. (79). The oxidative stress markers also were significantly higher in the elderly compared with the younger adults (76).
The mean incidence of infections per subject in 12 mo was significantly lower (P < 0.01) in the zinc-supplemented group (0.29 ± 0.46) than in the placebo group (1.4 ± 0.95; effect size 1.46). The plasma zinc increased, and ex vivo generation of TNF-α and IL-10 decreased significantly in the zinc group compared with the placebo group (76). Oxidative stress biomarkers in the plasma also decreased significantly in the zinc group compared with the placebo group (76).
In MNCs isolated from zinc-deficient elderly subjects, zinc supplementation increased the ex vivo PHA-induced IL-2 mRNA expression and plasma zinc concentration compared with the zinc-deficient subjects who received placebo.
Thus, our study showed that zinc supplementation (45 mg/d elemental zinc) in the elderly subjects decreased the incidence of infection by nearly 66%. After supplementation, we also observed that oxidative stress markers and the generation of inflammatory cytokines that were increased before supplementation, decreased significantly. These are highly significant effects of zinc supplementation in the elderly, and it may imply that zinc may also prove to be an excellent agent for the prevention of some of the chronic diseases.
Future implications of therapeutic use of zinc
Oxidative stress and increased generation of inflammatory cytokines have been implicated in the initiation and progression of many chronic diseases. These include atherosclerosis, diabetes mellitus type 2, neurodegenerative disorders, AD, and some malignancies. Inasmuch as zinc is not only required for cell-mediated immunity, it is also an effective antioxidant and anti-inflammatory agent, I hypothesize that zinc will prove to be an effective therapeutic agent in the management of some of these disorders. In this section, the present rationale for the use of therapeutic zinc in the management of atherosclerosis, diabetes mellitus type 2, and AD is presented.
Atherosclerosis and zinc
Atherosclerosis is a slowly progressive chronic inflammatory disease characterized by focal arterial lesions that ultimately block the blood vessels, which leads to angina, myocardial infarction, cerebrovascular ischemia and stroke, and even death (46, 78). Inflammation, oxidative stress, and/or endothelial dysfunction caused by known risk factors such as age, sex, smoking, hypertension, diabetes, and obesity are involved in the development and progression of atherosclerosis. Our previous studies showed that zinc deficiency increases the generation of inflammatory cytokines, increases oxidative stress, and induces endothelial cell dysfunction (46).
Our studies also showed that zinc deficiency may affect nearly 30%–40% of the well-off healthy ambulatory elderly subjects in the Detroit area (75, 76). In these subjects, decreased plasma zinc and increased plasma lipid peroxidation products and endothelial cell adhesion molecules compared with the healthy zinc-sufficient younger adults were observed.
In a randomized, placebo-controlled zinc supplementation trial in elderly subjects, we examined the effect of supplementation on plasma C-reactive protein (CRP), IL-6, macrophage chemoattractant protein 1, VCAM, and oxidative stress markers (46). Additionally, we examined the effect of zinc supplementation on A20, PPAR-α, and NF-κB activation in HAEC and monocytic cell lines [HL-60 and human monocytic leukemia cell line (THP-1)]. The zinc-supplemented group received 45 mg/d zinc as gluconate for 6 mo. At this level of zinc supplementation in the elderly, we have not observed any decrease in copper status (46).
The plasma zinc increased in the zinc-supplemented group, and no change was observed in the placebo group. We observed a significant decrease in lipid peroxidation product and a significant increase in antioxidant power [represented by ascorbate equivalent units (U/mL)] in the zinc-supplemented group compared with the placebo group.
Plasma high-sensitivity CRP in the zinc-supplemented group decreased significantly compared with the placebo group. Plasma IL-6, macrophage chemoattractant protein 1, secretory phospholipase A2, soluble VCAM-1, and soluble E-selectin decreased after zinc supplementation, and the mean changes before and after between the 2 groups were statistically significant (46). Plasma zinc concentrations in the elderly subjects inversely correlated with the changes in plasma concentrations of high-sensitivity CRP, VCAM-1, macrophage chemoattractant protein 1, and MDA+HAEs after 6 mo of supplementation (46).
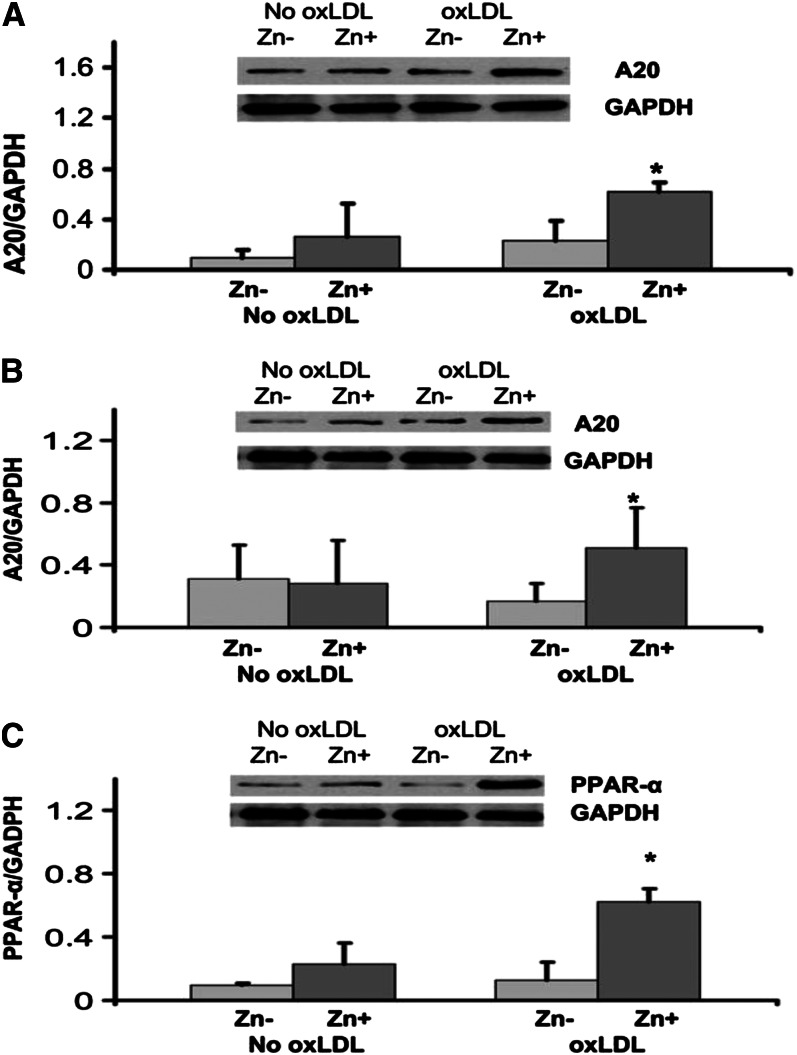
Zinc decreased the generation of TNF-α, IL-1β, VCAM-1, and MDA+HAEs in HL-60 and THP-1 and HAECs after incubation with oxidized LDL (oxLDL) for 24 h compared with zinc-deficient cells. Zinc increased A20 and PPAR-α proteins in oxLDL-stimulated THP-1 cells and HAECs compared with zinc-deficient cells.
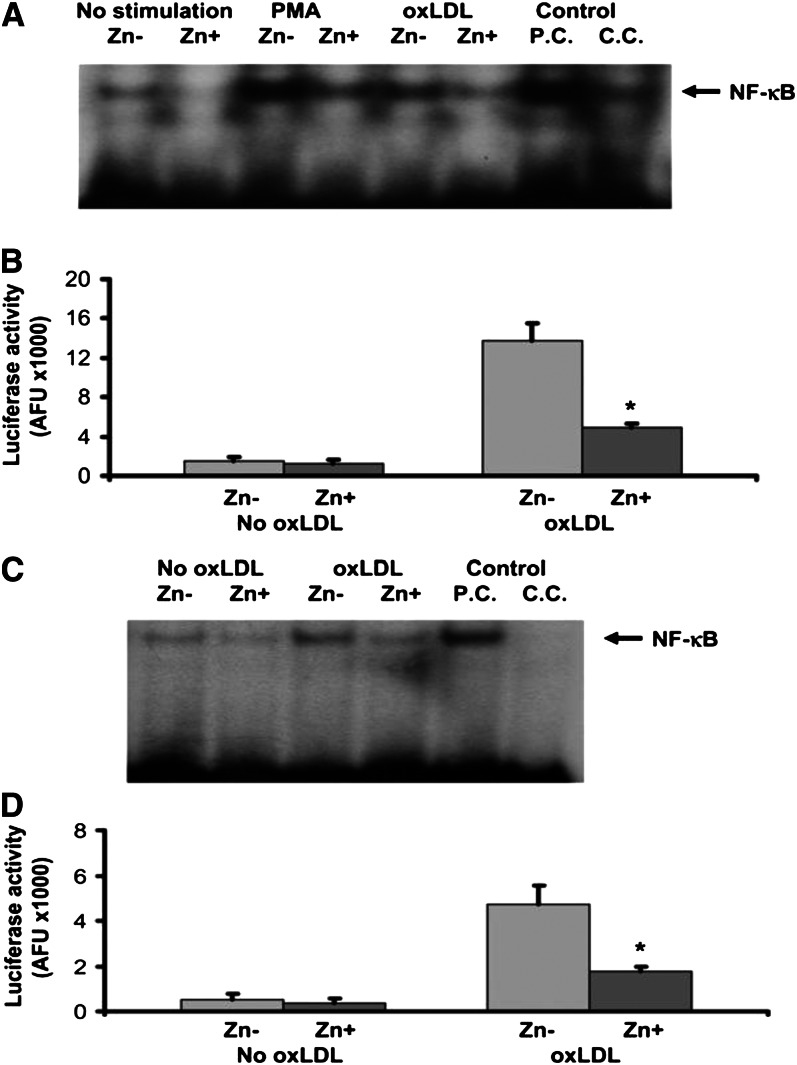
The effect of zinc on NF-κB activation in THP-1 cells and HAECs is shown in Figures 1 and 2. There was no significant difference in NF-κB activation by either NF-κB–driven luciferase reporter gene assay or electrophoretic mobility shift assay between the nonstimulated THP-1 cells and HAECs incubated in zinc-deficient and zinc-sufficient media. However, after 24 h of oxLDL stimulation, zinc-sufficient THP-1 cells and HAECs showed a significant decrease in NF-κB activation compared with zinc-deficient cells.
Figure 1.
The effect of zinc on A20 and peroxisome proliferator–activated receptor α (PPAR-α) in the human monocytic leukemia cell line (THP-1) (A) and human aortic endothelial cells (HAECs) (B and C) after oxidized LDL (oxLDL) stimulation. The cells were incubated either in zinc-deficient (Zn−, 1 μmol/L) or zinc-sufficient (Zn+, 15 μmol/L) medium for 8 d (for the THP-1) and for 6 d (for HAECs), followed by 24 h of stimulation with 50 μg oxLDL/mL. A20 and PPAR-α proteins were measured by Western blot analysis. *P < 0.05 for Zn− compared with Zn+ (Values are SD; n = 3). GADPH, glyceraldehyde 3-phosphate dehydrogenase. Reproduced with permission from (46).
Figure 2.
Effect of zinc on nuclear transcription factor κB (NF-κB) activation in the human monocytic leukemia cell line (THP-1) after oxidized LDL (oxLDL) or phorbol myristate acetate (PMA) stimulation. Zinc-deficient (Zn−) THP-1 cells and zinc-sufficient (Zn+) THP-1 cells were used for the measurement of NF-κB activation by electrophoretic mobility shift assay (EMSA) (A) and luciferase reporter gene assay (B). Effect of zinc on NF-κB activation in human aortic endothelial cells (HAECs) after oxLDL stimulation. Zn− HAECs and Zn+ HAECs were used for the measurement of NF-κB activation by EMSA (C) and luciferase reporter gene assay (D). *P < 0.05 for Zn− compared with Zn+ (Values are SD; n = 3). AFU, arbitrary fluorescent unit/β-galactosidase U/100 μg protein; P.C., positive control; C.C., competition control. Reproduced with permission from (46).
A high plasma CRP concentration is a risk factor that is independent of other risk factors such as total cholesterol, LDL, age, smoking, BMI, diabetes, and hypertension (46). CRP is a widely used marker for atherosclerosis and its clinical course, complication, and prognosis (46). In prospective studies, healthy men and women with an increased baseline level of CRP were at greater risk of coronary artery disease (46).
Our study showed that zinc supplementation (45 mg elemental zinc as gluconate) daily was effective in lowering plasma CRP concentration. This is the first documentation to show that zinc is effective in downregulating the plasma CRP level in the elderly.
The increased production of ROS and the activation of redox-dependent signaling cascades are involved in atherosclerosis (46, 78). ROS itself can initiate NF-κB–mediated transcriptional activation of inflammatory genes, thereby potentially acting as independent triggers of atherosclerosis. Zinc deficiency increased oxidative stress and zinc supplementation decreased oxidative stress in cell culture models and humans. We confirmed in this study that zinc supplementation decreased oxidative stress in elderly subjects and human vascular endothelial and monocytic cells. Thus, decreased oxidative stress by zinc may decrease the LDL oxidation and exhibit an atheroprotective effect.
NF-κB is one of the major immune response transcription factors involved in the initiation and development of atherosclerosis (46, 78). Zinc plays an important role in NF-κB activation. However, the regulation of NF-κB activation by zinc is cell specific. Zinc is required for NF-κB DNA binding in purified or recombinant NF-κB p50 protein of the T helper cell line. Additional studies also showed that zinc decreased LPS-, ROS-, or TNF-α-induced NF-κB activation in endothelial cells and cancer cells (46). We reported previously (44) that compared with controls, healthy volunteers who were supplemented with zinc (45 mg/d) had a significant decrease in TNF-α and IL-1β mRNA and TNF-α induced NF-κB DNA binding in isolated peripheral blood mononuclear cells, and zinc upregulated the expression of A-20 in HL-60 cells. In this study, we observed that zinc decreased oxLDL-induced generation of TNF-α, IL-1β, and VCAM-1, oxidative stress markers, and activation of NF-κB and increased A20 and PPAR-2 proteins in human monocytic and vascular endothelial cells. We propose that zinc inhibited NF-κB activation by inducing A20, a transactivating factor that played a role in reducing IL-1β– and TNF-α–induced NF-κB activation. A20 inhibits NF-κB signaling via TNF receptor–associated factor pathways in endothelial cells (45).
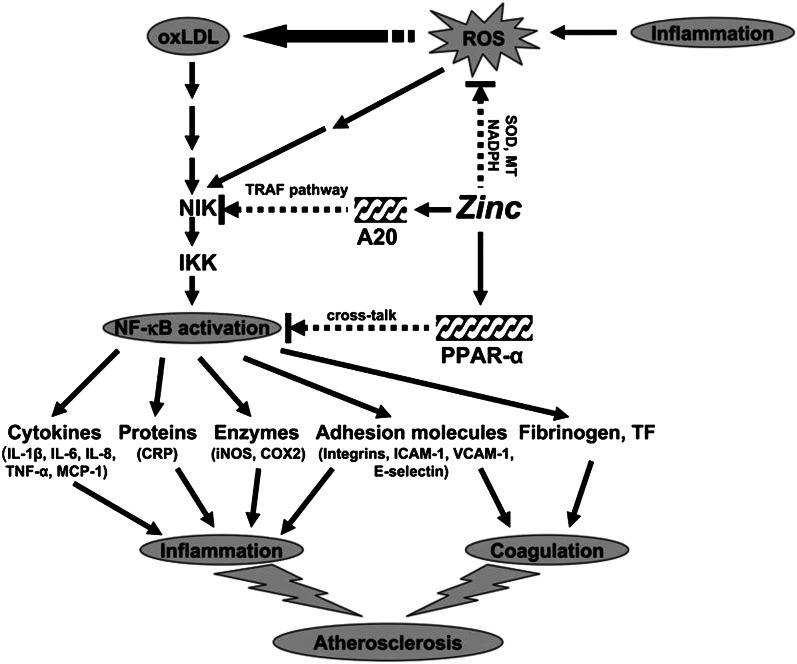
Our concept of the mechanism by which zinc may be beneficially regulating various pathways involved in the development of atherosclerosis is presented in Figure 3. Inflammation generates ROS, resulting in oxLDL. OxLDL activates the NF-κB inducible kinase/1κB kinase/NF-κB signaling pathway and upregulates the downstream target genes such as inflammatory cytokines, CRP, adhesion molecules, inducible nitric oxide synthase, cyclooxygenase 2, fibrinogen, and tissue factor. These cytokines and molecules attract blood cells and platelets leading to coagulation, which initiates the development of atherosclerosis. We showed that zinc supplementation increased the plasma concentration of antioxidant power and decreased the plasma concentration of inflammatory cytokines and lipid peroxidation biomarkers in elderly subjects.
Figure 3.
Signaling pathway for zinc prevention of atherosclerosis in monocytes/macrophages and vascular endothelial cells: a proposed hypothesis. Reactive oxygen species (ROS) induced by many stimuli modifies LDL into oxidized LDL (oxLDL) in macrophages and vascular endothelial cells. oxLDL or ROS can activate the apoptotic pathway via activation of proapoptotic enzymes and the nuclear transcription factor κB (NF-κB) pathway via NF-κB inducible kinase (NIK) activation, which eventually results in the development and progression of atherosclerosis. Zinc might have an atheroprotective function by the following mechanisms: 1) inhibition of ROS generation via metallothionein (MT), superoxide dismutase (SOD), and inhibition of NADPH oxidase and 2) downregulation of atherosclerotic cytokines/molecules such as inflammatory cytokines, adhesion molecules, inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX2), fibrinogen, and tissue factor (TF) through inhibition of NF-κB activation by A20-mediating TNF–receptor associated factor (TRAF) signaling and peroxisome proliferator–activated receptor α (PPAR-α)–mediating crosstalk signaling. The black arrows indicate upregulation; arrows with a broken line indicate downregulation or the inhibitory pathway. IKK, IκB kinase; MCP-1, macrophage chemoattractant protein 1; CRP, C-reactive protein; ICAM-1, intercellular adhesion molecule 1; VCAM-1, vascular cell adhesion molecule 1. Reproduced with permission from (46).
In view of these results, I recommend that a controlled, prospective trial of zinc supplementation in elderly subjects should be conducted to establish the atheroprotective effect of zinc. This will have an immense impact on the management of patients with coronary artery disease and patients with stroke.
Role of zinc in diabetes
Diabetes mellitus is one of the most common chronic diseases, and according to WHO, estimate nearly 300 million individuals are affected by this disorder. Diabetes is one of the major causes of blindness, increased risk of cardiovascular disorder, end-stage renal disease, and nontraumatic limb amputation (80).
Insulin, produced by the β cells of the pancreas, is essential for glucose clearance from the blood to muscle, fat, and liver cells. The hallmark of diabetes is a loss of control of glycemia due to lack of insulin, which may be relative or absolute depending on the type of diabetes.
Type 1 diabetes mellitus alone accounts for 5%–10% of all cases of diabetes. It is caused by autoimmune destruction of pancreatic β cells, resulting in virtually no production of insulin. Without insulin, carbohydrate cannot be used for energy; therefore, fats become the main intracellular source of energy. This results in generation of ketone bodies leading to ketoacidosis. Exogenous insulin administration is the main treatment for this situation.
Type 2 diabetes mellitus accounts for >90% of the cases of diabetes and is due to insulin resistance; there is no problem in insulin production initially by the β cells. Eventually, however, islet β cell function also declines, leading to overt diabetes. These patients ultimately also require exogenous insulin for treatment.
Zinc is crucial for the pancreas and the regulation of blood glucose. Insulin is stored in a crystalline form as a zinc insulin complex. Hence, the zinc concentration of the pancreatic β cells is among the highest in the body. Addition of zinc to insulin in vitro extended the duration of insulin action. In the 1930s, zinc ions were added in vitro to produce protamine zinc insulin to control the blood sugar in diabetic patients.
In the presence of zinc ions, both insulin and proinsulin dimers aggregate into hexamers containing bound zinc (80). ZnT8 mRNA and protein has been shown to be almost exclusively confined to pancreatic islets and to participate in the regulation of insulin secretion (80). ZnT8 is believed to be crucial for both zinc transport in the insulin granules and insulin crystallization, which could not occur unless zinc is present.
Zinc is a potent physiological regulator of insulin signal transduction, mainly through the inhibitory effect on protein tyrosine phosphatase 1β, the key phosphatase that dephosphorylates the insulin receptor. An adequate supply of zinc is crucial for insulin biosynthesis and storage, especially when there is hyperglycemia. In zinc-deficient states, there is a clear decrease in islet cell insulin content (80, 81). Recent studies by Jansen et al. (82) showed that zinc supplementation may be a potential treatment adjunct in type 2 diabetes because zinc also promotes insulin signaling.
The decrease in total body zinc in diabetic patients may be due to either hyperzincuria or decreased intestinal zinc absorption. Decreased zinc in plasma, lymphocytes, granulocytes, platelets, and hyperzincuria has been observed in diabetic patients (83). Zinc deficiency contributes to diabetic complications such as increased susceptibility to infections due to immune dysfunction, increased generation of inflammatory cytokines, and increased oxidative stress. In streptozotocin-induced diabetic rats, zinc supplementation (5 mg/kg zinc sulfate) attenuated diabetes-induced renal oxidative damage and inflammation and prevented the kidney from diabetes-induced proteinuria and pathological alterations (80).
A positive correlation between hyperzincuria and glycosylated hemoglobin has been observed (80, 84). Inflammatory cytokines such as IL-1β, IL-6, and TNF-α play important roles in the development and complications of type 2 diabetes. Insulin signaling interference by adipokines leads to insulin resistance, as demonstrated by serine phosphorylation of insulin receptor substrate by TNF-α (75, 78).
CRP and IL-6 were significantly increased in the diabetic group compared with the nondiabetic controls in 1 study (80, 84). In nested case-control and prospective trials, it was shown that IL-1β, IL-6, TNF-α, and CRP were elevated several years before the onset of type 2 diabetes (80, 84).
In our limited trial, we supplemented 9 Type 2 diabetes 45 mg/d elemental zinc (as gluconate) and 7 subjects received placebo for 3 mo. The plasma zinc increased significantly and HbA1 C decreased in the zinc-supplemented group (P = 0.03) Plasma IL-6, CRP, and ICAM-1 showed a statistically nonsignificant decrease in the zinc group. Obviously a large placebo-controlled trial is needed before any firm conclusion can be drawn.
A prospective study of zinc intake and risk of type 2 diabetes was assessed among 82,297 women 33–60 y of age at baseline in 1980 and followed until 2004 in the Nurses’ Health Study (85). During the 24 y of follow-up, they identified and confirmed 6030 cases of incident type 2 diabetes. In an age-adjusted analysis, intake of total zinc but not dietary zinc from food sources was significantly associated with a lower risk of type 2 diabetes. After adjustment for nondietary risk factors, including age, BMI, smoking, and other covariate, the highest quintiles of both total and dietary zinc intake were significantly associated with an ∼20% lower risk of type 2 diabetes.
At the cellular blood level, zinc increases total protein phosphorylation of the insulin receptor β subunit of both preadipocytes and adipocytes. Zinc inhibits tyrosine phosphatase 1B, leading to increased phosphorylation of the insulin receptor β subunit. An intracellular release of free zinc is a potent physiological regulator of insulin signal transduction through its inhibitory effect on tyrosine phosphatase 1B, the key phosphatase that dephosphorylates the insulin receptor (80). Thus, this evidence implicates an important role of zinc in diabetes management, and I recommend a clinical trial of zinc in this disease.
Zinc and AD
The first description of AD was published by Alois Alzheimer in 1906 (86). AD now represents the most prevalent form of dementia in modern society, accounting for 60%–80% of all dementia cases (87). Individuals with AD have a progressive cognitive decline that ultimately also erodes all higher order executive functioning. At autopsy, they exhibit a number of cardinal features within the brain. The most pronounced features are the presence of extracellular deposits known as plaques and intracellular accumulations known as neurofibrillary tangles. These 2 features are diagnostic of AD. Other gross manifestations are the thinning of the cortical gray matter, the enlargement of the ventricular spaces, and generalized atrophy of the brain (87).
The potential role of zinc in dementia was first proposed by Burnet (88). Since then, many reports have been published concerning the role of zinc in AD. The concentrations of zinc within the brain tend to increase from birth to adulthood and then remain stable throughout life. The serum/plasma zinc in AD patients has been reported to be increased, unchanged, and decreased (87, 89, 90); thus, there appears to be a large degree of variations between different study groups.
Molina et al. (91) examined 26 AD and 28 control subjects and found a significant decrease in cerebrospinal fluid zinc levels in AD patients. Consistent with this, Kapaki et al. (92) found a similar result in their study.
There is no difference in zinc level in frontal lobe tissue of brain between AD and controls. However, when these tissues were subfractionated, a significant decrease in zinc levels in the nuclear fraction (but not the mitochondrial or microsome fraction) of AD cases was observed (87). Decreased zinc levels were also seen in the neocortex, superior frontal and parietal gyri, medial temporal gyrus, thalamus, and hippocampus in AD patients (87).
Studies showed that senile plaque cores of AD patients consist primarily of β-amyloid protein (AB) derived from a larger transmembrane protein amyloid precursor protein (APP) with both zinc and copper coordinated to histidine residues located at the N-terminal end of the protein (87). It appears that a dysregulation in various metal storage and transport proteins within the AD brain may contribute to a failure in zinc homeostasis (87). There are several key proteins involved in the regulation of zinc in the brain. These include zinc transporting ZIP and ZNT and metallothionein. Furthermore, a number of these proteins such as ZNT family are found not only in the neuropil but also within the plaques (87).
APP is ubiquitously expressed. One function of APP is to participate in the maintenance of metal ion homeostasis. An abnormal expression of APP might result in dysregulation of metal ion homeostasis. Recently, it was reported that APP possesses iron-export ferroxidase activity that is inhibited by zinc (87). A disturbance in zinc homeostasis may result in decreased ferroxidase activity, resulting in iron accumulation.
The processing of APP to generate AB peptides of varying length involves different secretases, all of which appear to interact with different metals including zinc (87). After generation of AB, it too is capable of binding zinc. It appears that metals promote the AB aggregation pathway.
Another important consequence of the binding of zinc to AB is that it obscures the proteolytic cleavage site (87), thereby inhibiting its ability to be degraded by matrix metalloproteinase. Removal of the zinc with clioquinol, however, restored the sensitivity of AB degradation by matrix metalloproteinase (87).
This discussion suggests a deleterious effect of zinc binding to APP and AB; however, there are several studies that suggest a potentially protective effect of zinc under certain circumstances (87). Lovell (93) reported that AB/Zn ratios 1:0.1 and 1:0.01 result in a protective activity against AB toxicity. This effect is mediated in part by a modulation of Na+K+ATPase activity that prevents the typical calcium dyshomeostasis and cell death associated with AB toxicity (87).
Garai et al. (94) suggested that, in vitro, very low concentrations of zinc may eliminate oligomeric AB, thereby limiting the toxicity mediated by soluble oligomers. It has been suggested that zinc-AB aggregates may form so as to inhibit the reduction of Cu2+ and the production of hydrogen peroxide that arises from AB-Cu interaction (87). This represents the role of zinc as an antioxidant, which results by displacing pro-oxidant Cu2+.
Zinc as a therapeutic modality for AD.
Studies using Tg576 mice, which overexpress a mutant human form of APP and develop cerebral AB plaques (87), and ZnT3 knockout mice have suggested that zinc may hold amyloid load in a dissociable equilibrium and provide strong support for a role of synaptic zinc in the metabolism of AB. The study also showed a reduction in cerebrovascular amyloid loads in these animals. Genetic ablation of ZnT3 may generate a phenocopy for the cognitive and synaptic deficits present in transgenic mouse models of AD (87).
Recently, a number of metal-modulatory compounds have been developed that normalize cerebral zinc ion homeostasis (87). Clioquinol treatment in aged Tg 2576 mice showed a significant reduction in plaque burden parallel with a shift in brain content of AB toward more soluble species (87). Analysis of cerebral metal levels did not reveal a reduction in metals content but showed a significant increase in copper and zinc on treatment. These data obtained after clioquinol and PBT2 (a metal-modulatory compound) treatment thus showed that there is a redistribution of metals in the brain and do not chelate metals out of the system (87).
PBT2 showed profound effects in transgenic mouse models of AD (87). It was shown that the administration of PBT2 significantly decreased both insoluble and soluble burden, decreased levels of phosphorylated tau, increased levels of synaptophysin (a surrogate marker of synapses), and rapidly improved learning and memory performances in the Morris water maze (87). This drug is currently undergoing a clinical trial for the treatment of AD patients (87).
A placebo-controlled trial of zinc supplementation was conducted for 6 mo recently in AD patients in Florida (95). The subjects received 150 mg/d of elemental zinc away from food. The new zinc product, Reazin, was developed by Adeona Pharmaceuticals, Ann Arbor, MI. The product releases zinc slowly in the stomach and later in the small intestine, which maintained a sustained elevation of plasma zinc throughout the day. The sum of scores on 3 cognitive measures, Alzheimer’s Disease Assessment Scale-Cognitive Subscale, Mini-Mental State Examination, and Clinical Dementia Rating scale, were assessed.
The study showed that zinc had stabilized cognition, whereas the placebo group continued to deteriorate. In patients age 70 y and older, the study showed a statistically significant effect of zinc supplementation on cognitive function.
Recent studies showed that neuronal stress and consequent overexpression of proinflammatory proteins are the likely instigators of neuropathological changes, including both plaque and tangle formation in AD patients (96). It is believed that microglia cells are essentially the immune cells of the brain. It was shown that neurons were activating microglia to release IL-1, which in turn activated astrocytes and caused them to release S100, a soluble astrocyte Ca-binding protein with proinflammatory effects. Thus, it appears that IL-1 and S100 are associated with AD (96). IL-1 was shown to upregulate the production of βAPP in cord blood and brain; thus, excess inflammatory cytokine IL-1 was a driving force in neurodegeneration and genesis of amyloid plaques (96).
Brain with IL-1 pellets had elevated levels of the mRNA of a phosphorylating kinase protein mitogen-activated protein kinase 38. It was shown that IL-1 was driving both the production of tau protein and its hyperphosphorylation via IL-1 induction of a specific kinase: mitogen-activated protein kinase p38 (96). IL-1 may also contribute to the memory deficits in AD by decreasing the levels of neurotransmitter acetylcholine, a decrease often seen in AD patients. It was observed that the enzyme acetyl cholinesterase, which degrades acetylcholine, is upregulated by IL-1 (96).
We reported in the past that zinc deficiency in humans and in cell culture models activates macrophages and monocytes by increasing oxidative stress and via upregulation of NF-κB activation generates mRNA of IL-1β and the cytokine protein (37, 44, 46). I hypothesize that similar phenomenon may be taking place in zinc-deficient elderly subjects such that microglia cells are being oxidatively stressed due to zinc deficiency leading to upregulation of IL-1β in brain cells. Increased IL-1β in turn could upregulate the generation of AB and tau proteins and hyperphosphorylation of tau protein, leading to formation of neurofibrils and damage to neuronal cells. If this hypothesis is correct, one would see an effective prevention of AD in zinc-deficient elderly patients who are supplemented with zinc (45 mg/d elemental zinc) to correct their deficiency. This hypothesis needs to be tested. If correct, zinc supplementation may prove to be a very important therapeutic adjunct of AD.
Acknowledgments
The sole author had responsibility for all parts of the manuscript.
Footnotes
Abbreviations used: AB, β amyloid protein; AD, Alzheimer’s disease; AE, acrodermatitis enteropathica; APP, amyloid precursor protein; CRP, C-reactive protein; DC, dendritic cells; HAE, 4-hydroxyalkenal; HAEC, human vascular endothelial cell; HL-60, human promyelocytic leukemia cell line; ICAM-1, intercellular adhesion molecule 1; IFN, interferon; IGF-1, insulin-like growth factor 1; MDA, malondialdehyde; MNC, mononuclear cell; NF-κB, nuclear factor κB; oxLDL, oxidized LDL; PHA-P, phytohemagglutinin P; PMA, phorbol-12 myristate 13 acetate; ra, receptor antagonist; RDA, recommended daily allowance; ROS, reactive oxygen species; SCD, sickle cell disease; sIL-1 ra, soluble interleukin-1 receptor antagonist; THP-1, human monocytic leukemia cell line; TK, deoxythymidine kinase; VCAM-1, vascular cell adhesion molecule 1.
Literature Cited
- 1.Raulin J. Chemical studies on vegetation [in French]. Ann Sci Nat. 1869;11:93–9. [Google Scholar]
- 2.Todd WR, Elvehjem CA, Hart EB. Zinc in the nutrition of the rat. Am J Physiol. 1933;107:146–56 [Google Scholar]
- 3.Prasad AS, Halsted JA, Nadimi M. Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism, and geophagia. Am J Med. 1961;31:532–46 [DOI] [PubMed] [Google Scholar]
- 4.Prasad AS, Miale A, Farid Z, Schulert A, Sandstead HH. Zinc metabolism in patients with the syndrome of iron deficiency anemia, hypogonadism and dwarfism. J Lab Clin Med. 1963;61:537–49 [PubMed] [Google Scholar]
- 5.Sandstead HH, Prasad AS, Schulert AR, Farid Z, Miale A, Jr, Bassily S, Darby WJ. Human zinc deficiency, endocrine manifestations and response to treatment. Am J Clin Nutr. 1967;20:422–42 [DOI] [PubMed] [Google Scholar]
- 6.Prasad AS, Miale A, Farid Z, Sandstead HH, Schulert AR, Darby WJ. Biochemical studies on dwarfism, hypogonadism, and anemia. AMA Arch Intern Med. 1963;111:407–28 [DOI] [PubMed] [Google Scholar]
- 7.National Academy of Sciences. Trace elements: zinc. In: Recommended Dietary Allowances. 8th rev. ed. Washington, DC: National Academy of Sciences; 1974. p. 99–101.
- 8.Guidelines for essential trace Element preparation for parenteral use. A statement by an expert panel. AMA Department of Foods and Nutrition. JAMA. 1979;241:2051–4 [PubMed] [Google Scholar]
- 9.Eggleton WGE. The zinc content of epidermal structures in beriberi. Biochem J. 1939;33:403–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eggleton WGE. The zinc and copper contents of the organs and tissues of Chinese subjects. Biochem J. 1940;34:991–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lutz RE. The normal occurrence of zinc in biological materials: a review of the literature, and a study of the normal distribution of zinc in the rat, cat, and man. J Industr Hyg. 1926;7:177–207 [Google Scholar]
- 12.Bartholomay AF, Vallee BL, Wacker WEC, Robin ED. Zinc metabolism in hepatic dysfunction - serum zinc concentrations in Laennec’s cirrhosis and their validation by sequential analysis. N Engl J Med. 1956;255:403–8 [DOI] [PubMed] [Google Scholar]
- 13.McCance RA, Widdowson EM. The absorption and excretion of zinc. Biochem J. 1942;36:692–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vikbladh I. Studies on zinc in blood. Scand J Clin Lab Invest. 1950;2:143–8 [DOI] [PubMed] [Google Scholar]
- 15.Vikbladh I. Studies on zinc in blood. Scand J Clin Lab Invest. 1951;3:(Suppl 2):1–74 [PubMed] [Google Scholar]
- 16.Caggiano V, Schnitzler R, Strauss W, Baker RK, Carter AC, Josephson AS, Wallach S. Zinc deficiency in a patient with retarded growth, hypogonadism, hypogammaglobulinemia, and chronic infection. Am J Med Sci. 1969;257:305–19 [DOI] [PubMed] [Google Scholar]
- 17.Hambidge KM, Hambidge C, Jacobs M, Brown JD. Low levels of zinc in hair, anorexia, poor growth and hypogeusia in children. Pediatr Res. 1972;6:868–74 [DOI] [PubMed] [Google Scholar]
- 18.Halsted JA, Ronaghy HA, Abadi P, Haghshenass M, Amirhakimi GH, Barakat RM, Reinhold JG. Zinc deficiency in man: the Shiraz experiment. Am J Med. 1972;53:277–84 [DOI] [PubMed] [Google Scholar]
- 19.Barnes PM, Moynahan EJ. Zinc deficiency in acrodermatitis enteropathica. Proc R Soc Med. 1973;66:327–9 [PMC free article] [PubMed] [Google Scholar]
- 20.Wang K, Zhou B, Kuo YM, Zemansky J, Gitschier J. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am J Hum Genet. 2002;71:66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prasad AS. Biochemistry of zinc. New York: Plenum Press; 1993.
- 22.Cavdar AO, Babacan E, Arcasoy A, Ertein U. Effect of nutrition on serum zinc concentration during pregnancy in Turkish women. Am J Clin Nutr. 1980;33:542–4 [DOI] [PubMed] [Google Scholar]
- 23.Kay RG, Tasman-Jones C. Zinc deficiency and intravenous feeding. Lancet. 1975;2:605–6 [DOI] [PubMed] [Google Scholar]
- 24.Okada A, Takagi Y, Itakura T, Satani M, Manabe H. Skin lesions during intravenous hyperalimentation: Zinc deficiency. Surgery. 1976;80:629–35 [PubMed] [Google Scholar]
- 25.Arakawa T, Tamara T, Igarashi Y. Zinc deficiency in two infants during parenteral alimentation for diarrhea. Am J Clin Nutr. 1976;29:197–204 [DOI] [PubMed] [Google Scholar]
- 26.Obladen M, Loui A, Kampmann W, Renz H. Zinc deficiency in rapidly growing preterm infants. Acta Paediatr. 1998;87:685–91 [DOI] [PubMed] [Google Scholar]
- 27.Klingberg WG, Prasad AS, Oberleas D. Zinc deficiency following penicillamine therapy. In: Prasad AS, editor. Trace elements in human health and disease, vol. 1. New York: Academic Press; 1976. p. 51–65.
- 28.MacDonald RS. The role of zinc in growth and cell proliferation. J Nutr. 2000;130(Suppl):1500S–8S [DOI] [PubMed] [Google Scholar]
- 29.Ohisson C, Bengtsson BA, Isaksson OG, Andreassen TT, Slootweg MC. Growth hormone and bone. Endocrinol Rev. 1998;19:55–79 [DOI] [PubMed] [Google Scholar]
- 30.Cossack ZT. Decline in somatomedin-C, insulin-like growth factor-1, with experimentally induced zinc deficiency in human subjects. Clin Nutr. 1991;10:284–91 [DOI] [PubMed] [Google Scholar]
- 31.Ninh NX, Thissen JP, Maiter D, Adam E, Mulumba N, Ketelsiegers JM. Reduced liver insulin-like growth factor-1 gene expression in young zinc-deprived rats in associated with a decrease in liver growth hormone (GH) receptors and serum GH-binding protein. J Endocrinol. 1995;144:449–56 [DOI] [PubMed] [Google Scholar]
- 32.Wilson M, Hogstrand C, Maret W. Picomolar concentrations of free zinc (II) ions regulate receptor protein tyrosine phosphatase beta activity. J Biol Chem. 2012;287:9322–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacDonald RS, Wollard-Biddle LC, Browning JD, Thornton WH, O’Dell BL. Zinc deprivation of murine 3T3 cells by use of dieth-ylenetrinitrilopentaacetate impairs DNA synthesis upon stimulation with insulin-like growth factor-1 (IGF-I). J Nutr. 1998;128:1600–5 [DOI] [PubMed] [Google Scholar]
- 34.Prasad AS, Beck FW, Endre L, Handschu W, Kukuruga M, Kumar G. Zinc deficiency affects cell cycle and deoxythymidine kinase (TK) gene expression in HUT-78 cells. J Lab Clin Med. 1996;128:51–60 [DOI] [PubMed] [Google Scholar]
- 35.Prasad AS, Rabbani P, Abbasi A, Bowersox E, Spivey-Fox MR. Experimental zinc deficiency in humans. Ann Intern Med. 1978;89:483–90 [DOI] [PubMed] [Google Scholar]
- 36.Beck FW, Kaplan J, Fine N, Handschu W, Prasad AS. Decreased expression of CD73 (ecto-5′-nucleotidase) in the CD8+ subset is associated with zinc deficiency in human patients. J Lab Clin Med. 1997;130:147–56 [DOI] [PubMed] [Google Scholar]
- 37.Beck FW, Prasad AS, Kaplan J, Fitzgerald JT, Brewer GJ. Changes in cytokine production and T cell subpopulations in experimentally induced zinc deficient humans. Am J Physiol Endocrinol Metab. 1997;272:1002–7 [DOI] [PubMed] [Google Scholar]
- 38.Prasad AS, Meftah S, Abdallah J, Kaplan J, Brewer GJ, Bach JF, Dardenne M. Serum thymulin in human zinc deficiency. J Clin Invest. 1988;82:1202–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirano T, Murakami M, Fukada T, Nishida K, Yamasaki S, Suzuki T. Roles of zinc and zinc signaling in immunity: zinc as an intracellular signaling molecule. Adv Immunol. 2008;97:149–76 [DOI] [PubMed] [Google Scholar]
- 40.Kitamura H, Morikawa H, Kamon H, Iguchi M, Hojyo S, Fukada T, Yamashita S, Kaisho T, Akiron S, Murakami M, et al. Toll-like receptor-mediated regulation of zinc homeostasis influences dentritic cell function. Nat Immunol. 2006;7:971–7 [DOI] [PubMed] [Google Scholar]
- 41.Haase H, Rink L. Signal transduction in monocytes: the role of zinc ions. Biometals. 2007;20:579–85 [DOI] [PubMed] [Google Scholar]
- 42.Rosenkranz E, Prasad AS, Rink L. Immunobiology and hematology of zinc. In: Rink L, editor. Zinc in human health. Amsterdam, the Netherlands: IOS Press, 2011. p. 195–233.
- 43.Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68:447S–63S [DOI] [PubMed] [Google Scholar]
- 44.Prasad AS, Bao B, Beck FWJ, Kucuk O, Sarkar FH. Antioxidant effect of zinc in humans. Free Radic Biol Med. 2004;37:1182–90 [DOI] [PubMed] [Google Scholar]
- 45.Prasad AS, Bao B, Beck FWJ, Sarkar FH. Zinc-suppressed inflammatory cytokines by induction of A20-medated inhibition of nuclear factor-κB. Nutrition. 2011;27:816–23 [DOI] [PubMed] [Google Scholar]
- 46.Bao B, Prasad AS, Beck FWJ, Fitzgerald JT, Snell D, Bao GW, Singh T, Cardozo LJ. Zinc decreases C-Reactive protein, lipid peroxidation, and implication of zinc as an atheroprotective agent. Am J Clin Nutr. 2010;91:1634–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bao B, Prasad AS, Beck WJ, Bao GW, Singh T, Ali S, Sarkar FH. Intracellular free zinc up-regulates IFN-γ and T-bet essential for Th1 differentiation in Con-A stimulated HUT-78 cells. BBRC. 2011;407:703–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacMahon RA, Parker ML, McKinnon M. Zinc treatment in malabsorption. Med J Aust. 1968;2:210–2 [DOI] [PubMed] [Google Scholar]
- 49.Rabbani P, Prasad AS. Plasma ammonia and liver ornithine transcarbamoylase activity in zinc-deficient rats. Am J Physiol. 1978;235:E203–6 [DOI] [PubMed] [Google Scholar]
- 50.Brody MS, Steinberg JR, Svinger BA, Luecke RW. Increased purine nucleotide cycle activity associated with dietary zinc deficiency. Biochem Biophys Res Commun. 1977;78:144–50 [DOI] [PubMed] [Google Scholar]
- 51.Grungreiff K, Reinhold D. Zinc and the liver. In: Rink L, editor. Zinc in human health. Amsterdam, the Netherlands: IOS Press; 2011. p. 473–492.
- 52.Mahajan SK, Prasad AS, Rabbani P, Briggs WA, McDonald FD. Zinc metabolism in uremia. J Lab Clin Med. 1979;94:693–8 [PubMed] [Google Scholar]
- 53.Mahajan SK, Abbasi AA, Prasad AS, Rabbani P, Briggs WA, McDonald FD. Effect of oral zinc therapy on gonadal function in hemodialysis patients. Ann Intern Med. 1982;97:357–61 [DOI] [PubMed] [Google Scholar]
- 54.Prasad AS, Beck FWJ, Kaplan J, Chandrasekar PH, Ortega J, Fitzgerald JT, Swerdlow P. Effect of zinc supplementation on incidence of infections and hospital admissions in sickle cell disease (SCD). Am J Hematol. 1999;61:194–202 [DOI] [PubMed] [Google Scholar]
- 55.Bao B, Prasad AS, Beck FWJ, Snell D, Sunega A, Sarkar FH, Doshi N, Fitzgerald JT, Swerdlow P. Zinc supplementation decreased oxidative stress, incidence of infection and generation of inflammatory cytokines in sickle cell disease patients. Transl Res. 2008;152:67–80 [DOI] [PubMed] [Google Scholar]
- 56.Sazawal S, Black RE, Bhan MK, Bhandari N, Sinha A, Jalla S. Zinc supplementation in young children with acute diarrhea in India. N Engl J Med. 1995;333:839–44 [DOI] [PubMed] [Google Scholar]
- 57.Fisher Walker CL, Lamberti L, Roth D, Black RE. In: Rink L, editor. Zinc in human health. Amsterdam, the Netherlands: IOS Press; 2011. p. 234–53.
- 58.Bhatnagar S, Wadlora N, Areja S, Lodha R, Kabra SK, Natcha UCM, Sommerfelt H, Dutta AK, Chandra J, Rath B, et al. Zinc as adjunct treatment in infants aged between 1 and 120 d with probable serious bacterial infection: a randomized, double-blind, placebo controlled trial. Lancet. 2012;379:2072–8 [DOI] [PubMed] [Google Scholar]
- 59.Prasad AS, Fitzgerald JT, Bao B, Beck WJ, Chandrasekar PH. Duration of symptoms and plasma cytokine levels in patients with the common cold treated with zinc acetate. Ann Intern Med. 2000;133:245–52 [DOI] [PubMed] [Google Scholar]
- 60.Prasad AS, Beck FWJ, Bao B, Snell D, Fitzgerald T. Duration and severity of symptoms and levels of plasma interleukin-1 receptor antagonist, soluble tumor necrosis factor receptor, and adhesion molecule in patients with common cold treated with zinc acetate. J Infect Dis. 2008;197:795–802 [DOI] [PubMed] [Google Scholar]
- 61.Eby GA, Davis DR, Halcomb WW. Reduction in duration of common cold by zinc gluconate lozenges in a double-blind study. Antimicrob Agents Chemother. 1984;25:20–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh M, Das R. Zinc for the common cold. Cochrane Syst Rev. 2011;(2): 1–58. [DOI] [PubMed]
- 63.Hemilä H. Zinc lozenges may shorten the duration of colds: a systematic review. Open Respir Med J. 2011;5:51–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Science M, Johnston J, Roth DE, Guyatt G, Loeb M. Zinc for the treatment of the common cold: a systematic review and meta-analysis of randomized control trials. CMAJ. 2012;184:E551–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brewer GJ, Yuzbasiyan-Gurkan V. Wilson disease. Medicine. 1992;71:139–64 [DOI] [PubMed] [Google Scholar]
- 66.Brewer GJ. Practical recommendations and new therapies for Wilson’s disease. Drugs. 1995;50:240–9 [DOI] [PubMed] [Google Scholar]
- 67.Brewer GJ, Schoomaker EB, Leichtman DA, Kruckleberg WC, Brewer LF, Myers N. The uses of pharmacologic doses of zinc in the treatment of sickle cell anemia. In: Brewer GJ, Prasad AS, editors. Zinc metabolism: current aspects in health and disease. New York: Allan R. Liss; 1977. p. 241–58.
- 68.Brewer GJ, Hill GM, Prasad AS, Cossack ZT, Rabbani P. Oral zinc therapy for Wilson's disease. Ann Intern Med. 1983;99:314–9 [DOI] [PubMed] [Google Scholar]
- 69.Prasad AS, Brewer GJ, Schoomaker EB, Rabbani P. Hypocupremia induced by zinc therapy in adults. JAMA. 1978;240:2166–8 [PubMed] [Google Scholar]
- 70.Hall AC, Young BW, Bremner I. Intestinal metallothionein and the mutual antagonism between copper and zinc in the rat. J Inorg Biochem. 1979;11:57–66 [DOI] [PubMed] [Google Scholar]
- 71.Barzegar-Befroet N, Cahyaki S, Fango A, Peto T, Lengeyel I. Zinc and eye disease. In: Rink L, editor. Zinc and human health. Amsterdam, the Netherlands: IOS Press, 2011. p. 530–53.
- 72.Newsome DA, Miceli MV, Tats DJ, Alcock NW, Oliver PD. Zinc content of human retinal pigment epithelium decreases with age and macular degeneration but superoxide dismutase activity increases. J Trace Elem Exp Med. 1996;8:193–9 [Google Scholar]
- 73.Age-Related Eye Disease Study Research Group A randomized, placebo controlled, clinical trial of high-dose supplemented with vitamins C and E, beta-carotene, for age-related macular degeneration and vision loss. AREDS Report No. 8. Arch Ophthalmol. 2001;119:1417–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clemons TE. Association of mortality with ocular disorders and an intervention of high dose antioxidants and zinc in the age-related eye disease study. Arch Ophthalmol. 2004;122:716–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prasad AS, Fitzgerald JT, Hess JW, Kaplan J, Pelen F, Dardenne M. Zinc deficiency in the elderly patients. Nutrition. 1993;9:218–24 [PubMed] [Google Scholar]
- 76.Prasad AS, Beck FWJ, Bao B, Fitzgerald JT, Snell DC, Steinberg JD, Cardozo LJ. Zinc supplementation decreases incidence of infections in the elderly: Effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr. 2007;85:837–44 [DOI] [PubMed] [Google Scholar]
- 77.Briefel RR, Bialostosky K, Kennedy-Stephenson J, McDowell MA, Ervin RB, Wright JD. Zinc intake of US population Findings from the Third National Health and Nutrition Survey 1988–1994. J Nutr. 2000;130(5S Suppl):1367S–73S [DOI] [PubMed] [Google Scholar]
- 78.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74 [DOI] [PubMed] [Google Scholar]
- 79.Cakman I, Rohwer J, Rudolf MS, Kirchner H, Rink L. Dysregulation between Th1 and Th2 subpopulations in the elderly. Mech Ageing Dev. 1996;87:197–209 [DOI] [PubMed] [Google Scholar]
- 80.Chimienti F, Rutter GA, Wheeler MB, Wijesekara N. Zinc and diabetes. In: Zinc in human health. Rink L (ed) IOS Press, Netherlands. 2011, p. 493–513.
- 81.Sakurai H, Adachi Y. The pharmacology of the insulinomimetic effect of zinc complexes. Biometals. 2005;18:319–23 [DOI] [PubMed] [Google Scholar]
- 82.Jansen J, Rosenkranz E, Overbeck S, Warmuth S, Mocchegiani E, Giacconi R, Weiskirchen R, Karges W, Rink L. Disturbed zinc homeostasis in diabetic patients by in vitro and in vivo analysis of insulinomimetic activity of zinc. J Nutr Biochem. 2012;23:1458–66 [DOI] [PubMed] [Google Scholar]
- 83.Pai LH, Prasad AS. Cellular zinc in patients with diabetes mellitus. Nutr Res. 1988;8:889–97 [Google Scholar]
- 84.Stephens JM, Lee J, Pilch PF. Tumor necrosis factor-α induced insulin resistance in 3T3–L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT-4 expression without a loss of insulin receptor-mediated signal transduction. J Biol Chem. 1997;272:971–6 [DOI] [PubMed] [Google Scholar]
- 85.Sun Q, Van Dam RM, Willett WC, Hu FB. Prospective study of zinc intake and risk of type 2 diabetes in women. Diabetes Care. 2009;32:629–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Small DH, Cappai R. Alois Alzheimer and Alzheimer’s disease: a centennial perspective. J Neurochem. 1906;99:708–10 [DOI] [PubMed] [Google Scholar]
- 87.Adlard PA, Bush A. Zinc and Alzheimer’s disease. In: Rink L, editor. Zinc in human health. Amsterdam, the Netherlands: IOS Press; 2011. p. 417–31.
- 88.Burnet FM. A possible role of zinc in the pathology of dementia. Lancet. 1981;1:186–8 [DOI] [PubMed] [Google Scholar]
- 89.González C, Martin T, Cacho J, Brenas MT, Arroyo T, Garcia-Berrocal B, Navajo JA, Gonzalez-Buitrago JM. Serum zinc, copper, insulin and lipids in Alzheimer’s disease epsilon 4 apolipoprotein E allele carrier. Eur J Clin Invest. 1999;29:637–42 [DOI] [PubMed] [Google Scholar]
- 90.Dong J, Robertson JD, Markesbery WR, Lovell MA. Serum zinc in the progression of Alzheimer’s disease. J Alzheimers Dis. 2008;15:443–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Molina JA, Jimenez-Jimenez FJ, Aguilar MV, Meseguer I, Mateos-Vega CJ, Gonzalez-Munoz MJ, de Bustos F, Porta J, Orti-Pareja M, Zurdo M, et al. Cerebrospinal fluid levels of transition metal in patients with Alzheimer’s disease. J Neural Transm. 1998;105:479–88 [DOI] [PubMed] [Google Scholar]
- 92.Kapaki E, Segditsa J, Papageorgiou C. Zinc, copper and magnesium concentration in serum and CSF of patients with neurological disorders. Acta Neurol Scand. 1989;79:373–8 [DOI] [PubMed] [Google Scholar]
- 93.Lovell MA. A potential role for alterations of zinc and zinc transport proteins in the progression of Alzheimer’s disease. J Alzheimers Dis. 2009;16:471–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garai K, Sengupta P, Sahoo B, Maiti S. Selective destabilization of soluble amyloid beta oligomers by divalent metal ions. Biochem Biophys Res Commun. 2006;345:210–5 [DOI] [PubMed] [Google Scholar]
- 95.Brewer GJ. Copper excess, zinc deficiency, and cognition loss in Alzheimer’s disease. Biofactors. 2012;38:107–13 [DOI] [PubMed] [Google Scholar]
- 96.Griffin W, Sue T. What causes Alzheimer's? The Scientist. 2011;25:36–40 [Google Scholar]