Abstract
What do the Atkins Diet and the traditional Japanese diet have in common? The Atkins Diet is low in carbohydrate and usually high in fat; the Japanese diet is high in carbohydrate and usually low in fat. Yet both work to promote weight loss. One commonality of both diets is that they both eliminate the monosaccharide fructose. Sucrose (table sugar) and its synthetic sister high fructose corn syrup consist of 2 molecules, glucose and fructose. Glucose is the molecule that when polymerized forms starch, which has a high glycemic index, generates an insulin response, and is not particularly sweet. Fructose is found in fruit, does not generate an insulin response, and is very sweet. Fructose consumption has increased worldwide, paralleling the obesity and chronic metabolic disease pandemic. Sugar (i.e., fructose-containing mixtures) has been vilified by nutritionists for ages as a source of “empty calories,” no different from any other empty calorie. However, fructose is unlike glucose. In the hypercaloric glycogen-replete state, intermediary metabolites from fructose metabolism overwhelm hepatic mitochondrial capacity, which promotes de novo lipogenesis and leads to hepatic insulin resistance, which drives chronic metabolic disease. Fructose also promotes reactive oxygen species formation, which leads to cellular dysfunction and aging, and promotes changes in the brain’s reward system, which drives excessive consumption. Thus, fructose can exert detrimental health effects beyond its calories and in ways that mimic those of ethanol, its metabolic cousin. Indeed, the only distinction is that because fructose is not metabolized in the central nervous system, it does not exert the acute neuronal depression experienced by those imbibing ethanol. These metabolic and hedonic analogies argue that fructose should be thought of as “alcohol without the buzz.”
Introduction
We are in the midst of a global pandemic of chronic metabolic disease, 30 y in the making. The UN Secretary General in 2011 declared that metabolic syndrome (type 2 diabetes, hypertension, dyslipidemia, heart disease) and other noncommunicable diseases (e.g., cancer, dementia) are now a greater threat to both the developed and developing worlds than is acute infectious disease, including HIV (1). Most people blame obesity as the driver of these other diseases; however, 20% of obese subjects are metabolically normal, whereas as many as 40% of normal-weight people manifest specific components of metabolic syndrome (2–4). Obesity is not the cause of metabolic syndrome; rather, it is a marker for the metabolic dysfunction that is occurring worldwide. Furthermore, there are now >30% more obese people on the planet than those who are malnourished. Two decades ago, it was the opposite. Is it really possible, even in the most impoverished countries, that so many people became gluttons and sloths in such a short period of time? The ever-onward progression of these diseases in countries that also witness severe malnutrition is more reminiscent of an exposure than it is an alteration in behavior.
But, aside from caloric overconsumption, what kind of exposure could cause metabolic syndrome? One specific foodstuff that has increased in all countries during the pandemic and has the capacity to promote chronic metabolic disease is the monosaccharide fructose. Fructose is half of sucrose (cane or beet sugar) and 55% of high-fructose corn syrup (HFCS)4. In 1 century, Americans have increased fructose consumption from ∼15 g/d (4% of total energy) to 75 g/d (12% of total energy) (5). Currently, per capita consumption of fructose or fructose-containing disaccharides is at ∼130 lb/y (almost 60 kg/y) or 6.5 oz/d for the average American. Although America is the greatest sugar consumer, other countries are not far behind (6).
Although most people consider fructose, and sugar in general, as “empty calories,” there is nothing empty about these calories. First, there is not 1 human biochemical reaction that requires dietary fructose. The only place in the body that fructose is of physiologic import is in semen, and the fructose is manufactured de novo from glucose using the aldose reductase/sorbitol pathway (7). In other words, fructose is a vestigial nutrient for humans, held over from the differentiation between plants and animals. Indeed, patients with hereditary fructose intolerance, who are missing the enzyme fructose-1-phosphate aldolase B, and cannot consume fructose lest they become hypoglycemic, do not only have fewer dental caries (8), but they are quite healthy provided they continue to restrict their fructose exposure (9, 10).
Second, fructose exerts 3 different negative impacts on human metabolism, each of which is exclusive of its calories. Most people compare fructose with its isomer glucose, which is so essential for life that your liver will produce it when it is in short supply via the process of gluconeogenesis. Although fructose is an energy source, the actions of fructose on the body more closely resemble those of ethanol (grain alcohol), another nonessential energy source. This paper compares the metabolic actions of fructose with those of glucose and ethanol to make the point that fructose is “alcohol without the buzz.”
Hepatic insulin resistance and metabolic syndrome
The pathogenesis of metabolic syndrome remains a puzzle (11, 12). One reason for this puzzle is trying to explain the phenomenon of “selective hepatic insulin resistance” (13). Insulin normally exerts its effects on hepatic energy metabolism via 2 metabolic pathways. Insulin’s effects on maintaining euglycemia occurs through phosphorylation of the forkhead protein O1 (FoxO1), thus restricting it from entering the nucleus and preventing transcription of various gluconeogenic enzymes (14, 15). Insulin also activates the lipogenic pathway by stimulating sterol regulatory element binding protein 1c (SREBP-1c), which activates the enzymes of de novo lipogenesis (DNL) to turn excess mitochondrial energy substrate into fatty acids, which are then linked to apolipoprotein B100 and packaged into VLDL for hepatic export.
However, metabolic syndrome does not result from complete hepatic insulin resistance (16) because this would result in hyperglycemia (lack of FoxO1 phosphorylation) and low serum VLDL (lack of SREBP-1c activation). Rather, metabolic syndrome results from “selective” hepatic insulin resistance in which FoxO1 is not phosphorylated yet SREBP-1c is still activated to promote triglyceride synthesis and dyslipidemia. If there is only 1 insulin receptor, how can it activate 1 pathway and not the other (17)? To parse this dichotomy, the hepatic metabolism of glucose, ethanol, and fructose are considered in turn.
Hepatic glucose metabolism
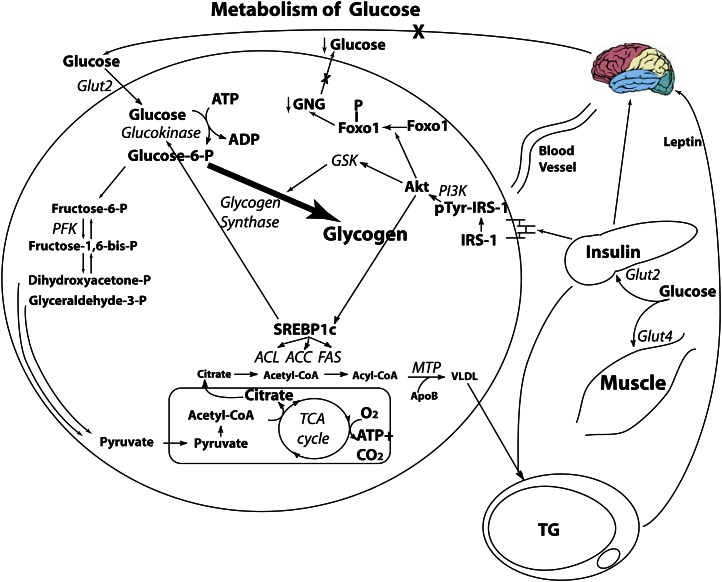
Glucose is unique in that every prokaryotic and eukaryotic cell on the planet has the capacity to use glucose for energy. After oral consumption of glucose (Fig. 1), the bolus enters the portal circulation. Approximately 20% of the glucose bolus enters the liver via the Glut2 glucose transporter, which is insulin independent (18). The rest (80%) appears in the peripheral circulation plasma glucose levels increase, and insulin is released by the β-cells in response. Insulin binds to its liver receptor, which promotes the tyrosine phosphorylation of insulin receptor substrate-1 (IRS-1), which increases the activity of phosphatidylinositol 3-kinase, inducing the transcription factor Akt responsible for insulin’s intracellular metabolic effects. 1) Akt phosphorylates FoxO1, downregulating gluconeogenesis, keeping blood glucose low (15). 2) Akt activates glycogen synthase kinase, which then activates glycogen synthase. This leads to the conversion of the majority of glucose molecules as hepatic glycogen for storage. The small amount that undergoes glycolysis reaches the mitochondria as pyruvate and is quickly esterified into acetyl-CoA. 3) Akt increases the activity of SREBP-1c. This allows any excess acetyl-CoA that cannot be β-oxidized for energy and exits the mitochondria to be rebuilt into FFAs, which then are packaged into VLDL for hepatic export and storage in adipocytes. This VLDL can promote atherogenesis and/or obesity, but only ∼2% of ingested glucose will find its way into VLDL; thus, glucose contributes extremely slowly to cardiovascular disease and other aspects of metabolic syndrome.
Figure 1.
Hepatic glucose metabolism. Of an ingested glucose load, 20% is metabolized by the liver. Under the action of insulin, glycogen synthase is increased, and the majority of the glucose load is stored as glycogen. Although insulin activation of sterol response element binding protein 1c (SREBP-1c) activates the lipogenic pathway, there is little citrate formed to act as a substrate for lipogenesis. In addition, insulin action on the liver phosphorylates forkhead protein O1 (FoxO1), excluding it from the nucleus, and suppressing the enzymes involved in gluconeogenesis (GNG). ACC, acetyl CoA carboxylase; ACL, ATP citrate lyase; ACSS2, acyl-CoA synthetase short-chain family member 2; ApoB, apolipoprotein B; ChREBP, carbohydrate response element binding protein; CPT-1, carnitine palmitoyl transferase 1; FAS, fatty acid synthase; Glut2, glucose transporter 2; Glut4, glucose transporter 4; Glut 5, glucose transporter 5; GSK, glycogen synthase kinase; IRS-1, insulin receptor substrate 1; MTP, microsomal transfer protein; PFK, phosphofructokinase; PI3K, phosphatidylinositol 3-kinase; SREBP-1c, sterol regulatory element binding protein 1c. Reproduced from (59) with permission.
Hepatic ethanol metabolism
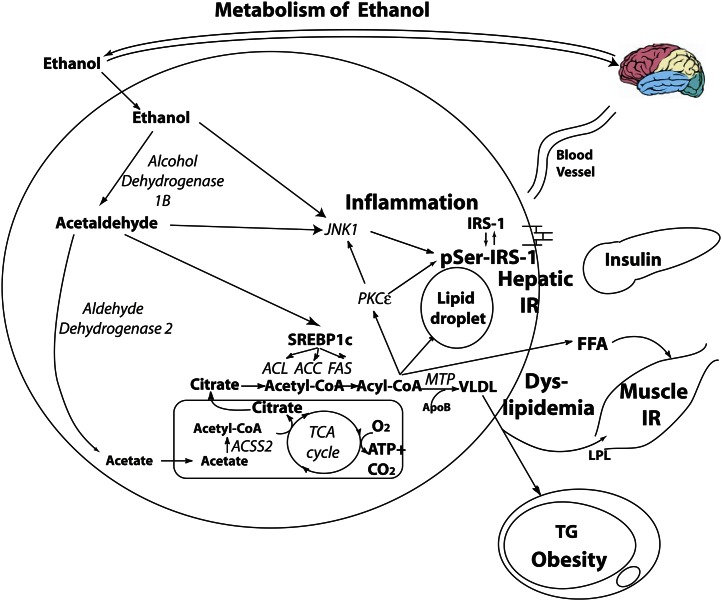
The hepatic pathway of ethanol metabolism is different from that of glucose in its regulation and the disposition of intermediary metabolites (Fig. 2). Ethanol enters the hepatocyte through osmosis, it does not require insulin for its metabolism, and it does not stimulate insulin secretion. Ethanol does not undergo glycolysis. Instead, it is converted by alcohol dehydrogenase 1B to form acetaldehyde, which, due to its free aldehyde, can generate reactive oxygen species (ROS) formation and toxic damage (19) if not quenched by hepatic antioxidants such as glutathione and ascorbic acid (see ROS formation and aging section) (20). Acetaldehyde is then quickly metabolized by the enzyme aldehyde dehydrogenase 2 to the intermediary acetic acid. From there, acetic acid is metabolized by the enzyme acyl-CoA synthetase short-chain family member 2 to form acetyl-CoA, which can then enter the mitochondrial tricarboxylic acid cycle (as per glucose). However, in the event of consumption of a large dose of ethanol producing a large amount of acetyl-CoA or due to the presence of other caloric substrate (i.e., as in beer in which ethanol and glucose are consumed together), the ethanol will more likely be converted to FFAs through DNL (21). Furthermore, acetaldehyde stimulates SREBP-1c, activating the enzymes of DNL (22) to increase rate of formation of FFAs. Although the absolute rate of DNL of ethanol (i.e., that which is metabolized to VLDL) is relatively small, fractional DNL increases from 1% at baseline to 31% after an ethanol bolus (21); thus, the liver is primed to convert ethanol to FFAs. Normally, intrahepatic lipid is exported as VLDL. Ethanol suppression of MTP alters VLDL production and lipid export machinery (23) to increase VLDL production and contribute to hypertriglyceridemia (24–26). By increasing intrahepatic lipid formation, ethanol drives hepatic insulin resistance (27, 28). Although the mechanism is still unclear, dyslipidemia and hepatic insulin resistance may be due to hepatic diacylglycerol and triglyceride accumulation seen in hepatic steatosis, with resultant activation of the enzyme c-jun N-terminal kinase 1 (JNK-1) (see the following) (29).
Figure 2.
Hepatic ethanol metabolism. Of an ingested load, 80% reaches the liver. Ethanol induces the following: 1) de novo lipogenesis and dyslipidemia; 2) c-jun N-terminal kinase (JNK1) activation, which serine phosphorylates hepatic insulin receptor substrate 1 (IRS-1), rendering it inactive and contributing to hepatic insulin resistance, which promotes hyperinsulinemia and influences substrate deposition into fat; 3) hepatic lipid droplet formation, leading to steatosis; and 4) stimulation of the reward pathway, promoting continuous consumption. ACC, acetyl CoA carboxylase; ACL, ATP citrate lyase; ACSS2, acyl-CoA synthetase short-chain family member 2; ApoB, apolipoprotein B; FAS, fatty acid synthase; IR, insulin resistance; IRS-1, insulin receptor substrate 1; LPL, lipoprotein lipase; MTP, microsomal transfer protein; PGC-PI3K, phosphatidylinositol 3-kinase; PKCɛ, protein kinase C-ɛ SREBP-1c, sterol regulatory element binding protein 1c. Reproduced from (59) with permission.
Hepatic fructose metabolism
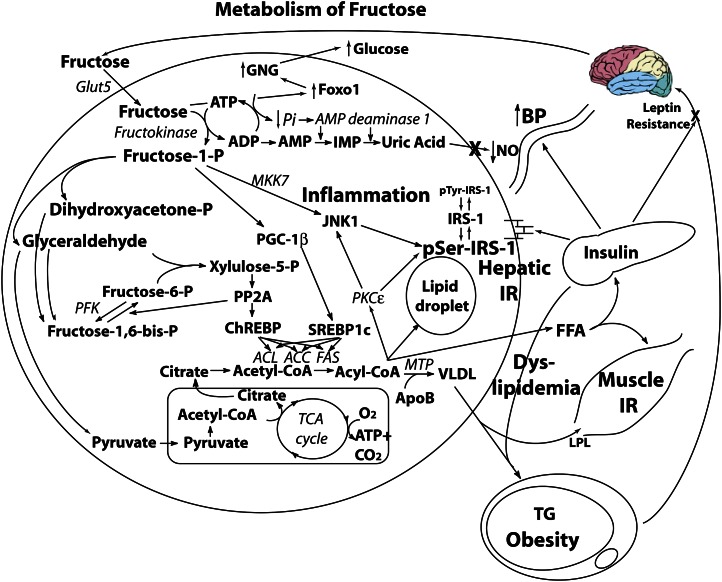
Only the liver possesses the Glut5 transporter (30), and the liver has a very high fructose extraction rate (31); thus, virtually an entire ingested fructose load finds its way to the liver. In contrast to the majority of hepatic glucose being converted to glycogen in the liver under the influence of insulin, fructose does not get converted to glycogen directly [although in case of glycogen depletion due to starvation or exercise, it can be converted to fructose-6-phosphate, which is isomerized to glucose-6-phosphate, which can rebuild glycogen (32)]. Rather, fructose is phosphorylated independently of insulin to fructose-1-phosphate by the enzyme fructokinase (Fig. 3), which undergoes glycolysis, and is metabolized to pyruvate, with the resultant large volume of acetyl-CoA entering the mitochondrial tricarboxylic acid cycle. Any extra intermediary will be available for DNL, similar to ethanol. Alternatively, a proportion of early glycolytic intermediaries will recombine to form fructose-1,6-bisphosphate, which then also combines with glyceraldehyde to form xylulose-5-phosphate (33). Xylulose-5-phosphate is a potent stimulator of protein phosphatase 2A (34), which activates carbohydrate response element binding protein (35), stimulating the activity of DNL. Furthermore, fructose also stimulates PPAR-γ coactivator 1β, a transcriptional coactivator for SREBP-1c, which further accentuates DNL enzymatic activity (36). In other words, fructose drives “double DNL” because carbohydrate response element binding protein and PPAR-γ coactivator 1β drive these enzymes additively. Human studies demonstrate a rate of fractional DNL of 2% with glucose, yet up to 10% after 6 d of high fructose feeding (37, 38) A recent human study demonstrated that fructose feeding increased fractional DNL to 17% (39). More importantly, when the liver receives glucose and fructose simultaneously, the glucose occupies the glycogenic pathway, forcing the fructose down the lipogenic pathway, thus tripling the rate of DNL compared with fructose alone (40). The attachment of hepatic triglyceride to apolipoprotein B by MTP completes its conversion to VLDL, which is exported out of the liver to contribute to fructose-induced hypertriglyceridemia (39, 41–43), along with the production of “small dense” LDL (44), which is particularly atherogenic because it can be oxidized rapidly and is small enough to get under the surface of vascular endothelial cells to start the foam cell process (39, 45–47). Some of the fatty acyl-CoA products from DNL escape packaging into VLDL for export and instead accumulate as lipid droplets in the hepatocyte (48), driving hepatic steatosis, similar to ethanol. In doing so, the enzyme JNK-1 (49) is activated, which induces serine phosphorylation of IRS-1 in the liver (50), thereby preventing normal insulin-mediated tyrosine phosphorylation of IRS-1 and promoting hepatic insulin resistance. This drives hyperinsulinemia (51), with resultant obesity causing worsening insulin resistance. Furthermore, fructose increases the expression of FoxO1 (52). In the face of hepatic insulin resistance, FoxO1 is not phosphorylated to maintain its exclusion from the nucleus, with resultant transcription of gluconeogenic enzymes and hyperglycemia, requiring an even greater β-cell insulin response. Eventually, in response to the hepatic insulin resistance, gluconeogenesis, and the phenomena of glucotoxicity, lipotoxicity, endoplasmic reticulum stress (53–56), and the unfolded protein response (57) at the β-cell, this leads to inadequate insulin secretion in relation to the degree of peripheral insulin resistance and type 2 diabetes (58).
Figure 3.
Hepatic fructose metabolism. Of an ingested sucrose load, 20% of the glucose and 100% of the fructose is metabolized by the liver. Fructose induces the following: 1) substrate-dependent phosphate depletion, which increases uric acid and contributes to hypertension via inhibition of endothelial nitric oxide synthase and reduction of nitric oxide (NO); 2) de novo lipogenesis and dyslipidemia; 3) hepatic lipid droplet formation and steatosis; 4) muscle insulin resistance; 5) c-jun N-terminal kinase (JNK1) activation, contributing to hepatic insulin resistance, which promotes hyperinsulinemia and influences substrate deposition into fat; 6) increased forkhead protein O1 (FoxO1), promoting gluconeogenesis (GNG) and hyperglycemia; and 7) central nervous system hyperinsulinemia, which antagonizes central leptin signaling and promotes continued energy intake. ACC, acetyl CoA carboxylase; ACL, ATP citrate lyase; ACSS2, acyl-CoA synthetase short-chain family member 2; ApoB, apolipoprotein B; ChREBP, carbohydrate response element binding protein; CPT-1, carnitine palmitoyl transferase 1; FAS, fatty acid synthase; Glut2, glucose transporter 2; Glut4, glucose transporter 4; Glut 5, glucose transporter 5; GSK, glycogen synthase kinase; IR, insulin resistance; IRS-1, insulin receptor substrate 1; LPL, lipoprotein lipase; MKK7, MAP kinase kinase 7; MTP, microsomal transfer protein; PFK, phosphofructokinase; PGC-1β, PPAR-γ coactivator-1β PI3K, phosphatidylinositol 3-kinase; PKCɛ, protein kinase C-ɛ PP2A, protein phosphatase 2a; SREBP-1c, sterol regulatory element binding protein 1c. Reproduced from (59) with permission.
Hepatic metabolic profile and substrate burden: fructose vs. ethanol
Thus, fructose and ethanol are analogous qualitatively in terms of hepatic metabolism. In small doses, neither will overwhelm hepatic mitochondrial capacity. However, as Paracelsus stated, “The dose determines the poison.” In a substrate overload/hypercaloric paradigm, excess energy substrate conversion to acetyl-CoA without any insulin regulation and with limited diversion to nontoxic intermediaries such as glycogen will occur. Both fructose and ethanol uniquely drive DNL, generating intrahepatic lipid, inflammation, and insulin resistance. Through the phenomena of enhanced DNL, JNK-1 activation, and hepatic insulin resistance, the hepatic metabolic profile of fructose is analogous to that of ethanol. Furthermore, fructose and ethanol are also analogous quantitatively. Table 1 demonstrates the hepatic burden of a can of beer vs. a can of soda. Both contain 150 kcal per 12-oz (355-mL) can (59). Both contain a concomitant glucose load combined with either the ethanol load (beer) or the fructose load (soda). The first-pass effect of ethanol in the stomach and intestine removes 10% of the ethanol. In the case of beer (3.6% ethanol and 6.6% maltose, a glucose disaccharide), ∼92 kcal reach the liver, whereas for soda, 90 kcal reach the liver. Indeed, the quantitative metabolic demand on the liver from beer and soda are analogous as well (59).
Table 1.
Similarities between soda and beer with respect to hepatic handling1
| Soda (12 oz can) | Beer (12 oz can) | |
| Calories | 150 | 150 |
| Percentage of carbohydrate | 10.5% (sucrose) | 3.6% (alcohol), 5.3% (other carbs) |
| Calories from | ||
| Fructose | 75 (4.1 kcal/g) | |
| Alcohol | 90 (7 kcal/g) | |
| Other carbohydrate | 75 (glucose) | 60 (maltose) |
| First-pass stomach-intestine metabolism | 0% | 10% |
| Calories reaching liver | 90 | 92 |
Reproduced with permission from (59).
ROS formation and aging
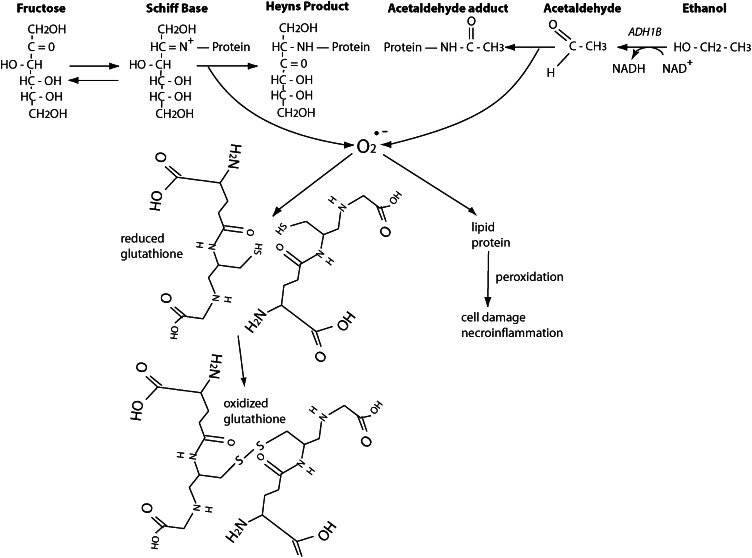
Any nutritional substrate with a free reactive aldehyde or ketone can induce ROS formation when that reactive moiety binds to an ε-amino group of lysine found in proteins or DNA bases or with a free hydroxyl group found in lipids. In the case of carbohydrate, that reactive moiety may be available in the linear form or hidden in the ring form. Because glucose forms a 6-member glucopyranose ring with only 1 hydroxymethyl group, the ring form is stable, thereby reducing the availability of the free aldehyde. However, fructose forms a 5-member fructofuranose ring with 2 axial hydroxymethyl groups, which forces fructose at greater frequency into its linear form with the free ketone moiety (60).
Effects of glucose
The aldehyde form of glucose can react with free amino groups on proteins in a nonenzymatic exothermic reaction, leading to nonenzymatic protein glycation (61), termed the Maillard or “browning” reaction (e.g., hemoglobin A1c). Each glycation generates 1 superoxide radical that must be quenched by an antioxidant or cellular damage will occur (62). However, at 37°C and pH 7.4, the majority of glucose molecules are found in the stable 6-member glucopyranose ring form, limiting aldehyde exposure and reducing ROS generation.
Effects of ethanol
After ethanol is metabolized by alcohol dehydrogenase 1B to the intermediary acetaldehyde, its free aldehyde moiety engages rapidly in ROS formation (63). Acetaldehyde induces hepatocellular damage through several different mechanisms (20), including mitochondrial damage, membrane effects, hypoxia, cytokine production, and iron mobilization. In the absence of antioxidant quenching (due to micronutrient malnutrition often seen in alcoholics), ROS formation may lead to lipid peroxidation, fibrogenesis, and ultimately cirrhosis (Fig. 4).
Figure 4.
Generation of reactive oxygen species (ROS) by fructose or ethanol. Fructose first forms an intermediate Schiff base with the ε-amino group of lysine, which then spontaneously hydrogenates to form an irreversible Heyns product (hydroxyamide linkage or fructose adduct), termed the Maillard reaction. The heat of formation of this reaction is −19 kcal/mol and is therefore exothermically favorable. Each protein fructation generates 1 superoxide radical (O2•−), which must be quenched by an antioxidant (such as glutathione with its reduced sulfhydryl groups). Conversely, ethanol is metabolized by alcohol dehydrogenase 1B (ADH1B), generating NADH, to acetaldehyde, which then participates in the same Maillard reaction to form acetaldehyde adducts, with generation of superoxide radicals that must also be quenched by antioxidants. In the absence of adequate antioxidant capacity, ROS production leads to peroxidation, hepatocellular damage, necroinflammation (nonalcoholic steatohepatitis), fibrosis, and ultimately cirrhosis. Reproduced from (59) with permission.
Effects of fructose
Because fructose forms a 5-member fructofuranose ring with steric hindrance from the 2 axial (abutting) hydroxymethyl groups, more molecules find themselves in the linear form, which exposes their reactive keto group and leads to fructation of proteins 7 times more rapidly than with glucose (64, 65). Thus, fructose-generated ROS species are abundant (66, 67) and require quenching by a hepatic antioxidant (e.g., glutathione) or hepatocellular damage will result (Fig. 4). The hepatotoxic effects of fructose via ROS formation have been demonstrated in both cultured hepatocytes (68) and animal models (69). Although mechanistic data in humans are difficult to obtain, case-control studies demonstrate that fructose consumption correlates with the development of hepatic steatosis and nonalcoholic steatohepatitis (70–72).
Central nervous system effects to increase consumption
The hedonic pathway that motivates the “reward” of food intake consists of the ventral tegmental area (VTA) (the home of the dopamine perikarya) and the nucleus accumbens (NA) (the destination of the dopamine axons, also referred to as the “pleasure center” of the brain). Food intake is a “readout” of the reward pathway; for example, administration of morphine to the NA increases food intake in a dose-dependent fashion (73). Dopamine neurotransmission from the VTA to the NA mediates the reward properties of food (74), whereas obesity results in decreased density of dopamine D2 receptors as measured by positron emission tomography scanning (75). Indeed, any process that reduces dopamine receptor density or occupancy can drive increased food intake and weight gain (76).
Effects of glucose
In a rat model, 30-d ad libitum administration of 25% glucose solution did not result in significantly altered dopaminergic or opioidergic tone versus chow-fed animals (77). However, when the glucose was instead administered in a cyclic food deprivation/supply paradigm designed to create dependency, reductions in dopamine neurotransmission in the NA, which were also exacerbated by naloxone administration, mimicked withdrawal. These data suggest that glucose by itself does not routinely alter dopamine neurotransmission in the NA, but can exert some degree of dependency in a susceptible animal.
Effects of ethanol
Ethanol is a known substance of abuse via its effects on fostering reward (78). By altering γ-aminobutyric acid and opioid transmission within the VTA and central area of the amygdala, acute ethanol exposure activates dopamine neurotransmission (79). However, after repeated exposure to ethanol, dopamine increases, but dopamine receptor levels are decreased due to downregulation, and peak effects relative to baseline are attenuated (80), leading to tolerance. Human genetic studies demonstrate that downregulation of dopamine transport (and the resultant inadequate neurotransmission) results in increased ethanol consumptive behavior (81), and human imaging studies show that dysfunction of dopamine neurotransmission is associated with withdrawal and relapse (82). Such downregulation of dopamine neurotransmission with long-term substrate exposure is a hallmark of the addictive state (83).
Effects of fructose
Fructose has direct effects on increasing caloric consumption. Increasing the palatability of food by the addition of sucrose undermines normal satiety signals and motivates energy intake independent of energy need (84, 85). For instance, sucrose infusion directly into the NA reduces D2 receptors and μ-opioid receptors similar to that of morphine (86). Both sweet and high-fat foods mobilize both opioids and dopamine within the NA and establish hard-wired pathways for craving in these areas that can be identified by functional magnetic resonance imaging (73, 87). Furthermore, animal models of intermittent sugar administration, over a 3-wk interval, can induce behavioral alterations consistent with dependence, i.e., bingeing, withdrawal and anxiety, craving, and cross-sensitization to other drugs of abuse (88). Neuropharmacologic analyses demonstrate a reduction in D2 receptors in the NA, consistent with the fostering of reward and behavioral changes seen in addiction.
There is also evidence that sugar may be addictive in humans. Anecdotal reports from self-identified food addicts describe withdrawal as feeling “irritable,” “shaky,” “anxious,” and “depressed” (89). Other studies show that subjects will use sugar to treat psychological symptoms. Although dysphoria is a psychological manifestation of withdrawal, the directionality of this relationship is unclear. It is not known whether the negative effect is purely a symptom of withdrawal or that these subjects more likely had some degree of dysphoria that preceded the dependence on sugar to medicate it. Other areas that warrant study of potential sugar addiction in humans are craving and tolerance. Benton (90) points out that sugar craving can vary widely by age, menstrual cycle, and time of day. We have examined the content of the “fast food” meal as it relates to addiction; of the various components, fat and salt increase the salience of the food, but only sugar and caffeine exhibit true dependence (91). Although anecdotal reports abound supporting human “sugar addiction,” whether this “vicious cycle” of fructose consumption is merely habituation or full-fledged dependence is not yet clear (92).
Response of the sugar industry
It should be pointed out that all of these physiological similarities are just that — similarities. True quantitative and mechanistic studies have not proved this qualitative analogy. It is also necessary to highlight that the food industry vehemently argues that HFCS is no different from sucrose (93) and that fructose is natural, benign, and certainly not a long-term dose-dependent hepatotoxin. However, a discussion of these points is clearly in order.
Animals are not humans
The food industry is quick to point out that most fructose studies are done in rodents with large doses over a short period of time. However, studies done in primates demonstrate similar detrimental effects (94). Furthermore, human studies are consistent with the analogies stated previously (39, 43).
Fructose does not increase blood glucose or hemoglobin A1c
The industry argues that fructose does not increase the blood glucose. It has a very low glycemic index of 19 (glucose is the index at 100), which is a measure of a food’s generation of an insulin response and used as a method for quantifying a food’s potential for weight gain. Indeed, fructose alone does not increase the blood glucose nor does it generate an insulin response, nor does alcohol (in fact, alcohol lowers the blood glucose). But adding alcohol to foods is not a rational way to make foods healthier. There is no fructose alone in nature; it is always found paired with glucose (either as sucrose or HFCS), and the glucose contribution generates quite a hefty insulin response. So the glucose is metabolized by the liver’s glycogenic pathway and drives up insulin, whereas the fructose is metabolized by the liver’s lipogenic pathway and causes liver insulin resistance.
The industry also argues that as fructose does not increase the serum glucose and also does not increase hemoglobin A1c levels (95). In the blood, fructose does not bind to the amino moiety at position 1 of hemoglobin to generate hemoglobin A1c (leading the food industry to wrongly assume that fructose is benign); rather, fructation occurs at positions 7, 66, and 127 of the hemoglobin molecule instead (67).
Fructose for glucose exchange studies show no detrimental effects
Meta-analyses of controlled isocaloric “fructose for glucose” exchange studies demonstrate no effects of weight gain or other morbidities (96). Perhaps 1 reason for this is because crystalline fructose is incompletely absorbed, and thus its effects on glucose and HbA1c may be minimal. If so, then the gastrointestinal symptoms of the residual fructose in the intestine are maximal, generating pain, bloating, and diarrhea (97). Furthermore, those meta-analyses in which fructose was supplied in excess showed weight gain, dyslipidemia, and insulin resistance (96). Thus, the dose determines the poison.
The food industry is fond of referring to a 1999 study showing that DNL of oral fructose occurs at a very low rate (<5%) (98). That is true if you are thin, insulin sensitive, fasting (and therefore glycogen depleted), and given just fructose alone (which is poorly absorbed). Conversely, if you are obese, insulin resistant, fed, and getting both fructose and glucose together (a sizable percentage of the population), then fructose gets converted to fat at a much higher rate, ∼30% (40). In other words, the toxicity of fructose depends on the context. If you are an elite athlete and glycogen depleted, fructose is not an issue. But for the rest of us, our current excess fructose consumption drives chronic metabolic disease.
A little fructose improves insulin secretion
Studies of ethanol use show that small doses are healthy because ethanol increases HDL and improves insulin sensitivity and longevity (99). Like alcohol, a small dose of fructose has been shown in some studies to have a beneficial effect on insulin secretion (100). The negative effects of fructose, just like alcohol, are dose dependent. For alcohol, we have empirical evidence that in most people, a maximum dose of 50 g/d is the threshold for toxicity. By analogy, that is likely the threshold for fructose as well. The problem is that the current average adult fructose consumption is 51 g/d (101). So the levels of half of all adults are likely above the threshold for fructose toxicity, and adolescents currently average 75 g/d.
Conclusions
Most people consider sugar (i.e., fructose-containing compounds) to be just “empty calories.” However, this paper reports 3 separate ways that fructose exerts negative effects beyond its caloric equivalent. First, in the hypercaloric state, fructose drives DNL, resulting in dyslipidemia, hepatic steatosis, and insulin resistance, akin to that seen with ethanol. This should not be surprising because fructose and ethanol are congruent evolutionarily and biochemically. Ethanol is manufactured by the fermentation of fructose — the big difference is that for ethanol, the yeast performs the glycolysis, whereas for fructose, we humans perform our own glycolysis. Second, through production of reactive carbonyl moieties, both fructose and ethanol generate excess ROS, which increases the risk of hepatocellular damage if not quenched by antioxidants. Last, by downregulation of D2 receptors in the reward pathway, chronic fructose exposure contributes to a paradigm of continuous food intake independent of energy need and exerts symptoms of tolerance and withdrawal, similar to chronic ethanol abuse. Therefore, it should not be surprising that the disease profile of fructose and ethanol overconsumption would also be similar (Table 2).
Table 2.
Phenotypes of long-term energy substrate exposure1
| Long-term ethanol exposure | Long-term fructose exposure |
| Hematologic disorders | |
| Electrolyte abnormalities | |
| Hypertension | Hypertension (uric acid) |
| Cardiac dilation | |
| Cardiomyopathy | Myocardial infarction (dyslipidemia, insulin resistance) |
| Dyslipidemia | Dyslipidemia (de novo lipogenesis) |
| Pancreatitis | Pancreatitis (hypertriglyceridemia) |
| Obesity (insulin resistance) | Obesity (insulin resistance) |
| Malnutrition | Malnutrition (obesity) |
| Hepatic dysfunction (ASH) | Hepatic dysfunction (NASH) |
| Fetal alcohol syndrome | |
| Addiction | Habituation, if not addiction |
Reproduced with permission from (59).
Fructose also exhibits notable social and market similarities with ethanol. Both have been “fetishized” by various cultures in times past. Of course, today both sugar and alcohol are legal commodities and are traded freely. The problems of overuse and related health harm tend to occur in lower socioeconomic groups. Those who overconsume either substance are stigmatized. Finally, within public health circles, alcohol clearly evinces the 4 criteria of unavoidability, toxicity, abuse, and negative impact on society, which warrant consideration for personal intervention (e.g., “rehab”) and societal intervention (e.g., “laws”). Sucrose/HFCS satisfies those same 4 criteria as well (6).
Although fructose does not exhibit the same acute toxic effects of ethanol (i.e., central nervous system depression and resultant auto accidents), it recapitulates all the chronic toxic effects on long-term health. It is time for a paradigm shift in our societal treatment of fructose, recognizing that fructose is “alcohol without the buzz.”
Acknowledgments
The sole author had responsibility for all parts of the manuscript.
Footnotes
Abbreviations used: DNL, de novo lipogenesis; Foxo1, forkhead protein O1; HFCS, high-fructose corn syrup; IRS-1, insulin receptor substrate 1; JNK-1, c-jun N-terminal kinase 1; MKK7, MAP kinase kinase 7; MTP, microsomal transfer protein; NA, nucleus accumbens; NO, nitric oxide; ROS, reactive oxygen species; SREBP-1c, sterol regulatory element binding protein 1c; VTA, ventral tegmental area.
Literature Cited
- 1.United Nations General Assembly. Prevention and control of non-communicable diseases. UN General Assembly. New York, 2010. [Google Scholar]
- 2.Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17:961–9 [DOI] [PubMed] [Google Scholar]
- 3.Abbasi F, Chu JW, Lamendola C, McLaughlin T, Hayden J, Reaven GM, Reaven PD. Discrimination between obesity and insulin resistance in the relationship with adiponectin. Diabetes. 2004;53:585–90 [DOI] [PubMed] [Google Scholar]
- 4.Voulgari C, Tentolouris N, Dilaveris P, Tousoulis D, Katsilambros N, Stefanadis C. Increased heart failure risk in normal-weight people with metabolic syndrome compared with metabolically healthy obese individuals. J Am Coll Cardiol. 2011;58:1343–50 [DOI] [PubMed] [Google Scholar]
- 5.Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J Med. 2008;10:160. [PMC free article] [PubMed] [Google Scholar]
- 6.Lustig RH, Schmidt LA, Brindis CD. Public health: the toxic truth about sugar. Nature. 2012;482\7:27–9 [DOI] [PubMed] [Google Scholar]
- 7.Frenette G, Thabet M, Sullivan R. Polyol pathway in human epididymis and semen. J Androl. 2006;27:233–9 [DOI] [PubMed] [Google Scholar]
- 8.Newbrun E, Hoover C, Mettraux G, Graf H. Comparison of dietary habits and dental health of subjects with hereditary fructose intolerance and control subjects. J Am Dent Assoc. 1980;101:619–26 [DOI] [PubMed] [Google Scholar]
- 9.Burmeister LA, Valdivia T, Nuttall FQ. Adult hereditary fructose intolerance. Arch Intern Med. 1991;151:773–6 [PubMed] [Google Scholar]
- 10.Yasawy MI, Folsch UR, Schmidt WE, Schwend M. Adult hereditary fructose intolerance. World J Gastroenterol. 2009;15:2412–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reaven GM. The metabolic syndrome: is this diagnosis necessary? Am J Clin Nutr. 2006;83:1237–47 [DOI] [PubMed] [Google Scholar]
- 12.Weiss R, Bremer AA, Lustig RH. What is metabolic syndrome, and why are children getting it? In: Braaten D, editor. Year in Diabetes and Obesity, 2012. New York: Annals of the New York Academy of Science; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–6 [DOI] [PubMed] [Google Scholar]
- 14.Naïmi M, Gautier N, Chaussade C, Valverde AM, Accili D, Van Obberghen E. Nuclear forkhead box O1 controls and integrates key signaling pathways in hepatocytes. Endocrinology. 2007;148:2424–34 [DOI] [PubMed] [Google Scholar]
- 15.Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, White MF. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008;8:65–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Aleman JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bremer AA, Mietus-Snyder ML, Lustig RH. Toward a unifying hypothesis of metabolic syndrome. Pediatrics. 2012;129:557–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bizeau ME, Pagliassotti MJ. Hepatic adaptations to sucrose and fructose. Metabolism. 2005;54:1189–201 [DOI] [PubMed] [Google Scholar]
- 19.Farfán Labonne BE, Gutiérrez M, Gómez-Quiroz LE, Konigsberg Fainstein M, Bucio L, Souza V, Flores O, Ortíz V, Hernández E, Kershenobich D, et al. Acetaldehyde-induced mitochondrial dysfunction sensitizes hepatocytes to oxidative damage. Cell Biol Toxicol. 2009;25:599–609 [DOI] [PubMed] [Google Scholar]
- 20.Dey A, Cedarbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43: Suppl 1:S63–74 [DOI] [PubMed] [Google Scholar]
- 21.Siler SQ, Neese RA, Hellerstein MK. De novo lipogenesis, lipid kinetics, and whole-body lipid balances in humans after acute alcohol consumption. Am J Clin Nutr. 1999;70:928–36 [DOI] [PubMed] [Google Scholar]
- 22.You M, Crabb DW. Molecular mechanisms of alcoholic fatty liver: role of sterol regulatory element-binding proteins. Alcohol. 2004;34:39–43 [DOI] [PubMed] [Google Scholar]
- 23.Sozio M, Crabb DW. Alcohol and lipid metabolism. Am J Physiol Endocrinol Metab. 2008;295:E10–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinberg D, Pearson TA, Kuller LH. Alcohol and atherosclerosis. Ann Intern Med. 1991;114:967–76 [DOI] [PubMed] [Google Scholar]
- 25.Suter PM, Schutz Y. doi: 10.1038/ijo.2008.206. The effect of exercise, alcohol or both combined on health and physical performance. Int J Obes 2008;32 Suppl 6:S48–52. [DOI] [PubMed] [Google Scholar]
- 26.Schneider J, Tesdorfpf M, Kaffarnik H, Hausmann L, Zöfel P, Zilliken F. Alteration of plasma lipids and intermediates of lipid metabolism in healthy fasting volunteers by ethanol and fructose. Res Exp Med (Berl). 1976;167:159–70 [DOI] [PubMed] [Google Scholar]
- 27.Yokoyama H, Hiroshi H, Ohgo H, Hibi T, Saito I. Effects of excessive ethanol consumption on the diagnosis of the metabolic syndrome using its clinical diagnostic criteria. Intern Med. 2007;46:1345–52 [DOI] [PubMed] [Google Scholar]
- 28.Onishi Y, Honda M, Ogihara T, Sakoda H, Anai M, Fujishiro M, Ono H, Shojima N, Fukushima Y, Inukai K, et al. Ethanol feeding induces insulin resistance with enhanced PI 3-kinase activation. Biochem Biophys Res Commun. 2003;303:788–94 [DOI] [PubMed] [Google Scholar]
- 29.Lee YJ, Aroor AR, Shukla SD. Temporal activation of p42/44 mitogen-activated protein kinase and c-Jun N-terminal kinase by acetaldehyde in rat hepatocytes and its loss after chronic ethanol exposure. J Pharmacol Exp Ther. 2002;301:908–14 [DOI] [PubMed] [Google Scholar]
- 30.Douard V, Ferraris RP. Regulation of the fructose transporter Glut5 in health and disease. Am J Physiol Endocrinol Metab. 2008;295:E227–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HS, Paik HY, Lee KU, Lee HK, Min HK. Effects of several simple sugars on serum glucose and serum fructose levels in normal and diabetic subjects. Diabetes Res Clin Pract. 1988;4:281–7 [DOI] [PubMed] [Google Scholar]
- 32.Décombaz J, Jentjens R, Ith M, Scheurer E, Buehler T, Jeukendrup A, Boesch C. Fructose and galactose enhance postexercise human liver glycogen synthesis. Med Sci Sports Exerc. 2011;43:1964–71 [DOI] [PubMed] [Google Scholar]
- 33.Bonsignore A, Pontremoli S, Mangiarotti G, De Flora A, Mangiarotti M. A direct interconversion: D-fructose 6-phosphate to sedoheptulose 7-phosphate and D-xylulose 5-phosphate catalyzed by the enzymes transketolase and transaldolase. J Biol Chem. 1962;237:3597–602 [Google Scholar]
- 34.Kabashima T, Kawaguchi T, Wadzinski BE, Uyeda K. Xylulose 5-phosphate mediates glucose-induced lipogenesis by xylulose 5-phosphate-activated protein phosphatase in rat liver. Proc Natl Acad Sci U S A. 2003;100:5107–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dentin R, Benhamed F, Hainault I, Fauveau V, Foufelle F, Dyck JRB, Girard J, Postic C. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes. 2006;55:2159–70 [DOI] [PubMed] [Google Scholar]
- 36.Nagai Y, Yonemitsu S, Erion DM, Iwasaki T, Stark R, Weismann D, Dong J, Zhang D, Jurczak MJ, Löffler MG, et al. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab. 2009;9:252–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwarz JM, Noworolski SM, Lee GA, Wen M, Dyachenko A, Prior J, Weinberg M, Herraiz L, Rao M, Mulligan K, editors. Effects of short-term feeding with high- vs low-fructose isoenergetic diets on hepatic de novo lipogenesis, liver fat content and glucose regulation. Diabetes. 2009;58 suppl 1:4382, abstract 1476P
- 38.Faeh D, Minehira K, Schwarz JM, Periasami R, Seongsu P, Tappy L. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes. 2005;54:1907–13 [DOI] [PubMed] [Google Scholar]
- 39.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, et al. Consuming fructose-, not glucose-sweetened beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hudgins LC, Parker TS, Levine DM, Hellerstein MK. A dual sugar challenge test for lipogenic sensitivity to dietary fructose. J Clin Endocrinol Metab. 2011;96:861–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teff KL, Elliott SS, Tschop M, Kieffer TJ, Rader D, Heiman M, Townsend RR, Keim NL, D'Alessio D, Havel PJ. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89:2963–72 [DOI] [PubMed] [Google Scholar]
- 42.Chong MF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr. 2007;85:1511–20 [DOI] [PubMed] [Google Scholar]
- 43.Teff KL, Grudziak J, Townsend RR, Dunn TN, Grant RW, Adams SH, Keim NL, Cummings BP, Stanhope KL, Havel PJ. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab. 2009;94:1562–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aeberli I, Zimmermann MB, Molinari L, Lehmann R, l'Allemand D, Spinas GA, Berneis K. Fructose intake is a predictor of LDL particle size in overweight schoolchildren. Am J Clin Nutr. 2007;86:1174–8 [DOI] [PubMed] [Google Scholar]
- 45.Hellerstein MK, Schwarz JM, Neese RA. Regulation of hepatic de novo lipogenesis in humans. Annu Rev Nutr. 1996;16:523–57 [DOI] [PubMed] [Google Scholar]
- 46.Schwarz JM, Linfoot P, Dare D, Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr. 2003;77:43–50 [DOI] [PubMed] [Google Scholar]
- 47.Lê KA, Ith M, Kreis R, Faeh D, Bortolotti M, Tran C, Boesch C, Tappy L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr. 2009;89:1760–5 [DOI] [PubMed] [Google Scholar]
- 48.Cave M, Deaciuc I, Mendez C, Song Z, Joshi-Barve S, Barve S, McClain C. Nonalcoholic fatty liver disease: predisposing factors and the role of nutrition. J Nutr Biochem. 2007;18:184–95 [DOI] [PubMed] [Google Scholar]
- 49.Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–53 [DOI] [PubMed] [Google Scholar]
- 50.Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2006;103:10741–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim SP, Ellmerer M, Van Citters GW, Bergman RN. Primacy of hepatic insulin resistance in the development of the metabolic syndrome induced by an isocaloric moderate-fat diet in the dog. Diabetes. 2003;52:2453–60 [DOI] [PubMed] [Google Scholar]
- 52.Qu S, Su D, Altomonte J, Kamagate A, He J, Perdomo G, Tse T, Jiang Y, Dong HH. PPARα mediates the hypolipidemic action of fibrates by antagonizing FoxO1. Am J Physiol Endocrinol Metab. 2007;292:E421–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cnop M, Igoillo-Esteve M, Cunha DA, Ladrière L, Eizirik DL. An update on lipotoxic endoplasmic reticulum stress in pancreatic beta-cells. Biochem Soc Trans. 2008;36:909–15 [DOI] [PubMed] [Google Scholar]
- 55.Liu M, Hodish I, Rhodes CJ, Arvan P. Proinsulin maturation, misfolding, and proteotoxicity. Proc Natl Acad Sci U S A. 2007;104:15841–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hotamisligil GS. doi: 10.1038/ijo.2008.238. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int J Obes. 2008;32 Suppl 7:S52–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Merksamer PI, Trusina A, Papa FR. Real-time redox measurements during endoplasmic reticulum stress reveal interlinked protein folding functions. Cell. 2008;135:933–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes. 2002;51: Supp 1:S212–20 [DOI] [PubMed] [Google Scholar]
- 59.Lustig RH. Fructose: metabolic, hedonic, and societal parallels with ethanol. J Am Diet Assoc. 2010;110:1307–21 [DOI] [PubMed] [Google Scholar]
- 60.Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010;7:251–64 [DOI] [PubMed] [Google Scholar]
- 61.Dills WL. Protein fructosylation: fructose and the Maillard reaction. Am J Clin Nutr. 1993;58:779S–87S [DOI] [PubMed] [Google Scholar]
- 62.Figueroa-Romero C, Sadidi M, Feldman EL. Mechanisms of disease: the oxidative stress theory of diabetic neuropathy. Rev Endocr Metab Disord. 2008;9:301–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niemelä O, Parkkila S, Ylä-Herttuala S, Villanueva J, Ruebner B, Halsted CH. Sequential acetaldehyde production, lipid peroxidation, and fibrogenesis in micropig model of alcohol-induced liver disease. Hepatology. 1995;22:1208–14 [PubMed] [Google Scholar]
- 64.Ahmed N, Furth AJ. Failure of common glycation assays to detect glycation by fructose. Clin Chem. 1992;38:1301–3 [PubMed] [Google Scholar]
- 65.Schalkwijk CG, Stehouwer CD, van Hinsbergh VW. Fructose-mediated non-enzymatic glycation: sweet coupling or bad modification. Diabetes Metab Res Rev. 2004;20:369–82 [DOI] [PubMed] [Google Scholar]
- 66.Bunn HF, Higgins PJ. Reaction of monosaccharides with proteins: possible evolutionary significance. Science. 1981;213:222–4 [DOI] [PubMed] [Google Scholar]
- 67.Bose T, Chakraborti AS. Fructose-induced structural and functional modifications of hemoglobin: implication for oxidative stress in diabetes mellitus. Biochim Biophys Acta. 2008;1780:800–8 [DOI] [PubMed] [Google Scholar]
- 68.Lee O, Bruce WR, Dong Q, Bruce J, Mehta R, O'Brien PJ. Fructose and carbonyl metabolites and endogenous toxins. Chem Biol Interact. 2009;178:332–9 [DOI] [PubMed] [Google Scholar]
- 69.Pickens MK, Yan JS, Ng RK, Ogata H, Grenert JP, Beysen C, Turner SM, Maher JJ. Dietary sucrose is essential to the development of liver injury in the MCD model of steatohepatitis. J Lipid Res. 2009;50:2072–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Assy N, Nasser G, Kamayse I, Nseir W, Beniashvili Z, Djibre A, Grosovski M. Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can J Gastroenterol. 2008;22:811–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abid A, Taha O, Nseir W, Farah R, Grosovski M, Assy N. Soft drink consumption is associated with fatty liver disease independent of metabolic syndrome. J Hepatol. 2009;51:918–24 [DOI] [PubMed] [Google Scholar]
- 72.Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, Diehl AM, Nonalcoholic Steatohepatitis Clinical Research Network NSCR. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1961–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–77 [DOI] [PubMed] [Google Scholar]
- 74.Carr KD, Tsimberg Y, Berman Y, Yamamoto N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neuroscience. 2003;119:1157–67 [DOI] [PubMed] [Google Scholar]
- 75.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357:354–7 [DOI] [PubMed] [Google Scholar]
- 76.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10:478–88 [DOI] [PubMed] [Google Scholar]
- 78.Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytiä P, Merlo-Pich E, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9 [PubMed] [Google Scholar]
- 79.Melis M, Diana M, Enrico P, Marinelli M, Brodie MS. Ethanol and acetaldehyde action on central dopamine systems: mechanisms, modulation, and relationship to stress. Alcohol. 2009;43:531–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Philpot RM, Wecker L, Kirstein CL. Repeated ethanol exposure during adolescence alters the developmental trajectory of dopaminergic output from the nucleus accumbens septi. Int J Dev Neurosci. 2009;27:805–15 [DOI] [PubMed] [Google Scholar]
- 81.Lind PA, Eriksson CJ, Wilhelmsen KC. Association between harmful alcohol consumption behavior and dopamine transporter (DAT1) gene polymorphisms in a male Finnish population. Psychiatr Genet. 2009;19:117–25 [DOI] [PubMed] [Google Scholar]
- 82.Heinz A, Beck A, Grüsser SM, Grace AA, Wrase J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict Biol. 2009;14:108–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tupala E, Tiihonen J. Dopamine and alcoholism: neurobiological basis of ethanol abuse. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1221–47 [DOI] [PubMed] [Google Scholar]
- 84.Erlanson-Albertsson C. How palatable food disrupts appetite regulation. Basic Clin Pharmacol Toxicol. 2005;97:61–73 [DOI] [PubMed] [Google Scholar]
- 85.Pelchat ML. Of human bondage: food craving, obsession, compulsion, and addiction. Physiol Behav. 2002;76:347–52 [DOI] [PubMed] [Google Scholar]
- 86.Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz S. F. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Brain Res Mol Brain Res. 2004;124:134–42 [DOI] [PubMed] [Google Scholar]
- 87.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. Neuroimage. 2004;23:1486–93 [DOI] [PubMed] [Google Scholar]
- 88.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ifland JR, Preuss HG, Marcus MT, Rourke KM, Taylor WC, Burau K, Jacobs WS, Kadish W, Manso G. Refined food addiction: a classic substance use disorder. Med Hypotheses. 2009;72:518–26 [DOI] [PubMed] [Google Scholar]
- 90.Benton D. The plausibility of sugar addiction and its role in obesity and eating disorders. Clin Nutr. 2010;29:288–303 [DOI] [PubMed] [Google Scholar]
- 91.Garber AK, Lustig RH. Is fast food addictive? Curr Drug Abuse Rev. 2011;4:146–62 [DOI] [PubMed] [Google Scholar]
- 92.Ziauddeen H, Farooqi ISFP. Obesity and the brain: how convincing is the addiction model? Nat Rev Neurosci. 2012;13:279–86 [DOI] [PubMed] [Google Scholar]
- 93.Stanhope KL, Griffen SC, Bair BR, Swarbrick MM, Keim NL, Havel PJ. . Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. Am J Clin Nutr. 2008;87:1194–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bremer AA, Stanhope KL, Graham JL, Cummings BP, Wang W, Saville BR, Havel, PJ. Fructose-fed rhesus monkeys: a nonhuman primate model of insulin resistance, metabolic syndrome, and type 2 diabetes. Clin Transl Sci. 2011;4:243–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cozma AI, Sievenpiper JL, de Souza RJ, Chiavaroli L, Ha V, Wang DD, Mirrahimi A, Yu ME, Carleton AJ, Di Buono M, et al. Effect of fructose on glycemic control in diabetes: a systematic review and meta-analysis of controlled feeding trials. Diabetes Care. 2012;35:1611–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sievenpiper JL, de Souza RJMA, Yu ME, Carleton AJ, Beyene J, Chiavaroli L, Di Buono M, Jenkins AL, Leiter LA, Wolever TM, et al. Effect of fructose on body weight in controlled feeding trials: a systematic review and meta-analysis. Ann Intern Med. 2012;156:291–304 [DOI] [PubMed] [Google Scholar]
- 97.Rumessen JJ. E. G-H. Absorption capacity of fructose in healthy adults. Comparison with sucrose and its constituent monosaccharides. Gut. 1986;27:1161–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Parks EJ, Krauss RM, Christiansen MP, Neese RA, Hellerstein MK. Effects of a low-fat, high-carbohydrate diet on VLDL-triglyceride assembly, production, and clearance. J Clin Invest. 1999;104:1087–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Facchini F, Chen YD, Reaven GM. Light-to-moderate alcohol intake is associated with enhanced insulin sensitivity. Diabetes Care. 1994;17:115–119 [DOI] [PubMed] [Google Scholar]
- 100.Moore MC, Davis SN, Mann SL, Cherrington AD. Acute fructose administration improves oral glucose tolerance in adults with type 2 diabetes. Diabetes Care. 2001;24:1882–7 [DOI] [PubMed] [Google Scholar]
- 101.Marriott BP, Olsho L, Hadden L, Connor P. Intake of added sugars and selected nutrients in the United States, National Health and Nutrition Examination Survey, 2003–2006. Crit Rev Food Sci Nutr. 2010;50:228–58 [DOI] [PubMed] [Google Scholar]