Abstract
Dicalcium phosphate dihydrate (DCPD) cements are attractive biomaterials for bone repair, and a number of different DCPD cement formulations have been proposed in the literature. In this study we have specifically compared monocalcium phosphate monohydrate (MCPM)/hydroxyapatite (HA) and MCPM/β-tricalcium phosphate (β-TCP) formulations to test the hypothesis that DCPD cement chemistry affects the degradation properties and cytocompatibility of the cement. Using simple in vitro models we found that MCPM/β-TCP formulations degraded primarily by DCPD dissolution, which was associated with a slight pH drop and relatively low mass loss. Cytocompatibility testing of cement conditioned culture media revealed no significant change in cell viability relative to the negative control for all of the MCPM/β-TCP formulations. In contrast, the MCPM/HA formulations were prone to undergo rapid conversion of DCPD to HA, resulting in a sharp pH drop and extensive mass loss. A stoichiometric excess of HA in the cement was found to accelerate the conversion process, and significant cytotoxicity was observed for the MCPM/HA formulations containing excess HA. Collectively, these results show that, although the product of the setting reaction is the same, DCPD cements produced with MCPM/HA and MCPM/β-TCP formulations differ significantly in their degradation properties and cytocompatibility. These differences may have important implications for the selection of a DCPD cement formulation for clinical application.
Keywords: calcium phosphate, cement, dicalcium phosphate dihydrate, brushite, degradation, cytocompatibility
1. INTRODUCTION
Dicalcium phosphate dihydrate (DCPD; also known as brushite) cements are attractive biomaterials for bone repair. Calcium phosphate cements in general are osteoconductive and bioactive due to their compositional similarity to bone mineral [1,2]. Furthermore, because of their cementitious nature, they can be molded intraoperatively to conform to irregularly shaped bone defects, which is particularly advantageous for cranio- and maxillofacial reconstruction [3,4], and even fabricated into complex 3D scaffold architechtures for tissue engineering [5–7]. The principal advantage of DCPD cements, however, is the excellent solubility of DCPD at physiologic pH. This property translates to superior biodegradability and resorbability compared to calcium phosphate cements that set to form hydroxyapatite [8].
DCPD cements have conventionally been prepared by mixing β-tricalcium phosphate (β-TCP) with water and an acidic phosphate source, such as monocalcium phosphate monohydrate (MCPM) or phosphoric acid [9–11]. The reaction governing the formation of DCPD from MCPM and β-TCP is
| (Eq. 1) |
Alternatively, our group and others have shown that β-TCP can be replaced with hydroxyapatite (HA) [12–14]. The reaction governing the formation of DCPD from MCPM and HA is
| (Eq. 2) |
Using HA instead of β-TCP has some potential advantages. For example, the solubility differences between HA and β-TCP could make a wider range of setting and resorption properties accessible [15]. Tuning DCPD cement resorption rate by incorporating more basic calcium phosphates is of particular interest, as rapid resorption of DCPD can limit bone apposition [8] and potentially result in sub-optimal bone healing. Finally, HA has superior mechanical properties compared to β-TCP [16], which could be leveraged to improve the mechanical properties of biphasic DCPD based cements.
Because the principal advantage of DCPD is its degradability under physiologic conditions, there has been considerable interest in studying the degradation properties of DCPD cements. For example, Bohner et al. and Grover et al. both studied the degradation properties of β-TCP based DCPD cement formulations in vitro using methods based on soaking in solutions simulating the conditions of the in vivo environment [17–19]. The in vivo degradation properties of β-TCP based DCPD cement formulations have also been studied, with an emphasis on understanding compositional changes [20–24]. Of particular interest in these studies is whether the DCPD dissolves, is resorbed through cell-mediated degradation, or is converted to HA. Conversion of DCPD to HA occurs because DCPD dissolution produces a solution that is supersaturated with respect to HA, which is the most stable calcium orthophosphate phase at pH greater than about 4, thereby leading to HA precipitation [25]. While this process typically occurs slowly due to the slow crystal growth kinetics of HA, it has important implications. First and foremost, because HA has low solubility at physiologic pH and is slowly resorbed [8], conversion of DCPD to HA negates the advantage of biodegradability [9,26]. In addition, conversion of DCPD to HA produces phosphoric acid [25], which may have ramifications for biocompatibility.
The degradation properties of DCPD cements prepared with HA have not been well characterized compared to β-TCP based formulations. We previously studied the in vitro degradation properties of DCPD cements prepared from MCPM and HA and found conversion of DCPD to HA to be a key mechanism [27]. This result was in stark contrast to what has been reported for DCPD cements prepared using β-TCP [17–19], suggesting that, even though the reaction product is the same, the chemistry plays an important role in determining the final properties of the cement. To test this hypothesis, the objective of this study was to directly assess the effects of the cement chemistry on degradation properties through a head-to-head comparison of DCPD cements prepared with MCPM/HA and MCPM/β-TCP formulations. To this end, we subjected cement specimens from both formulations to static degradation in PBS for 14 days and monitored changes in pH, mass loss, and composition. In addition, because conversion of DCPD to HA produces an acidic environment that could potentially be cytotoxic, we used an in vitro assay to directly compare the cytocompatibility of MCPM/HA and MCPM/β-TCP formulations.
2. MATERIALS AND METHODS
2.1. DCPD cement preparation
DCPD cements were prepared with the MCPM/β-TCP and MCPM/HA systems according to Table 1. Briefly, for the MCPM/HA system, MCPM (crystallinity = 91.5%; Strem Chemicals, Newburyport, MA) was dry mixed with poorly crystalline HA (percent crystallinity = 74.8%; Strem Chemicals) in MCPM:HA molar ratios of 4:1, 4:1.5, 4:2, and 4:3, and then combined with 100 mM sodium citrate (Fisher Scientific, Pittsburgh, PA) in a powder to liquid mass ratio (P/L) of 1.0 g/g. Sodium citrate was added as a setting regulator to retard cement hardening during sample preparation [13,14]. Similarly, MCPM and β-TCP (percent crystallinity = 91.8%; Fluka Chemical Corporation, Ronkonkoma, NY) were dry mixed in MCPM:β-TCP molar ratios of 1:1, 1:1.5, 1:2, and 1:3 and mixed with 100 mM sodium citrate in a P/L of 1.0 g/g. The molar ratio groups for the MCPM/β-TCP system were chosen in order to have an equivalent molar excess of base reactant compared to the MCPM/HA groups, as can be seen from equations 1 and 2. It should be noted, however, that this experimental design resulted in similar but not identical Ca:P ratios due to the different basicities of HA and β-TCP. The percent crystallinity of all reactant powders was determined by x-ray diffraction analysis. The data were acquired using a Bruker D8 Focus instrument equipped with a Cu Kα source and 1D high speed Lynxeye detector (Bruker AXS, Madison, WI). Analysis was performed using Bruker DiffracPlus EVA software and the ICDD PDF2 database for indexing patterns.
Table 1.
DCPD cement formulations.a
| MCPM:HA | MCPM:β-TCP | Equivalents of excess baseb |
|---|---|---|
| 4:1 | 1:1 | 0 |
| 4:1.5 | 1:1.5 | 0.5 |
| 4:2 | 1:2 | 1 |
| 4:3 | 1:3 | 2 |
Presented as molar ratios.
Calculated according to equations 1 and 2.
Cylindrical specimens for each experimental group were prepared by manually pressing the unhardened cement paste into Teflon® molds which had nominal dimensions of 3 mm diameter and 7 mm height (mold tolerance: diameter = 3.05 ± 0.06 mm). After allowing the cements to set at room temperature in air for approximately 30 min, the specimens were removed from the mold and dried under vacuum in a dessicator chamber for 2 days prior to use in order to obtain initial masses prior to degradation.
2.2. Evaluation of degradation properties
Cement degradation was characterized using an in vitro model based on static soaking in PBS (pH = 7.4; from Fisher Scientific). To characterize changes in pH and mass, individual samples from each experimental group were placed separately into glass vials containing 3 ml of PBS, resulting in a cement surface area to liquid volume ratio of approximately 50 mm2/ml. The submerged cement samples were then incubated at 37°C for up to 14 days with no soaking media changes. Three specimens from each group were removed from the incubator at 2, 4, 6, 8, 10, 12, and 14 days. The pH of the PBS was measured with a pH meter (Denver Instruments, Arvada, CO), which was calibrated with the appropriate buffer solutions prior to each use. Cement specimens were then dried under vacuum in a dessicator chamber for 2 days and weighed to determine the percent change in mass.
In addition, to characterize changes in composition, samples of each molar ratio were removed at 6 and 14 days, dried for 2 days in a vacuum dessicator chamber, and then analyzed by powder x-ray diffraction. Day 0 cement specimens were also analyzed for comparison. Briefly, the dried cement was crushed to a fine powder using a mortar and pestle, dispersed on a glass slide in acetone, and then analyzed on a Siemens D5000 automated powder x-ray diffractometer equipped with a Cu tube and graphite monochromator (Bruker AXS). The powder was scanned at 40 kV and 30 mA from 5° to 40° (2θ) in 0.02° increments at a scan speed of 1°/min. The resultant x-ray diffraction patterns were compared to Joint Committee on Powder Diffraction Standards – Powder Diffraction Files (JCPDSPDF) in order to determine the phases present.
2.3. Cytocompatibility assay
To test the effects of cement chemistry on cytocompatibility, cylindrical specimens from each experimental group were sterilized by soaking in 70% ethanol for 30 min, dried, and then soaked individually in cell culture medium (Dulbecco’s modified eagles medium (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA)). As in the degradation study, each cement specimen was soaked in 3 ml of media to maintain a consistent cement surface area to liquid volume ratio between experiments (~ 50 mm2/ml). After 24 hours, the conditioned media was removed with a pipette. The effects of this conditioned media on cell viability were then evaluated on murine mesenchymal stem cells, which were isolated and cultured as we previously described [28]. The cells were added to a 96 well plate at 5,000 cells per well. After the cells had adhered to plate, 100 μL of the conditioned media was added to each well. Non-conditioned medium which had not been exposed to DCPD cement was used as a negative control. After 24 hours, the media was removed, the plate was rinsed with PBS, and the Cell Titer-Glo Luminescent Cell Viability Assay (Promega) was used to assess viability after treatment. Importantly, this assay measures intracellular ATP, which is an indirect measure of cell number. Thus, the luminescence data are presented as a percentage of the negative control (n = 5).
2.4. Statistical analysis
Quantitative data for pH and mass loss are presented as the mean plus or minus the standard deviation. An analysis of variance (ANOVA) two factor mixed effects model was used to determine the effects of base reactant and equivalents of excess base reactant on the pH and mass loss at day 14, and cytocompatibility. Significance between experimental groups was determined by post hoc comparisons using Tukey’s method (α = 0.05).
3. RESULTS
3.1. Evolution of pH and Mass Loss During Degradation
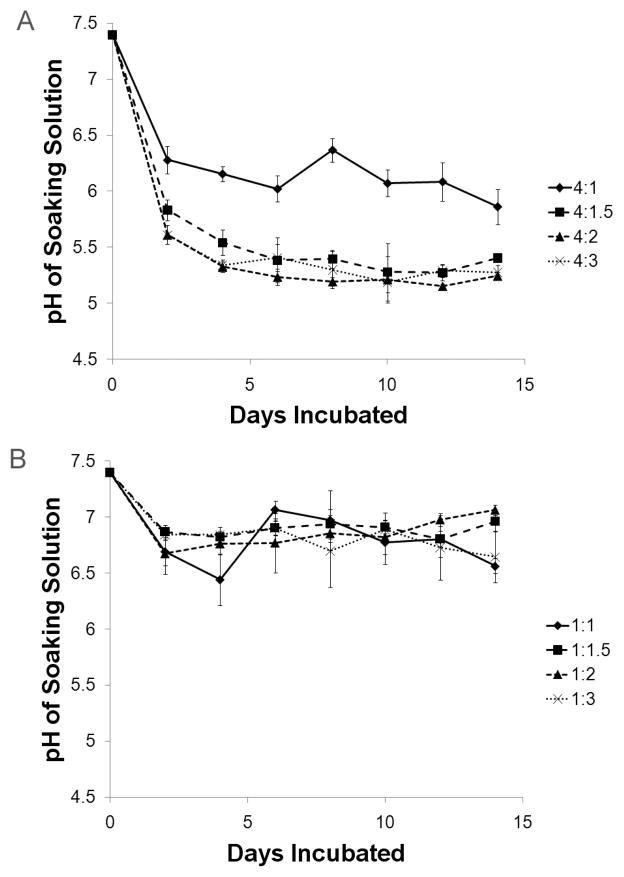
The MCPM/HA and MCPM/β-TCP cements showed markedly different pH profiles over the 14 day degradation period (Figure 1), and the effect of base reactant (i.e. HA or β-TCP) on final pH was statistically significant (p < 0.05 from ANOVA). On day 2 the 4:1, 4:1.5, 4:2, and 4:3 MCPM:HA groups had pHs of 6.28 ± 0.12, 5.83 ± 0.09, 5.61 ± 0.09, and 5.61 ± 0.02, respectively. After this sharp initial drop, the pH leveled off to the day 14 values of 5.86 ± 0.16, 5.41 ± 0.02, 5.25 ± 0.04, and 5.28 ± 0.07 for the 4:1, 4:1.5, 4:2, and 4:3 groups, respectively. The 4:1 and 4:1.5 groups were significantly different from all other groups (p < 0.05). In contrast, the pH values for the MCPM/β-TCP cements were much higher and showed no trend with the amount of excess base reactant. The final pH values for the 1:1, 1:1.5, 1:2, and 1:3 groups were 6.56 ± 0.07, 6.96 ± 0.09, 7.06 ± 0.05, and 6.64 ± 0.23, respectively. These values were significantly higher compared to MCPM/HA groups with equivalent amounts of excess base (p < 0.05).
Figure 1.
Comparison of MCPM/HA and MCPM/β-TCP cement pH change during in vitro degradation. (A) MCPM/HA cements. (B) MCPM/β-TCP cements.
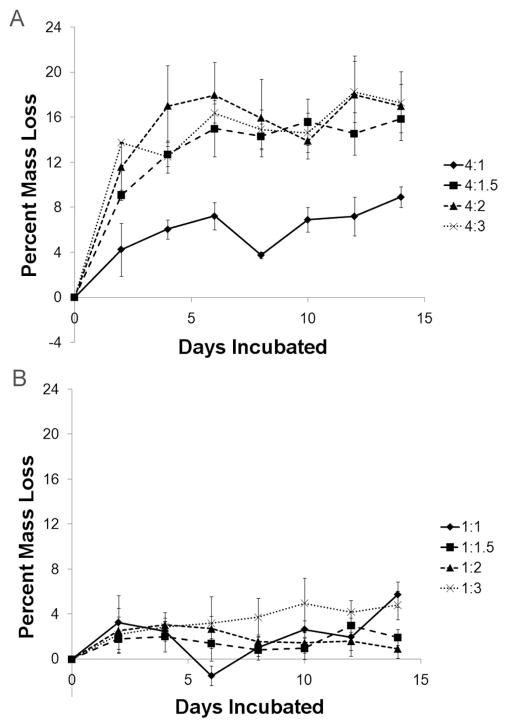
Mass loss followed a nearly identical trend to pH (Figure 2), with the effect of base reactant on final mass loss being statistically significant (p < 0.05 from ANOVA). After 2 days the 4:1, 4:1.5, 4:2, and 4:3 MCPM:HA groups had lost 4.27 ± 2.34 %, 9.10 ± 0.45 %, 11.58 ± 2.18 %, and 13.77 ± 0.05 % of their masses, respectively. As with pH, the values leveled off after a sharp initial change, with the final mass loss values being 8.94 ± 0.91 %, 15.87 ± 1.22 %, 17.01 ± 3.05 %, and 17.32 ± 1.64 % for the 4:1, 4:1.5, 4:2, and 4:3 groups, respectively. The 4:1 group was significantly different from all other groups (p < 0.05). Mass loss in the MCPM/β-TCP cements was much lower and showed no trend with the amount of excess base reactant. The final mass loss values for the 1:1, 1:1.5, 1:2, and 1:3 groups were 5.75 ± 1.11 %, 1.93 ± 0.70 %, 0.93 ± 0.84 %, and 4.79 ± 1.27 %, respectively. These values were significantly lower compared to MCPM/HA groups with equivalent amounts of excess base (p < 0.05).
Figure 2.
Comparison of MCPM/HA and MCPM/β-TCP cement mass loss during in vitro degradation. (A) MCPM/HA cements. (B) MCPM/β-TCP cements.
3.2. Compositional Changes During Degradation
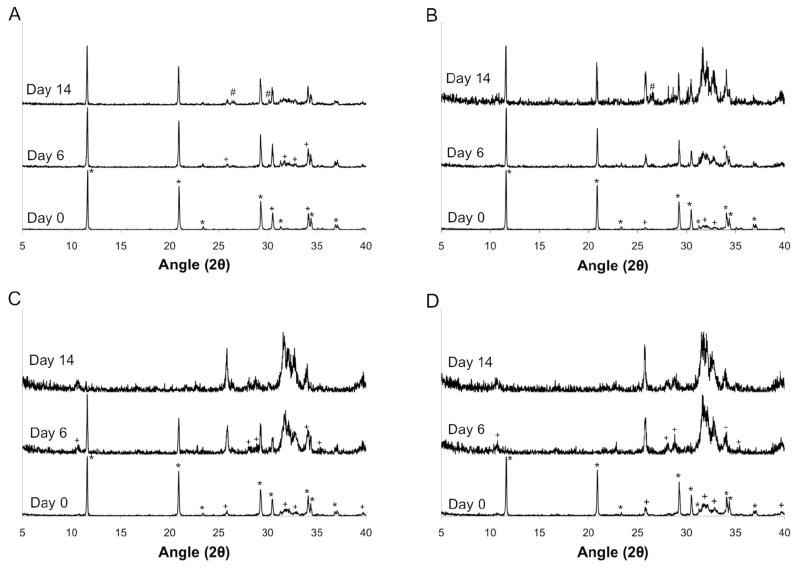
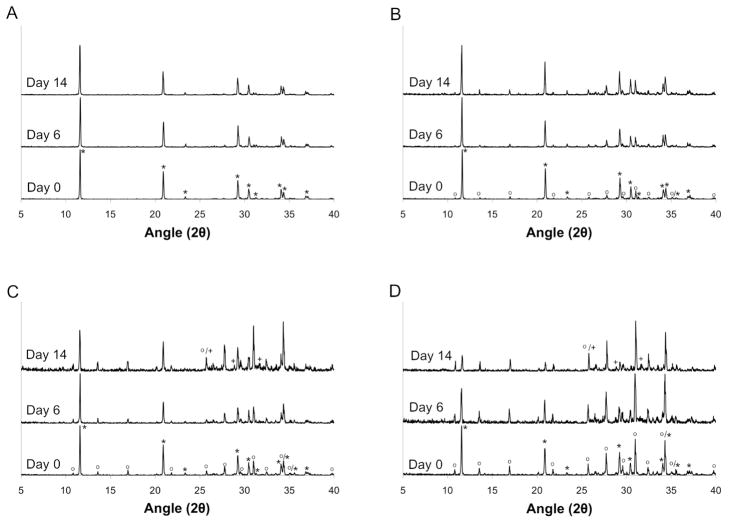
Phase composition of the MCPM/HA and MCPM/β-TCP cements was evaluated at day 0 to determine the phases initially present and their relative amounts. Initially, the 4:1 MCPM:HA group consisted of pure DCPD. The other MCPM/HA groups were biphasic, with the relative amount of HA increasing with increasing stoichiometric excess, as expected (Figure 3). Similarly, the 1:1 MCPM/β-TCP group consisted of only DCPD, whereas the other MCPM/β-TCP groups contained increasing amounts of β-TCP with increasing stoichiometric excess (Figure 4).
Figure 3.
Powder x-ray diffraction patterns for MCPM/HA cements at days 0, 6, and day 14 of the degradation study. (A) 4:1, (B) 4:1.5, (C) 4:2, and (D) 4:3 MCPM:HA molar ratios. Note: * = DCPD (00-009-0077), + = HA (00-009-0432), # = DCP (00-009-0080).
Figure 4.
Powder x-ray diffraction patterns for MCPM/β-TCP cements at days 0, 6, and day 14 of the degradation study. (A) 1:1, (B) 1:1.5, (C) 1:2, and (D) 1:3 MCPM:β-TCP molar ratios. Note: * = DCPD (00-009-0077), o = β-TCP (00-009-0169), + = HA (00-009-0432).
All experimental groups were analyzed again at days 6 and 14 in order to qualitatively evaluate compositional changes during degradation. First considering the MCPM/HA cements, it is clear that by day 6 the relative fraction of HA in all four experimental groups was increased compared to the day 0 results. As expected, a greater amount of HA was present as the stoichiometric excess of HA increased. Interestingly, the 4:3 MCPM:HA group consisted of pure HA at day 6. By day 14, the relative fraction of HA had increased further. DCPD was still the predominant phase in the 4:1 group, although small amounts of HA and anhydrous dicalcium phosphate (DCP) were present. Similarly, DCPD and a small amount of DCP were also present in the 4:1.5 group at 14 days, but the relative amount of HA was markedly increased compared to day 6. The 4:2 and 4:3 groups consisted of pure HA at day 14.
While the appearance of substantial amounts of poorly crystalline HA was observed in the MCPM/HA cements, the MCPM/β-TCP cements consisted of predominantly DCPD and unreacted β-TCP at both day 6 and day 14. The major change compared to the day 0 results was a decrease in the percent of DCPD and a concomitant increase in the percent of β-TCP. Small amounts of HA were noted in the 1:2 and 1:3 groups at day 14, but the relative fraction of HA was substantially lower than in the MCPM/HA cements.
3.3. Cell Viability After Exposure to Conditioned Media
The pH of the cell culture media was clearly affected by the MCPM/HA cements. The media used to soak the 4:1.5, 4:2, and 4:3 MCPM:HA cements was orange-yellow in color. Cell culture media contains phenol red as a pH indicator. Thus, this color change was indicative of a drop in pH. In contrast, no color change in the media used to soak the 4:1 MCPM:HA or any of the MCPM:β-TCP formulations was apparent, suggesting minimal change in pH.
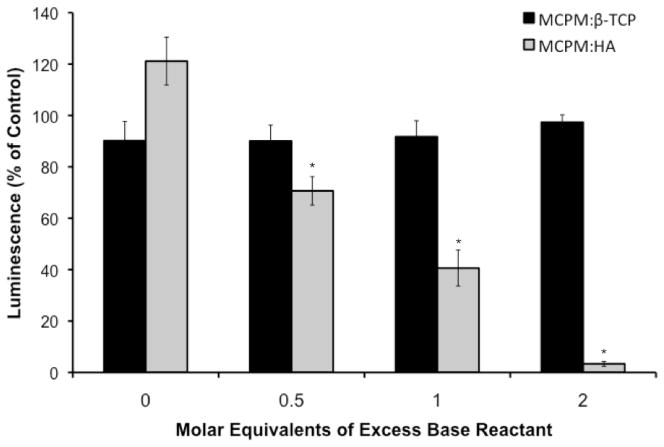
Addition of the DCPD cement conditioned media to murine mesenchymal stem cells for 24 h had a significant effect on cell viability (Figure 5). The luminescent signals measured for cells treated with 1:1, 1:1.5, 1:2, and 1:3 MCPM:β-TCP were 90.16 ± 7.50, 90.03 ± 6.24, 91.72 ±6.21, and 97.37 ± 2.88 percent of the negative control, indicating minimal cytotoxicity. The differences between these experimental groups were not statistically significant. The results for the MCPM:HA cements were markedly different. The 4:1, 4:1.5, 4:2, and 4:3 MCPM:HA groups had relative luminescent signals of 121.12 ± 9.29, 70.57 ± 5.53, 40.61 ± 7.00, and 3.33 ± 0.96 percent compared to the negative control. The differences between the four MCPMA:HA experimental groups were statistically significant (p < 0.05). The values for the 4:1.5, 4:2, and 4:3 MCPM:HA cements were also significantly lower compared to the stoichiometrically equivalent MCPM:β-TCP formulations (i.e. 1:1.5, 1:2, and 1:3 MCPM:β-TCP, respectively; p < 0.05).
Figure 5.
Comparison of MCPM/HA and MCPM/β-TCP cement cytocompatibility. Note: * indicates a statistically significant decrease compared to the other MCPM/HA formulations, as well as the equivalent MCPM/β-TCP formulation (p < 0.05).
4. DISCUSSION
Characterizing and understanding the degradation properties of calcium phosphate cements is critical to assess their utility as biomaterials for bone repair. In this study we tested the hypothesis that DCPD cement chemistry affects the in vitro degradation properties and cytocompatibility of the cement product. We specifically investigated the effects of the base reactant in the formulation by directly comparing MCPM/β-TCP and MCPM/HA formulations.
In order to evaluate the results of this study, it is important to recognize that three mechanisms can contribute to the degradation of DCPD cements in our in vitro system: dissolution, disintegration, and conversion [18,29,30]. Dissolution occurs when DCPD is placed in a solution undersaturated in calcium and phosphate ions. It directly leads to mass loss in the cement and proceeds according to the following equation:
| (Eq. 3) |
High degrees of dissolution can also lead to disintegration of the cement because of microstructural changes [18,19], which further increases mass loss and can also be detrimental to mechanical properties. Once the solubility limit is reached and the solution is saturated with respect to DCPD, further dissolution can only proceed if calcium and phosphate are removed from the solution. For a closed system, such as our in vitro static degradation model, this removal process can only occur via a precipitation reaction. Precipitation can occur, since DCPD is only metastable at pH greater than about 4 and its dissolution supersaturates the solution with respect to HA [31]. As a result, the third potential degradation mechanism for DCPD cements is conversion of DCPD to a more basic species such as HA. Importantly, after conversion to HA begins to occur in the cement, DCPD dissolution can resume, which again supersaturates the solution with respect to HA, leading to further conversion. The overall reaction for the conversion of DCPD to HA is
| (Eq. 4) |
Importantly, this process results in both a progressive pH drop and a net decrease in mass (HA/DCPD mass ratio for conversion is ≈ 0.58), and will proceed until the DCPD-HA singularity point is reached. This process can be understood by considering the solubility isotherms of DCPD and HA (for more in depth discussion we recommend references [15] and [25]).
Analyzing the pH and mass loss data in Figures 1 and 2, differences between the MCPM/HA cements and MCPM/β-TCP cements are clear. Notably, pH drop and mass loss were relatively low for the MCPM/β-TCP cements, and there was no trend with molar excess of β-TCP. This data indicates dissolution as the key degradation mechanism, as all four MCPM/β-TCP groups appear to have reached the solubility limit, with no further dissolution occurring. This conclusion is supported by the powder x-ray diffraction data presented in Figure 4. All groups showed an increase in the fraction of β-TCP at days 6 and 14 due to DCPD dissolution. Only very small amounts of HA were found to be present in the 1:2 and 1:3 groups at day 14. Overall, these results are consistent with published literature on in vitro degradation of β-TCP based DCPD cement formulations. For example, Grover et al. studied the degradation of DCPD cements prepared from β-TCP and orthophosphoric acid and reported roughly 8% mass loss and minimal conversion to HA after 14 days of static soaking in PBS [18]. In a subsequent study of DCPD cements prepared from β-TCP and pyrophosphoric acid published by the same group, conversion of DCPD to HA was not noted over a 90 day period in either PBS or serum [19].
In contrast to what was observed for the MCPM/β-TCP, the MCPM/HA cements showed significant pH drop and underwent extensive mass loss, suggesting accelerated conversion of DCPD to HA as the key degradation mechanism in this formulation. As alluded to earlier, the only way for pH drop and mass loss to be increased in the MCPM/HA cements, assuming that saturation was reached for the MCPM/β-TCP cements, is if conversion to HA were occurring. Indeed, powder x-ray diffraction revealed the presence of substantial amounts of HA in all of the MCPM/HA cements at days 6 and 14. The 4:1 MCPM:HA group initially consisted of pure DCPD. The presence of a comparatively larger fraction of HA in this group alone compared to the MCPM/β-TCP cements is indicative of accelerated conversion to HA in the MCPM/HA system. However, further consideration of the other MCPM/HA cements provides additional confirmation of accelerated conversion of DCPD to HA compared to MCPM/β-TCP cements and also provides insight to the mechanism.
The 4:1.5, 4:2, and 4:3 MCPM:HA cements had a significantly lower pH and significantly higher mass loss compared to the 4:1 group. Furthermore, they contained an even greater fraction of HA at days 6 and 14, with the 4:3 group consisting of pure HA after just 6 days (Figure 3). Importantly, the approximately 15% mass loss observed over the two week degradation period cannot account for the extent of disappearance of DCPD observed by XRD. Although no significant differences in pH or mass loss were observed between the 4:1.5, 4:2, and 4:3 MCPM:HA groups, these results suggest that conversion to HA is accelerated by the presence of unreacted HA in the cement. Recognizing that crystal nucleation is the rate limiting step in the conversion of DCPD to HA [21], it is likely that unreacted HA facilitates conversion by providing nucleation sites for HA precipitation. It is possible that a small amount of unreacted HA, undetectable by x-ray diffraction, was present in the 4:1 MCPM:HA group and led to the accelerated conversion to HA compared to the MCPM/β-TCP cements, which lacked inherent nucleation sites for HA precipitation, but at a slower rate compared to the 4:1.5, 4:2, and 4:3 MCPM:HA groups. Interestingly, a small amount of HA formation was observed in the MCPM/β-TCP cements, but did not lead to rapid conversion to HA over the course of our experiments. While epitaxial crystal growth of HA from DCPD does not occur, HA precipitation is thought to slow conversion by creating a barrier to further dissolution [32]. Thus, it appears that that in MCPM/HA cements the presence of unreacted HA crystals throughout the cement provides readily accessible nucleation sites for precipitation without hindering further DCPD dissolution.
Accelerated conversion of DCPD to HA in the MCPM/HA formulations compared to the MCPM/β-TCP formulations is an important finding, as rapid conversion to HA could potentially nullify the principal advantage of DCPD cements, which is there excellent resorbability [19]. Additionally, the observation that excess HA in MCPM/HA formulations further accelerates conversion of DCPD to HA is also important. Currently available commercial DCPD cement formulations are biphasic and contain excess base reactant. For example, ChronOS Inject™ (Synthes, Inc., West Chester, PA), which is an MCPM/β-TCP formulation, consists of approximately 15 wt % of unreacted β-TCP powder plus an additional 30 wt % β-TCP granules after the setting reaction is complete [26]. Excess base reactant is desirable for modulating cement properties such as acidity of the paste during setting, mechanical properties, and resorption rate. However, tuning of the cement properties through a biphasic approach may not be feasible in the MCPM/HA system due to the role of unreacted HA in accelerating the conversion of DCPD to HA.
In addition to affecting the cement properties during degradation, a propensity towards rapid conversion to HA in the MCPM/HA cements could have significant implications for biocompatibility because of the phosphoric acid produced. We hypothesized that the increased acidity resulting from accelerated conversion of DCPD to HA in the MCPM/HA system would have a cytotoxic effect compared to MCPM/β-TCP formulations. To test this hypothesis, we used an in vitro biocompatibility assay to directly compare the effects of MCPM/HA and MCPM/β-TCP cements on the viability of murine mesenchymal stem cells. After 24 hours in cell culture media conditioned with DCPD cement prepared with the MCPM/β-TCP system, the percent of viable cells was virtually unaffected (Figure 5). These results agree with published data on MCPM/β-TCP formulations for DCPD cements, which have shown good cytocomatibility with osteoblast and macrophage cell lines [32–34]. In contrast, while the 4:1 MCPM:HA group did not negatively impact cell viability, the 4:1.5, 4:2, and 4:3 MCPM:HA groups showed significantly lower luminescence value compared to the negative control (p < 0.05 for comparisons between all MCPM/HA groups and for comparisons between the groups with equivalent excess of base). Cell viability was reduced to nearly zero for the 4:3 group. This sharp decline in cell viability is most likely the result of rapid phosphoric acid production and acidification of the cell culture media. Considering that this acidification occurred over just 24 h in vitro, this result suggests that the propensity towards rapid conversion of DCPD to HA in MCPM/HA formulations may have an effect on in vivo biocompatibility.
5. CONCLUSIONS
In this study we have used simple in vitro models to evaluate the effects of DCPD cement chemistry on the cement degradation properties and cytocompatibility. We have specifically tested MCPM/HA and MCPM/β-TCP formulations for DCPD cements in a head-to-head comparison. Our results clearly show that major differences exist between the MCPM/HA and MCPM/β-TCP formulations. Specifically, while conversion to HA occurs in DCPD cements prepared with β-TCP, it is slow due to the slow crystal growth kinetics of HA. In contrast, our results definitively show that rapid conversion of DCPD to HA is a key factor in the MCPM/HA cement system, and that excess HA further accelerates the conversion process. Because conversion to HA could limit the resorption of the cement and potentially have a negative effect on biocompatibility, future in vivo characterization of MCPM/HA cement formulations will be essential in assessing their potential for clinical use as bone repair materials.
Acknowledgments
We thank Prof. Jeffery Swope of the IUPUI Department of Geological Science for the use of his x-ray diffractometer, and Ms. Patricia Metcalf of the Purdue University School of Materials Science and Engineering for her assistance in determining the percent crystallinity of the calcium phosphate powders. This work was supported in part by the Indiana University School of Dentistry Professional Development Fund (TGC) and by National Institutes of Health grant K08 HL75253 (WSG). WSG is Medical Director for General BioTechnology, LLC; The Genesis Bank, LLC; and Renovocyte, LLC.
References
- 1.Bohner M. Calcium orthophosphates in medicine: from ceramics to calcium phosphate cements. Injury. 2000;31 (Suppl 4):37–47. doi: 10.1016/s0020-1383(00)80022-4. [DOI] [PubMed] [Google Scholar]
- 2.LeGeros RZ. Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop Relat Res. 2002;395:81–98. doi: 10.1097/00003086-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Gilardino MS, Cabiling DS, Bartlett SP. Long-term follow-up experience with carbonated calcium phosphate cement (Norian) for cranioplasty in children and adults. Plast Reconstr Surg. 2009;123:983–994. doi: 10.1097/PRS.0b013e318199f6ad. [DOI] [PubMed] [Google Scholar]
- 4.Gómez E, Martín M, Arias J, Carceller F. Clinical applications of Norian SRS (calcium phosphate cement) in craniofacial reconstruction in children: our experience at Hospital La Paz since 2001. J Oral Maxillofac Surg. 2005;63:8–14. doi: 10.1016/j.joms.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Alge DL, Bennet J, Treasure T, Voytik-Harbin S, Goebel WS, Chu T-MG. Poly(propylene fumarate) reinforced dicalcium phosphate dihydrate cement composites for bone tissue engineering. J Biomed Mater Res A. doi: 10.1002/jbm.a.34130. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alge DL, Chu T-MG. Calcium phosphate cement reinforcement by polymer infiltration and in situ curing: a method for 3D scaffold reinforcement. J Biomed Mater Res A. 2010;94:547–555. doi: 10.1002/jbm.a.32742. [DOI] [PubMed] [Google Scholar]
- 7.Habibovic P, Gbureck U, Doillon CJ, Bassett DC, van Blitterswijk CA, Barralet JE. Osteoconduction and osteoinduction of low-temperature 3D printed bioceramic implants. Biomaterials. 2008;29:944–953. doi: 10.1016/j.biomaterials.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Apelt D, Theiss F, El-Warrak AO, Zlinszky K, Bettschart-Wolfisberger R, Bohner M, et al. In vivo behavior of three different injectable hydraulic calcium phosphate cements. Biomaterials. 2004;25:1439–1451. doi: 10.1016/j.biomaterials.2003.08.073. [DOI] [PubMed] [Google Scholar]
- 9.Tamimi F, Sheikh Z, Barralet J. Dicalcium phosphate cements: brushite and monetite. Acta Biomater. 2012;8:474–487. doi: 10.1016/j.actbio.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Mirtchi AA, Lemaitre J, Terao N. Calcium phosphate cements: study of the beta-tricalcium phosphate/monocalcium phosphate system. Biomaterials. 1989;10:475–480. doi: 10.1016/0142-9612(89)90089-6. [DOI] [PubMed] [Google Scholar]
- 11.Bohner M, Van Landuyt P, Merkle HP, Lemaitre J. Composition effects on the pH of a hydraulic calcium phosphate cement. J Mater Sci Mater Med. 1997;8:675–681. doi: 10.1023/a:1018583706335. [DOI] [PubMed] [Google Scholar]
- 12.Barralet JE, Lilley KJ, Grover LM, Farrar DF, Ansell C, Gbureck U. Cements from nanocrystalline hydroxyapatite. J Mater Sci Mater Med. 2004;15:407–411. doi: 10.1023/b:jmsm.0000021111.48592.ab. [DOI] [PubMed] [Google Scholar]
- 13.Alge DL, Santa Cruz G, Goebel WS, Chu T-MG. Characterization of dicalcium phosphate dihydrate cements prepared using a novel hydroxyapatite-based formulation. Biomed Mater. 2009;4:025016. doi: 10.1088/1748-6041/4/2/025016. [DOI] [PubMed] [Google Scholar]
- 14.Santa Cruz Chavez G, Alge DL, Chu T-MG. Additive concentration effects on dicalcium phosphate dihydrate cements prepared using monocalcium phosphate monohydrate and hydroxyapatite. Biomed Mater. 2011;6:065007. doi: 10.1088/1748-6041/6/6/065007. [DOI] [PubMed] [Google Scholar]
- 15.Dorozhkin SV. Self-setting calcium orthophosphate formulations: cements, concretes, pastes and putties. Int J Mat Chem. 2011;1:1–48. [Google Scholar]
- 16.Wagoner Johnson AJ, Herschler BA. A review of the mechanical behavior of CaP and CaP/polymer composites for applications in bone replacement and repair. Acta Biomater. 2011;7:16–30. doi: 10.1016/j.actbio.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Bohner M, Merkle HP, Lemaître J. In vitro aging of a calcium phosphate cement. J Mater Sci Mater Med. 2000;11:155–162. doi: 10.1023/a:1008927624493. [DOI] [PubMed] [Google Scholar]
- 18.Grover LM, Knowles JC, Fleming GJP, Barralet JE. In vitro ageing of brushite calcium phosphate cement. Biomaterials. 2003;24:4133–4141. doi: 10.1016/s0142-9612(03)00293-x. [DOI] [PubMed] [Google Scholar]
- 19.Grover LM, Gbureck U, Wright AJ, Tremayne M, Barralet JE. Biologically mediated resorption of brushite cement in vitro. Biomaterials. 2006;27:2178–2185. doi: 10.1016/j.biomaterials.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Klammert U, Ignatius A, Wolfram U, Reuther T, Gbureck U. In vivo degradation of low temperature calcium and magnesium phosphate ceramics in a heterotopic model. Acta Biomater. 2011;7:3469–3475. doi: 10.1016/j.actbio.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Bohner M, Theiss F, Apelt D, Hirsiger W, Houriet R, Rizzoli G, et al. Compositional changes of a dicalcium phosphate dihydrate cement after implantation in sheep. Biomaterials. 2003;24:3463–3474. doi: 10.1016/s0142-9612(03)00234-5. [DOI] [PubMed] [Google Scholar]
- 22.Frayssinet P, Gineste L, Conte P, Fages J, Rouquet N. Short-term implantation effects of a DCPD-based calcium phosphate cement. Biomaterials. 1998;19:971–977. doi: 10.1016/s0142-9612(97)00163-4. [DOI] [PubMed] [Google Scholar]
- 23.Penel G, Leroy N, Van Landuyt P, Flautre B, Hardouin P, Lemaître J, et al. Raman microspectrometry studies of brushite cement: in vivo evolution in a sheep model. Bone. 1999;25:81S–84S. doi: 10.1016/s8756-3282(99)00139-8. [DOI] [PubMed] [Google Scholar]
- 24.Constantz BR, Barr BM, Ison IC, Fulmer MT, Baker J, McKinney L, et al. Histological, chemical, and crystallographic analysis of four calcium phosphate cements in different rabbit osseous sites. J Biomed Mater Res. 1998;43:451–461. doi: 10.1002/(sici)1097-4636(199824)43:4<451::aid-jbm13>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 25.Fernández E, Gil FJ, Ginebra MP, Driessens FC, Planell JA, Best SM. Calcium phosphate bone cements for clinical applications. Part I: solution chemistry. J Mater Sci Mater Med. 1999;10:169–176. doi: 10.1023/a:1008937507714. [DOI] [PubMed] [Google Scholar]
- 26.Theiss F, Apelt D, Brand B, Kutter A, Zlinszky K, Bohner M, et al. Biocompatibility and resorption of a brushite calcium phosphate cement. Biomaterials. 2005;26:4383–4394. doi: 10.1016/j.biomaterials.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 27.Alge DL, Goebel WS, Chu T-MG. In vitro degradation and cytocompatibility of dicalcium phosphate dihydrate cements prepared using the monocalcium phosphate monohydrate/hydroxyapatite system reveals rapid conversion to HA as a key mechanism. J Biomed Mater Res B Appl Biomater. 2012;100:595–602. doi: 10.1002/jbm.b.31938. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Chen S, Yuan J, Yang Y, Li J, Ma J, et al. Mesenchymal stem/progenitor cells promote the reconstitution of exogenous hematopoietic stem cells in Fancg−/− mice in vivo. Blood. 2009;113:2342–2351. doi: 10.1182/blood-2008-07-168138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu J, Descamps M, Dejou J, Koubi G, Hardouin P, Lemaitre J, et al. The biodegradation mechanism of calcium phosphate biomaterials in bone. J Biomed Mater Res. 2002;63:408–412. doi: 10.1002/jbm.10259. [DOI] [PubMed] [Google Scholar]
- 30.Alkhraisat MH, Mariño FT, Retama JR, Jerez LB, López-Cabarcos E. Beta-tricalcium phosphate release from brushite cement surface. J Biomed Mater Res A. 2008;84:710–717. doi: 10.1002/jbm.a.31381. [DOI] [PubMed] [Google Scholar]
- 31.Fernández E, Gil FJ, Ginebra MP, Driessens FC, Planell JA, Best SM. Calcium phosphate bone cements for clinical applications. Part II: precipitate formation during setting reactions. J Mater Sci Mater Med. 1999;10:177–183. doi: 10.1023/a:1008989525461. [DOI] [PubMed] [Google Scholar]
- 32.Fulmer MT, Brown PW. Hydrolysis of dicalcium phosphate dihydrate to hydroxyapatite. J Mater Sci Mater Med. 1998;9:197–202. doi: 10.1023/a:1008832006277. [DOI] [PubMed] [Google Scholar]