Abstract
Cystic Echinococcosis also known as cystic hydatid disease is a parasitic infection endemic in many parts of the world. Humans are accidental intermediate hosts with cysts most commonly developing in the liver. This case describes a rare presentation of hydatid disease following trauma to the liver. Intraparenchymal cyst rupture led to haemodynamic instability with release of the parasites protoscolices into hepatic venules producing severe life threatening anaphylaxis.
INTRODUCTION
Cystic Echinococcosis (CE), also known as cystic hydatid disease is a parasitic infection endemic in many parts of the world. This tapeworm requires two mammalian hosts to complete its life cycle. These are dogs (definitive host) and sheep or other domestic herbivores (intermediate hosts) with humans being accidental intermediate hosts. The adult worm develops in the small intestine of the definitive host, producing eggs which are excreted in faeces. Egg ingestion leads to release of the embryo into the duodenum of the intermediate host with penetration of the intestinal wall leading to spread via the portal circulation. Development of the larval stage occurs most frequently in the liver with protoscolices being produced. These are then ingested by the carnivorous definitive host. Humans become infected by ingesting eggs of the parasite via infected foodstuffs or via contact with definitive hosts. Patients with liver involvement are often asymptomatic, but where symptoms do occur they may present with vague abdominal pain, a secondary liver abscess, cholestatic jaundice or cyst rupture with or without anaphylaxis.
We present a case of anaphylaxis secondary to trauma in previously unrecognised cystic hydatid disease.
CASE REPORT
A 24 year old Turkish male presented to his local emergency department after being assaulted. He was previously well and had lived in the UK for 6 years. He had no known allergies.
On admission he was tachycardic and had increasingly severe abdominal pain. His haemoglobin (Hb) decreased from 15 to 10.7g/dl. A CT scan revealed a large mass in the right lobe of the liver with associated haemorrhage. Urgent transfer to our Liver intensive care unit (LITU) was arranged with packed cells, fresh frozen plasma (FFP) and platelets all being transfused. He was noted to be erythematous and was suspected to have had a drug or transfusion reaction and thus received chlorphenamine.
Repeat CT scan and angiography (Figures 1&2) revealed a 17cm mass occupying the right lobe of the liver with evidence of intrahepatic arterial and portal venous contrast extravasation. There was disruption of the normal right portal venous anatomy with the left portal vein being visible but displaced. Two arterial bleeding points were embolized. The underlying nature of the mass was unknown.
Figure 1.
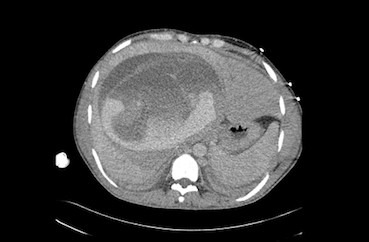
CT scan showing a large liver mass with associated haemorrhage. Detached laminated membrane is visualised.
Figure 2.
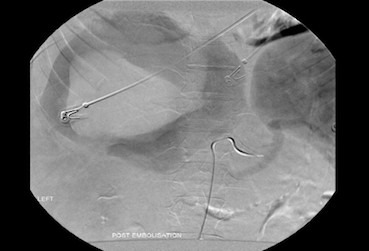
Indirect portography demonstrating a disruption of the intra hepatic right portal vein with extravasation of contrast into a hepatic mass.
On return to LITU he developed acute respiratory distress with a respiratory rate of 40 breaths per minute, saturation of 88-90% and was again erythematous. He was intubated and ventilated, but despite this he continued to deteriorate with a ‘silent chest’ on auscultation. Intravenous and nebulised adrenaline, salbutamol, magnesium sulphate and ketamine were all required to stabilise his condition. IgA deficient FFP was ordered, antibiotics changed and latex and contrast allergy considered.
He subsequently underwent laparotomy. Approximately 1.5 litres of blood stained fluid were drained. The right and left lobes of liver were grossly abnormal with a haematoma behind the porta hepatis and an attenuated portal vein (PV). On inspection, the liver mass/ cyst had not ruptured into the peritoneal cavity. Multiple biopsies were taken for histology. Attempted PV dissection precipitated significant bleeding and was abandoned.
Over the next 48 hours vasopressors were weaned and he was extubated. One further short episode of wheeze was effectively managed and he was transferred to the ward. Biopsies showed irregular liver architecture with fibrosis indicating a chronic process (Figure 3). Higher magnification identified a parasite (a hydatid protoscolex) within a hepatic venule (Figures 4&5) and a diagnosis of hydatid disease (Echinococcus granulosus) was made. He was treated with albendazole 400mg bd and discharged home on day 16.
Figure 3.
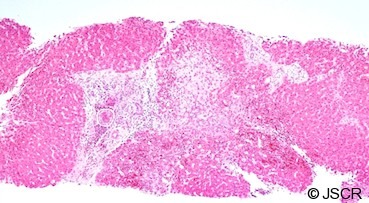
A liver biopsy showing fibrotic change within and outside the portal tracts. Note the organism further magnified in Figures 4&5.
Figure 4.
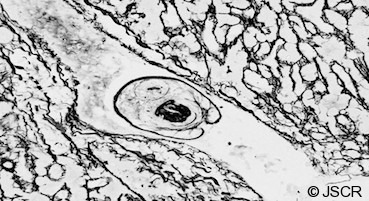
Silver impregnated slide showing a hydatid protoscolex.
Figure 5.
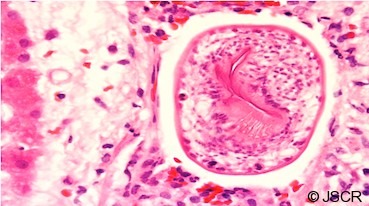
Higher magnification H&E stained image showing a hepatic venule containing a characteristic echinococcal protoscolex with hooklets visible in section.
Elective endocystectomy was performed 6 months after discharge with histology showing no viable germinal membrane or protoscolices.
DISCUSSION
The diagnosis of hydatid was not recognised at initial presentation. Our local population is ethnically diverse but the patient had lived in the UK for six years prior to presentation and had been asymptomatic throughout. Accidental traumatic peritoneal rupture with associated acute hypersensitivity reactions have been reported (1,2) with upper abdominal pain, a mass or symptoms related to mass effect being frequent presentations. Incidental discovery is not uncommon.
Management of hydatid disease of the liver involves both medical and surgical treatment. Total pericystectomy is considered the treatment of choice but anatomical difficulties, as exemplified in this case, can make this option difficult (2). Ruptured hydatid cysts require surgery to provide peritoneal toilet. Ozturk (1) et al have advocated a ‘conservative’ approach of de-roofing the cyst and either draining the residual cavity or obliterating it using omentoplasty or capitonage. Percutaneous aspiration, injection and reaspiration (PAIR) is another technique employed (3). A Cochrane review of the PAIR technique with or without benzimidazole coverage concluded that it may be comparable or superior to surgery (4). Increasing experience of laparoscopic therapy in combination with albendazole therapy has been shown to be safe and efficient (5). Optimal treatment should involve pre and post resection albendazole (6) but complications such as abdominal pain, nausea, vomiting, jaundice, leucopenia and anaemia are described (7). No consensus on duration of treatment has been made.
This is a rare case of anaphylaxis to cystic hydatid disease initiated by leakage into the vasculature following trauma. Cases of anaphylactic shock have been reported as the only manifestation of hydatid disease in cases of rupture into the peritoneal cavity and is considered in endemic regions (1,2,8). Buyuk et al (9) reported a case where hydatid disease led to death with cyst content spreading into the bloodstream. Peritoneal rupture of the cyst did not occur in our case but the hydatid protoscolex found within a hepatic venule (Figures 4&5) indicates that the patient had intravascular exposure to the parasite. Intra portal venous rupture and probable manipulation led to anaphylaxis.
Lewall and McCorkell (10) classified echinococcal cyst rupture into direct, contained and communicating types. The communicating type consists of a tear of the endocyst with escape of contents via biliary radicles or bronchioles. No description of communication with the portal vein exists but the findings in this case and the autopsy report from Buyuk et al (9) suggest the classification should be extended to include this scenario.
REFERENCES
- 1.Ozturk G, Aydinli B, Yildirgan I, Basoglu M, Atamanalp SS, Polat Y, Alper F, Guvendi B, et al. Postraumatic free intraperitoneal rupture of liver cystic echinococcosis: a case series and review of literature. Am J Surg 2007. 194(3): 313–316 [DOI] [PubMed] [Google Scholar]
- 2.Gunay K, Taviloglu K, Berber E, et al. Traumatic Rupture of Hydatid Cysts: a 12 year experience from an endemic region, J Trauma (1999) 46: 164–167 [DOI] [PubMed] [Google Scholar]
- 3.Felice C, Pirola F, Brunetti E, Dughetti S, Strosselli M, Foglieni CS. A new therapeutic approach for hydatid liver cysts: Aspiration and alcohol injection under sonographic guidance. Gastroenterology 1990;98:1366–1368 [DOI] [PubMed] [Google Scholar]
- 4.Nasseri Moghaddam S, Abrishami A, Malekzadeh R. Percutaneous needle aspiration, injection, and reaspiration with or without benzimidazole coverage for uncomplicated hepatic hydatid cysts. Cochrane Database Syst Rev. 2006. Apr 19;(2):CD003623 [DOI] [PubMed] [Google Scholar]
- 5.Dziri C, Haouet K. Fingerhut A Treatment of hydatid Cyst of the liver: where is the evidence? World J Surg (2004) 28: 731–736 [DOI] [PubMed] [Google Scholar]
- 6.Wen H, New RRC, Craig PS. Diagnosis and Treatment of human hydatidosis. British Journal of Clinical Pharmacology (1993) 35: 565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen H, Zou PF, Yao PL, Lu J. Research on chemotherapy of hydatidosis with albendazole. Chin Med J. 1990; 70, 47–49 [Google Scholar]
- 8.Maqbool B, Anwar MS. Hepatic hydatid cyst presenting as anaphylaxis. J Coll Physicians Surg Pak. 2007. Apr;17(4) : 224–5 [PubMed] [Google Scholar]
- 9.Buyuk Y, Turan AA, Uzan I, Aybar Y, Cin O, Kurnaz G. Non-ruptured hydatid cyst can lead to death by spread of cyst content into bloodstream: an autopsy case. Eur J Gastroenterol Hepatol 2005. Jun;17(6):671–3 [DOI] [PubMed] [Google Scholar]
- 10.Lewall DB, McCorkell SJ. Rupture of echinococcal cysts: diagnosis, classification, and clinical implications. Am J Roentgenol 1986; 146: 391–394 [DOI] [PubMed] [Google Scholar]


