Abstract
Background. Black women with breast cancer (BC) in Nigeria have higher mortality rate compared with British women. This study investigated prognostic features of cell proliferation biomarker (Ki-67) in Nigerian breast cancer women. Materials and Methods. The protein expression of Ki-67 was investigated in series of 308 Nigerian women, prepared as a tissue microarray (TMA), using immunohistochemistry. Clinic-pathological parameters, biomarkers, and patient outcome of tumours expressing Ki-67 in Nigerian women were correlated with UK grade-matched series. Results. A significantly larger proportion of breast tumours from Nigerian women showed high Ki-67 expression. Those tumours were significantly correlated with negative expression of the steroid hormone receptors (ER and PgR), p21, p27, E-cadherin, BRCA-1, and Bcl-2 (all P < 0.001), but positively associated with EGFR (P = 0.003), p53, basal cytokeratins: CK56, CK14, triple negative, and basal phenotype using Nielsen's classification (all P < 0.001) compared to UK women. Multivariate analyses showed that race was also associated with BCSS independent of tumour size, lymph node status, and ER status. Conclusion. Ki-67 expression was observed to have contributed to the difference in the BCSS in Nigerian compared with British BC women. Therefore, targeting Ki-67 in the indigenous black women with BC might improve the patient outcome in the black women with BC.
1. Introduction
There are discrepancies in mortality rates among the nationalities with Caucasian women having a low mortality rate compared with black women [1–6]. In African-American women, the effect is more pronounced in younger women compared with European-American women [3, 7]. Although there are international variations in the mortality rates of breast cancer, the explanations that predispose the cells to aggressive tumour phenotype and patient survival are not clear.
Cell proliferation is one of the important driving steps in patients with aggressive tumour phenotype and patient survival [8, 9]. Cell proliferation is controlled by regulatory proteins that ensure orderly progression of the cells through the check points of the cell cycle [10–12]. The cell cycle checkpoints comprise of 4 important phases which are arranged sequentially: G1 phases that prepare their machinery for duplication, S phase is responsible for the genomic materials' duplication, G2 phase is known as intervention phase, and the M phase that controls mitosis [13]. Abnormal cell cycle regulatory protein activities are central to increase cell proliferation, poor maintenance of chromosomal integrity, and therefore encouraging tumour development [11, 12].
KI-67 is one of the proliferative markers strongly linked to cell cycle control. During mitosis, this protein is tightly regulated by protease upon completion of its activity, such that its half life is less than 90 minutes [14, 15]. It is expressed in all proliferative cells both in normal and malignant cells. In addition, it represents easy and reliable methods of assessing the cell cycle pathways particularly in breast cancer [14, 15]. The prognostic significance of this protein in BC of western women has been reported [8, 9, 16, 17]. There is a positive correlation between KI-67 protein expression, cell proliferation rate, and the active phase of the cell cycle in invasive breast carcinoma. High KI-67 is associated with tumours of high grade, large size, and lymph node involvement, basal phenotype, and ER and PR negative and HER-2 positive tumours [18, 19]. KI-67 is also highly expressed among breast tumours from African-American women compared with Caucasian women, most especially among younger women [20, 21].
Although few studies have been reported on prognostic significance of KI-67 on African-American women with breast cancer as one of the reasons for increased likelihood of African-American women presenting with advance stage at diagnosis, these findings might not completely explain the roles of KI-67 in the indigenous African women with BC, because of the differences in the environmental factors. Up until now, the tumour biology that may contribute to the differences observed in the ethnic nationalities is not completely understood especially on the indigenous black women with breast cancer [22, 23]. We therefore, hypotheses that alteration of KI-67 expression may contribute to the tumour biology observed among the ethnic nationalities with BC.
Thus, the aim of this study is to investigate the KI-67 expression using immunohistochemistry in breast tumours from Nigerian women and to compare them to a well-characterised series of BC from Caucasian women living in the UK, in order to establish whether the differences between the two nationalities are due to KI-67 tumour biology.
2. Material and Methods
2.1. Patients
The Nigerian patient cohort comprised formalin-fixed paraffin embedded (FFPE) breast cases from 308 women presenting at the Olabisi Onabanjo University Teaching Hospital, Sagamu, and Histopathology Specialist laboratory, Idi-Araba, Lagos, Nigerian, from January 2002 to December 2008. Clinical history and tumour characteristics including age, menopausal status, tumour type, histological grade, tumour size, lymph node status, and vascular invasion were assessed in a standardised manner for all the patients.
The tissue sections were reevaluated for histological features such as tumour grade and type. Patient outcome and treatment data were retrieved from the patient's records. All patients were treated with combination of classical chemotherapy cyclophosphamide, methotrexate 5FU, and hormonal therapy (tamoxifen). Eighty-five out of the patients (42.5%) received radiotherapy. Patients were followed up for at least 60 months (260 weeks).
A UK patient cohort of 1,902 primary operable invasive breast carcinoma cases was from the well-characterised Nottingham-Tenovus Primary Breast Carcinoma Series consisting of women presenting between 1986 and 1998. All patients were assessed in a standardised manner for clinical history and tumour characteristics [24–27]. Patient management was based on tumour characteristics by the Nottingham Prognostic Index (NPI) and hormone receptor status. Patients with an NPI score ≤3.4 received no adjuvant therapy, and those with a NPI score >3.4 received Tamoxifen if estrogen receptor (ER) positive (±Zoladex if premenopausal) or classical cyclophosphamide, methotrexate, and 5-fluorouracil if ER negative and fit enough to tolerate chemotherapy [28]. Survival data including breast cancer specific survival (BCSS) and disease-free interval (DFI) were maintained on a prospective basis. DFI was defined as the interval (in months) from the date of the primary surgical treatment to the first locoregional (including invasive malignancy and ductal carcinoma in situ) or distant recurrence. BCSS was taken as the time (in months) from the date of the primary surgical treatment to the time of death from breast cancer.
Of this series, 841 cases had complete data on the following immunohistochemical markers: estrogene receptor (ER), progesterone receptor (PgR), cytokeratins (CK5/6, CK14,) EGFR (HER1), HER2, BRCA1, placental-cadherin (P_-cadherin), Epithelial-cadherin (E-cadherin), p53, p21, Bcl-2, MDM2, and MDM4 [24–27]. In order to compare the Nigerian and UK series with respect to biomarkers and patient outcome, a grade-matched UK control groups to the Nigerian cohort, comprising of 308 patients, was generated from the previous patients. Within the UK tumour grade-matched cohort, 202 were selected for patient outcome 122/202 (60.4%), 53/202 (26.2%) had died at 60 months and 80/202 (39.6%), 149/202 (73.8) remained alive in Nigeria and UK respectively.
The Reporting Recommendations for Tumour Marker Prognostic Studies (REMARK) criteria, recommended by McShane et al. [29], were followed. This study was approved by the Medical Advisory Committee, Olabisi Onabanjo University Teaching Hospital and by the Nottingham Research Ethics Committee 2 under the title of “Development of a molecular genetics classification of breast cancer.”
2.2. Tissue Microarray Array Construction
Three hundred and eight samples from Nigerian cohort were constructed as tissue microarrays (TMA) as previously described [30]. Breast tumour cores were taken from each FFPE donor tissue block that has been marked for the most representative points of tumour (both peripherally and centrally). A precision instrument (ALPHELYS MiniCore) was used to take representative cores of tissue (0.6 mm diameter, 3 mm height) from each sample, which was then arrayed into a recipient paraffin block in 11 × 15 core format.
2.2.1. Immunohistochemistry Method
All the biomarkers required antigen retrieval except HER-2 and EGFR. Antigen retrieval was performed by microwaving the slides at 800 W for 10 minutes followed by 560 W for 10 minutes in citrate buffer (1 M sodium citrate at pH of 6.0) followed by cooling in running water immediately. The primary antibody for each biomarker (Table 1) was incubated for 60 minutes at room temperature. Diaminobenzidine tetrahydrochloride (DAB) solution was incubated for 10 minutes after which copper-sulphate solution (0.5% copper sulphate in 0.8% sodium chloride) were applied to the slides and incubated for 10 minutes each and counter stained with haematoxylin for 2-3 minutes, followed by rinsing in tap water. Slides were de-hydrated by immersing in three alcohol baths for 10 seconds and cleared in two xylene baths followed by application of cover slip. Negative and positive controls were performed by omitting the primary antibody and including control tissues as specified by the antibody supplier, respectively. Immunoreactivity expression of the biomarker in the nucleus was considered positive (Figure 1).
Table 1.
Sources, dilution, distribution, cut-offs point, and pretreatment used for revalidation.
| Antibody | Clone | Source | Dilution | Distribution | Scoring System | Cut-offs | Pretreatment | Positive control | Negative control |
|---|---|---|---|---|---|---|---|---|---|
| Bcl-2 | 124 | Dako-Cytomation | 1 : 100 | Cytoplasm | % of positive cells | >10% (positive) | Antigen retrieval microwave | Normal breast acini | Omitting the antibody |
| BRCA1 | Ab-1 (MS110) | Calbiochem | 1 : 150 | Nuclear | % of positive cells | <25% (negative) |
Antigen retrieval microwave | MCF 7 cells | Omitting the antibody |
| Ck5/6 | M7237 | Dako-Cytomation | 1 : 60 | Cytoplasm | % of positive cells | ≥10% (positive) | Antigen retrieval microwave | Known case of CK56 BC | Omitting the antibody |
| E-cadherin | NCH-38 | Dako-Cytomation | 1 : 100 | Cytoplasm and membrane | % of positive cells | ≥100 H score (positive) | Antigen retrieval microwave | Normal gastric mucosa | Omitting the antibody |
| EGFR | 31G7 | Novocastra | 1 : 30 | Membrane | % of positive cells | ≥10% (positive) | Not required | Myoepithelial cells of normal duct in normal mammary gland | Omitting the antibody |
| erbB2 | Polyclonal | Dako-Cytomation | 1 : 100 | Membrane | Table 2.1 | Table 2.1 | Not required | Known case of erbB2 strong BC expression | Omitting the antibody |
| ER | 1D5 | Dako-Cytomation | 1 : 200 | Nuclear | % of positive cells | ≥0 (positive) | Antigen retrieval microwave | Normal breast acini | Omitting the antibody |
| Ki-67 | MIB1 | Dako-Cytomation | 1 : 25 | Nuclear | % of positive cells | <10% (low) |
Antigen retrieval microwave | Human tonsil tissue | Omitting the antibody |
| P-cadherin | NCL-P-cad |
Novocastra |
1 : 200 | Cytoplasm | % of positive cells | ≥5% (positive) | Antigen retrieval microwave | Known case of P-cadherin strong BC expression | Omitting the antibody |
| PgR | PgR | Dako-Cytomation | 1 : 150 | Nuclear | % of positive cells | ≥0 (positive) | Antigen retrieval microwave | Normal breast acini | Omitting the antibody |
| p21 | EA10 | Abcam | 1 : 25 | Nuclear | % of positive cells | ≥10% (positive) | Antigen retrieval microwave | Normal breast acini | Omitting the antibody |
| p53 | DO7 | Novocastra | 1 : 50 | Nuclear | % of positive cells | >10% (negative) |
Antigen retrieval microwave | Normal breast acini | Omitting the antibody |
Figure 1.
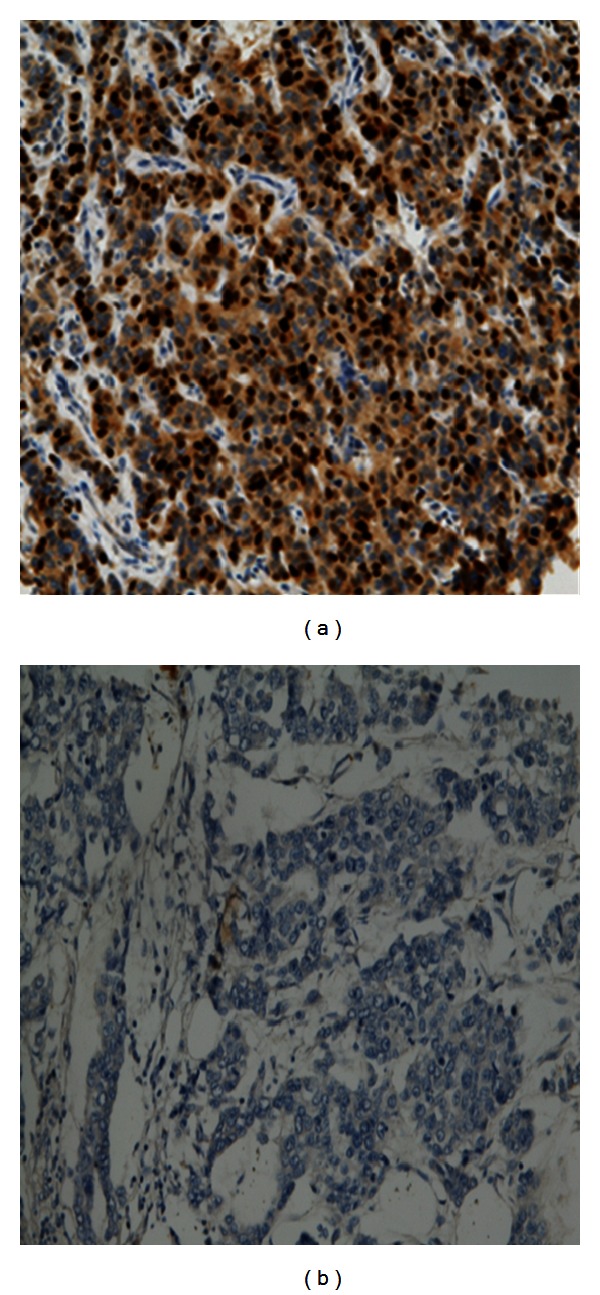
(a) and (b) show positive and negative immunoreactivity of KI-67 in Nigerian breast cancer magnification ×20.
2.2.2. Immunohistochemical Scoring
The scoring was performed using the modified histochemical score (H-score), a semiquantitative assessment. Staining intensity was scored from 0, 1, and 2 to 3, and the percentage of positive cells was determined for each score to produce a final score in the range 0–300. The cases were scored without knowledge of the patient outcome. The TMA samples were scored twice by one observer (JA). The mean of the scores were calculated to reach a final score. A proportion of these were counter scored by an observer (AG) to ensure reproducibility.
Table 1 shows the cut-off points used for the biomarkers analysis [31, 32]. For c-erbB2, the American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer was used for assessment [33]. Equivocal (2+) cases were confirmed by CISH as previously described [34].
For molecular classification, Nielsen's method [35] was used. This comprises of Luminal A (ER, PR positive, and HER 2 negative), Luminal B (ER, PR HER 2 positive), basal (ER, PR, HER-2 negative, and CK5/6 and, or EGFR positive), HER2 (ER negative and HER2 positive), and an unclassified group (ER, PR, HER2 CK5/6, and EGFR negative).
2.3. Statistical Analysis
Statistical analysis was performed using SPSS 16.0 statistical software. Chi-squared analyses were used for interrelationships between the Nigerian and UK series and for comparison with clinicopathological parameters. The Kaplan–Meier survival method and the log-rank test were used for survival curves. Multivariate analyses using Cox proportional hazard regression models were performed, and from the model both the risk factor and 95% confidence intervals were generated. A two-sided P value of <0.05 was considered significant.
3. Results
The relationship between KI-67 expression in grade-matched breast cancer between Nigerian and UK women is summarised in Table 2. A significantly large proportion of breast tumours from Nigerian women showed high KI-67 expression compared with UK women, which accounted for 82.6% and 66.7%, respectively (P < 0.001).
Table 2.
Relationship between KI-67 marker expression in Nigeria and UK series.
| KI-67 | Nigeria (%) | UK (%) | χ 2 value | P value |
|---|---|---|---|---|
| Negative | 46 (17.4) | 82 (33.3) | 85.40 | <0.001 |
| Positive | 218 (82.6) | 164 (66.7) |
In those tumours showing positive KI-67, a significant proportion of breast cancers from the Nigerian series was from patients that were premenopausal (P < 0.001) and diagnosed before 50 years (P < 0.001). Also, the tumours were significantly larger in size (P < 0.01), ductal carcinoma histological type (P < 0.001) with evidence of metastasis into lymph node (P < 0.001) and vascular invasion (P < 0.001) compared with the UK series (Table 3).
Table 3.
Relationship between clinicopathological parameters in Nigerian and UK tumours expressing KI-67.
| Variables | KI-67 positive expression | |||
|---|---|---|---|---|
| Nigeria (%) | UK (%) | χ 2 value | P value | |
| Age (years) | ||||
| ≤50 | 136 (62.4) | 62 (37.8) | 22.65 | <0.001 |
| >50 | 82 (37.6) | 102 (62.2) | ||
| Menopausal | ||||
| Pre | 157 (72.0) | 60 (36.8) | 47.15 | <0.001 |
| Post | 61 (28.0) | 103 (63.2) | ||
| Sizes (cm) | ||||
| ≤2 | 18 (8.3) | 73 (44.5) | 67.79 | <0.001 |
| >2 | 200 (91.7) | 91 (55.5) | ||
| Lymph node involvement | ||||
| Negative | 16 (7.3) | 97 (59.1) | 120.58 | <0.001 |
| Positive | 202 (92.7) | 67 (40.9) | ||
| Vascular invasion | ||||
| Negative | 51 (23.4) | 86 (52.4) | 34.32 | <0.001 |
| Positive | 167 (76.6) | 78 (47.6) | ||
| Tumour type | ||||
| Typical medullary | 3 (1.4) | 1 (0.6) | 43.10 | <0.001 |
| Atypical medullary | 4 (1.8) | 7 (4.3) | ||
| Tubular | 1 (0.5) | 0 (0.0) | ||
| Lobular | 3 (1.4) | 8 (4.9) | ||
| Ductal NST | 189 (86.7) | 105 (64.8) | ||
| Mucinous | 4 (1.8) | 0 (0.0) | ||
| Tubulolobular | 0 (0.0) | 7 (4.3) | ||
| Mixed NST | 14 (6.4) | 30 (18.5) | ||
| Others | 0 (0.0) | 2 (1.2) | ||
NST: no special type. Others: metaplastic, spindle, and alveolar lobular histological type.
In the Nigerian series, positive expression of KI-67 biomarker was significantly correlated with negative expression of the steroid hormone receptors (ER and PgR), p21, p27, E-cadherin, BRCA-1, and Bcl-2 (all P < 0.001). In addition, a significantly larger proportion of Nigerian tumours that showed a positive KI-67 expression had positive relationship with EGFR (P = 0.003), p53, basal cytokeratins: CK56, CK14, triple negative, and basal phenotype using Nielsen's classification (all P < 0.001) compared to UK women. There was no positive correlation between the series in relation to HER-2 and P-cadherin (Table 4).
Table 4.
Relationship between biomarker expression in UK and Nigerian tumours expressing KI-67.
| Variables | KI-67 positive expression | |||
|---|---|---|---|---|
| Nigeria (%) | UK (%) | χ 2 value | P value | |
| BRCA1 | ||||
| Negative | 143 (80.8) | 31 (21.5) | 112.34 | <0.001 |
| Positive | 34 (19.2) | 113 (78.5) | ||
| Bcl-2 | ||||
| Negative | 101 (57.4) | 53 (37.6) | 13.15 | <0.001 |
| Positive | 75 (42.6) | 88 (62.4) | ||
| CK5/6 | ||||
| Negative | 108 (58.7) | 137 (84.6) | 27.90 | <0.001 |
| Positive | 76 (41.3) | 25 (15.4) | ||
| CK14 | ||||
| Negative | 97 (58.8) | 148 (90.8) | 44.5 | <0.001 |
| Positive | 68 (41.2) | 15 (9.2) | ||
| ER | ||||
| Negative | 169 (84.5) | 45 (28.0) | 118.17 | <0.001 |
| Positive | 31 (15.5) | 116 (72.0) | ||
| EGFR | ||||
| Negative | 119 (66.5) | 123 (80.4) | 8.08 | 0.003 |
| Positive | 60 (33.5) | 30 (19.6) | ||
| E-cadherin | ||||
| Negative | 119 (73.5) | 65 (40.1) | 36.7 | <0.001 |
| Positive | 43 (26.5) | 97 (59.9) | ||
| HER-2 | ||||
| Negative | 148 (81.8) | 140 (87.5) | 2.13 | 0.14 |
| Positive | 33 (18.2) | 20 (12.5) | ||
| PgR | ||||
| Negative | 126 (79.7) | 68 (41.7) | 48.53 | <0.001 |
| Positive | 32 (20.3) | 95 (58.3) | ||
| p27 | ||||
| Negative | 131 (68.9) | 45 (46.4) | 13.77 | <0.001 |
| Positive | 59 (31.1) | 52 (53.6) | ||
| p21 | ||||
| Negative | 152 (83.1) | 65 (61.9) | 16.07 | <0.001 |
| Positive | 31 (16.9) | 40 (38.1) | ||
| p53 | ||||
| Negative | 1 (1.0) | 10 (14.7) | 13.11 | <0.001 |
| Positive | 104 (99.0) | 58 (85.3) | ||
| P-cadherin | ||||
| Negative | 81 (41.5) | 66 (44.9) | 0.38 | 0.54 |
| Positive | 114 (58.5) | 81 (55.1) | ||
| Triple negative | ||||
| No | 68 (45.6) | 128 (79.0) | 37.10 | <0.001 |
| Yes | 81 (54.4) | 34 (21.0) | ||
| Classification | ||||
| Basal | 56 (49.1) | 15 (10.2) | 149.02 | <0.001 |
| HER-2 | 26 (22.8) | 9 (6.2) | ||
| Luminal A | 27 (23.7) | 113 (77.4) | ||
| Luminal B | 5 (4.4) | 9 (6.2) | ||
Survival analyses were performed comparing the Nigerian and UK series in relation to both DFI and BCSS in the tumour expressing KI-67 biomarker. Nigerian women were significantly associated with BCSS (P = 0.001), but no significant association with DFI was observed (Figure 2). Cox multivariate analyses showed that race was not only associated with OS, but also its predictive power was independent of tumour size, lymph node status, and ER status (Table 5).
Figure 2.
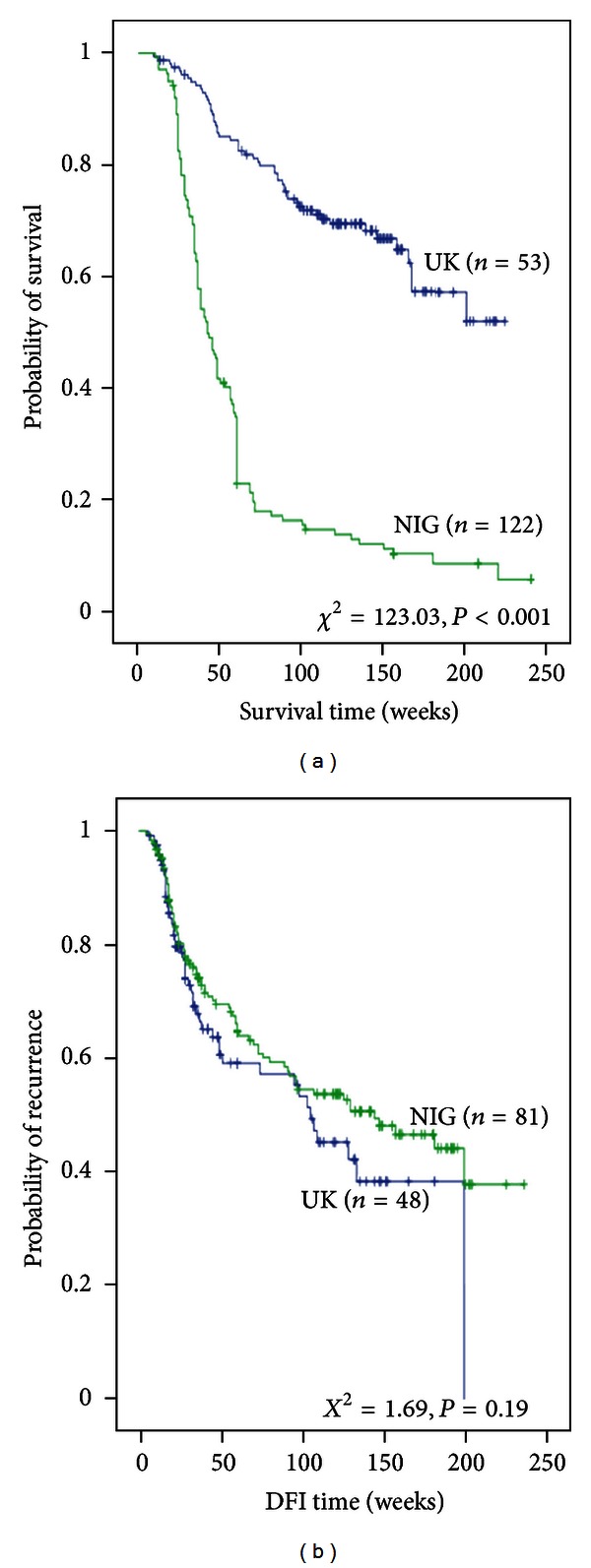
(a) and (b) show KI-67 positive expression in relation to BCSS and DFI between UK and Nigerian series.
Table 5.
Cox multivariate analysis of probability of survival in tumours expressing KI-67 in Nigerian and UK breast cancer.
| KI-67 positive expression | ||||
|---|---|---|---|---|
| Variables | P value | Hazard ratio | 95% CI | |
| Lower | Upper | |||
| Racial difference Nigeria versus UK |
<0.001 | 4.18 | 2.68 | 6.53 |
| Lymph node | 0.69 | 1.09 | 0.71 | 1.67 |
| Tumour size | 0.12 | 1.12 | 0.96 | 1.31 |
| ER | 0.21 | 0.79 | 0.55 | 1.14 |
4. Discussion
KI-67 has been described as one of the important regulators of cell cycle for the maintenance of chromosomal integrity, and therefore any abnormalities in this cell proliferation marker expression might also contribute into passing deformed genes or chromosomes into daughter cells which may serve as impetus for carcinogenesis development with severe implications on tumour behaviour [36–38]. The prognostic significance of KI-67 is currently undergoing intense debate, in view of their importance in the development, proliferation, cell migration, and clinical outcome [39–41]. Also, up until now, there is no consensus regarding the significance of this marker in BC among different ethnicities.
In this study, protein expression of the KI-67 in 308 breast cancer cases from Nigerian women was evaluated and compared with histological tumour grade matched British women BC. Currently, there is a paucity of information on the protein expression of KI-67 in Nigerian breast cancer, and the results presented in this study showed that the tumour specimens obtained from Nigerian compared with British women were more likely to express KI-67. This is in support of other data on KI-67 protein expression at diagnosis in black women [39–41]. In ethnicity studies, KI-67 expression was higher in breast cancers from African-American compared with Caucasian women [21]. In addition high level of flow cytometric S phase measurement and mitotic count was also reported in African-American [23, 42, 43]. Previous studies on KI-67 expression in Nigerian and Saudi Arabian women also showed that KI-67 was overexpressed [39–41]. In addition, a strong relationship was established between poor prognostic indicators and KI-67 expression in this study, where Nigerian women with KI-67 expression were associated with patient diagnosed earlier in life, premenopausal, larger tumour size, lymph node involvement, vascular invasion, exhibited a basal phenotype, and triple negative, lacked hormone receptors, BRCA1, and shorter BCSS compared with British women. This is in line with previous studies on Caucasian women, where aggressive tumours are associated with KI-67 expression [8, 16, 44, 45]. Similar aggressive features observed in this study were also reported on high KI-67 expression in African-American compared with Caucasian women [20, 21]. In Nigerian series, the high expression of this proliferative marker might have contributed to their aggressiveness, having been previously associated with aneuploidy that are responsible for high cell division turnover rate, which has been linked with aggressive tumours [11, 12]. It may also be involved in the metastatic potential of this BC, because of its association with majority of tumour that had already invaded both the lymphatic and blood vessels at diagnosis. In addition, this marker may also be involved in the lack of response to endocrine therapy and conventional chemotherapy in Nigerian tumours. KI-67 overexpression would have also contributed to high mortality rate observed in Nigerian compared to UK women as a result of its relationship with basal phenotype, lack of hormonal receptors and BRCA1. Low KI-67 expression and high hormone receptors expression are found to be associated with improved endocrine therapy [46], and only few basal phenotype and BRCA1 are likely to response to chemotherapy [47]. Furthermore, KI-67 has also been reported to be a predictor of poor outcome in aggressive BC [45, 48], in line with this, Nigerian BC had a shorter BCSS compared with British women. Race was observed to be independent prognostic factor in the tumours expressing KI-67, and therefore this marker might have also contributed to the difference in the tumour biology observed between the black and Caucasian women, and also using KI-67 expression to stratify black women with BC may improve the prognostic significance of clinical response to treatment.
In conclusion, KI-67 expression was found to have contributed to the difference in the tumour biology and poor overall survival in Nigerian BC women compared with British grade counterpart. Therefore, targeting this marker in the indigenous black women with BC might reduce the dismay outcome in the black women with BC.
Conflict of Interests
The authors declared no competing interest.
Authors' Contribution
Ayodeji Johnson Agboola (AJA) participated in the design of the study and performed the immunohistochemistry and paper write up; Adewale A. Musa (AAM) and Babatunde Salami (AS) contributed to patients' management and outcome follow up; Adekumbiola A Banjo ( AAB) and Charles C Anunobi (CCA) performed the histological diagnosis of the samples in Nigeria; Emad A Rakha (ERK) and Ian O Ellis (IOE) contributed reevaluation of the histological diagnosis of the samples from Nigeria at UK histopathology laboratory; IOE also contributed immensely towards the design of the study and paper development. Christopher C Nolan (CCN) and Andrew R Green (ARG) performed the TMA, and Additionally, ARG participated in the design of the study, immunohistochemical scoring and also edited the paper. Anotu Mopelola Deji-Agboola (AMD) performed the statistical analysis.
Acknowledgments
The authors wish to acknowledge the efforts of the staffs of the Histopathology Department, Olabisi Onabanjo University Teaching Hospital Sagamu, Nigeria, and University of Nottingham City Hospital for the completion of this project. The authors appreciation also goes to my colleagues for their team spirit and the University of Nottingham, where this study was carried out.
References
- 1.Amend K, Hicks D, Ambrosone CB. Breast cancer in African-American women: differences in tumor biology from European-American women. Cancer Research. 2006;66(17):8327–8330. doi: 10.1158/0008-5472.CAN-06-1927. [DOI] [PubMed] [Google Scholar]
- 2.Ijaduola TG, Smith EB. Pattern of breast cancer among White-American, African-American, and nonimmigrant West-African women. Journal of the National Medical Association. 1998;90(9):547–551. [PMC free article] [PubMed] [Google Scholar]
- 3.Smigal C, Jemal A, Ward E, et al. Trends in breast cancer by race and ethnicity: update 2006. Ca-A Cancer Journal for Clinicians. 2006;56(3):168–183. doi: 10.3322/canjclin.56.3.168. [DOI] [PubMed] [Google Scholar]
- 4.Bray F, McCarron P, Parkin DM. The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Research. 2004;6(6):229–239. doi: 10.1186/bcr932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. Journal of Clinical Oncology. 2006;24(14):2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 6.Agboola AJ, Musa AA, Wanangwa N, et al. Molecular characteristics and prognostic features of breast cancer in Nigerian compared with UK women. Breast Cancer Research and Treatment. 2012;135(2):555–569. doi: 10.1007/s10549-012-2173-7. [DOI] [PubMed] [Google Scholar]
- 7.Ghafoor A, Jemal A, Ward E, Cokkinides V, Smith R, Thun M. Trends in breast cancer by race and ethnicity. Ca-A Cancer Journal for Clinicians. 2003;53(6):342–355. doi: 10.3322/canjclin.53.6.342. [DOI] [PubMed] [Google Scholar]
- 8.Aleskandarany MA, Green AR, Rakha EA, et al. Growth fraction as a predictor of response to chemotherapy in node-negative breast cancer. International Journal of Cancer. 2010;126(7):1761–1769. doi: 10.1002/ijc.24860. [DOI] [PubMed] [Google Scholar]
- 9.Dai H, Van’t Veer L, Lamb J, et al. A cell proliferation signature is a marker of extremely poor outcome in a subpopulation of breast cancer patients. Cancer Research. 2005;65(10):4059–4066. doi: 10.1158/0008-5472.CAN-04-3953. [DOI] [PubMed] [Google Scholar]
- 10.Porter PL, Lund MJ, Lin MG, et al. Racial differences in the expression of cell cycle-regulatory proteins in breast carcinoma: study of young African American and white women in Atlanta, Georgia. Cancer. 2004;100(12):2533–2542. doi: 10.1002/cncr.20279. [DOI] [PubMed] [Google Scholar]
- 11.Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79(4):551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 12.Hunter T, Pines J. Cyclins and cancer II: cyclin D and CDK inhibitors come of age. Cell. 1994;79(4):573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 13.Grana X, Reddy EP. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs) Oncogene. 1995;11(2):211–219. [PubMed] [Google Scholar]
- 14.Wu Y, Luo H, Kanaan N, Wu J. The proteasome controls the expression of a proliferation-associated nuclear antigen Ki-67. Journal of Cellular Biochemistry. 2000;76(4):596–604. [PubMed] [Google Scholar]
- 15.Endl E, Gerdes J. The Ki-67 protein: fascinating forms and an unknown function. Experimental Cell Research. 2000;257(2):231–237. doi: 10.1006/excr.2000.4888. [DOI] [PubMed] [Google Scholar]
- 16.Finek J, Holubec L, Jr., Topolcan O, Elgrova L, Skalova A, Pecen L. The importance of prognostic factors in premenopausal women with breast cancer. Anticancer Research. 2007;27(4):1893–1896. [PubMed] [Google Scholar]
- 17.De Azambuja E, Cardoso F, De Castro G, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12 155 patients. British Journal of Cancer. 2007;96(10):1504–1513. doi: 10.1038/sj.bjc.6603756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruiz C, Seibt S, Al Kuraya K, et al. Tissue microarrays for comparing molecular features with proliferation activity in breast cancer. International Journal of Cancer. 2006;118(9):2190–2194. doi: 10.1002/ijc.21581. [DOI] [PubMed] [Google Scholar]
- 19.Irigoyen MA, Garcia FV, Iturriagagoitia AC, Beroiz BI, Martinez MS, Grima FG. Molecular subtypes of breast cancer: prognostic implications and clinical and immunohistochemical characteristics. Anales del Sistema Sanitario de Navarra. 34(2):219–233. doi: 10.4321/s1137-66272011000200008. [DOI] [PubMed] [Google Scholar]
- 20.Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101(1):3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 21.Morris GJ, Naidu S, Topham AK, et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the national cancer institute’s surveillance, epidemiology, and end results database. Cancer. 2007;110(4):876–884. doi: 10.1002/cncr.22836. [DOI] [PubMed] [Google Scholar]
- 22.Eloy JW, Hill HA, Chen VW, et al. Racial differences in survival from breast cancer: results of the National Cancer Institute Black/White Cancer Survival Study. Journal of the American Medical Association. 1994;272(12):947–954. doi: 10.1001/jama.272.12.947. [DOI] [PubMed] [Google Scholar]
- 23.Elledge RM, Clark GM, Chamness GC, Osborne CK. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. Journal of the National Cancer Institute. 1994;86(9):705–712. doi: 10.1093/jnci/86.9.705. [DOI] [PubMed] [Google Scholar]
- 24.Abd El-Rehim DM, Ball G, Finder SE, et al. High-throughput protein expression analysis using tissue microarray technology of a large well-characterised series identifies biologically distinct classes of breast cancer confirming recent cDNA expression analyses. International Journal of Cancer. 2005;116(3):340–350. doi: 10.1002/ijc.21004. [DOI] [PubMed] [Google Scholar]
- 25.Abd El-Rehim DM, Pinder SE, Paish CE, et al. Expression and co-expression of the members of the epidermal growth factor receptor (EGFR) family in invasive breast carcinoma. British Journal of Cancer. 2004;91(8):1532–1542. doi: 10.1038/sj.bjc.6602184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rakha EA, El-Rehim DA, Paish C, et al. Basal phenotype identifies a poor prognostic subgroup of breast cancer of clinical importance. European Journal of Cancer. 2006;42(18):3149–3156. doi: 10.1016/j.ejca.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Rakha EA, El-Sayed ME, Green AR, Lee AHS, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109(1):25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 28.Madjd Z, Pinder SE, Paish C, Ellis IO, Carmichael J, Durrant LG. Loss of CD59 expression in breast tumours correlates with poor survival. Journal of Pathology. 2003;200(5):633–639. doi: 10.1002/path.1357. [DOI] [PubMed] [Google Scholar]
- 29.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies. Journal of Clinical Oncology. 2005;23(36):9067–9072. doi: 10.1200/JCO.2004.01.0454. [DOI] [PubMed] [Google Scholar]
- 30.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nature Medicine. 1998;4(7):844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 31.Abdel-Fatah TM, Powe DG, Agboola J, et al. The biological, clinical and prognostic implications of p53 transcriptional pathways in breast cancers. Journal of Pathology. 2010;220(4):419–434. doi: 10.1002/path.2663. [DOI] [PubMed] [Google Scholar]
- 32.Rakha EA, Abd El Rehim D, Pinder SE, Lewis SA, Ellis IO. E-cadherin expression in invasive non-lobular carcinoma of the breast and its prognostic significance. Histopathology. 2005;46(6):685–693. doi: 10.1111/j.1365-2559.2005.02156.x. [DOI] [PubMed] [Google Scholar]
- 33.Wolff AC, Hammond MEH, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Journal of Clinical Oncology. 2007;25(1):118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 34.García-Caballero T, Grabau D, Green AR, et al. Determination of HER2 amplification in primary breast cancer using dual-colour chromogenic in situ hybridization is comparable to fluorescence in situ hybridization: a European multicentre study involving 168 specimens. Histopathology. 2010;56(4):472–480. doi: 10.1111/j.1365-2559.2010.03503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clinical Cancer Research. 2004;10(16):5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 36.Keyomarsi K, Tucker SL, Buchholz TA, et al. Cyclin E and survival in patients with breast cancer. The New England Journal of Medicine. 2002;347(20):1566–1575. doi: 10.1056/NEJMoa021153. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen NH, Lodén M, Cajander J, Emdin SO, Landberg G. G1-S transition defects occur in most breast cancers and predict outcome. Breast Cancer Research and Treatment. 1999;56(2):105–112. doi: 10.1023/a:1006208419350. [DOI] [PubMed] [Google Scholar]
- 38.Porter PL, Malone KE, Heagerty PJ, et al. Expression of cell-cycle regulators p27(Kip1) and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nature Medicine. 1997;3(2):222–225. doi: 10.1038/nm0297-222. [DOI] [PubMed] [Google Scholar]
- 39.Ghebeh H, Tulbah A, Mohammed S, et al. Expression of B7-H1 in breast cancer patients is strongly associated with high proliferative Ki-67-expressing tumor cells. International Journal of Cancer. 2007;121(4):751–758. doi: 10.1002/ijc.22703. [DOI] [PubMed] [Google Scholar]
- 40.Al Tamimi DM, Shawarby MA, Ahmed A, Hassan AK, AlOdaini AA. Protein expression profile and prevalence pattern of the molecular classes of breast cancer—a Saudi population based study. BMC Cancer. 2010;10(article 223) doi: 10.1186/1471-2407-10-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huo D, Ikpatt F, Khramtsov A, et al. Population differences in breast cancer: survey in indigenous african women reveals over-representation of triple-negative breast cancer. Journal of Clinical Oncology. 2009;27(27):4515–4521. doi: 10.1200/JCO.2008.19.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shavers VL, Harlan LC, Stevens JL. Racial/ethnic variation in clinical presentation, treatment, and survival among breast cancer patients under age 35. Cancer. 2003;97(1):134–147. doi: 10.1002/cncr.11051. [DOI] [PubMed] [Google Scholar]
- 43.Weiss SE, Tartter PI, Ahmed S, et al. Ethnic differences in risk and prognostic factors for breast cancer. Cancer. 1995;76(2):268–274. doi: 10.1002/1097-0142(19950715)76:2<268::aid-cncr2820760217>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 44.Niemiec JA, Adamczyk A, Malecki K, Majchrzyk K, Rys J. Relationships between immunophenotype, Ki-67 index, microvascular density, Ep-CAM/P-cadherin, and MMP-2 expression in early-stage invasive ductal breast cancer. Applied Immunohistochemistry & Molecular Morphology. 2012;20(6):550–560. doi: 10.1097/PAI.0b013e31824f21af. [DOI] [PubMed] [Google Scholar]
- 45.Konsti J, Lundin M, Joensuu H, et al. Development and evaluation of a virtual microscopy application for automated assessment of Ki-67 expression in breast cancer. BMC Clinical Pathology. 2011;11(article 3) doi: 10.1186/1472-6890-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Endo Y, Toyama T, Takahashi S, et al. High estrogen receptor expression and low Ki67 expression are associated with improved time to progression during first-line endocrine therapy with aromatase inhibitors in breast cancer. International Journal of Clinical Oncology. 2011:1–7. doi: 10.1007/s10147-011-0215-5. [DOI] [PubMed] [Google Scholar]
- 47.Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. Journal of Clinical Oncology. 2005;23(28):7212–7220. doi: 10.1200/JCO.2005.07.501. [DOI] [PubMed] [Google Scholar]
- 48.Railo M, Lundin J, Haglund C, Von Smitten K, Nordling S. Ki-67, p53, ER receptors, ploidy and S phase as long-term prognostic factors in T1 node-negative breast cancer. Tumor Biology. 2006;28(1):45–51. doi: 10.1159/000097702. [DOI] [PubMed] [Google Scholar]


