Abstract
New discoveries in the field of neurophysiology and neuropharmacology have revealed the role of (n-3) fatty acids in controlling inflammation and protecting neuron cells from oxidative damage, preserving their function. It has also been thought that their psychoactive properties could be beneficial in certain psychiatric illnesses. This article discusses the newest discoveries of the affected activities by these fats in the cerebral cortex and the efforts that have been made to put them in practice in clinical trials in humans. In general, we were able to detect certain discord in the scientific community when designing placebo-based studies (mainly in establishing the appropriate therapeutic dose of (n-3) fatty acids, varying from the recommended dietary dose to an amount that may be 3 or 4 times higher), and in interpreting results. Although many studies have had the validity of their results questioned because of their small sample size, several studies seem to indicate that the (n-3) fatty acids are useful therapeutic tools in treating psychiatric conditions such as major depression, bipolar disorder, and several other disorders. Larger sample size studies are still required to better analyze the treatment potential of these agents.
Introduction
(N-3) fatty acids are long-chained and unsaturated molecules only obtained by dietary intake of certain grains, such as flaxseed, canola, and walnuts, and sea fish (1). All varieties of (n-3) acids (mainly alpha-linoleic, DHA, and EPA) are essential components in mammalian metabolism, whether it be as anti-inflammatory molecules in the elongase-desnaturase pathways that synthesize the different subtypes of (n-3) fatty acids (also directly inhibiting of the (n-6)–derived eicosanoids), inhibitors of excessive platelet activity, immune-modulating agents, and the main components in guaranteeing cell membrane stability (2–5). Unfortunately, as the scientific community finds, at an alarming rate, more and more evidence that these fats have cardioprotective, psychoactive, and cancer-fighting properties (6, 7), the general population has decreased daily intake of (n-3)-rich foods, amounting to what has been generally called the Western diet, rich in sugars and with severe deficiencies in several micronutrients—vitamins, minerals, and (n-3) fatty acids (8, 9). The western diet, compared with the (n-3)-rich foods of the Mediterranean diet, has been correlated with greater incidence of rectal cancer, cardiovascular diseases, and psychiatric illnesses (8).
It is widely accepted that the PUFA have an important role in many neural pathways and that their deficiency may be correlated with the occurrence of several psychiatric illnesses, such as major depression, bipolar disorder, obsessive-compulsive disorder, and anxiety disorders (10–14). Exploration of these mechanisms of action has inspired the pursuit of new treatment protocols that feature PUFA as an adjunctive or as a monotherapy for treatment of these diseases, with many surprising results.
Current status of knowledge
(N-3) fatty acids as membrane components
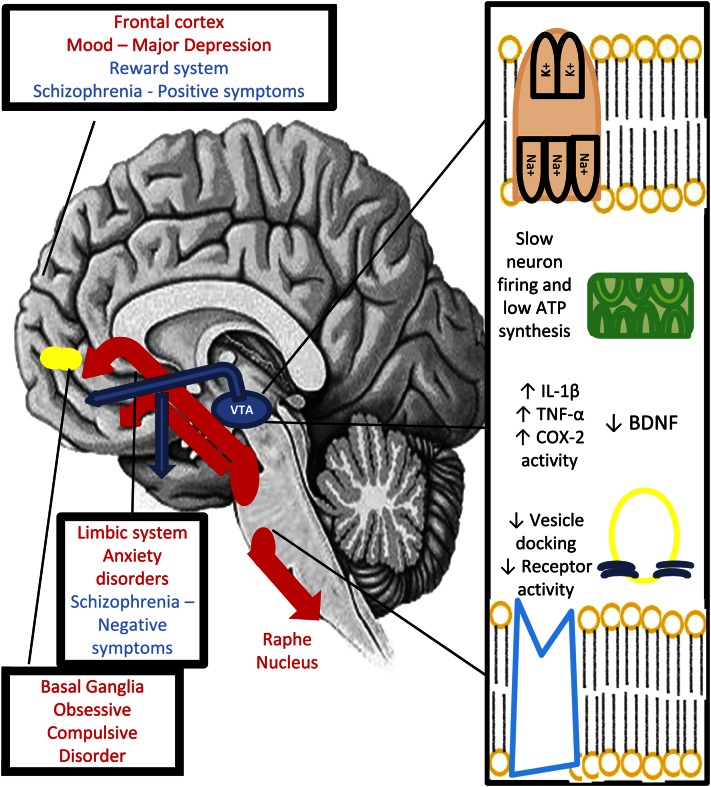
(N-3) fatty acids are responsible for almost 20% of the brain’s dry weight, and one third of all fats in the central nervous system belong to the PUFA class (15, 16). In the neuron membrane, they are responsible for the maintenance of stability and conformity of receptors and structural ligands such as the Na+/K+ ATPase, calcium, sodium and chloride ion channels, and caveolin proteins. Lack of these essential components can alter cell function in many ways. The molecular role of omega 3 fatty acids is synthetized in Figure 1.
Figure 1.
Neuron activity and affected neural pathways in a low (n-3) fatty acid concentration environment. The molecular role of (n-3) fatty acids vary in the neuron. They are responsible for maintaining membrane stability and the conformity and function of proteins, whether they be ion receptors, the complex Na+/K+ ATPase, or vesicle-docking peptides necessary for neurotransmission. They have also anti-inflammatory properties, lowering the concentration of proinflammatory cytokines, which may provoke neuron damage and death. (N-3) fatty acids are also necessary for the synthesis of brain-derived neurotrophic factor, a substance involved in the process of neurogenesis and synaptic plasticity. In lacking these components, the neurological pathways malfunction and may contribute to the start of certain psychiatric diseases. There are recorded experiences using (n-3) fatty acids in many psychiatric conditions in the literature, ranging from major depression to borderline personality disorder, each with a particular theoretical justification and generally positive outcomes when compared with the control/placebo group.
Neuron membrane potential depends of an even flow of Na+ cations (through Na+ channels), which promotes depolarization and rapid repolarization of the neuron, Fig. 1 by the protein Na+/K+ ATPase. This electric current is responsible for the release of neurotransmitters in the synaptic cleft and signal transmission throughout the cortex. Any malfunction in this system can lead to neuron hypofunctioning, slower responses, and decreased cognitive and limbic function (17–20).
Release of neurotransmitters from their intracytoplasmic vesicles is dependent on the docking of Ca2+ ions through vesicle-associated membrane proteins (21). Difficulty in ion transport, such as caused by membrane instability, can make the signal transmission more difficult in the cleft, therefore causing decreased neuron function in the central nervous system.
Proton leak is an essential process in which the neuron expends ∼20% of its energy and is essential for the normal aerobic respiratory activity in these cells. This process depends on the integrity of membrane proteins in the mitochondria, which are, in their turn, dependent on the dietary intake of essential fats (21–24).
GABAergic function, promoted by a class of chloride receptors, is important to decrease the firing rate of other neuron systems, thus not permitting them to hyperfunction and to cause neuron damage. Its malfunction is also correlated with the occurrence of certain anxiety disorders such as generalized anxiety disorder. (N-3) fatty acid depletion can alter the conformation of the chloride ion channels, which may derail GABAergic receptor activity (25).
Caveolae are a special class of proteins that can help dock or separate certain membrane proteins and promote signal exchange between the nucleus and the cytosolic environment, increasing receptor exchange between both compartments, preventing membrane protein senescence and loss of function, and increasing gene transcription of membrane receptors (25,26). Several studies show their enforcing role in the signal transduction via the type 2 serotonin membrane receptor, enabling serotonergic activity in the prefrontal, parietal, and somatosensory cortex, having a protective effect against depression (27–29). Their promotion of glutamatergic activity via the L-type Ca2+ channel/glutamate receptor subunit N-methyl-d-aspartate A2B/voltage-dependent anion channel signaling is also responsible for preventing neuron death (30, 31) and preventing several pathological processes that may lead to major depression, attention-deficit disorder, and dementia (31). Their action against dopamine receptors type D1 has proved to be useful in preventing the negative symptoms of schizophrenia (32).
In sum, the normal activity of the proteins involved in cell metabolism (transporters in the cell membrane and the mitochondria, voltage-dependent anion channels, Na+/K+ ATPase) is intrinsically connected to the (n-3) fatty acid composition of the cell membrane, and any decrease in the concentration of these fats can lead to neuron hypofunctioning, serotonin depletion, increased neuron death, and decreased energy metabolism in the neurons (22–24), which may be responsible for causing more pernicious diseases such as dementia.
(N-3) fatty acids in cytosolic pathways
The physiological balance between concentrations of (n-6) and (n-6) fatty acids maintains lower synthesis of proinflammatory cytokines (33–37) (mainly IL-1β and TNF-α), lower synthesis of prostaglandins, leukotrienes, and phosphodiesterase type 4 (38, 39). When there is low concentration of the (n-3) PUFA, the increase in tissue inflammation can lead to cAMP cleavage, lower levels of cAMP response element binding factor, and brain-derived neurotrophic factor, which reduces synaptic plasticity and neurotransmission and increases neuron damage and death (40).
The (n-3) PUFA are also responsible for interacting with several nuclear transcription factor receptors such as sterol regulatory element binding protein 1c, PPAR receptor type α, retinoic acid receptors, and retinoid X receptor, all of which have important roles in memory, cognition, and problem-solving (41, 42). Recent studies have also indicated the role of (n-3) fatty acids in controlling RNA transcription of fatty acid synthase, an important regulator of lipid metabolism in the neuron, and cyclooxygenase-2, an enzyme necessary in the inflammation cascades (42). The discovery of these pathways have shed light to certain pathological processes that occur in the brain, such as the decrease of brain-derived neurotrophic factor production and activity in major depressive disorder (40) and the decreased production of nuclear binding elements in age-related cognitive decline (42,43) and dementia (44).
(N-3) fatty acid deficiency, inflammation, and the etiology of mental illness
It has been demonstrated that inflammation may be key to the development of mental illnesses by causing increased production of cytokines, migration of inflammatory cells, and activation of glial cells, mainly astrocytes (45–47). It is believed that this continuous inflammation can lead to neuron malfunction and eventually to neuron death because of the increase in oxidative stress in the brain (47) via several molecular pathways that include, but are not limited to, glutathione peroxidase, glial fibrillary acidic protein, and TNF-α/IL-18 (46–48). This damage may be reflected in decreased neurogenesis in the hippocampus, causing several symptoms correlating with mood disorders (48), such as major depression and obsessive-compulsive disorder, and when conducted for a long period, long-term and irreversible effects can be observed in the prefrontal cortex and dopaminergic nuclei, causing dementia and Parkinson’s disease (49).
The lack of (n-3) fatty acids can lead to neuron malfunction and death by other means, whether by affecting the energy metabolism of the neuron (17) or mRNA translation of important components for the cell—second messengers, transduction receptors, and survival signals (41–43). Other membrane transporters may be affected by the lack of these essential fats, which are involved in maintaining hydroelectrolytic balance in the neuron, and the continuous exchange of substrates and products of cytosolic metabolism (26). It has been shown that (n-3) fatty acid depletion can decrease the rate of production of neurotransmitters in the monoaminergic pathways and decrease the rate of neuron firing—in a process similar to that observed in depressed patients—with decreased production of serotonin and decreased activity in serotoninergic pathways (49).
Major depression
Rationale.
There is solid research highlighting the importance of the (n-3) fatty acids in maintaining membrane integrity for the transport of tryptophan, the precursor of serotonin in the raphe nucleus, the maintenance of serotonin type 2 receptors in the prefrontal cortex, which are responsible for mood state, and the control of inflammatory and oxidative damage to serotoninergic neurons. Maes et al. (50) verified that there is abnormal (n-3) fatty acid metabolism in depressed patients, with rapid increase in monounsaturated fatty acids and inflammatory response, and that these alterations were not reversed in traditional antidepressant therapy in some volunteers. Peet et al. (51) determined the red blood cell membrane concentration of (n-3) fatty acids in depressed patients compared with controls. These measurements are thought to relate directly to the neuron membrane concentration of these same PUFA. This study revealed that not only are (n-3) fatty acid concentrations lower in depressed patients, but that this correlated with greater inflammatory activity, matched only when the control group samples were immersed in a peroxide solution.
Studies.
Major depression has been considered a successful example of a treatable disease either by monotherapy or combination of these fatty acids with traditional selective serotonin uptake inhibitors. Nemets et al. (52) designed a placebo-controlled study of subjects already undergoing antidepressant therapy and found better outcome results in the treatment group with ethyl-EPA (E-EPA), a modified component of (n-3) fatty acid formulations. Jazayeri et al. (53) studied a group of 60 outpatients, divided in 3 subgroups: 1 group received fluoxetine 20 mg/d, the second group received EPA at a dose of 1 g/d, and the third received a combination of these 2 treatments. The result was a similar improvement in general symptoms of the monotherapy groups compared with baseline and a significantly greater improvement in the combined therapy group. Peet et al. (54) conducted a study that showed improvement in the patients treated with E-EPA, and who were refractory to selective serotonin uptake inhibitor monotherapy for major depression. Other clinical trials have found positive correlations between an increase in (n-3) fatty acid concentrations and improvement in symptoms, whether in major isolated depression (54–57), pediatric unipolar depression (58), and depression associated with Parkinson’s disease (59), with menopausal transition (60), and with aging in women (61).
However, certain trials could not find any correlation between administration of fatty acid doses and improvement of symptoms. Even though well conducted, there should be certain reservations when interpreting their results, mainly because of disagreement in the literature concerning the appropriate therapeutic daily dose of these substances, and the appropriate DHA:EPA ratio that should be prescribed for these patients (56–62), making administrations unique. One example is Rogers et al. (63), who could not find any correlation between supplementation and improvement, although there were reservations about their use of oleic acids as an appropriate placebo, and their preparations with low concentrations of EPA, which is thought to be the most active component in relieving mood symptoms.
Highlighting postpartum depression, there was a great division of opinion concerning the effectiveness of treatment. Four studies found no correlation (61–64) between supplementation and improvement, and two others found a positive correlation (65, 66), not yet clarifying whether there is any benefit in this approach.
Bipolar disorder
Rationale.
(N-3) fatty acids appear to be natural membrane stabilizers, stabilizing calcium voltage-dependent channels, neuron firing, and inflammatory processes that possibly damage this cell layer (17–21, 71). Noaghiul and Hibbeln (72), compiling epidemiological data from other studies on the prevalence of bipolar disorder (types I and II) found that the occurrence of these diseases was inversely correlated with high seafood consumption. Biochemical analysis of different age sectors in U.S. and European populations established the correlation between low concentration of (n-3) fatty acids and the higher prevalence of mood disorders and mood instability (73–75).
Studies.
Studies on pediatric patients have shown promising results, although they are limited due to being open label and with a limited sample size (76–80). Fragou (81) conducted a placebo-controlled study in adults using 1–2 g/d of E-EPA and observed a modest improvement in severity of illness. This same author observed an increase in the concentration of N-acetylaspartate in supplemented patients, a substance with proven neurotrophic properties (75).
Other authors have shown that supplementation with EPA is both well tolerated and effective, although its use is considered optimal in adult patients with moderately intense symptoms (82–84) because 1 study revealed that even with doses almost 3 times the dietary recommendation (6 g/d), patients with severe symptoms or rapid cycling disorder did not present any improvement after treatment compared with placebo (85).
Dementia
Rationale.
There is a clear link between the aging brain, cognitive decline, and decrease in the total concentration of DHA, EPA, and lipid markers (86). Long-term potentiation, an activity that permits the conversion of short-time memory into long-term memory, is also shown to be a process closely linked to DHA levels in the hippocampus (87). This potentiation is deficient in certain conditions such as Alzheimer’s disease, and supplementation with DHA in selected animals was able to restore neuron function to normal. DHA supplementation was also able to reduce concentration of β-amyloid protein, plaque burden, and tau protein, which are thought to be disease activity markers in Alzheimer’s disease (82, 83, 88, 89).
Clinical trials
Studies are divided in their findings of the effectiveness of essential fatty acids in improving symptoms. Although some clinical trials have shown that age-related cognitive decline can be partially reversed by supplementation with DHA and EPA and that patients with organic brain damage or Alzheimer’s disease can have improvement in immediate and delayed memory function (90, 91), others found no effect of supplementation (92–95). Other studies found mixed results, with improvement only in a cognitively impaired population and not in Alzheimer’s patients (96), in depressive symptoms associated with this disease (97), or only improving cognitive function in very mildly symptomatic patients (98).
Attention-deficit hyperactivity disorder
Rationale.
There are no clear mechanisms that involve the role of essential fatty acid deficiency in generating hyperactive symptoms, although it can be inferred that their role as anti-inflammatory molecules and as membrane-stabilizing components may be responsible for such an effect (100–103). Population studies revealed that children with attention-deficit hyperactivity disorder had lower serum concentrations of PUFA, particularly EPA (100, 102), and had a higher rate of oxidation of (n-3) fatty acids, which results in a lower serum concentration of these fats (103).
Clinical trials
Generally, trials were successful in confirming (n-3) fatty acid supplementation as feasible in reducing hyperactive symptoms (104–107), improving student-teacher relations, impulsive action (105), visual memory acquisition (106), and learning performance as a whole (107). There were certain exceptions to these studies (108, 109), in which no effects were found.
Schizophrenia and first-episode psychosis
Rationale.
First-episode psychotic patients show a change in the composition of N-acetylaspartate in the brain (110), with a rapid decrease in concentration of this substance. This is also correlated with development of more serious and lasting schizophrenic symptoms, greater neuron death, and decrease in dopaminergic function (111). There are studies that show that N-acetylaspartate is linked to neuron membrane integrity, and its concentrations are dependent on (n-3) fatty acid serum concentrations (110).
Clinical trials
Supplementation with E-EPA reduced markers for neuron death in first-episode psychosis (112), mainly glutathione, and the tolerability of neuroleptic medication in patients (113), also showing improvement in negative symptoms of schizophrenia and neuroprotective effects in hippocampal neurons (114). Another study, conducted by Amminger et al. (115), showed that in a sample of 81 individuals with an ultrahigh risk of the development of a psychotic disorder, there was prevention of the development of negative symptoms and slowing of the progression to full schizophrenia in the intervention group with 1.2 g/d for a 12-wk period, followed by a 40-wk observation period compared with placebo. There was also effectiveness in reducing positive and negative symptoms in schizophrenic patients, whether in monotherapy or in association with neuroleptics, even when used in low doses (2 g/d) for brief periods of intervention (12 wk) (116–118). Only 1 study did not find any improvement when (n-3) fatty acids were added to the treatment (119).
Autism
Rationale.
There are no described molecular mechanisms that are affected by (n-3) fatty acids in this disorder, and the evidence that any nutritional or pharmacological component can produce any improvement is derived from a group of pilot studies (120–122).
Clinical trials
Amminger et al. (123) conducted a pilot trial with 13 individuals with a diagnosis of autism for 6 wk in which 1.5 g total (n-3) fatty acid composition (7 g of DHA and 0.84 g of EPA) were given to the intervention group. They observed a statistically significant change in hyperactivity and stereotypy of behavior in individuals compared with the placebo group. Both Bent et al. (124) and Politi et al. (125) conducted similar placebo-controlled studies with autistic patients in childhood and adulthood, respectively, yet neither group could find statistically significant differences between groups. They observed that there was a tendency toward a small improvement of symptoms in the intervention group, however, which justified conducting studies with larger samples of individuals.
Anorexia nervosa
Rationale.
Two studies with female patients with anorexia revealed that these patients had an alteration in the EPA/arachidonic acid profile, a decrease in all fractions of (n-3) fatty acids, and a decrease in liver protein metabolism (126, 127). It is thought that this disease has certain similarities to obsessive-compulsive disorder, with malfunction of serotonin pathways in the limbic system and prefrontal cortex and that the PUFA act in the same way as in depressive disorders, as an anti-inflammatory molecule, preventing neuron damage, stabilizing neuron membrane, and signal transduction throughout the affected pathways (3, 5, 12).
Clinical trials
Only 2 trials have been conducted that used (n-3) fatty acids as therapeutic tools, in 1 trial as the only intervention and in another trial as an adjunctive therapy with fluoxetine. Ayton et al. (128) worked with only 7 patients, all of whom showed significant improvement in anxiety symptoms, yet there was no control group with which to make the necessary comparisons. Barbarich et al. (129) could not find any correlation between improvements in weight gain in the intervention group compared with PUFA placebo.
Obsessive-compulsive disorder
Rationale.
The change in membrane permeability and neuron activity in serotoninergic pathways and increased inflammatory activity in the brain are thought to be a connection between a deficiency of essential fatty acids and the development of compulsive symptoms (46).
Clinical trials
Only 1 clinical trial was conducted with a placebo-controlled crossover model for 6 wk for EPA, in which no correlation was found between intervention and improvement in symptoms (130).
Borderline personality disorder
Rationale.
Neural mechanisms have not yet been discovered in this psychiatric condition. There is 1 study that revealed a correlation between DHA supplementation and a decrease in aggression in otherwise healthy young adults (131). Sudden and usually unmotivated aggression is one of the main symptoms of bipolar disorder, and a similar improvement was thought to occur in these patients.
Clinical trials
Only 1 trial concerning this disorder was conducted by Zanarini et al. (132). In it, 30 female subjects were divided in a 2:1 ratio for EPA:placebo treatment, for an 8-wk period. There were significant improvements in aggressive symptoms and depression scores; therapy was well tolerated and presented no serious side effects.
Drug dependence
Rationale.
Drug-dependent patients go through a period of great anxiety and individual psychological suffering, with combined physical symptoms that include, but are not limited to, tremors and pain. It is the phase of most relapses in drug users and is commonly referred to as “craving.” New theories clarify that the role of proinflammatory cytokines is indeed important in the establishment of this syndrome and that the EPA fraction of essential fats counteracts the toxic effects of these substances in the brain (133–135). The neuroprotective effect of (n-3) fatty acids in serotonin production and action in the prefrontal cortex may also be responsible for the preservation of the ability of planning and task execution, both of which are impaired during withdrawal and the establishment of craving symptoms.
Clinical trials
Only 2 trials have tested the effects of PUFA in drug abusers, both conducted by Buydens-Branchey et al. (135, 136). In 2006, 1 trial tested anxiety symptoms in substance abusers before and after supplementation with an EPA+DHA mixture, total 3 g PUFA/d for 3 mo (135). The improvement in the supplementation group was statistically different compared with placebo patients, and even after discontinuing therapy, the intervention group maintained a lower score of anxiety symptoms than the placebo group after 3 mo (135). The other study, a pilot, included analyses of anger and anxiety symptoms in drug abusers and found that the (n-3) fatty acids group showed a greater improvement compared with placebo (136).
Conclusions
Most evidence suggests that (n-3) fatty acids act on many mechanisms involved in the physiopathology of mental illnesses. However, it is impossible, at the moment, to state emphatically whether the deficiency of essential fatty acids is the cause of these problems themselves or whether these molecules simply can counterbalance the effect of other causes of mental disorders.
The essential fatty acids, according to the knowledge gathered so far, present themselves as new viable agents in psychiatric treatment because they are generally well tolerated, with minimal or no side effects. The studies that have proved a positive correlation between improvement in symptoms and the intake of (n-3) fatty acids are validity. However, further studies are necessary to establish of they are viable in larger and more complex placebo-controlled studies because the previous work done with (n-3) fatty acids lacked standardization of therapeutic doses and were generally conducted with small samples.
Acknowledgments
Both authors have read and approved the final manuscript.
Footnotes
Supported by Associação Fundo de Incentivo à Pesquisa, Fundação de Amparo à pesquisa do Estado de São Paulo, and Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Author disclosures: P. L. Prior and J. C. F. Garduróz, no conflicts of interest.
Literature Cited
- 1.Michaelsen KF, Dewey KG, Perez-Exposito AB, Nurhasan M, Lauritzen L, Roos N. Food sources and intake of n-6 and n-3 fatty acids in low-income countries with emphasis on infants, young children (6–24 months), and pregnant and lactating women. Matern Child Nutr. 2011;7: Suppl 2:124–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serebruany VL, Miller M, Pokov AN, Lynch D, Jensen JK, Hallén J, Atar D. Early impact of prescription omega-3 fatty acids on platelet biomarkers in patients with coronary artery disease and hypertriglyceridemia. Cardiology. 2011;118:187–94 [DOI] [PubMed] [Google Scholar]
- 3.Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68:280–9 [DOI] [PubMed] [Google Scholar]
- 4.Akhtar Khan N. Polyunsaturated fatty acids in the modulation of T-cell signalling. Prostaglandins Leukot Essent Fatty Acids. 2010;82:179–87 [DOI] [PubMed] [Google Scholar]
- 5.Hulbert AJ, Turner N, Storlien LH, Else PL. Dietary fats and membrane function: implications for metabolism and disease. Biol Rev Camb Philos Soc. 2005;80:155–69 [DOI] [PubMed] [Google Scholar]
- 6.Holub BJ. Clinical nutrition: 4. Omega-3 fatty acids in cardiovascular care. CMAJ. 2002;166:608–15 [PMC free article] [PubMed] [Google Scholar]
- 7.van der Meij BS, van Bokhorst-de van der Schueren MA, Langius JA, Brouwer IA, van Leeuwen PA. N-3 PUFAs in cancer, surgery, and critical care: a systematic review on clinical effects, incorporation, and washout of oral or enteral compared with parenteral supplementation. Am J Clin Nutr. 2011;94:1248–65 [DOI] [PubMed] [Google Scholar]
- 8.Heidemann C, Schulze MB, Franco OH, van Dam RM, Mantzoros CS, Hu FB. Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all causes in a prospective cohort of women. Circulation. 2008;118:230–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young SN. Clinical nutrition: 3. The fuzzy boundary between nutrition and psychopharmacology. CMAJ. 2002;166:205–9 [PMC free article] [PubMed] [Google Scholar]
- 10.Wurtman RJ, O'Rourke D, Wurtman JJ. Nutrient imbalances in depressive disorders. Possible brain mechanisms. Ann N Y Acad Sci. 1989;575:75–82 [DOI] [PubMed] [Google Scholar]
- 11.Young SN. Folate and depression–a neglected problem. J Psychiatry Neurosci. 2007;32:80–2 [PMC free article] [PubMed] [Google Scholar]
- 12.Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351:1213. [DOI] [PubMed] [Google Scholar]
- 13.Rudin DO. The major psychoses and neuroses as omega-3 essential fatty acid deficiency syndrome: substrate pellagra. Biol Psychiatry. 1981;16:837–50 [PubMed] [Google Scholar]
- 14.Rudin DO. The dominant diseases of modernized societies as omega-3 essential fatty acid deficiency syndrome: substrate beriberi. Med Hypotheses. 1982;8:17–47 [DOI] [PubMed] [Google Scholar]
- 15.Bourre JM, Dumont O, Piciotti M, Clément M, Chaudière J, Bonneil M, Nalbone G, Lafont H, Pascal G, Durand G. Essentiality of omega 3 fatty acids for brain structure and function. World Rev Nutr Diet. 1991;66:103–17 [DOI] [PubMed] [Google Scholar]
- 16.Yehuda S, Rabinovitz S, Mostofsky DI. Essential fatty acids are mediators of brain biochemistry and cognitive functions. J Neurosci Res. 1999;56:565–70 [DOI] [PubMed] [Google Scholar]
- 17.Brookes PS, Buckingham JA, Tenreiro AM, Hulbert AJ, Brand MD. The proton permeability of the inner membrane of liver mitochondria from ectothermic and endothermic vertebrates and from obese rats: correlations with standard metabolic rate and phospholipid fatty acid composition. Comp Biochem Physiol B Biochem Mol Biol. 1998;119:325–34 [DOI] [PubMed] [Google Scholar]
- 18.Turner N, Else PL, Hulbert AJ. Docosahexaenoic acid (DHA) content of membranes determines molecular activity of the sodium pump: implications for disease states and metabolism. Naturwissenschaften. 2003;90:521–3 [DOI] [PubMed] [Google Scholar]
- 19.Else PL, Wu BJ. What role for membranes in determining the higher sodium pump molecular activity of mammals compared to ectotherms? J Comp Physiol B. 1999;169:296–302 [DOI] [PubMed] [Google Scholar]
- 20.Wu BJ, Hulbert AJ, Storlien LH, Else PL. Membrane lipids and sodium pumps of cattle and crocodiles: an experimental test of the membrane pacemaker theory of metabolism. Am J Physiol Regul Integr Comp Physiol. 2004;287:R633–41 [DOI] [PubMed] [Google Scholar]
- 21.Leaf A. The electrophysiologic basis for the antiarrhythmic and anticonvulsant effects of n-3 polyunsaturated fatty acids: heart and brain. Lipids. 2001;36: Suppl:S107–10 [DOI] [PubMed] [Google Scholar]
- 22.Stanley WC, Khairallah RJ, Dabkowski ER. Update on lipids and mitochondrial function: impact of dietary n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care. 2012;15:122–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids. 2003;126:1–27 [DOI] [PubMed] [Google Scholar]
- 24.Stillwell W, Jenski LJ, Crump FT, Ehringer W. Effect of docosahexaenoic acid on mouse mitochondrial membrane properties. Lipids. 1997;32:497–506 [DOI] [PubMed] [Google Scholar]
- 25.El-Ansary AK, Al-Daihan SK, El-Gezeery AR. On the protective effect of omega-3 against propionic acid-induced neurotoxicity in rat pups. Lipids Health Dis. 2011;10:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol. 2008;48:359–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhatnagar A, Sheffler DJ, Kroeze WK, Compton-Toth B, Roth BL. Caveolin-1 interacts with 5–HT2A serotonin receptors and profoundly modulates the signaling of selected Galphaq-coupled protein receptors. J Biol Chem. 2004;279:34614–23 [DOI] [PubMed] [Google Scholar]
- 28.Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27:9941–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKenna NJ, Lanz RB, O'Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–44 [DOI] [PubMed] [Google Scholar]
- 30.Head BP, Patel HH, Tsutsumi YM, Hu Y, Mejia T, Mora RC, Insel PA, Roth DM, Drummond JC, Patel PM. Caveolin-1 expression is essential for N-methyl-D-aspartate receptor-mediated Src and extracellular signal-regulated kinase 1/2 activation and protection of primary neurons from ischemic cell death. FASEB J. 2008;22:828–40 [DOI] [PubMed] [Google Scholar]
- 31.Couet J, Sargiacomo M, Lisanti MP. Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J Biol Chem. 1997;272:30429–38 [DOI] [PubMed] [Google Scholar]
- 32.Kong MM, Hasbi A, Mattocks M, Fan T, O'Dowd BF, George SR. Regulation of D1 dopamine receptor trafficking and signaling by caveolin-1. Mol Pharmacol. 2007;72:1157–70 [DOI] [PubMed] [Google Scholar]
- 33.Maes M, Smith RS. Fatty acids, cytokines, and major depression. Biol Psychiatry. 1998;43:313–4 [DOI] [PubMed] [Google Scholar]
- 34.Suarez EC, Krishnan RR, Lewis JG. The relation of severity of depressive symptoms to monocyte-associated proinflammatory cytokines and chemokines in apparently healthy men. Psychosom Med. 2003;65:362–8 [DOI] [PubMed] [Google Scholar]
- 35.Xia Z, DePierre JW, Nassberger L. Tricyclic antidepressants inhibit IL-6, IL-1, and TNF-alpha release in human blood monocytes and IL-2 and interferon-gamma in T cells. Immunopharmacology. 1996;34:27–37 [DOI] [PubMed] [Google Scholar]
- 36.James MJ, Cleland LG. Dietary n-3 fatty acids and therapy for rheumatoid arthritis. Semin Arthritis Rheum. 1997;27:85–97 [DOI] [PubMed] [Google Scholar]
- 37.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71:343S–8S [DOI] [PubMed] [Google Scholar]
- 38.Kuwamori M, Wada M, Takita T, Tadokoro T, Maekawa A, Innami S. Effect of dietary n-3/n-6 fatty acid ratio on the total count, fatty acid composition, and histamine and leukotriene concentrations of mast cells in tunica mucosa bronchiorum of type I allergic guinea pig. Biosci Biotechnol Biochem. 1997;61:763–7 [DOI] [PubMed] [Google Scholar]
- 39.Lieb J, Karmali R, Horrobin D. Elevated levels of prostaglandin E2 and thromboxane B2 in depression. Prostaglandins Leukot Med. 1983;10:361–7 [DOI] [PubMed] [Google Scholar]
- 40.Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada S, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003;54:70–5 [DOI] [PubMed] [Google Scholar]
- 41.Chiang MY, Misner D, Kempermann G, Schikorski T, Giguère V, Sucov HM, Gage FH, Stevens CF, Evans RM. An essential role for retinoid receptors RARbeta and RXRgamma in long-term potentiation and depression. Neuron. 1998;21:1353–61 [DOI] [PubMed] [Google Scholar]
- 42.Rao JS, Ertley RN, Lee HJ, DeMar JC, Jr, Arnold JT, Rapoport SI, Bazinet RP. N-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry. 2007;12:36–46 [DOI] [PubMed] [Google Scholar]
- 43.Dyall SC, Michael GJ, Michael-Titus AT. Omega3 fatty acids reverse age related decreases in nuclear receptors and increase neurogenesis in old rats. J Neurosci Res. 2010;88:2091–102 [DOI] [PubMed] [Google Scholar]
- 44.Kawakita E, Hashimoto M, Shido O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience. 2006;139:991–7 [DOI] [PubMed] [Google Scholar]
- 45.Gupta S, Knight AG, Gupta S, Keller JN, Bruce-Keller AJ. Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. J Neurochem. 2012;120:1060–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calder PC. Fatty acids and inflammation: the cutting edge between food and pharma. Eur J Pharmacol. 2011;668: Suppl 1:S50–8 [DOI] [PubMed] [Google Scholar]
- 47.Mythri RB, Venkateshappa C, Harish G, Mahadevan A, Muthane UB, Yasha TC, Srinivas Bharath MM, Shankar SK. Evaluation of markers of oxidative stress, antioxidant function and astrocytic proliferation in the striatum and frontal cortex of Parkinson's disease brains. Neurochem Res. 2011;36:1452–63 [DOI] [PubMed] [Google Scholar]
- 48.Freeman MP, Rapaport MH. Omega-3 fatty acids and depression: from cellular mechanisms to clinical care. J Clin Psychiatry. 2011;72:258–9 [DOI] [PubMed] [Google Scholar]
- 49.Kiecolt-Glaser JK, Belury MA, Porter K, Beversdorf DQ, Lemeshow S, Glaser R. Depressive symptoms, n-6:n-3 fatty acids, and inflammation in older adults. Psychosom Med. 2007;69:217–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maes M, Christophe A, Delanghe J, Altamura C, Neels H, Meltzer HY. Lowered omega3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Res. 1999;85:275–91 [DOI] [PubMed] [Google Scholar]
- 51.Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry. 1998;43:315–9 [DOI] [PubMed] [Google Scholar]
- 52.Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry. 2002;159:477–9 [DOI] [PubMed] [Google Scholar]
- 53.Jazayeri S, Tehrani-Doost M, Keshavarz SA, Hosseini M, Djazayery A, Amini H, Jalali M, Peet M. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42:192–8 [DOI] [PubMed] [Google Scholar]
- 54.Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002;59:913–9 [DOI] [PubMed] [Google Scholar]
- 55.Su KP, Huang SY, Chiu CC, Shen WW. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol. 2003;13:267–71 [DOI] [PubMed] [Google Scholar]
- 56.Mischoulon D, Best-Popescu C, Laposata M, Merens W, Murakami JL, Wu SL, Papakostas GI, Dording CM, Sonawalla SB, Nierenberg AA, et al. A double-blind dose-finding pilot study of docosahexaenoic acid (DHA) for major depressive disorder. Eur Neuropsychopharmacol. 2008;18:639–45 [DOI] [PubMed] [Google Scholar]
- 57.Lespérance F, Frasure-Smith N, St-André E, Turecki G, Lespérance P, Wisniewski SR. The efficacy of omega-3 supplementation for major depression: a randomized controlled trial. J Clin Psychiatry. 2011;72:1054–62 [DOI] [PubMed] [Google Scholar]
- 58.Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry. 2006;163:1098–100 [DOI] [PubMed] [Google Scholar]
- 59.da Silva TM, Munhoz RP, Alvarez C, Naliwaiko K, Kiss A, Andreatini R, Ferraz AC. Depression in Parkinson's disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J Affect Disord. 2008;111:351–9 [DOI] [PubMed] [Google Scholar]
- 60.Freeman MP, Hibbeln JR, Silver M, Hirschberg AM, Wang B, Yule AM, Petrillo LF, Pascuillo E, Economou NI, Joffe H, et al. Omega-3 fatty acids for major depressive disorder associated with the menopausal transition: a preliminary open trial. Menopause. 2011;18:279–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rondanelli M, Giacosa A, Opizzi A, Pelucchi C, La Vecchia C, Montorfano G, Negroni M, Berra B, Politi P, Rizzo AM. Effect of omega-3 fatty acids supplementation on depressive symptoms and on health-related quality of life in the treatment of elderly women with depression: a double-blind, placebo-controlled, randomized clinical trial. J Am Coll Nutr. 2010;29:55–64 [DOI] [PubMed] [Google Scholar]
- 62.Marangell LB, Martinez JM, Zboyan HA, Kertz B, Kim HF, Puryear LJ. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry. 2003;160:996–8 [DOI] [PubMed] [Google Scholar]
- 63.Rogers PJ, Appleton KM, Kessler D, Peters TJ, Gunnell D, Hayward RC, Heatherley SV, Christian LM, McNaughton SA, Ness AR. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99:421–31 [DOI] [PubMed] [Google Scholar]
- 64.Mischoulon D, Papakostas GI, Dording CM, Farabaugh AH, Sonawalla SB, Agoston AM, Smith J, Beaumont EC, Dahan LE, Alpert JE, et al. Double-blind, randomized controlled trial of ethyl-eicosapentaenoate for major depressive disorder. J Clin Psychiatry. 2009;70:1636–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Freeman MP, Hibbeln JR, Wisner KL, Brumbach BH, Watchman M, Gelenberg AJ. Randomized dose-ranging pilot trial of omega-3 fatty acids for postpartum depression. Acta Psychiatr Scand. 2006;113:31–5 [DOI] [PubMed] [Google Scholar]
- 66.Su KP, Huang SY, Chiu TH, Huang KC, Huang CL, Chang HC, Pariante CM. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69:644–51 [DOI] [PubMed] [Google Scholar]
- 67.Makrides M, Gibson RA, McPhee AJ, Yelland L, Quinlivan J, Ryan P;DOMInO Investigative Team Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: a randomized controlled trial. JAMA. 2010;304:1675–83 [DOI] [PubMed] [Google Scholar]
- 68.Doornbos B, van Goor SA, Dijck-Brouwer DA, Schaafsma A, Korf J, Muskiet FA. Supplementation of a low dose of DHA or DHA+AA does not prevent peripartum depressive symptoms in a small population based sample. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:49–52 [DOI] [PubMed] [Google Scholar]
- 69.Rees AM, Austin MP, Parker GB. Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust N Z J Psychiatry. 2008;42:199–205 [DOI] [PubMed] [Google Scholar]
- 70.Llorente AM, Jensen CL, Voigt RG, Fraley JK, Berretta MC, Heird WC. Effect of maternal docosahexaenoic acid supplementation on postpartum depression and information processing. Am J Obstet Gynecol. 2003;188:1348–53 [DOI] [PubMed] [Google Scholar]
- 71.Stahl LA, Begg DP, Weisinger RS, Sinclair AJ. The role of omega-3 fatty acids in mood disorders. Curr Opin Investig Drugs. 2008;9:57–64 [PubMed] [Google Scholar]
- 72.Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. Am J Psychiatry. 2003;160:2222–7 [DOI] [PubMed] [Google Scholar]
- 73.Conklin SM, Manuck SB, Yao JK, Flory JD, Hibbeln JR, Muldoon MF. High omega-6 and low omega-3 fatty acids are associated with depressive symptoms and neuroticism. Psychosom Med. 2007;69:932–4 [DOI] [PubMed] [Google Scholar]
- 74.Féart C, Peuchant E, Letenneur L, Samieri C, Montagnier D, Fourrier-Reglat A, Barberger-Gateau P. Plasma eicosapentaenoic acid is inversely associated with severity of depressive symptomatology in the elderly: data from the Bordeaux sample of the Three-City Study. Am J Clin Nutr. 2008;87:1156–62 [DOI] [PubMed] [Google Scholar]
- 75.Mamalakis G, Tornaritis M, Kafatos A. Depression and adipose essential polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2002;67:311–8 [DOI] [PubMed] [Google Scholar]
- 76.Gracious BL, Chirieac MC, Costescu S, Finucane TL, Youngstrom EA, Hibbeln JR. Randomized, placebo-controlled trial of flax oil in pediatric bipolar disorder. Bipolar Disord. 2010;12:142–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. Eur J Clin Nutr. 2009;63:1037–40 [DOI] [PubMed] [Google Scholar]
- 78.Wozniak J, Biederman J, Mick E, Waxmonsky J, Hantsoo L, Best C, Cluette-Brown JE, Laposata M. Omega-3 fatty acid monotherapy for pediatric bipolar disorder: a prospective open-label trial. Eur Neuropsychopharmacol. 2007;17:440–7 [DOI] [PubMed] [Google Scholar]
- 79.Marangell LB, Suppes T, Ketter TA, Dennehy EB, Zboyan H, Kertz B, Nierenberg A, Calabrese J, Wisniewski SR, Sachs G. Omega-3 fatty acids in bipolar disorder: clinical and research considerations. Prostaglandins Leukot Essent Fatty Acids. 2006;75:315–21 [DOI] [PubMed] [Google Scholar]
- 80.Frangou S, Lewis M, McCrone P. Efficacy of ethyl eicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. Br J Psychiatry. 2006;188:46–50 [DOI] [PubMed] [Google Scholar]
- 81.Frangou S, Lewis M, Wollard J, Simmons A. Preliminary in vivo evidence of increased N-acetyl-aspartate following eicosapentanoic acid treatment in patients with bipolar disorder. J Psychopharmacol. 2007;21:435–9 [DOI] [PubMed] [Google Scholar]
- 82.Chiu CC, Huang SY, Chen CC, Su KP. Omega-3 fatty acids are more beneficial in the depressive phase than in the manic phase in patients with bipolar I disorder. J Clin Psychiatry. 2005;66:1613–4 [DOI] [PubMed] [Google Scholar]
- 83.Osher Y, Bersudsky Y, Belmaker RH. Omega-3 eicosapentaenoic acid in bipolar depression: report of a small open-label study. J Clin Psychiatry. 2005;66:726–9 [DOI] [PubMed] [Google Scholar]
- 84.Stoll AL, Severus WE, Freeman MP, Rueter S, Zboyan HA, Diamond E, Cress KK, Marangell LB. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56:407–12 [DOI] [PubMed] [Google Scholar]
- 85.Keck PE, Jr, Mintz J, McElroy SL, Freeman MP, Suppes T, Frye MA, Altshuler LL, Kupka R, Nolen WA, Leverich GS, et al. Double-blind, randomized, placebo-controlled trials of ethyl-eicosapentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biol Psychiatry. 2006;60:1020–2 [DOI] [PubMed] [Google Scholar]
- 86.Samieri C, Féart C, Letenneur L, Dartigues JF, Pérès K, Auriacombe S, Peuchant E, Delcourt C, Barberger-Gateau P. Low plasma eicosapentaenoic acid and depressive symptomatology are independent predictors of dementia risk. Am J Clin Nutr. 2008;88:714–21 [DOI] [PubMed] [Google Scholar]
- 87.Martin DS, Spencer P, Horrobin DF, Lynch MA. Long-term potentiation in aged rats is restored when the age-related decrease in polyunsaturated fatty acid concentration is reversed. Prostaglandins Leukot Essent Fatty Acids. 2002;67:121–30 [DOI] [PubMed] [Google Scholar]
- 88.Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, Salem N, Jr, Frautschy SA, Cole GM. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci. 2005;25:3032–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Green KN, Martinez-Coria H, Khashwji H, Hall EB, Yurko-Mauro KA, Ellis L, LaFerla FM. Dietary docosahexaenoic acid and docosapentaenoic acid ameliorate amyloid-beta and tau pathology via a mechanism involving presenilin 1 levels. J Neurosci. 2007;27:4385–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yurko-Mauro K, McCarthy D, Rom D, Nelson EB, Ryan AS, Blackwell A, Salem N, Jr, Stedman M. MIDAS Investigators. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 2010;6:456–64 [DOI] [PubMed] [Google Scholar]
- 91.Kotani S, Sakaguchi E, Warashina S, Matsukawa N, Ishikura Y, Kiso Y, Sakakibara M, Yoshimoto T, Guo J, Yamashima T. Dietary supplementation of arachidonic and docosahexaenoic acids improves cognitive dysfunction. Neurosci Res. 2006;56:159–64 [DOI] [PubMed] [Google Scholar]
- 92.Quinn JF, Raman R, Thomas RG, Yurko-Mauro K, Nelson EB, Van Dyck C, Galvin JE, Emond J, Jack CR, Jr, Weiner M, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010;304:1903–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Freund-Levi Y, Hjorth E, Lindberg C, Cederholm T, Faxen-Irving G, Vedin I, Palmblad J, Wahlund LO, Schultzberg M, Basun H, et al. Effects of omega-3 fatty acids on inflammatory markers in cerebrospinal fluid and plasma in Alzheimer's disease: the OmegAD study. Dement Geriatr Cogn Disord. 2009;27:481–90 [DOI] [PubMed] [Google Scholar]
- 94.van de Rest O, Geleijnse JM, Kok FJ, van Staveren WA, Dullemeijer C, Olderikkert MG, Beekman AT, de Groot CP. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology. 2008;71:430–8 [DOI] [PubMed] [Google Scholar]
- 95.Boston PF, Bennett A, Horrobin DF, Bennett CN. Ethyl-EPA in Alzheimer's disease–a pilot study. Prostaglandins Leukot Essent Fatty Acids. 2004;71:341–6 [DOI] [PubMed] [Google Scholar]
- 96.Chiu CC, Su KP, Cheng TC, Liu HC, Chang CJ, Dewey ME, Stewart R, Huang SY. The effects of omega-3 fatty acids monotherapy in Alzheimer's disease and mild cognitive impairment: a preliminary randomized double-blind placebo-controlled study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1538–44 [DOI] [PubMed] [Google Scholar]
- 97.Freund-Levi Y, Basun H, Cederholm T, Faxén-Irving G, Garlind A, Grut M, Vedin I, Palmblad J, Wahlund LO, Eriksdotter-Jönhagen M. Omega-3 supplementation in mild to moderate Alzheimer's disease: effects on neuropsychiatric symptoms. Int J Geriatr Psychiatry. 2008;23:161–9 [DOI] [PubMed] [Google Scholar]
- 98.Freund-Levi Y, Eriksdotter-Jönhagen M, Cederholm T, Basun H, Faxén-Irving G, Garlind A, Vedin I, Vessby B, Wahlund LO, Palmblad J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006;63:1402–8 [DOI] [PubMed] [Google Scholar]
- 99.Mitchell EA, Aman MG, Turbott SH, Manku M. Clinical characteristics and serum essential fatty acid levels in hyperactive children. Clin Pediatr (Phila). 1987;26:406–11 [DOI] [PubMed] [Google Scholar]
- 100.Stevens LJ, Zentall SS, Deck JL, Abate ML, Watkins BA, Lipp SR, Burgess JR. Essential fatty acid metabolism in boys with attention-deficit hyperactivity disorder. Am J Clin Nutr. 1995;62:761–8 [DOI] [PubMed] [Google Scholar]
- 101.Chen JR, Hsu SF, Hsu CD, Hwang LH, Yang SC. Dietary patterns and blood fatty acid composition in children with attention-deficit hyperactivity disorder in Taiwan. J Nutr Biochem. 2004;15:467–72 [DOI] [PubMed] [Google Scholar]
- 102.Stevens LJ, Zentall SS, Abate ML, Kuczek T, Burgess JR. Omega-3 fatty acids in boys with behavior, learning, and health problems. Physiol Behav. 1996;59:915–20 [DOI] [PubMed] [Google Scholar]
- 103.Ross BM, McKenzie I, Glen I, Bennett CP. Increased levels of ethane, a non-invasive marker of n-3 fatty acid oxidation, in breath of children with attention deficit hyperactivity disorder. Nutr Neurosci. 2003;6:277–81 [DOI] [PubMed] [Google Scholar]
- 104.Young GS, Conquer JA, Thomas R. Effect of randomized supplementation with high dose olive, flax or fish oil on serum phospholipid fatty acid levels in adults with attention deficit hyperactivity disorder. Reprod Nutr Dev. 2005;45:549–58 [DOI] [PubMed] [Google Scholar]
- 105.Gustafsson PA, Birberg-Thornberg U, Duchén K, Landgren M, Malmberg K, Pelling H, Strandvik B, Karlsson T. EPA supplementation improves teacher-rated behaviour and oppositional symptoms in children with ADHD. Acta Paediatr. 2010;99:1540–9 [DOI] [PubMed] [Google Scholar]
- 106.Vaisman N, Kaysar N, Zaruk-Adasha Y, Pelled D, Brichon G, Zwingelstein G, Bodennec J. Correlation between changes in blood fatty acid composition and visual sustained attention performance in children with inattention: effect of dietary n-3 fatty acids containing phospholipids. Am J Clin Nutr. 2008;87:1170–80 [DOI] [PubMed] [Google Scholar]
- 107.Johnson M, Ostlund S, Fransson G, Kadesjö B, Gillberg C. Omega-3/omega-6 fatty acids for attention deficit hyperactivity disorder: a randomized placebo-controlled trial in children and adolescents. J Atten Disord. 2009;12:394–401 [DOI] [PubMed] [Google Scholar]
- 108.Hirayama S, Hamazaki T, Terasawa K. Effect of docosahexaenoic acid-containing food administration on symptoms of attention-deficit/hyperactivity disorder - a placebo-controlled double-blind study. Eur J Clin Nutr. 2004;58:467–73 [DOI] [PubMed] [Google Scholar]
- 109.Voigt RG, Llorente AM, Jensen CL, Fraley JK, Berretta MC, Heird WC. A randomized, double-blind, placebo-controlled trial of docosahexaenoic acid supplementation in children with attention-deficit/hyperactivity disorder. J Pediatr. 2001;139:189–96 [DOI] [PubMed] [Google Scholar]
- 110.Hirashima F, Parow AM, Stoll AL, Demopulos CM, Damico KE, Rohan ML, Eskesen JG, Zuo CS, Cohen BM, Renshaw PF. Omega-3 fatty acid treatment and T(2) whole brain relaxation times in bipolar disorder. Am J Psychiatry. 2004;161:1922–4 [DOI] [PubMed] [Google Scholar]
- 111.Nakamura K, Wang W, Kang UJ. The role of glutathione in dopaminergic neuronal survival. J Neurochem. 1997. 69: 1850–1858 [DOI] [PubMed] [Google Scholar]
- 112.Wood SJ, Cocchi L, Proffitt TM, McConchie M, Jackson GD, Takahashi T, Pantelis C, McGorry PD, Berger GE. Neuroprotective effects of ethyl-eicosapentaenoic acid in first episode psychosis: a longitudinal T2 relaxometry pilot study. Psychiatry Res. 2010;182:180–2 [DOI] [PubMed] [Google Scholar]
- 113.Berger GE, Proffitt TM, McConchie M, Yuen H, Wood SJ, Amminger GP, Brewer W, McGorry PD. Ethyl-eicosapentaenoic acid in first-episode psychosis: a randomized, placebo-controlled trial. J Clin Psychiatry. 2007;68:1867–75 [DOI] [PubMed] [Google Scholar]
- 114.Berger GE, Proffitt TM, McConchie M, Yuen H, Wood SJ, Amminger GP, Brewer W, McGorry PD. Ethyl-eicosapentaenoic acid in first-episode psychosis. A 1H-MRS study. Neuropsychopharmacology. 2008;33:2467–73 [DOI] [PubMed] [Google Scholar]
- 115.Amminger GP, Schäfer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, Mackinnon A, McGorry PD, Berger GE. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–54 [DOI] [PubMed] [Google Scholar]
- 116.Emsley R, Myburgh C, Oosthuizen P, van Rensburg SJ. Randomized, placebo-controlled study of ethyl-eicosapentaenoic acid as supplemental treatment in schizophrenia. Am J Psychiatry. 2002;159:1596–8 [DOI] [PubMed] [Google Scholar]
- 117.Peet M, Horrobin DF. E-E Multicentre Study Group. A dose-ranging exploratory study of the effects of ethyl-eicosapentaenoate in patients with persistent schizophrenic symptoms. J Psychiatr Res. 2002;36:7–18 [DOI] [PubMed] [Google Scholar]
- 118.Peet M, Brind J, Ramchand CN, Shah S, Vankar GK. Two double-blind placebo-controlled pilot studies of eicosapentaenoic acid in the treatment of schizophrenia. Schizophr Res. 2001;49:243–51 [DOI] [PubMed] [Google Scholar]
- 119.Fenton WS, Dickerson F, Boronow J, Hibbeln JR, Knable M. A placebo-controlled trial of omega-3 fatty acid (ethyl eicosapentaenoic acid) supplementation for residual symptoms and cognitive impairment in schizophrenia. Am J Psychiatry. 2001;158:2071–4 [DOI] [PubMed] [Google Scholar]
- 120.Chez MG, Burton Q, Dowling T, Chang M, Khanna P, Kramer C. Memantine as adjunctive therapy in children diagnosed with autistic spectrum disorders: an observation of initial clinical response and maintenance tolerability. J Child Neurol. 2007;22:574–9 [DOI] [PubMed] [Google Scholar]
- 121.Dosman CF, Brian JA, Drmic IE, Senthilselvan A, Harford MM, Smith RW, Sharieff W, Zlotkin SH, Moldofsky H, Roberts SW. Children with autism: effect of iron supplementation on sleep and ferritin. Pediatr Neurol. 2007;36:152–8 [DOI] [PubMed] [Google Scholar]
- 122.Miral S, Gencer O, Inal-Emiroglu FN, Baykara B, Baykara A, Dirik E. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur Child Adolesc Psychiatry. 2008;17:1–8 [DOI] [PubMed] [Google Scholar]
- 123.Amminger GP, Berger GE, Schäfer MR, Klier C, Friedrich MH, Feucht M. Omega-3 fatty acids supplementation in children with autism: a double-blind randomized, placebo-controlled pilot study. Biol Psychiatry. 2007;61:551–3 [DOI] [PubMed] [Google Scholar]
- 124.Bent S, Bertoglio K, Ashwood P, Bostrom A, Hendren RL. A pilot randomized controlled trial of omega-3 fatty acids for autism spectrum disorder. J Autism Dev Disord. 2011;41:545–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Politi P, Cena H, Comelli M, Marrone G, Allegri C, Emanuele E, Ucelli di Nemi S. Behavioral effects of omega-3 fatty acid supplementation in young adults with severe autism: an open label study. Arch Med Res. 2008;39:682–5 [DOI] [PubMed] [Google Scholar]
- 126.Holman RT, Adams CE, Nelson RA, Grater SJ, Jaskiewicz JA, Johnson SB, Erdman JW., Jr Patients with anorexia nervosa demonstrate deficiencies of selected essential fatty acids, compensatory changes in nonessential fatty acids and decreased fluidity of plasma lipids. J Nutr. 1995;125:901–7 [DOI] [PubMed] [Google Scholar]
- 127.Langan SM, Farrell PM. Vitamin E, vitamin A and essential fatty acid status of patients hospitalized for anorexia nervosa. Am J Clin Nutr. 1985;41:1054–60 [DOI] [PubMed] [Google Scholar]
- 128.Ayton AK, Azaz A, Horrobin DF. A pilot open case series of ethyl-EPA supplementation in the treatment of anorexia nervosa. Prostaglandins Leukot Essent Fatty Acids. 2004;71:205–9 [DOI] [PubMed] [Google Scholar]
- 129.Barbarich NC, McConaha CW, Halmi KA, Gendall K, Sunday SR, Gaskill J, La Via M, Frank GK, Brooks S, Plotnicov KH, et al. Use of nutritional supplements to increase the efficacy of fluoxetine in the treatment of anorexia nervosa. Int J Eat Disord. 2004;35:10–5 [DOI] [PubMed] [Google Scholar]
- 130.Fux M, Benjamin J, Nemets B. A placebo-controlled cross-over trial of adjunctive EPA in OCD. J Psychiatr Res. 2004;38:323–5 [DOI] [PubMed] [Google Scholar]
- 131.Hamazaki T, Sawazaki S, Itomura M, Asaoka E, Nagao Y, Nishimura N, Yazawa K, Kuwamori T, Kobayashi M. The effect of docosahexaenoic acid on aggression in young adults. A placebo-controlled double-blind study. J Clin Invest. 1996;97:1129–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zanarini MC, Frankenburg FR. Omega-3 Fatty acid treatment of women with borderline personality disorder: a double-blind, placebo-controlled pilot study. Am J Psychiatry. 2003;160:167–9 [DOI] [PubMed] [Google Scholar]
- 133.Song C, Li X, Leonard BE, Horrobin DF. Effects of dietary n-3 or n-6 fatty acids on interleukin-1beta–induced anxiety, stress, and inflammatory response in rats. J Lipid Res. 2003;44:1984–91 [DOI] [PubMed] [Google Scholar]
- 134.Song C, Leonard BE, Horrobin DF. Dietary ethyl-eicosapentaenoic acid but not soybean oil reverses central interleukin-1–induced changes in behavior, corticosterone and immune response in rats. Stress. 2004;7:43–54 [DOI] [PubMed] [Google Scholar]
- 135.Buydens-Branchey L, Branchey M. n-3 polyunsaturated fatty acids decrease anxiety feelings in a population of substance abusers. J Clin Psychopharmacol. 2006;26:661–5 [DOI] [PubMed] [Google Scholar]
- 136.Buydens-Branchey L, Branchey M, Hibbeln JR. Associations between increases in plasma n-3 polyunsaturated fatty acids following supplementation and decreases in anger and anxiety in substance abusers. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:568–75 [DOI] [PMC free article] [PubMed] [Google Scholar]