Abstract
Regulation of mRNA translation is a rapid and effective means to couple changes in the cellular environment with global rates of protein synthesis. In response to stresses, such as nutrient deprivation and accumulation of misfolded proteins in the endoplasmic reticulum, phosphorylation of the α subunit of eukaryotic initiation factor 2 (eIF2α~P) reduces general translation initiation while facilitating the preferential translation of select transcripts, such as that encoding activating transcription factor 4 (ATF4), a transcriptional activator of genes subject to the integrated stress response (ISR). In this review, we highlight the translational control processes regulated by nutritional stress, with an emphasis on the events triggered by eIF2α~P, and describe the family of eukaryotic initiation factor 2 kinases and the mechanisms by which each sense different stresses. We then address 3 questions. First, what are the mechanisms by which eIF2α~P confers preferential translation on select mRNA and what are the consequences of the gene expression induced by the ISR? Second, what are the molecular processes by which certain stresses can differentially activate eIF2α~P and ATF4 expression? The third question we address is what are the modes of cross-regulation between the ISR and other stress response pathways, such as the unfolded protein response and mammalian target of rapamycin, and how do these regulatory schemes provide for gene expression programs that are tailored for specific stresses? This review highlights recent advances in each of these areas of research, emphasizing how eIF2α~P and the ISR can affect metabolic health and disease.
Introduction
The process of mRNA translation is dynamic and a primary level of control of protein abundance in mammalian cells (1). As such, regulation at the level of translation is a rapid and effective means for the cell to respond to many different stresses, including those affecting nutrition, such as deficiencies of amino acids or glucose and high-fat diets. A central mechanism for translational control involves phosphorylation of the α subunit of eukaryotic initiation factor (eIF) 2 (eIF2α~P),3 which represses the initiation phase of protein synthesis, allowing cells to conserve resources while a new gene expression program is adopted to prevent stress damage. Accompanying this global translational control, eIF2α~P selectively enhances the translation of activating transcription factor (ATF) 4, a transcriptional activator of genes involved in metabolism and nutrient uptake, the redox status of cells, and the regulation of apoptosis (2–5). The idea that ATF4 is a common downstream target that integrates signaling from multiple eIF2α kinases has led to the eIF2α~P/ATF4 pathway being referred to as the integrated stress response (ISR) (5). The ISR shares many features with induced eIF2α~P and general control nonderepressible (GCN) 4 translational control in the general amino acid control pathway in yeast, highlighting its evolutionary conserved role in ameliorating nutritional deficiencies (6, 7).
Highlights of this review
This review begins with a brief overview of translation initiation and the processes controlled by nutrition, with an emphasis on the events triggered by eIF2α~P. Additionally, we describe the family of eIF2α kinases. Each serves as a sensor for different stress arrangements, standing guard for disturbances in cellular homeostasis. Enhanced eIF2α~P initiates a gradient of translational control of preexisting mRNAs, in which most mRNAs are translationally repressed, whereas a cadre of stress-related mRNAs are preferentially translated. We then focus on 3 key topics concerning translational control elicited by eIF2α~P. First, we highlight the mechanisms by which eIF2α~P confers preferential translation on select mRNAs and its effect on the gene expression programs induced by the ISR. One mechanism described for ATF4 involves delayed translation reinitiation, which allows for scanning ribosomes to selectively enhance ATF4 expression in response to eIF2α~P. In addition to ATF4, many other mRNAs are suggested to be subject to preferential translation during eIF2α~P, some via alternative mechanisms (8–11). Our second topic concerns the molecular processes by which stress signals can differentially activate eIF2α~P and ATF4 expression. ATF4 expression is controlled by both transcriptional and translational mechanisms, and certain stresses can repress ATF4 transcription, reducing the levels of ATF4 mRNA available for translation despite robust eIF2α~P (12). In this situation, eIF2α~P and translational control are invoked without activating ATF4 and its downstream targets. The third topic addresses the cross-regulation of the ISR with other stress response pathways, such as the unfolded protein response (UPR) and mammalian target of rapamycin (mTOR), and the role that these regulatory networks can play in health and disease, with a focus on diabetes and related metabolic disorders. This review highlights recent advances in these areas of research, emphasizing an understanding of how eIF2α~P and key metabolic processes are intricately linked.
Nutritional stresses regulate translation initiation
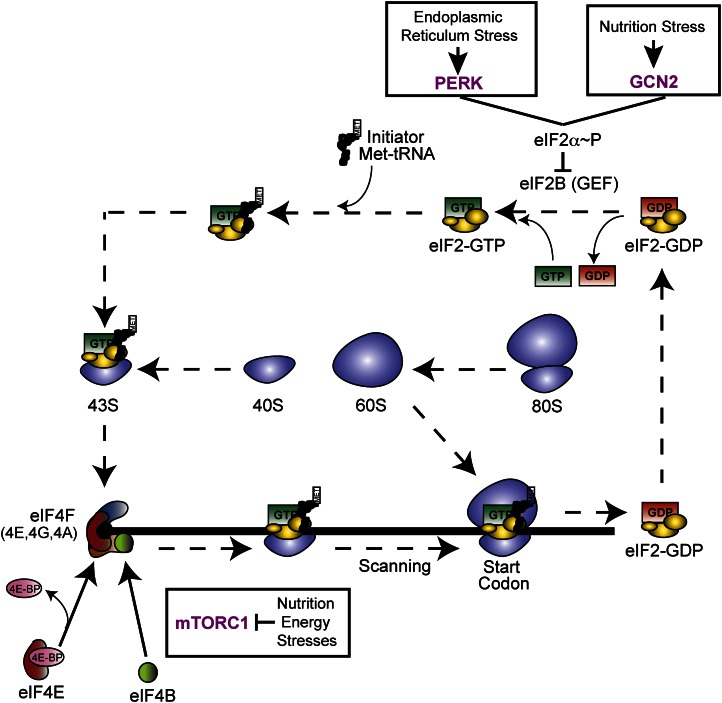
The regulation of eukaryotic protein synthesis occurs predominantly during translation initiation, and multiple associated proteins, each designated as eIFs, are required to assemble a translationally competent 80S ribosome. Although many of these initiation factors are indispensable for initiation, the nutritional status primarily regulates translation initiation at 2 steps involving the eIF4F cap-binding complex and eIF2, which delivers initiator methionyl tRNA (Met-tRNAi) to the translational machinery. Translational control facilitated by eIF2α~P is a primary focus of this review (Fig. 1). For in-depth reviews of the mechanisms underlying protein synthesis and additional regulatory schemes, see References 7, 13, and 14.
Figure 1.
Regulation of translation initiation is a rapid means for coupling nutrient deprivation and other stress conditions with levels of protein synthesis. This illustration shows the dissociation of the 80S ribosome complex into the individual 40S and 60S ribosomal subunits, which participate in translation initiation in conjunction with additional translation factors to initiate protein synthesis. Cap-dependent initiation of translation can be divided into 2 key events: the binding of the eukaryotic initiation factor 4f complex to the 7-methyl guanosine 5′ cap and the subsequent recruitment and scanning of the 43S complex, composed of eIF2-GTP-Met-tRNAMeti and other translation initiation factors attached to the 40S ribosomal subunit. After recognition of the start codon by the scanning 43S preinitiation complex, a 60S subunit joins to form an actively translating 80S ribosome. During conditions of low stress and high nutrient availability, an abundance of active eukaryotic initiation factor (eIF) 4F and eIF2 ternary complexes promotes high levels of cap-dependent translation. Nutritional stresses, such as amino acid or glucose deprivation, signal for a rapid reduction in global translation through phosphorylation of eIF2α (eIF2α~P) and repression of mammalian target of rapamycin (mTORC)1. Enhanced eIF2α~P leads to inhibition of eIF2B and lowered exchange of eIF2-GDP to eIF2-GTP. mTORC1 can enhance cap-dependent translation by 2 mechanisms. First, mTORC1 enhances phosphorylation of 4E-BP1 and 4E-BP2, leading to release of this inhibitory protein from eIF4E, the cap-binding subunit of eIF4F. Second, mTORC1 triggers S6 kinase phosphorylation of eIF4B, which then associates with the eIF4A subunit of eIF4F, enhancing eIF4A helicase function that expedites ribosome scanning during translation. In addition to nutritional stresses, perturbations in ER function activates PERK-induced eIF2α~P, effectively reducing the influx of nascent peptides to the overloaded protein-folding machinery. GCN2, general control nonderepressible; GEF, guanine nucleotide exchange factor; mTORC1, mammalian target of rapamycin complex 1; PERK, PKR-like endoplasmic reticulum kinase; tRNA, transfer RNA.
All eukaryotic mRNA have 5′-leader structures proximal to the primary coding sequence that are required for recruiting the translation initiation machinery. It is important to note that the distal 3′ noncoding portion of the mRNA can also contribute to enhanced translation efficiency and, in some cases, repress protein synthesis via the closed loop model of ribosome recycling (13). The mechanisms involving the 3′ noncoding portion of the mRNA are not discussed in detail in this review. Individual 5′ leaders vary in length and can regulate expression of the downstream coding sequence via complex secondary structures and upstream open reading frames (uORF) located 5′ to the primary coding sequence of mRNA. In a sense, these 5′ leaders serve as bar codes by which ribosomes will identify which transcripts are to be repressed or preferentially translated by enhanced eIF2α~P.
Once in the cytoplasm, the 7-methyl guanosine 5′ cap structure of the mRNA to be translated is bound by eIF4F, consisting of the eIF4E subunit that binds to the cap, the helicase eIF4A, and scaffolding protein eIF4G, which facilitates the closed loop between the 5′ and 3′ ends of the mRNA (Fig. 1). With the eIF4F complex effectively bound to the 5′ cap, the next step of translation involves the recruitment of a 43S preinitiation complex (PIC) composed of the 40S ribosomal subunit bound to eIF3, eIF1, eIF1A, eIF5, and the eIF2-GTP-Met-tRNAi ternary complex (eIF2-TC). The 43S PIC scans the 5′ leader in a processive 5′ to 3′ manner until it encounters an initiation codon, at which point Pi is released from the hydrolyzed GTP associated with eIF2, and the anticodon loop of the initiator methionyl tRNA base pairs with the initiation codon in the P site of the 40S subunit (13, 15). After the recognition of the start codon and the joining of the 40S and 60S ribosomal subunits, the 80S ribosome is primed for translation elongation and subsequent polypeptide synthesis.
The recycling of eIF2-GDP to its translationally active eIF2-GTP form by the guanine nucleotide exchange factor (GEF) eIF2B is a key regulatory switch in the modulation of protein synthesis (Fig. 1). eIF2B is a complex GEF consisting of 5 different subunits, 2 participating in catalytic function and the other 3 facilitating regulation (6, 16–19). During nutrient deprivation and other stress conditions, eIF2α is phosphorylated at serine 51, which then directly engages with the regulatory subcomplex of eIF2B, transforming eIF2 from a member of the 43S PIC into a competitive inhibitor of the GEF. As a consequence, there are reduced eIF2-GTP levels and decreased global protein synthesis.
During conditions of low nutrient availability, eIF4E can also be sequestered by the eIF4E-binding proteins (4E-BP), thus limiting assembly of the eIF4F complex (Fig. 1) (20–22). Once nutrient availability returns to optimal levels, mTORC1, consisting of mTOR complexed with Raptor and Lst8 (GβL), signals for increased protein synthesis by phosphorylating 4E-BP1 and 4E-BP2, preventing their binding to eIF4E and effectively promoting cap-dependent translation. Additionally, mTORC1 can phosphorylate and activate the S6 kinases, which in turn phosphorylate eIF4B, thus enhancing the affinity of eIF4B for the helicase eIF4A (20, 21, 23, 24). As a consequence, eIF4A has enhanced binding to ATP and increased processivity of the helicase, which promotes ribosome scanning of mRNA, especially those with structured 5′ leaders. Therefore, mTORC1 enhances cap-dependent translation by multiple mechanisms involving eIF4F.
eIF2#x03B1 kinases: sentinels against cellular stress
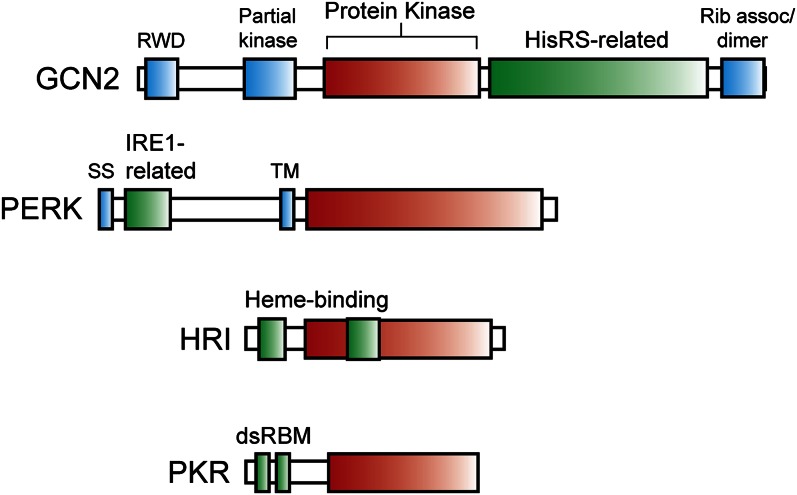
Mammals express 4 different eIF2α kinases, each serving as a cellular sentry that monitors for different exogenous and endogenous stresses. Family members and their respective stress signals include GCN2 (EIF2AK4), an eIF2α kinase induced in response to nutritional stresses (6, 25), PKR-like endoplasmic reticulum kinase (PERK) (EIF2AK3/PEK), which responds to perturbations in the endoplasmic reticulum (ER) (ER stress) (26, 27); heme-regulated eIF2α kinase (HRI) (EIF2AK1), which is activated by heme deprivation in erythroid cells (28, 29); and protein kinase R (PKR) (EIF2AK2), which participates in an antiviral defense pathway involving interferon (Fig. 2) (30–33). Dysfunctions in each of these eIF2α kinases are linked with pathologies in multiple organs, emphasizing their critical roles in the recognition and alleviation of environmental stress.
Figure 2.
A family of eukaryotic initiation factor (eIF) 2α kinases are activated in response to diverse stress conditions. The eIF2α kinases possess a related protein kinase domain (red box) that is flanked by distinct regulatory sequences, which facilitate induction of phosphorylation of the α subunit of eukaryotic initiation factor 2 (eIF2α~P) in response to different stress conditions. Due to differences in the length of the characteristic insert sequences shared among eukaryotic initiation fact (eIF) 2α kinases, the size of the protein kinase domain differs among family members. The eIF2α kinase general control nonderepressible (GCN) 2 contains a RWD sequence that associates with the activator protein GCN1, a partial kinase domain required for GCN2 activation, histidyl-tRNA synthetase–related sequences (HisRS) that directly bind uncharged tRNA, which accumulate during nutritional stress, and a carboxy terminal region that facilitates GCN2 dimerization and its ribosome association. Note many of the functional features of these domains are based on studies for yeast GCN2, which shares the same domain arrangement (6). PKR-like endoplasmic reticulum kinase (PERK) contains an endoplasmic reticulum (ER) transmembrane segment (TM) that divides this eukaryotic initiation factor (eIF) 2α kinase in 2. The carboxy terminal protein kinase domain catalyzes eIF2α~P. The amino terminal portion features a signal sequence (SS), facilitating translocation of this portion of PERK into the lumen of the ER, and sequences related to the unfolded protein response sensor IRE1, which are suggested to monitor accumulation of unfolded protein in this organelle. HRI contains 2 regions that bind to heme, 1 at the amino-terminal portion of HRI and the second in the insert region of the protein kinase domain, which can repress this eIF2α kinase (29). Low levels of iron lead to reduced amounts of heme in erythroid cells, which triggers a release from this repressing mechanism and enhanced eIF2α~P. As a consequence, the availability of heme is tightly coupled to globin synthesis, the predominant translation product in erythroid tissues. PKR participates in an antiviral defense mechanism triggered by interferon. Two double-stranded RNA binding motifs (dsRBM) associate with double-stranded RNA that can accumulate in cells infected by viruses, leading to PKR autophosphorylation and enhanced eIF2α~P (30). Lowered protein synthesis would reduce viral replication and proliferation. HisRS, histidyl-tRNA synthetase; Rib assoc, ribosome association; TM, transmembrane.
GCN2 is the primary responder to nutritional deprivation and is the only eIF2α kinase conserved among virtually all eukaryotes. The mechanism of activation during amino acid depletion involves the binding of accumulating uncharged tRNA in the cytoplasm to a region of GCN2 homologous with histidyl-tRNA synthetases (6, 34–36) (Fig. 2). GCN2 binding to uncharged tRNA ultimately triggers a conformational change that relieves inhibitory interactions within the protein kinase domain, resulting in autophosphorylation in the activation loop of the enzyme (6, 37–39).
Activation of GCN2 involves not only histidine starvation, but limitations for other essential amino acids as well as some nonessential (6, 25, 34, 40–42). Furthermore, this eIF2α kinase was reported to be activated by genetic disruptions of aminoacyl-tRNA synthetases or amino acid transporters and drugs that diminish the uptake or synthesis of amino acids or charging of tRNA (41, 43–48). These findings suggest that the aminoacylation levels of many different tRNA, including tRNAHis, can be used by GCN2 to measure the availability of amino acids. Loss of GCN2 in mice subject to leucine starvation diminishes eIF2α~P in the liver, which can occur in wild-type mice within 1 h of a leucine-deprived diet (42). However, protein synthesis was reduced to the same extent in both the wild-type and GCN2−/− mice during short-term administration of the leucine-deficient diet. Conversely, after 6 d of leucine deprivation, there were significant differences in the levels of translation between the wild-type and GCN2−/− mice. In wild-type mice, eIF2α~P continued to be high, accompanied by significant lowering of protein synthesis in the liver and shrinkage of this organ. By contrast, in GCN2-deficient mice, there were high levels of liver protein synthesis despite deficiencies for the essential amino acids (42). As a consequence, there was extensive muscle breakdown in GCN2−/− mice in a futile attempt to replenish amino acids and quench the liver translation system. Furthermore, whereas lipid synthesis is repressed in livers of wild-type mice during longer periods of leucine starvation, the production of lipids occurs unabated in GCN2-deficient mice, ultimately contributing to liver steatosis (49). An underlying rationale for the dysregulated lipid metabolism in the livers of GCN2−/− mice was suggested to be persistent activation of SREBP-1c and its target genes involved in the production and transport of fatty acids.
GCN2 can also be activated by glucose deprivation and exposure to high salt and stresses not directly related to nutrients, such as UV irradiation and anticancer drugs that inhibit proteasomes or histone deacetylases (12, 50–57). Currently, it is unclear whether uncharged tRNA are the activating ligand for GCN2 during these diverse stresses. In the yeast model system, mutations that disrupt GCN2 binding to uncharged tRNA block induced eIF2α~P in response to stresses involving amino acid starvation, as well as those not directly linked to nutrients (34, 35, 58, 59). This finding suggests that changes in tRNA charging may be a common activating signal for GCN2 in response to many different stresses. To directly test this idea, tRNA charging was measured genome-wide in yeast using a microarray-based approach (60). In response to deprivation of histidine, leucine, or tryptophan, there was a decrease in the charging of the cognate tRNA. In some cases, tRNA not charged with the starved amino acid became rapidly deacylated despite there being no reduction in the intracellular levels for these nonstarved amino acids (60). Additionally, high salinity stress also triggered transient changes in the charging of several different tRNAs. These studies suggest that GCN2 can be activated by many different tRNA species and that changes in the charging of tRNA can serve as a broad sensor of metabolic homeostasis in cells.
In addition to uncharged tRNA, regulatory proteins can alter the activity of GCN2. For example, GCN1 is a ribosome-associated protein that directly binds to the amino-terminal RWD segment of GCN2 (61–65). GCN1 is suggested to facilitate passage and binding of uncharged tRNA to the HisRS related domain of GCN2, thus enhancing eIF2α~P during nutrient stress (6, 66). Interestingly, IMPACT (yeast YIH1) is a protein that also contains an RWD (RING finger and WD repeat containing) that can compete with GCN2 for binding to the activator GCN1, thus blocking activation of the eIF2α kinase (67–69). IMPACT is variably expressed among cell types, with highest abundance in the central nervous system, suggesting that IMPACT can differentially repress GCN2 in selected tissues during dietary limitations for essential amino acids. Finally, stress signaling pathways are suggested to regulate GCN2 and eIF2α~P. For example, the DNA damage checkpoint protein kinase, DNA-PKc, was reported to directly or indirectly phosphorylate GCN2 in response to UV irradiation, facilitating translational control and cell survival (11).
The other eIF2α kinase that has a major role in nutrient stress and metabolism is PERK. PERK is an ER transmembrane protein that contains a regulatory region that resides in the lumen of the ER and a cytosolic eIF2α kinase domain (26, 27, 70–73). Calcium dysregulation, oxidative damage, and increased secretory loads or perturbations in posttranslational modification of proteins can lead to accumulation of misfolded protein that can cause ER stress (26). Regarding nutritional stresses, fluctuations in glucose levels and high-fat diets are linked to ER stress. In addition to PERK and the ISR, ER stress activates 2 additional transmembrane proteins, IRE1 (ERN1) and ATF6, which collectively induce the UPR. The UPR features translational control by PERK phosphorylation of eIF2α, which reduces the influx of nascent proteins into the ER, along with activating a program of gene expression designed to expand the processing capacity of the ER and enhance ER-associated protein degradation, a mechanism for the clearance and degradation of misfolded proteins from the secretory pathway. The UPR is linked to the progression and treatment of many diseases, including diabetes and related metabolic disorders, renal disorders, neuropathologies, and cancers (27, 74–78). The importance of PERK in diabetes is highlighted by the discovery that mutations disrupting this eIF2α kinase result in Wolcott-Rallison syndrome (WRS), which is characterized by neonatal diabetes, atrophy of the exocrine pancreas, skeletal dysplasia, growth retardation, and hepatic complications resulting in morbidity (79–82).
Activation of PERK during ER stress is thought to occur in parallel with the other UPR sensors, but the timing or duration of each may differ. Although the mechanistic details are not yet resolved, it has been proposed that the ER luminal portion of PERK can be bound and repressed by the ER chaperone BiP/GRP78 (72, 73). Misfolded proteins that accumulate in the ER lumen during stress are suggested to compete with PERK for BiP binding, triggering the release of the ER chaperone, thus leading to PERK oligomerization, which facilitates PERK autophosphorylation and enhanced eIF2α~P. It has been suggested that because of the abundance of BiP in the ER, this regulatory scheme would be too coarse to trigger a rapid titration of BiP from UPR sensors such as PERK (83, 84). This concern assumes that PERK and BiP are distributed equally across the ER, as opposed to being localized in some form of regulatory hub. An alternative mechanism proposed for the activation of the ER sensor IRE1 suggests that unfolded proteins can directly interact with the luminal regulatory region of IRE1, triggering its oligomerization and activation (84–87). This direct unfolded protein binding model is supported by genetic, biochemical, and structural studies and addresses the rapidity with which the UPR is activated on disruptions of the ER (84, 85). Because PERK and IRE1 share sequence homology in their luminal regulatory domains, features of the latter model are also germane to the regulation of PERK. Note that these models are not necessarily mutually exclusive, but rather may represent activation mechanisms invoked at different stages of the UPR.
Preferential translation of ATF4 during eIF2#x03B1~P
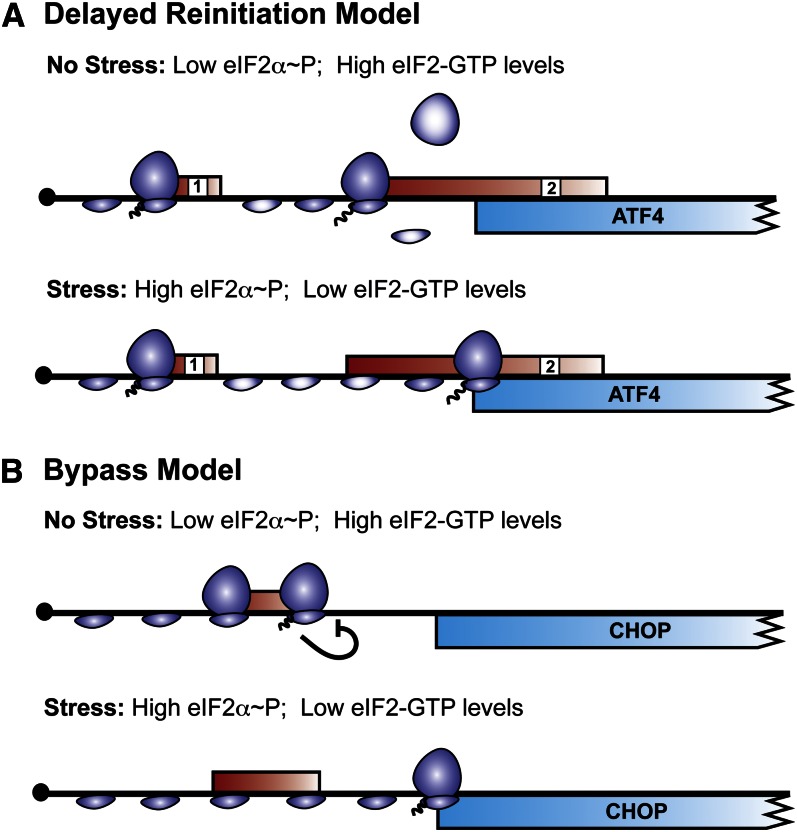
ATF4 is a basic leucine zipper (bZIP) transcription factor that is preferentially translated in response to eIF2α~P. The 5′ leader of ATF4 mRNA contains 2 uORF that orchestrate a mechanism by which ATF4 expression is paradoxically enhanced during eIF2α~P (Fig. 3) (2–4, 13). Increased ATF4 synthesis can subsequently activate the transcription of target genes in the ISR, which can collectively alleviate the nutritional stress. Preferential translation of ATF4 begins with the joining of the 43S PIC to the 5′-cap complex of the ATF4 mRNA. The 43S PIC then scans along the transcript in a 5′ to 3′ manner, and translation initiation occurs at the start codon of the 5′-proximal uORF1 of ATF4. After termination of translation at uORF1, the small ribosomal subunit is not disengaged from the ATF4 mRNA, but rather resumes scanning processively along the leader of the ATF4 transcript. To initiate translation once again, the 40S ribosomal subunit must reacquire the eIF2-TC. Under nonstressed conditions and high levels of eIF2-GTP, reinitiation occurs rapidly at the next available initiation codon, which corresponds to that of the inhibitory uORF2 (Fig. 3). The uORF2 overlaps out-of-frame with the primary ATF4 coding region, and after translation of uORF2, ribosomes dissociate from the ATF4 mRNA, and therefore ATF4 synthesis is diminished.
Figure 3.
Mechanisms of preferential translation during phosphorylation of the α subunit of eukaryotic initiation factor 2 (eIF2α~P). A, In the delayed translation reinitiation model, 2 upstream open reading frames (uORF) (red boxes) in the 5′ leader of the ATF4 mRNA direct preferential translation (4). The 5′-proximal uORF1 is a positive-acting element that facilitates retention of the scanning 40S subunit and resumption of scanning 5′ to 3′, leading inevitably to reinitiation at a downstream start codon. During nonstressed conditions, when eIF2α~P is low and eukaryotic initiation factor (eIF) 2-GTP levels are abundant, the scanning ribosome readily acquires the eIF2 ternary complex (eIF-TC) and reinitiates translation at the next available uORF, i.e., uORF2. The reacquisition of eIF2-TC is indicated by the darker shading in the scanning 40S ribosome. The uORF2 overlaps out-of-frame with the coding sequence (blue box) and, when translated, prevents synthesis of ATF4, as depicted by the dissociation of the small and large subunits after termination of uORF2 translation. During nutrient deprivation and other stressful events, there is an increase in eIF2α~P, which lowers the levels of eIF2-GTP. As a consequence, the 40S ribosome, which continues scanning after the translation of uORF1, needs additional time to reacquire the limiting eIF2-TC. This delay in reinitiation of translation allows for the 40S ribosome to scan through the uORF2 initiation codon. During the interval between the initiation codons of the uORF2 and the ATF4 coding region, the 40S ribosome obtains the limiting eIF2-TC (dark shading) and translates the ATF4 open reading frame. B, Translation of CHOP mRNA is inhibited during nonstress conditions by the presence of a single inhibitory uORF, which, when translated, functions to block translation elongation or termination, as illustrated by the bar symbol. This inhibitory uORF encodes a 34-amino acid residue sequence that is well conserved among vertebrates (10). In the bypass model of translational control, stress-induced eIF2α~P facilitates leaky ribosome scanning through the inhibitory uORF, which is suggested to result from the poor Kozak context of the start codons in the uORF. Consequently, the scanning ribosome initiates translation at the CHOP coding region, which features an initiation codon containing a strong Kozak consensus sequence.
On amino acid depletion and enhanced eIF2α~P, there is reduced eIF2-GTP recycling, and therefore the levels of the eIF2-TC are lowered. Consequently, after termination of translation at the positive-acting uORF1, the scanning 40S ribosomal subunit is unable to attain a new eIF2-TC in sufficient time to recognize the start codon of the inhibitory uORF2. Instead, as the small ribosomal subunit scans the interval between the initiation codons of uORF2 and the ATF4 coding region, the eIF2-TC is reacquired and the ribosome initiates translation at the ATF4 open reading frame (ORF) (Fig. 3) (4). This mechanism, deemed delayed translation reinitiation, thus relies on a sparsity of eIF2-TC during eIF2α~P for preferential translation of ATF4 mRNA and subsequent enhanced expression of ATF4 protein (4).
The key features of the model for preferential translation of ATF4 are shared with those elegantly studied by Hinnebusch (6) for yeast GCN4 translation control. In the GCN4 transcript, there are 3 inhibitory uORF that the reinitiating ribosomes bypass during the delayed reacquisition of eIF2-TC. Therefore, this mode of translational control induced by eIF2α~P can accommodate ≥2 uORF and is shared among diverse eukaryotes. Furthermore, recent studies on yeast GCN4 have begun to provide mechanistic insight as to how ribosomes can reinitiate after translation of uORF1. The multisubunit initiation factor eIF3 is suggested to be retained on ribosomes for the duration of the translation of uORF1. On termination of translation of uORF1, eIF3 can stabilize mRNA association with small ribosomal subunits and facilitate resumption of ribosomal scanning for subsequent recruitment of the eIF2-TC and reinitiation of translation at a downstream ORF (88, 89).
ATF4 directs transcription of ISR genes
Elevated synthesis of ATF4 during eIF2α~P facilitates transcriptional regulation of genes subject to the ISR. In this process, ATF4 can form homodimers or heterodimers with several other bZIP transcription factors, including the C/EBP isoforms, FOS, JUN, NRF2, and C/EBP-homologous protein (CHOP) (DDIT3/GADD153). ATF4 can then bind to the ISR-targeted promoters via CARE elements, which contain a half-site for members of the C/EBP family and a half-site for ATF transcription factors (90, 91). Microarray analyses and other functional studies of ATF4-dependent gene expression identified target genes involved in diverse cellular functions, including the synthesis and import of amino acids, maturation and degradation of proteins, glutathione synthesis and the control of the cellular redox status, autophagy, mitochondrial function, control of apoptosis, signaling and expression of additional transcription factors, and feedback regulation of the ISR (5, 92–95). Although ATF4 triggers the transcription of many common target genes during diverse stresses, activation of many other genes can be specific to a given stress condition or to a selected tissue.
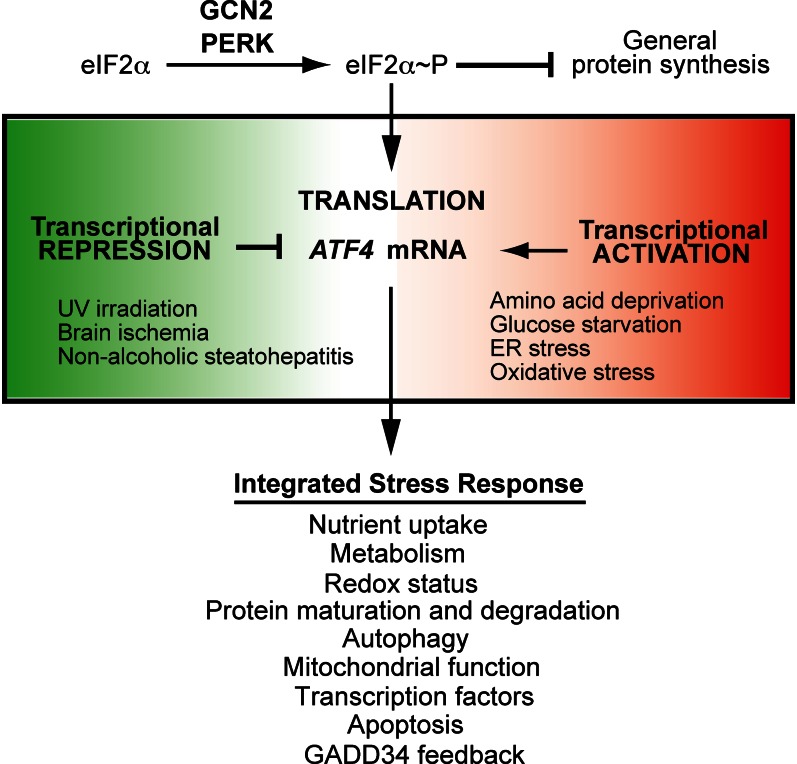
One of the best characterized promoters activated by ATF4 is asparagine synthetase (ASNS), which catalyzes the conversion of aspartate to asparagine (90, 96, 97). During limitations for essential amino acids, ATF4 complexed with C/EBPβ binds to an element in the ASNS promoter, leading to localized histone acetylation. The resulting chromatin remodeling recruits general transcription factors and RNA polymerase II, leading to increased ASNS mRNA synthesis. After several hours of amino acid deprivation, ATF4 can be displaced at the ASNS promoter by another transcription factor induced by the ISR, ATF3, coinciding with diminished ASNS transcription (97). This illustrates the dynamic regulation of ATF4-targeted genes during dietary stress and highlights the importance of feedback systems in the control of gene expression of the ISR. In addition to the displacement of ATF4 at target promoters, eIF2α~P itself is subject to feedback control. ATF4 and the ISR activates the transcription of GADD34 (Ppp1r15a), encoding a regulatory subunit of the type 1 serine/threonine protein phosphatase that dephosphorylates eIF2α. Therefore, protein synthesis can be restored once a new transcriptome is implemented by the ISR (Fig. 4) (78, 98–103).
Figure 4.
Transcriptional regulation of ATF4 enables differential expression of integrated stress response (ISR) genes. In response to nutritional deprivation and other diverse stress conditions, phosphorylation of eukaryotic initiation factor (eIF) 2α by general control nonderepressible (GCN) 2 or PKR-like endoplasmic reticulum kinase (PERK) represses global translation. Additionally, phosphorylation of the α subunit of eukaryotic initiation factor 2 (eIF2α~P) preferentially enhances the translation of ATF4. Increased levels of the ATF4 transcription factor triggers the transcription of a gene expression program collectively referred to as the ISR. Expression of ATF4 is also subject to transcriptional regulation. Transcriptional activation in response to the indicated stress conditions serves to provide high levels of mRNA available for preferential translation during eIF2α~P, thus enhancing the ISR. Alternatively, transcriptional repression reduces the levels of ATF4 mRNA available for translation. In this case, there is discordant induction of the ISR, with eIF2α~P reducing global protein synthesis, but low expression levels of ATF4 and its target genes. ER, endoplasmic reticulum.
Mice homozygous for an ATF4 knockout exhibit defects in ocular, skeletal, pancreatic, and hematopoietic development, as well as significant changes in glucose and insulin homeostasis (104, 105). The hallmark feature of the ATF4−/− mice is microphthalmia due to the absence of the lens of the eye (105, 106). ER stress accompanies eye development, and loss of ATF4, which is required for full implementation of the UPR, was reported to lead to massive and synchronous apoptosis of cells of the epithelial lens. Furthermore, mice deleted for ATF4 exhibit bone deformities due to decreased synthesis and secretion of type I collagen (107). Given the important role of ATF4 in amino acid synthesis and uptake, it was proposed that low levels of amino acids in ATF4-deficient osteoblasts would decrease translation, thus reducing the major biosynthetic product, collagen. Consistent with this idea, providing ATF4−/− mice a high protein diet helped to alleviate developmental defects and low bone mass (108). Finally, ATF4-deficient mice were reported to have enhanced energy expenditure and decreased diet-induced diabetes, along with lowered hyperlipidemia and hepatosteatosis (109). These findings reflect the changes that the ISR can elicit in lipid and glucose metabolism, which are a consequence of differences in nutrient utilization, changes in protein synthesis, and direct and indirect modulation of key transcription factors, such as PPARγ, PGC1α, SREBP1, and CHOP, which can regulate expression of metabolic genes (49, 110–113).
Multiple mechanisms regulate gene-specific translation during eIF2#x03B1~P
It was reported that there are hundreds of different mRNA, ~3% of protein-coding genes, which are subject to preferential translation in response to GCN2 phosphorylation of eIF2α (8). In this study, livers from wild-type and GCN2−/− mice were perfused with medium lacking methionine, and then lysates were subjected to sucrose gradient centrifugation to identify mRNA that show enhanced association with large polysomes specifically in wild-type mice upon the nutrient limitation. The mRNA association with large polysomes is a predictor of preferential translation. Transcripts participating in metabolism and energy production were prevalent among those genes suggested to be preferentially translated. This indicates that enhanced translation is central to not only ATF4 expression, but also to many other genes in the ISR. Included among those genes that were preferentially translated was ATF5, a bZIP transcription factor most closely related to ATF4 (114). The ATF5 mRNA contains 2 uORF and is induced by eIF2α~P by the mechanism of delayed translation reinitiation described earlier for ATF4 (115, 116). ATF5 expression is enhanced by multiple stress conditions, and ATF5 has been reported to be important for both neural differentiation and the formation of gliomas (115, 117, 118).
Other members of the ISR are also subject to preferential translation during eIF2α~P, albeit by alternative mechanisms. CHOP is a bZIP transcription factor and an ATF4-targeted gene that is important for triggering apoptosis during chronic stress (5, 91, 119, 120). CHOP mRNA is poorly translated under basal conditions as the result of a single uORF (10, 121–123). Translation of the uORF during nonstressed conditions serves as a barrier that prevents translation of the downstream CHOP coding region. However, upon stress, eIF2α~P facilitates the 43S PIC bypass of the uORF and instead the scanning ribosome translates CHOP (Fig. 3) (10).
We still do not fully understand the nature by which eIF2α~P mediates the bypass of the inhibitory uORF in the CHOP mRNA. This uORF has 2 AUG codons at codon positions 1 and 4 in the uORF. The second initiation codon is dominant, although both are suggested to be able to serve as the initiator of translation of the uORF (10, 121). The frequency of initiation during scanning of the 43S PIC is influenced by the nucleotide sequence surrounding the start AUG codon. Kozak (124) first described the importance of this consensus sequence (termed the Kozak context) in the late 1980s, where an optimal context in mammalian mRNA is considered 5′-GCC(A/G)CC AUG (G)-3′ (7). Deviations from this context, particularly at the −3 and +4 positions, can reduce the efficiency of translation initiation. In the bypass mechanism of CHOP translation control, both of the initiation codons in the inhibitory uORF are in poor context, whereas the start codon of the CHOP coding region is optimal. Under nonstressed conditions and low eIF2α~P, translation initiation at the uORF leads to a block in translation elongation or termination, preventing further ribosome scanning and translation at the downstream CHOP ORF. Critical to this inhibitory function of the uORF is the synthesis of the carboxy terminal portion of the 34-residue uORF. However, as a consequence of the poor Kozak context of the uORF, stress-induced eIF2α~P is suggested to facilitate leaky scanning through the inhibitory uORF and instead initiation occurs downstream at the optimal context of the CHOP coding sequence. In support of this idea, substitution of an optimal Kozak context for the initiation codon of the uORF substantially reduces CHOP expression even during induced eIF2α~P (10). The bypass model for CHOP translational control helps explain how expression of CHOP and the fate of cells are tightly linked to the levels of eIF2α~P and stress damage (Fig. 3) (10).
Another mRNA in the ISR that is subject to preferential translation in response to eIF2α~P, is GADD34 (125). As noted earlier, GADD34 is a stress-inducible factor responsible for facilitating the dephosphorylation of eIF2α~P (125). The GADD34 mRNA contains 2 uORF, and it is currently unclear whether the delayed reinitiation or bypass models underlie GADD34 translational control. Nonetheless, the preferential translation of GADD34 provides an explanation for how the accompanying feedback mechanism is induced during a global repression of protein synthesis.
Another means by which mRNAs can be preferentially translated during a global decrease in eIF2-GTP levels is through cap-independent processes. Although the overwhelming majority of cellular mRNA rely on the scanning mechanism for translation initiation, noncanonical cap-independent initiation via internal ribosome entry sites (IRES) is suggested to be important for the expression of several cellular proteins including HIF1α, BCL2, CAT-1, and XIAP (126–129). First described in viral mRNA, IRES are RNA elements that can directly recruit components of the translational machinery to the mRNA independent of eIF4E cap binding, and many are suggested to be resistant to reductions in eIF2-TC levels as a result of eIF2α~P (9, 130). The delayed reinitiation, ribosomal bypass, and IRES-mediated mechanisms of translational control each provide a means by which protein expression for an individual gene is enhanced during a global reduction in mRNA translation. Given that only a handful of mRNA have thus far been characterized among the hundreds of genes suggested to be subject to preferential translation during eIF2α~P, there are likely additional translational control mechanisms that contribute to the ISR.
Discordant induction of eIF2#x03B1~P and ATF4
Although eIF2α~P elicits translational control in response to many different stresses, there are selected stresses, such as exposure to UV irradiation, that do not increase ATF4 expression despite robust eIF2α~P (12, 50, 51). The molecular basis for this discordant induction of ATF4 expression and eIF2α~P is that ATF4 is subject to both translational and transcriptional regulation (Fig. 4). In response to UV irradiation, transcription of ATF4 is repressed, and therefore ATF4 mRNA is not readily available for preferential translation (12).
Transcriptional regulation of ATF4 provides an important regulatory hub for the cellular implementation of the ISR. The half-life of ATF4 mRNA and protein are short, from 2 to 4 h (12, 131); therefore, the activity of ATF4 is tightly linked to its synthesis, namely the transcription of ATF4 and its translation, which is dictated by the status of eIF2α~P. Activation of ATF4 transcription leads to more mRNA available for preferential translation induced by eIF2α~P (Fig. 4) (12). Alternatively, repression of ATF4 leads to lower mRNA, thus decreasing synthesis of ATF4. Elevated eIF2α~P and accompanying translational control enhance the resistance of cultured cells to UV treatment, whereas forced expression of ATF4 with the UV insult substantially reduces survival (12). In the case of UV stress, eIF2α~P was reported to lead to preferential translation of alternative mRNA, those encoding key members of the nucleotide excision repair pathway, thus facilitating the repair of DNA damage (11). In addition to UV irradiation, brain ischemia (132) and nonalcoholic steatohepatitis (133) were reported to trigger eIF2α~P but not increased ATF4 expression. Therefore, transcription repression and the discordant induction of ATF4 and eIF2α~P are suggested to occur during diverse stress conditions.
We are only beginning to understand the full mechanistic features of transcriptional regulation of ATF4. The transcription factor C/EBPβ is suggested to be a potent repressor of ATF4 transcription in response to UV irradiation (S Dey and R Wek, unpublished results). Expression of different isoforms of C/EBPβ are controlled by a range of developmental and differentiation processes, along with environmental stresses, providing these cellular processes a vehicle for controlling a key step in the ISR. Stresses shown to enhance ATF4 mRNA levels include ER stress (3, 134, 135), starvation for amino acids (136), oxidative stress (137, 138), and resistance to anticancer agents (139, 140). During oxidative stress, the transcription factor NRF2 can bind to the ATF4 promoter and enhance its transcription, which serves to alleviate oxidative damage and facilitate angiogenesis (Fig. 4) (137, 138). The transcription factor CLOCK can also associate with the ATF4 promoter, leading to increased ATF4 expression, which facilitates resistance to the anticancer drugs cisplatin and etoposide (141). Similarly PDX1, a pancreas-specific transcription factor was reported to regulate ER stress responses in islet β cells by binding to the ATF4 promoter and increasing its expression (142). These studies suggest that many different transcription factors can bind to the ATF4 promoter and modulate the levels of ATF4 mRNA. Some of these transcription factors are repressors, triggering discordant induction of eIF2α~P and ATF4 expression upon selected environmental stresses, whereas others are activators, accentuating ATF4 levels in the ISR. As a consequence, multiple stress pathways can control the induction ATF4 by eIF2α~P, ensuring that the expression of ATF4 and its ISR-target genes are tailored for a given stress condition.
Cross-regulation between the ISR and other signaling pathways
Translational and transcriptional control induced by the ISR can be integrated with additional stress signal pathways to direct gene expression dedicated for specific stresses and control cell fate. An example of this integration can be seen in cells responding to ER stress, where PERK functions in conjunction with the 2 other stress sensors, ATF6 and IRE1, to induce the UPR. Upon ER stress, ATF6 transports from the ER to the Golgi apparatus, where ATF6 is subject to intramembrane proteolysis, allowing for the release of the amino-terminal cytoplasmic portion of ATF6 (143, 144). This portion of ATF6 functions as a bZIP transcription factor that enters the nucleus and targets UPR genes involved in protein folding and the ER-associated protein degradation pathway for clearance of unfolded protein from the ER. IRE1 is a riboendonuclease that cleaves XBP1 mRNA in the cytoplasm, leading to translation of another active bZIP transcription factor of the UPR (145, 146).
Although activation of the 3 UPR sensors by ER stress can be viewed as occurring in parallel, PERK-mediated eIF2α~P was recently shown to trigger not only translational control, but was also central to the transcriptional phase of the UPR (92). In mice subjected to tunicamycin, a potent inducer of ER stress, PERK was shown to be required for 74% of the UPR genes induced in livers by 2-fold or greater. Furthermore, PERK deficiency in the livers of these mice led to increased triglycerides and apoptosis within 24 h of the onset of ER stress. The rationale for the broad impact of PERK on the UPR transcriptome is that ATF4 facilitates activation of ATF6 during ER stress by at least 2 mechanisms. First, ATF4 enhances the transcription of ATF6, ensuring that newly synthesized ATF6 is available for continued processing and activation (92). Second, ATF4 contributes to the trafficking of ATF6 from the ER to the Golgi for subsequent proteolysis and activation (92). ATF4 enhances the expression of numerous genes that facilitate protein passage from the ER to Golgi, and it was proposed that 1 or more of these genes are critical for ATF6 processing and activation. Therefore, the PERK/eIF2α~P/ATF4 pathway is integrated with additional ER stress sensors to activate a collection of UPR genes critical for alleviating the accumulation of unfolded protein in the secretory pathway.
The function of the ISR can also be integrated with mTOR. Central to the signaling pathways controlling mTOR is the tuberous sclerosis complex (TSC), which consists of TSC1 and TSC2 subunits that inhibit RHEB, a small GTPase that binds and activates mTORC1 (20). Loss of TSC triggers constitutive activation of mTORC1, leading to neoplasms that are characterized by high levels of protein synthesis. Interestingly, it was reported that disruption of TSC also causes ER stress, activating PERK and the UPR (147). Induction of the UPR with loss of TSC was observed in cell culture and mouse models, as well as in cortical tubers, the most common kind of tumors arising in tuberous sclerosis patients. It was proposed that increased protein synthesis resulting from hyperactivation of mTORC1 in the TSC-deficient cells can increase the influx of nascent proteins entering the secretory system and, as a consequence, overload the ER. Supporting this idea, treatment of TSC-deficient cells with cycloheximide, an inhibitor of translation elongation, prevents activation of the UPR (147). Additional targets altered by hyperactivation of TORC1, including changes in transcriptional networks and disruption of autophagy, may also work in conjunction with high levels of protein synthesis to create ER stress in the TSC mutant cells.
Dysregulated activation of mTORC1 can interfere with insulin signaling, and the induced UPR in TSC-deficient cells plays a critical role in the inhibitory process. IRE1 signaling via recruitment of TRAF2 and subsequent activation of JNK can lead to inhibitory phosphorylation and degradation of IRS1 (148, 149). Therefore, the key signaling events that stem from the UPR help explain the insulin resistance associated with TSC. From a protein synthesis perspective, the UPR diminishment of insulin activity would make sense, in that insulin signaling would enhance translation, which would further exacerbate the ER stress. Finally, loss of TSC renders cells sensitive to drugs that can elicit ER stress, such as the proteasome inhibitor bortezomib, which is currently approved for the treatment of multiple myeloma (147, 150, 151). Although the UPR is generally viewed as cytoprotective, accentuated ER stress can alter this stress response pathway to become one that is proapoptotic (77, 152, 153). Therefore, drugs that can trigger ER stress may provide a potent treatment strategy for treating tuberous sclerosis.
The ISR is also suggested to direct gene expression that can control mTORC1 function. For example, in response to ER and oxidative stress, ATF4 can reduce mTORC1 activity by enhancing expression of REDD1, which interfaces with TSC to inhibit mTOR signaling (154–156). ATF4 was also reported to directly increase the expression of a downstream effector of mTORC1, 4E-BP1, in islet β-cells. Loss of 4E-BP1 leads to deregulated translational control, contributing to loss of β cells and exacerbating hyperglycemia in mouse models (157).
Role of eIF2#x03B1~P in diabetes
As the acronym UPR implies, it is suggested that accumulation of unfolded or misfolded protein is the critical signal that activates the ER stress sensors. However, it is important to emphasize that measuring ER stress directly is problematic, and most studies infer ER stress by assaying for activation of the UPR sensors, such as PERK. There may be many different ER signals directly triggering the UPR, some effecting other cellular compartments. Tissues specialized for secretion, such as the pancreas and liver, are suggested to encounter fluctuating ER stress under normal physiological conditions, and the UPR allows cells to adapt to these physiological stresses, as well as to overcome stress caused by disease or environmental perturbations. These ideas can be illustrated in islet β cells exposed to transient high blood glucose, leading to an increase in proinsulin mRNA translation and the protein processing workload of the ER, thereby activating the UPR. Furthermore, in the obese state, chronic elevated levels of free fatty acids and glucose, along with inflammatory cytokines, can trigger ER stress in many different tissues.
Our understanding of the stresses activating the UPR and the role this pathway plays in alleviating these cellular insults is largely based on the characterization of gene mutations disrupting key steps in the UPR. As noted earlier in this review, mutations have been identified in patients that disrupt PERK, resulting in WRS, and alterations in upstream regulators and downstream effectors have also been shown to have a major impact on the UPR and cell fate. Importantly, mouse models containing these precise mutations result in pathologies that closely mirror the human condition.
Missense or truncation mutations in PERK (EIF2AK3) lead to WRS, which features neonatal diabetes due to the loss of β cells. PERK-deficient mice also display hyperglycemia due to loss of these islet cells (158, 159). It was suggested that β-cell death is a consequence of unresolvable ER stress that can occur as the result of unregulated translation and excessive proinsulin targeting to the ER. Conditional mutations in PERK suggest that this eIF2α kinase is also required for the development of the pancreas, including β cells (160). Loss of PERK, or its downstream target ATF4, also leads to atrophy of the acinar cells of the pancreas, which is associated with inflammatory responses and pancreatitis (161). Given our earlier discussion of the role of PERK/eIF2α~P/ATF4 in the activation of ATF6, it is likely that the absence of PERK also broadly compromises the UPR transcriptome, preventing the appropriate expansion of the ER processing capacity.
The Akita mouse strain features a dominant missense mutation, C96Y, in the insulin 2 gene that eliminates a disulfide linkage and impairs protein folding (162, 163). As a consequence, there is dysfunction and death of β cells, and accompanying diabetes. The same mutation in the insulin gene was recently identified in patients with early-onset diabetes (164). The dysfunctional insulin folding would result in an unresolvable ER stress that would lead to constant activation of the UPR sensors. Significant levels of ER stress in β cells can also be the consequence of continued exposure to free fatty acids and cytokines (165–168). Although the UPR is important for resolution of ER stress, chronic induction of PERK is suggested to trigger maladaptive responses and cell death. Consistent with this idea, deletion of CHOP in the Akita mouse, as well as in mouse obesity models, delayed the onset of hyperglycemia and β-cell death (113, 169). CHOP is suggested to elicit apoptosis through repression of BCL2 and the induction of proapoptotic genes, such as BIM, DR5, TRB3, and those tied to autophagy (91, 93, 113, 121, 170–174). Additionally, continued induction of CHOP can lead to oxidative stress and promotion of protein synthesis by feedback regulation of GADD34, which would further exacerbate preexisting ER stress (78, 98).
WFS1 is a target gene of the UPR, whose disruption leads to Wolfram syndrome, characterized by juvenile-onset diabetes, optic atrophy, and later onset of neurodegeneration, ultimately leading to patient death (175, 176). WFS1 protein is localized to the ER and is highly expressed in β cells. WFS1 is predicted to be a multipass transmembrane protein that may function as an ion channel to facilitate calcium mobilization and ER homeostasis (177, 178). Another function attributed to WFS1 is feedback control of ATF6, facilitating ATF6 ubiquitination and degradation by the proteasome (179). In mouse β cells and lymphocytes derived from Wolfram syndrome patients, loss of WFS1 leads to hyperactivation of ATF6. Therefore, disruption of a downstream effector of the UPR can also lead to its dysfunction, triggering maladaptive responses.
Behavior, memory, and neurological degeneration
GCN2 and tRNA charging also have a role in animal behavior. Animals have an innate ability to detect imbalanced diets lacking essential amino acids and, upon doing so reject the deficient diet to forage for a balanced food source. This “food aversion” response is not regulated by peripheral sensations such as taste and smell, but rather is modulated by tRNA charging in the anterior piriform cortex, an area of the cerebral cortex responsible for detecting amino acid homeostasis (180). When levels of an essential amino acid are low, uncharged cognate tRNA accumulate and subsequently activate the GCN2/eIF2α~P pathway (180–182). GCN2 is central to this regulated behavior because GCN2-deficient mice have an impaired ability to both avoid imbalanced diets and phosphorylate eIF2α in brain tissues when deprived of essential amino acids. Thus, GCN2 triggers a mechanism shared among metazoans in which amino acid availability is coupled to foraging behavior to ensure the maintenance of amino acid homeostasis. GCN2 and ATF4 signaling in the hippocampus have also been shown to regulate memory and learning. GCN2−/− mice were reported to have aberrant induction of long-term potentiation and impaired spatial memory patterns when compared with wild-type littermates (183).
Disruptions in the ISR can also lead to neural degeneration. Missense mutations in any of the 5 subunits of the GEF eIF2B reduce eIF2-GTP recycling independent of stress and result in an inherited neurodegenerative disorder termed vanishing white matter leukoencephalopathy (also known as childhood ataxia with central nervous system hypomyelination) (184–189). These genetic alterations cause reduced eIF2B activity and lowered eIF2-TC levels independent of eIF2α~P. In the event of a provoking stress, such as head trauma and fever, there is induced eIF2α~P, which, when combined with underlying eIF2B lesions, is suggested to lead to hyperactivation of the ISR. The net result is that the ISR, which typically serves to alleviate stress damage, is altered to one that becomes maladaptive. Consequently, astrocytes and oligodendrocytes in the brain of vanishing white matter leukoencephalopathy patients die, ultimately resulting in rapid neurological deterioration and death.
Nutrient availability, hypoxia, and tumorigenesis
Recent studies emphasized the importance of the eIF2α~P/ATF4 axis in tumor proliferation. The tumor microenvironment is often characterized by hypoxic regions limited for nutrients due to vasculature restrictions. An important means by which solid tumors survive and proliferate in these stressful conditions is the induction of eIF2α~P and ATF4 expression. Knockdown of ATF4 mRNA causes reduced cell survival and increased G1/S arrest in human fibrosarcoma and colorectal adenocarcinoma cells, and nude mice injected with K-RasV12–transformed GCN2−/− MEF cells develop substantially smaller tumors than those injected with the transformed wild type (56). The mechanistic basis for this reliance on elevated levels of ATF4 involves a dependence on the aforementioned ATF4 downstream target ASNS, encoding asparagine synthetase. The reliance of multiple cancer types on asparagine and its synthesis is already being targeted in the clinic as a means of treating hematological neoplasms. l-asparaginase depletes asparagine from the bloodstream, effectively inhibiting tumorigenesis and malignancy in patients with acute lymphoblastic leukemia (190).
In addition to nutrient limitation, tumor cells must also cope with both acute and chronic episodes of extreme hypoxia. One mechanism by which transformed cells survive the hypoxic microenvironment is through PERK activation, which allows for the conservation of resources via the global reduction in protein synthesis and preferential translation of ATF4 that is important in combating oxidative stress (191). Constitutive activation of the ISR in the tumor microenvironment provides a selective advantage in transformed cancer cells; therefore, eIF2α kinases and downstream target genes offer attractive new targets for cancer therapies.
Acknowledgments
We are grateful to Sheree Wek for help with figure illustrations. Both authors have read and approved the final manuscript.
Footnotes
Supported by National Institutes of Health grant GM049164 to R.C.W.
Author disclosures: T.D. Baird and R.C. Wek, no conflicts of interest.
Abbreviations used: ASNS, asparagine synthetase; ATF, activating transcription factor; bZIP, basic leucine zipper; CHOP, C/EBP-homologous protein; eIF, eukaryotic initiation factor; eIF2α~P, phosphorylation of the α subunit of eukaryotic initiation factor 2; eIF2-TC, eukaryotic initiation factor 2 ternary complex; ER, endoplasmic reticulum; GCN, general control nonderepressible; GEF, guanine nucleotide exchange factor; IRES, internal ribosome entry site; ISR, integrated stress response; mTOR, mammalian target of rapamycin; ORF, open reading frame; 43S PIC, 43S preinitiation complex; PERK, PKR-like endoplasmic reticulum kinase; tRNA, transfer RNA; TSC, tuberous sclerosis complex; uORF, upstream open reading frame; UPR, unfolded protein response; WRS, Wolcott-Rallison syndrome.
Literature Cited
- 1.Schwanhäusser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M. Global quantification of mammalian gene expression control. Nature. 2011;473:337–42 [DOI] [PubMed] [Google Scholar]
- 2.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–108 [DOI] [PubMed] [Google Scholar]
- 3.Lu PD, Jousse C, Marciniak SJ, Zhang Y, Novoa I, Scheuner D, Kaufman RJ, Ron D, Harding HP. Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J. 2004;23:169–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A. 2004;101:11269–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–33 [DOI] [PubMed] [Google Scholar]
- 6.Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol. 2005;59:407–50 [DOI] [PubMed] [Google Scholar]
- 7.Hinnebusch AG. Molecular mechanism of scanning and start codon selection in eukaryotes. Microbiol Mol Biol Rev. 2011;75:434–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dang Do AN, Kimball SR, Cavener DR, Jefferson LS. eIF2alpha kinases GCN2 and PERK modulate transcription and translation of distinct sets of mRNAs in mouse liver. Physiol Genomics. 2009;38:328–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez J, Yaman I, Sarnow P, Snider MD, Hatzoglou M. Regulation of internal ribosomal entry site-mediated translation by phosphorylation of the translation initiation factor eIF2alpha. J Biol Chem. 2002;277:19198–205 [DOI] [PubMed] [Google Scholar]
- 10.Palam LR, Baird TD, Wek RC. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J Biol Chem. 2011;286:10939–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powley IR, Kondrashov A, Young LA, Dobbyn HC, Hill K, Cannell IG, Stoneley M, Kong YW, Cotes JA, Smith GC, et al. Translational reprogramming following UVB irradiation is mediated by DNA-PKcs and allows selective recruitment to the polysomes of mRNAs encoding DNA repair enzymes. Genes Dev. 2009;23:1207–20 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Dey S, Baird TD, Zhou D, Palam LR, Spandau DF, Wek RC. Both transcriptional regulation and translational control of ATF4 are central to the integrated stress response. J Biol Chem. 2010;285:33165–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol. 2010;11:113–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Algire MA, Maag D, Lorsch JR. Pi release from eIF2, not GTP hydrolysis, is the step controlled by start-site selection during eukaryotic translation initiation. Mol Cell. 2005;20:251–62 [DOI] [PubMed] [Google Scholar]
- 16.Pavitt GD, Ramaiah KV, Kimball SR, Hinnebusch AG. eIF2 independently binds two distinct eIF2B subcomplexes that catalyze and regulate guanine-nucleotide exchange. Genes Dev. 1998;12:514–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez E, Mohammad SS, Pavitt GD. Characterization of the minimal catalytic domain within eIF2B: the guanine-nucleotide exchange factor for translation initiation. EMBO J. 2002;21:5292–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnamoorthy T, Pavitt GD, Zhang F, Dever TE, Hinnebusch AG. Tight binding of the phosphorylated alpha subunit of initiation factor 2 (eIF2alpha) to the regulatory subunits of guanine nucleotide exchange factor eIF2B is required for inhibition of translation initiation. Mol Cell Biol. 2001;21:5018–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimball SR, Fabian JR, Pavitt GD, Hinnebusch AG, Jefferson LS. Regulation of guanine nucleotide exchange through phosphorylation of eukaryotic initiation factor eIF2alpha. Role of the alpha- and delta-subunits of eIF2b. J Biol Chem. 1998;273:12841–5 [DOI] [PubMed] [Google Scholar]
- 20.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–18 [DOI] [PubMed] [Google Scholar]
- 22.Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416–22 [DOI] [PubMed] [Google Scholar]
- 23.Shahbazian D, Parsyan A, Petroulakis E, Hershey J, Sonenberg N. eIF4B controls survival and proliferation and is regulated by proto-oncogenic signaling pathways. Cell Cycle. 2010;9:4106–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shahbazian D, Roux PP, Mieulet V, Cohen MS, Raught B, Taunton J, Hershey JW, Blenis J, Pende M, Sonenberg N. The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation and activity. EMBO J. 2006;25:2781–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11 [DOI] [PubMed] [Google Scholar]
- 26.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–6 [DOI] [PubMed] [Google Scholar]
- 27.Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–89 [DOI] [PubMed] [Google Scholar]
- 28.Han AP, Yu C, Lu L, Fujiwara Y, Browne C, Chin G, Fleming M, Leboulch P, Orkin SH, Chen JJ. Heme-regulated eIF2alpha kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. EMBO J. 2001;20:6909–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen JJ. Regulation of protein synthesis by the heme-regulated eIF2alpha kinase: relevance to anemias. Blood. 2007;109:2693–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dey M, Cao C, Dar AC, Tamura T, Ozato K, Sicheri F, Dever TE. Mechanistic link between PKR dimerization, autophosphorylation, and eIF2alpha substrate recognition. Cell. 2005;122:901–13 [DOI] [PubMed] [Google Scholar]
- 31.García MA, Meurs EF, Esteban M. The dsRNA protein kinase PKR: virus and cell control. Biochimie. 2007;89:799–811 [DOI] [PubMed] [Google Scholar]
- 32.Rothenburg S, Seo EJ, Gibbs JS, Dever TE, Dittmar K. Rapid evolution of protein kinase PKR alters sensitivity to viral inhibitors. Nat Struct Mol Biol. 2009;16:63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pindel A, Sadler A. The role of protein kinase R in the interferon response. J Interferon Cytokine Res. 2011;31:59–70 [DOI] [PubMed] [Google Scholar]
- 34.Wek SA, Zhu S, Wek RC. The histidyl-tRNA synthetase-related sequence in the eIF-2 alpha protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol Cell Biol. 1995;15:4497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong J, Qiu H, Garcia-Barrio M, Anderson J, Hinnebusch AG. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol Cell. 2000;6:269–79 [DOI] [PubMed] [Google Scholar]
- 36.Wek RC, Jackson BM, Hinnebusch AG. Juxtaposition of domains homologous to protein kinases and histidyl-tRNA synthetases in GCN2 protein suggests a mechanism for coupling GCN4 expression to amino acid availability. Proc Natl Acad Sci U S A. 1989;86:4579–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gárriz A, Qiu H, Dey M, Seo EJ, Dever TE, Hinnebusch AG. A network of hydrophobic residues impeding helix alphaC rotation maintains latency of kinase Gcn2, which phosphorylates the alpha subunit of translation initiation factor 2. Mol Cell Biol. 2009;29:1592–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Padyana AK, Qiu H, Roll-Mecak A, Hinnebusch AG, Burley SK. Structural basis for autoinhibition and mutational activation of eukaryotic initiation factor 2alpha protein kinase GCN2. J Biol Chem. 2005;280:29289–99 [DOI] [PubMed] [Google Scholar]
- 39.Qiu H, Hu C, Dong J, Hinnebusch AG. Mutations that bypass tRNA binding activate the intrinsically defective kinase domain in GCN2. Genes Dev. 2002;16:1271–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palii SS, Kays CE, Deval C, Bruhat A, Fafournoux P, Kilberg MS. Specificity of amino acid regulated gene expression: analysis of genes subjected to either complete or single amino acid deprivation. Amino Acids. 2009;37:79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reinert RB, Oberle LM, Wek SA, Bunpo P, Wang XP, Mileva I, Goodwin LO, Aldrich CJ, Durden DL, McNurlan MA, et al. Role of glutamine depletion in directing tissue-specific nutrient stress responses to L-asparaginase. J Biol Chem. 2006;281:31222–33 [DOI] [PubMed] [Google Scholar]
- 42.Anthony TG, McDaniel BJ, Byerley RL, McGrath BC, Cavener DR, McNurlan MA, Wek RC. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J Biol Chem. 2004;279:36553–61 [DOI] [PubMed] [Google Scholar]
- 43.Bunpo P, Dudley A, Cundiff JK, Cavener DR, Wek RC, Anthony TG. GCN2 protein kinase is required to activate amino acid deprivation responses in mice treated with the anti-cancer agent L-asparaginase. J Biol Chem. 2009;284:32742–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Habibi D, Ogloff N, Jalili RB, Yost A, Weng AP, Ghahary A, Ong CJ. Borrelidin, a small molecule nitrile-containing macrolide inhibitor of threonyl-tRNA synthetase, is a potent inducer of apoptosis in acute lymphoblastic leukemia. Invest New Drugs. Epub 2011 Jun 17 [DOI] [PubMed] [Google Scholar]
- 45.Usui T, Nagumo Y, Watanabe A, Kubota T, Komatsu K, Kobayashi J, Osada H. Brasilicardin A, a natural immunosuppressant, targets amino acid transport system L. Chem Biol. 2006;13:1153–60 [DOI] [PubMed] [Google Scholar]
- 46.Bröer A, Juelich T, Vanslambrouck JM, Tietze N, Solomon PS, Holst J, Bailey CG, Rasko JE, Broer S. Impaired nutrient signaling and body weight control in a Na+ neutral amino acid cotransporter (Slc6a19)-deficient mouse. J Biol Chem. 2011;286:26638–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marion V, Sankaranarayanan S, de Theije C, van Dijk P, Lindsey P, Lamers MC, Harding HP, Ron D, Lamers WH, Kohler SE. Arginine deficiency causes runting in the suckling period by selectively activating the stress kinase GCN2. J Biol Chem. 2011;286:8866–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lanker S, Bushman JL, Hinnebusch AG, Trachsel H, Mueller PP. Autoregulation of the yeast lysyl-tRNA synthetase gene GCD5/KRS1 by translational and transcriptional control mechanisms. Cell. 1992;70:647–57 [DOI] [PubMed] [Google Scholar]
- 49.Guo F, Cavener DR. The GCN2 eIF2alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab. 2007;5:103–14 [DOI] [PubMed] [Google Scholar]
- 50.Deng J, Harding HP, Raught B, Gingras AC, Berlanga JJ, Scheuner D, Kaufman RJ, Ron D, Sonenberg N. Activation of GCN2 in UV-irradiated cells inhibits translation. Curr Biol. 2002;12:1279–86 [DOI] [PubMed] [Google Scholar]
- 51.Jiang HY, Wek RC. GCN2 phosphorylation of eIF2alpha activates NF-kappaB in response to UV irradiation. Biochem J. 2005;385:371–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang HY, Wek RC. Phosphorylation of the alpha-subunit of the eukaryotic initiation factor-2 (eIF2alpha) reduces protein synthesis and enhances apoptosis in response to proteasome inhibition. J Biol Chem. 2005;280:14189–202 [DOI] [PubMed] [Google Scholar]
- 53.Peidis P, Papadakis AI, Rajesh K, Koromilas AE. HDAC pharmacological inhibition promotes cell death through the eIF2alpha kinases PKR and GCN2. Aging. 2010;2:669–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neznanov N, Dragunsky EM, Chumakov KM, Neznanova L, Wek RC, Gudkov AV, Banerjee AK. Different effect of proteasome inhibition on vesicular stomatitis virus and poliovirus replication. PLoS One. 2008;3:e1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang R, Wek SA, Wek RC. Glucose limitation induces GCN4 translation by activation of Gcn2 protein kinase. Mol Cell Biol. 2000;20:2706–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, De Panis DN, Bobrovnikova-Marjon E, Diehl JA, Ron D, Koumenis C. The GCN2–ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 2010;29:2082–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai Q, Brooks HL. Phosphorylation of eIF2alpha via the general control kinase, GCN2, modulates the ability of renal medullary cells to survive high urea stress. Am J Physiol Renal Physiol. 2011;301:F1202–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu S, Sobolev AY, Wek RC. Histidyl-tRNA synthetase-related sequences in GCN2 protein kinase regulate in vitro phosphorylation of eIF-2. J Biol Chem. 1996;271:24989–94 [DOI] [PubMed] [Google Scholar]
- 59.Narasimhan J, Staschke KA, Wek RC. Dimerization is required for activation of eIF2 kinase Gcn2 in response to diverse environmental stress conditions. J Biol Chem. 2004;279:22820–32 [DOI] [PubMed] [Google Scholar]
- 60.Zaborske JM, Narasimhan J, Jiang L, Wek SA, Dittmar KA, Freimoser F, Pan T, Wek RC. Genome-wide analysis of tRNA charging and activation of the eIF2 kinase Gcn2p. J Biol Chem. 2009;284:25254–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia-Barrio M, Dong J, Ufano S, Hinnebusch AG. Association of GCN1–GCN20 regulatory complex with the N-terminus of eIF2alpha kinase GCN2 is required for GCN2 activation. EMBO J. 2000;19:1887–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kubota H, Ota K, Sakaki Y, Ito T. Budding yeast GCN1 binds the GI domain to activate the eIF2alpha kinase GCN2. J Biol Chem. 2001;276:17591–6 [DOI] [PubMed] [Google Scholar]
- 63.Kubota H, Sakaki Y, Ito T. GI domain-mediated association of the eukaryotic initiation factor 2alpha kinase GCN2 with its activator GCN1 is required for general amino acid control in budding yeast. J Biol Chem. 2000;275:20243–6 [DOI] [PubMed] [Google Scholar]
- 64.Marton MJ, Crouch D, Hinnebusch AG. GCN1, a translational activator of GCN4 in Saccharomyces cerevisiae, is required for phosphorylation of eukaryotic translation initiation factor 2 by protein kinase GCN2. Mol Cell Biol. 1993;13:3541–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marton MJ, Vazquez de Aldana CR, Qiu H, Chakraburtty K, Hinnebusch AG. Evidence that GCN1 and GCN20, translational regulators of GCN4, function on elongating ribosomes in activation of eIF2alpha kinase GCN2. Mol Cell Biol. 1997;17:4474–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sattlegger E, Hinnebusch AG. Separate domains in GCN1 for binding protein kinase GCN2 and ribosomes are required for GCN2 activation in amino acid-starved cells. EMBO J. 2000;19:6622–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bittencourt S, Pereira CM, Avedissian M, Delamano A, Mello LE, Castilho BA. Distribution of the protein IMPACT, an inhibitor of GCN2, in the mouse, rat, and marmoset brain. J Comp Neurol. 2008;507:1811–30 [DOI] [PubMed] [Google Scholar]
- 68.Pereira CM, Sattlegger E, Jiang HY, Longo BM, Jaqueta CB, Hinnebusch AG, Wek RC, Mello LE, Castilho BA. IMPACT, a protein preferentially expressed in the mouse brain, binds GCN1 and inhibits GCN2 activation. J Biol Chem. 2005;280:28316–23 [DOI] [PubMed] [Google Scholar]
- 69.Sattlegger E, Swanson MJ, Ashcraft EA, Jennings JL, Fekete RA, Link AJ, Hinnebusch AG. YIH1 is an actin-binding protein that inhibits protein kinase GCN2 and impairs general amino acid control when overexpressed. J Biol Chem. 2004;279:29952–62 [DOI] [PubMed] [Google Scholar]
- 70.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–4 [DOI] [PubMed] [Google Scholar]
- 71.Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998;18:7499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma K, Vattem KM, Wek RC. Dimerization and release of molecular chaperone inhibition facilitate activation of eukaryotic initiation factor-2 kinase in response to endoplasmic reticulum stress. J Biol Chem. 2002;277:18728–35 [DOI] [PubMed] [Google Scholar]
- 73.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–32 [DOI] [PubMed] [Google Scholar]
- 74.Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev. 2008;29:317–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oslowski CM, Urano F. The binary switch between life and death of endoplasmic reticulum-stressed beta cells. Curr Opin Endocrinol Diabetes Obes. 2010;17:107–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–49 [DOI] [PubMed] [Google Scholar]
- 79.Julier C, Nicolino M. Wolcott-Rallison syndrome. Orphanet J Rare Dis. 2010;5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wolcott CD, Rallison ML. Infancy-onset diabetes mellitus and multiple epiphyseal dysplasia. J Pediatr. 1972;80:292–7 [DOI] [PubMed] [Google Scholar]
- 81.Delépine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000;25:406–9 [DOI] [PubMed] [Google Scholar]
- 82.Senée V, Vattem KM, Delepine M, Rainbow LA, Haton C, Lecoq A, Shaw NJ, Robert JJ, Rooman R, Diatloff-Zito C, et al. Wolcott-Rallison Syndrome: clinical, genetic, and functional study of EIF2AK3 mutations and suggestion of genetic heterogeneity. Diabetes. 2004;53:1876–83 [DOI] [PubMed] [Google Scholar]
- 83.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29 [DOI] [PubMed] [Google Scholar]
- 84.Credle JJ, Finer-Moore JS, Papa FR, Stroud RM, Walter P. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2005;102:18773–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gardner BM, Walter P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science. 2011;333:1891–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li H, Korennykh AV, Behrman SL, Walter P. Mammalian endoplasmic reticulum stress sensor IRE1 signals by dynamic clustering. Proc Natl Acad Sci U S A. 2010;107:16113–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Korennykh AV, Egea PF, Korostelev AA, Finer-Moore J, Zhang C, Shokat KM, Stroud RM, Walter P. The unfolded protein response signals through high-order assembly of Ire1. Nature. 2009;457:687–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Munzarová V, Panek J, Gunisova S, Danyi I, Szamecz B, Valasek LS. Translation reinitiation relies on the interaction between eIF3a/TIF32 and progressively folded cis-acting mRNA elements preceding short uORFs. PLoS Genet. 2011;7:e1002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Szamecz B, Rutkai E, Cuchalova L, Munzarova V, Herrmannova A, Nielsen KH, Burela L, Hinnebusch AG, Valasek L. eIF3a cooperates with sequences 5′ of uORF1 to promote resumption of scanning by post-termination ribosomes for reinitiation on GCN4 mRNA. Genes Dev. 2008;22:2414–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab. 2009;20:436–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J. 1999;339:135–41 [PMC free article] [PubMed] [Google Scholar]
- 92.Teske BF, Wek SA, Bunpo P, Cundiff JK, McClintick JN, Anthony TG, Wek RC. The eIF2 kinase PERK and the integrated stress response facilitate activation of ATF6 during endoplasmic reticulum stress. Mol Biol Cell. 2011;22:4390–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rouschop KM, van den Beucken T, Dubois L, Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W, Voncken JW, et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest. 2010;120:127–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rzymski T, Milani M, Pike L, Buffa F, Mellor HR, Winchester L, Pires I, Hammond E, Ragoussis I, Harris AL. Regulation of autophagy by ATF4 in response to severe hypoxia. Oncogene. 2010;29:4424–35 [DOI] [PubMed] [Google Scholar]
- 95.Jiang HY, Wek SA, McGrath BC, Lu D, Hai T, Harding HP, Wang X, Ron D, Cavener DR, Wek RC. Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol Cell Biol. 2004;24:1365–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kilberg MS, Pan YX, Chen H, Leung-Pineda V. Nutritional control of gene expression: how mammalian cells respond to amino acid limitation. Annu Rev Nutr. 2005;25:59–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen H, Pan YX, Dudenhausen EE, Kilberg MS. Amino acid deprivation induces the transcription rate of the human asparagine synthetase gene through a timed program of expression and promoter binding of nutrient-responsive basic region/leucine zipper transcription factors as well as localized histone acetylation. J Biol Chem. 2004;279:50829–39 [DOI] [PubMed] [Google Scholar]
- 98.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kojima E, Takeuchi A, Haneda M, Yagi A, Hasegawa T, Yamaki K, Takeda K, Akira S, Shimokata K, Isobe K. The function of GADD34 is a recovery from a shutoff of protein synthesis induced by ER stress: elucidation by GADD34-deficient mice. FASEB J. 2003;17:1573–5 [DOI] [PubMed] [Google Scholar]
- 100.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153:1011–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Novoa I, Zhang Y, Zeng H, Jungreis R, Harding HP, Ron D. Stress-induced gene expression requires programmed recovery from translational repression. EMBO J. 2003;22:1180–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brush MH, Weiser DC, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol. 2003;23:1292–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ma Y, Hendershot LM. Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. J Biol Chem. 2003;278:34864–73 [DOI] [PubMed] [Google Scholar]
- 104.Masuoka HC, Townes TM. Targeted disruption of the activating transcription factor 4 gene results in severe fetal anemia in mice. Blood. 2002;99:736–45 [DOI] [PubMed] [Google Scholar]
- 105.Hettmann T, Barton K, Leiden JM. Microphthalmia due to p53-mediated apoptosis of anterior lens epithelial cells in mice lacking the CREB-2 transcription factor. Dev Biol. 2000;222:110–23 [DOI] [PubMed] [Google Scholar]
- 106.Firtina Z, Duncan MK. Unfolded Protein Response (UPR) is activated during normal lens development. Gene Expr Patterns. 2011;11:135–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, Li L, Brancorsini S, Sassone-Corsi P, Townes TM, et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell. 2004;117:387–98 [DOI] [PubMed] [Google Scholar]
- 108.Elefteriou F, Benson MD, Sowa H, Starbuck M, Liu X, Ron D, Parada LF, Karsenty G. ATF4 mediation of NF1 functions in osteoblast reveals a nutritional basis for congenital skeletal dysplasiae. Cell Metab. 2006;4:441–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seo J, Fortuno ES, 3rd, Suh JM, Stenesen D, Tang W, Parks EJ, Adams CM, Townes T, Graff JM. Atf4 regulates obesity, glucose homeostasis, and energy expenditure. Diabetes. 2009;58:2565–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Back SH, Scheuner D, Han J, Song B, Ribick M, Wang J, Gildersleeve RD, Pennathur S, Kaufman RJ. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009;10:13–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, Clark R, Miao H, Hassler JR, Fornek J, et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hansen MB, Mitchelmore C, Kjaerulff KM, Rasmussen TE, Pedersen KM, Jensen NA. Mouse Atf5: molecular cloning of two novel mRNAs, genomic organization, and odorant sensory neuron localization. Genomics. 2002;80:344–50 [DOI] [PubMed] [Google Scholar]
- 115.Zhou D, Palam LR, Jiang L, Narasimhan J, Staschke KA, Wek RC. Phosphorylation of eIF2 directs ATF5 translational control in response to diverse stress conditions. J Biol Chem. 2008;283:7064–73 [DOI] [PubMed] [Google Scholar]
- 116.Watatani Y, Ichikawa K, Nakanishi N, Fujimoto M, Takeda H, Kimura N, Hirose H, Takahashi S, Takahashi Y. Stress-induced translation of ATF5 mRNA is regulated by the 5′-untranslated region. J Biol Chem. 2008;283:2543–53 [DOI] [PubMed] [Google Scholar]
- 117.Arias A, Lamé MW, Santarelli L, Hen R, Greene LA, Angelastro JM. Regulated ATF5 loss-of-function in adult mice blocks formation and causes regression/eradication of gliomas. Oncogene. 2013;31:739–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Greene LA, Lee HY, Angelastro JM. The transcription factor ATF5: role in neurodevelopment and neural tumors. J Neurochem. 2009;108:11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol. 2002;318:1351–65 [DOI] [PubMed] [Google Scholar]
- 120.Averous J, Bruhat A, Jousse C, Carraro V, Thiel G, Fafournoux P. Induction of CHOP expression by amino acid limitation requires both ATF4 expression and ATF2 phosphorylation. J Biol Chem. 2004;279:5288–97 [DOI] [PubMed] [Google Scholar]
- 121.Jousse C, Bruhat A, Carraro V, Urano F, Ferrara M, Ron D, Fafournoux P. Inhibition of CHOP translation by a peptide encoded by an open reading frame localized in the chop 5′UTR. Nucleic Acids Res. 2001;29:4341–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee HC, Chen YJ, Liu YW, Lin KY, Chen SW, Lin CY, Lu YC, Hsu PC, Lee SC, Tsai HJ. Transgenic zebrafish model to study translational control mediated by upstream open reading frame of human chop gene. Nucleic Acids Res. 2011;39:e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen YJ, Tan BC, Cheng YY, Chen JS, Lee SC. Differential regulation of CHOP translation by phosphorylated eIF4E under stress conditions. Nucleic Acids Res. 2010;38:764–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee YY, Cevallos RC, Jan E. An upstream open reading frame regulates translation of GADD34 during cellular stresses that induce eIF2alpha phosphorylation. J Biol Chem. 2009;284:6661–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sherrill KW, Byrd MP, Van Eden ME, Lloyd RE. BCL-2 translation is mediated via internal ribosome entry during cell stress. J Biol Chem. 2004;279:29066–74 [DOI] [PubMed] [Google Scholar]
- 127.Riley A, Jordan LE, Holcik M. Distinct 5′ UTRs regulate XIAP expression under normal growth conditions and during cellular stress. Nucleic Acids Res. 2010;38:4665–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lang KJ, Kappel A, Goodall GJ. Hypoxia-inducible factor-1alpha mRNA contains an internal ribosome entry site that allows efficient translation during normoxia and hypoxia. Mol Biol Cell. 2002;13:1792–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fernandez J, Yaman I, Huang C, Liu H, Lopez AB, Komar AA, Caprara MG, Merrick WC, Snider MD, Kaufman RJ, et al. Ribosome stalling regulates IRES-mediated translation in eukaryotes, a parallel to prokaryotic attenuation. Mol Cell. 2005;17:405–16 [DOI] [PubMed] [Google Scholar]
- 130.Wilson JE, Pestova TV, Hellen CU, Sarnow P. Initiation of protein synthesis from the A site of the ribosome. Cell. 2000;102:511–20 [DOI] [PubMed] [Google Scholar]
- 131.Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kumar R, Krause GS, Yoshida H, Mori K, DeGracia DJ. Dysfunction of the unfolded protein response during global brain ischemia and reperfusion. J Cereb Blood Flow Metab. 2003;23:462–71 [DOI] [PubMed] [Google Scholar]
- 133.Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, Sanyal AJ. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134:568–76 [DOI] [PubMed] [Google Scholar]
- 134.Adachi Y, Yamamoto K, Okada T, Yoshida H, Harada A, Mori K. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct Funct. 2008;33:75–89 [DOI] [PubMed] [Google Scholar]
- 135.Armstrong JL, Flockhart R, Veal GJ, Lovat PE, Redfern CP. Regulation of endoplasmic reticulum stress-induced cell death by ATF4 in neuroectodermal tumor cells. J Biol Chem. 2010;285:6091–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Siu F, Bain PJ, LeBlanc-Chaffin R, Chen H, Kilberg MS. ATF4 is a mediator of the nutrient-sensing response pathway that activates the human asparagine synthetase gene. J Biol Chem. 2002;277:24120–7 [DOI] [PubMed] [Google Scholar]
- 137.Afonyushkin T, Oskolkova OV, Philippova M, Resink TJ, Erne P, Binder BR, Bochkov VN. Oxidized phospholipids regulate expression of ATF4 and VEGF in endothelial cells via NRF2-dependent mechanism: novel point of convergence between electrophilic and unfolded protein stress pathways. Arterioscler Thromb Vasc Biol. 2010;30:1007–13 [DOI] [PubMed] [Google Scholar]
- 138.Miyamoto N, Izumi H, Miyamoto R, Bin H, Kondo H, Tawara A, Sasaguri Y, Kohno K. Transcriptional regulation of activating transcription factor 4 under oxidative stress in retinal pigment epithelial ARPE-19/HPV-16 cells. Invest Ophthalmol Vis Sci. 2011;52:1226–34 [DOI] [PubMed] [Google Scholar]
- 139.Levenson VV, Davidovich IA, Roninson IB. Pleiotropic resistance to DNA-interactive drugs is associated with increased expression of genes involved in DNA replication, repair, and stress response. Cancer Res. 2000;60:5027–30 [PubMed] [Google Scholar]
- 140.Tanabe M, Izumi H, Ise T, Higuchi S, Yamori T, Yasumoto K, Kohno K. Activating transcription factor 4 increases the cisplatin resistance of human cancer cell lines. Cancer Res. 2003;63:8592–5 [PubMed] [Google Scholar]
- 141.Igarashi T, Izumi H, Uchiumi T, Nishio K, Arao T, Tanabe M, Uramoto H, Sugio K, Yasumoto K, Sasaguri Y, et al. Clock and ATF4 transcription system regulates drug resistance in human cancer cell lines. Oncogene. 2007;26:4749–60 [DOI] [PubMed] [Google Scholar]
- 142.Sachdeva MM, Claiborn KC, Khoo C, Yang J, Groff DN, Mirmira RG, Stoffers DA. Pdx1 (MODY4) regulates pancreatic beta cell susceptibility to ER stress. Proc Natl Acad Sci U S A. 2009;106:19090–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.DeBose-Boyd RA, Brown MS, Li WP, Nohturfft A, Goldstein JL, Espenshade PJ. Transport-dependent proteolysis of SREBP: relocation of site-1 protease from Golgi to ER obviates the need for SREBP transport to Golgi. Cell. 1999;99:703–12 [DOI] [PubMed] [Google Scholar]
- 144.Shen J, Prywes R. Dependence of site-2 protease cleavage of ATF6 on prior site-1 protease digestion is determined by the size of the luminal domain of ATF6. J Biol Chem. 2004;279:43046–51 [DOI] [PubMed] [Google Scholar]
- 145.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–6 [DOI] [PubMed] [Google Scholar]
- 146.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–91 [DOI] [PubMed] [Google Scholar]
- 147.Ozcan U, Ozcan L, Yilmaz E, Duvel K, Sahin M, Manning BD, Hotamisligil GS. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell. 2008;29:541–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61 [DOI] [PubMed] [Google Scholar]
- 149.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–6 [DOI] [PubMed] [Google Scholar]
- 150.Kane RC, Bross PF, Farrell AT, Pazdur R. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003;8:508–13 [DOI] [PubMed] [Google Scholar]
- 151.Kang YJ, Lu MK, Guan KL. The TSC1 and TSC2 tumor suppressors are required for proper ER stress response and protect cells from ER stress-induced apoptosis. Cell Death Differ. 2011;18:133–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rutkowski DT, Hegde RS. Regulation of basal cellular physiology by the homeostatic unfolded protein response. J Cell Biol. 2010;189:783–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jin HO, Seo SK, Woo SH, Kim ES, Lee HC, Yoo DH, An S, Choe TB, Lee SJ, Hong SI, et al. Activating transcription factor 4 and CCAAT/enhancer-binding protein-beta negatively regulate the mammalian target of rapamycin via Redd1 expression in response to oxidative and endoplasmic reticulum stress. Free Radic Biol Med. 2009;46:1158–67 [DOI] [PubMed] [Google Scholar]
- 155.Sofer A, Lei K, Johannessen CM, Ellisen LW. Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol Cell Biol. 2005;25:5834–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Whitney ML, Jefferson LS, Kimball SR. ATF4 is necessary and sufficient for ER stress-induced upregulation of REDD1 expression. Biochem Biophys Res Commun. 2009;379:451–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yamaguchi S, Ishihara H, Yamada T, Tamura A, Usui M, Tominaga R, Munakata Y, Satake C, Katagiri H, Tashiro F, et al. ATF4-mediated induction of 4E–BP1 contributes to pancreatic beta cell survival under endoplasmic reticulum stress. Cell Metab. 2008;7:269–76 [DOI] [PubMed] [Google Scholar]
- 158.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in PERK−/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–63 [DOI] [PubMed] [Google Scholar]
- 159.Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002;22:3864–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang W, Feng D, Li Y, Iida K, McGrath B, Cavener DR. PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab. 2006;4:491–7 [DOI] [PubMed] [Google Scholar]
- 161.Iida K, Li Y, McGrath BC, Frank A, Cavener DR. PERK eIF2 alpha kinase is required to regulate the viability of the exocrine pancreas in mice. BMC Cell Biol. 2007;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Izumi T, Yokota-Hashimoto H, Zhao S, Wang J, Halban PA, Takeuchi T. Dominant negative pathogenesis by mutant proinsulin in the Akita diabetic mouse. Diabetes. 2003;52:409–16 [DOI] [PubMed] [Google Scholar]
- 163.Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, Izumi T. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest. 1999;103:27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Støy J, Edghill EL, Flanagan SE, Ye H, Paz VP, Pluzhnikov A, Below JE, Hayes MG, Cox NJ, Lipkind GM, et al. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci U S A. 2007;104:15040–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cardozo AK, Ortis F, Storling J, Feng YM, Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz A, Eizirik DL. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes. 2005;54:452–61 [DOI] [PubMed] [Google Scholar]
- 166.Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology. 2006;147:3398–407 [DOI] [PubMed] [Google Scholar]
- 167.Kharroubi I, Ladriere L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology. 2004;145:5087–96 [DOI] [PubMed] [Google Scholar]
- 168.Oyadomari S, Takeda K, Takiguchi M, Gotoh T, Matsumoto M, Wada I, Akira S, Araki E, Mori M. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci U S A. 2001;98:10845–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Avivar-Valderas A, Salas E, Bobrovnikova-Marjon E, Diehl JA, Nagi C, Debnath J, Aguirre-Ghiso JA. PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Mol Cell Biol. 2011;31:3616–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 2005;24:1243–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–49 [DOI] [PubMed] [Google Scholar]
- 173.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem. 2004;279:45495–502 [DOI] [PubMed] [Google Scholar]
- 174.Yoshida T, Shiraishi T, Nakata S, Horinaka M, Wakada M, Mizutani Y, Miki T, Sakai T. Proteasome inhibitor MG132 induces death receptor 5 through CCAAT/enhancer-binding protein homologous protein. Cancer Res. 2005;65:5662–7 [DOI] [PubMed] [Google Scholar]
- 175.Inoue H, Tanizawa Y, Wasson J, Behn P, Kalidas K, Bernal-Mizrachi E, Mueckler M, Marshall H, Donis-Keller H, Crock P, et al. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome). Nat Genet. 1998;20:143–8 [DOI] [PubMed] [Google Scholar]
- 176.Strom TM, Hortnagel K, Hofmann S, Gekeler F, Scharfe C, Rabl W, Gerbitz KD, Meitinger T. Diabetes insipidus, diabetes mellitus, optic atrophy and deafness (DIDMOAD) caused by mutations in a novel gene (wolframin) coding for a predicted transmembrane protein. Hum Mol Genet. 1998;7:2021–8 [DOI] [PubMed] [Google Scholar]
- 177.Osman AA, Saito M, Makepeace C, Permutt MA, Schlesinger P, Mueckler M. Wolframin expression induces novel ion channel activity in endoplasmic reticulum membranes and increases intracellular calcium. J Biol Chem. 2003;278:52755–62 [DOI] [PubMed] [Google Scholar]
- 178.Hofmann S, Philbrook C, Gerbitz KD, Bauer MF. Wolfram syndrome: structural and functional analyses of mutant and wild-type wolframin, the WFS1 gene product. Hum Mol Genet. 2003;12:2003–12 [DOI] [PubMed] [Google Scholar]
- 179.Fonseca SG, Gromada J, Urano F. Endoplasmic reticulum stress and pancreatic beta-cell death. Trends Endocrinol Metab. 2011;22:266–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gietzen DW, Hao S, Anthony TG. Mechanisms of food intake repression in indispensable amino acid deficiency. Annu Rev Nutr. 2007;27:63–78 [DOI] [PubMed] [Google Scholar]
- 181.Hao S, Sharp JW, Ross-Inta CM, McDaniel BJ, Anthony TG, Wek RC, Cavener DR, McGrath BC, Rudell JB, Koehnle TJ, et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science. 2005;307:1776–8 [DOI] [PubMed] [Google Scholar]
- 182.Maurin AC, Jousse C, Averous J, Parry L, Bruhat A, Cherasse Y, Zeng H, Zhang Y, Harding HP, Ron D, et al. The GCN2 kinase biases feeding behavior to maintain amino acid homeostasis in omnivores. Cell Metab. 2005;1:273–7 [DOI] [PubMed] [Google Scholar]
- 183.Costa-Mattioli M, Gobert D, Harding H, Herdy B, Azzi M, Bruno M, Bidinosti M, Ben Mamou C, Marcinkiewicz E, Yoshida M, et al. Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2. Nature. 2005;436:1166–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hanefeld F, Holzbach U, Kruse B, Wilichowski E, Christen HJ, Frahm J. Diffuse white matter disease in three children: an encephalopathy with unique features on magnetic resonance imaging and proton magnetic resonance spectroscopy. Neuropediatrics. 1993;24:244–8 [DOI] [PubMed] [Google Scholar]
- 185.Schiffmann R, Moller JR, Trapp BD, Shih HH, Farrer RG, Katz DA, Alger JR, Parker CC, Hauer PE, Kaneski CR, et al. Childhood ataxia with diffuse central nervous system hypomyelination. Ann Neurol. 1994;35:331–40 [DOI] [PubMed] [Google Scholar]
- 186.Bugiani M, Boor I, Powers JM, Scheper GC, van der Knaap MS. Leukoencephalopathy with vanishing white matter: a review. J Neuropathol Exp Neurol. 2010;69:987–96 [DOI] [PubMed] [Google Scholar]
- 187.Li W, Wang X, Van Der Knaap MS, Proud CG. Mutations linked to leukoencephalopathy with vanishing white matter impair the function of the eukaryotic initiation factor 2B complex in diverse ways. Mol Cell Biol. 2004;24:3295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Leegwater PA, Vermeulen G, Konst AA, Naidu S, Mulders J, Visser A, Kersbergen P, Mobach D, Fonds D, van Berkel CG, et al. Subunits of the translation initiation factor eIF2B are mutant in leukoencephalopathy with vanishing white matter. Nat Genet. 2001;29:383–8 [DOI] [PubMed] [Google Scholar]
- 189.Richardson JP, Mohammad SS, Pavitt GD. Mutations causing childhood ataxia with central nervous system hypomyelination reduce eukaryotic initiation factor 2B complex formation and activity. Mol Cell Biol. 2004;24:2352–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Richards NG, Kilberg MS. Asparagine synthetase chemotherapy. Annu Rev Biochem. 2006;75:629–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Blais JD, Addison CL, Edge R, Falls T, Zhao H, Wary K, Koumenis C, Harding HP, Ron D, Holcik M, et al. Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Mol Cell Biol. 2006;26:9517–32 [DOI] [PMC free article] [PubMed] [Google Scholar]