Abstract
Proteins are suspected to have a greater satiating effect than the other 2 macronutrients. After protein consumption, peptide hormones released from the gastrointestinal tract (mainly anorexigenic gut peptides such as cholecystokinin, glucagon peptide 1, and peptide YY) communicate information about the energy status to the brain. These hormones and vagal afferents control food intake by acting on brain regions involved in energy homeostasis such as the brainstem and the hypothalamus. In fact, a high-protein diet leads to greater activation than a normal-protein diet in the nucleus tractus solitarius and in the arcuate nucleus. More specifically, neural mechanisms triggered particularly by leucine consumption involve 2 cellular energy sensors: the mammalian target of rapamycin and AMP-activated protein kinase. In addition, reward and motivation aspects of eating behavior, controlled mainly by neurons present in limbic regions, play an important role in the reduced hedonic response of a high-protein diet. This review examines how metabolic signals emanating from the gastrointestinal tract after protein ingestion target the brain to control feeding, energy expenditure, and hormones. Understanding the functional roles of brain areas involved in the satiating effect of proteins and their interactions will demonstrate how homeostasis and reward are integrated with the signals from peripheral organs after protein consumption.
Introduction
Protein is an indispensable nutrient, and protein ingestion as a source of amino acids is necessary for almost all biological processes. Accordingly, food intake is sensitive to protein, and the response to protein content of meals and diets is controlled at different levels from peripheral organs to the brain. Protein intake induces complex signals including neuropeptides secreted in the gut, metabolic hormones such as insulin produced in response to nutrient absorption, and blood amino acids plus derived metabolites released in the blood. These signals converge on the central nervous system either through the activation of afferent fibers of the vagus nerve projecting to the brainstem or more frequently by acting directly on brain receptors located mostly in the arcuate nucleus (ARC5) of the hypothalamus and in the area postrema of the brainstem via the incomplete blood-brain barrier in these areas. In the past decade, many studies in humans have been conducted to examine differences in pre- and postprandial hormone profiles that could be the cause of satiety induced by proteins, but very often no clear correlation emerged between these hormones and satiety. The specificity of peripheral hormones secreted after protein intake deserves further investigation (1). However, we know that the peripheral hormones cholecystokinin (CCK) and glucagon-like peptide 1 (GLP-1) acting via the vagus nerve, ghrelin via the bloodstream, and peptide YY (PYY), which can transmit signals to the brain via the vagal afferent pathway (2) or the bloodstream, are involved in the mechanism of protein-induced satiety (3, 4). These signals are integrated by the brain and participate in the homeostatic control of feeding. These pathways are often separated from nonhomeostatic hedonic components of feeding that involve peripheral sensory components and brain regions playing a role in reward and motivation such as the mesolimbic system and nucleus accumbens. All are involved in different functions of feeding control such as meal termination, appetite, and motivation for food. The neuronal pathways linking them together exhibit great plasticity. These complex interactions between neurons having different functions make it a challenge to understand the precise role of each and their modulation by a high-protein diet.
Food intake is sensitive to protein content in the diet
The human body controls protein ingestion during a meal. Although the percentage of ingested energy from protein in the diet is relatively constant in animals and humans, it also seems that protein intake is adapted to protein needs. A very low protein (2%) diet is aversive in the rodent (5, 6), a low-protein diet tends to increase food intake to meet protein requirements (7, 8) and an increase in protein content of the diet usually reduces energy intake (8–12). Interestingly, after protein deficiency in humans, food intake and food preferences show adaptive changes (higher intake of protein for the same total energy intake and enhanced preferences for savory high-protein food), suggesting that compensatory mechanisms are induced to restore adequate protein status (13). This indicates that animals and humans have behavioral strategies to avoid protein shortage.
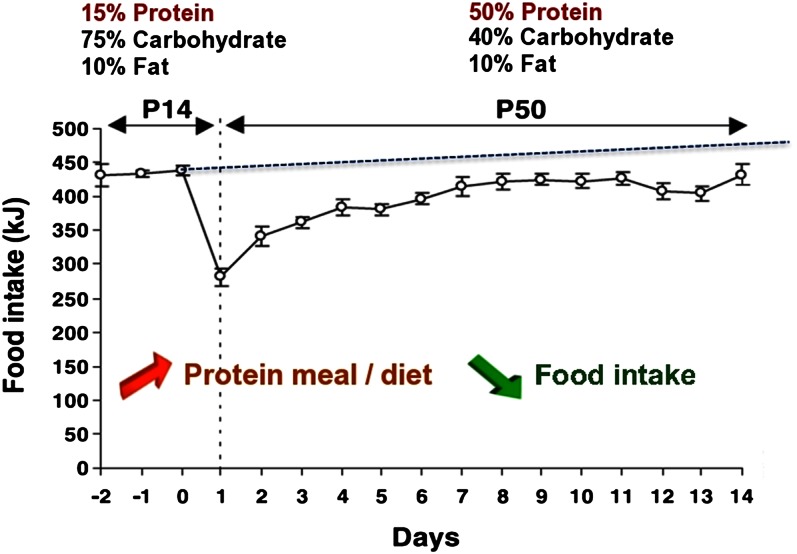
In the rat, the transition from a normal- to a high-protein diet induces a transient decrease in total food intake over the first few days; food intake then increases progressively but stabilizes below the level sustained on the normal-protein diet (control diet) (Fig. 1) (11). The rapid and transient decrease in food intake is not due to a conditioned food aversion, but rather to both a lower initial palatability of the diet and its enhanced satiety effects. The longer term food intake depression induced by a high-protein diet in the rat is independent of the palatability of the diet. The consumption of a sugar-sweetened high-protein diet or a high-protein diet leads to the same decrease in energy intake compared with a normal-protein diet, whether sugar sweetened or not (14). Compared with a high-carbohydrate diet, a high-protein diet is more potent at inducing satiety and decreasing food intake in animals (15). Moreover, increasing protein in the diet dose dependently reduces energy intake in the rat, independent of the carbohydrate:fat ratio of the diet (16). In the long term, ingestion of a high-protein diet often leads to reduced body weight and body fat mass in wild-type rats (17–19) and ob/ob mice (19). Feeding normal rats or obese mice a high-protein diet for 1 wk leads to a 2-fold increase in uncoupling protein expression in brown adipose tissue (19). This result was confirmed when increased dietary protein was combined with either a high-fat or low-fat diet in comparison with the corresponding isocaloric normal-protein diets (16). This has led to new strategies against overweight and obesity. In humans, a diet high in protein seems to provide a good long-term maintenance of reduced intra-abdominal fat stores (20). Additionally, the effect of weight loss induced by exercise and energy-restricted diets in overweight women was greater with higher protein and increased dairy product content, with a greater total and visceral fat loss and lean mass gain (21). This improvement in body composition could be explained by the decrease in food intake, but also by an increase in energy expenditure, fat oxidation (22), or thermogenesis (23). In humans, the effect of a high-protein intake on satiety is more ambiguous than in rodents (Table 1). In short-term studies, a higher protein intake increases feelings of satiety. Protein appears more potent than fat at inducing satiety, whereas results are more variable for carbohydrate (22, 24–36). However, comparison of these studies is not easy because of the diversity of protocols used. Several parameters appear to greatly influence the results such as the type of charges administered (texture of the load, type of food), its energy, its macronutrient composition (nature, proportion, and amount of each macronutrient) and the time between the administration of the load and the test meal.
Figure 1.
A high-protein diet decreases energy intake without conditioned taste aversion in the rat. Daily energy intake of rats receiving a normal-protein diet (P14) and thereafter a high-protein diet (P50) for 14 d. Results presented are ± SEM. Adapted from Reference 11 with permission.
TABLE 1.
Protein intake and satiety in humans
| Effect on satiety | Reference | Population | Macronutrient | Duration |
| Proteins > carbohydrates | Porrini et al., 1995 (24) | 12 normal males | 56% protein, 25% fat, 19% carbohydrates | 2 h |
| Proteins > carbohydrates > lipids | Johnstone et al., 1996 (25) | 6 normal males | 60% protein, 20% fat, 20% carbohydrates | 15 d |
| Proteins > carbohydrates = lipids | Poppitt et al., 1998 (26) | 12 normal females | 37% protein, 29% fat, 34% carbohydrates | 90 min |
| Stubbs et al., 1999 (27) | 16 normal males | 60% protein, 20% fat, 20% carbohydrates | 24 h | |
| Proteins = carbohydrates > lipids | Potier et al., 2010 (28) | 56 normal subjects | drink containing proteins only | Preload |
| Westerterp-Plantenga et al., 1999 (29) | 8 normal females | 29% protein, 10% fat, 61% carbohydrates | 24 h | |
| Proteins > lipids | Porrini et al., 1997 (30) | 14 normal males | 54% protein, 45% fat, 1% carbohydrates | 2 h |
| Weigle et al., 2005 (31) | 19 normal subjects | 30% protein, 20% fat, 50% carbohydrates | 4 wk | |
| Proteins > lipids > carbohydrates | Batterham et al., 2006 (32) | 10 normal males | 65.3% protein, 17.4% fat, 17.3% carbohydrates | 25 min |
| Whey = soy > egg = sucrose | Anderson et al., 2004 (33) | 13 normal males | egg, whey, soy, sucrose in beverages | 1 h |
| Whey > soy = casein (10% protein) | Veldhorst et al., 2009 (34) | 25 normal subjects | 10% protein, 35% fat, 55% carbohydrates | 20 min |
| Whey = soy = casein (25% protein) | Veldhorst et al., 2009 (34) | 25 normal subjects | 25% protein, 20% fat, 55% carbohydrates | 20 min |
| Soy = casein > whey | Acheson et al., 2011 (22) | 23 normal subjects | 50% protein, 10% fat, 40% carbohydrates | 330 min |
A high-protein diet generates signals that activate the nucleus tractus solitarius
The response to protein content during a meal is controlled at the level of the brain. Protein and amino acid ingestion induces the secretion of neuropeptides in different parts of the small intestine, such as CCK in the duodenum or peptide YY (PYY) and GLP-1 in the ileum. Some of these gut hormones (mainly CCK and GLP-1) then activate the vagus nerve. The involvement of this pathway in protein sensing and signaling to the brain goes along with the finding that infusion of proteins into the duodenum activates vagal afferent fibers in rats in a CCK-dependent manner (37). Some data indicate that enteroendocrine cells of the gut express receptors for glutamate, the most prevalent amino acid in almost all dietary proteins and for other amino acids. More precisely, recent advances highlight that amino acid receptors such as T1R1/T1R3 heterodimer, CaR, and GPR93, which are expressed in the apical face of the gut, sense glutamate and other amino acids in the lumen (38–41). These receptors could exert direct control on the secretion of gastrointestinal peptides (such as CCK, GLP-1, and PYY) in the lamina propria in response to amino acid transit and absorption in the gut (42). The infusion of glutamate into the stomach, duodenum, and portal vein increases afferent activity in the vagal gastric, celiac, and hepatic nerves. Glutamate sensors are present in the gastric wall, intestinal wall, and hepatoportal region (43), and this sensing conveys information to the brain via the vagus nerve. In STC-1 cells, free amino acid sensing by CaR led to CCK and GLP-1 secretion (44, 45). Additionally, blocking CaR by NPS2143 abolished mobilization of intracellular Ca2+ and CCK secretion (46). Protein hydrolysates seem to be more potent at stimulating enteroendocrine function than free amino acids, and in an earlier study, peptone treatment induced the secretion of CCK in isolated jejunoileal cells (47). In STC-1 cells, peptone has been demonstrated to elicit the release of GLP-1 (48) and CCK dependent on GPR93 protein receptor activation (49). In addition, the transporter PEPT1 also appeared to be involved, albeit indirectly, in CCK secretion (50, 51) by inducing membrane depolarization and an increase in intracellular Ca2+ (52).
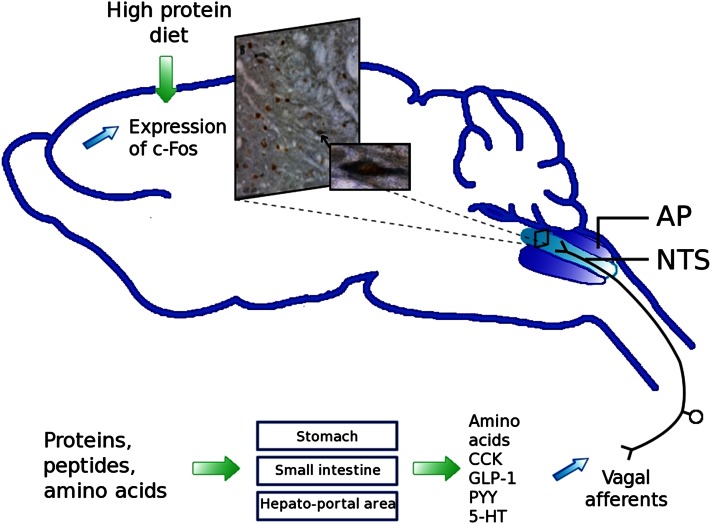
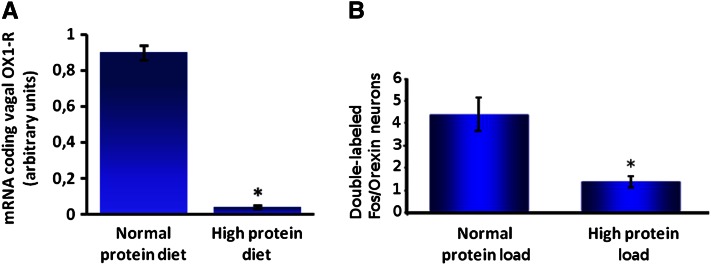
The vagus nerve conveys satiety signals through afferent fibers to the brainstem, more specifically to the nucleus tractus solitarius (NTS) (Fig. 2) (53). An alteration in food intake, such as intake of a high-protein diet, can lead to structural and functional changes in neuronal circuits controlling food intake. In fact, protein intake can initiate short- and long-term changes in neuronal organization by influencing the brain’s signal transduction pathways. The modulation of the satiety pathway in the NTS by a long-term ingestion of protein reflects this synaptic plasticity. This neuronal plasticity was observed in a c-Fos immunochemistry study in mice, where neuronal activity in the NTS was detected after an intragastric protein or sucrose load. Protein activated a different subpopulation of neurons in the NTS compared with sucrose (54). The decrease in energy ingested and the enhanced satiety due to protein intake could involve an increased sensitivity to anorexigenic hormones such as CCK, a key peripheral mediator in satiation. In rats, the activation of noradrenergic neurons and the increased expression of c-Fos in the NTS have been observed after high-protein feeding compared with normal protein (53). In another study, high-protein feeding in mice potentiated the vagally mediated NTS response to CCK, as shown by increased c-Fos activation (1). In addition, a high-protein load compared with a normal-protein load leads to decreased messenger RNA expression of the vagal receptor of orexin-1 in nodose ganglia (Fig. 3A) (55). CCK has been shown to activate orexin neurons (56), which reverse the CCK-induced loss of appetite. Thus, in the case of protein intake, the inhibitory effect of orexin on CCK signaling could be decreased, leading to increased CCK-induced satiety and decreased food intake.
Figure 2.
Vagal signaling by proteins and amino acids induces neuronal activation in the nucleus tractus solitarius (NTS). Photomicrograph of the rostral part of the NTS. Double-labeled Fos/GLP-1 neurons (brown nuclei and blue/gray cytoplasm, magnification ×20). Zoom (magnification ×40) shows 1 double-labeled neuron. 5-HT, serotonin; AP, area postrema; CCK, cholecystokinin; GLP-1, glucagon-like particle 1; PYY, peptide YY. Adapted from Reference 53 with permission.
Figure 3.
A high-protein load (55% protein as energy) compared with a normal protein (NP) load (14% protein as energy) leads to decreased messenger RNA expression of orexin-1 receptor in nodose ganglia (A) and decreased activity of orexin neurons in the lateral hypothalamus (B). A, Effect of a high-protein (HP) diet on messenger RNA expression of orexin-1 receptor (OX1-R) in nodose ganglia. Male mice were adapted for 15 d to their respective diets: NP or HP diet (n = 6). Mice were fasted overnight and killed 2 h after receiving an intragastric load of their respective diets (4.07 kcal). To measure orexin-1 receptor expression, 4 nodose ganglia were pooled in each observation. Primers used in this experiment (5′-3′) are OX1-R sense (ACGGCGAGCTGTGCTCTT), OX1-R antisense (CCTGGACCGCTGGTATGC), 18S-sense (ACGGAAGGGCACCACCAGGAG), and 18S antisense (GACCCACCACCCACGGAAACG). Results represent relative expression compared with 18S (2−ΔCT; CT = CTORX1-R − CT18S) ± SEM. *Significant effect of diet (P ≤ 0.05). Adapted from Reference 55 with permission. B, Effect of an intragastric load of protein on the activity of orexin neurons in the LH in rats. Male rats (n = 18) adapted to an NP diet were separated into 2 groups (n = 9), fasted overnight, and killed 90 min after receiving an intragastric load (10.5 kcal) of an NP or HP diet. Rats were perfused intracardially with saline and 4% paraformaldehyde in PBS, and brains were then cryoprotected in 30% sucrose. Transverse 20-μm thick lateral hypothalamus sections were cut with a cryostat (Bregma −3,70; −1,30). Briefly, sections were mounted on slides, dried overnight, and frozen (−20°C). For immunochemistry, slides were rinsed in PBS, incubated in 2% bovine serum albumin for 60 min, incubated for 24 h with rabbit anti–c-Fos antibody (1:1000) (Calbiochem) at room temperature. Sections were then placed for 3 h at room temperature with a biotyinlated goat anti-rabbit secondary antibody (1:200) diluted in PBS-bovine serum albumin, and revealed with diaminobenzidine (Sigma). c-Fos staining was followed by neuronal phenotype staining [primary antibody rabbit anti-orexin (Oncogene), 1/100; anti-rabbit secondary antibody, 1:200 (Vector)]. Orexin neurons were revealed by reaction with an Elite Vectastain SG kit (Vector). After washing and drying overnight, sections were cleared in ethanol and xylene. Results are presented as means ± SEM per section. *Significant effect of the load P ≤ 0.01. Adapted from Reference 65 with permission.
Protein modulates the activity of satiety hypothalamic pathways
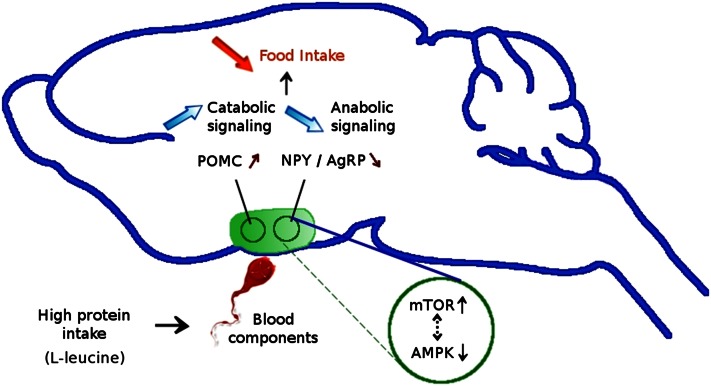
The brain plays a key role in the control of energy homeostasis, and complex neuronal pathways contribute to balancing food intake and energy expenditure to maintain body weight and adipose tissue mass. The ARC is a key area of the hypothalamus that integrates satiety and adiposity signals, relaying the information to other areas of the brain. This region is mainly composed of 2 types of neurons, anabolic neurons [synthesizing 2 peptides, neuropeptide Y (NPY) and agouti-related protein] and catabolic neurons [synthesizing the peptide pro-opiomelanocortin (POMC)]. These 2 neuronal circuits are in balance to control food intake, including the sensing of protein and energy. High-protein diets have been shown to regulate both catabolic and anabolic neuronal pathways in the ARC. Proteins inhibit anabolic neuronal signaling (decreased NPY and agouti-related protein mRNA levels) and activate the catabolic signaling (POMC neurons producing α-melanocyte-stimulating hormone) in the hypothalamus (12, 18, 19). In rats, we have shown that after ingestion of a high-protein meal, the numbers of double-labeled Fos and α-melanocyte stimulating hormone (a marker of POMC neuron activation) marked cells increased, concomitantly with a reduction in the activation of non-POMC neurons. This confirmed the up-regulation of the anorexigenic POMC satiety pathway in the ARC due to protein intake (53). The balance between the 2 parallel hypothalamic circuits is dependent on hormonal signaling, such as by the anorectic hormone PYY. In normal-weight and obese human subjects, a high-protein intake leads to a release of PYY. In mice, long-term ingestion of a high-protein diet also increases plasma PYY levels and PYY null mice are resistant to the satiating and weight-reducing effects of high-protein diets (32). Intracellular pathways responsible for the anabolic and catabolic signaling in the ARC involve mammalian target of rapamycin (mTOR) and AMP-activated protein kinase (AMP-APK), where they colocalize with NPY and POMC neurons (19). AMP-APK and mTOR were recently found to be associated with brain mechanisms responding to protein intake and seem to play a major role in the hypothalamic control of energy balance, mostly in the ARC. These kinases involved in energy sensing are both dependent on the intracellular AMP:ATP ratio, but in opposite directions. Orexigenic agents such as agouti-related protein activate hypothalamic AMP-APK and inhibit mTOR (57), whereas anorexigenic agents such as leptin have opposite effects on this pathway in the hypothalamus (58). Thus, AMP-APK overexpression in the hypothalamus increases food intake and body weight (57), and mTOR overexpression in the same brain area decreases food intake and body weight (58). The hypothalamic ATP level is increased by a high-protein diet and the AMP:ATP ratio is reduced, leading to an activation of mTOR signaling and inhibition of AMP-PK signaling in the ARC, resulting in a reduction of food intake and weight loss (19) (Fig. 4). Leucine seems to be a key element in the satiety function of high-protein diets. A leucine-supplemented diet led to a similar decrease in food intake and body weight as a high-protein diet, and leucine administration directly in the central nervous system decreased food intake in a dose-dependent manner, something not observed with other amino acids (19). Injection of leucine in the third ventricle of rats suppressed hypothalamic AMP-APK and acetyl CoA carboxylase phosphorylation (downstream target of AMP-APK) and activated mTOR signaling. Additionally, intracerebroventricular administration of leucine increased POMC mRNA levels and decreased those of NPY, consistent with the key role of this protein. These effects were blocked by rapamycin, illustrating the major role of mTOR in this regulation. l-Leucine, having both the ability to activate mTOR in the hypothalamus and to inhibit food intake, would be the principal modulator of mTOR and AMP-APK pathways stimulated by a high-protein diet. This regulation by the mTOR pathway is also modulated by growth factors and hormones, mainly by leptin. The anorectic actions of leucine were extended to AMP-APK, confirming the strong relationship between mTOR and AMP-APK (19).
Figure 4.
Proteins up-regulate pro-opiomelanocortin (POMC) and down-regulate neuropeptide Y (NPY) and agouti-related protein (AgRP) in the rat hypothalamus, via a phosphorylated mammalian target of rapamycin (mTOR) and phosphorylated AMP-activated protein kinase (AMPK)–dependent mechanism.
Protein modulates the activity of the brain reward system
Although many studies have examined the effect of various dietary proteins on the homeostatic hormonal control of food intake, more recent approaches looked into the nonhomeostatic mechanisms underlying ingestive behavior. One of these nonhomeostatic appetite centers is the central mesolimbic reward system, whose stimulation generates a sensation of pleasure and an increased motivation for food. In contrast, the inhibition of this neuronal system generates a decrease in motivation for food. Mechanisms of reward are influenced not only by the taste, smell, and texture of a meal, but also by its energy composition and notably its protein content. A very low protein diet induces an aversive response, and a high-protein diet seems to be less rewarding than a normal-protein diet in the rat (59, 60). In fact, rats acquire comparable preferences for flavors paired with the consumption of isocaloric solutions of casein and polycose solutions. These nutrients have equivalent postingestive effects, providing the same energy benefit to the animals (61). In this case, the flavor preference is mostly due to the nutrient energy value and not its orosensory properties.
Proteins are thought to stimulate satiety centers (the NTS and the ARC), but also to reduce reward mechanisms in the brain. Magnetic resonance imaging studies in humans or animals addressing the brain neural responses to food stimuli and the link between homeostatic and nonhomeostatic neural pathways indicated that the neuronal activation after a meal depends on its macronutrient composition. Some studies have shown that the consumption of a standardized meal reduces activation in the amygdala compared with the fasted state (62, 63). A manganese-enhanced magnetic resonance imaging study showed that mice adapted to a high-protein diet compared with a high carbohydrate diet and have lower basal activation in the hypothalamus, particularly in the paraventricular nucleus and the lateral hypothalamus (64). This is associated with lower orexin neuron activity in the lateral hypothalamus (Fig. 3B) (65). In the rat, the satiety effect of proteins is associated with a decrease in blood-oxygen-level-dependent signal (the blood-oxygen-level-dependent contrast imaging the change in blood flow related to energy use by brain cells), specifically in the amygdala, which is a part of the limbic system and involved in the memory of emotional reactions, including sensory stimuli and appetitive conditioning (66). In overweight “breakfast-skipping” adolescent girls, the addition of breakfast led to reductions in brain activation responses to food stimuli in limbic regions previously associated with food motivation and reward (notably in the hippocampus, amygdala, anterior cingulated, and parahippocampus) before lunch, with increased reductions in brain responses in those brain areas after a higher protein breakfast (67). These data suggest that activation of specific brain regions in the corticolimbic system is involved in the response to protein intake. Overall, a high-protein diet seems to reduce reward-driven eating behavior.
The circuitry of reward involves specific neuropeptides, transmitters such as dopamine secreted in the ventral tegmental area, opioid receptors, and γ-aminobutyric acid in the accumbens nucleus and also serotoninergic pathways. Thus, it was postulated that dietary protein could influence the brain availability of their amino acid precursors. The synthesis of serotonin (5-HT) in brain neurons could, for instance, vary with the supply of its precursor, tryptophan. This is an indispensable amino acid provided from dietary protein, and the increase of tryptophan availability could lead to a greater 5-HT concentration in rat brain. Because of this relationship, brain 5-HT could be sensitive to the presence of specific proteins in a meal (68, 69) and because brain 5-HT has a key role in the regulation of stress, mood, and feeding behavior (70), protein ingestion could affect these processes. The rates of dopamine synthesis and its release are also directly modified by the brain concentrations of its amino acid precursors, tyrosine and phenylalanine (71). Different sources of protein in a meal change cortical tyrosine concentration in rats but on a much smaller scale than tryptophan (68), and the influence of dietary proteins on catecholamines as well as opioid peptides is not obvious. Moreover, if the orosensory and energy properties of proteins do play a role in the secretion of brain reward mediators, other signals are likely involved, from neural signals stimulating the brain via the vagus nerve to metabolic signals mediated by gastrointestinal hormones. The interactions between the homeostatic and hedonic controls of protein intake are poorly understood and complex. Noninvasive measurements of neuronal activity with very good spatial and temporal resolution, such as those of functional magnetic resonance imaging, present powerful tools with which to study these interactions, allowing the mapping of brain activation after a meal or food stimuli.
Conclusions
Protein seems to play an important role in the emergence of satiety. Long-term ingestion of a high-protein diet not only decreases food intake but also lowers animals’ body weight and reduces body adiposity in animals and humans. The understanding of the effects of a high-protein diet on the modulation of satiety involves multiple pathways. Globally, to meet the body’s demand for energy continuously, the organism requires various signals at different levels and the overall regulation of these processes relies on opposing effector systems (Fig. 5). This could result from complex integration of signals coming from the periphery but also from multiple areas in the brain. At the peripheral level, after the consumption of a high-protein diet, the gut produces different hormones (mostly anorexigenic hormones such as CCK) stimulating the vagus nerve, which conveys neuronal stimuli, mostly to the NTS. Other neurons are involved in the homeostatic control of protein intake centrally, such as in the ARC. Neurons in the ARC (mainly those expressing POMC and NPY) receive distal and proximal signals related to available and stored energy. The modulation of these neuronal pathways by a high-protein diet is widely studied and can be partly explained at the cellular level. In particular, neurons can sense nutrients to control their own metabolism or to generate signals that are transmitted to other cells. mTOR and AMP-APK are involved in this cell energy sensing, notably in the ARC where exposure to a high-protein diet leads to the inhibition of AMP-APK and the activation of mTOR signaling, further increasing synaptic plasticity in the neuronal pathways controlling food intake. The importance of reward and motivation is a new aspect of our understanding of protein-enhanced satiety signaling. Thus, the reward system deserves further investigation aimed at understanding the mechanisms that allow the brain to differentiate between low- and high protein foods, as well as the integration of homeostatic and hedonic systems in the control of food intake.
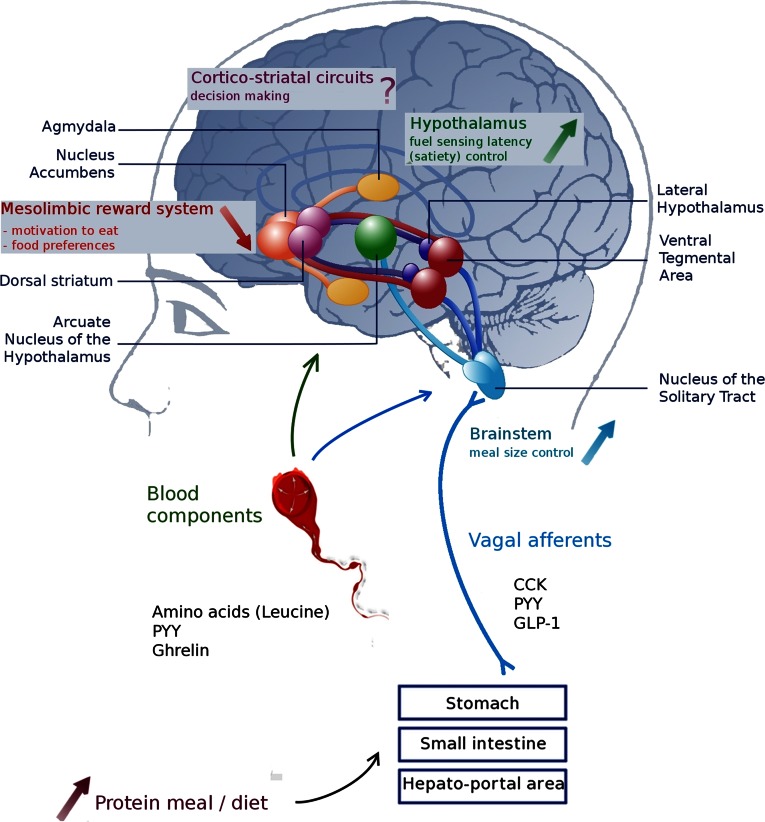
Figure 5.
Mechanisms responsible for the protein-induced reduction in food intake. Protein intake leads to the production of specifics hormones that reach the brain via the vagus nerve or bloodstream. Centrally, hormonal signaling reaches different regions of the brain: the nucleus tractus solitarius and the arcuate nucleus (ARC) would be responsible for increased satiety, and protein ingestion would decrease the motivation to eat in the mesolimbic reward system (including the nucleus accumbens). The role of decision-making areas is not yet well understood. CCK, cholecystokinin; GLP-1, glucagon-like peptide 1; PYY, peptide YY.
Acknowledgments
The authors thank Nachiket Nadkarni for editing of the manuscript and Wahiba Nefti and Mylène Potier for carrying out experiments concerning orexin activity in the lateral hypothalamus (see Figure 3). All authors have read and approved the final manuscript.
Footnotes
Supported by AgroParisTech - INRA.
Author disclosures: M. Journel, C. Chaumontet, N. Darcel, G. Fromentin and D. Tomé, no conflicts of interest.
Abbreviations used: 5-HT, serotonin; AMP-APK, AMP-activated protein kinase;, ARC, arcuate nucleus; CCK, cholecystokinin;, GLP-1, glucagon-like peptide 1; mTOR, mammalian target of rapamycin; NPY, neuropeptide Y; NTS, nucleus tractus solitarius; POMC, pro-opiomelanocortin; PYY, peptide YY.
Literature Cited
- 1.Fromentin G, Darcel N, Chaumontet C, Marsset-Baglieri A, Nadkarni N, Tome D. Peripheral and central mechanisms involved in the control of food intake by dietary amino acids and proteins. NRR. 2012; in press. [DOI] [PubMed] [Google Scholar]
- 2.Koda S, Date Y, Murakami N, Shimbara T, Hanada T, Toshinai K, Niijima A, Furuya M, Inomata N, Osuye K, et al. The role of the vagal nerve in peripheral PYY3–36-induced feeding reduction in rats. Endocrinology. 2005;146:2369–75 [DOI] [PubMed] [Google Scholar]
- 3.Veldhorst M, Smeets A, Soenen S, Hochstenbach-Waelen A, Hursel R, Diepvens K, Lejeune M, Luscombe-Marsh N, Westerterp-Plantenga M. Protein-induced satiety: effects and mechanisms of different proteins. Physiol Behav. 2008;94:300–7 [DOI] [PubMed] [Google Scholar]
- 4.Moran TH, Dailey MJ. Intestinal feedback signaling and satiety. Physiol Behav. 2011;105:77–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feurte S, Nicolaidis S, Even PC, Tome D, Mahe S, Fromentin G. Rapid fall in plasma threonine followed by increased intermeal interval in response to first ingestion of a threonine-devoid diet in rats. Appetite. 1999;33:329–41 [DOI] [PubMed] [Google Scholar]
- 6.Hao S, Sharp JW, Ross-Inta CM, McDaniel BJ, Anthony TG, Wek RC, Cavener DR, McGrath BC, Rudell JB, Koehnle TJ, et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science. 2005;307:1776–8 [DOI] [PubMed] [Google Scholar]
- 7.Whitedouble dagger BD, Porter MH, Martin RJ. Protein selection, food intake, and body composition in response to the amount of dietary protein. Physiol Behav. 2000;69:383–9 [DOI] [PubMed] [Google Scholar]
- 8.Sorensen A, Mayntz D, Raubenheimer D, Simpson SJ. Protein-leverage in mice: the geometry of macronutrient balancing and consequences for fat deposition. Obesity (Silver Spring). 2008;16:566–71. [DOI] [PubMed] [Google Scholar]
- 9.Harper AE, Peters JC. Protein intake, brain amino acid and serotonin concentrations and protein self-selection. J Nutr. 1989;119:677–89 [DOI] [PubMed] [Google Scholar]
- 10.Du F, Higginbotham DA, White BD. Food intake, energy balance and serum leptin concentrations in rats fed low-protein diets. J Nutr. 2000;130:514–21. [DOI] [PubMed] [Google Scholar]
- 11.Bensaïd A, Tome D, L'Heureux-Bourdon D, Even P, Gietzen D, Morens C, Gaudichon C, Larue-Achagiotis C, Fromentin G. A high-protein diet enhances satiety without conditioned taste aversion in the rat. Physiol Behav. 2003;78:311–20 [DOI] [PubMed] [Google Scholar]
- 12.Morrison CD, Xi X, White CL, Ye J, Martin RJ. Amino acids inhibit Agrp gene expression via an mTOR-dependent mechanism. Am J Physiol Endocrinol Metab. 2007;293:E165–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffioen-Roose S, Mars M, Siebelink E, Finlayson G, Tome D, de Graaf C. Protein status elicits compensatory changes in food intake and food preferences. Am J Clin Nutr. 2012;95:32–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.L'Heureux-Bouron D, Tome D, Bensaid A, Morens C, Gaudichon C, Fromentin G. A very high 70%-protein diet does not induce conditioned taste aversion in rats. J Nutr. 2004;134:1512–5 [DOI] [PubMed] [Google Scholar]
- 15.Bensaid A, Tome D, Gietzen D, Even P, Morens C, Gausseres N, Fromentin G. Protein is more potent than carbohydrate for reducing appetite in rats. Physiol Behav. 2002;75:577–82 [DOI] [PubMed] [Google Scholar]
- 16.Pichon L, Potier M, Tome D, Mikogami T, Laplaize B, Martin-Rouas C, Fromentin G. High-protein diets containing different milk protein fractions differently influence energy intake and adiposity in the rat. Br J Nutr. 2008;99:739–48 [DOI] [PubMed] [Google Scholar]
- 17.Jean C, Rome S, Mathe V, Huneau JF, Aattouri N, Fromentin G, Achagiotis CL, Tome D. Metabolic evidence for adaptation to a high protein diet in rats. J Nutr. 2001;131:91–8 [DOI] [PubMed] [Google Scholar]
- 18.Kinzig KP, Hargrave SL, Hyun J, Moran TH. Energy balance and hypothalamic effects of a high-protein/low-carbohydrate diet. Physiol Behav. 2007;92:454–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ropelle ER, Pauli JR, Fernandes MFA, Rocco SA, Marin RM, Morari J, Souza KK, Dias MM, Gomes-Marcondes MC, Gontijo JAR, et al. A central role for neuronal AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) in high-protein diet–induced weight loss. Diabetes. 2008;57:594–605 [DOI] [PubMed] [Google Scholar]
- 20.Skov AR, Toubro S, Ronn B, Holm L, Astrup A. Randomized trial on protein vs carbohydrate in ad libitum fat reduced diet for the treatment of obesity. Int J Obes Relat Metab Disord. 1999;23:528–36 [DOI] [PubMed] [Google Scholar]
- 21.Josse AR, Atkinson SA, Tarnopolsky MA, Phillips SM. Increased consumption of dairy foods and protein during diet- and exercise-induced weight loss promotes fat mass loss and lean mass gain in overweight and obese premenopausal women. J Nutr. 2011;141:1626–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acheson KJ, Blondel-Lubrano A, Oguey-Araymon S, Beaumont M, Emady-Azar S, Ammon-Zufferey C, Monnard I, Pinaud S, Nielsen-Moennoz C, Bovetto L. Protein choices targeting thermogenesis and metabolism. Am J Clin Nutr. 2011;93:525–34 [DOI] [PubMed] [Google Scholar]
- 23.Westerterp-Plantenga MS, Lejeune MP, Nijs I, van Ooijen M, Kovacs EM. High protein intake sustains weight maintenance after body weight loss in humans. Int J Obes Relat Metab Disord. 2004;28:57–64 [DOI] [PubMed] [Google Scholar]
- 24.Porrini M, Crovetti R, Testolin G, Silva S. Evaluation of satiety sensations and food intake after different preloads. Appetite. 1995;25:17–30 [DOI] [PubMed] [Google Scholar]
- 25.Johnstone AM, Stubbs RJ, Harbron CG. Effect of overfeeding macronutrients on day-to-day food intake in man. Eur J Clin Nutr. 1996;50:418–30 [PubMed] [Google Scholar]
- 26.Poppitt SD, McCormack D, Buffenstein R. Short-term effects of macronutrient preloads on appetite and energy intake in lean women. Physiol Behav. 1998;64:279–85 [DOI] [PubMed] [Google Scholar]
- 27.Stubbs RJ, O'Reilly LM, Johnstone AM, Harrison CL, Clark H, Franklin MF, Reid CA, Mazlan N. Description and evaluation of an experimental model to examine changes in selection between high-protein, high-carbohydrate and high-fat foods in humans. Eur J Clin Nutr. 1999;53:13–21 [DOI] [PubMed] [Google Scholar]
- 28.Potier M, Fromentin G, Lesdema A, Benamouzig R, Tome D, Marsset-Baglieri A. The satiety effect of disguised liquid preloads administered acutely and differing only in their nutrient content tended to be weaker for lipids but did not differ between proteins and carbohydrates in human subjects. Br J Nutr. 2010;104:1406–14 [DOI] [PubMed] [Google Scholar]
- 29.Westerterp-Plantenga MS, Rolland V, Wilson SA, Westerterp KR. Satiety related to 24 h diet-induced thermogenesis during high protein/carbohydrate vs high fat diets measured in a respiration chamber. Eur J Clin Nutr. 1999;53:495–502 [DOI] [PubMed] [Google Scholar]
- 30.Porrini M, Santangelo A, Crovetti R, Riso P, Testolin G, Blundell JE. Weight, protein, fat, and timing of preloads affect food intake. Physiol Behav. 1997;62:563–70 [DOI] [PubMed] [Google Scholar]
- 31.Weigle DS, Breen PA, Matthys CC, Callahan HS, Meeuws KE, Burden VR, Purnell JQ. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr. 2005;82:41–8 [DOI] [PubMed] [Google Scholar]
- 32.Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, Le Roux CW, Thomas EL, Bell JD, Withers DJ. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4:223–33 [DOI] [PubMed] [Google Scholar]
- 33.Anderson GH, Tecimer SN, Shah D, Zafar TA. Protein source, quantity, and time of consumption determine the effect of proteins on short-term food intake in young men. J Nutr. 2004;134:3011–5 [DOI] [PubMed] [Google Scholar]
- 34.Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, van Vught AJ, Westerterp KR, Engelen MP, Brummer RJ, Deutz NE, Westerterp-Plantenga MS. Dose-dependent satiating effect of whey relative to casein or soy. Physiol Behav. 2009;96:675–82 [DOI] [PubMed] [Google Scholar]
- 35.Lejeune MP, Westerterp KR, Adam TC, Luscombe-Marsh ND, Westerterp-Plantenga MS. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am J Clin Nutr. 2006;83:89–94 [DOI] [PubMed] [Google Scholar]
- 36.Hochstenbach-Waelen A, Veldhorst MA, Nieuwenhuizen AG, Westerterp-Plantenga MS, Westerterp KR. Comparison of 2 diets with either 25% or 10% of energy as casein on energy expenditure, substrate balance, and appetite profile. Am J Clin Nutr. 2009;89:831–8 [DOI] [PubMed] [Google Scholar]
- 37.Tomé D, Schwarz J, Darcel N, Fromentin G. Protein, amino acids, vagus nerve signaling, and the brain. Am J Clin Nutr. 2009;90:838S–43S [DOI] [PubMed] [Google Scholar]
- 38.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–66 [DOI] [PubMed] [Google Scholar]
- 39.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature. 2002;416:199–202 [DOI] [PubMed] [Google Scholar]
- 40.Chaudhari N, Landin AM, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nat Neurosci. 2000;3:113–9 [DOI] [PubMed] [Google Scholar]
- 41.Blackshaw LA, Page AJ, Young RL. Metabotropic glutamate receptors as novel therapeutic targets on visceral sensory pathways. Front Neurosci. 2011;5:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rasoamanana R, Darcel N, Fromentin G, Tome D. Nutrient sensing and signalling by the gut. Proc Nutr Soc. 2012;1–10. [DOI] [PubMed] [Google Scholar]
- 43.Niijima A. Reflex effects of oral, gastrointestinal and hepatoportal glutamate sensors on vagal nerve activity. J Nutr. 2000;130(4S Suppl):971S–3S. [DOI] [PubMed] [Google Scholar]
- 44.Leech CA, Habener JF. Regulation of glucagon-like peptide-1 receptor and calcium-sensing receptor signaling by L-histidine. Endocrinology. 2003;144:4851–8 [DOI] [PubMed] [Google Scholar]
- 45.Hira T, Nakajima S, Eto Y, Hara H. Calcium-sensing receptor mediates phenylalanine-induced cholecystokinin secretion in enteroendocrine STC-1 cells. FEBS J. 2008;275:4620–6 [DOI] [PubMed] [Google Scholar]
- 46.Nakajima S, Hira T, Eto Y, Asano K, Hara H. Soybean beta 51–63 peptide stimulates cholecystokinin secretion via a calcium-sensing receptor in enteroendocrine STC-1 cells. Regul Pept. 2010;159:148–55 [DOI] [PubMed] [Google Scholar]
- 47.Cordier-Bussat M, Bernard C, Haouche S, Roche C, Abello J, Chayvialle JA, Cuber JC. Peptones stimulate cholecystokinin secretion and gene transcription in the intestinal cell line STC-1. Endocrinology. 1997;138:1137–44 [DOI] [PubMed] [Google Scholar]
- 48.Cordier-Bussat M, Bernard C, Levenez F, Klages N, Laser-Ritz B, Philippe J, Chayvialle JA, Cuber JC. Peptones stimulate both the secretion of the incretin hormone glucagon-like peptide 1 and the transcription of the proglucagon gene. Diabetes. 1998;47:1038–45 [DOI] [PubMed] [Google Scholar]
- 49.Choi S, Lee M, Shiu AL, Yo SJ, Hallden G, Aponte GW. GPR93 activation by protein hydrolysate induces CCK transcription and secretion in STC-1 cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1366–75 [DOI] [PubMed] [Google Scholar]
- 50.Darcel NP, Liou AP, Tome D, Raybould HE. Activation of vagal afferents in the rat duodenum by protein digests requires PepT1. J Nutr. 2005;135:1491–5 [DOI] [PubMed] [Google Scholar]
- 51.Liou AP, Chavez DI, Espero E, Hao S, Wank SA, Raybould HE. Protein hydrolysate-induced cholecystokinin secretion from enteroendocrine cells is indirectly mediated by the intestinal oligopeptide transporter PepT1. Am J Physiol Gastrointest Liver Physiol. 2011;300:G895–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsumura K, Miki T, Jhomori T, Gonoi T, Seino S. Possible role of PEPT1 in gastrointestinal hormone secretion. Biochem Biophys Res Commun. 2005;336:1028–32 [DOI] [PubMed] [Google Scholar]
- 53.Faipoux R, Tome D, Gougis S, Darcel N, Fromentin G. Proteins activate satiety-related neuronal pathways in the brainstem and hypothalamus of rats. J Nutr. 2008;138:1172–8 [DOI] [PubMed] [Google Scholar]
- 54.Schwarz J, Burguet J, Rampin O, Fromentin G, Andrey P, Tome D, Maurin Y, Darcel N. Three-dimensional macronutrient-associated Fos expression patterns in the mouse brainstem. PLoS ONE. 2010;5:e8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nefti W.Les modifications de la sensibilité du nerf vague aux neuro-peptides gastro-intestinaux induites par des situations nutritionnelles chez la souris: bases cellulaires et conséquences sur le comportement alimentaire. Thesis Paris: Agroparistech; 2009. 138 p. [Google Scholar]
- 56.Tsujino N, Yamanaka A, Ichiki K, Muraki Y, Kilduff TS, Yagami K, Takahashi S, Goto K, Sakurai T. Cholecystokinin activates orexin/hypocretin neurons through the cholecystokinin A receptor. J Neurosci. 2005;25:7459–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–74 [DOI] [PubMed] [Google Scholar]
- 58.Cota D, Proulx K, Blake S, Kathi A, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–30. [DOI] [PubMed] [Google Scholar]
- 59.McArthur LH, Kelly WF, Gietzen DW, Rogers QR.The role of palatability in the food intake response of rats fed high-protein diets Appetite. 1993;20:181–96 [DOI] [PubMed] [Google Scholar]
- 60.Semon BA, Leung PM, Rogers QR, Gietzen DW. Effect of type of protein on food intake of rats fed high protein diets. Physiol Behav. 1987;41:451–8 [DOI] [PubMed] [Google Scholar]
- 61.Pérez C, Ackroff K, Sclafani A. Carbohydrate- and protein-conditioned flavor preferences: effects of nutrient preloads. Physiol Behav. 1996;59:467–74 [DOI] [PubMed] [Google Scholar]
- 62.Holsen LM, Zarcone JR, Thompson TI, Brooks WM, Anderson MF, Ahluwalia JS, Nollen NL, Savage CR. Neural mechanisms underlying food motivation in children and adolescents. Neuroimage. 2005;27:669–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldstone AP, Prechtl de Hernandez CG, Beaver JD, Muhammed K, Croese C, Bell G, Durighel G, Hughes E, Waldman AD, Frost G, et al. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci. 2009;30:1625–35 [DOI] [PubMed] [Google Scholar]
- 64.Zeeni N, Nadkarni N, Bell JD, Even PC, Fromentin G, Tome D, Darcel N. Peripherally injected cholecystokinin-induced neuronal activation is modified by dietary composition in mice. Neuroimage. 2010;50:1560–5 [DOI] [PubMed] [Google Scholar]
- 65.Potier M. Thesis. Paris: Agroparistech; 2009. Caractérisation de l'effet satiétogène des protéines et mécanismes impliqués: application aux protéines laitières. [Google Scholar]
- 66.Min DK, Tuor UI, Koopmans HS, Chelikani PK. Changes in differential functional magnetic resonance signals in the rodent brain elicited by mixed-nutrient or protein-enriched meals. Gastroenterology. 2011;141:1832–41 [DOI] [PubMed] [Google Scholar]
- 67.Leidy HJ, Lepping RJ, Savage CR, Harris CT.Neural responses to visual food stimuli after a normal vs. higher protein breakfast in breakfast-skipping teens: a pilot fMRI study. Obesity (Silver Spring) 2011;19:2019–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choi S, Disilvio B, Fernstrom MH, Fernstrom JD. Meal ingestion, amino acids and brain neurotransmitters: effects of dietary protein source on serotonin and catecholamine synthesis rates. Physiol Behav. 2009;98:156–62 [DOI] [PubMed] [Google Scholar]
- 69.Choi S, DiSilvio B, Fernstrom MH, Fernstrom JD. The chronic ingestion of diets containing different proteins produces marked variations in brain tryptophan levels and serotonin synthesis in the rat. Neurochem Res. 2011;36:559–65 [DOI] [PubMed] [Google Scholar]
- 70.Akana SF. Feeding and stress interact through the serotonin 2C receptor in developing mice. Physiol Behav. 2008;94:569–79 [DOI] [PubMed] [Google Scholar]
- 71.Fernstrom JD, Fernstrom MH. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J Nutr. 2007;137:1539S–47S [DOI] [PubMed] [Google Scholar]