Abstract
There are increasing reports of rickets and vitamin D deficiency worldwide. Breastfeeding without adequate sunlight exposure and vitamin D supplementation are the major risk factors. In view of the drive to promote and increase the rate of exclusive breastfeeding, the relationship among maternal vitamin D status, vitamin D concentration of human milk, and hence vitamin D status of breastfeeding infants deserves reassessment. This review provides current information on the interrelationship between maternal vitamin D status and the vitamin D status of the breastfeeding infant. It also reviews the results of ongoing research on the effect of high-dose maternal vitamin D supplementation alone as a possible option to prevent vitamin D deficiency in the breastfeeding mother-infant dyad.
Introduction
There are global efforts to promote breastfeeding as optimal nutrition in early infancy. There are, however, increasing reports worldwide of vitamin D deficiency and rickets among breast-fed infants who lack adequate sunlight exposure and do not receive vitamin D supplementation (1–4). Maternal vitamin D status and human milk vitamin D concentration significantly affect infant vitamin D status and, therefore, should be of global concern. This review focuses on the interrelationship among maternal vitamin D status and intake, human milk vitamin D concentration, and vitamin D status of the breastfeeding infants. It also presents the findings of recent research indicating that vitamin D deficiency could be an underrecognized global mother-infant health problem and that maternal vitamin D supplementation alone could increase milk vitamin D concentration significantly and possibly provide an option for optimizing the vitamin D status of the mother and her breast-fed infant.
Vitamin D physiology and functions
Vitamin D is produced after skin exposure to UVB radiation with the conversion of pre–vitamin D-3 to vitamin D-3 (cholecalciferol). This process accounts for 90% of vitamin D in the body in unsupplemented individuals (5). Very few foods contain vitamin D, and the main dietary sources include fortified milk or juice and fatty fish (5). Sunshine deprivation and lack of adequate vitamin D intake have been reported in studies and reviews to account for the high prevalence of vitamin D deficiency in children (1–3, 6).
Vitamin D is transported to the liver where it is hydroxylated to 25-hydroxyvitamin D [25(OH)D6; calcidiol], which is the major circulating vitamin D used in assessing body vitamin D status. The 25(OH)D is hydroxylated in the kidney to produce the most active metabolite, 1,25-dihydroxyvitamin D (calcitriol), which is responsible for calcium and phosphorous absorption from the gut and resorption of calcium from bone to maintain calcium and phosphorous homeostasis and bone mineralization (5). It is now known that immune cells and many organs can synthesize 1,25-dihydroxyvitamin D and express vitamin D receptors. Therefore, in addition to maintaining bone health, vitamin D may be important in immunomodulation, regulation of cell growth, and cardiovascular health (7).
Vitamin D deficiency in children
In children, long-term overt vitamin D deficiency leads to rickets with significant skeletal deformities and poor growth. Additionally, low vitamin D status at birth and in infancy has recently been linked to an increased risk of acute lower respiratory tract infections including respiratory syncytial virus infections in studies from India (8), Bangladesh (9), and the Netherlands (10).
In a review from the United States (US) of 22 case reports during the period 1986–2003, which included 166 patients with nutritional rickets, it was found that most (96%) of the children with rickets were breast-fed, and only 5% were reported to have received vitamin D supplementation. The infants in the studies were reported to have had minimal sunlight exposure, although this was not quantified (4). In a case-control study of 38 children with vitamin D deficiency rickets and 50 nonrachitic children from the United Arab Emirates (1), the authors found that 92% of the rachitic children compared with 58% of nonrachitic children (P = 0.004) were still breastfeeding at presentation, and the prevalence of vitamin D supplementation among rachitic compared with nonrachitic children was 8% vs. 38%, respectively (P = 0.001). Furthermore, the reported median number of minutes of sunlight exposure per day was significantly lower in rachitic than nonrachitic infants (0 vs. 45, P = 0.001). Taken together, these studies showed that breastfeeding without adequate sunlight exposure and vitamin D supplementation are important risk factors for vitamin D deficiency rickets in breast-fed infants. It was also noted that mothers of infants with vitamin D deficiency rickets had a higher prevalence of vitamin D deficiency compared with mothers of nonrachitic children (1). There appears to be a relationship between maternal vitamin D status and the vitamin D status of the unsupplemented breastfeeding infant. The connection could be that maternal vitamin D deficiency during lactation may increase the likelihood of low human milk vitamin D concentration (11).
Sources of vitamin D in breastfeeding infants
The natural sources of vitamin D in breastfeeding infants are previous placental transfer, human milk, and sunlight exposure. The vitamin D stores in the infant at birth depend on maternal vitamin D status during pregnancy and start physiologically with transplacental transfer of vitamin D as 25(OH)D (12). It is well-known that there is a positive correlation between maternal and cord blood 25(OH)D concentrations, and, generally, the cord blood 25(OH)D level is between 50% and 80% of the maternal value (12). After birth, the source of vitamin D for an exclusively breastfeeding infant will depend on sunlight exposure and vitamin D intake from the human milk, mostly as parent vitamin D and 25(OH)D (13–15). The milk of healthy lactating women contains relatively small amounts of vitamin D and 25(OH)D and is usually considered insufficient to prevent vitamin D deficiency in exclusively breast-fed infants if sunlight exposure is limited (16, 17). However, recent studies showed that high-dose vitamin D supplementation of 4000 IU/d and 6400 IU/d of vitamin D of healthy lactating mothers can increase the vitamin D concentration of milk to a level that supplies adequate vitamin D intake for the breastfeeding infant even though both mother and infant were limited in sunlight exposure (18, 19). Therefore, reassessment of the human milk vitamin D concentration and maternal factors that affect its quantity is timely to investigate a strategy to optimize vitamin D intake from human milk and thus vitamin D status of breast-fed infants (13, 14, 18).
Effect of currently recommended maternal vitamin D intake and sun exposure, and season on human milk vitamin D content
Most of the studies of human milk vitamin D concentration were reported >2 decades ago from North America and Europe. The vitamin D concentration of the milk was expressed as antirachitic activity (ARA) based on the biological activity values of the vitamin D and the vitamin D metabolite [25(OH)D] in human milk (14, 20). In general, the mean ARA of the human milk in healthy lactating women, unsupplemented or supplemented with existing recommended vitamin D intake, ranges from 10 to 80 IU/L (14–16, 18, 19, 21, 22) (Table 1). These values lead to low vitamin D intake in the breast-fed infant compared with the recommended intake of 400 IU/d of vitamin D (23, 24) if human milk is sole source of vitamin D.
TABLE 1.
Studies of vitamin D concentration of human milk1
| Reference | Country/race | Maternal Vitamin D intake | Time Postpartum | No. | Season | Mean Antirachitic activity, IU/L1 |
| Hollis et al., 1981 (21) | US/white | NSP | 1–21 d | 5 | NSP | 25 |
| Hollis et al., 1986 (15) | US/NSP | 400 IU/d | 14–90 d | 51 | All | 33–68 |
| Ala-Houhala et al. ,1988 (16) | Finland/white | No supplement | 8 wk | 20 | Winter | 14 (8–30) |
| 8 wk | 20 | Summer | 124 (19–332) | |||
| Atkinson et al., 1987 (22) | Canada | |||||
| 400 IU/d | 14–21 d2 | 8 | NSP | 80 ± 9 | ||
| 400 IU/d | 14–21 d3 | 9 | NSP | 60 ± 7 | ||
| Specker et al., 1985 (14) | US | |||||
| White | 512 IU/d | NSP | 15 | All | 68 | |
| Black | 365 IU/d | NSP | 10 | 35 | ||
| Hollis et al., 2004 (18) | US | |||||
| White (n = 7), black (n = 2) | NSP | 28 d | 9 | All | 40.4 ± 3.7 | |
| White (n = 6), black (n = 3) | NSP | 28 d | 9 | All | 35.5 ± 3.5 | |
| Wagner et al., 2006 (19) | US | |||||
| White (n = 6), Hispanic (n = 2), black (n = 2) | 273 ± 274 IU/d | 28 d | 10 | All | 59.6 |
Values are mean (range) or mean ± SD. Antirachitic activity was calculated from measurement of milk vitamin D and 25-hydroxyvitamin D concentrations and converted to biological activity, from reference data (20,21). NSP, not specified.
Preterm mothers.
Term mothers.
A study from the United States (14) showed that the total milk vitamin D concentration correlated with vitamin D intake and that human milk vitamins D-3 and D-2 (ergocalciferol) and 25-hydroxycholecalciferol were lower in black than in white women (Table 2). The authors suggested that this was because of the lower vitamin D intake and sun exposure among black women than white women. In another study of the effect of a 1.5 minimal erythemic dose of total-body UVB radiation on milk vitamin D concentration in 5 white lactating women (13), the serum total vitamin D-3 concentration increased from 1.0 μg/L (2.5 nmol/L) at baseline to 23 μg/L (57.5 nmol/L) and 22.5 μg/L (56.3 nmol/L) at 24 and 48 h and then decreased to 4 μg/L (10 nmol/L) and 3 μg/L (7.5 nmol/L) at 7 and 14 d, respectively. Similarly, the human milk vitamin D concentration at baseline was 150 pg/mL and increased to 1100 and 1600 pg/mL at 24 and 48 h, respectively, and then decreased to 400 and 350 pg/mL at 7 and 14 d, respectively. In a study from Finland (16), the mean (range) of milk ARA of 124 IU/L (19–332 IU/L) in summer was significantly higher than the value of 14 IU/L (8–30 IU/L) in winter. These studies indicate that maternal sunlight exposure will be expected to increase human milk vitamin D concentration.
TABLE 2.
Breast milk vitamin D and 25-hydroxyvitamin D concentrations by race1
| Mean concentration, pg/mL (95% CI) |
|||||
| Breast milk metabolites | No. | White | No. | Black | P Value |
| Vitamin D-3 (cholecalciferol) | 14 | 268 (127–567) | 10 | 36 (22–58) | 0.002 |
| Vitamin D-2 (ergocalciferol) | 14 | 290 (164–512) | 10 | 54 (22–134) | 0.001 |
| 25-Hydroxyvitamin D-3 (25-hydroxycholecalciferol) | 15 | 124 (97–159) | 10 | 87 (75–101) | 0.03 |
| 25-Hydroxyvitamin D-2 (25-hydroxyergocalciferol) | 15 | 82 (64–105) | 10 | 66 (52–85) | 0.21 |
| Total antirachitic activity, IU/L 2 | 64 | 34 | |||
Reproduced with permission from (14).
Conversions to antirachitic activity in international units/liter were based on the following: 25 pg/mL of vitamins D-3 and D-2 = 1 IU/L and 5 pg/mL of 25-hydroxyvitamins D-3 and D-2 = 1 IU/L.
Despite the association between sunlight exposure and human milk vitamin D concentration, there are no reports of the effect of long-term sunlight exposure of the mother on her milk vitamin D concentration. In addition, there are no comparative studies of the human milk vitamin D concentration in mothers living in geographic areas of low and high sunlight exposure behaviors. Such studies would provide valuable information on the effect of natural long-term sun exposure or lack of exposure on human milk vitamin D concentration.
Despite an abundance of sunshine, studies from the Middle East show that there is a high prevalence of vitamin D deficiency in women of childbearing age, mainly attributed to cultural avoidance of sunlight and traditional dress style that cover most of the body while outdoors, and inadequate vitamin D intake (25, 26). We showed that there is a high prevalence of vitamin D deficiency in exclusively breastfeeding Arab mothers and their infants in the United Arab Emirates and postulated that very low milk vitamin D in these vitamin D–deficient mothers contributed to the vitamin D deficiency in their infants (27, 28). In view of the lack of data on human milk vitamin D concentration in Arab women, we tested this hypothesis recently by examining the vitamin D status of human milk in a subset of Arab mothers participating in an open randomized parallel group trial of either 2000 IU/d or 60,000 IU/mo of vitamin D supplementation. The study design and the sociodemographic characteristics of subjects in the main study have been previously reported (28). Briefly, the lactating mothers reported a low average vitamin D intake of 192 IU/d and very low mean sunlight exposure of 7 min/wk. Eight of the 45 lactating women participating in the main study donated milk at 2 wk postpartum (baseline) during the period September 2005 to February 2006. Previous study showed no seasonal variation in vitamin D status in this community (29). The human milk vitamin D concentration in the 8 lactating women was measured as previously described (15). The 8 mothers who donated milk were not different from the main study population with respect to age, vitamin D intake, and sun exposure (data not shown). At 2 wk postpartum, all the 8 mothers were vitamin D deficient, defined as serum 25(OH)D <50 nmol/L (30). The median (range) serum 25(OH)D level was 16.5 nmol/L (6.5–44 nmol/L). The human milk vitamin D concentration (expressed as ARA) was below detectable level of the assay (<20 IU/L) in all the 8 women (11). After 3 mo of supplementation with 2000 IU/d or 60,000 IU/mo of vitamin D-2 supplementation, the milk ARA only increased to a median (range) of 51 IU/L (10–63 IU/L), which is similar to values reported in women taking recommended vitamin D supplementation of 400 IU/d in the US (15). To our knowledge, this is the first reported study of undetectable human milk vitamin D concentration related to long-term sunshine deprivation in lactating women (11).
Taken together, the reported studies indicate that human milk vitamin D concentration correlates with maternal vitamin D intake and UVB exposure and varies with season. Vitamin D concentration of milk in healthy women is low, even with currently recommended vitamin D intake, and would theoretically provide inadequate vitamin D intake compared with recommended intake for infants.
Maternal vitamin D status during pregnancy and vitamin D status of infants at birth
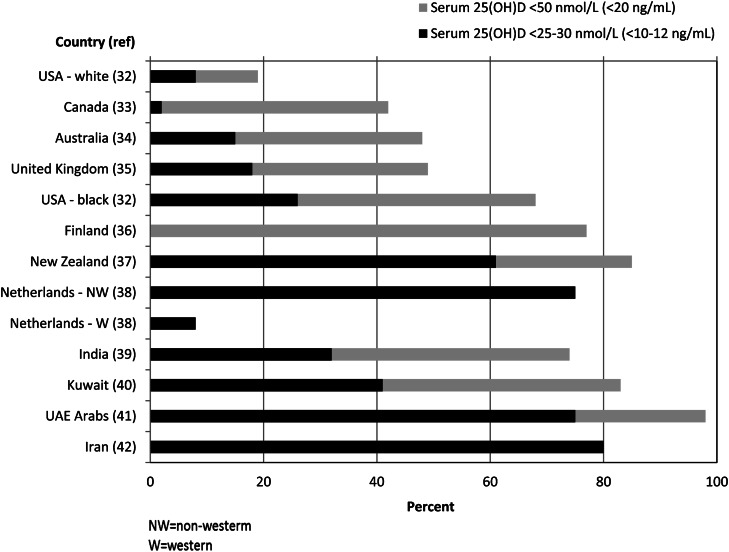
Because vitamin D status of the mother during pregnancy is important in determining the vitamin D status of the infant at birth, the strong relationship between maternal and cord blood vitamin D status (12), vitamin D deficiency during pregnancy should be of concern, especially if the mother is planning to exclusively breastfeed. Review of recent studies (31) indicates that vitamin D deficiency is highly prevalent in pregnancy in many countries and is related to limited sun exposure and inadequate vitamin D intake (32–42). Figure 1 shows that vitamin D deficiency, defined as serum 25(OH)D <50 nmol/L in the Institute of Medicine’s most recent report (24), is a global health problem during pregnancy. The reported prevalence of vitamin D deficiency in pregnancy ranges from 18% in non-Hispanic whites in the United States (32) to 42–48% in Canada (33), Australia (34), and United Kingdom (35) and 68–82% in African-American mothers in the United States (32), and in white mothers in Finland (36) and New Zealand (37). The prevalence ranges from 72% in India (39) to 98% in the Middle East (43). More alarming is the very high prevalence of severe deficiency [serum 25(OH)D <25 nmol/L], which could be detrimental to the health of the mother by increasing the risk of maternal osteomalacia, and low bone mineral content of her infant later in childhood (35). The high prevalence of very low vitamin D stores in the mother indicates that a significant proportion of infants worldwide could be born with low vitamin D stores.
Figure 1.
International comparison of recent studies of vitamin D deficiency using recommended cutoff values (24). There is a high prevalence of vitamin D deficiency (serum 25-hydroxyvitamin D <50 nmol/L) globally. Very low vitamin D status (serum 25-hydroxyvitamin D <25–30 nmol/L) appears also to be widespread. Adapted with permission from (31).
Vitamin D status of exclusively breastfeeding mothers and their nursing infants
Recent studies (27, 43–47) indicate that vitamin D deficiency in exclusively breast-fed infants appears to be an underdiagnosed public health problem in many countries and that rickets may be an underrepresentation of the magnitude of vitamin D deficiency. The reported prevalence of vitamin D deficiency in unsupplemented exclusively breast-fed infants is summarized in Table 3. Because the current recommended cutoff value for vitamin D deficiency in children is defined as serum 25(OH)D <50 nmol/L (<20 μg/L) (23, 24), the cutoff values of serum 25(OH)D levels <25–37.5 nmol/L (<10–12 μg/L) used in most studies in the table are very conservative. The prevalence of serum 25(OH)D <25–37.5 nmol/L in exclusively breast-fed infants was 18% in a study from the US (43), 27% in Greece (45), 43–51% in India (46), and 82% in the United Arab Emirates (27). In recent studies from Cincinnati, Ohio (43) and Charleston, South Carolina (47) in the US, the prevalence of vitamin D deficiency in breast-fed infants based on the current cutoff value of serum 25(OH)D <50 nmol/L was 76% and 72% at 4 wk of age, respectively. In 3 studies in which maternal vitamin D status of breastfeeding infants was evaluated, maternal vitamin D deficiency [serum 25(OH)D <50 nmol/L] was found in 10% of white and 43% of black mothers in the United States (43) and 62% of Arab mothers in the United Arab Emirates (27).
TABLE 3.
Prevalence of vitamin D deficiency in exclusively breast-fed infants from recent studies1
| Reference | Location | Study participants, no. | Age at study | Time of year | Prevalence, % | Cutoff serum 25(OH)D, nmol/L |
| Challaet al., 2005 (45) | Ioannina, Greece | 66 | 6 mo | All | 27 | <25 |
| Bhalala et al., 2007 (44) | Mumbai, India | 35 | 3 mo | NS | 51 | <37.5 |
| Seth et al., 2009 (46) | New Delhi, India | 180 | 2–24 wk | All | 43 | <25 |
| Dawodu et al., 2003 (27) | Al Ain , United Arab Emirates | 78 | 1–4 mo | All | 82 | <25 |
| Dawodu et al., 2010 (43) | Cincinnati, Ohio, United States | 87 | 1 mo | All | 18 76 | <25 <50 |
| Wagner et al., 2010 (47) | Charleston, South Carolina, United States | 33 | 1 mo | All | 72 | <50 |
Divide values by 2.5 to convert to μg/L. 25(OH)D, 25-hydroxyvitamin D.
Previous studies from the US (48, 49) and New Zealand (50) showed that during the first 4 mo of life, unsupplemented exclusively breast-fed white infants who had adequate vitamin D stores at birth or during the first 2 wk of life and whose lactating mothers had normal mean serum 25(OH)D levels had mean serum 25(OH)D levels 2- to 4-fold higher than unsupplemented exclusively breast-fed infants in at-risk populations such as United Arab Emirates and Pakistan (27). A high prevalence of vitamin D deficiency in lactating mothers contributed to the low vitamin D status in the Arab (27) and Pakistani (51) infants.
It would appear that in at-risk populations, a combination of a high prevalence of maternal vitamin D deficiency in pregnancy and during lactation and the expected low human milk vitamin D concentration contributes to the high prevalence of vitamin D deficiency in unsupplemented exclusively breast-fed infants who lack sun exposure. Therefore, preventive strategies starting from pregnancy theoretically should target both mother and infant.
Strategies for prevention of vitamin D deficiency in exclusively breast-fed infants
Multiple studies from the US and Europe show that supplementation of breastfeeding infants with 400 IU/d of vitamin D is sufficient to prevent vitamin D deficiency when sun exposure is limited (16, 19, 47, 52). It is, therefore, recommended that all breastfeeding infants in North America and the United Kingdom receive 400 IU of vitamin D supplement orally, assuming minimal sunlight exposure (23, 24, 53). It is, however, unknown whether such intake is adequate in dark-skinned infants and in parts of the world where there is a high prevalence of severe vitamin D deficiency (11). There is also the issue of poor compliance with vitamin D supplementation of breastfeeding infants (54, 55). Furthermore, the strategy of vitamin D supplementation of the breastfeeding infant does not address the concomitant high prevalence of vitamin D deficiency in their mothers. Current areas of research have, therefore, focused on whether maternal vitamin D supplementation alone can improve the vitamin D status of the mother and increase the vitamin D concentrationof the human milk to a level that would meet the requirements of her nursing infant.
Increasing maternal and breast milk vitamin D status
Although maternal UVB exposure can increase vitamin D concentration of milk (13), the effect of long-term maternal UVB exposure on milk vitamin D concentration has not been reported, probably because of the concern of an association between long-term sunlight exposure and skin cancer (56). Ongoing research activities to increase human milk vitamin D concentration have focused on high-dose maternal vitamin D supplementation alone.
Maternal vitamin D supplementation of at least 2000 IU/d is required to provide a significant amount of vitamin D in the breast milk for breastfeeding infants. In an older study from Finland (16), the authors studied 3 groups of healthy, well-nourished exclusively breastfeeding white Finnish mothers during the winter months of January to April. In group 1, 17 mothers were supplemented with 2000 IU/d of vitamin D-3 and the infants were not supplemented. In group 2, 16 mothers were given 1000 IU/d of vitamin D-3 and the infants were not supplemented. In group 3, 16 mothers were not supplemented, but their breast-fed infants were supplemented with 400 IU/d of vitamin D-3. At delivery, the cord blood 25(OH)D concentrations were similar in the 3 groups. At 8 wk of age, the serum 25(OH)D concentration in infants in groups 1 and 3 was similar, but significantly lower in group 2 (P < 0.01), and 3 of the infants in the latter group had serum 25(OH)D levels ≤12.5 nmol/L, described as the risk limit for rickets. At 15 wk of age, the serum 25(OH) D concentrations were still significantly lower in group 2 than in groups 1 and 3 (P < 0.01). The maternal milk vitamin D concentration was not measured in this study. The authors concluded that 1) vitamin D supplementation of 400 IU/d to breast-fed infants is adequate to prevent vitamin D deficiency during winter in northern latitudes, 2) breast milk does not have enough ARA even when the mothers are supplemented with 1000 IU/d of vitamin D-3, and 3) an adequate supply of vitamin D in the breast milk to the breast-fed infants is achieved only by increasing maternal vitamin D supplementation to 2000 IU/d of vitamin D-3, and 4) the safety of such supplementation on a long-term basis should be investigated.
After a lag of 2 decades and because of increasing reports of rickets (3, 4) and a high prevalence of vitamin D deficiency in breastfeeding infants (27, 45) and new data from adults on the safety of intake of up to 10,000 IU/d of vitamin D for 5 mo (57), a pilot study was undertaken in Charleston, South Carolina in the US to expand the earlier Finnish study by examining the effect of 2000 IU/d and 4000 IU/d of vitamin D-2 supplementation alone in lactating women on maternal vitamin D status, human milk vitamin D concentration, and vitamin D status of nursing infants (18). In the study, 2 groups of fully lactating women were enrolled at 1 mo postpartum. In group 1, 9 mothers (3 African American and 6 white) were randomly assigned to receive 2000 IU/d of vitamin D (as prenatal vitamin D-3, 400 IU and 1600 IU vitamin D-2). In group 2, 9 mothers (2 African American and 7 white) were randomized to receive 4000 IU/d of vitamin D (as prenatal vitamin D-3 of 400 IU and 3600 IU of vitamin D-2). Vitamin D-2 was used to track the contribution of vitamin D-2 supplement to the increase in milk vitamin D concentration and the transfer from the mother to the infant. The subjects were instructed to limit sun exposure for mothers and infants during the study period.
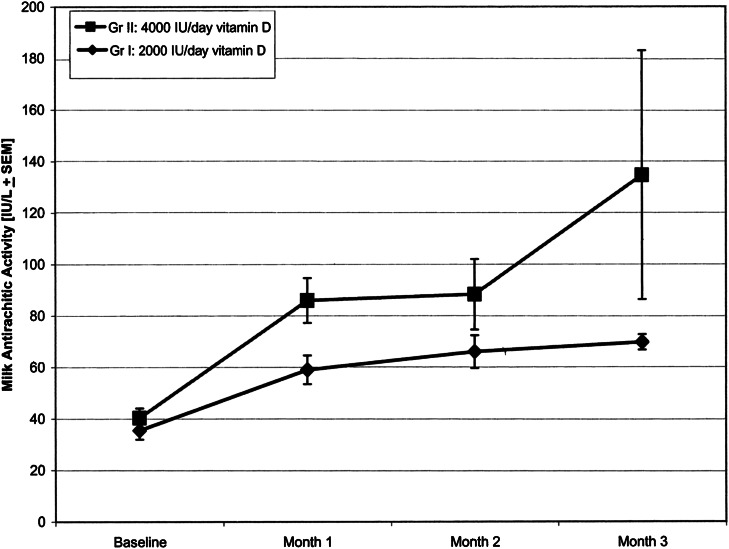
After 3 mo of supplementation, the high-dose 3600 IU/d of vitamin D-2 increased maternal serum 25-hydroxyergocalciferol from baseline significantly by 23.2 μg/L (58 nmol/L) compared with a mean increase of 17.0 μg/L (42.5 nmol/L) in mothers receiving 1600 IU/d of vitamin D-2 (P < 0.01). At enrollment, the ARA in the milk of mothers was similar in the 2 groups (35.5 vs. 40.4 IU/L) and was comparable to milk ARA of 33–68 IU/L in an earlier report (15). Mothers receiving 4000 IU/d of vitamin D had higher ARA of milk than those receiving 2000 IU/d of vitamin D (Fig. 2). The average increase in ARA of milk in mothers receiving 4000 IU/d of vitamin D was 94.2 IU/L compared with 34.2 IU/L in mothers receiving 2000 IU/d of vitamin D. The increase in the milk vitamin D concentration was associated with both vitamin D-2 and the metabolite 25-hydroxyvitamin D-2 as expected (14, 15).
Figure 2.
Milk antirachitic activity (mean ± SEM) over time in lactating mothers receiving 2000 IU/d (n = 9) or 4000 IU/d (n = 9) of vitamin D. Whole-milk samples were evaluated for vitamin D antirachitic activity from measurements of vitamin D-2, vitamin D-3, 25-hydroxyvitamin D-2, and 25-hydroxyvitamin D-3 concentrations in the milk. The values were converted to antirachitic activity (IU/L) using reference from biological activity assays. Reproduced with permission from (18).
The vitamin D status of the breastfeeding infants reflected vitamin D intake from the milk. After 3 mo of supplementation, the infants of mothers in the 2000 IU/d group had significantly lower serum 25-hydroxyvitamin D-2 levels than infants of mothers on 4000 IU/d (6.0 ± 1.1 μg/L (15.0 ± 2.7 nmol/L) vs. 12.0 ± 1.4 μg/L (30.0 ± 3.5 nmol/L), P = 0.003). Infant serum 25(OH)D at the end of the study was 27.8 μg/L (69.5 nmol/L) in the 2000 IU/d group compared with 30.8 μg/L (77.0 nmol/L) in the 4000 IU/d group. The authors observed no evidence of hypercalcemia or hypercalciuria in the mothers, indicating no evidence of vitamin D toxicity.
The study indicates that maternal vitamin D intake of 2000 IU/d increases the vitamin D status of the mother and her milk and the vitamin D status of the infant, and maternal vitamin D intake of 4000 IU/d may ensure adequate vitamin D status of both the mother and nursing infant. The authors suggested additional detailed studies to determine optimal vitamin D supplementation for lactating women.
Randomized, controlled trial of high-dose maternal vitamin D supplementation of breastfeeding mothers
Another recent study from the US investigated the effect of high-dose maternal vitamin D-3 supplementation on the vitamin D status of breastfeeding mothers and their infants in a pilot randomized, controlled trial (19). Two groups of fully lactating women were enrolled at 1 mo postpartum and followed for 6 mo. In group 1, 9 mothers received 400 IU/d of vitamin D-3, and the infants were given 300 IU/d of vitamin D-3. In group 2, ten mothers were given 6400 IU/d of vitamin D-3, and the infants received placebo. The mothers were advised to limit sun exposure for themselves and their infants. On enrollment at 1 mo postpartum, the mothers receiving 400 IU/d of vitamin D-3 had mean serum 25(OH)D of 32.2 μg/L (80.5 nmol/L), which was similar to 34.0 μg/L (85 nmol/L) in mothers receiving 6400 IU/d of vitamin D-3.
At 7 mo postpartum, mothers receiving 6400 IU/d of vitamin D-3 increased their mean serum 25(OH)D levels to 58.8 μg/L (147 nmol/L) compared with 38.4 μg/L (96.0 nmol/L) in mothers receiving 400 IU/d of vitamin D-3. The mean ARA in the milk of mothers receiving 6400 IU/d vitamin D-3 varied significantly from 82.4–873.5 IU/L, whereas the mean milk ARA in mothers receiving 400 IU/d varied little during the study, ranging from 45.7 to 78.6 IU/L. After the 6 mo of vitamin D-3 supplementation, the mean ARA in milk was significantly higher in the 6400 IU/d group than the 400 IU/d group (873 vs. 76.3 IU/L, P = 0.0003). The result indicates that maternal vitamin D supplementation of 6400 IU/d (19) was more effective in increasing milk ARA than 4000 IU/d (18).
At baseline, the mean serum 25(OH)D concentration in infants in both groups 1 and 2 (13.0 μg/L) (32.5 nmol/L) vs. 14.0 μg/L (35 nmol/L), respectively, were similar and low. At the end of the study, the mean serum 25(OH)D level of 46 μg/L (115 nmol/L) in infants whose sole dietary vitamin D intake was from the breast milk of the mother was similar to the level of 43 μg/L (108 nmol/L) in infants supplemented with 300 IU/d of vitamin D-3 orally. Although the high upper limit of ARA of milk of mothers receiving 6400 IU/d of vitamin D may be of concern, there was no evidence of early signs of vitamin D toxicity in infants, and the mothers, based on serum calcium levels and urinary calcium-to-creatinine ratio measurements. The authors concluded that maternal intake of 400 IU/d of vitamin D-3 will provide limited vitamin D intake for breastfeeding infants. However, maternal intake of 6400 IU/d of vitamin D-3 increased milk vitamin D significantly and provided vitamin D intake to the nursing infant similar to oral supplementation of 300 IU/d of vitamin D-3, which was considered sufficient to prevent vitamin D deficiency in the infant. It would appear from this study that it is possible to increase the milk vitamin D concentration to a level that will provide adequate intake for the nursing infant. The recommended dietary allowance of 600 IU of vitamin D for lactating women in the new Institute of Medicine report (24) did not address the vitamin D dietary intake of lactating mothers to meet the need of both mother and her nursing infant. Based on the results of high-dose maternal vitamin D supplementation (18, 19), the Endocrine Society recommended a daily intake of 4000–6000 IU of vitamin D for the lactating mother if the infant is not receiving 400 IU/d (58). If the objective of vitamin D supplementation during lactation is to optimize maternal vitamin D status and achieve vitamin D sufficiency in both mother and infant through maternal supplementation alone, there is a need for larger studies to identify the appropriate safe and effective dose. Therefore, if the safety and effectiveness of the 6400 IU/d of vitamin D maternal supplementation is confirmed in large studies in different locations, it could be a step forward as an option to prevent vitamin D deficiency in breastfeeding mothers and their nursing infants.
Conclusions
Human milk vitamin D reflects maternal vitamin D intake and the amount of maternal UVB exposure. Vitamin D concentration of human milk is low in subjects on existing recommended maternal vitamin D intake and is insufficient to meet the needs of breastfeeding infants. Vitamin D deficiency is widespread during pregnancy and lactation and may increase the likelihood of vitamin D deficiency in breastfeeding infants who do not receive adequate sunlight exposure and vitamin D supplementation. The results of infant supplementation studies indicate that oral vitamin D supplementation of 400 IU/d meets the requirements of breastfeeding infants, assuming limited sun exposure. Recent studies show that high-dose maternal vitamin D supplementation during lactation with 4000–6400 IU/d of vitamin D substantially increases mother and infant vitamin D status without adverse events and provides a possible option to prevent vitamin D deficiency in the mother and her nursing infant.
Future directions
There is a need for larger global comparative data of human milk vitamin D concentration from different geographic locations with different sunlight exposure behaviors and pattern of dietary vitamin D intake. The results might provide data on geographic differences in human vitamin D concentration caused by variation in UVB exposure and dietary vitamin D intake. Studies are warranted to determine whether there is a safe UVB exposure to prevent vitamin D deficiency without increasing the risk of skin cancer. Larger studies are warranted to compare the effect of high-dose supplementation of lactating mothers alone versus oral supplementation of the infant to prevent vitamin D deficiency and rickets in breastfeeding infants, assuming limited sun exposure. Future research should investigate the effect of adequate maternal supplementation in pregnancy on optimal vitamin D requirements during lactation to prevent vitamin D deficiency in mother-infant pairs.
Acknowledgments
All authors have read and approved the final manuscript.
Footnotes
Published as a supplement to Advances in Nutrition. Presented as part of the symposium entitled “Impact of Maternal Status on Breast Milk Quality and Infant Outcomes: An Update on Key Nutrients,” given at the Experimental Biology 2011 meeting, April 12, 2011, in Washington, DC. The symposium was sponsored by the American Society for Nutrition and supported by an unrestricted educational grant from Medela. The symposium was chaired by Laurie Nommsen-Rivers and Donna J. Chapman. Guest Editors for this symposium publication were Donna J. Chapman and Shelley McGuire. Guest Editor disclosure: Donna J. Chapman received travel support and compensation for editorial services provided for this symposium from the International Society for Research on Human Milk and Lactation. Shelley McGuire had no conflicts to disclose.
Supported in part by Thrasher Research Fund #02826-4 (A.D.).
Author disclosures: A. Dawodu and R. C. Tsang, no conflicts of interest.
Abbreviations: ARA, antirachitic activity; 25(OH)D, 25-hydroxyvitamin D; US, United States.
Literature Cited
- 1.Dawodu A, Agarwal M, Sankarankutty M, Hardy D, Kochiyil J, Badrinath P. Higher prevalence of vitamin D deficiency in mothers of rachitic than nonrachitic children. J Pediatr. 2005;147:109–11 [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thacher TD, Fischer PR, Strand MA, Pettifor JM. Nutritional rickets around the world: causes and future directions. Ann Trop Paediatr. 2006;26:1–16 [DOI] [PubMed] [Google Scholar]
- 4.Weisberg P, Scanlon KS, Li R, Cogswell ME. Nutritional rickets among children in the United States: review of cases reported between 1986 and 2003. Am J Clin Nutr. 2004; 80(6, Suppl)1697S–705S [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–71 [DOI] [PubMed] [Google Scholar]
- 6.Wharton B, Bishop N. Rickets. Lancet. 2003;362:1389–400 [DOI] [PubMed] [Google Scholar]
- 7.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81 [DOI] [PubMed] [Google Scholar]
- 8.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58:563–7 [DOI] [PubMed] [Google Scholar]
- 9.Roth DE, Shah R, Black RE, Baqui AH. Vitamin D status and acute lower respiratory infection in early childhood in Sylhet, Bangladesh. Acta Paediatr. 2010;99:389–93 [DOI] [PubMed] [Google Scholar]
- 10.Belderbos ME, Houben ML, Wilbrink, Lentjes E, Bloemen EM, Kimpen JL, Rovers M, Bont L. Cord blood vitamin d deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics. 2011;127:e1513–20 [DOI] [PubMed] [Google Scholar]
- 11.Saadi HF, Dawodu A, Afandi B, Zayed R, Benedict S, Nagelkerke N, Hollis BW. Effect of combined maternal and infant vitamin D supplementation on vitamin D status of exclusively breastfed infants. Matern Child Nutr. 2009;5:25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nutr. 2004;79:717–26 [DOI] [PubMed] [Google Scholar]
- 13.Greer FR, Hollis BW, Cripps DJ, Tsang RC. Effects of maternal ultraviolet B irradiation on vitamin D content of human milk. J Pediatr. 1984;105:431–3 [DOI] [PubMed] [Google Scholar]
- 14.Specker BL, Tsang RC, Hollis BW. Effect of race and diet on human-milk vitamin D and 25-hydroxyvitamin D. Am J Dis Child. 1985;139:1134–7 [DOI] [PubMed] [Google Scholar]
- 15.Hollis BW, Pittard WB, 3rd, Reinhardt TA. Relationships among vitamin D, 25-hydroxyvitamin D, and vitamin D-binding protein concentrations in the plasma and milk of human subjects. J Clin Endocrinol Metab. 1986;62:41–4 [DOI] [PubMed] [Google Scholar]
- 16.Ala-Houhala M, Koskinen T, Parviainen MT, Visakorpi JK. 25-Hydroxyvitamin D and vitamin D in human milk: effects of supplementation and season. Am J Clin Nutr. 1988;48:1057–60 [DOI] [PubMed] [Google Scholar]
- 17.Hoogenboezem T, Degenhart HJ, de Muinck Keizer-Schrama SM, Bouillon R, Grose WF, Hackeng WH, Visser HK. Vitamin D metabolism in breast-fed infants and their mothers. Pediatr Res. 1989;25:623–8 [DOI] [PubMed] [Google Scholar]
- 18.Hollis BW, Wagner CL. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am J Clin Nutr. 2004; 80(6, Suppl)1752S–8S [DOI] [PubMed] [Google Scholar]
- 19.Wagner CL, Hulsey TC, Fanning D, Ebeling M, Hollis BW. High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: a 6-month follow-up pilot study. Breastfeed Med. 2006;1:59–70 [DOI] [PubMed] [Google Scholar]
- 20.Reeve LE, Chesney RW, DeLuca HF. Vitamin D of human milk: identification of biologically active forms. Am J Clin Nutr. 1982;36:122–6 [DOI] [PubMed] [Google Scholar]
- 21.Hollis BW, Roos BA, Draper HH, Lambert PW. Vitamin D and its metabolites in human and bovine milk. J Nutr. 1981;111:1240–8 [DOI] [PubMed] [Google Scholar]
- 22.Atkinson S, Reinhardt T, Hollis B. Vitamin D activity in maternal plasma and milk in relation to gestational stage at delivery. Nutr Res. 1987;7:1005–11 [Google Scholar]
- 23.Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–52 [DOI] [PubMed] [Google Scholar]
- 24. Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed]
- 25.Dawodu A, Absood G, Patel M, Agarwal M, Ezimokhai M, Abdulrazzaq Y, Khalayli G. Biosocial factors affecting vitamin D status of women of childbearing age in the United Arab Emirates. J Biosoc Sci. 1998;30:431–7 [DOI] [PubMed] [Google Scholar]
- 26.Gannagé-Yared MH, Chemali R, Yaacoub N, Halaby G. Hypovitaminosis D in a sunny country: relation to lifestyle and bone markers. J Bone Miner Res. 2000;15:1856–62 [DOI] [PubMed] [Google Scholar]
- 27.Dawodu A, Agarwal M, Hossain M, Kochiyil J, Zayed R. Hypovitaminosis D and vitamin D deficiency in exclusively breast-feeding infants and their mothers in summer: a justification for vitamin D supplementation of breast-feeding infants. J Pediatr. 2003;142:169–73 [DOI] [PubMed] [Google Scholar]
- 28.Saadi HF, Dawodu A, Afandi BO, Zayed R, Benedict S, Nagelkerke N. Efficacy of daily and monthly high-dose calciferol in vitamin D-deficient nulliparous and lactating women. Am J Clin Nutr. 2007;85:1565–71 [DOI] [PubMed] [Google Scholar]
- 29.Saadi HF, Nagelkerke N, Benedict S, Qazaq HS, Zilahi E, Mohamadiyeh MK, Al-Suhaili AI. Predictors and relationships of serum 25 hydroxyvitamin D concentration with bone turnover markers, bone mineral density, and vitamin D receptor genotype in Emirati women. Bone. 2006;39:1136–43 [DOI] [PubMed] [Google Scholar]
- 30.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–6 [DOI] [PubMed] [Google Scholar]
- 31.Dawodu A, Wagner C. Prevention of vitamin D deficiency in mothers and infants worldwide-a paradigm shift. Pediatr Int Child Health. 2012;;1:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamilton SA, McNeil R, Hollis BW, Davis DJ, Winkler j, Cook C, Warner G, Bivens B, McShane P, Wagner CL. Profound vitamin D deficiency in a diverse group of women during pregnancy living in a sun-rich environment at latitude 32°N. Int J Endocrinol. 2010;2010:917428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newhook LA, Sloka S, Grant M, Randell E, Kovacs CS, Twells LK. Vitamin D insufficiency common in newborns, children and pregnant women living in Newfoundland and Labrador, Canada. Matern Child Nutr. 2009;5:186–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowyer L, Catling-Paull C, Diamond T, Homer C, Davis G, Craig ME. Vitamin D, PTH and calcium levels in pregnant women and their neonates. Clin Endocrinol (Oxf). 2009;70:372–7 [DOI] [PubMed] [Google Scholar]
- 35.Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, Arden NK, Godfrey KM, Cooper C; Princess Anne Hospital Study Group Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43 [DOI] [PubMed] [Google Scholar]
- 36.Viljakainen HT, Saarnio E, Hytinantti T, Miettinen M, Surcel H, Mäkitie O, Andersson S, Laitinen K, Lamberg-Allardt C. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab. 2010;95:1749–57 [DOI] [PubMed] [Google Scholar]
- 37.Judkins A, Eagleton C. Vitamin D deficiency in pregnant New Zealand women. N Z Med J. 2006;119:U2144. [PubMed] [Google Scholar]
- 38.van der Meer IM, Karamali NS, Boeke AJ, Lips P, Middelkoop BJ, Verhoeven I, Wuister JD. High prevalence of vitamin D deficiency in pregnant non-Western women in The Hague, Netherlands. Am J Clin Nutr. 2006;84:350–3, quiz 468–9 [DOI] [PubMed] [Google Scholar]
- 39.Sahu M, Bhatia V, Aggarwal A, Rawat V, Saxena P, Pandey A, Das V. Vitamin D deficiency in rural girls and pregnant women despite abundant sunshine in northern India. Clin Endocrinol (Oxf). 2009;70:680–4 [DOI] [PubMed] [Google Scholar]
- 40.Molla AM, Al Badawi M, Hammoud MS, Molla AM, Shukkur M, Thalib L, Eliwa MS. Vitamin D status of mothers and their neonates in Kuwait. Pediatr Int. 2005;47:649–52 [DOI] [PubMed] [Google Scholar]
- 41.Dawodu A, Saadi HF, Bakdache G, Altaye M, Hollis BW. Extraordinarily high prevalence and lack of season variation of vitamin D deficiency in pregnant Arab Women. Presented at the Pediatric Academic Societies Annual Meeting, 2010, Vancouver [Google Scholar]
- 42.Bassir M, Laborie S, Lapillonne A, Claris O, Chappuis MC, Salle BL. Vitamin D deficiency in Iranian mothers and their neonates: a pilot study. Acta Paediatr. 2001;90:577–9 [PubMed] [Google Scholar]
- 43.Dawodu A, Zalla L, Woo J, Herbers P, Heubi J, Morrow A. Vitamin D deficiency in breast feeding mothers and their infants. Presented at the Pediatric Academic Societies Annual Meeting, 2010, Vancouver [Google Scholar]
- 44.Bhalala U, Desai M, Parekh P, Mokal R, Chheda B. Subclinical hypovitaminosis D among exclusively breastfed young infants. Indian Pediatr. 2007;44:897–901 [PubMed] [Google Scholar]
- 45.Challa A, Ntourntoufi A, Cholevas V, Bitsori M, Galanakis E, Andronikou S. Breastfeeding and vitamin D status in Greece during the first 6 months of life. Eur J Pediatr. 2005;164:724–9 [DOI] [PubMed] [Google Scholar]
- 46.Seth A, Marwaha RK, Singla B, Aneja S, Mehrotra P, Sastry A, Khurana ML, Mani K, Sharma B, Tandon N. Vitamin D nutritional status of exclusively breast fed infants and their mothers. J Pediatr Endocrinol Metab. 2009;22:241–6 [DOI] [PubMed] [Google Scholar]
- 47.Wagner CL, Howard C, Hulsey TC, Lawrence RA, Taylor SN, Will H, Ebeling M, Hutson J, Hollis BW. Circulating 25-hydroxyvitamin d levels in fully breastfed infants on oral vitamin d supplementation. Int J Endocrinol. 2010;2010:235035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greer FR, Marshall S. Bone mineral content, serum vitamin D metabolite concentrations, and ultraviolet B light exposure in infants fed human milk with and without vitamin D2 supplements. J Pediatr. 1989;114:204–12 [DOI] [PubMed] [Google Scholar]
- 49.Chan GM, Roberts CC, Folland D, Jackson R. Growth and bone mineralization of normal breast-fed infants and the effects of lactation on maternal bone mineral status. Am J Clin Nutr. 1982;36:438–43 [DOI] [PubMed] [Google Scholar]
- 50.Birkbeck JA, Scott HF. 25-Hydroxycholecalciferol serum levels in breast-fed infants. Arch Dis Child. 1980;55:691–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atiq M, Suria A, Nizami SQ, Ahmed I. Vitamin D status of breastfed Pakistani infants. Acta Paediatr. 1998;87:737–40 [DOI] [PubMed] [Google Scholar]
- 52.Ala-Houhala M, Koskinen T, Terho A, Koivula T, Visakorpi J. Maternal compared with infant vitamin D supplementation. Arch Dis Child. 1986;61:1159–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shaw NJ, Pal BR. Vitamin D deficiency in UK Asian families: activating a new concern. Arch Dis Child. 2002;86:147–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perrine CG, Sharma AJ, Jefferds ME, Serdula MK, Scanlon KS. Adherence to vitamin D recommendations among US infants. Pediatrics. 2010;125:627–32 [DOI] [PubMed] [Google Scholar]
- 55.Taylor JA, Geyer LJ, Feldman KW. Use of supplemental vitamin D among infants breastfed for prolonged periods. Pediatrics. 2010;125:105–11 [DOI] [PubMed] [Google Scholar]
- 56. National Coalition for Skin Cancer Prevention, National Forum for Skin Cancer Prevention in Health, Physical Education, Recreation, and Youth Sports. Reston, VA: American Association for Health Education. 1998.
- 57.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–10 [DOI] [PubMed] [Google Scholar]
- 58.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM; Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30 [DOI] [PubMed] [Google Scholar]