Abstract
Human milk contains more than a hundred structurally distinct oligosaccharides. In this review, we provide examples of how the structural characteristics of these human milk oligosaccharides (HMO) determine functionality. Specific α1–2-fucosylated HMO have been shown to serve as antiadhesive antimicrobials to protect the breast-fed infant against infections with Campylobacter jejuni, one of the most common causes of bacterial diarrhea. In contrast, α1–2-fucosylation may abolish the beneficial effects of HMO against Entamoeba histolytica, a protozoan parasite that causes colitis, acute dysentery, or chronic diarrhea. In a different context, HMO need to be both fucosylated and sialylated to reduce selectin-mediated leukocyte rolling, adhesion, and activation, which may protect breast-fed infants from excessive immune responses. In addition, our most recent data show that a single HMO that carries not 1 but 2 sialic acids protects neonatal rats from necrotizing enterocolitis, one of the most common and often fatal intestinal disorders in preterm infants. Oligosaccharides currently added to infant formula are structurally different from the oligosaccharides naturally occurring in human milk. Thus, it appears unlikely that they can mimic some of the structure-specific effects of HMO. Recent advances in glycan synthesis and isolation have increased the availability of certain HMO tri- and tetrasaccharides for in vitro and in vivo preclinical studies. In the end, intervention studies are needed to confirm that the structure-specific effects observed at the laboratory bench translate into benefits for the human infant. Ultimately, breastfeeding remains the number one choice to nourish and nurture our infants.
Introduction
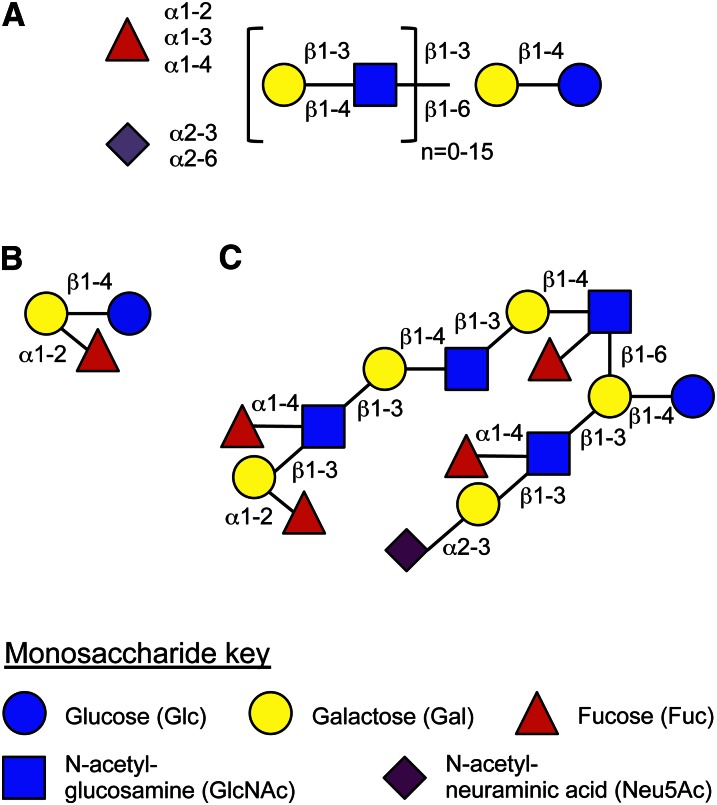
HMO4 are unconjugated glycans that are highly abundant in human milk but not in infant formula (1–4). HMO synthesis follows a basic blueprint (Fig. 1A). All HMO contain the disaccharide lactose at their reducing end. Lactose can be fucosylated either at the terminal Gal in α1–2 linkage to form 2′-fucosyllactose (Fig. 1B) or at the reducing end Glc in α1–3 linkage to yield 3-fucosyllactose. Alternatively, lactose can be sialylated at the terminal Gal in α2–3 or α2–6 linkage to generate 3′-sialyllactose and 6′-sialyllactose, respectively. In addition to these human milk trisaccharides, which are traditionally included as HMO, the nonreducing end of lactose can be elongated and branched with lacto-N-biose I (Galβ1–3GlcNAc) or N-acetyl-lactosamine (Galβ1–4GlcNAc) in β1–3 or β1–6 linkages. Complex HMO with more than a dozen of these disaccharide repeats have been identified. Furthermore, the HMO backbone can be modified with one or more Fuc residues bound in α1–2, α1–3, and/or α1–4 linkage or with one or more sialic acid residues (N-acetyl-neuraminic acid) bound in an α2–3 or α2–6 linkage (Fig. 1C). More than one hundred structurally distinct HMO have been identified so far that follow this basic blueprint and carry various potentially bioactive glycan epitopes.
Figure 1.
HMO blueprint. (A) Structural composition of HMO. If n = 0, the lactose backbone is either sialylated or fucosylated to form human milk trisaccharides such as 2′-fucosyllactose (B). If n > 0, complex HMO are formed that can be branched and also modified like the sialylated and fucosylated iso-lacto-N-decaose (C). The monosaccharide key is shown on the bottom and used throughout this article. HMO, human milk oligosaccharide.
Inter- and intrapersonal variations
Although HMO synthesis generally follows the same basic blueprint, there are important inter- and intrapersonal variations in HMO composition. Different women synthesize different subsets of oligosaccharides, and the total amount and relative abundance of HMO changes over the course of lactation.
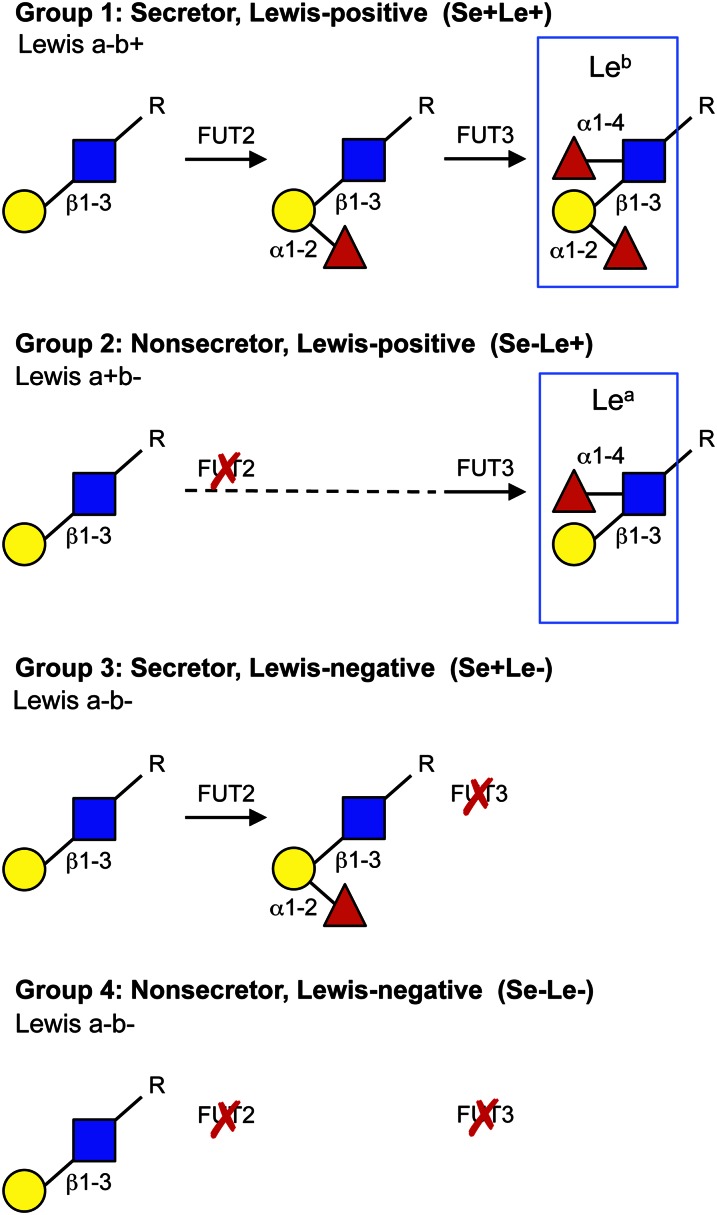
The most extreme interpersonal variations are found with respect to HMO fucosylation and are based on women’s Secretor and Lewis blood group status (Fig. 2). The majority of women express the fucosyltransferase FUT2 that connects Fuc to terminal Gal in an α1–2 linkage (5). 2′-Fucosyllactose and LNFP I are some of the most abundant HMO in milk of the so-called Secretor women (6–9). In contrast, these HMO are virtually absent in the milk of Nonsecretor women who do not express FUT2 (Fig. 3). An independent variable in HMO fucosylation is the expression of another fucosyltransferase, FUT3 (10). This enzyme connects Fuc with subterminal GlcNAc of type I chains in α1–4 linkage, which generates the Lewis b antigen in Secretors (Lewis a-b+) and the Lewis a antigen in Nonsecretors (Lewis a+b-). These epitopes are absent in the milk of Lewis negative women (Lewis a-b-), who can be either Secretors or Nonsecretors. Thus, based on the expression of the FUT2 and FUT3 fucosyltransferases, HMO profiles can be divided into 4 groups: Se+Le+, Se-Le+, Se+Le-, and Se-Le-, where Se stands for Secretor and Le stands for Lewis (Fig. 2). In terms of fucosylation, Se+Le+ women secrete milk with the most complex HMO composition, whereas Se-Le- women secrete milk with the least complex HMO composition. However, the HMO concentration in milk of Se-Le- women is still on the order of several g/L.
Figure 2.
Genetically determined variations in HMO. Composition of HMO fucosylation highly depends on the expression of the Secretor gene that encodes the α1–2-fucosyltransferase FUT2 and the Lewis gene that encodes for the α1–3/4-fucosyltransferase FUT3. Based on the expression of these fucosyltransferases, 4 different HMO groups can be distinguished. If both fucosyltransferases are expressed, the milk contains oligosaccharides with the Leb. If FUT3, but not FUT2, is expressed, the milk contains oligosaccharides with the Lea. Neither Lea nor Leb epitopes are present on HMO of women that do not express FUT3, but the HMO profile can be different depending on whether or not FUT2 is expressed. HMO, human milk oligosaccharide; Lea, Lewis a blood group antigen; Leb, Lewis b blood group antigen.
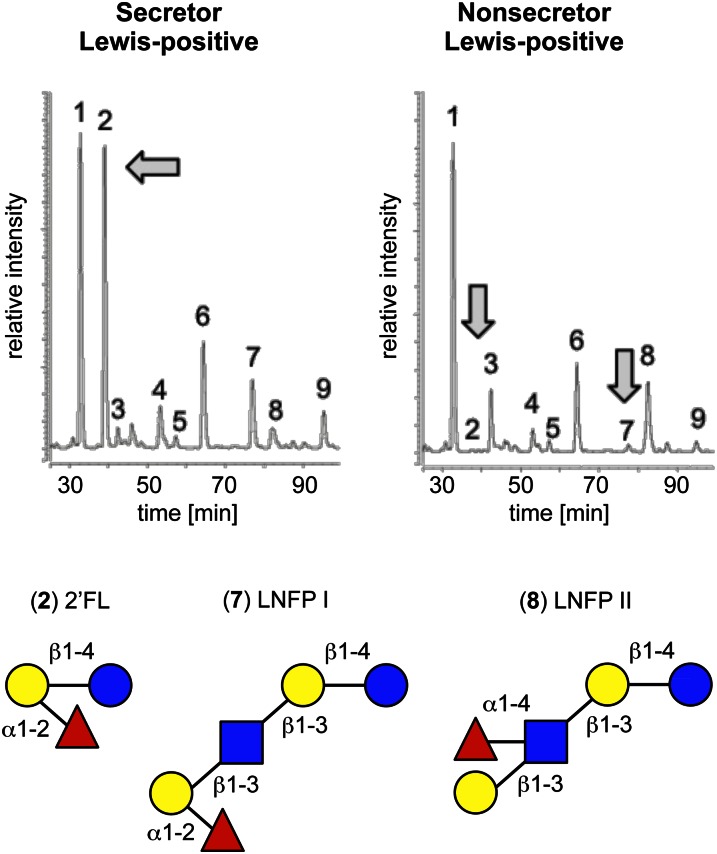
Figure 3.
Individual HMO are absent in the milk of Nonsecretor women. The HMO profiles of a Secretor and a Nonsecretor woman are strikingly different as shown by the 2 HPLC-FL chromatograms on the top left and right, respectively. Peak 1 represents the internal standard that allows for accurate inter-chromatogram comparison. 2′FL (peak 2) and LNFP I (peak 7) are some of the most abundant HMO in the milk from the Secretor woman but are absent in the milk from the Nonsecretor woman. Instead, the concentration of the isomer LNFP II (peak 8) increases. 2′FL, 2′-fucosyllactose; HMO, human milk oligosaccharide; LNFP, lacto-N-fucopentaose.
Interpersonal variations in HMO sialylation are more subtle and not based on an all-or-nothing expression of individual sialyltransferases. Differential sialyltransferase expression and regulation may, however, contribute to variations in the relative abundance of sialylated HMO.
Next to these genetically determined differences in HMO composition, environmental factors may also affect HMO synthesis. Our preliminary data shows that the total amount of HMO is significantly lower in women with a BMI between 14 and 18 than in women with a BMI between 24 and 28 (L. Bode, C. Nissan, R. Raqib, unpublished data). The study was, however, not powered to determine whether or not a reduced secretion of individual HMO contributes to the overall lower amount of total HMO. Whether general malnutrition or specific nutrient deficits affect HMO synthesis remains to be elucidated.
In addition to HMO variations between different women, there are also variations in different milk samples from the same woman depending on the stage of lactation. The total amount of HMO is highest in colostrum and decreases through transitional to mature milk. The concentration of sialylated HMO is higher in milk from the first weeks postpartum and decreases over the course of lactation. The milk of mothers delivering preterm infants has been reported to have significantly higher oligosaccharide concentrations compared to term milk (11).
In conclusion, inter- and intrapersonal variations in HMO synthesis determine the composition and relative abundance of individual HMO in a given milk sample. Each HMO is structurally unique and structure often determines biological function, as outlined below.
α1–2-Fucosylated HMO are antiadhesive antimicrobials against Campylobacter jejuni
Bacterial, viral, and protozoan infections remain the most common causes of infant mortality. Many of these pathogens use lectins, glycan-binding proteins, to attach to specific glycans on epithelial surfaces, which is often an essential first step to colonize the host and cause disease. HMO resemble some of these cell surface glycans and serve as soluble decoy receptors that block pathogen attachment and reduce subsequent infection and disease. Thus, HMO that intercept pathogen attachment are antiadhesive antimicrobials and part of the innate immune protection provided with human milk.
Lectin binding is structure specific. For example, some lectins require their glycan binding partners to be sialylated; other lectins depend on specifically linked Fuc as part of their glycan ligand. Different pathogens use different lectins and some pathogens are able to express multiple lectins to increase their chances to colonize the host. One HMO alone cannot block all different lectins and fend off the wide variety of intruders that infants are exposed to and have been exposed to throughout evolution. This selection pressure might provide one explanation for why human milk contains not one, but more than 100 structurally distinct HMO.
The most consistent and comprehensive data on antiadhesive antimicrobial effects have been reported for α1–2-fucosylated HMO and their protection against C. jejuni, one of the most common causes of bacterial diarrhea and infant mortality (12–14). In vitro, 2′-fucosylated HMO reduce C. jejuni attachment to 2′-fucosylated glycans on epithelial cells (15). These results were confirmed ex vivo showing that preincubation of C. jejuni with pooled HMO or 2′-fucosyllactose alone reduced colonization on freshly isolated human intestinal mucosa. In vivo, C. jejuni colonization was significantly reduced in mice that were orally gavaged with pooled HMO. In addition, intestinal clearance of C. jejuni was significantly faster in mouse pups nursing on transgenic dams that secreted 2′-fucosyllactose, which is normally not present in mouse milk. The combination of these in vitro, ex vivo, and in vivo studies showed that 2′-fucosylated HMO in general and 2′-fucosyllactose alone serve as decoy receptors to inhibit C. jejuni attachment and colonization.
The physiological relevance of these results was later confirmed in 93 breastfeeding mother-infant pairs (16). The total incidence of diarrhea (given per 100 child months) was significantly lower in infants that received intermediate to high levels of α1–2-fucosylated HMO with their mother’s milk. More specifically, Campylobacter diarrhea occurred less often in infants who received high levels of 2′-fucosyllactose with the mother’s milk, which was consistent with previous reports that 2′-fucosylated HMO can block C. jejuni attachment in vitro and in mice. In addition, Calicivirus diarrhea occurred less often when the milk contained high levels of lacto-N-difucohexaose, another α1–2-fucosylated HMO.
α1–2-Fucosylated HMO may not always be the most effective
In contrast to the previous example where α1–2-fucosylated HMO block C. jejuni attachment and prevent disease, α1–2-fucosylation may actually counteract the benefits of HMO in the context of another type of enteric infection, as our laboratory recently showed (17). In addition to certain bacteria and viruses, protozoan parasites like Toxiplasma gondi or Entamoeba histolytica also employ lectins as virulence factors to attach to the host’s intestinal epithelial cell surface. E. histolytica causes amebiasis, the 3rd leading cause of death by parasitic diseases after malaria and schistosomiasis (18). Approximately 50 million people are infected with this parasite worldwide, which results in nearly 100,000 deaths each year. E. histolytica is ingested as a dormant cyst that survives the harsh environment in the host’s stomach and small intestine and reaches the colon, where it differentiates into mobile trophozoites (18). E. histolytica employs the Gal/GalNAc lectin to attach to glycans on the host’s epithelium. But the lectin is also involved in the killing and phagocytosis of the host’s intestinal epithelial cells and is considered one of the parasite’s major virulence factors (19, 20). The lectin binds to Gal, GalNAc, and lactose, which can block E. histolytica attachment and cytotoxicity in vitro (21). In vivo, however, these mono- and disaccharides may not be able to provide a physiologically relevant protection. The majority of the mono- and disaccharides have already been digested and absorbed in the small intestine long before the parasite reaches the colon and differentiates from its protected cyst stage into the active trophozoite. The E. histolytica lectin rarely encounters the sugars that had been known to inhibit attachment and cytotoxicity. Guided by epidemiological data that breast-fed infants are at lower risk to acquire E. histolytica infections (22), we hypothesized that HMO also block the lectin and reduce E. histolytica attachment and cytotoxicity. If confirmed, blocking the lectin with HMO would be physiologically more relevant, because HMO reach the colon undigested and thus actually colocalize with the parasite’s active trophozoite form. In addition, HMO contain Gal, which might facilitate an interaction with the Gal/GalNAc lectin. Our preliminary data strongly support this hypothesis (17). HMO isolated from pooled human milk blocked E. histolytica attachment to the surface of culture vials as well as human intestinal epithelial cells, which were also protected from E. histolytica-induced cytotoxicity.
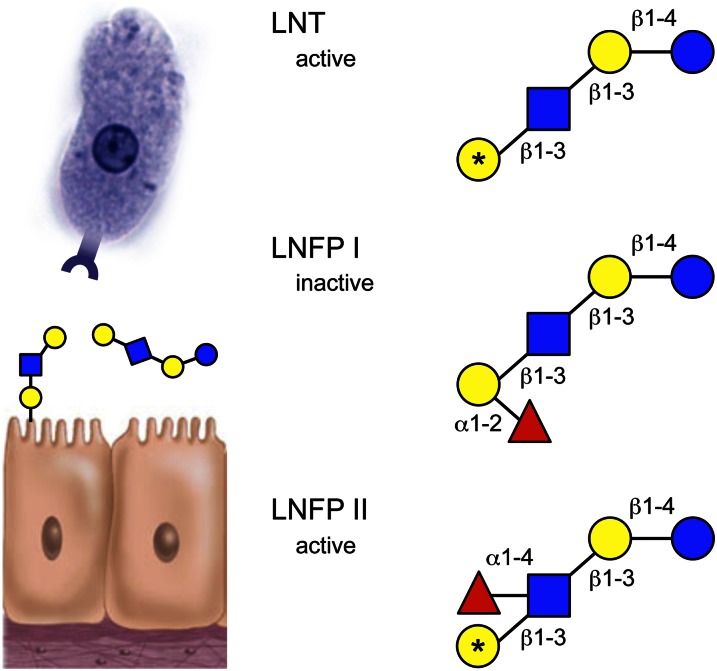
We then used individual, commercially available HMO to determine which HMO are responsible for these protective effects and whether there is a certain structural motif required to confer protection. Although none of the fucosylated or sialylated milk trisaccharides had an effect, lacto-N-tetraose blocked attachment and cytotoxicity at physiologically relevant concentrations. To our surprise, LNFP I had no effect, even at very high concentrations. LNFP II and III, however, were effective. Although LNFP I, II, and III are all monofucosylated LN(n)T isomers, only LNFP I carries its Fuc in an α1–2 linkage at the terminal Gal. LNFP II and III carry their Fuc in an α1–4 and α1–3 linkage, respectively, at the subterminal GlcNAc (Fig. 4). We speculated that the “Fuc cap” masks the terminal Gal and that an uncovered terminal Gal is essential to interact with the E. histolytica lectin. Removal of the terminal α1–2-linked Fuc with a linkage-specific fucosidase indeed restored HMO activity, which led us to conclude that some HMO reduce E. histolytica attachment and cytotoxicity, but only if the HMO are not α1–2-fucosylated. If these results translated to human infants, the milk of Secretor women with high relative abundance of α1–2-fucosylated HMO would potentially be less effective in preventing E. histolytica infections. These findings would be in sharp contrast to previous studies showing that the milk of Secretor women lowers the incidence of infections with C. jejuni or Calicivirus (15, 16). The antiadhesive antimicrobial effects of HMO depend on HMO structure but also on the pathogens the infant is exposed to. Field studies in regions with high E. histolytica incidence will be needed to confirm this hypothesis.
Figure 4.
LNT and LNFP II, but not LNFP I, block E. histolytica attachment and cytotoxicity. The protozoan parasite E. histolytica uses a lectin as a major virulence factor that facilitates attachment to and killing and phagocytosis of the host’s intestinal epithelial cells. Whereas LNT reduces E. histolytica attachment and cytotoxicity, LNFP I has no effect, because the terminal Gal is covered by α1–2-linked Fuc. The structural isomer LNFP II does not contain a terminal “Fuc cap.” Instead, Fuc is linked α1–4 to the subterminal GalNAc and the terminal Gal (indicated with an asterisk) is available for binding to the E. histolytica lectin. Fuc, fucose; Gal, galactose; HMO, human milk oligosaccharide; LNFP, lacto-N-fucopentaose; LNT, lacto-N-tetraose.
Fucosylation is required but not sufficient to block selectins
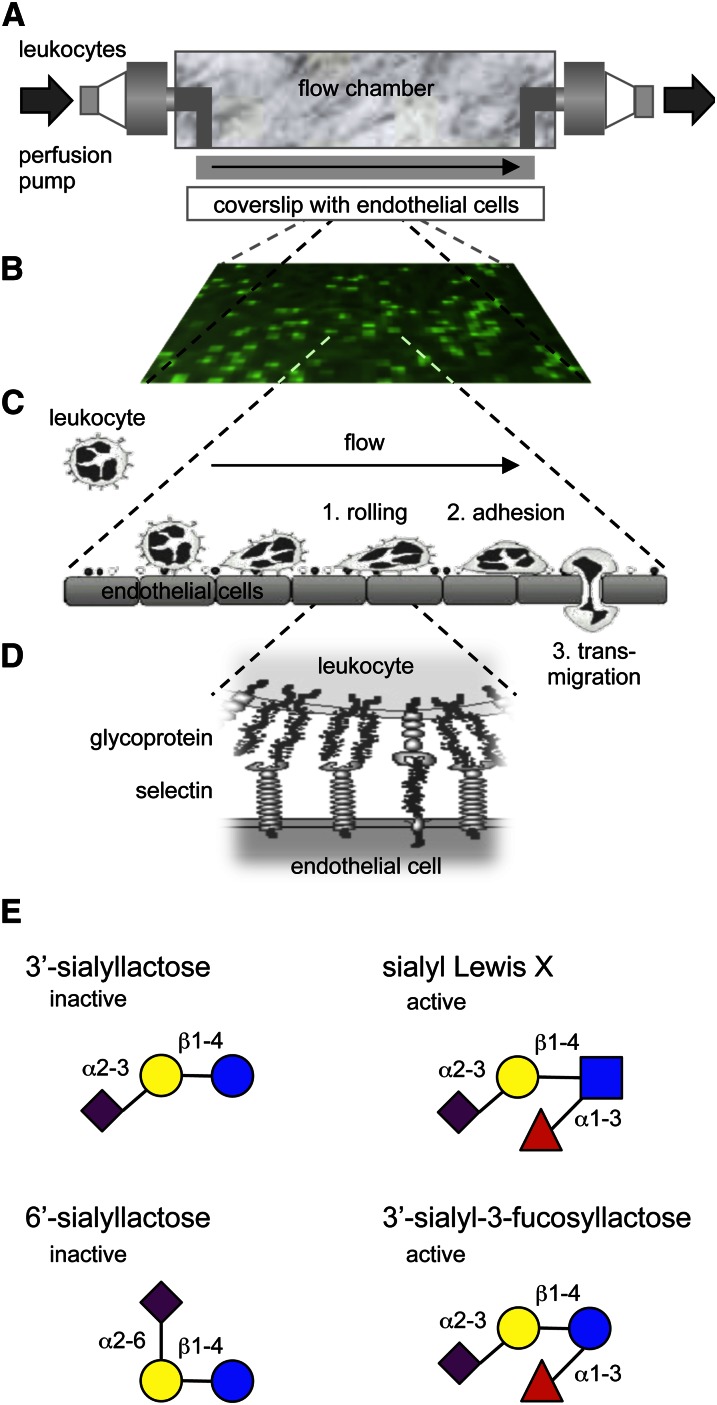
Although the presence or absence of Fuc appears to determine whether individual HMO are protective against some of the enteric pathogens, Fuc is an essential but not sufficient constituent of HMO that interfere with selectin-mediated cell-cell interactions in the host’s immune system. Selectins are C-type lectins (23) that facilitate the initial step of leukocyte extravasation at sites of inflammation (24, 25). Inflammatory mediators induce the expression of E- and P-selectin on the apical surface of endothelial cells. The selectins protrude into the blood vessel lumen and interact with glycoconjugate ligands on leukocytes that pass by in the bloodstream (24, 25). These initial selectin-ligand interactions decelerate the leukocytes and they start rolling over the endothelial surface before they tightly adhere and finally transmigrate to reach subendothelial regions (24, 25). Based on reports that sialyl-Lewis x (sialylated and fucosylated N-acetyllactosamine) is the minimum glycan epitope required to facilitate selectin-ligand interactions, Kunz and Rudloff were the first to hypothesize that HMO interfere with leukocyte rolling and potentially reduce leukocyte extravasation. HMO contain Lewis epitopes (26) and were at least indirectly shown to reach the systemic circulation (27–29). We used an in vitro parallel flow chamber model with human leukocytes passing over primary human endothelial cells and confirmed that sialylated but not nonsialylated HMO reduced leukocyte rolling and adhesion (30) (Fig. 5). In addition, we used individual, commercially available HMO to determine the minimum structural requirements for HMO to be effective. The physiological epitope sialyl-Lewis x (3′-sialyl-3-fucosyllactosamine) but also 3′-sialyl-3-fucosyllactose reduced leukocyte rolling and adhesion. The only difference between sialyl-Lewis x and 3′-sialyl-3-fucosyllactose is that their reducing end monosaccharide is either GlcNAc or Glc. However, the acetylamine at the C2 position of GlcNAc appears irrelevant for HMO to interact with selectins. The nonfucosylated HMO 3′-sialyllactose and 6′-sialyllactose had no effect on leukocyte rolling and adhesion, which was consistent with previous reports that Fuc is an essential part of selectin ligand epitopes. A genetic defect in the intracellular Fuc metabolism in patients with Leukocyte Adhesion Deficiency II (Congenital Disorder of Glycosylation IIc) significantly reduces the fucosylation of cell surface glycans, which include selectin ligands (31–34). As a consequence, leukocyte motility and extravasation are impaired and lead to recurrent infections. The molecular etiology of this rare inherited glycosylation disorder demonstrates the importance of fucosylation for selectin-mediated cell-cell interactions in the immune system and provides an explanation for why some HMO reduce leukocyte rolling and adhesion while others are ineffective. It is important to note that the fraction of pooled sialylated HMO was more effective than the sialylated and fucosylated tetrasaccharides sialyl-Lewis x and 3′-sialyl-3-fucosyllactose alone. We speculate that some of the more complex HMO carry more than one sialyl-Lewis x epitope and facilitate multivalent binding to selectins. Thus, the more complex HMO might be of physiological relevance even though their relative abundance in human milk is lower than the less complex tri- and tetrasaccharides.
Figure 5.
In vitro flow model reveals specific sialylated and fucosylated HMO that reduce leukocyte rolling and adhesion. (A) Leukocytes isolated from human blood were pumped over coverslips with cultured human umbilical vein endothelial cells. (B) Leukocytes (light green) that were rolling over or adhered to endothelial cells (dark green) were counted from video-microscope images. (C) The leukocyte extravasation cascade starts with the deceleration of leukocytes from the blood stream. After this initial rolling, leukocytes tightly adhere to the endothelium and eventually transmigrate to subendothelial regions. (D) Leukocyte rolling is mediated by selectins and their glycoprotein ligands. (E) Examples of HMO that reduce leukocyte rolling and adhesion and HMO that had no effect. HMO, human milk oligosaccharide.
Comparable results were obtained when we studied the effects of HMO on another selectin-mediated cell-cell interaction, the formation of platelet-neutrophil complexes (35). Activated platelets increase their expression of P-selectin, which binds to the glycoprotein PSGL-1 on neutrophils (36). PSGL-1 then triggers a signaling cascade that leads to neutrophil activation with an increase in reactive oxygen production, phagocytosis activity, and expression of adhesion molecules that facilitate neutrophil extravasation (37, 38). Our ex vivo studies with human platelets and neutrophils confirmed that only sialylated HMO reduce platelet-neutrophil complexes formation and neutrophil activation, that HMO fucosylation was required, and that complex HMO were more effective than the monovalent tetrasaccharides sialyl-Lewis x and 3′-sialyl-3-fucosyllactose (35).
The combined results from our in vitro and ex vivo studies on selectin-mediated leukocyte rolling, adhesion, and activation show that only very specific HMO interfere with these cell-cell interactions in the immune system. It remains unclear whether these observations translate to in vivo, whether they are physiologically relevant to human infants, and whether a reduction in leukocyte extravasation and activation has positive or negative consequences for the breast-fed infant.
Not 1 but 2 sialic acids are required to protect from NEC
Mucosal neutrophil infiltration is one of the hallmarks of NEC (39), one of the most common and often fatal intestinal disorders in preterm infants (40–42). More than 5% of all very-low–birth weight infants develop NEC (43). About one-quarter of them die and the survivors often face long-term neurological complications (44). Based on the importance of neutrophil infiltration in NEC pathogenesis and our findings that HMO inhibit leukocyte rolling, adhesion, and activation, we hypothesized that HMO reduce mucosal neutrophil infiltration and prevent NEC. If confirmed, it could provide one explanation for the intriguing finding that breast-fed infants are at a 6- to 10-fold lower risk to develop NEC than formula-fed infants that do not receive HMO with their formula (45–49).
Intervention studies in human preterm infants to test this hypothesis are unfeasible for several reasons. Hundreds of very-low–birth weight, preterm infants would need to be recruited to power the study. NEC usually develops within the first 2–4 wk postpartum and during this period, infants would need to be fed with formula that either contains or does not contain HMO. Formula feeding is a known risk factor for NEC, which raises ethical concerns for this kind of intervention study. In addition, the amount of HMO needed to feed hundreds of infants with HMO-supplemented formula for several weeks far exceeds the amount of currently available HMO. Instead, we used an established neonatal rat NEC model (50–53) to test the hypothesis that HMO reduce NEC incidence (54). Rat pups were separated from the dam immediately after birth and housed in a temperature- and humidity-controlled environment. Pups were orally gavaged with formula that resembled the caloric and protein content of rat milk. Some of the pups were left with the dam to serve as breast-fed controls. All rat pups were exposed to hypoxia for 10 min thrice per day and killed on day-of-life 4 to collect their intestines for macroscopic and microscopic evaluation. Whereas all of the breast-fed pups survived, almost 25% of the formula-fed pups died before reaching the end of the 4-d study period. Most remarkably, the addition of pooled HMO to the very same formula increased the survival rate to almost 100%. Evaluation of H&E-stained ileum sections showed a significant increase in pathology scores in formula-fed pups compared to breast-fed pups. Pathology scores for the HMO-fed pups were significantly lower than those in the formula-fed group and similar to those in the breast-fed group. Additional experiments showed that HMO needed to be fed to the pups during the first 24 h postpartum to be protective against NEC. However, exposure to HMO for the first 24 h alone was not sufficient.
In the next set of experiments, we asked whether all HMO are beneficial or whether there are specific structural requirements for HMO to protect against NEC. Instead of testing dozens of individual HMO alone, we used a 2-dimensional approach to identify the most effective group of HMO starting from pooled HMO. In the first dimension, we used anion exchange chromatography and separated the pooled HMO by charge. This first step generated 5 different HMO fractions with oligosaccharides that contained either no sialic acid (neutral HMO) or contained 1, 2, 3, or 4 sialic acids, respectively. We then tested the different HMO fractions in the rat NEC model and found that the disialylated HMO fraction was most effective in reducing NEC pathology scores. In the second dimension, we used gel filtration chromatography to separate the disialylated HMO fraction by size, tested these HMO subfractions in the rat model, and found that one particular HMO subfraction reduced NEC pathology scores. In a combination of matrix-assisted laser desorption/ionization time-of-flight MS analysis, HPLC analysis after sequential exoglycosidase digestions as well as GC-MS linkage analysis of partially methylated alditol acetates, we were able to unambiguously characterize the protective HMO as a specific isomer of DSLNT. Digests with linkage-specific neuraminidases revealed that both sialic acids are critical for the HMO to be protective against NEC. It is important to note that DSLNT is not fucosylated, which makes it unlikely that HMO prevent NEC by reducing selectin-mediated neutrophil extravasation and activation as originally hypothesized. In fact, we did not observe any difference in mucosal neutrophil infiltration in our rat model. At this point, it is unknown whether DSLNT interacts with other host lectins like Siglecs, which specifically bind to sialylated glycans (55), or whether DSLNT interacts with intestinal bacteria, which could indirectly protect the host from NEC. It is also important to note that GOS, which are currently added to some of the commercially available infant formula as a prebiotic, had no effect on survival or pathology scores. Overall, the protective effects of HMO in the context of NEC appear to be highly structure-dependent.
Although it was unfeasible to conduct a human intervention study to test our hypothesis that HMO protect against NEC, the rat model also clearly has its limitations (56) and it needs to be confirmed whether or not the results translate to human preterm infants. If confirmed, DSLNT could become a valuable supplement to prevent NEC in formula-fed infants. But our results could also benefit breast-fed infants, because the DSLNT concentration in the mother’s milk could become a noninvasive biomarker to identify breast-fed infants at risk to develop NEC.
Infant formula oligosaccharides are structurally different
The emerging evidence for the biological effects of HMO on the breast-fed infant created a rationale to add oligosaccharides to infant formula to provide formula-fed infants with the same benefits that breast-fed infants receive with their mother’s milk. The challenge has been to identify ingredients that provide HMO. Some HMO are also found in the milk of other mammals but usually at much lower concentrations. Other HMO are unique to human milk, especially the ones with more complex structures. In other words, HMO were simply not available to be added to infant formula. The search for alternatives led to GOS and Fructooligosaccharides (FOS) (Fig. 6), which are synthesized by bacteria and plants, respectively. It is important to note that Gal and fructose oligomers do not naturally occur in human milk. In fact, the fructose monomer itself is not found in human milk. Despite their structural differences compared to HMO, ingestion of GOS and FOS has been reported to influence the microbiota composition in the infant’s feces and provide other benefits like softer stools. A defined mixture of GOS and FOS has been shown to reduce the incidence of atopic dermatitis during the first 6 mo of life (57) and reduce the incidence of allergic manifestations and infections during the first 2 y of life (58). The long-term health benefits or risks of providing infants with a significant amount of these nonhuman milk glycans have not yet been described.
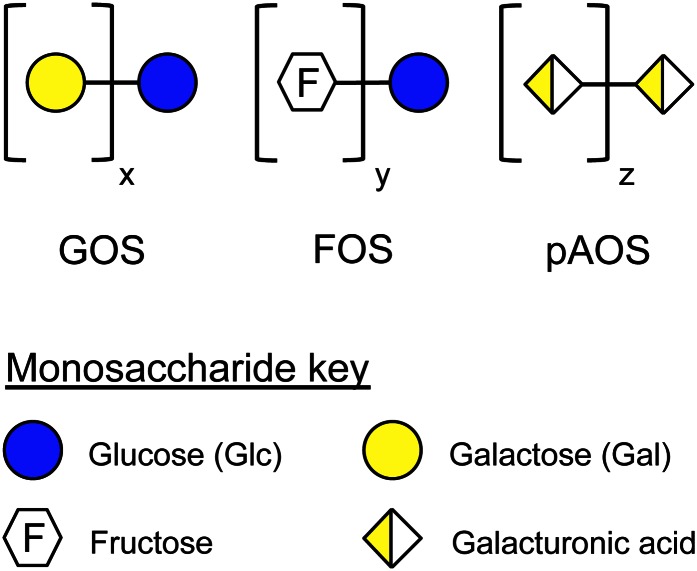
Figure 6.
Infant formula oligosaccharides are structurally different from HMO. GOS and FOS are currently added to infant formula. pAOS are currently studied as novel ingredients to mimic some of the effects of HMO. GOS, FOS, and pAOS are structurally very different than HMO and are not present in human milk. Fructose or its polymers as well as galacturonic acid and its homo- or heteropolymers are not found in human milk. In contrast, Fuc and sialic acid are important components of HMO, but they are not part of any of the infant formula oligosaccharides. FOS, fructooligosaccharide; Fuc, fucose; GOS, galactooligosaccharide; HMO, human milk oligosaccharide; pAOS, pectin-derived acidic oligosaccharide.
Although some HMO are sialylated, GOS and FOS do not contain any sialic acid at all. The negatively charged carboxyl-group of sialic acid is critical for some of the HMO effects as outlined above, and it seems most unlikely that GOS or FOS can mimic these effects due to their lack of sialic acid. In an attempt to more closely resemble the composition of oligosaccharides naturally occurring in human milk, a pectin hydrolysate consistent of galacturonic acid oligomers has recently been studied as an additional infant formula oligosaccharide (59–61). Although galacturonic acid provides a negatively charged carboxyl-group, its overall structure is very different from sialic acid, and it is not surprising that the inclusion of these pAOS in a formula containing a mixture of GOS and FOS did not add specific advantages to the formula in terms of stool viscosity, frequency, pH as well as feeding tolerance (61). Whether pAOS have an effect as antiadhesive antimicrobials, protect against NEC, or have any other benefits has not been reported. Short- and long-term adverse effects of introducing nonhuman milk galacturonic oligomers in early infant feeding have not been studied or at least not been reported.
In the context of structure-function relationships, it is important to emphasize that GOS, FOS pAOS, and other “prebiotic” glycans are structurally very different from those oligosaccharides that naturally occur in human milk. Because most of the biological effects described for HMO are structure-specific, it seems unlikely that structurally inconsistent oligosaccharides can provide the very same variety of health benefits as HMO.
Lately, several companies have been able to synthesize individual HMO tri- and tetrasaccharides on a large scale. These oligosaccharides resemble some of the smaller and most abundant HMO naturally occurring in human milk, which now become available for in vitro and in vivo experiments as well as clinical studies. The structurally more complex HMO, however, remain unavailable.
Although the synthesis of individual HMO is a very significant step away from the nonhuman oligosaccharides toward the naturally occurring oligosaccharides, their potential use in infant formula raises additional questions. Which HMO should be added and at what concentrations? If we followed the concept of modeling infant formula after the “gold standard” human milk, which human milk would we use to guide us, and what would be the consequences for the formula-fed infant? For example, infants fed by Nonsecretor mothers receive very little or no 2′-fucosyllactose and other 2′-fucosylated HMO. What were the potential consequences if these infants now received a formula with a generic mixture of HMO that contains substantial amounts of 2′-fucosyllactose? In other words, how important is the match between the HMO composition in the mother’s milk and the infant’s HMO “requirements”? Although intriguing to ask, these questions are not new. They also arise for infants that receive donor milk instead of their mother’s milk. A batch of donor milk, either from single or multiple donors, is often distributed to more than one infant in the unit and a match to the mother’s HMO profile rather unlikely. Whether or not an infant benefits from receiving “matched” oligosaccharides from its own mother’s milk remains unclear.
Summary and Conclusion
Over the past decades, more than 100 structurally distinct oligosaccharides have been identified in human milk. Accumulating evidence suggests that these HMO benefit the breast-fed infant, protect from infections and other diseases, and may contribute to brain development and cognition (62). Most of these beneficial effects depend on specific HMO structures or epitopes. Most studies on structure-function relationships of HMO distinguish at least between nonsialylated (neutral) and sialylated (acidic) HMO. In addition, some studies use individual, commercially available HMO to determine structural requirements (30, 35, 63, 64). Other studies start out with pooled HMO and use multi-dimensional chromatography to narrow the effects down to structurally similar groups (15) or even structurally distinct individual HMO as in the case of NEC (54).
Oligosaccharides currently added to most infant formula are structurally different from those naturally occurring in human milk. Therefore, it seems most unlikely that these non-HMO are able to mimic the structure-specific effects of HMO. The trend appears to go from using non-HMO to synthesizing oligosaccharides that are actually present in human milk. Future preclinical and clinical studies will reveal whether or not the currently available tri- and tetrasaccharides are indeed beneficial. In the end, human milk contains not one or just a handful of tri- and tetrasaccharides, but more than 100 structurally complex and distinct HMO. A better understanding of how HMO are synthesized in the human mammary gland may provide valuable information to guide new technologies to produce a diverse array of complex HMO that most closely resembles the oligosaccharide composition in human milk. Ultimately, breastfeeding remains the number one choice to nourish and nurture our infants.
Acknowledgments
Both authors have read and approved the final manuscript.
Footnotes
Published in a supplement to Advances in Nutrition. Presented at the conference “The First International Conference on Glycobiology of Human Milk Oligosaccharides” held in Copenhagen, Denmark, May 16–17, 2011. The conference was supported by Glycom A/S, Lyngby, Denmark. The supplement coordinators for this supplement were Clemens Kunz, University of Giessen, Germany, and Sharon M. Donovan, University of Illinois, Urbana, IL. Supplement Coordinator disclosures: Sharon Donovan has received human milk oligosaccharides from Glycom through their HMO biology donations program. Clemens Kunz has received human milk oligosaccharides from Glycom through their HMO biology donation program. The supplement is the responsibility of the Guest Editor to whom the Editor of Advances in Nutrition has delegated supervision of both technical conformity to the published regulations of Advances in Nutrition and general oversight of the scientific merit of each article. The Guest Editor for this supplement was Mark A. McGuire, University of Idaho. Guest Editor disclosure: Mark A. McGuire consults with Abbott Nutrition, a division of Abbott Laboratories, and provides professional opinions on matters related to human lactation and infant nutrition. Further, he collaborates with Dr. Lars Bode, University of California, San Diego, in research related to human milk oligosaccharides. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
Supported in part by NIH K99/R00 DK078668 (L.B.) and a Max Kade Koepfe Fellowship (E.J-K.).
Author disclosures: L. Bode and E. Jantscher-Krenn, no conflicts of interest.
Abbreviations used: DSLNT, disialyllacto-N-tetraose; FOS, fructooligosaccharide; Fuc, fucose; Gal, galactose; Glc, glucose; GlcNAc, N-acetyl-glucosamine; GOS, galactooligosaccharide; HMO, human milk oligosaccharide; LNFP, lacto-N-fucopentaose; NEC, necrotizing enterocolitis; pAOS, pectin-derived acidic oligosaccharide.
Literature Cited
- 1.Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr. 2000;20:699–722 [DOI] [PubMed] [Google Scholar]
- 2.Kobata A. Structures and application of oligosaccharides in human milk. Proc Jpn Acad, Ser B, Phys Biol Sci. 2010;86:731–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.German JB, Freeman SL, Lebrilla CB, Mills DA. Human milk oligosaccharides: evolution, structures and bioselectivity as substrates for intestinal bacteria. Nestle Nutr Workshop Ser Pediatr Program. 2008;62:205–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bode L. Recent advances on structure, metabolism, and function of human milk oligosaccharides. J Nutr. 2006;136:2127–30 [DOI] [PubMed] [Google Scholar]
- 5.Kumazaki T, Yoshida A. Biochemical evidence that secretor gene, Se, is a structural gene encoding a specific fucosyltransferase. Proc Natl Acad Sci USA. 1984;81:4193–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thurl S, Munzert M, Henker J, Boehm G, Muller-Werner B, Jelinek J, Stahl B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br J Nutr. 2010;104:1261–71 [DOI] [PubMed] [Google Scholar]
- 7.Thurl S, Henker J, Siegel M, Tovar K, Sawatzki G. Detection of four human milk groups with respect to Lewis blood group dependent oligosaccharides. Glycoconj J. 1997;14:795–9 [DOI] [PubMed] [Google Scholar]
- 8.Egge H, Dell A, Von Nicolai H. Fucose containing oligosaccharides from human milk. I. Separation and identification of new constituents. Arch Biochem Biophys. 1983;224:235–53 [DOI] [PubMed] [Google Scholar]
- 9.Coppa GV, Gabrielli O, Zampini L, Galeazzi T, Ficcadenti A, Padella L, Santoro L, Soldi S, Carlucci A, Bertino E, et al. Oligosaccharides in 4 different milk groups, Bifidobacteria, and Ruminococcus obeum. J Pediatr Gastroenterol Nutr. 2011;53:80–7 [DOI] [PubMed] [Google Scholar]
- 10.Johnson PH, Watkins WM. Purification of the Lewis blood-group gene associated alpha-3/4-fucosyltransferase from human milk: an enzyme transferring fucose primarily to type 1 and lactose-based oligosaccharide chains. Glycoconj J. 1992;9:241–9 [DOI] [PubMed] [Google Scholar]
- 11.Gabrielli O, Zampini L, Galeazzi T, Padella L, Santoro L, Peila C, Giuliani F, Bertin E, Fabris C, Coppa GV. Preterm milk oligosaccharides during the first month of lactation. Pediatrics. 2011;128:e1520–31 [DOI] [PubMed] [Google Scholar]
- 12.Samuel MC, Vugia DJ, Shallow S, Marcus R, Segler S, McGivern T, Kassenborg H, Reilly K, Kennedy M, Angulo F, et al. Epidemiology of sporadic Campylobacter infection in the United States and declining trend in incidence, FoodNet 1996–1999. Clin Infect Dis. 2004; 15(38 Suppl 3):S165–74 [DOI] [PubMed] [Google Scholar]
- 13.Marcus R. New information about pediatric foodborne infections: the view from FoodNet. Curr Opin Pediatr. 2008;20:79–84 [DOI] [PubMed] [Google Scholar]
- 14.Fullerton KE, Ingram LA, Jones TF, Anderson BJ, McCarthy PV, Hurd S, Shiferaw B, Vugia D, Haubert N, Hayes T, et al. Sporadic campylobacter infection in infants: a population-based surveillance case-control study. Pediatr Infect Dis J. 2007;26:19–24 [DOI] [PubMed] [Google Scholar]
- 15.Ruiz-Palacios GM, Cervantes LE, Ramos P, Chavez-Munguia B, Newburg DS. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J Biol Chem. 2003;278:14112–20 [DOI] [PubMed] [Google Scholar]
- 16.Morrow AL, Ruiz-Palacios GM, Altaye M, Jiang X, Guerrero ML, Meinzen-Derr JK, Farkas T, Chaturvedi P, Pickering LK, Newburg DS. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J Pediatr. 2004;145:297–303 [DOI] [PubMed] [Google Scholar]
- 17.Jantscher-Krenn E, Lauwaet T, Bliss LA, Reed SL, Gillin FD, Bode L. Human milk oligosaccharides reduce Entamoeba histolytica attachment and cytotoxicity in vitro. Br J Nutr. Epub 2012 Jan 23 [DOI] [PubMed] [Google Scholar]
- 18.Pritt BS, Clark CG. Amebiasis. Mayo Clin Proc. 2008;83:1154–9 [DOI] [PubMed] [Google Scholar]
- 19.Saffer LD, Petri WA., Jr Entamoeba histolytica: recognition of alpha- and beta-galactose by the 260-kDa adherence lectin. Exp Parasitol. 1991;72:106–8 [DOI] [PubMed] [Google Scholar]
- 20.Saffer LD, Petri WA., Jr Role of the galactose lectin of Entamoeba histolytica in adherence-dependent killing of mammalian cells. Infect Immun. 1991;59:4681–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cano-Mancera R, Lopez-Revilla R. Inhibition of the adhesion of Entamoeba histolytica trophozoites to human erythrocytes by carbohydrates. Parasitol Res. 1987;74:18–22 [DOI] [PubMed] [Google Scholar]
- 22.Islam A, Stoll BJ, Ljungstrom I, Biswas J, Nazrul H, Huldt G. The prevalence of Entamoeba histolytica in lactating women and in their infants in Bangladesh. Trans R Soc Trop Med Hyg. 1988;82:99–103 [DOI] [PubMed] [Google Scholar]
- 23.Cummings RD, McEver RP. C-type lectins. : Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, et al., Essentials of glycobiology. 2nd ed. New York: Cold Spring Harbor Laboratory Press, 2009 [PubMed] [Google Scholar]
- 24.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14 [DOI] [PubMed] [Google Scholar]
- 25.Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 1990;62:3–6 [DOI] [PubMed] [Google Scholar]
- 26.Rudloff S, Stefan C, Pohlentz G, Kunz C. Detection of ligands for selectins in the oligosaccharide fraction of human milk. Eur J Nutr. 2002;41:85–92 [DOI] [PubMed] [Google Scholar]
- 27.Rudloff S, Pohlentz G, Diekmann L, Egge H, Kunz C. Urinary excretion of lactose and oligosaccharides in preterm infants fed human milk or infant formula. Acta Paediatr. 1996;85:598–603 [DOI] [PubMed] [Google Scholar]
- 28.Rudloff S, Pohlentz G, Borsch C, Lentze MJ, Kunz C. Urinary excretion of in vivo 13C-labelled milk oligosaccharides in breastfed infants. Br J Nutr. Epub 2011 Sept 5:1–7 [DOI] [PubMed] [Google Scholar]
- 29.Obermeier S, Rudloff S, Pohlentz G, Lentze MJ, Kunz C. Secretion of 13C-labelled oligosaccharides into human milk and infant's urine after an oral [13C]galactose load. Isotopes Environ Health Stud. 1999;35:119–25 [DOI] [PubMed] [Google Scholar]
- 30.Bode L, Kunz C, Muhly-Reinholz M, Mayer K, Seeger W, Rudloff S. Inhibition of monocyte, lymphocyte, and neutrophil adhesion to endothelial cells by human milk oligosaccharides. Thromb Haemost. 2004;92:1402–10 [DOI] [PubMed] [Google Scholar]
- 31.Lühn K, Wild MK, Eckhardt M, Gerardy-Schahn R, Vestweber D. The gene defective in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat Genet. 2001;28:69–72 [DOI] [PubMed] [Google Scholar]
- 32.Lübke T, Marquardt T, Etzioni A, Hartmann E, von Figura K, Korner C. Complementation cloning identifies CDG-IIc, a new type of congenital disorders of glycosylation, as a GDP-fucose transporter deficiency. Nat Genet. 2001;28:73–6 [DOI] [PubMed] [Google Scholar]
- 33.Etzioni A, Frydman M, Pollack S, Avidor I, Phillips ML, Paulson JC, Gershoni-Baruch R. Brief report: recurrent severe infections caused by a novel leukocyte adhesion deficiency. N Engl J Med. 1992;327:1789–92 [DOI] [PubMed] [Google Scholar]
- 34.Becker DJ, Lowe JB. Leukocyte adhesion deficiency type II. Biochim Biophys Acta. 1999;1455:193–204 [DOI] [PubMed] [Google Scholar]
- 35.Bode L, Rudloff S, Kunz C, Strobel S, Klein N. Human milk oligosaccharides reduce platelet-neutrophil complex formation leading to a decrease in neutrophil beta 2 integrin expression. J Leukoc Biol. 2004;76:820–6 [DOI] [PubMed] [Google Scholar]
- 36.Evangelista V, Manarini S, Sideri R, Rotondo S, Martelli N, Piccoli A, Totani L, Piccardoni P, Vestweber D, de Gaetano G, et al. Platelet/polymorphonuclear leukocyte interaction: P-selectin triggers protein-tyrosine phosphorylation-dependent CD11b/CD18 adhesion: role of PSGL-1 as a signaling molecule. Blood. 1999;93:876–85 [PubMed] [Google Scholar]
- 37.Peters MJ, Heyderman RS, Hatch DJ, Klein NJ. Investigation of platelet-neutrophil interactions in whole blood by flow cytometry. J Immunol Methods. 1997;209:125–35 [DOI] [PubMed] [Google Scholar]
- 38.Peters MJ, Dixon G, Kotowicz KT, Hatch DJ, Heyderman RS, Klein NJ. Circulating platelet-neutrophil complexes represent a subpopulation of activated neutrophils primed for adhesion, phagocytosis and intracellular killing. Br J Haematol. 1999;106:391–9 [DOI] [PubMed] [Google Scholar]
- 39.Stefanutti G, Lister P, Smith VV, Peters MJ, Klein NJ, Pierro A, Eaton S. P-selectin expression, neutrophil infiltration, and histologic injury in neonates with necrotizing enterocolitis. J Pediatr Surg. 2005;40:942–7 [DOI] [PubMed] [Google Scholar]
- 40.Uauy RD, Fanaroff AA, Korones SB, Phillips EA, Phillips JB, Wright LL. Necrotizing enterocolitis in very low birth weight infants: biodemographic and clinical correlates. National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1991;119:630–8 [DOI] [PubMed] [Google Scholar]
- 41.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364:255–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holman RC, Stoll BJ, Clarke MJ, Glass RI. The epidemiology of necrotizing enterocolitis infant mortality in the United States. Am J Public Health. 1997;87:2026–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holman RC, Stoll BJ, Curns AT, Yorita KL, Steiner CA, Schonberger LB. Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr Perinat Epidemiol. 2006;20:498–506 [DOI] [PubMed] [Google Scholar]
- 44.Rees CM, Pierro A, Eaton S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2007;92:F193–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawoger R, Kiechl-Kohlendorfer U, Chan GM, Blanco CL, Abrams S, Cotton CM, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. 2010;156:562–7 e1 [DOI] [PubMed] [Google Scholar]
- 46.Sisk PM, Lovelady CA, Dillard RG, Gruber KJ, O'Shea TM. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J Perinatol. 2007;27:428–33 [DOI] [PubMed] [Google Scholar]
- 47.Schanler RJ, Shulman RJ, Lau C. Feeding strategies for premature infants: beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics. 1999;103:1150–7 [DOI] [PubMed] [Google Scholar]
- 48.Schanler RJ, Lau C, Hurst NM, Smith EO. Randomized trial of donor human milk versus preterm formula as substitutes for mothers’ own milk in the feeding of extremely premature infants. Pediatrics. 2005;116:400–6 [DOI] [PubMed] [Google Scholar]
- 49.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336:1519–23 [DOI] [PubMed] [Google Scholar]
- 50.Upperman JS, Potoka D, Grishin A, Hackam D, Zamora R, Ford HR. Mechanisms of nitric oxide-mediated intestinal barrier failure in necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:159–66 [DOI] [PubMed] [Google Scholar]
- 51.Nadler EP, Dickinson E, Knisely A, Zhang XR, Boyle P, Beer-Stolz D, Watkins SC, Ford HR. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res. 2000;92:71–7 [DOI] [PubMed] [Google Scholar]
- 52.Guner YS, Franklin AL, Chokshi NK, Castle SL, Pontarelli E, Wang J, Prasadarao NV, Upperman JS, Grishin AV, Ford HR. P-glycoprotein induction by breast milk attenuates intestinal inflammation in experimental necrotizing enterocolitis. Lab Invest. 2011;91:1668–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barlow B, Santulli TV. Importance of multiple episodes of hypoxia or cold stress on the development of enterocolitis in an animal model. Surgery. 1975;77:687–90 [PubMed] [Google Scholar]
- 54.Jantscher-Krenn E, Zherebtsov M, Nissan C, Goth K, Guner YS, Naidu N, Choudhury B, Grishin AV, Ford HR, Bode L. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut. Epub 2011 Dec 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–66 [DOI] [PubMed] [Google Scholar]
- 56.Sodhi C, Richardson W, Gribar S, Hackam DJ. The development of animal models for the study of necrotizing enterocolitis. Dis Model Mech. 2008;1:94–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moro G, Arslanoglu S, Stahl B, Jelinek J, Wahn U, Boehm G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child. 2006;91:814–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arslanoglu S, Moro GE, Schmitt J, Tandoi L, Rizzardi S, Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr. 2008;138:1091–5 [DOI] [PubMed] [Google Scholar]
- 59.Westerbeek EA, van den Berg A, Lafeber HN, Fetter WP, van Elburg RM. The effect of enteral supplementation of a prebiotic mixture of non-human milk galacto-, fructo- and acidic oligosaccharides on intestinal permeability in preterm infants. Br J Nutr. 2011;105:268–74 [DOI] [PubMed] [Google Scholar]
- 60.Westerbeek EA, Morch E, Lafeber HN, Fetter WP, Twisk JW, Van Elburg RM. Effect of neutral and acidic oligosaccharides on fecal IL-8 and fecal calprotectin in preterm infants. Pediatr Res. 2011;69:255–8 [DOI] [PubMed] [Google Scholar]
- 61.Westerbeek EA, Hensgens R, Mihatsch W, Boehm G, Lafeber H, van Elburg R. The effect of neutral and acidic oligosaccharides on stool viscosity, stool frequency and stool pH in preterm infants. Acta Paediatr. 2011;100:1426–31 [DOI] [PubMed] [Google Scholar]
- 62.Wang B. Sialic acid is an essential nutrient for brain development and cognition. Annu Rev Nutr. 2009;29:177–222 [DOI] [PubMed] [Google Scholar]
- 63.Kuntz S, Rudloff S, Kunz C. Oligosaccharides from human milk influence growth-related characteristics of intestinally transformed and non-transformed intestinal cells. Br J Nutr. 2008;99:462–71 [DOI] [PubMed] [Google Scholar]
- 64.Kuntz S, Kunz C, Rudloff S. Oligosaccharides from human milk induce growth arrest via G2/M by influencing growth-related cell cycle genes in intestinal epithelial cells. Br J Nutr. 2009;101:1306–15 [DOI] [PubMed] [Google Scholar]