Abstract
Since the discovery of human milk oligosaccharides (HMO) >60 y ago, research has faced major challenges including (i) the development of methods to identify and characterize these components, (ii) the need to use HMO fractions for functional studies because single HMO were not available, (iii) the uncertainty of the purity of HMO fractions that were often “contaminated” by remainders of lactose, proteins, or glycoproteins, and (iv) the low availability of large quantities of a single HMO for animal and human studies. In the past 10 years, there has been tremendous progress in all of these areas, particularly in the development of methods for detailed structural analysis in extremely low milk volumes. The greatest success, however, is that biotechnological means are available today to produce large amounts even of a single HMO in a purity that allows human studies to be performed in the future. In this review, we summarize the current knowledge about the metabolic aspects of HMO in infants starting with the first studies by Lundblad and co-workers in the early 1980s. After discussing newer observations in recent years, the review closes with a perspective on some important questions regarding metabolic and functional aspects of HMO.
Introduction
Human milk contains a large variety of complex oligosaccharides in concentrations ranging from 10 to 20 g/L (1–4). The quantity of these components does not only depend on the lactational stage of the mother but is also affected by the expression of specific glycosyltransferases in the mammary glands (1–5). Genes encoding for blood group H and Lewis antigens as well as the secretor status determine the presence of α1–2, α1–3, and/or α1–4 fucosylated core structures of oligosaccharides (5). In addition, different patterns of sialylation, i.e., the attachment of α2–3- and/or α2–6-linked N-acetylneuraminic acid (NeuAc)5 increase the variability of human milk oligosaccharides (HMO) to >100 structures characterized so far (2, 6). In addition to lactose and fat, HMO belong to the third most abundant group of milk components. However, due to previous difficulties in characterizing these structures and the lack of available substrates even today, only little is known about their metabolic fate in infants.
Large amounts of HMO, i.e., several grams per day, rinse the gastrointestinal tract of a human milk-fed infant, thereby potentially preventing pathogen adhesion to the intestinal epithelium or influencing gut maturation processes (7–10). On the one hand, HMO are considered not to be degraded by human digestive enzymes and transported into the lower parts of the intestine where they may be metabolized by the intestinal microbiota or get excreted with feces (11); on the other hand, ∼1–2% of some HMO seem to be excreted via infants’ urine, as is shown later (12, 13). Hence, several hundred milligrams per day circulate in the infants’ blood. Therefore, we can also expect systemic functions such as anti-inflammatory or anti-infective effects of HMO.
Background information on the blood group A, B, O, and Lewis-specific components in milk and feces
To better understand the variety of components that may be excreted in infants’ feces, some background information is given on the potential influence of the blood groups A, B, O, and Lewis and the secretor status of the respective mothers and their infants. It has been known for a long time that in milk, hardly any blood group A, B, or H-active oligosaccharides can be detected, whereas in the feces of infants, those components can often be identified (14). Just the opposite is true for Lewis-specific components.
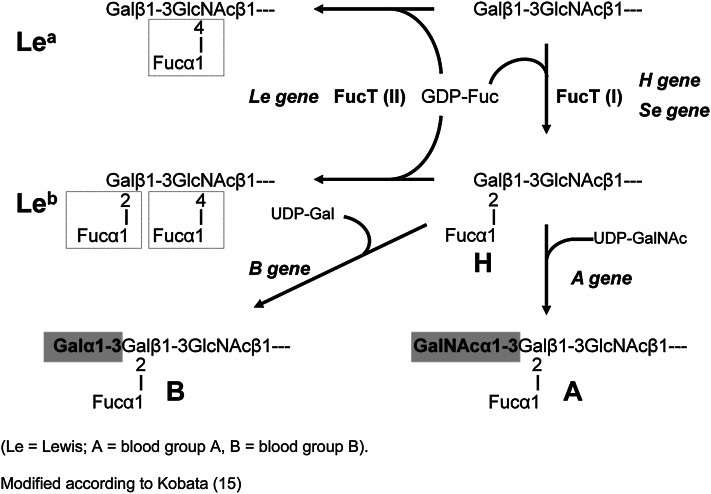
The presence of different neutral oligosaccharides in human milk depends on the activity of specific fucosyltransferases (FucTs) in the lactating gland (Fig. 1) (5, 15, 16). Milk of women of the so-called secretor type is characterized by the presence of α1–2-fucosylated galactose (Gal) forming Fucα1–2Galβ1–3N-acetylglucosamine (GlcNAc) units (compound 1, Table 1) like lacto-N-fucopentaose I (compound 3, Table 1) or in 2′-fucosyllactose (compound 2, Table 1). These linkages are caused by FucT II.
Figure 1.
Biosynthetic pathways of A,B,O, and Lewis blood group determinants. Fuc, fucose; FucT, fucosyltransferase; Gal, galactose. Adapted from Reference (15).
Table 1.
Structural elements of human milk oligosaccharide prototypes and Lewis/secretor specific components1
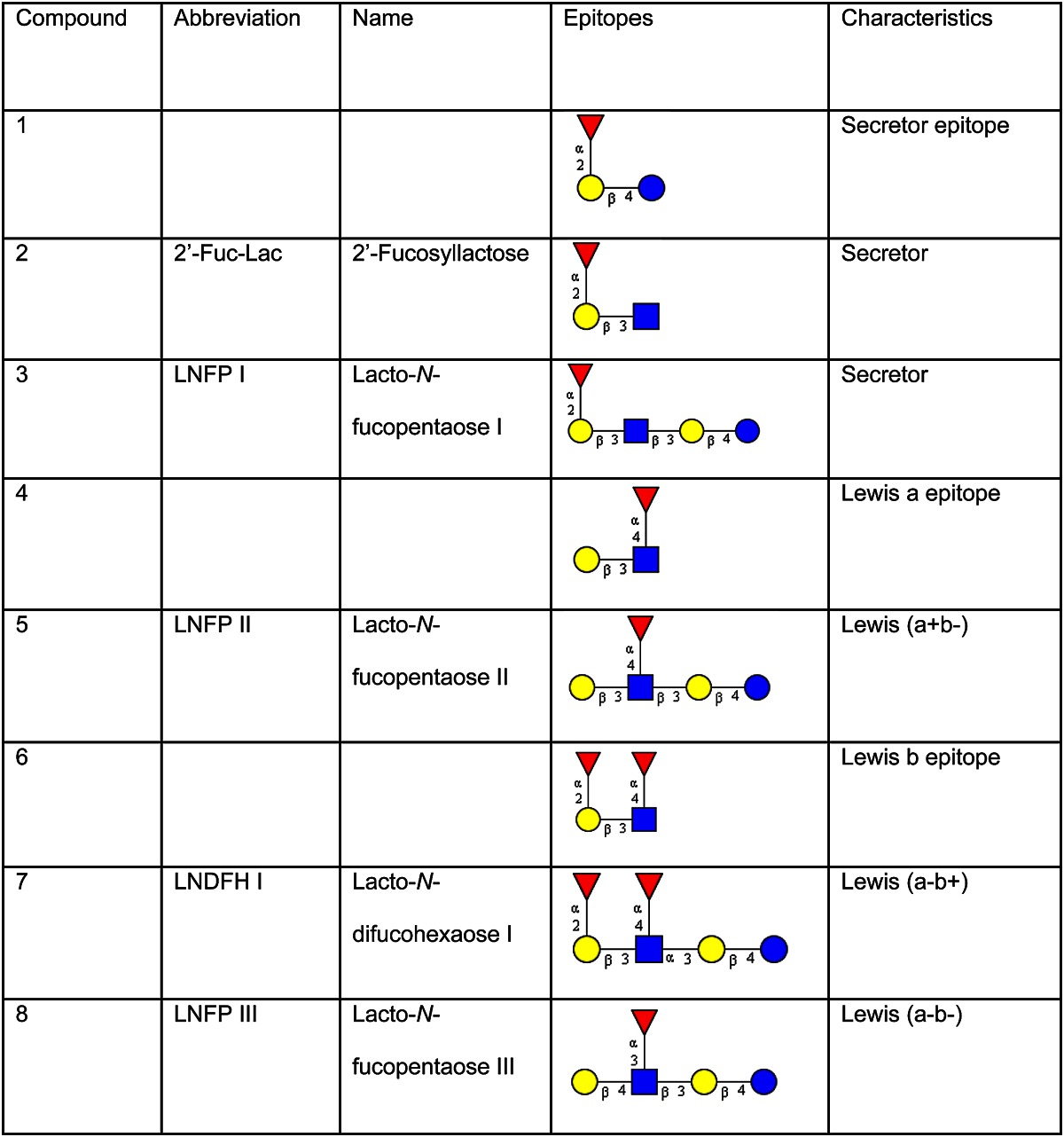
Glycan structures are depicted according to the recommendations of the Consortium of Functional Glycomics using the GlycoWorkbench software tool (40). Yellow circle, galactose; blue circle, glucose; blue square, N-acetylglucosamine; red triangle, fucose.
In Lewis (a+b−) individuals, FucT III attaches fucose (Fuc) residues in α1–4 linkages to a subterminal GlcNAc residue of type 1 chains. Therefore, in Lewis (a+b−) nonsecretor milk, the major fucosylated oligosaccharide is lacto-N-fucopentaose II [Galβ1–3(Fucα1–4)GlcNAcβ1–3Galβ1–4Glc; component 5, Table 1]. This characteristic component is found in ∼20% of the population.
In Lewis (a+b−) donors who represent ∼70% of the population, both FucT II and III, the secretor gene and the Lewis gene–dependent form, are expressed. Here, one of the major milk oligosaccharides is lacto-N-difucohexaose I [Fucα1–2Galβ1–3(Fucα1–4)GlcNAcβ1–3Galβ1–4Glc; component 7, Table 1].
In ∼5–10% of the population that belong to blood group Lewis (a−b−), both FucT II and III are not active, but another FucT, not belonging to the Lewis system, attaches Fucα1–3 linked to GlcNAc in type 2 chains. The major oligosaccharide in the milk of these donors is lacto-N-fucopentaose III [Galβ1–4(Fucα1–3)GlcNAcβ1–3Galβ1–4Glc, component 8 Table 1].
First fecal excretion studies in term and preterm infants fed human milk
In the 1980s, Lundblad and co-workers started a series of experiments analyzing the feces of breast-fed infants to investigate metabolic aspects of HMO (17–20). Apart from full-term infants, preterm infants were also examined, comparing their fecal oligosaccharide composition with the HMO pattern of the milk. In the following, a few examples are given demonstrating the unique and distinct excretion pattern depending on various factors such as the blood group and secretor status of the lactating mother, the gestational age of the infant, and the time after birth when the feces were collected. Although the number of samples analyzed was rather small, the results raised many questions that are still important for current research activities (21, 22).
From the feces of an infant of blood group A, Lundlad’s group isolated only 4 different blood group A–active oligosaccharides (A-tetrasaccharide, A-pentasaccharide, A-hexasaccharide, and A-heptasaccharide) in high amounts (Table 2) (17). In the feces of other infants with blood group A and secretor status, these components were also detected, but in considerably lower amounts. Because those blood group–active components were not detected in the milk of the respective mothers, the authors’ conclusion was that HMO will be modified within the gut through their transit and that the processes involved most likely take place within the enterocytes. As it is shown later, today it is more likely that the modifications are caused by the prevailing intestinal microbiota.
Table 2.
Structures and amounts of compounds isolated from feces of a blood group A breast-fed infant1
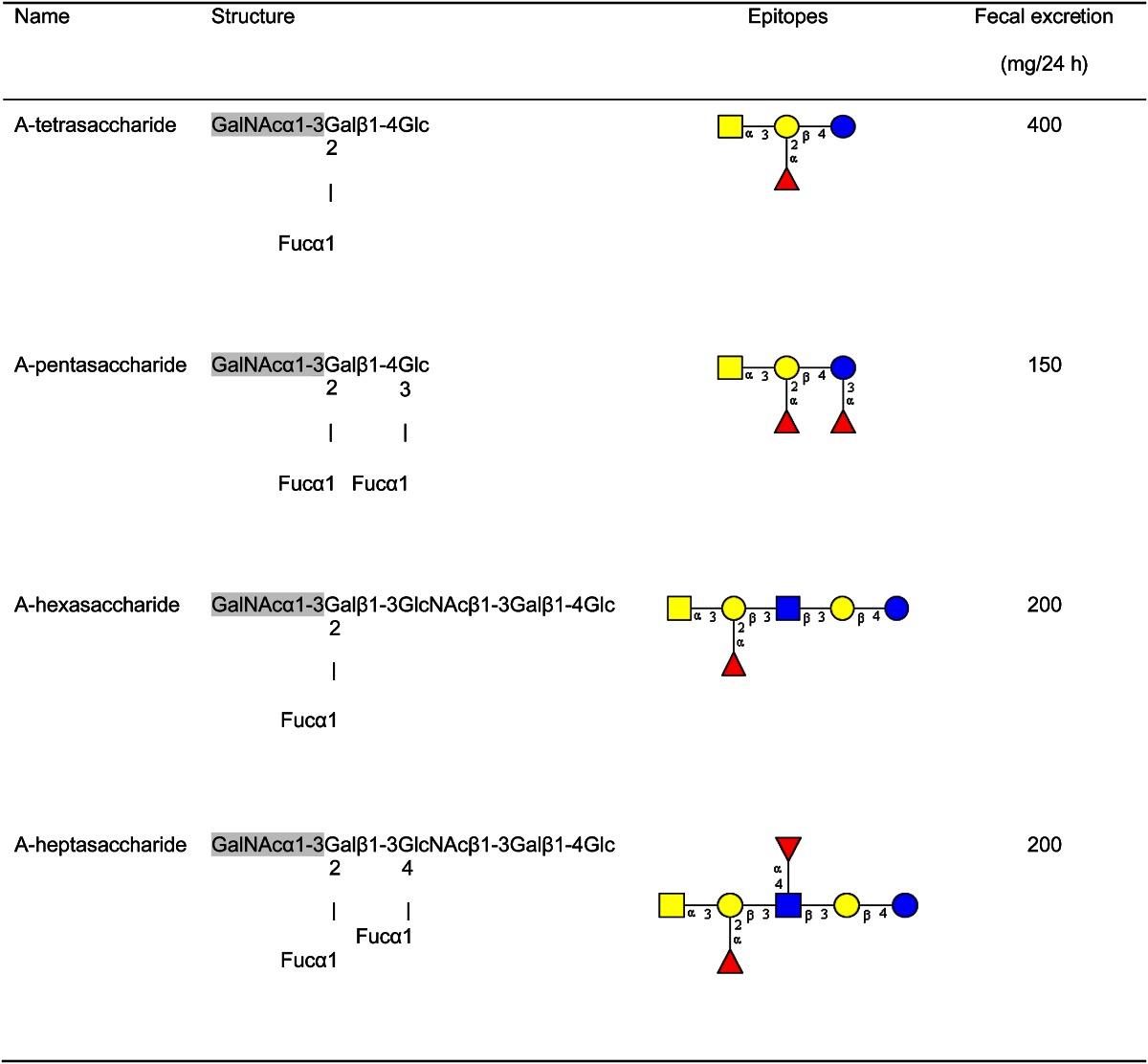
Modified from Reference 17.
Interestingly, in the feces of infants with blood group B–secretor status, analogue blood group B–active oligosaccharides such as B-pentasaccharides and B-hexasaccharides could not be detected, not even if the mother herself was a B-secretor (18).
Fecal samples from another infant with B-secretor status whose mother was blood group O (H-antigens) and a nonsecretor were examined in detail. Eight oligosaccharides were isolated and characterized; 7 of them were identical to oligosaccharide structures that can be found in human milk, whereas 1 of them, a trifucosylated component, had been unknown so far. Unexpectedly, oligosaccharides with Fuc(α1–2)Gal moieties were found in the infant’s feces, although these components were not found in the milk of the mother because she was a nonsecretor. Hence, the modification must have been caused by some unknown processes in the infant because the infant was a secretor and was therefore able to produce H-active substances (18).
Oligosaccharides in feces of preterm infants
The feces of a preterm infant who was blood group A, nonsecretor, and fed pooled mother’s milk were examined with respect to their HMO composition. Nine neutral and 5 acidic oligosaccharides were identified. Among the neutral oligosaccharides, only those characteristic for nonsecretor milk were found. Interestingly, secretor gene–dependent oligosaccharides were not detected in the feces. This nonsecretor infant fed pooled human milk obviously catabolized oligosaccharides from pooled milk in which, although not analyzed, secretor-specific components must have been present as is the case for 70% of milk donors (19, 20).
In the feces of another blood group–A preterm infant but with secretor status who also had been fed pooled human milk and from whom several fecal samples were analyzed, the same HMO were found as those found in the feces from the nonsecretor infant mentioned previously. The lack of A-active oligosaccharides in feces obtained early postpartum was obvious. However, the analyses of a fecal sample collected 8 wk thereafter contained characteristic blood group A–active oligosaccharides (19). These data indicate that very intensive HMO metabolism occurs in the gut, which is supported by recent observations from Gruppen’s group (see later). Apart from that, it is interesting that no blood group A–active oligosaccharides are found in the feces of preterm infants, whereas in the feces of full-term infants, there are. This indicates an inadequately developed expression of the intestinal blood group A–glycosyltransferase or a lack of α1–2-FucT.
To summarize, these first intriguing observations by Lundblad and co-workers demonstrate a very complex metabolism of HMO in the infants’ gastrointestinal tract, and we are still attempting to better understand the underlying mechanisms. Today, in particular, the methodological developments for characterizing minute amounts of HMO and degradation products allow some of the questions that were already raised a long time ago to be addressed. At that time, the importance of the gut microbiota for human health had not been realized. Also, today we know that the serological typing of the Lewis blood group and secretor status is not always sufficient to draw strong conclusions about the HMO composition to be expected in milk (23, 24).
Fecal analysis with new analytical tools
Recently, Gruppen and co-workers reported new data on the fecal excretion of HMO in breast-fed infants by applying capillary electrophoresis with laser-induced fluorescence detection (21, 22, 25). In breast-fed infants, they observed a gradual change in the fecal oligosaccharide profile during the first 6 mo postpartum. Hence, from their data, they hypothesize 3 stages of a different fecal oligosaccharide profile:
Stage 1: occurrence of neutral or acidic HMO. Fecal oligosaccharides obtained within the first 2 mo postpartum can be subdivided according to their pattern dominated by either neutral or acidic HMO.
Stage 2: Predominance of metabolic products. Fecal samples collected within ∼3–6 mo postpartum can be characterized by the absence of HMO and the presence of a few, individual-dependent, dominant carbohydrate structures. These were described as gastrointestinal metabolic products of HMO carrying blood group determinant epitopes. These metabolic products may be conjugates using HMO as building blocks on which blood group–specific antigenic determinants (A, B, or H), which are known to be present in the gastrointestinal mucous layer, may be attached.
Stage 3: Appearance of oligosaccharides characteristic of follow-up diet. After exclusive breastfeeding when complementary food is introduced, predominantly oligosaccharides characteristic of the follow-up diet are found in infant’s feces. Monitoring the gastrointestinal fate of diet-related oligosaccharides pointed to an individual gastrointestinal adaptation to enteral food during the postnatal period, depending on the oligosaccharide composition of breast milk, still given to some extent, and complementary food. The authors conclude from their data that there is a strong need to link the individual HMO metabolism to the composition and activity of the intestinal microbiota.
The hypothesis of Gruppen and co-workers proposing 3 different stages of HMO metabolism is an interesting concept that certainly will lead to further studies to prove the concept.
In vivo 13C-labeling of HMO and subsequent metabolization
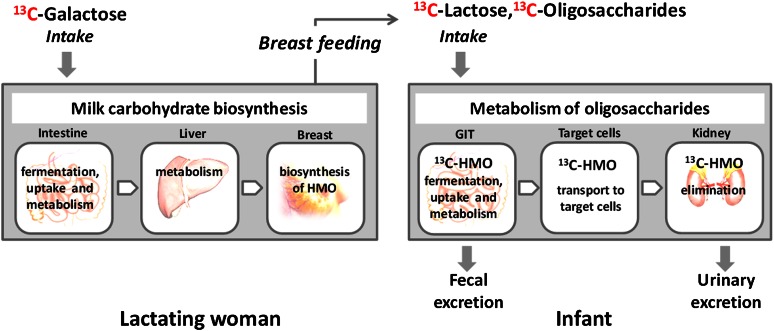
Some years ago, we started to study the fate of HMO in breast-fed infants by endogenously labeling HMO after applying 13C-labeled glucose or 13C-Gal as an oral bolus given to their lactating mothers (Fig. 2) (4, 26).
Figure 2.
Schematic of the studies with 13C-labeled monosaccharides (13C-galactose and 13C-glucosamine) to investigate questions with regard to milk carbohydrate biosynthesis and oligosaccharide metabolism in infants. HMO, human milk oligosaccharides.
We showed that a major part of a 13C-Gal bolus is immediately transported to the lactating gland and is directly incorporated into HMO (26). There is an immediate increase of the 13C-enrichment of milk fractions after the oral 13C-Gal bolus was given to the mothers, followed by a decrease at the end of the day and a second increase in the morning of the next day. This pattern can be found in milk of all women whom we investigated so far. Thus, using stable isotopes, an in vivo 13C-labeling of HMO can be achieved to address questions with regard to their metabolic fate. We observed that in the urine of infants’ HMO are found in all samples collected within 36 h after a 13C-Gal bolus was given to their mothers (13). An overview of identified HMO by fast atom bombardment MS is shown in Table 3. Because we found native, unmodified HMO in the infants’ urine, we conclude that these components can only be derived from milk because the biosynthesis of HMO occurs exclusively in the lactating mammary gland. Also, the urinary excretion of intact HMO in term infants at 3–6 mo post partum verifies our earlier data in preterm infants showing very similar results without using stable isotopes (12).
Table 3.
Neutral and acidic oligosaccharides identified in infants’ urine 1
| Oligosaccharides | Composition |
| Lac | Hex2 |
| FucLacNAc | FucHexHexNAc |
| FucLac | FucHex2 |
| Fuc2Lac | Fuc2Hex2 |
| Fuc1LNTri | FucHex2HexNAc |
| LNT | Hex3HexNAc |
| Fuc1LNT | FucHex3HexNAc |
| Fuc2LNT | Fuc2Hex3HexNAc |
| — | Hex6 |
| Fuc2LNH | Fuc2Hex4HexNAc2 |
| Fuc3LNH | Fuc3Hex4HexNAc2 |
| NeuAcLac | NeuAcHex2 |
| NeuAcLNT | NeuAcHex3HexNAc |
| NeuAcFuc1GlcNAcLNT | NeuAcFucHex3HexNAc2 |
Modified from Reference 17.
In addition to intact HMO, we also detected cleavage products in which not, as expected, the terminal Gal but the glucose moiety on the reducing end had been split off. At the moment, no plausible explanation can be given for such an unusual metabolic degradation step. There is only 1 congress contribution from Lundblad’s group on the renal excretion of oligosaccharides in a few preterm and full-term infants using MS methods for analysis (27). The authors found that small typical milk oligosaccharides were excreted by human milk–fed infants. Most of the analyzed urine samples contained 2′-fucosyllactose and 3-fucosyllactose as well as difucosyllactose. They could not detect these components in the urine of nonbreast-fed infants. A further comparison with our data regarding complex oligosaccharides such as LNT and fucosylated and/or sialylated derivatives as well as N-acetylneuraminyl-lactose (NeuAcLac) is not feasible because Lundblad and co-workers did not report the analysis of such components.
There are 2 possible explanations for the occurrence of modified milk oligosaccharides in urine: they may either originate from human milk itself or they are synthesized endogenously from smaller precursors. Although there is no evidence from in vivo studies yet, these modifications may derive from bacterial activity within the gut. Also, if such HMO modifications occur within the colon, the underlying mechanism of the succeeding absorptive process needs to be demonstrated as well.
Currently, we are investigating whether the infants’ urinary oligosaccharide profile resembles the oligosaccharide pattern in their mothers’ milk with regard to Lewis-specific HMO (28). In addition, we want to obtain information on the 13C-enrichment of individual urinary HMO. Our data so far suggest that the HMO patterns in the urine from breast-fed infants reflect those in their mothers’ milk, suggesting a strong association with the mothers’ Lewis blood group and secretor phenotype.
Amount of HMO through suckling and urinary excretion
In our ongoing studies, we determined the infants’ intake of lacto-N-tetraose and its monofucosylated derivative lacto-N-fucopentaose II. Our data show that with each suckling, an infant receives between 50 to 160 mg of each component, respectively (Table 4). The renal excretion of both components varied between 1 and 3 mg/d (Table 5). Because the intake of individual HMO by the infants is in the range of several hundred milligrams per suckling, i.e., several grams per day, and because some of these components are excreted in milligram amounts as intact HMO with the infants’ urine, not only local but also systemic effects might be expected. Such effects, however, remain to be demonstrated in future studies. The enormous biotechnological progress in HMO production in recent years will enable these studies in vivo.
TABLE 4.
Intake of LNT and its monofucosylated derivative LNFP II in an infant at one feeding 1
| Milk sample | Volume, mL | Concentration in mg/ml2 |
Total intake through milk in mg |
||
| LNT | LNFP II | LNT | LNFP II | ||
| 1 | 97 | 0.77 ± 0.01 | 1.10 ± 0.03 | 74.7 | 106.3 |
| 2 | 126 | 0.78 ± 0.01 | 1.25 ± 0.04 | 98.2 | 157.2 |
| 3 | 58 | 0.79 ± 0.00 | 1.09 ± 0.01 | 46.2 | 63.5 |
| 4 | 97 | 0.77 ± 0.09 | 1.07 ± 0.11 | 75.1 | 103.8 |
| 5 | 97 | 0.57 ± 0.00 | 0.85 ± 0.01 | 55.6 | 82.3 |
LNFP, Lacto- -fucopentaose; LNT, Lacto-N-tetraose.
Concentration mg/ml is given as mean ± SD. Modified from Reference 13.
TABLE 5.
Urinary excretion of LNT and its monofucosylated derivative LNFP II in one infant 1
| Urine sample | Urine volume, mL | Concentration, μg/mL 2 |
Total exretion, mg |
||
| LNT | LNFP II | LNT | LNFP II | ||
| 1 | 35.0 | 13.4 ± 0.2 | 13.1 ± 0.4 | 0.47 | 0.46 |
| 2 | 25.0 | 21.2 ± 05 | 22.6 ± 1.5 | 0.53 | 0.56 |
| 3 | 22.8 | 15.2 ± 0.8 | 17.1 ± 0.1 | 0.35 | 0.39 |
| 4 | 20.0 | 16.6 ± 0.9 | 18.2 ± 0.4 | 0.33 | 0.36 |
| 6 | 26.3 | 11.4 ± 0.2 | 12.4 ± 0.5 | 0.30 | 0.33 |
| 7 | 19.7 | 7.3 ± 0.1 | 7.0 ± 0.1 | 0.14 | 0.14 |
| 8 | 23.2 | 11.6 ± 0.3 | 13.3 ± 0.1 | 0.27 | 0.31 |
| 9 | 27.0 | 6.7 ± 0.1 | 6.6 ± 0.3 | 0.18 | 0.18 |
LNFP, Lacto- -fucopentaose; LNT, Lacto-N-tetraose.
Concentrations are given as mean ± SD ( = 4). Modified from Reference 13.
Functional importance and perspective
The biological importance of the predominance of type I milk oligosaccharides in humans has become an interesting focus in the comparative study of milk oligosaccharides and may help to elucidate some aspects of human evolution (29–31). From our metabolic observations that intact HMO and degradation products can be detected in infants’ urine, it is most likely that in addition to local functions of HMO within the gastrointestinal tract, systemic effects like the adhesion of leukocytes to endothelial cells or the interaction of platelets with neutrophils may be influenced by HMO as well (7, 32–34). An impact of HMO on brain glycoconjugate composition has also been discussed (35). In 1986, Carlson and House compared an intraperitoneal administration with an intragastric application of NeuAc on rat brain composition and found that both oral and systemic routes resulted in considerably more cerebral and cerebellar glycolipid and glycoprotein NeuAc than did glucose injections (36). Compared with free NeuAc, orally given NeuAcLac, the major acidic oligosaccharide in human milk, was even more effective on brain composition. These data supported an earlier observation by Witt et al. (37) comparing radiolabeled free NeuAc and NeuAcLac. The authors showed a preferential incorporation of 14C-NeuAcLac in rat brain gangliosides. Our data suggest that the absorption of HMO in breast-fed infants supports the possibility that sialic acid from HMO is used as a substrate for the biosynthesis of components including gangliosides and glycoproteins, for example, for the brain. The importance of individual monosaccharides for humans, either as a precursor to the production of HMO or as components themselves having an effect on specific processes can be demonstrated by recent data of Duncan et al. (38). They reported that, based on recent observations in animals, one can speculate that during the neonatal suckling period, de novo sialic acid production may not be sufficient to meet the needs of all tissues in the rapidly developing newborn and that sialic acid could serve as a conditionally essential nutrient for the suckling neonate (39). The data from Duncan et al. support the views that (i) when milk sialic acid (SA) levels are high, in the colon, SA is catabolized to GlcNAc that in turn may be used as such or as substrate for SA synthesis and (ii) when milk SA levels are low, the endogenous SA synthetic machinery in the colon is activated. These observations are supported by studying the metabolism of SA through gene expression profiling, with results supporting the view that the high concentration of SA found in milk early during lactation favors its catabolism in the colon of the suckling pup, whereas the low concentration seen at weaning stimulates the expression of genes for its synthesis and subsequent use (38).
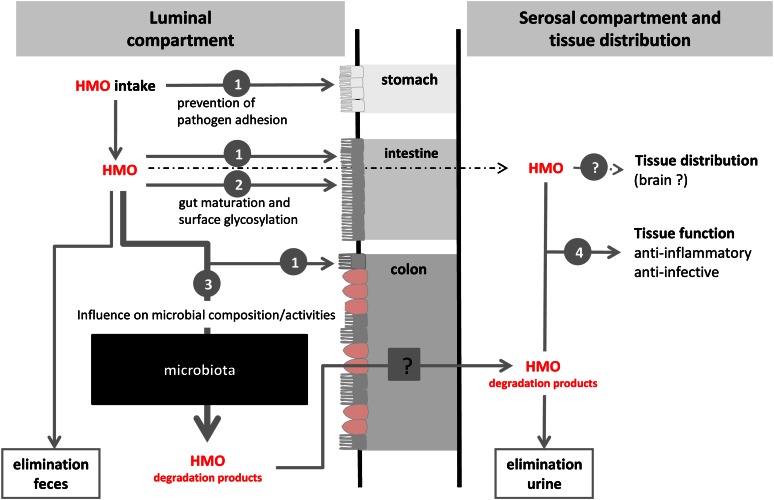
These interesting new data and knowledge on HMO functions and metabolism are schematically summarized in Fig. 3, which gives an overview of the current status of HMO research and raises many questions to be addressed in the near future. A few examples are as follows: (i) Are HMO differently metabolized in term and preterm infants and what is the underlying mechanism? (ii) Do single HMO affect the microbial composition and/or activities more efficiently than a mixture of various components and how can health effects be investigated in humans? (iii) Which specific HMO have a direct impact on intestinal or tissue target cells, e.g., on cell maturation, cell surface glycosylation, or brain functions? (iv) Are there specific HMO or their precursors that are preferentially absorbed and transported into target cells (brain and others)?
Figure 3.
Overview of metabolic processes in infants after the intake of human milk oligosaccharides (HMO). The numbers 1 to 4 indicate specific functions: 1, prevention of pathogen adhesion; 2, direct effects on epithelial cells; 3, influence on the microbiota composition and/or activity; and 4, systemic effects. Intensities of the arrows reflect the current focus in HMO functions.
In recent years there has been a tremendous increase in our knowledge regarding the specific effects of HMO. Concomitantly with these studies, progress in biotechnology today allows the production of at least some of the major HMO to potentially be added to infant formula or other food at reasonable costs. However, to decide which compound should be added, in which concentrations or combinations, and how long it should be given, studies are needed regarding the metabolic fate, fecal and urinary excretion, and the local and systemic effects of HMO or their degradation products.
Acknowledgments
Both authors have read and approved the final manuscript.
Footnotes
Published in a supplement to Advances in Nutrition. Presented at the conference “The First International Conference on Glycobiology of Human Milk Oligosaccharides” held in Copenhagen, Denmark, May 16–17, 2011. The conference was supported by Glycom A/S, Lyngby, Denmark. The supplement coordinators for this supplement were Clemens Kunz, University of Giessen, Germany, and Sharon M. Donovan, University of Illinois, Urbana, IL. Supplement Coordinator disclosures: Sharon Donovan has received human milk oligosaccharides from Glycom through their HMO biology donations program. Clemens Kunz has received human milk oligosaccharides from Glycom through their HMO biology donation program. The supplement is the responsibility of the Guest Editor to whom the Editor of Advances in Nutrition has delegated supervision of both technical conformity to the published regulations of Advances in Nutrition and general oversight of the scientific merit of each article. The Guest Editor for this supplement was Mark A. McGuire, University of Idaho. Guest Editor disclosure: Mark A. McGuire consults with Abbott Nutrition, a division of Abbott Laboratories, and provides professional opinions on matters related to human lactation and infant nutrition. Further, he collaborates with Dr. Lars Bode, University of California, San Diego, in research related to human milk oligosaccharides. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
Author disclosures: S. Rudloff, C. Kunz, no conflicts of interest.
Abbreviations used: Fuc, fucose; FucT, fucosyltransferase; Gal, galactose; GlcNAc, N-acetylglucosamine; NeuAc, N-acetylneuraminic acid; NeuAcLac, N-acetylneuraminyl-lactose; HMO, human milk oligosaccharides; SA, sialic acid.
Literature Cited
- 1.Egge H, Dell A, von Nicolai H. Fucose containing oligosaccharides from human milk. I. Separation and identification of new constituents. Arch Biochem Biophys. 1983;224:235–53 [DOI] [PubMed] [Google Scholar]
- 2.Urashima T, Kitaoka M, Terabayashi T, Fukuda K, Ohnishi M, Kobata A. Milk oligosaccharides. In: Oligosaccharides: Sources, properties and applications. Gordon NG, editor New York: Nova Science Publishers; 2011. p. 1–77 [Google Scholar]
- 3.Thurl S, Munzert M, Henker J, Boehm G, Müller-Werner B, Jelinek J, Stahl B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br J Nutr. 2010;104:1261–71 [DOI] [PubMed] [Google Scholar]
- 4.Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk. Structural, functional and metabolic aspects. Annu Rev Nutr. 2000;20:699–722 [DOI] [PubMed] [Google Scholar]
- 5.Le Pendu J. Histo-blood group antigen and human milk oligosaccharides: genetic polymorphism and risk of infectious diseases. Adv Exp Med Biol. 2004;554:135–43 [DOI] [PubMed] [Google Scholar]
- 6.Ruhaak LR, Lebrilla CL. High throughput analysis and quantitation of free oligosaccharides in mammalian milk. Adv Nutr. (these Proceedings)
- 7.Bode L, Rudloff S, Kunz C, Strobel S, Klein N. Human milk oligosaccharides reduce platelet-neutrophil complex formation leading to a decrease in neutrophil β 2 integrin expression. J Leukoc Biol. 2004;76:820–6 [DOI] [PubMed] [Google Scholar]
- 8.Newburg DS, Ruiz-Palacios GM. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr. 2005;25:37–58 [DOI] [PubMed] [Google Scholar]
- 9.Kuntz S, Rudloff S, Kunz C. Oligosaccharides from human milk influence growth-related characteristics of intestinally transformed and non-transformed intestinal cells. Br J Nutr. 2008;99:462–71 [DOI] [PubMed] [Google Scholar]
- 10.Kuntz S, Kunz C, Rudloff S. Oligosaccharides from human milk induce growth arrest via G2/M by influencing growth-related cell cycle genes in intestinal epithelial cells. Br J Nutr. 2009;101:1306–15 [DOI] [PubMed] [Google Scholar]
- 11.Gnoth MJ, Kunz C, Kinne-Saffran E, Rudloff S. Human milk oligosaccharides are minimally digested in vitro. J Nutr. 2000;130:3014–20 [DOI] [PubMed] [Google Scholar]
- 12.Rudloff S, Pohlentz G, Diekmann L, Egge H, Kunz C. Urinary excretion of lactose and oligosaccharides in preterm infants fed human milk or infant formula. Acta Paediatr. 1996;85:598–603 [DOI] [PubMed] [Google Scholar]
- 13.Rudloff S, Pohlentz G, Borsch C, Lentze MJ, Kunz C. Urinary excretion of in vivo 13C-labelled milk oligosaccharides in breastfed infants. Br J Nutr. 2011;5:1–7 [DOI] [PubMed] [Google Scholar]
- 14.Egge H. The diversity of oligosaccharides in human milk. In Renner, B. and Sawatzki, G, editors. New perspectives in infant nutrition. Stuttgart, New York: Thieme; 1993. p. 12–26. [Google Scholar]
- 15.Kobata A. A journey to the world of glycobiology. Glycoconj J. 2000;17:443–64 [DOI] [PubMed] [Google Scholar]
- 16.Oriol R, Le Pendu J, Mollicone R. Genetics of ABO, H, Lewis, X and related antigens. Vox Sang. 1986;51:161–71 [DOI] [PubMed] [Google Scholar]
- 17.Sabharwal H, Nilsson B, Chester MA, Sjöblad S, Lundblad A. Blood group specific oligosaccharides from faeces of a blood group A breast-fed infant. Mol Immunol. 1984;21:1105–12 [DOI] [PubMed] [Google Scholar]
- 18.Sabharwal H, Nilsson B, Chester MA, Lindh F, Grönberg G, Sjöblad S, Lundblad A. Oligosaccharides from faeces of a blood-group B, breast-fed infant. Carbohydr Res. 1988;178:145–54 [DOI] [PubMed] [Google Scholar]
- 19.Sabharwal H, Nilsson D, Grönberg G, Chester MA, Dakour J, Sjöblad S, Lundblad A. Oligosaccharides from feces of preterm infants fed on breast milk. Arch Biochem Biophys. 1988;265:390–406 [DOI] [PubMed] [Google Scholar]
- 20.Sabharwal H, Sjöblad S, Lundblad A. Sialylated oligosaccharides in human milk and feces of preterm, full-term and weaning infants. J Pediatr Gastroenterol Nutr. 1991;12:480–4 [DOI] [PubMed] [Google Scholar]
- 21.Albrecht S, Schols HA, Van den Heuvel EGHM, Voragen AGJ, Gruppen H. CE-LIF-MSn profiling of oligosaccharides in human milk and feces of breast-fed babies. Electrophoresis. 2010;31:1264–73 [DOI] [PubMed] [Google Scholar]
- 22.Albrecht S, Schols HA, Van den Heuvel EGHM, Voragen AGJ, Gruppen H. Occurrence of oligosaccharides in feces of breast-fed babies in their first six months of life and the corresponding breast milk. Carbohydr Res. 2011;346:2540–50 [DOI] [PubMed] [Google Scholar]
- 23.Blank D, Gebhardt S, Maass K, Lochnit G, Dotz V, Blank J, Geyer R, Kunz C. High-throughput mass finger printing and Lewis blood group assignment of human milk oligosaccharides. Anal Bioanal Chem. 2011;401:2495–510 [DOI] [PubMed] [Google Scholar]
- 24.Blank D, Dotz V, Geyer R, Kunz C. Human milk oligosaccharides and Lewis blood group - Individual high-throughput sample profiling to enhance conclusions from functional studies. Adv Nutr. 2012. [DOI] [PMC free article] [PubMed]
- 25.Albrecht S, Schols HA, Van Zoeren D, Van Lingen RA, Groot Jebbink LJM, Van den Heuvel GHM, Voragen AGJ, Gruppen H. Oligosaccharides in feces of breast- and formula-fed babies. Carbohydr Res. 2011;346:2173–81 [DOI] [PubMed] [Google Scholar]
- 26.Rudloff S, Obermeier S, Borsch C, Pohlentz G, Hartmann R, Brösicke H, Lentze MJ, Kunz C. Incorporation of orally applied 13C-galactose into milk lactose and oligosaccharides. Glycobiology. 2006;16:477–87 [DOI] [PubMed] [Google Scholar]
- 27.Chester MA, Lundblad A, Renlund M, Sjöblad S. Urinary excretion of oligosaccharides by premature and full-term babies and adults. In: Yamakawa T, Osawa T, Handa S, editors. Proceedings of the 6th International Symposium on Glycoconjugates. Tokyo: Japan Scientific Societies Press; 1981. p. 213A.
- 28.Dotz V, Rudloff S, Blank D, Gebhardt S, Maass K, Geyer R, Kunz C. Oligosaccharide MALDI-MSn profiles in milk and urine from mother-child pairs. 1st Int. Conference on the Glycobiology of Human Oligosaccharides, May 2011, Copenhagen, Denmark.
- 29.Urashima T, Sadaki Asakuma S, Fiame Leo F, Kenji Fukuda K, Messer M, Oftedal OT. Possible significance of the predominance of type I oligosaccharides, a feature specific to human breast milk. Adv Nutr. (these Proceedings) [DOI] [PMC free article] [PubMed]
- 30.Sela DA, Chapman J, Adeuya A, Kim JH, Chen F, Whitehead TR, Lapidus A, Rokhsar DS, Lebrilla CB, German JB, et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci U S A. 2008;105:18964–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sela DA, Mills DA. Nursing our microbiota: molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol. 2010;18:298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunz C, Rudloff S. Health promoting aspects of milk oligosaccharides. Int Dairy J. 2006;16:1341–6 [Google Scholar]
- 33.Donovan SM. Human milk oligosaccharides – the plot thickens. Br J Nutr. 2009;101:1267–9 [DOI] [PubMed] [Google Scholar]
- 34.Bode L, Kunz C, Muhly-Reinholz M, Mayer K, Seeger W, Rudloff S. Inhibition of monocyte, lymphocyte, and neutrophil adhesion to endothelial cells by human milk oligosaccharides. Thromb Haemost. 2004;92:1402–10 [DOI] [PubMed] [Google Scholar]
- 35.Wang B. Sialic acid is an essential nutrient for brain development and cognition. Annu Rev Nutr. 2009;29:177–222 [DOI] [PubMed] [Google Scholar]
- 36.Carlson SE, House SG. Oral and intraperitoneal administration of N-acetylneuraminic acid: effect on rat cerebral and cerebrellar N-acetylneuraminic acid. J Nutr. 1986;116:881–6 [DOI] [PubMed] [Google Scholar]
- 37.Witt W, von Nicolai H, Zilliken F. Uptake and distribution of orally applied N-acetyl-(14C)-neuraminyllactose and N-acetyl-(14C)-neuraminic acid in the organs of newborn rats. Nutr Metab. 1979;23:51–61 [DOI] [PubMed] [Google Scholar]
- 38.Duncan PI, Raymond F, Fuerholz A, Sprenger N. Sialic acid utilisation and synthesis in the neonatal rat revisited. PLoS ONE 2009;4:e8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang B, Brand-Miller J, McVeagh P, Petocz P. Concentration and distribution of sialic acid in human milk and infant formulas. Am J Clin Nutr. 2001;74:510–5 [DOI] [PubMed] [Google Scholar]
- 40.Ceroni A, Maass K, Geyer H, Geyer R, Dell A, Haslam SM. GlycoWorkbench: a tool for the computer-assisted annotation of mass spectra of glycans. J Proteome Res. 2008;7:1650–9 [DOI] [PubMed] [Google Scholar]