Abstract
This review focuses on important observations regarding infant health around 1900 when breastfeeding was not considered a matter of importance. The discovery of lactobacilli and bifidobacteria and their relevance for health and disease was an important milestone leading to a decrease in infant mortality in the first year of life. At the same time, pediatricians realized that the fecal composition of breast-fed and bottle-fed infants differed. Observations indicated that this difference is linked to milk composition, particularly due to the milk carbohydrate fraction. Circa 1930, a human milk carbohydrate fraction called gynolactose was identified. This was the starting point of research on human milk oligosaccharides (HMO). In the following years, the first HMO were identified and their functions investigated. Studies after 1950 focused on the identification of various HMO as the bifidus factor in human milk. In the following 30 years, a tremendous amount of research was done with regard to the characterization of individual HMO and HMO patterns in milk. In this short introduction to the history of HMO research, which ends circa 1980, some outstanding scientists in pediatrics and chemistry and their pioneering contributions to research in the field of HMO are presented.
Introduction
Interest in milk carbohydrates started around the beginning of the past century, influenced by observations that the survival rate of breast-fed infants compared with bottle-fed infants was much higher and that this might be attributed to the unique composition of human milk. Two parallel routes of research on carbohydrates and later on human milk oligosaccharides (HMO) could be observed, one was based on physicochemical, the other on nutritional observations (1). Table 1 shows the time periods and important investigations regarding HMO that are covered in this review.
Table 1.
Milestones in milk carbohydrate research1
HMO, human milk oligosaccharides.
Situation around 1900, infant mortality, and feeding
Around the turn of the century, the mortality rate ranging from 20% to 30% in the first year of an infant’s life was alarmingly high. Breastfeeding was not considered a matter of importance. It was found, for example, that the mortality risk would increase dramatically if the infants could not be breast-fed. In bottle-fed infants compared with breast-fed infants, the mortality rate was 7 times higher (2).
The importance of carbohydrates in milk is intrinsically tied to the growing awareness toward the end of the 19th century that nutrition plays an essential role in the health of infants. At that time, infants were fed pure carbohydrates, chewed food, and mixtures of milk and cereals with or without added meat, or they were given native animal’s milk (2). One of the first infant formulas consisting of modified cow’s milk was developed by Justus Liebig (1803–1870) for his daughter’s children and termed “soup for infants” (“Kindersuppe”) in 1864 (3). It was based on the use of milk and cereals. This soup for infants induced many others to produce infant formula to improve the overall health of infants. It was Justus von Liebig who provided a scientific basis for nutritional investigations by his technical and analytical innovations. But at the beginning, the alternative products to human milk were not very successful. This is not surprising because only later, due to scientific innovations, it was possible, for example, to perform the first balance studies (Max von Pettenkofer and C. von Voit, 1881), to determine the energy content of food (Max Rubner, 1889), or the protein content in food, after Johann Kjeldahl (1883) had developed a method for the analysis of nitrogen.
At the same time, pediatrics developed into an independent medical field, and in Europe (e.g., Vienna, Graz, Zurich, Berlin, Munich), the first professorships and chairs were established held by internationally esteemed persons (2, 4). Among them, Adalbert Czerny, the author of the textbook Des Kindes Ernährung (The Child’s Nutrition), which was for a long time one of the leading books in this area. The tremendeous impact of this book in pediatrics at that time was summarized in 1933 in a Festschrift by Henry F. Helmholtz in the Journal of Pediatrics as follows: “this work is a bible. … At a time when pediatrics in America was still in its swaddling clothes, German masters took us into their clinics as students and by their inspiration and training played a part in the development of pediatrics in America of which they may feel proud” (5).
Discovery of the importance of microorganisms for health and disease
A major breakthrough for infant survival was the discovery of microorganisms and their importance to health and, at the same time, the observation that milk carbohydrates play an important role in the growth of these microorganisms. The pioneering work in this area was done by Theodor Escherich, one of the most respected pediatricians in Europe (Table 2) (4). By 1886, Escherich (6) had published a monograph on the relationship between intestinal bacteria and physiology of digestion in the infant. This research established him as the leading bacteriologist in pediatrics.
Table 2.
Theodor Escherich: a pioneer in pediatrics and microbiology

Photograph reproduced with permission from the (Austrian National Library).
Another pioneer in pediatrics at that time was Ernst Moro, who was born in 1874 in Slovenia (Table 3). Moro studied in Graz, and his pediatric career started in the laboratory of Escherich, who held the first Chair of Pediatrics in Graz (4). In 1900, Moro presented the first bacteriological characterization of Lactobacillus acidophilus (7). He attracted worldwide attention in various fields, e.g., for developing a simple percutaneous skin test for tuberculosis, which was used until the 1960s, for recommending a carrot soup as treatment for children with diarrhea, and for describing a milestone in the infant’s neurological development (the “embracing reflex”) (4). Being a student of Escherich explains Moros’ strong interest in bacteria and their importance for infant health.
Table 3.
Ernst Moro: a pioneer in pediatrics

Photograph reproduced with permission from Reference 4.
The intimate link between basic and applied research circa 1900 is demonstrated in Figure 1, which shows Escherich at his last lecture, with Moro on the left, giving an injection to an animal, while Meinhard von Pfaundler, another renowned pediatrician, is examining a child (4).
Figure 1.
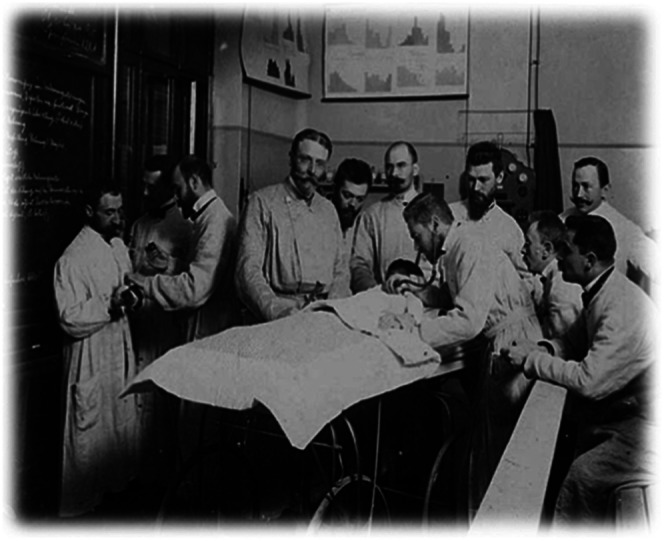
Escherich circa 1900 at his last lecture, with Moro on the left giving an injection to an animal while Meinhard von Pfaundler, another renowned pediatrician, is examining a child. Reproduced from Reference 4 with permission.
Being fascinated by Escherich’s success in the field of bacteriology and others, it was mainly Moro who stimulated research to find the growth factors of microorganisms in milk. In particular, Paul György, a student of Moro, was inspired by these ideas, as is discussed later. From then on, those who conducted research on milk carbohydrates can be divided into those with a chemical background or those with a physiological/nutritional background.
Chemical observations as a background for HMO research
It was observed very early that there was a not yet specified fraction detectable in human milk that could not be found in bovine milk (1). In 1888, Eschbach was the first to find that animal milk and human milk do not contain that type of lactose that is typically found in bovine milk (cited in Reference 1). He observed that in both types of milk, a mixture of various forms of lactose with different properties could be found and concluded that bovine milk contained the most homogeneous type of lactose, whereas human milk contained the most heterogeneous type.
However, Deniges observed that both types of lactose in human and bovine milk were identical, but that mother’s milk contained a carbohydrate fraction still to be identified (cited in Reference 1). Only 40 years later Michel Polonowski and Albert Lespagnol in Lille, France, established a method to specify the so far unknown carbohydrate fraction. They called this fraction, which was insoluble in methanol, gynolactose (8, 9). The authors themselves, however, pointed out that this gynolactose was not homogeneous, but consisted of various components. They also found that nitrogen was an essential component of the hexosamines and that this gynolactose showed a very strong bile reaction. As we know today, this reaction is due to the presence of N-acetylneuraminic acid, not yet known then. Later on, Polonowski and Lespagnol together with Montreuil, by applying 2-dimensional paper chromatography for the first time, identified the first 2 fucosyllactoses (2′-fucosyllactose and 3-fucosyllactose) in the fraction that had been unknown so far (10).
Jean Montreuil was a charismatic personality of sciences and one of the pioneers in the field of carbohydrates and glycoconjugates (Table 4). In addition to other important discoveries, he proposed that all N-glycans have the same core to which are attached various branches that he named antennae to suggest their mobility and potential recognition roles (11). After having built molecular models, he proposed interconvertible conformations and coined first the terms Y and T conformations, later called bird or umbrella conformations. These original studies on the structures of glycoconjugates stimulated many researchers to study their biological roles in health and disease. As Bratosin and Mignon emphasized, enthusiasm is the key word to characterize Jean Montreuil (11).
Table 4.
Jean Montreuil: a pioneer in HMO research1

HMO, human milk oligosaccharides.
Photograph reproduced with permission from Reference 11.
It was Montreuil’s ambition to describe this complex mixture in gynolactose in more detail and to specify its structures (12; see also later), although he did not realize that he would strongly compete with Richard Kuhn by doing so. Concurrent research work in Heidelberg by Kuhn and co-workers and by Montreuil and his team in Lille led to the first clear description of HMO, namely, 2′- and 3-fucosyllactose and difucosyllactose and to the identification of lacto-N-tetraose, lacto-N-fucopentaose I and II, difucosyllactose, and many others (12–16).
Since then, research in the field of HMO has continued, above all by Jean Montreuil together with L. Grimmonprez, G. Strecker, J. M. Wieruszeski, and others; Richard Kuhn and his many co-workers; Viktor Ginsburgh; Akira Kobata; Heinz Egge; and many others (see later).
Kuhn himself had already had numerous publications together with his co-workers Bär, Brossmer, Gaue, and later Egge, identifying various oligosaccharides (for reviews, see References 1, 17–19). The starting point of Kuhn’s work on glycolipids in general and HMO research completely differs from that of the research of Polonowski, Lespagnol, and Montreuil.
Physiological observations as background to HMO research
To understand the physiological background, we need to go back to the turn of the 19th century. By then it was found that breast-fed infants showed a significantly greater resistance against various diseases, including infectious diarrhea, than infants fed bovine milk. This is the reason why at the end of the past century, Henri Tissier of the Pasteur Institute in Paris tried to compare the intestinal microbiota of breast-fed infants with that of infants fed bovine milk (20). At the same time, Moro in Heidelberg independently found a unique predominance of bifidobacteria in the feces of breast-fed infants (4, 7). In the following years, these observations led to an intensive search for microbial growth factors, mainly in human milk but also in milk from a variety of animals.
In 1926, it was Herbert Schönfeld who significantly contributed to research in the field of growth factors in human milk by proving that the bifidus factor was thermoresistant and was not contained in the nonprotein fraction of milk (21). Schönfeld drew the conclusion that this bifidus factor was a vitamin; vitamins were only just being discovered at that time.
At this point, another leading pediatrician with worldwide reputation needs to be introduced (Table 5).
Table 5.
Paul György: a pioneer in pediatrics

Photograph reproduced with permission from Reference 22.
The name of Paul György is associated by most of us with a period around the turn of the past century when the first vitamins were discovered and isolated (22). György was a pediatrician with a strong interest in basic sciences, especially in the field of nutrition in general and nutrition of children and adolescents in particular. From the beginning, he had promoted the benefit of breast milk for children, although at that time, those recommendations were not at all very popular. György was born on April 7, 1893. Shortly after World War I, he joined Moro’s group in Heidelberg. Therefore, it is not surprising that György had all his life been engaged in research on the intestinal microbiota and its relevance for the health of breast-fed infants. Due to the Nazi regime, György had to leave Germany in 1933. Via Cambridge (England), he finally came to Cleveland, Ohio, and later to Pennsylvania (22).
In the 1950s, György and Kuhn started their impressive collaboration on HMO research. György, in Philadelphia since 1936, contacted Kuhn, and thus ∼30 years after the beginning of their joint research on lactoflavines in Heidelberg, the 2 research groups renewed their collaboration (Table 6). They started to investigate the importance of human milk compared with cow’s milk in the infant’s resistance against infections. The cooperation between the 2, being extremely successful, led to the proof that the growth factors for Lactobacillus bifidus in human milk consist of oligosaccharides containing N-acetylglucosamine and polysaccharides (1, 17–19). In contrast, the nitrogen-free fraction in human milk did not show any bifidogenous effect.
Table 6.
Major discoveries by Paul György and Richard Kuhn
Photographs reproduced with permission from References 22 and the Österreichische Nationalbibliothek ÖNB (Austrian National Library).
Kuhn was interested in science at a very young age (Table 7) (23). He did not go to elementary school but was educated by his mother before entering secondary school. When he was 13 years old, he helped a family friend who was a professor of chemistry in Zurich to prepare the experimental classes for his chemistry students. At this time he received as a present the newest chemistry textbook, about which Richard Kuhn was very excited. Therefore, it is not that surprising that at the age of 21, he started his PhD under the supervision of Richard Willstätter (the recipient of the Nobel Prize in Chemistry in 1915) in 1921 and finished 1 year later. The title of his PhD thesis was “The Specificity of Enzymes in Carbohydrate Metabolism.”
Table 7.
Richard Kuhn - a pioneer in Chemistry and in HMO research1

HMO, human milk oligosaccharides.
Photograph reproduced with permission from the Österreichische Nationalbibliothek ÖNB (Austrian National Library)]
The 1950s were a second period of enormous research by Richard Kuhn, consisting of investigations on immune factors and associated research on oligosaccharides in human milk and gangliosides in the brain (23). Based on studies of bifidus factors in milk and the virus-inhibiting effects of human milk, Kuhn, together with Bär, Gaue, Brossmer, and others, focused on the identification and characterization of oligosaccharides in human milk (1, 16, 17, 23). Since 1954, numerous oligosaccharides have been purely presented and structurally elucidated by excellent techniques. Isolated in 1954, lacto-N-tetraose was identified as a key substance of HMO, and during the following years, further components were identified (16).
Kuhn had always been interested in the biological significance of isolated compounds. The findings that oligosaccharides containing lactaminic acid (called sialic acid today) are cleavable by influenza virus was of great interest. 3′- and 6′-lactaminyl lactose were found to be the most easily cleavable component. It showed, for example, that influenza virus cleaves the glycosidic linkage of lactaminic acid, and in the following years, lactaminyl oligosaccharides were identified as receptors for influenza virus, thus explaining the virus-inhibiting effect of human milk (23, 24). In his lecture on resistance problems at the 100th Meeting of the Society of German Natural and Medical Scientists in 1958, Kuhn explained that cells not developing such lactaminyl oligosaccharide structures on the surface had to be resistant to influenza virus (16, 23, 24), an observation with long-lasting influence on research even until today in the fields of chemistry, biology, and medicine.
From the beginning of their collaboration on HMO research, György in Philadelphia and Kuhn in Heidelberg found out that there was a connection between the work of Tissier on bacteria and the milk fraction research of Polonowski and Lespagnol, called gynolactose (8). In the course of their investigations, György found a Lactobacillus bifidus mutant solely growing in the presence of milk, which he named after its place of discovery L. bifidus var. pennsilvanicus (25).
György also realized that the bifidus factor activity is somehow related to the intestinal defense system. In 1953, he published one of his key papers “A Hitherto Unrecognized Biochemical Difference Between Human Milk and Cow’s Milk” (26). He, together with Kuhn, Rose, and Zilliken, found the greatest activity in human colostrum, followed by rat colostrum, human milk, rat milk, and cow colostrum. The milk of ruminants (i.e., cows, ewes, goats) showed only weak activity, if any. A large number of organic compounds including known microbial growth factors, yeast extract, vitamins not present in the original Lactobacillus growth medium, carbohydrates, and several vegetable extracts were all ineffective in replacing the bifidus factor. In this close collaboration between Philadelphia and Heidelberg, many HMO such as lacto-N-tetraose, lacto-N-fucopentaose I, lacto-N-fucopentaose II, and lacto-N-difucohexaose have been characterized by classic methods (1, 17, 19).
On the basis of their observations that L. bifidus grew only in the presence of N-acetylglucosamine, they postulated that those bacteria were not able to synthesize acetylglucosamine themselves (23). They concluded at that time that if an infant is fed N-acetylglucosamine containing oligosaccharides, growth of L. bifidus will increase, thus inhibiting the invasion of pathogenic bacteria in 2 ways. One was is the production of milk and acetic acid by which the activity of L. bifidus leads to a decrease in the intestinal pH, inhibiting the growth of various pathogenic bacteria. The other way is to support the growth of L. bifidus, which will lead to a lack of nutrients for pathogenic microorganisms. These underlying mechanisms had been discovered in the mid-1950s, but are still research topics, as the current strong interest in investigating probiotics and prebiotics and their importance in health and disease shows.
Elucidation of the ABO and secretor pathway of HMO
This topic is intimately linked to Akira Kobata, another pioneer HMO investigator (Table 8).
Table 8.
Akira Kobata: a pioneer in HMO research1

HMO, human milk oligosaccharides.
Photograph reproduced with permission from Reference 19.
Beginning at about 1960, the blood group ABO and secretor system were investigated by Watkins (27) and others. Due to their large amounts in human milk, HMO had been used at that time as an optimal source for the elucidation of the blood group specificity. In 1967, Grollmann and Ginsburg (28) proved that 2′-fucosyllactose is not detectable in milk samples of nonsecretor women. These women express ABO blood group epitopes on their erythrocytes corresponding to their genetic ABO background, but do not express them in glycoproteins secreted by epithelial cells of mucus-producing gland.
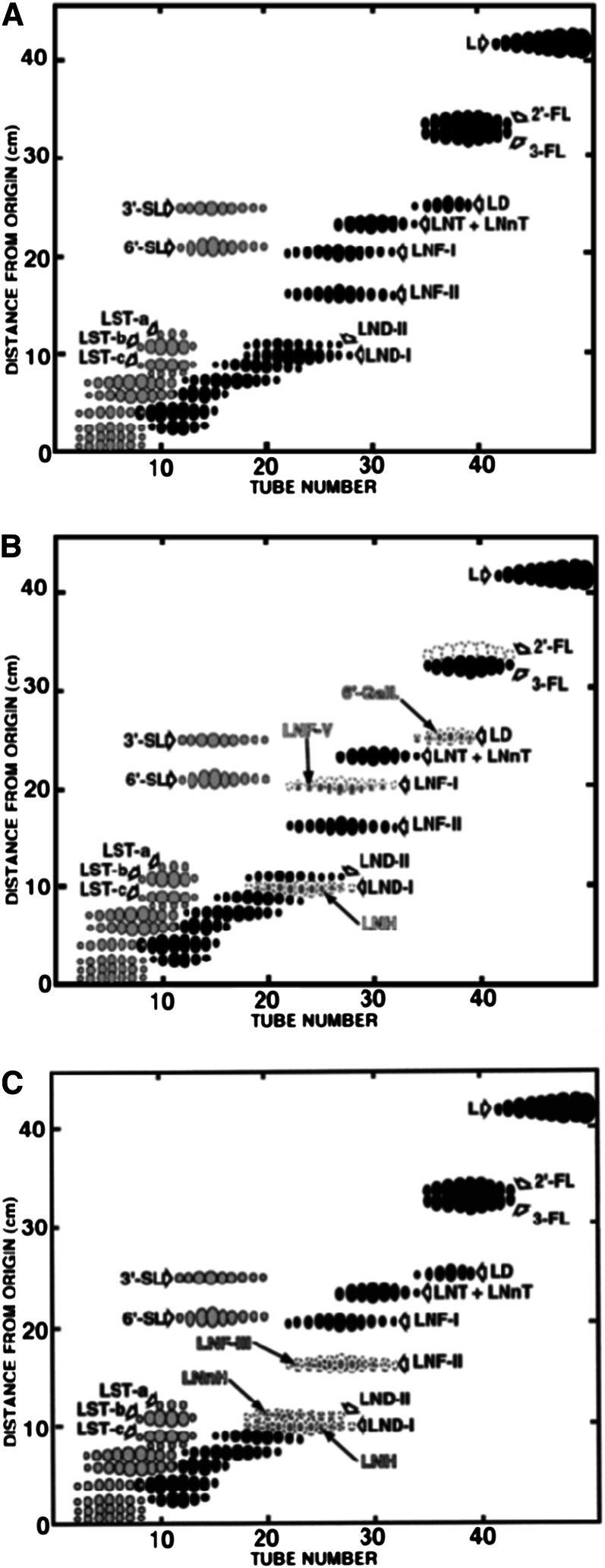
Kobata, first in Ginsburg’s laboratory in Washington and later with his co-workers in Japan, developed a new method to determine the oligosaccharide pattern using only a small amount of milk (reviewed in Reference 19). This method consisted of a combination of gel filtration and paper chromatography, which offered the possibility to show 14 oligosaccharides at the same time in the form of fingerprinting using ∼10 mL of milk (Fig. 2A). They could prove that 3 different oligosaccharide patterns were detectable using this method. Eighty percent of all milk samples showed 14 spots corresponding to the 14 oligosaccharides shown in Figure 2A. In contrast, 15% of the milk samples showed a pattern shown in Figure 2B. The lack of 4 oligosaccharides is characteristic of this pattern. A further interesting observation was that all mothers whose milk showed this pattern were nonsecretors, which means that they neither expressed ABO blood group determinants nor Lewis determinants in their secreted glycoproteins. The structures of the 4 lacking oligosaccharides clearly showed that they were all lacking fucose linked to galactose in the α1,2 position. This means that the secretor organs of these nonsecretor persons did not have a fucosyltransferase, which is responsible for the generation of the disaccharide. The remaining 5% of the milk samples showed the pattern in Figure 2C, in which 3 oligosaccharides are lacking. The examination of those women’s blood group types revealed that they were all Lewis negative, which means that they had neither Lewis A nor Lewis B antigens in their secreted glycoproteins or on their erythrocytes (19).
Figure 2.
A, Fingerprinting of the oligosaccharide fraction obtained from milk samples of Lea+b+ individuals (19). Fraction numbers as indicated by TUBE NUMBER in abscissa were obtained by Sephadex G-25 column chromatography of human milk oligosaccharide fraction. Aliquots of the fractions were spotted at the origin of a sheet of a filter paper and subjected to chromatography using ethyl acetate/pyridine/acetic acid/water (5:5:1:3) as solvent. Black dots represent oligosaccharides visualized by alkaline-AgNO3 reagent, and hatched ones encircled by black line represent those detected by both alkaline-AgNO3 reagent and thiobarbituric acid reagent (Kobata). Reproduced from Reference 19 with permission. B, Fingerprinting of the oligosaccharide fraction obtained from milk samples of nonsecretor individuals. The condition of fingerprinting was the same as in the legend for A. Dots shown by broken lines were missing in the pattern. Gray dots detected at the positions of missing oligosaccharides are minor oligosaccharides hidden under the major oligosaccharides (19). C, Fingerprinting of the oligosaccharide fraction obtained from milk samples of Le a−b− individuals. The condition of fingerprinting and the descriptions of various spots were the same in the legend for A and B (19).
As shown in Table 8, Kobata and co-workers have a tremendous list of classic publications on the development of methods to separate and characterize HMO (18, 19, 29, 30).
Development of new methods for the analysis of complex HMO
Gain in knowledge of HMO has only been possible by establishing advanced methods for the identification and characterization of HMO within the past 60 years of research. One of the leading pioneers in this field is Heinz Egge (Table 9).
Table 9.
Heinz Egge: a pioneer in HMO research1

HMO, human milk oligosaccharides.
Photograph from the author’s private collection.
Egge was involved in glycoconjugate research throughout his entire career, starting as a graduate student with Richard Kuhn at the Max Planck Institute for Medical Research in Heidelberg (31). In the late 1950s, Kuhn’s laboratory elucidated the first structure of a ganglioside, a major glycolipid component of neuronal plasma membranes. Classic methods of the time required large amounts of material; hence, Egge started his structural analysis of gangliosides from 350 kg of bovine brain! Later, following Fritz Zilliken to Bonn and then being appointed Director of the Institute of Physiological Chemistry, he was able to follow his interests in glycoconjugate research, focusing on spectroscopic methods for structural elucidation. His collaboration with others (e.g., H. von Nicolai, P. Hanfland, J. and U. Dabrowski) resulted in a number of publications, which became classics for glycosphingolipid analysis (17, 32, 33). He also was one of the first to introduce fast atom bombardment MS for HMO and to establish it as a general method for mapping and sequencing of native and derivatized glycoconjugates.
In his publication on fucose-containing HMO in 1983, Egge and co-workers used 10 L of pooled human milk and applied classic chromatographic separation methods together with high-performance thin-layer liquid chromatography, HPLC, MS, and 1H-NMR spectroscopy to analyze reduced neutral and peracetylated HMO (34). Compared with conventional methods of determination of carbohydrate constituents, this method was highly reliable, sensitive, and specific. It allowed an exact determination of the molecular composition and also the analysis of mixtures not resolved by chromatographic methods. Because the determinations could be performed with microgram quantities of substance, it was the method of choice. In view of the enormous variability of higher carbohydrate structures, of which only the tip of the iceberg was identified, Egge recommended in 1973 that it would certainly be necessary to analyze the milk of single donors to reduce the number of possible isomers. In the following years, some of Egge’s ideas about HMO have been addressed and are still investigated by his previous co-workers (35–42).
Final remarks
This short review on the development of HMO research ends in the 1980s. However, there is still a continuing strong fascination with these components, which are, together with other components, the reason for the special character of human milk. It is impressive how clearly 50 years ago many of the HMO could be characterized by classic analytical methods, and functions like the influence on microbial composition or the effects on the immune system were investigated. Still today, these main areas of research are given top priority (e.g., see contributions in these proceedings). I would like to stress that apart from the mentioned individuals who influenced HMO research substantially, there are numerous others not mentioned who have also made important contributions, leading to our current knowledge of HMO.
This brief review ends with a quote from Jean Montreuil who wrote in 1993: “History demonstrates the importance of the knowledge of the primary structure to approach the metabolism, molecular biology and mechanisms of the biological activity of HMO. Only the collaboration of scientists belonging to different domains allows to solve the problems” (1).
Acknowledgments
The sole author had responsibility for all parts of the manuscript.
Footnotes
Published in a supplement to Advances in Nutrition. Presented at the conference “The First International Conference on Glycobiology of Human Milk Oligosaccharides” held in Copenhagen, Denmark, May 16–17, 2011. The conference was supported by Glycom A/S, Lyngby, Denmark. The supplement coordinators for this supplement were Clemens Kunz, University of Giessen, Germany, and Sharon M. Donovan, University of Illinois, Urbana, IL. Supplement Coordinator disclosures: Sharon Donovan has received human milk oligosaccharides from Glycom through their HMO biology donations program. Clemens Kunz has received human milk oligosaccharides from Glycom through their HMO biology donation program. The supplement is the responsibility of the Guest Editor to whom the Editor of Advances in Nutrition has delegated supervision of both technical conformity to the published regulations of Advances in Nutrition and general oversight of the scientific merit of each article. The Guest Editor for this supplement was Mark A. McGuire, University of Idaho. Guest Editor disclosure: Mark A. McGuire consults with Abbott Nutrition, a division of Abbott Laboratories, and provides professional opinions on matters related to human lactation and infant nutrition. Further, he collaborates with Dr. Lars Bode, University of California, San Diego, in research related to human milk oligosaccharides. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
Supported by the German Research Foundation (DFG, KU 781/2-2; KU 781/7-2; KU 781/8-2; RU 529/4-3).
Author disclosure: C. Kunz, no conflicts of interest.
Dedicated to Prof. Dr. Heinz Egge on the occasion of his 81st birthday.
Literature Cited
- 1.Montreuil J. The saga of human milk gynolactose. : Renner B, Sawatzki G, New Perspectives in Infant Nutrition. Stuttgart, New York: Georg Thieme Verlag, 1992; pp 3–11 [Google Scholar]
- 2.Nützenadel W. Des Kindes Ernährung - Ein Rückblick in Entwicklung und Perspektiven der Kinder-und Jugendmedizin. 150 Jahre Pädiatrie in Heidelberg S.191–209 Eds. Georg F. Hoffmann, Wolfgang U. Eckart, Philipp Osten. Mainz: Kirchheim Verlag 2010. [Google Scholar]
- 3.Liebig J. Suppe für Säuglinge. Braunschweig: Friedrich Vieweg und Sohn, 1866. [Google Scholar]
- 4.Weirich A, Hoffmann GF. Ernst Moro (1874–1951) – A great pediatric career started at the rise of university-based pediatric research but was curtailed in the shadows of Nazi laws. Eur J Pediatr. 2005;164:599–606 [DOI] [PubMed] [Google Scholar]
- 5.Helmholtz HF. Festschrift Adalbert Czerny–an appreciation. J Pediatr. 1933;3:5–6 [Google Scholar]
- 6.Escherich T. Die Darmbakterien des Säuglings und ihre Beziehungen zur Physiologie der Verdauung. Stuttgart: Enke Verlag, 1886. [Google Scholar]
- 7.Moro E. Über den Bacillus acidophilus n. spec. Ein Beitrag zur Kenntnis der normalen Darmbakterien des Säuglings. Jahrbuch Kinderh. 1900;52:38–55 [Google Scholar]
- 8.Polonowski M, Lespagnol A. Sur deux nouveaux sucres du lait de femme, le gynolactose et l’allolactose. C R Acad Sci. 1931;192:1319 [Google Scholar]
- 9.Polonowski M, Lespagnol A. Sur la nature glucidique de la substance lévogyre du lait de Femme. Bull Soc Biol. 1929;101:61–3 [Google Scholar]
- 10.Polonowski M, Montreuil J. Etude chromatographique des polyosides du lait de Femme. C R Acad Sci Paris. 1954;238:2263–4 [PubMed] [Google Scholar]
- 11.Bratosin D, Mignot C. In memoriam Professor Emeritus Jean Montreul (1920–2010). A lifetime dedicated to the progress of science and education. Cytometry B Clin Cytom. Epub 2011 Jan 14 [DOI] [PubMed] [Google Scholar]
- 12.Montreuil J. Les glucides du lait. Bull Soc Chim Biol (Paris). 1960;42:1399–427 [PubMed] [Google Scholar]
- 13.Grimmonprez L, Montreuil J. Etude des fractions glycanniques des glycosphingolipides totaux de la membrane des globules lipidiques du lait de femme. Biochimie. 1977;59:899–907 [PubMed] [Google Scholar]
- 14.Aminozucker KR. Angew Chem. 1957;69:23–33 [Google Scholar]
- 15.Kuhn R, Baer HH. Die Konstitution der Lacto-N-tetraose. Chem Ber. 1956;89:504–11 [Google Scholar]
- 16.Kuhn R. Les oligosaccharides du lait. Bull Soc Chim Biol (Paris). 1958;40:297–314 [PubMed] [Google Scholar]
- 17.Egge H. The diversity of oligosaccharides in human milk. In: Renner B, Sawatzki G, editors. New Perspectives in Infant Nutrition. Stuttgart, New York: Georg Thieme Verlag, 1992, pp 12–26 [Google Scholar]
- 18.Kobata A. A journey to the world of glycobiology. Glycoconj J. 2000;17:443–64 [DOI] [PubMed] [Google Scholar]
- 19.Kobata A. Possible application of milk oligosaccharides for drug development. Chang Gung Med J. 2003;26:621–36 [PubMed] [Google Scholar]
- 20.Tissier H. 1900 Recherches sur la flore intestinale des nourrissons (état normal et pathologique). G. Carre and C. Naud, Paris, France. [Google Scholar]
- 21.Schönfeld H. Über die Beziehungen der einzelnen Bestandteile der Frauenmilch zur Bifidusflora. Jahrbuch der Kinderh. 1926;113:19–60 [Google Scholar]
- 22.Barness LA, Tomarelli RM. Paul György (1893–1976). A biographical sketch. J Nutr. 1979;109:19–23 [PubMed] [Google Scholar]
- 23.Westphal O. Richard Kuhn zum Gedächtnis. Angew Chem. 1968;80:501–40 [Google Scholar]
- 24.Kuhn R, Brossmer R. Über die O-Acetyl-lactamin-säure-lactose aus Kuh-Colostrum und ihre Spaltbarkeit durch Influenza-Virus. Chem Ber. 1956;98:2013–35 [Google Scholar]
- 25.György P, Norris RF, Rose CS. Bifidus factor I: A variant of lactobacillus bifidus requiring a special growth factor. Arch Biochem Biophys. 1954;48:193–201 [DOI] [PubMed] [Google Scholar]
- 26.György P. A hitherto unrecognized biochemical difference between human milk and cow’s milk. Pediatrics. 1953;11:98–108 [PubMed] [Google Scholar]
- 27.Watkins WM. Blood group specific substances. : Gottschalk A, editor Glycoproteins. Amsterdam: Elsevier, 1966; pp 462–515 [Google Scholar]
- 28.Grollman EF, Ginsburg V. Correlation between secretor status and the occurrence of 2′-fucosyllactose in human milk. Biochem Biophys Res Commun. 1967;28:50–3 [DOI] [PubMed] [Google Scholar]
- 29.Kobata A. Milk glycoproteins and oligosaccharides. : Horowitz MI, Pigman W, The Glycoconjugates. New York: Academic Press, 1977; pp 423–40 [Google Scholar]
- 30.Yamashita K, Mizuochi T, Kobata A. Oligosaccharides of human milk. : Ginsburg V, editor Methods in Enzymology. New York: Academic Press, 1982, pp 105–26 [DOI] [PubMed] [Google Scholar]
- 31.Peter-Katalinic J, Sandhoff K. Highlight: Glycobiology. Biol Chem. 2001;381:141–2 [Google Scholar]
- 32.Egge H, Peter-Katalinic J, Karas M, Stahl B. The use of fast atom bombardment and laser desorption mass spectrometry in the analyses of complex carbohydrates. Pure Appl Chem. 1991;63:491–8 [Google Scholar]
- 33.Egge H, Peter-Katalinic J. Fast atom bombardment mass spectrometry for the structural elucidation of glycoconjugates. Mass Spectrom Rev. 1987;6:331–93 [Google Scholar]
- 34.Egge H, Dell A, von Nicolai H. Fucose containing oligosaccharides from human milk. I. Separation and identification of new constituents. Arch Biochem Biophys. 1983;224:235–53 [DOI] [PubMed] [Google Scholar]
- 35.Wieruszeski JM, Cekkor A, Bouquelet S, Montreuil J, Strecker G, Peter-Katalinic J, Egge H. Structure of two new oligosaccharides isolated from human milk: sialylated lacto-N-fucopentaoses I and II. Carbohydr Res. 1985;137:127–38 [DOI] [PubMed] [Google Scholar]
- 36.Bruntz R, Dabrowski U, Dabrowski J, Ebersold A, Peter-Katalinic J, Egge H. Fucose-containing oligosaccharides from human milk from a donor of blood group O, Le a nonsecretor. Biol Chem Hoppe Seyler. 1988;369:257–73 [PubMed] [Google Scholar]
- 37.Kunz C, Rudloff S, Hintelmann A, Pohlentz G, Egge H. High-pH anion-exchange chromatography with pulsed amperometric detection and molar response factors of human milk oligosaccharides. J Chromatogr B Biomed Appl. 1996;685:211–21 [DOI] [PubMed] [Google Scholar]
- 38.Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk. Structural, functional and metabolic aspects. Annu Rev Nutr. 2000;20:699–722 [DOI] [PubMed] [Google Scholar]
- 39.Dreisewerd K, Kölbl S, Peter-Katalinic J, Berkenkamp S, Pohlentz G. Analysis of native milk oligosaccharides directly from thin-layer chromatography plates by matrix-assisted laser desorption/ionization orthogonal-time-of-flight mass spectrometry with a glycerol matrix. J Am Soc Mass Spectrom. 2006;17:139–50 [DOI] [PubMed] [Google Scholar]
- 40.Rudloff S, Obermeier S, Borsch C, Pohlentz G, Hartmann R, Brösicke H, Lentze MJ, Kunz C. Incorporation of orally applied 13C-galactose into milk lactose and oligosaccharides. Glycobiology. 2006;16:477–87 [DOI] [PubMed] [Google Scholar]
- 41.Kuntz S, Kunz C, Rudloff S. Oligosaccharides from human milk induce growth arrest via G2/M by influencing growth-related cell cycle genes in intestinal epithelial cells. Br J Nutr. 2009;101:1306–15 [DOI] [PubMed] [Google Scholar]
- 42.Rudloff S, Pohlentz G, Borsch C, Lentze MJ, Kunz C. Urinary excretion of in vivo 13C-labelled milk oligosaccharides in breastfed infants. Br J Nutr. 2011;5:1–7 [DOI] [PubMed] [Google Scholar]