Abstract
Human milk oligosaccharides (HMO) are discussed to play a crucial role in an infant’s development. Lewis blood group epitopes, in particular, seem to remarkably contribute to the beneficial effects of HMO. In this regard, large-scale functional human studies could provide evidence of the variety of results from in vitro investigations, although increasing the amount and complexity of sample and data handling. Therefore, reliable screening approaches are needed. To predict the oligosaccharide pattern in milk, the routine serological Lewis blood group typing of blood samples can be applied due to the close relationship between the biosynthesis of HMO and the Lewis antigens on erythrocytes. However, the actual HMO profile of the individual samples does not necessarily correspond to the serological determinations. This review demonstrates the capabilities of merging the traditional serological Lewis blood group typing with the additional information provided by the comprehensive elucidation of individual HMO patterns by means of state-of-the-art analytics. Deduced from the association of the suggested HMO biosynthesis with the Lewis blood group, the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry profiles of oligosaccharides in individual milk samples exemplify the advantages and the limitations of sample assignment to distinct groups.
Introduction
Free lactose-derived oligosaccharides in human milk (HMO)6 are present at concentrations ranging approximately from 10 to 20 g/L; hence, several grams of these unique components pass through the breast-fed infant’s gut daily (1–3). Various health protective actions have been deduced from in vitro investigations, i.e., prebiotic, anti-infective, or immune effects (4, 5), which might partially be associated with the presence of fucosylated oligosaccharide structures. Those are determined by the expression of the secretor (Se) and Lewis (Le) genes in the mammary gland. Hence, distinct patterns of milk oligosaccharides according to the Le/Se types Le(a+b−) non-Se, Le(a−b+) Se, and Le(a−b−) Se or non-Se genes have been described by several investigators (2, 6, 7). The prevalence in white individuals averages 22%, 72%, and 6%, respectively (8), whereas 80% of the Europeans secrete ABH substances in saliva and other secretions; thus, they are typed as Se (9, 10). Observational studies indicate that certain HMO from Secretor are associated with various preventive effects, such as reducing diarrhea and promoting intestinal maturation in preterm neonates (11, 12).
The final evidence of the functionality of the Le-related HMO compounds in humans has to be confirmed by intervention studies with large numbers of participants. Therefore, reliable high-throughput screening methods are needed to determine the oligosaccharide profiles in individual milk samples. Due to high inter- and intraindividual variations in HMO expression, the relationship between the serologically detectable Le blood group and the corresponding oligosaccharide pattern in milk can only be used for a crude milk classification, even though serological detection as a routine method is rapid and highly practicable. Nevertheless, detailed information on the HMO composition in milk samples provides a more accurate indication of the substances actually ingested by the infant. For this purpose, new developments in the field of glycomics have great potential for facilitating the handling of large sample and data sets from follow-up investigations on the correlation of Le-active components in infants’ feeding and their health (13–15).
In this review, we demonstrate the correlation of the serologically detected Le blood group and the expressed HMO pattern in the milk of the donors and show how this information can be enhanced by recent high-throughput HMO screening methods. On the basis of the suggested biosynthesis of HMO, specific variances in the HMO pattern are taken as examples to highlight the necessity of careful individual milk sample analysis.
Biosynthesis of Le and Se gene-related oligosaccharides in milk
In the past years, the structures of the major HMO have been thoroughly characterized (16, 17). From these data, some structural rules have been deduced and the biosynthetic pathways of the neutral HMO have been proposed (Fig. 1). Unfortunately, experimental data on the biosynthesis of HMO are lacking to date.
Figure 1.
Biosynthesis of neutral complex human milk oligosaccharides (HMO). The assumed biosynthetic pathway starts from the activated monosaccharides and includes the most important enzymes only [N-acetylglucosaminyltransferases (GlcNAcT)]: iβ3GlcNAcT attaches N-acetylglucosamine (GlcNAc) in the β1–3 position to terminal galactose (Gal), Iβ6GlcNAcT attaches GlcNAc in β1–6 position to terminal Gal. Galactosyltransferases (GalT): β3GalT attaches Gal in the β1–3 position to GlcNAc and β4GalT attaches Gal in the β1–4 position to GlcNAc. Fucosyltransferases (FucT): α2FucT attaches fucose (Fuc) in the α1–2 position to terminal Gal, secretor (Se) enzyme, α3FucT attaches Fuc in the α1–3 position to GlcNAc, α3/4FucT attaches Fuc in the α1–3/4 position to GlcNAc and in the α1–3 position to Glc of the lactose core, Lewis (Le) enzyme . The no entry signs mean that no further elongation takes place. Fucosylation is indicated exemplarily for terminal type 1 and type 2 chains. Glycan structures are depicted according to the recommendations of the Consortium of Functional Glycomics using the GlycoWorkbench software tool (94).
Because the reducing end of the unbound oligosaccharides from milk consistently contains lactose, which is the major macronutrient in human milk, this disaccharide is assumed to be the initial substrate for HMO synthesis. Lactose is formed in the Golgi apparatus by the action of the lactose synthase complex containing α-lactalbumin and β1–4-galactosyltransferase (18). UDP-activated galactose is attached to Glc-I-P with high affinity due to the presence of α-lactalbumin, which is only expressed in the lactating mammary gland of mammals.
We speculate that analogous to the O-glycosylation of proteins in the Golgi of submaxillary and gastrointestinal secreting cells, the glycosyltransferases for HMO synthesis might occur as membrane-bound glycoproteins and process the oligosaccharide sequentially by the addition of a single monosaccharide from sugar nucleotides. Those are synthesized in the cytosol and conveyed to the Golgi lumen via specific membrane antiporters, e.g., GLUT1 for monophosphorylated glucose (19–21).
Thus, elongation (a), branching (b), and fucosylation (c)of lactose and derived structures might be performed by the concerted action of (a) iβ1–3-N-acetylglucosaminyltransferase and β1–3- and β1–4-galactosyltransferase for type 1 and type 2 chains, respectively, and (b) Iβ1–6-N-acetylglucosaminyltransferase as depicted in Figure 1. Following the suggested rules of HMO synthesis, no further elongation is observed for a terminal type 1 chain (indicated by a no entry sign in Figure 1) (7, 22, 23). The final fucosylation and Le antigen formation is achieved by the consecutive action of α1–2-, α1–3-, or α1–3/4-fucosyltransferases (FucT)(c), as summarized in Figure 2.
Figure 2.
The Lewis (Le) and Secretor (Se) gene–related glycan epitopes. The Le and Se epitopes, which are characteristic for the Le phenotype in red blood cells and in human milk, are synthesized by the listed fucosyltransferases (FucTs). The Le and Se genes code for the active FucTs in presence of at least 1 functional allele (heterozygous with Lele or Sese, homozygous with LeLe or SeSe). The prevalence of the Le phenotypes is conferred to Europeans (8). Fuc, fucose; Gal, galactose; GlcNAc, N-acetylglucosamine.
The presence of at least 1 functional allele of the Le gene results in the expression of an α1–3/4-FucT (FucTIII), which is able to attach GDP-activated fucose (Fuc) in the O-4 position to N-acetylglucosamine in type 1 (Galβ1–3GlcNAc) chains, resulting in Le epitopes, as shown in Figure 1. The same enzyme forms O-3-Fuc units at the N-acetylglucosamine residue of type 2 (Galβ1–4GlcNAc) chains yielding Lex and, in Secretors, Ley epitopes, however with lower specificity than for type 1 substrates due to steric aspects (24). The O-3-fucosylation of the reducing glucose residue is known to be accomplished by the Le-gene–dependent FucTIII as well (25). Furthermore, the formation of Lex and Ley epitopes can also be performed by different α1–3-FucTs, i.e., FucTIII–VII and FucTIX (26).
Although secretory tissues and fluids have predominantly FucTII activity encoded by the Se gene, i.e., milk (27–29), saliva, or stomach tissue (30), in human serum, both FucTII and the H gene–controlled FucTI are present (31). Both enzymes transfer GDP-activated Fuc in α1–2-position to β-D-galactosides prior to the formation of Leb and Ley epitopes (Fig. 1).
The Le and Se gene–encoded FucTs compete for the substrates so that in Secretors Lea structures are also found in milk and other secretions, but not on erythrocytes or in plasma (29, 32).
Individuals with mutations resulting in the nonfunctional FucTs FucTII and FucTIII are usually typed as non-Se and Le negative or Le(a−b−), respectively (26, 33) and therefore should not secrete α1–2- and/or α1–4-fucosylated structures into milk.
Functional aspects of Le blood group#x2013related HMO
Despite the fact that the Le histo-blood group system was discovered more than half a century ago, our knowledge about its biological functions is based mainly on speculations. In contrast, the role of the α1–3-FucTs IV and VII, which synthesize Lex and Ley epitopes, seems to be proven because their corresponding genes are highly conserved among mammals and contribute to the formation of selectin ligands (34).
Considering that the Le and Se genes are mainly expressed in secretory tissues, which are in contact with the environment and, therefore, with a large number of various microorganisms, the manifold carbohydrate antigens in secretions and epithelial cells might provide protection against pathogens (32, 34). Marionneau et al. (34) suggested that providing different cell surface receptors for several pathogens, Lea antigens accomplish resistance against Leb-binding pathogens and vice versa. Microbial lectins recognize host glycans in the gut, which are presented by mucins and glycolipids, enclosing ABH and Le blood group recognition sites. This promotes colonization, which may have adverse health effects in case of pathogen adhesion (35). HMO bearing Le epitopes and other recognition sites attach to the pathogens and inhibit their adherence to intestinal cell surfaces. Subsequently, the pathogen-HMO complex can be excreted (36, 37).
Many attempts have been made to investigate the functions of HMO, including Le-specific structures; however, most of these studies were conducted in vitro. A brief overview is given in Table 1. HMO have antiadhesive properties, possibly resulting in the reduction of infections with Campylobacter jejuni, Escherichia coli, Vibrio cholerae, Shigella, and Salmonella species or in a decrease in HIV-1 mother-to-child transmission. The bifidogenic effect of HMO, which has been known for decades, might also be assisted by the interaction of bifidobacteria with the Le epitopes because Bifidobacterium infantis is able to both use and bind different glycans from human milk and intestinal cells in vitro, including Le-specific structures (37) (see also other symposium papers).
Table 1.
Effects of Lewis and secretor gene–related factors1
| Effect | Factor | Investigated in | Method | Reference |
| In vitro | ||||
| Bifidogenic | Lewis a, type 1 H-trisaccharide | Bifidobacterium longum ssp. infantis | Genotyping; glycan array | 37 |
| Antiadhesive | HMO, i.a. Lewis-epitope bearing | Campylobacter jejuni, Escherichia coli, Vibrio cholerae, Shigella,Salmonella, HIV-1 | Various | 5 |
| Antiadhesive vs. C. jejuni | α1–2-Fuc-HMO | Carcinoma-derived human epithelial cells | Bacterial adherence assay | 38 |
| Ex vivo | ||||
| Antiadhesive vs. C. jejuni | α1–2-Fuc-Lac, neutral HMO | Fresh human intestinal mucosa | Bacterial adherence assay | 38 |
| In vivo | ||||
| Colonization with C. jejuni reduced | α1–2-Fuc-oligosaccharides | Pups of transgenic mice | CFU counting after intestinal resection | 38 |
| Preventive vs. diarrhea from C. jejuni, calicivirus | α1–2-Fuc-HMO | Infants | Serological blood group classification; HPLC (HMO quantification) | 39 |
| Preventive vs. diarrhea from E. coli | α1–2-Fuc-HMO | Infants | Serological blood group classification; HPLC (HMO quantification) | 12 |
| Association with mortality, gram-negative sepsis, and necrotizing enterocolitis | Low or nonsecretor status | Preterm infants | Genotyping; phenotyping (enzyme immunoassay in saliva) | 11 |
| Association with Crohn’s disease | Nonsecretor status | Pediatric/adult individuals | Genotyping | 40 |
CFU, colony-forming units; Fuc, fucose; HMO, human milk oligosaccharides; Lac, lactose.
Because the availability of HMO compounds adequate for interventional clinical trials has been limited to date, in vivo functional studies are rare. Nevertheless, data from observational investigations give an insight into the possible associations between the Le or Se phenotypes and diseases. α1–2-fucosylated HMO decreased Campylobacter jejuni infections in mice in vivo (38) and significantly prevented diarrhea in breast-fed infants in a dose-dependent manner (12, 39). Furthermore, low or non-Se status was strongly associated with adverse outcomes in preterm infants, e.g., mortality and necrotizing enterocolitis (11), and with Crohn’s disease (40), giving further indication for the involvement of α1–2-fucosylated structures in the immune-related processes of gut development and health.
Considering the potentially important role of Le and Se epitopes in the infant’s digestive tract, it is noteworthy that infants are typed Le(a−b−) in the first months of life, as discussed in the following section. This could be due to a reduced Le and Se antigen expression in the immature gut of neonates because gastrointestinal epithelial cells are suggested to be the main source for Le-specific glycolipids in blood after reabsorption (41, 42). Nevertheless, strong Lea activity has been detected consistently in the fecal samples collected after birth (meconium) as well as at the 6-mo follow-up. Leb reactivity, if present, was complementary to Lea activity. Even though the investigation was not representative, it is striking that the feces of the formula-fed neonates exhibited slightly lower Le reactivity than their exclusively breast-fed counterparts (43).
Because several grams of HMO pass through the breast-fed infant’s gut daily, they may compensate for the initial lack of Le and Se antigens in the neonate’s intestine.
In addition to the lower production of Le and Se antigens in the newborn, there are also observations of decreased Fuc content of fecal glycans in younger infants (44) and formula-fed infants compared with older and breast-fed infants, respectively (45). Fucosylated HMO in milk of Se but not in Lea non-Se decrease steadily in the first 3 mo of lactation (2), which might be due to an adaptation of the oligosaccharide composition in milk to the infant’s gut maturity. Interestingly, HMO seem to be involved in the infant’s intestinal cell maturation (46). In a recent study, the fecal oligosaccharides of mixed-fed infants resembled the breast milk oligosaccharide patterns with few modifications. In contrast, the fecal oligosaccharide profiles from exclusively breast-fed preterm infants were substantially different from those of their mothers’ milk, showing an intense metabolism in the digestive tract. Interestingly, the authors reported the additional modification of the HMO with ABH epitopes several weeks postpartum (47), a phenomenon first described by Lundblad (48) as well. Albrecht et al. (47) explained these time-dependent variations in the infant’s individual gastrointestinal adaptation to enteral food.
Le-specific HMO might also contribute to the protective effect of breastfeeding against urinary tract infections (49) because uropathogenic E. coli has been found to attach to glycolipids in non-Se women, presumably causing more urinary tract infections than in Se, in whom the receptor is masked by the additional α1–2-Fuc (50). Some of the Le- and Se-specific HMO structures have been detected in the urine of lactating women (51, 52) and, more recently, also in the urine of breast-fed infants. The data suggest that the intact compounds reach the circulation after absorption in the infant’s gut and might, therefore, display systemic and local effects in the infant (3, 53).
Identification of Le blood group#x2013related compounds
Traditionally, Le blood group determination is performed by serological methods, which can become a challenging task. Cross-reactions of the commonly used antibodies are described as well as the presence of side products leading to misinterpretation of results (54). For example, healthy Le(a−b−) and Le(a+b−) individuals can show a slight expression of Leb epitopes in plasma, which may be due to mutation with incomplete inactivation of FucTIII and FucTII, respectively (55–57). The saliva of Lea non-Se can also contain Leb antigens as a result of slight FucTI activity in the salivary glands (58).
Red blood cells (RBC) do not synthesize Le antigens themselves, but acquire them secondarily from tissues (59, 60). Therefore, the serological phenotyping of RBCs does not necessarily describe the Le genotype of a donor because the Le epitope expression may differ in various tissues (61–63). Genetic factors and several conditions such as diseases, infections, transfusions, and bone marrow transplantations can lead to alterations in the Le phenotype, hence, leading to misinterpretation of the blood group determination (64–68).
In pregnancy, the prevalence of Le(a−b−)-typed women can increase threefold, which is most likely due to an increased attachment of Le-active glycolipids to plasma lipoproteins with a subsequent decrease in the antigen quantity on erythrocytes (63, 69). In these individuals, discordant Le phenotypes can be detected on RBC and saliva.
A similar situation can be observed in neonates. Most of them are typed Le(a−b−) by serological detection on RBC, whereas in saliva, Le- and Se-related epitopes are already expressed, according to the genotype of the infant (70). Several weeks after birth, Lea antigen can also be detected on erythrocytes, whereas Leb antigens are fully present in blood only at the age of 6 y due to delayed activation of the Se gene–controlled FucTII (71).
Because of the discrepancies and the numerous influencing factors of the Le phenotype determination in various tissues and body fluids, the data obtained from RBC phenotyping for Le blood group identification need to be regarded with suspicion (72). Nevertheless, as routine method in the clinical sector, it is convenient for screening purposes. For the assignment of milk samples to the distinct Le groups, the serological RBC-based typing of the milk donors remains a valuable tool to roughly estimate the HMO pattern expressed in milk. The link between the Le blood group and the oligosaccharide profile in milk is explained by the correspondence between the biosynthesis of the Le-active glycans present on RBC and the formation of free fucosylated oligosaccharides in the mammary gland (see previously). However, because of the described variations in serological phenotyping as well as individual and lactation time-specific alterations in HMO expression, serology by itself is not sufficient to predict the relative amount of single Le-type oligosaccharides in milk. Detailed information on the expressed oligosaccharide patterns might be a more appropriate basis for future research on HMO, especially for functional clinical studies and investigations on the metabolism and the biological activity of HMO.
HMO monitoring of individual samples and sample mixtures is an important research topic about which excellent studies were conducted in the past. Various methods starting from paper chromatography, HPLC, and high-pH anion-exchange chromatography over mass spectrometric methods to latest developments in HPLC chip mass spectrometry (MS) and capillary gel electrophoresis laser–induced fluorescence techniques have been applied (17, 73–82). All these powerful methods provide detailed insights into the oligosaccharide pattern of individual milk samples, frequently paired with further information about the relative amount of single isomers. Some of these techniques require sophisticated and time-consuming sample preparation procedures and/or large quantities of sample material, which is a drawback for large sample sets. The combination of a simple, automated, and standardized sample workup procedure combined with standard matrix-assisted laser desorption/ionization time-of-flight MS (MALDI-TOF-MS) analysis provides the analytical power needed for a high-throughput glycomics approach for HMO profiling (74).
Variation of the HMO pattern
Automated MALDI-TOF-MS(/MS) as an HMO screening method enables the Le blood group correlation of a large milk sample set and delivers, at the same time, the distinct HMO pattern of each milk sample with high reliability (74).
Recent findings in the oligosaccharide pattern of single milk samples show the necessity of individual sample monitoring. The discrepancy described between the serologically detected blood group and the expression of Le antigens in other body fluids and tissues seems to be conferrable to Le-type oligosaccharides in human milk.
From the genetic point of view, a more consistent oligosaccharide pattern might be expected within a distinct Le blood group (83). However, several studies showed that the proportion of distinct oligosaccharide structures can vary greatly among individual milk samples, depending on the lactation period and/or Le status (2, 4, 74, 75, 81, 84).
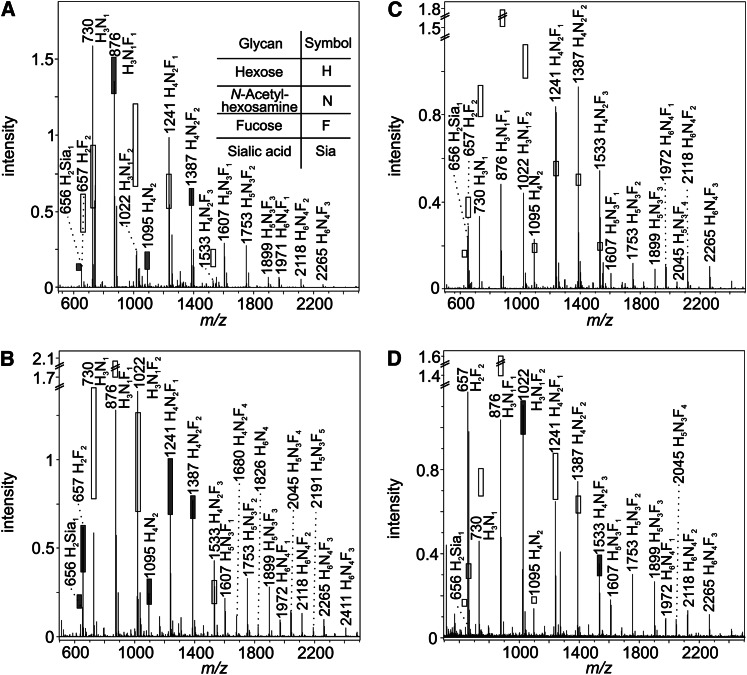
The results of the MALDI-TOF-MS(/MS) screening of 40 milk samples from mothers with serologically determined Le blood group underlined these findings. Figure 3 displays mass spectra from 2 Le(a−b−) and 2 Le(a−b+) donors, which differ markedly in their HMO pattern. The 95% CIs calculated for each signal within an Le blood group are indicated by shaded and open bars. In case of coincidence between the measured signal intensity and the predicted interval, the bar is shaded; otherwise, the CI is shown by an open bar. Each signal represents a distinct oligosaccharide composition with several structural isomers.
Figure 3.
Matrix-assisted laser desorption/ionization time-of-flight MS profile spectra of 4 individual milk samples. Spectra displayed were obtained from 4 women: 2 serologically typed as Le(a−b−) (A,B) and 2 as Le(a−b+) (C,D), respectively. The signals represent sodium adducts. The 95% CI, calculated for each human milk oligosaccharide composition, base on data from 40 individual milk samples. CI are illustrated by open bars if the measured signal intensity is not in the expected range and by shaded bars if it is. Relative CIs are described in Reference 74 and are applied to the measured signal intensities. Due to high variance in the signal intensities of the high molecular weight HMO, CIs were only calculated for signals up to m/z 1533. Compositions are calculated using GlycoPeakfinder software (95).
The HMO pattern of a serologically Le(a−b−)-typed woman is depicted in Figure 3 A. A strong overexpression of lacto-N-(neo)tetraose [LN(n)T] (m/z 730), as well as the monofucosylated lacto-N-(neo)hexaose [LN(n)H] (m/z 1241) was apparent. The multiply fucosylated species, such as difucosyllactose (m/z 657), difucosylated LN(n)T (m/z 1022) and the trifucosylated LN(n)H (m/z 1533) were substantially lower than expected or even absent. All of these 3 structures should contain a remarkable amount of α1–2-Fuc, whereas monofucosylated and difucosylated LN(n)H might contain mainly α1–3/4 fucosylated compounds. This leads to the conclusion that the donor might have an inefficient α1–2-FucT in the mammary gland, which is Se-gene dependent. Two explanations are possible for the anomaly in the depicted mass spectrum: i) this donor might belong to the rare subgroup of Le(a−b−) non-Se and ii) the donor’s Le phenotype is actually Le(a+b−) because the HMO profile resembles that of an Le(a+b−) pattern with regard to the calculated CIs (data not shown).
The second Le(a−b−) sample showed the opposite phenomenon (Fig. 3 B). The nonfucosylated precursor for the complex HMO, LN(n)T (m/z 730) and its monofucosylated form redundant (m/z 876) were underexpressed, whereas the multiply fucosylated species difucosylated LN(n)T (m/z 1022) and trifucosylated LN(n)H (m/z 1533) were overexpressed. Moreover, unusually intensive signals up to a mass of 2500 Da were detected in high abundance in this specific sample. This suggests that this donor expressed several highly efficient glycosyltransferases as well as FucTs forming a diverse HMO pattern also in the higher mass range, conforming to an Le(a−b+) HMO pattern rather than the expected Le(a−b−)-specific one. Because the HMO spectra shown in Figure 3A and B are atypical Le(a−b−) and resemble those characteristic of Le(a+b−) and Le(a−b+), respectively, the suspicion is raised that the milk HMO profiles may also reflect the partial discordance of the serological RBC- and saliva-based Le phenotyping in pregnant women, as discussed previously.
Figure 3C shows the HMO spectrum of an Le(a−b+) donor expressing an atypical HMO pattern, which cannot be assigned to any Le blood group. All signals from m/z 657 to 1022 were expressed in substantially lower intensity than expected, whereas the signals in the mass range from m/z 1095 to 1533 were overexpressed. Hence, a shift to high molecular weight HMO can be observed for this particular sample. As already discussed for Figure 3B, also in this example highly efficient glycosyltransferases might be responsible for the observed variation.
Only slight deviations in the Le(a−b+) spectrum are seen in Figure 3D, except for the unexpectedly high proportion of difucosyllactose (m/z 657).
The demonstrated variations in the MALDI-TOF-MS profile spectra confirm the conclusion of Thurl et al. (2) that each lactating woman expresses an individual HMO pattern, even though an assignment of the HMO profiles to the distinct Le blood groups was applicable for the majority of the milk samples in our investigations (see later).
The importance of screening methods to detect the individual oligosaccharide profile is further emphasized by the tandem mass spectrometry analysis of the precursor m/z 1022 in a milk sample from a woman typed Le(a+b−) (Fig. 4). As a major isomer, an LN(n)T core bearing 1 Fuc at the reducing end and the Lea/x epitope at the nonreducing end is expected in a milk sample from an Le(a+b−) donor. The most intensive fragment signals at m/z 730 (Y4βB4α) and m/z 876 (Y4β) result from the dissociation of 1 and 2 Fuc residues, respectively [fragment ions are designated in accordance with the nomenclature of Domon and Costello (85)]. In addition, the signals m/z 696 (B3α), m/z 511 (Y2α), and m/z 365 (Y2αB4α) underline the presence of the likeliest precursor structure. Strikingly, an indication for the presence of an Leb/y epitope is given by the signal m/z 680 (B2α), a difucosylated N-acetyllactosamine unit. The serologically detected Le blood group does not explain the presence of an Leb/y epitope because of the lack of the Se gene–dependent FucTII in Le(a+b−) individuals. Nevertheless, the difucosylation of either a terminal type 1 or 2 N-acetyllactosamine unit in this milk sample is an indication for α1–2-FucT activity. This finding confirms the unexpected presence of α1–2-fucosyl HMO in 2 serologically typed Le(a+b−) donors by Newburg et al. (12). These findings might be explained by a slight activity of the H gene–controlled FucTI, which has been detected at least in the saliva of Le(a+b−)-typed individuals (58).
Figure 4.
Matrix-assisted laser desorption/ionization time-of-flight MS/MS analysis of purified human milk oligosaccharides of a Lewis (a+b−) donor. Inset shows range from m/z 650 to m/z 720 at 50× magnification. The obtained fragment ions were assigned according to the recommendations of the Consortium of Functional Glycomics using GlycoWorkbench (94). Fragment ions are designated in accordance with the nomenclature of Domon and Costello (85). In some cases, fragments may be formed by different fragmentation pathways, only 1 of which is illustrated. All fragment ions represent sodium adducts. The unexpected signal is circled in red.
The application of the fragmentation analysis of individual oligosaccharides for structural characterization is described for various MS techniques (17, 73, 82, 86–93). Hence, tandem MS analysis can also provide additional structural information for individual HMO compositions in the case of HMO screening.
The presented examples demonstrate the drawbacks of serological Le blood group classification and emphasize the need for individual sample mapping. Le phenotyping in human milk by MALDI-TOF-MS(/MS) analysis and subsequent statistical data evaluation provide the opportunity not only to assign the specimens to definite groups, but also to reveal unusual tendencies for each individual milk sample, including its unique HMO profile (74).
Figure 5 shows the results from a discriminant analysis of 40 individual milk samples measured threefold. Using the new screening approach, 95% of the samples were correctly assigned to the serologically detected Le phenotype in blood by at least 2 of 3 measurements. Specifically, 99% of all serologically Le(a−b+), 100% of the Le(a+b−), and 68% of the Le(a−b−) typed samples were assigned to the previously determined Le blood group. Nevertheless, the partial inhomogeneity of the Le-specific HMO profiles described previously is also evident from the wide distribution of the samples within the Le blood groups. The overlapping area of the Le(a−b+) and Le(a−b−) typed samples, in particular, displays the similarity of the oligosaccharide profiles in several specimens with a different Le phenotype, most likely resulting from their Se gene activity. However, the fact that the majority of the milk samples were matched to the serologically detected Le blood group shows that a classification using both approaches can be useful despite the variations in the expression level of single oligosaccharide composition in milk. The location of each breakpoint in the coordinate plan therefore provides information about the Le phenotype tendency of a distinct milk sample and at the same time information about its actual oligosaccharide pattern. Consequently, the new MS screening approach provides a fast and material-saving option for individual milk sample mapping with detailed information on the expression level of individual oligosaccharide compositions.
Figure 5.
Discriminant analysis. The results obtained for 113 single matrix-assisted laser desorption/ionization time-of-flight MS and redundant MS/MS measurements of 40 milk samples underwent discriminant analysis. Discriminant function 1 is plotted on the x-axis and discriminant function 2 on the y-axis. Open diamonds, red squares, and green triangles represent HMO samples from Le(a−b+), Le(a+b−), and Le(a−b−) donors, respectively. The distribution of each group is indicated by colored shading. Reproduced with kind permission from Springer Science+Business Media (74), Figure 7.
CONCLUSION
Serological Le blood group determination can only offer a first indication of the expressed oligosaccharide pattern in human milk. Modern high-throughput screening methods can support the traditional serological RBC analysis and provide detailed information on the relative abundance for each oligosaccharide composition, thus, enhancing or qualifying the conclusions of functional studies. In particular, the capability of individual glycan epitope recognition and its relationship to observable, biologically relevant effects will be of great benefit. Furthermore, reliable Le phenotype screening, e.g., by the MALDI-TOF-MS approach presented here, can replace serological determination on erythrocytes if no blood sample is available.
The variations between the serologically detected Le blood group and the Le phenotype in other body fluids and tissues, which are frequently reported in literature, were also detectable in human milk using the novel screening approach. Based on the determined individual HMO patterns, some speculations about the activities of certain glycosyltransferases in the milk donors were deduced by taking into consideration the information from the suggested HMO biosynthetic pathway.
The combination of the traditional serological Le blood group detection supported by modern milk screening methods will lead to a solid glycan characterization as the basis for future research on the effects of HMO from native milk.
Acknowledgments
We thank Professor G. Bein and Professor H. Jomaa (Institute of Immunology and Transfusion Medicine, University Hospital Giessen-Marburg, Germany) for performing the Lewis blood group determination of blood samples as well as Dr. P. Gilbert and his team (St. Josef’s Hospital Giessen) for the collection of milk and blood samples. All authors read and approved the final manuscript.
Footnotes
Published in a supplement to Advances in Nutrition. Presented at the conference “The First International Conference on Glycobiology of Human Milk Oligosaccharides” held in Copenhagen, Denmark, May 16–17, 2011. The conference was supported by Glycom A/S, Lyngby, Denmark. The supplement coordinators for this supplement were Clemens Kunz, University of Giessen, Germany, and Sharon M. Donovan, University of Illinois, Urbana, IL. Supplement Coordinator disclosures: Sharon Donovan has received human milk oligosaccharides from Glycom through their HMO biology donations program. Clemens Kunz has received human milk oligosaccharides from Glycom through their HMO biology donation program. The supplement is the responsibility of the Guest Editor to whom the Editor of Advances in Nutrition has delegated supervision of both technical conformity to the published regulations of Advances in Nutrition and general oversight of the scientific merit of each article. The Guest Editor for this supplement was Mark A. McGuire, University of Idaho. Guest Editor disclosure: Mark A. McGuire consults with Abbott Nutrition, a division of Abbott Laboratories, and provides professional opinions on matters related to human lactation and infant nutrition. Further, he collaborates with Dr. Lars Bode, University of California, San Diego, in research related to human milk oligosaccharides. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
Author disclosures: D. Blank, V. Dotz, R. Geyer, and C. Kunz, no conflicts of interest.
Authors contributed equally to this work.
Abbreviations used: Fuc, fucose; FucT, fucosyltransferase; HMO, human milk oligosaccharide; Le, Lewis; LN(n)H, lacto-N-(neo)hexaose; LN(n)T, lacto-N-(neo)tetraose; MALDI-TOF-MS, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; RBC, red blood cell; Se, secretor.
Literature Cited
- 1.Urashima T, Asakuma S, Leo F, Fukuda K, Messer M, Oftedal OT. Possible significance of the predominance of type I oligosaccharides, a feature specific to human breast milk. Adv Nutr. [DOI] [PMC free article] [PubMed]
- 2.Thurl S, Munzert M, Henker J, Boehm G, Müller-Werner B, Jelinek J, Stahl B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br J Nutr. 2010;104:1261–71 [DOI] [PubMed] [Google Scholar]
- 3.Rudloff S, Pohlentz G, Borsch C, Lentze MJ, Kunz C. Urinary excretion of in vivo 13C-labelled milk oligosaccharides in breastfed infants. Br J Nutr. 2011 [DOI] [PubMed] [Google Scholar]
- 4.Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr. 2000;20:699–722 [DOI] [PubMed] [Google Scholar]
- 5.Bode L. Human milk oligosaccharides: prebiotics and beyond. Nutr Rev. 2009;67: Suppl 2:S183–91 [DOI] [PubMed] [Google Scholar]
- 6.Erney R, Hilty M, Pickering L, Ruiz-Palacios G, Prieto P. Human milk oligosaccharides: a novel method provides insight into human genetics. Adv Exp Med Biol. 2001;501:285–97 [PubMed] [Google Scholar]
- 7.Kobata A. Structures and application of oligosaccharides in human milk. Proc Jpn Acad, Ser B, Phys Biol Sci. 2010;86:731–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Race RR, Sanger R. Blood groups in man. 6th ed Oxford: Blackwell Scientific Publications; 1975. p. 585–8 [Google Scholar]
- 9.Grubb R. Correlation between Lewis blood group and secretor character in man. Nature. 1948;162:933. [DOI] [PubMed] [Google Scholar]
- 10.Schiff F, Sasaki H. Der Ausscheidungstypus, ein auf serologischem Wege nachweisbares mendelndes Merkmal. Klin Wochenschr. 1932;11:1426–9 [Google Scholar]
- 11.Morrow AL, Meinzen-Derr J, Huang P, Schibler KR, Cahill T, Keddache M, Kallapur SG, Newburg DS, Tabangin M, Warner BB, et al. Fucosyltransferase 2 non-secretor and low secretor status predicts severe outcomes in premature infants. J Pediatr. 2011;158:745–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newburg DS, Ruiz-Palacios GM, Altaye M, Chaturvedi P, Meinzen-Derr J, Guerrero Mde L, Morrow AL. Innate protection conferred by fucosylated oligosaccharides of human milk against diarrhea in breastfed infants. Glycobiology. 2004;14:253–63 [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Telford JE, Knezevic A, Rudd PM. High-throughput glycoanalytical technology for systems glycobiology. Biochem Soc Trans. 2010;38:1374–7 [DOI] [PubMed] [Google Scholar]
- 14.Rakus JF, Mahal LK. New technologies for glycomic analysis: toward a systematic understanding of the glycome. Annu Rev Anal Chem (Palo Alto Calif). 2011;4:367–92 [DOI] [PubMed] [Google Scholar]
- 15.Ruhaak LR, Hennig R, Huhn C, Borowiak M, Dolhain RJ, Deelder AM, Rapp E, Wuhrer M. Optimized workflow for preparation of APTS-labeled N-glycans allowing high-throughput analysis of human plasma glycomes using 48-channel multiplexed CGE-LIF. J Proteome Res. 2010;9:6655–64 [DOI] [PubMed] [Google Scholar]
- 16.Urashima T, Fukuda K, Kitaoka M, Ohnighi M, Terabayashi T, Kobata A. Milk oligosaccharides. New York: Nova Science Publishers, Inc.; 2011 [Google Scholar]
- 17.Wu S, Tao N, German JB, Grimm R, Lebrilla CB. The development of an annotated library of neutral human milk oligosaccharides. J Proteome Res. 2010;9:4138–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brew K, Hill RL. Lactose biosynthesis. Rev Physiol Biochem Pharmacol. 1975;72:105–58 [DOI] [PubMed] [Google Scholar]
- 19.Brockhausen I. Biosynthesis. Biosynthesis of O-glycans of the N-acetylglucosamine-α-Ser/Thr linkage type. In: Glycoproteins. Montreuil J, Vliegenthart J, Schachter H, editors. Amsterdam: Elsevier Ltd.; 1995. p. 201–50.
- 20.Roth J. Biosynthesis 4c. Compartmentation of glycoprotein biosynthesis. In: Glycoproteins. Montreuil J, Vliegenthart J, Schachter H, editors. Amsterdam: Elsevier Ltd.; 1995. p. 287–312.
- 21.McManaman JL, Neville MC. Mammary physiology and milk secretion. Adv Drug Deliv Rev. 2003;55:629–41 [DOI] [PubMed] [Google Scholar]
- 22.Almeida R, Amado M, David L, Levery SB, Holmes EH, Merkx G, van Kessel AG, Rygaard E, Hassan H, Bennett E, et al. A family of human β4-galactosyltransferases. Cloning and expression of two novel UDP-galactose:β-N-acetylglucosamine β1, 4-galactosyltransferases, β4Gal-T2 and β4Gal-T3. J Biol Chem. 1997;272:31979–91 [DOI] [PubMed] [Google Scholar]
- 23.Sasaki K, Kurata-Miura K, Ujita M, Angata K, Nakagawa S, Sekine S, Nishi T, Fukuda M. Expression cloning of cDNA encoding a human β-1,3-N-acetylglucosaminyltransferase that is essential for poly-N-acetyllactosamine synthesis. Proc Natl Acad Sci U S A. 1997;94:14294–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khare DP, Ole H, Lemieux RU. The synthesis of monodeoxy derivatives of lacto-N-biose I and N-acetyl-lactosamine to serve as substrates for the differentiation of α-l-fucosyl transferases. Carbohydr Res. 1985;136:285–308 [Google Scholar]
- 25.Eppenberger-Castori S, Lötscher H, Finne J. Purification of the N-acetylglucosaminide α(1–3/4)fucosyltransferase of human milk. Glycoconj J. 1989;6:101–14 [DOI] [PubMed] [Google Scholar]
- 26.Koda Y, Soejima M, Kimura H. The polymorphisms of fucosyltransferases. Leg Med (Tokyo). 2001;3:2–14 [DOI] [PubMed] [Google Scholar]
- 27.Betteridge A, Watkins WM. Variant forms of α-2-L-fucosyltransferase in human submaxillary glands from blood group ABH “Secretor” and "Non-secretor" individuals. Glycoconj J. 1985;2:61–78 [Google Scholar]
- 28.Shen L, Grollman EF, Ginsburg V. An enzymatic basis for secretor status and blood group substance specificity in humans. Proc Natl Acad Sci U S A. 1968;59:224–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watkins WM. Molecular basis of antigenic specificity in the ABO, H and Lewis blood-group systems. In: Glycoproteins. Montreuil J, Vliegenthart J, Schachter H, editors. Amsterdam: Elsevier Science B.V.; 1995. p. 313–90.
- 30.Chester MA, Watkins WM. α-L-fucosyltransferases in human submaxillary gland and stomach tissues associated with the H, Lea and Leb blood-group characters and ABH secretor status. Biochem Biophys Res Commun. 1969;34:835–42 [DOI] [PubMed] [Google Scholar]
- 31.Le Pendu J, Cartron JP, Lemieux RU, Oriol R. The presence of at least two different H-blood-group-related β-D-gal α-2-L-fucosyltransferases in human serum and the genetics of blood group H substances. Am J Hum Genet. 1985;37:749–60 [PMC free article] [PubMed] [Google Scholar]
- 32.Daniels G. Human blood Groups. 2nd ed Hoboken: Wiley-Blackwell; 2002. p. 7–98 [Google Scholar]
- 33.Bhende YM, Deshpande CK, Bhatia HM, Sanger R, Race RR, Morgan WT, Watkins WMA. "new" blood group character related to the ABO system. Lancet. 1952;1:903–4 [PubMed] [Google Scholar]
- 34.Marionneau S, Cailleau-Thomas A, Rocher J, Le Moullac-Vaidye B, Ruvoën N, Clemént M, Le Pendu J. ABH and Lewis histo-blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochimie. 2001;83:565–73 [DOI] [PubMed] [Google Scholar]
- 35.Lane JA, Mehra RK, Carrington SD, Hickey RM. The food glycome: a source of protection against pathogen colonization in the gastrointestinal tract. Int J Food Microbiol. 2010;142:1–13 [DOI] [PubMed] [Google Scholar]
- 36.Kunz C, Bode L, Rudloff S. Genetic variability of human milk oligosaccharides: Are there biological consequences? In: Nestlé nutrition workshop series, Genetic expression and nutrition. Bachmann C, and Koletzko B. Vevey: Les Presses de la Venoge; 2003. p 21–2.
- 37.Garrido D, Kim JH, German JB, Raybould HE, Mills DA. Oligosaccharide binding proteins from Bifidobacterium longum subsp. infantis reveal a preference for host glycans. PLoS ONE. 2011;6:e17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruiz-Palacios GM, Cervantes LE, Ramos P, Chavez-Munguia B, Newburg DS. Campylobacter jejuni binds intestinal H(O) antigen (Fucα1, 2Galß1,4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J Biol Chem. 2003;278:14112–20 [DOI] [PubMed] [Google Scholar]
- 39.Morrow AL, Ruiz-Palacios GM, Altaye M, Jiang X, Guerrero ML, Meinzen-Derr JK, Farkas T, Chaturvedi P, Pickering LK, Newburg DS. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J Pediatr. 2004;145:297–303 [DOI] [PubMed] [Google Scholar]
- 40.McGovern DP, Jones MR, Taylor KD, Marciante K, Yan X, Dubinsky M, Ippoliti A, Vasiliauskas E, Berel D, Derkowski C, et al. Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn's disease. Hum Mol Genet. 2010;19:3468–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanfland P, Graham HA. Immunochemistry of the Lewis-blood-group system: partial characterization of Le(a)-, Le(b)-, and H-type 1 (LedH)-blood-group active glycosphingolipids from human plasma. Arch Biochem Biophys. 1981;210:383–95 [DOI] [PubMed] [Google Scholar]
- 42.Ramsey G, Sherman LA. Blood component recalls in the United States, 1998. Transfusion. 2000;40:253–4 [DOI] [PubMed] [Google Scholar]
- 43.Larson G, Falk P, Hynsjö P, Midtvedt A, Midtvedt T. Faecal excretion of glycosphingolipids of breast-fed and formula-fed infants. Microb Ecol Health Dis. 1990;3:305–19 [Google Scholar]
- 44.Pang KY, Bresson JL, Walker WA. Development of gastrointestinal surface. VIII. Lectin identification of carbohydrate differences. Am J Physiol. 1987;252:G685–91 [DOI] [PubMed] [Google Scholar]
- 45.Albrecht S, Schols HA, van Zoeren D, van Lingen RA, Groot Jebbink LJ, van den Heuvel EG, Voragen AG, Gruppen H. Oligosaccharides in feces of breast- and formula-fed babies. Carbohydr Res. 2011;346:2173–81 [DOI] [PubMed] [Google Scholar]
- 46.Kuntz S, Kunz C, Rudloff S. Oligosaccharides from human milk induce growth arrest via G2/M by influencing growth-related cell cycle genes in intestinal epithelial cells. Br J Nutr. 2009;101:1306–15 [DOI] [PubMed] [Google Scholar]
- 47.Albrecht S, Schols HA, van den Heuvel EG, Voragen AG, Gruppen H. Occurrence of oligosaccharides in feces of breast-fed babies in their first six months of life and the corresponding breast milk. Carbohydr Res. 2011;346:2540–50 [DOI] [PubMed] [Google Scholar]
- 48.Lundblad A. The persistence of milk oligosaccharides in the gastrointestinal tract of infants. In: New perspectives in infant nutrition. Renner B, Sawatzki G, editors. Stuttgart/New York: Thieme; 1993. p. 66–73.
- 49.Mårild S, Jodal U, Hanson LA. Breastfeeding and urinary-tract infection. Lancet. 1990;336:942. [DOI] [PubMed] [Google Scholar]
- 50.Stapleton A, Nudelman E, Clausen H, Hakomori S, Stamm WE. Binding of uropathogenic Escherichia coli R45 to glycolipids extracted from vaginal epithelial cells is dependent on histo-blood group secretor status. J Clin Invest. 1992;90:965–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lundblad A. Oligosaccharides from human urine. Methods Enzymol. 1978;50:226–35 [DOI] [PubMed] [Google Scholar]
- 52.Zopf DA, Ginsburg V, Hallgren P, Jonsson AC, Lindberg BS, Lundblad A. Determination of Leb-active oligosaccharides in urine of pregnant and lactating women by radioimmunoassay. Eur J Biochem. 1979;93:431–5 [DOI] [PubMed] [Google Scholar]
- 53.Rudloff S, Pohlentz G, Diekmann L, Egge H, Kunz C. Urinary excretion of lactose and oligosaccharides in preterm infants fed human milk or infant formula. Acta Paediatr. 1996;85:598–603 [DOI] [PubMed] [Google Scholar]
- 54.Henry SM, Jovall PA, Ghardashkhani S, Gustavsson ML, Samuelsson BE. Structural and immunochemical identification of Leb glycolipids in the plasma of a group O Le(a-b-) secretor. Glycoconj J. 1995;12:309–17 [DOI] [PubMed] [Google Scholar]
- 55.Björk S, Breimer ME, Hansson GC, Karlsson KA, Leffler H. Structures of blood group glycosphingolipids of human small intestine. A relation between the expression of fucolipids of epithelial cells and the ABO, Le and Se phenotype of the donor. J Biol Chem. 1987;262:6758–65 [PubMed] [Google Scholar]
- 56.Henry SM, Oriol R, Samuelsson BE. Detection and characterization of Lewis antigens in plasma of Lewis-negative individuals. Evidence of chain extension as a result of reduced fucosyltransferase competition. Vox Sang. 1994;67:387–96 [DOI] [PubMed] [Google Scholar]
- 57.Miller EB, Rosenfield RE, Vogel P, Haber G, Gibbel N. The Lewis blood factors in American Negroes. Am J Phys Anthropol. 1954;12:427–43 [DOI] [PubMed] [Google Scholar]
- 58.Wang B, Akiyama K, Kimura H. Quantitative analysis of Lea and Leb antigens in human saliva. Vox Sang. 1994;66:280–6 [DOI] [PubMed] [Google Scholar]
- 59.Marcus DM, Cass LE. Glycosphingolipids with Lewis blood group activity: uptake by human erythrocytes. Science. 1969;164:553–5 [DOI] [PubMed] [Google Scholar]
- 60.Sneath JS, Sneath PH. Transformation of the Lewis groups of human red cells. Nature. 1955;176:172. [DOI] [PubMed] [Google Scholar]
- 61.Mollicone R, Reguigne I, Fletcher A, Aziz A, Rustam M, Weston BW, Kelly RJ, Lowe JB, Oriol R. Molecular basis for plasma α(1,3)-fucosyltransferase gene deficiency (FUT6). J Biol Chem. 1994;269:12662–71 [PubMed] [Google Scholar]
- 62.Ørntoft TF, Holmes EH, Johnson P, Hakomori S, Clausen H. Differential tissue expression of the Lewis blood group antigens: enzymatic, immunohistologic, and immunochemical evidence for Lewis a and b antigen expression in Le(a-b-) individuals. Blood. 1991;77:1389–96 [PubMed] [Google Scholar]
- 63.Yazawa S, Oh-kawara H, Nakajima T, Hosomi O, Akamatsu S, Kishi K. Histo-blood group Lewis genotyping from human hairs and blood. Jpn J Hum Genet. 1996;41:177–88 [DOI] [PubMed] [Google Scholar]
- 64.Hirano K, Kawa S, Oguchi H, Kobayashi T, Yonekura H, Ogata H, Homma T. Loss of Lewis antigen expression on erythrocytes in some cancer patients with high serum CA19–9 levels. J Natl Cancer Inst. 1987;79:1261–8 [PubMed] [Google Scholar]
- 65.Langkilde NC, Wolf H, Ørntoft TF. Lewis negative phenotype and bladder cancer. Lancet. 1990;335:926. [DOI] [PubMed] [Google Scholar]
- 66.Makni S, Dalix AM, Caillard T, Compagnon B, Le Pendu J, Ayed K, Oriol R. Discordance between red cell and saliva Lewis phenotypes in patients with hydatid cysts. Exp Clin Immunogenet. 1987;4:136–43 [PubMed] [Google Scholar]
- 67.Needs ME, McCarthy DM, Barrett J. ABH and Lewis antigen and antibody expression after bone marrow transplantation. Acta Haematol. 1987;78:13–6 [DOI] [PubMed] [Google Scholar]
- 68.Stigendal L, Olsson R, Rydberg L, Samuelsson BE. Blood group Lewis phenotype on erythrocytes and in saliva in alcoholic pancreatitis and chronic liver disease. J Clin Pathol. 1984;37:778–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hammar L, Månsson S, Rohr T, Chester MA, Ginsburg V, Lundblad A, Zopf D. Lewis phenotype of erythrocytes and Leb-active glycolipid in serum of pregnant women. Vox Sang. 1981;40:27–33 [DOI] [PubMed] [Google Scholar]
- 70.Lawler SD, Marshall R. Lewis and secretor characters in infancy. Vox Sang. 1961;6:541–54 [DOI] [PubMed] [Google Scholar]
- 71.Jordal K. The Lewis blood groups in children. Acta Pathol Microbiol Scand. 1956;39:399–406 [PubMed] [Google Scholar]
- 72.Henry S, Oriol R, Samuelsson B. Lewis histo-blood group system and associated secretory phenotypes. Vox Sang. 1995;69:166–82 [DOI] [PubMed] [Google Scholar]
- 73.Albrecht S, Schols HA, van den Heuvel EG, Voragen AG, Gruppen H. CE-LIF-MSn profiling of oligosaccharides in human milk and feces of breast-fed babies. Electrophoresis. 2010;31:1264–73 [DOI] [PubMed] [Google Scholar]
- 74.Blank D, Gebhardt S, Maass K, Lochnit G, Dotz V, Blank J, Geyer R, Kunz C. High-throughput mass finger printing and Lewis blood group assignment of human milk oligosaccharides. Anal Bioanal Chem. 2011;401:2495–510 [DOI] [PubMed] [Google Scholar]
- 75.Kobata A, Ginsburg V, Tsuda M. Oligosaccharides of human milk. I. Isolation and characterization. Arch Biochem Biophys. 1969;130:509–13 [DOI] [PubMed] [Google Scholar]
- 76.Kunz C, Rudloff S, Hintelmann A, Pohlentz G, Egge H. High-pH anion-exchange chromatography with pulsed amperometric detection and molar response factors of human milk oligosaccharides. J Chromatogr B Biomed Appl. 1996;685:211–21 [DOI] [PubMed] [Google Scholar]
- 77.Leo F, Asakuma S, Nakamura T, Fukuda K, Senda A, Urashima T. Improved determination of milk oligosaccharides using a single derivatization with anthranilic acid and separation by reversed-phase high-performance liquid chromatography. J Chromatogr A. 2009;1216:1520–3 [DOI] [PubMed] [Google Scholar]
- 78.Mariño K, Lane JA, Abrahams JL, Struwe W, Harvey DJ, Marotta M, Hickey RM, Rudd PM. Method for milk oligosaccharide profiling by 2-aminobenzamide labelling and hydrophilic interaction chromatography (HILIC). Glycobiology. 2011;21:1317–30 [DOI] [PubMed] [Google Scholar]
- 79.Niñonuevo MR, Perkins PD, Francis J, Lamotte LM, LoCascio RG, Freeman SL, Mills DA, German JB, Grimm R, Lebrilla CB. Daily variations in oligosaccharides of human milk determined by microfluidic chips and mass spectrometry. J Agric Food Chem. 2008;56:618–26 [DOI] [PubMed] [Google Scholar]
- 80.Thurl S, Henker J, Siegel M, Tovar K, Sawatzki G. Detection of four human milk groups with respect to Lewis blood group dependent oligosaccharides. Glycoconj J. 1997;14:795–9 [DOI] [PubMed] [Google Scholar]
- 81.Viverge D, Grimmonprez L, Cassanas G, Bardet L, Solere M. Discriminant carbohydrate components of human milk according to donor secretor types. J Pediatr Gastroenterol Nutr. 1990;11:365–70 [DOI] [PubMed] [Google Scholar]
- 82.Yang H, Yu Y, Song F, Liu S. Structural Characterization of Neutral Oligosaccharides by Laser-Enhanced In-Source Decay of MALDI-FTICR MS. J Am Soc Mass Spectrom. 2011;22:845–55 [DOI] [PubMed] [Google Scholar]
- 83.Mollicone R, Candelier JJ, Reguigne I, Couillin P, Fletcher A, Oriol R. Molecular genetics of α-L-fucosyltransferase genes (H, Se, Le, FUT4, FUT5 and FUT6). Transfus Clin Biol. 1994;1:91–7 [DOI] [PubMed] [Google Scholar]
- 84.Stahl B, Thurl S, Henker J, Siegel M, Finke B, Sawatzki G. Detection of four human milk groups with respect to Lewis-blood-group-dependent oligosaccharides by serologic and chromatographic analysis. Adv Exp Med Biol. 2001;501:299–306 [DOI] [PubMed] [Google Scholar]
- 85.Domon B, Costello CE. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj J. 1988;5:397–409 [Google Scholar]
- 86.Amano J, Osanai M, Orita T, Sugahara D, Osumi K. Structural determination by negative-ion MALDI-QIT-TOFMSn after pyrene derivatization of variously fucosylated oligosaccharides with branched decaose cores from human milk. Glycobiology. 2009;19:601–14 [DOI] [PubMed] [Google Scholar]
- 87.Broberg A. High-performance liquid chromatography/electrospray ionization ion-trap mass spectrometry for analysis of oligosaccharides derivatized by reductive amination and N,N-dimethylation. Carbohydr Res. 2007;342:1462–9 [DOI] [PubMed] [Google Scholar]
- 88.Ferreira JA, Domingues MR, Reis A, Monteiro MA, Coimbra MA. Differentiation of isomeric Lewis blood groups by positive ion electrospray tandem mass spectrometry. Anal Biochem. 2010;397:186–96 [DOI] [PubMed] [Google Scholar]
- 89.Niñonuevo MR, Park Y, Yin H, Zhang J, Ward RE, Clowers BH, German JB, Freeman SL, Killeen K, Grimm R, et al. A strategy for annotating the human milk glycome. J Agric Food Chem. 2006;54:7471–80 [DOI] [PubMed] [Google Scholar]
- 90.Pfenninger A, Karas M, Finke B, Stahl B. Structural analysis of underivatized neutral human milk oligosaccharides in the negative ion mode by nano-electrospray MSn (part 1: methodology). J Am Soc Mass Spectrom. 2002a;13:1331–40 [DOI] [PubMed] [Google Scholar]
- 91.Pfenninger A, Karas M, Finke B, Stahl B. Structural analysis of underivatized neutral human milk oligosaccharides in the negative ion mode by nano-electrospray MSn (part 2: application to isomeric mixtures). J Am Soc Mass Spectrom. 2002;13:1341–8 [DOI] [PubMed] [Google Scholar]
- 92.Wu S, Grimm R, German JB, Lebrilla CB. Annotation and structural analysis of sialylated human milk oligosaccharides. J Proteome Res. 2011;10:856–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamagaki T, Suzuki H, Tachibana K. A comparative study of the fragmentation of neutral lactooligosaccharides in negative-ion mode by UV-MALDI-TOF and UV-MALDI ion-trap/TOF mass spectrometry. J Am Soc Mass Spectrom. 2006;17:67–74 [DOI] [PubMed] [Google Scholar]
- 94.Ceroni A, Maass K, Geyer H, Geyer R, Dell A, Haslam SM. GlycoWorkbench: a tool for the computer-assisted annotation of mass spectra of glycans. J Proteome Res. 2008;7:1650–9 [DOI] [PubMed] [Google Scholar]
- 95.Maass K, Ranzinger R, Geyer H, von der Lieth CW, Geyer R. “Glyco-peakfinder”--de novo composition analysis of glycoconjugates. Proteomics. 2007;7:4435–44 [DOI] [PubMed] [Google Scholar]