Abstract
The infant intestinal microbiota is shaped by genetics and environment, including the route of delivery and early dietary intake. Data from germ-free rodents and piglets support a critical role for the microbiota in regulating gastrointestinal and immune development. Human milk oligosaccharides (HMO) both directly and indirectly influence intestinal development by regulating cell proliferation, acting as prebiotics for beneficial bacteria and modulating immune development. We have shown that the gut microbiota, the microbial metatranscriptome, and metabolome differ between porcine milk–fed and formula-fed (FF) piglets. Our goal is to define how early nutrition, specifically HMO, shapes host-microbe interactions in breast-fed (BF) and FF human infants. We an established noninvasive method that uses stool samples containing intact sloughed epithelial cells to quantify intestinal gene expression profiles in human infants. We hypothesized that a systems biology approach, combining i) HMO composition of the mother’s milk with the infant’s gut gene expression and fecal bacterial composition, ii) gene expression, and iii short-chain fatty acid profiles would identify important mechanistic pathways affecting intestinal development of BF and FF infants in the first few months of life. HMO composition was analyzed by HLPC Chip/time-of-flight MS and 3 HMO clusters were identified using principle component analysis. Initial findings indicated that both host epithelial cell mRNA expression and the microbial phylogenetic profiles provided strong feature sets that distinctly classified the BF and FF infants. Ongoing analyses are designed to integrate the host transcriptome, bacterial phylogenetic profiles, and functional metagenomic data using multivariate statistical analyses.
Introduction
A mother’s microbiota and breast milk and the infant’s microbiome represent an intricately linked triad that is central to an infant’s intestinal and immune development (1). Almost immediately after birth (2), the infant acquires an intestinal microbiota that is seeded by maternally derived microbes (3), nurtured by components in human milk (HM)9 (4), and shaped by the infant’s genetic background (5). Key to establishing the microbiota are the infant’s route of delivery (3), which dictates the degree of exposure to the mother’s vaginal and fecal microbes, and early nutrition, which determines the infant’s exposure to dietary oligosaccharides (2, 5, 6). Nutrients and bioactive components in HM directly influence the development of the infant’s immune system (7, 8), actively protect the infant from pathogenic infection (7, 9), and facilitate the establishment of the microbiota (5, 6), the latter of which is required to activate the mucosal immune system (10). As such, HM provides a means whereby a mother can nourish and protect her infant by promoting immune development and decreasing the incidence and/or severity of infectious diseases (11–13).
It has long been appreciated that the composition of the microbiota differs between breast-fed (BF) and formula-fed (FF) infants with a higher proportion of bifidobacteria species in the BF infant (14). In the past decade, nucleic acid–based approaches have been applied to define the succession of the neonatal microbiota (2–4, 15, 16). These culture-independent approaches have uncovered a greater diversity of microbes in the neonatal intestine than had been previously appreciated, while confirming that bifidobacteria constitute 60% to 91% and ∼50% of the fecal bacterial community of BF and FF infants, respectively (4, 15, 16). This preponderance of bifidobacteria led to the speculation of the presence of bifidus factor in HM, which was initially identified as N-acetylglycosamine–containing oligosaccharides (17). The application of modern analytical techniques over the past decade has revealed that HM contains a rich diversity of oligosaccharides (HMO) (18). Furthermore, the HMO concentration exceeds that of any other species’ milk by 10- to 100-fold (19, 20), suggesting a potentially unique role of HMO in human infant development. Indeed, HMO exert broad-spectrum benefit for the infant, including acting as a component of the immunity of HM by modulating the infant’s immune development, blocking the attachment of pathogens, and serving as prebiotics to promote colonization by a healthy gut microbiota (9, 21, 22), including bifidobacteria (23).
The neonatal gastrointestinal tract undergoes pronounced structural and functional changes in response to feeding (7). Although HM is generally considered to be trophic for the gut, proper gut development must balance proliferation, differentiation, and apoptosis (7). Kuntz et al. (24, 25) reported that HMO are strong inhibitors of proliferation and inducers of differentiation in intestinal cells in vitro by altering cell cycle dynamics via corresponding regulator genes and mitogen-activated protein kinase signaling (25). Thus, HMO have the potential to modulate neonatal gut growth through direct interactions with epithelial cells as well as indirectly, by modulating the microbiota and their fermentation products, such as butyrate, which may be trophic for gut epithelial cells (26).
Our long-term goal is to elucidate host-microbe interactions within the neonatal intestine in which the impact of diet on microbial colonization, bacterial metabolites, and gene expression and host neonatal intestinal gene expression are being measured concurrently. In this review, the current evidence linking HMO to host-microbe interactions in the infant gut is discussed as well as initial findings from our endeavor to systematically integrate genomic data from both the infant (host mucosa) and gut microbiota to define host gene-diet interactions within the context of the structure and operations of gut microbial communities.
Current status of knowledge
What components in the diet affect the intestinal microbiota?
The composition of the intestinal microbiota is highly variable in early infancy and largely stabilizes by the end of the first year of life (27). In BF infants, Bifidobacterium spp. become the predominant group of organisms by ∼3 mo of age (4), whereas FF infants develop a microbial community composed of some bifidobacteria, but also Bacteroides, Clostridia, Enterococcus, and Staphylococcus (14). HM promotes the growth of Bifidobacterium spp. This bifidogenic activity is likely attributed to both the protein and carbohydrate components in HM. For example, growth of bifidobacteria is promoted by lactoferrin both in vitro (28) and in vivo (29). In addition, peptides produced by in vitro proteolytic digestion of lactoferrin and secretory component are bifidogenic (30). However, most recent studies have focused on HMO as the primary bifidogenic components of HM (22). Indeed, findings from the laboratories of David Miller, Carlito Lebrilla, and colleagues at the University of California at Davis provide evidence that Bifidobacterium longum subsp. infantis is uniquely adapted for the BF infant (6, 22). Parallel glycoprofiling of HMO established that B. longum subsp. infantis ATCC15697 efficiently consumes several predominant small-mass HMO isomers (31). Genome sequencing of Bifidobacterium confirmed that B. infantis strains share several large clusters containing all of the genes necessary for transport and enzymatic degradation of HMO (32). Thus, the microbiota of the BF infant contains bacteria that are specialized to metabolize HMO. The implications of these findings is described in greater detail in other articles in this supplement (33, 34).
What bacteria and their genes are involved in the host-microbe interaction?
Mammals exist in a mutalistic relationship with their microbiota in which the host provides nutrients and a stable environment for the microbiota, which in turn stimulates development and metabolic activity of the host (35–37). There is abundant evidence from mice and humans documenting that microbes adapt to their environmental niche and concomitantly influence host phenotype (38–42). For example, it was recently demonstrated that crosstalk between lymphocytes, microbiota, and the intestinal epithelium governs immunity versus metabolism in the gut (43). However, precisely how diet shapes the structure and function of gut microbial communities in newborn infants is poorly understood. Given that the microbiota and dietary composition differ between BF and FF infants, it is reasonable to hypothesize that the microbial metatranscriptome would also differ. To address this hypothesis, pyrosequencing-based whole-transcriptome shotgun sequencing was used to evaluate community-wide gut microbial gene expression in the cecal contents of 21-day-old piglets fed either with porcine milk (PM) or formula (44). PM contains both acidic (sialylated) and neutral oligosaccharides (PMO) (45). Using MS, 29 distinct PMO structures were detected by in PM, of which most were sialylated (e.g., sialyllactose). Six fucosylated PMO were also detected (45). The presence of oligosaccharides was not measured in the PM replacer formula fed to the piglets in this study; however, most cow milk–based formulas contain only very low concentrations of sialyllactose (46). Microbial DNA and RNA were harvested, and both cDNA libraries and 16S recombinant DNA amplicons were sequenced. Communities were similar at the phylum level, but were dissimilar at the genus level; Prevotella was the dominant genus in PM samples and Bacteroides was most abundant in formula samples. The functional annotations of screened cDNA sequences were assigned by the metagenomics RAST annotation pipeline and based on best BLASTX hits to the NCBI COG database. Patterns of gene expression were very similar in PM-fed and FF animals; all samples were enriched with transcripts encoding proteins involved in carbohydrate and protein metabolism, stress response, binding to host epithelium and lipopolysaccharide metabolism. However, expression of genes involved in amino acid metabolism (e.g., arginine metabolism) and oxidative stress were enriched in PM-fed versus FF piglets (44). This finding may have clinical relevance because low serum arginine, a precursor of nitric oxide production, confers an increased risk of necrotizing enterocolitits (NEC) (47), and the incidence of NEC is lower in preterm infants fed HM (48). To further define the impact of diet on intraluminal metabolites, cecal contents of the same piglets were analyzed by GC/MS (49). Sugars, amino sugars, fatty acids, especially unsaturated fatty acids, and sterols were identified as being among the most important metabolites for distinguishing between PM-fed and FF groups. Joint analysis of microbiota and metabolomics pinpointed specific sets of metabolites associated with the dominant bacterial taxa. Therefore, we concluded that diet composition has a significant role in defining the microbiota and metabolic products in the developing gut. Interestingly, recent studies indicate that our piglet data are translatable to human infant stool samples (49, 50). In summary, tandem analysis of intestinal microbial, transcriptomic, and metabolic profiles is a powerful tool for delineating of the role of diet in health and disease and, ultimately, designing specific strategies to alter microbial behavior to improve clinical outcome.
Which host genes are involved in the host-microbe interaction and respond to bacterial signals?
Gnotobiotic animals provide a unique model in which to dissect the influence of a single bacteria or a complex microbiota on the host (51). Studies in germ-free animals have confirmed that the commensal microbiota is required for normal intestinal epithelial cell proliferation and migration and maintenance of villus morphology (35), immune development (36), energy balance, regulation of blood pressure, and risk of disease (37–39). A now common experimental approach is to colonize germ-free animals with a complex microbiota or to monoassociate with a single microbe (36–41, 51). Colonization of gnotobiotic piglets with a conventional microbiota (CM) induced the expression of genes contributing to intestinal epithelial cell turnover, mucus biosynthesis, and priming of the immune system (52, 53). Gut growth (53), digestive enzyme mRNA expression activities (54), and chemokines and their receptors (55) were differentially affected when gnotobiotic piglets were colonized with CM versus gram-negative Escherichia coli or gram-positive Lactobacillus fermentum. The CM and E. coli, but not L. fermentum, increased overall intestinal cell turnover in the piglets by stimulating increased apoptosis through the expression of Fas ligand and TNF-α and by increasing cell proliferation (53). Similarly, the ratio of enzyme mRNA expression to activity differed between CM and germ-free piglets and piglets colonized by a single microbe (54). The authors hypothesized that enterocyte upregulation of aminopeptidase N expression occurred as either a direct response to microbial colonization or as a feedback mechanism in response to reduced enzyme activity through microbial degradation. This mechanism may play a role in ensuring effective competition of the host with the intestinal microbiota for available nutrients (54).
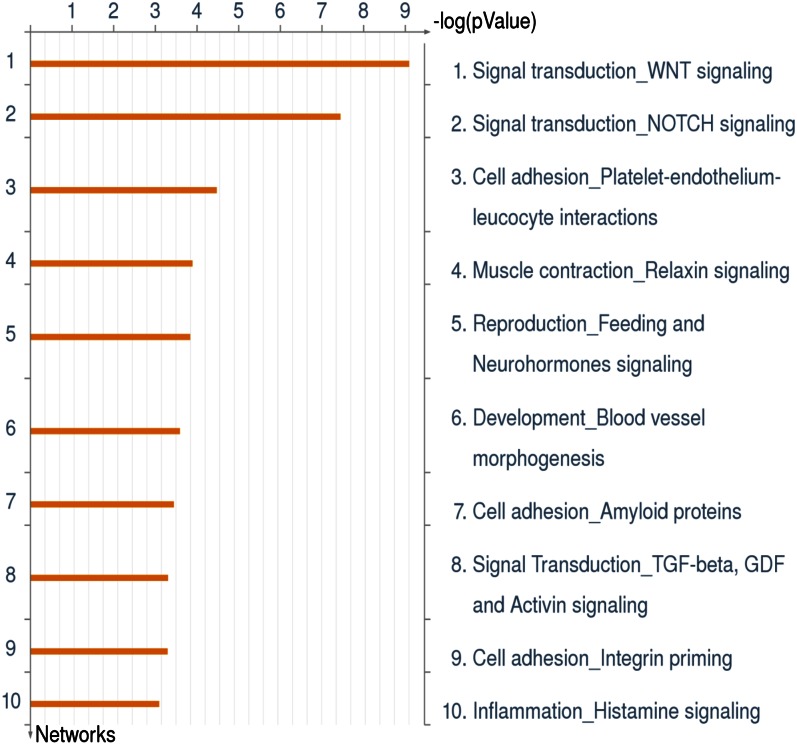
Thus, data obtained by our laboratory (44, 49) and others (52–55) using the piglet model have provided insight into host-microbe interactions in the neonatal gut. Stool samples are easy to obtain from infants, but it is extremely difficult to obtain intestinal biopsy samples from healthy infants to measure mucosal gene expression. To overcome this limitation, we established a noninvasive molecular methodology that uses stool samples containing intact sloughed epithelial cells to study intestinal gene expression in human infants (56). A total 1214 probe sets were differentially expressed in epithelial cells of BF and FF infants, which were compared with a database of 529 genes known to be involved in intestinal biology. Of these, 146 were differentially expressed in BF and FF infants. These genes were functionally analyzed using Metacore (GeneGo). Differentially expressed genes were fit into the following gene networks: signal transduction, cytoskeleton remodeling, cell migration, cell adhesion, barrier function, immune response, inflammation, and histamine (Figure 1). Linear discriminant analysis was used to identify the single genes and the 2- to 3-gene combinations that best distinguished BF from FF infants. In addition, putative “master” regulatory genes were identified using coefficient of determination (CoD) analysis. The best-performing gene using linear discriminant analysis was endothelial PAS domain-containing protein 1 (EPAS1), which was significantly (3-fold) upregulated in BF compared with FF infants. The EPAS1 protein is a basic helix-loop-helix PAS transcription factor that is activated by hypoxia leading to subsequent induction of vascular development (57). This is noteworthy because hypoxia-triggered angiogenesis may affect predisposition to proinflammatory states, including NEC (58). Although the role of EPAS1 in the developing human intestine has not been studied, CoD analyses confirmed its importance as a master regulatory gene that appears to drive the expression of other genes; thus, it is worthy of further investigation. The power of the CoD approach lies in its ability to find unexpected links between processes not previously known to be coordinated by signaling pathways. In summary, we showed for the first time that 2- and 3-gene combinations provide classifiers with potential to noninvasively identify discriminative signatures for the development of molecular markers to explore nutritional effects on maturation of intestinal function (56).
Figure 1.
Gene networks differentially expressed in exfoliated epithelial cells from breast- and formula-fed infants. Gene expression was determined in exfoliated intestinal epithelial cells from 3-mo-old breast- and formula-fed infants. Differentially expressed genes were analyzed using Metacore (GeneGo). Genes were associated with a number of key biological networks regulating intestinal function. The top 10 networks encompassed signal transduction, cytoskeleton remodeling, cell migration, cell adhesion, barrier function, immune response, inflammation, and histamine. GDF, growth differentiation factor. Additional information is provided in Chapkin et al. (56).
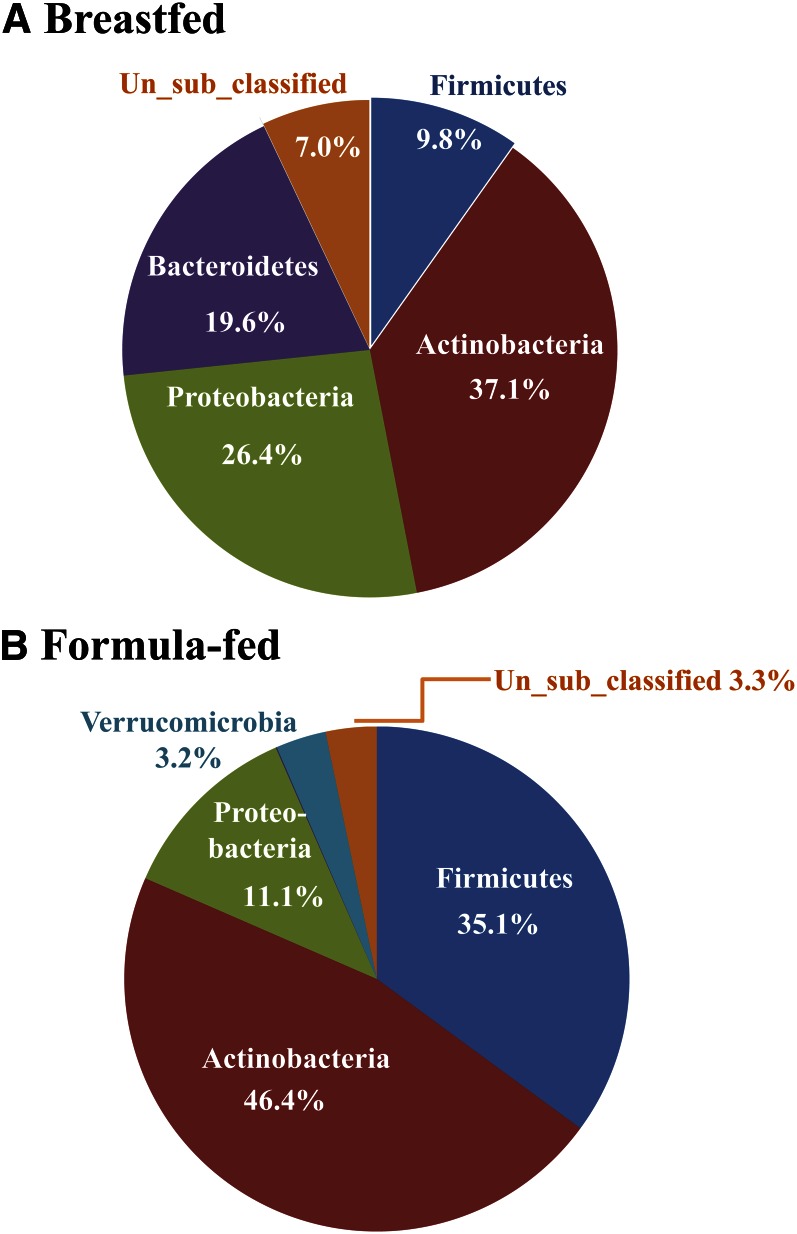
We recently expanded this data set to include the HMO content of the mother’s milk and the infant’s fecal short-chain fatty acid content, microbial composition, and the bacterial microbiome by 454 pyrosequencing of DNA libraries. HMO were profiled in the laboratory of Dr. Carlito Lebrilla (University of California, Davis) using HLPC Chip/time-of-flight MS and were identified using an annotated HMO library (59, 60). HMO profiles were analyzed using principal components analysis, which identified 3 clusters that primarily separated based on the abundance of 2 predominant HMO, 2′-fucosyllactose and lacto-N-tetraose (Figure 2). These data demonstrate that genetic variation in “secretor” status exists among the mothers because 2′-fucosyllactose is a prominent oligosaccharide in the milk of secretor donors (61). Data on the bacterial phyla in stool of the 3-mo-old BF and FF infants are presented in Figure 3. Consistent with previous reports (2, 3), the bacterial phylogenetic profiles differentiated the 2 groups of infants; BF infants had a higher percentage of Bacterodetes and lower Firmicutes and Verrucomicrobia compared with FF infants. Ongoing analyses aim to systematically integrate genomic data from both the infant (host mucosa) and gut microbiome to assess host gene-diet interactions within the context of the structure and operations of gut microbial communities.
Figure 2.
Principal components analysis of human milk oligosaccharides (HMO) profiles. The HMO profiles in samples collected at 3-mo of lactation were determined using HLPC chip/time-of-flight MS and identified compared with annotated HMO libraries (57, 58). HMO profiles were compared using principal components analysis, which identified 3 clusters that primarily separated based on the content of 2 predominant HMO, 2′-fucosyllactose and lacto-N-tetraose. The 2′-fucosyllactose content increases and the lacto-N-tetraose content decreases from left to right. We conclude that genetic variation in secretor status (61) exists in the mothers enrolled in our study, which will facilitate establishing associations between milk HMO and infant microbiota.
Figure 3.
Bacterial phyla in stool of 3-mo-old breast- and formula-fed infants. Genomic DNA was extracted from stool and the bacterial 16S recombinant RNA gene V1 to V3 regions were amplified with primers 27F RegS and 534R. Primer 27F RegS contained sequencing primer and barcode (MID). PCR products were purified and quantified, and amplicons were mixed in equimolar concentration and sequenced using 454 Life Sciences Genome Sequencer FLX with GS FLX Titanium series reagents at the Keck Center for Comparative and Functional Genomics at the University of Illinois, Urbana. 16S recombinant RNA gene sequences were processed and analyzed using the Mothur program (63). Sequences were classified to phylum by comparing with RDP training set. Breast-fed infants had a higher percentage of Bacterodetes and lower Firmicutes and Verrucomicrobia compared with formula-fed infants.
Conclusions and future directions
Clinical and epidemiological data support differences in the development, microbiota, and incidence of disease between BF and FF infants (1, 13). These data are consistent with a growing body of data indicating that perinatal diet orchestrates mucosal homeostasis (62). Given the abundance and diversity of HMO and their broad physiological actions and their absence in infant formula (6, 22), it is tempting to speculate that HMO are in part responsible for these differences between BF and FF infants. Systematic evaluation of the impact of HMO on infant development has been limited by the lack of sufficient quantities of pure HMO to conduct animal or human feeding studies. However, in the near future, this limitation will be overcome through improved synthetic approaches, opening avenues of investigation into the biology of HMO. Additionally, the availability of noninvasive methods of assessing outcomes in human infants (56) and high throughput methods for measuring HMO and infant microbiome will facilitate our understanding of the role of HMO in host-microbe interactions in the developing infant.
Acknowledgments
The authors thank Shuai Wu and Dr. Carlito Lebrilla at the University of California, Davis for performing the HMO analysis. All authors have read and approved the final manuscript.
Footnotes
Published in a supplement to Advances in Nutrition. Presented at the conference “The First International Conference on Glycobiology of Human Milk Oligosaccharides” held in Copenhagen, Denmark, May 16–17, 2011. The conference was supported by Glycom A/S, Lyngby, Denmark. The supplement coordinators for this supplement were Clemens Kunz, University of Giessen, Germany, and Sharon M. Donovan, University of Illinois, Urbana, IL. Supplement Coordinator disclosures: Sharon Donovan has received human milk oligosaccharides from Glycom through their HMO biology donations program. Clemens Kunz has received human milk oligosaccharides from Glycom through their HMO biology donation program. The supplement is the responsibility of the Guest Editor to whom the Editor of Advances in Nutrition has delegated supervision of both technical conformity to the published regulations of Advances in Nutrition and general oversight of the scientific merit of each article. The Guest Editor for this supplement was Mark A. McGuire, University of Idaho. Guest Editor disclosure: Mark A. McGuire consults with Abbott Nutrition, a division of Abbott Laboratories, and provides professional opinions on matters related to human lactation and infant nutrition. Further, he collaborates with Dr. Lars Bode, University of California, San Diego, in research related to human milk oligosaccharides. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
Supported by National Institutes of Health R01 HD061929 (to S.M.D.), R01 CA129444, R25T-CA090301 (to R.S.C.), and Hatch project #ILLU-971-346 provided through the Division of Nutritional Sciences Vision 20/20 program (to S.M.D.).
Author disclosures: S. M. Donovan, M. Wang, M. Li, I. Friedberg, S. L. Schwartz, and R. S. Chapkin, no conflicts of interest.
Abbreviations used: BF, breast-fed; CoD, coefficient of determination analysis; CM, conventional microbiota; EPAS1, endothelial PAS domain–containing protein 1; FF, formula-fed; HM, human milk; HMO, human milk oligosaccharides; NEC, necrotizing enterocolitis; PM, porcine milk; PMO, porcine milk oligosaccharides.
Literature Cited
- 1.Murgas Torrazza R, Neu J. The developing intestinal microbiome and its relationship to health and disease in the neonate. J Perinatol. 2011;31: Suppl 1:S29–34 [DOI] [PubMed] [Google Scholar]
- 2.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, Welling GW. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification band detection methods. J Pediatr Gastroenterol Nutr. 2000;30:61–7 [DOI] [PubMed] [Google Scholar]
- 5.Spor A, Koren O, Ley R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol. 2011;9:279–90 [DOI] [PubMed] [Google Scholar]
- 6.Zivkovic AM, German JB, Lebrilla CB, Mills DA. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc Natl Acad Sci U S A. 2011;108: Suppl 1:4653–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donovan SM. Role of human milk components in gastrointestinal development: current knowledge and future needs. J Pediatr. 2006;149:S49–61 [Google Scholar]
- 8.Palmer AC. Nutritionally mediated programming of the developing immune system. Adv Nutr. 2011;2:377–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newburg DS. Neonatal protection by an innate immune system of human milk consisting of oligosaccharides and glycans. J Anim Sci. 2009;87:13 Suppl:26–34 [DOI] [PubMed] [Google Scholar]
- 10.Kaplan JL, Shi HN, Walker WA. The role of microbes in developmental immunologic programming. Pediatr Res. 2011;69:465–72 [DOI] [PubMed] [Google Scholar]
- 11.Gartner LM, Morton J, Lawrence RA, Naylor AJ, O’Hare D, Schanler RJ, Eidelman AI; American Academy of PediatricsSection on Breastfeeding Breastfeeding and the use of human milk. Pediatrics. 2005;115:496–506 [DOI] [PubMed] [Google Scholar]
- 12.Dewey KG, Heinig MJ, Nommsen-Rivers LA. Differences in morbidity between breast fed and formula-fed infants. J Pediatr. 1995;126:696–702 [DOI] [PubMed] [Google Scholar]
- 13.Heinig MJ. Host defense benefits of breastfeeding for the infant. Effect of breastfeeding duration and exclusivity. Pediatr Clin North Am. 2001;48:105–23 [DOI] [PubMed] [Google Scholar]
- 14.Yoshioka H, Iseki K, Fujita K. Development and differences of intestinal flora in the neonatal period in breast-fed and bottle-fed infants. Pediatrics. 1983;72:317–21 [PubMed] [Google Scholar]
- 15.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2011;108: Suppl 1:4578–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boesten R, Schuren F, Ben Amore K, Haarman M, Know J, deVos WM. Bifidobacterium population analysis in the infant gut by direct mapping of genomic hybridization patterns: potential for monitoring temporal development and effects of dietary regimens. Microb Biotechnol. 2011;4:417–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gauhe A, Gyorgy P, Hoover JR, Kuhn R, Rose CS, Ruelius HW, Zilliken F. Bifidus factor. IV. Preparations obtained from human milk. Arch Biochem Biophys. 1954;48:214–24 [DOI] [PubMed] [Google Scholar]
- 18.Niñonuevo MR, Park Y, Yin H, Zhang J, Ward RE, Clowers BH, German JB, Freeman SL, Killeen K, Grimm R, et al. A strategy for annotating the human milk glycome. J Agric Food Chem. 2006;54:7471–80 [DOI] [PubMed] [Google Scholar]
- 19.Urashima T, Saito T, Nakamura T, Messer M. Oligosaccharides of milk and colostrum in non-human mammals. Glycoconj J. 2001;18:357–71 [DOI] [PubMed] [Google Scholar]
- 20.Warren CD, Chaturvedi P, Newburg AR, Oftedal OT, Tilden CD, Newburg DS. Comparison of oligosaccharides in milk specimens from humans and twelve other species. Adv Exp Med Biol. 2001;501:325–32 [DOI] [PubMed] [Google Scholar]
- 21.Bode L. Recent advances on structure, metabolism, and function of human milk oligosaccharides. J Nutr. 2006;136:2127–30 [DOI] [PubMed] [Google Scholar]
- 22.Sela DA, Mills DA. Nursing our microbiota: molecular linkages between biofidobacteria and milk oligosaccharides. Trends Microbiol. 2010;18:298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donovan SM. Promoting bifidobacteria in the human infant intestine: why, how, and which one? J Pediatr Gastroenterol Nutr. 2011;52:648–9 [DOI] [PubMed] [Google Scholar]
- 24.Kuntz S, Rudloff S, Kunz C. Oligosaccharides from human milk influence growth-related characteristics of intestinally transformed and non-transformed intestinal cells. Br J Nutr. 2008;99:462–71 [DOI] [PubMed] [Google Scholar]
- 25.Kuntz S, Rudloff S, Kunz C. Oligosaccharides from human milk induce growth arrest via G2/M by influencing growth-related cell cycle genes in intestinal epithelial cells. Br J Nutr. 2009;101:1306–15 [DOI] [PubMed] [Google Scholar]
- 26.Shen Q, Tuohy KM, Gibson GR, Ward RE. In vitro measurement of the impact of human milk oligosaccharides on the faecal microbiota of weaned formula-fed infants compared to a mixture of prebiotic fructooligosaccharies and galactooligosaccharides. Lett Appl Microbiol. 2011;52:337–43 [DOI] [PubMed] [Google Scholar]
- 27.Vael C, Desager K. The importance of the development of the intestinal microbiota in infancy. Curr Opin Pediatr. 2009;21:794–800 [DOI] [PubMed] [Google Scholar]
- 28.Rahman MM, Kim WS, Ito T, Kumura H, Shimazaki K. Growth promotion and cell binding ability of bovine lactoferrin to Bifidobacterium longum. Anaerobe. 2009;15:133–7 [DOI] [PubMed] [Google Scholar]
- 29.Roberts AK, Chierici R, Sawatzki G, Hill MJ, Volpato S, Vigi V. Supplementation of an adapted formula with bovine lactoferrin: 1. Effect on the infant faecal flora. Acta Paediatr. 1992;81:119–24 [DOI] [PubMed] [Google Scholar]
- 30.Liepke C, Adermann K, Raida M, Mägert HJ, Forssmann WG, Zucht HD. Human milk provides peptides highly stimulating the growth of bifidobacteria. Eur J Biochem. 2002;269:712–8 [DOI] [PubMed] [Google Scholar]
- 31.LoCascio RG, Niñonuevo MR, Kronewitter SR, Freeman SL, German JB, Lebrilla CB, Mills DA. A versatile and scalable strategy for glycoprofiling bifidobacterial consumption of human milk oligosaccharides. Microb Biotechnol. 2009;2:333–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LoCascio RG, Desai P, Sela DA, Weimer B, Mills DA. Broad conservation of milk utilization genes in Bifidobacterium longum subsp. infantis as revealed by comparative genomic hybridization. Appl Environ Microbiol. 2010;76:7373–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruhaak LR, Lebrilla CB. Advances in the analysis of free milk oligosaccharides. Adv Nutr. 2012;3:406S–14S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garrido D, Barile D, Mills DA. A molecular basis for bifidobacterial enrichment in the infant gastrointestinal tract. Adv Nutr. 2012;3:415S–21S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leser TD, Mølbak L. Better living through microbial action: the benefits of the mammalian gastrointestinal microbiota on the host. Environ Microbiol. 2009;11:2194–206 [DOI] [PubMed] [Google Scholar]
- 36.Hrncir T, Stepankova R, Kozakova H, Hudcovic T, Tlaskalova-Hogenova H. Gut microbiota and lipopolysaccharide content of the diet influence development of regulatory T cells: studies in germ-free mice. BMC Immunol. 2008;9:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Claus SP, Ellero SL, Berger B, Krause L, Bruttin A, Molina J, Paris A, Want EJ, de Waziers I, Cloarec O, et al. Colonization-induced host-gut microbial metabolic interaction. MBio. 2011;2:e00271–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore AM, Munck M, Sommer MOA, Dantas G. Functional metagenomic investigations of the human intestinal microbiota. Front Microbiol. 2011;2:188 doi: 10.3389/fmicb.2011.00188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31 [DOI] [PubMed] [Google Scholar]
- 40.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 2009;1(6):6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McNulty NP. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci Transl Med 2011;3:106ra106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shulzhenko N, Morgun A, Hsiao W, Battle M, Yao M, Gavrilova O, Orandle M, Mayer L, Macpherson AJ, McCoy KD, et al. Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nat Med. 2011;17:1585–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poroyko V, White JR, Wang M, Donovan SM, Alverdy JC, Liu DC, Morowitz MJ. Gut microbial gene expression in mother-fed and formula fed neonatal piglets. PLoS ONE. 2010;5:e12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tao N, Ochonicky KL, German JB, Donovan SM, Lebrilla CB. Structural determination and daily variations of porcine milk oligosaccharides. J Agric Food Chem. 2010;58:4653–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martín-Sosa S, Martín MJ, García-Pardo LA, Hueso P. Sialyloligosaccharides in human and bovine milk and in infant formulas: variations with the progression of lactation. J Dairy Sci. 2003;86:52–9 [DOI] [PubMed] [Google Scholar]
- 47.Shah P, Shah V. Arginine supplementation for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2007;3:CD004339. [DOI] [PubMed] [Google Scholar]
- 48.Morales Y, Schanler RJ. Human milk and clinical outcomes in VLBW infants: How compelling is the evidence of benefit? Semin Perinatol. 2007;31:83–8 [DOI] [PubMed] [Google Scholar]
- 49.Poroyko V, Morowitz M, Bell T, Ulanov A, Wang M, Donovan S, Bao N, Gu A, Hong L, Alverdy JC, et al. Diet creates metabolic niches in the “immature gut” that shape microbial communities. Nutr Hosp. 2011;26:1283–95 [DOI] [PubMed] [Google Scholar]
- 50.Morowitz MJ, Poroyko V, Caplan M, Alverdy JC, Liu DC. Redefining the role of intestinal microbes in the pathogenesis of necrotizing enterocolitis. Pediatrics. 2010;125:777–85 [DOI] [PubMed] [Google Scholar]
- 51.Falk PG, Hooper LV, Midtvedt T, Gordon JI. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol Mol Biol Rev. 1998;62:1157–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chowdhury SR, King DE, Willing BP, Band MR, Beever JE, Lane AB, Loor JJ, Marini JC, Rund LA, Schook LB, et al. Transcriptome profiling of the small intestinal epithelium in germfree versus conventional piglets. BMC Genomics. 2007;8:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Willing BP, Van Kessel AG. Enterocyte proliferation and apoptosis in the caudal small intestine is influenced by the composition of colonizing commensal bacteria in the neonatal gnotobiotic pig. J Anim Sci. 2007;85:3256–66 [DOI] [PubMed] [Google Scholar]
- 54.Willing BP, Van Kessel AG. Intestinal microbiota differentially affect brush border enzyme activity and gene expression in the neonatal gnotobiotic pig. J Anim Physiol Anim Nutr (Berl). 2009;93:586–95 [DOI] [PubMed] [Google Scholar]
- 55.Meurens F, Berri M, Siggers RH, Willing BP, Salmon H, Van Kessel AG, Gerdts V. Commensal bacteria and expression of two major intestinal chemokines, TECK/CCL25 and MEC/CCL28, and their receptors. PLoS ONE. 2007;2:e677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chapkin RS, Zhao C, Ivanov I, Davidson LA, Goldsby JS, Lupton JR, Mathai RA, Monaco MH, Rai D, Russell WM, et al. Stool-based detection of infant gastrointestinal development using gene expression profiles from exfoliated epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2010;298:G582–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Favier J, Kempf H, Corvol P, Gasc JM. Coexpression of endothelial PAS protein 1 with essential angiogenic factors suggests its involvement in human vascular development. Dev Dyn. 2001;222:377–88 [DOI] [PubMed] [Google Scholar]
- 58.Zamora R, Vodovotz Y, Betten B, Wong C, Zuckerbraun B, Gibson KF, Ford HR. Intestinal and hepatic expression of BNIP3 in necrotizing enterocolitis: regulation by nitric oxide peroxynitrite. Am J Physiol Gastrointest Liver Physiol. 2005;289:G822–30 [DOI] [PubMed] [Google Scholar]
- 59.Wu S, Tao N, German JB, Grimm R, Lebrilla CB. Development of an annotated library of neutral human milk oligosaccharides. J Proteome Res. 2010;9:4138–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu S, Wu S, Grimm R, German JB, Lebrilla CB. Annotation and structural analysis of sialylated human milk oligosaccharides. J Proteome Res. 2011;10:856–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erney RM, Malone WT, Skelding MB, Marcon AA, Kleman-Leyer KM, O'Ryan ML, Ruiz-Palacios G, Hilty MD, Pickering LK, Prieto PA. Variability of human milk neutral oligosaccharides in a diverse population. J Pediatr Gastroenterol Nutr. 2000;30:181–92 [DOI] [PubMed] [Google Scholar]
- 62.Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol. 2012;12:9–23 [DOI] [PubMed] [Google Scholar]
- 63. Mothur project. [Cited 2012 Jan 31. Available from: http://www.mothur.org/