Abstract
The early stages of neurodevelopment in infants are crucial for establishing neural structures and synaptic connections that influence brain biochemistry well into adulthood. This postnatal period of rapid neural growth is of critical importance for cell migration, neurite outgrowth, synaptic plasticity, and axon fasciculation. These processes thus place an unusually high demand on the intracellular pool of nutrients and biochemical precursors. Sialic acid (Sia), a family of 9-carbon sugar acids, occurs in large amounts in human milk oligosaccharides and is an essential component of brain gangliosides and sialylated glycoproteins, particularly as precursors for the synthesis of the polysialic acid (polySia) glycan that post-translationally modify the cell membrane-associated neural cell adhesion molecules (NCAM). Human milk is noteworthy in containing exceptionally high levels of Sia-glycoconjugates. The predominate form of Sia in human milk is N-acetylneuraminic acid (Neu5Ac). Infant formula, however, contains low levels of Sia consisting of both Neu5Ac and N-glycolyneuraminic acid (Neu5Gc). Current studies implicate Neu5Gc in several human inflammatory diseases. Polysialylated NCAM and neural gangliosides both play critical roles in mediating cell-to-cell interactions important for neuronal outgrowth, synaptic connectivity, and memory formation. A diet rich in Sia also increases the level of Sia in the brains of postnatal piglets, the expression level of 2 learning-related genes, and enhances learning and memory.
Introduction
Brain development is a complex process that involves the anatomic growth of the brain and many biochemical, physiological, and psychological processes. The rate of initial brain growth exceeds that of any other organ or body tissue, and by 2 y of age, the brain reaches ∼80% of its adult weight. Children are born with all of their neurons already formed. However, the synaptic connections between these neurons are in large part established and elaborated after birth. Important neurodevelopmental processes that occur throughout this critical period include synaptic networking, dendritic arborization, and cell multiplication and migration (1). In general, synapses are formed at a very rapid rate during the early months of life, usually achieving maximum density between 6 and 12 mo after birth. Subsequent to cell division, proliferation of many supporting cells (e.g., glial cells) occurs within the cerebrum, cerebellum, and brainstem, increasing synapse and myelination processes throughout postnatal growth of the brain. Infants born preterm or small for their gestational age are particularly vulnerable in early life to defects in early neural development (2). Studies have shown that preterm birth alters brain structure with reduced total cerebral tissue volumes compared with full-term control babies (3). It is not surprising, therefore, that up to 50% of very preterm infants have neurobehavioral problems, including lowered IQ, attention deficit hyperactivity disorder, anxiety disorders, and learning disabilities (2, 4).
Brain development is time dependent (5, 6). The different developmental processes follow a highly ordered sequence of events that are under strict genetic control but can also be influenced by epigenetic factors. Once the sequence of growth events has occurred, brain growth cannot be restarted and this may have large impact on cognitive function in adulthood. Hence, if a child is undernourished throughout the period of synapse proliferation, formation, and branching, brain growth will be impaired and nutritional rehabilitation after this time will have little, if any, beneficial effect on brain growth and cognition (5). Thus, recovery appears to be possible only if the nutritional status is improved before the end of the vulnerable period of growth. Neural growth and development continue throughout childhood and adolescence, in particular myelination in the frontal lobe. Nutrient intake may influence the micro- and macrostructure of brain anatomy throughout different phases of brain growth. Consequently, the optimal nutrition is critical to support neural growth until the brain has reached its maximum development potential.
Nutrients can affect multiple brain development processes by regulating neurotransmitter pathways, synaptic transmission, signal-transduction pathways, and synaptic plasticity. Subsequent studies showed that the best nutritional support for healthy nervous system development comes from a balance of the protein and energy nutrients with other essential micronutrients (6–8). These nutrients can serve as precursors or as preformed building blocks for newly synthesized tissues and cell growth. Such precursors can also be obtained via endogenous biosynthetic pathways. Biosynthesis usually occurs at maximal rates when tissue growth is accelerated during the pre- and postnatal life. Suboptimal nutrition during this phase has well-recognized, irreversible consequences for cognitive function (6). The optimal nutrition in early life affects brain developmental processes ranging from neuroanatomy, neurochemistry, neurophysiology, and neuropsychology to long-lasting influence on cognitive events well into adulthood. Long-chain PUFA, particularly DHA, have been the focus of most research in this field (9), but there are other factors that have been shown to enhance brain growth in animals. Our interest is in Sia3 (sialic acid), a 9-carbon sugar that is an integral structural and functional component of the nervous system. One of nature’s richest sources of Sia happens to be found in human milk. The purpose of this review is to summarize the evidence showing the importance of dietary Sia as an essential nutrient for optimal brain development and cognition.
Expression of Sia and polysialic acid in mammals
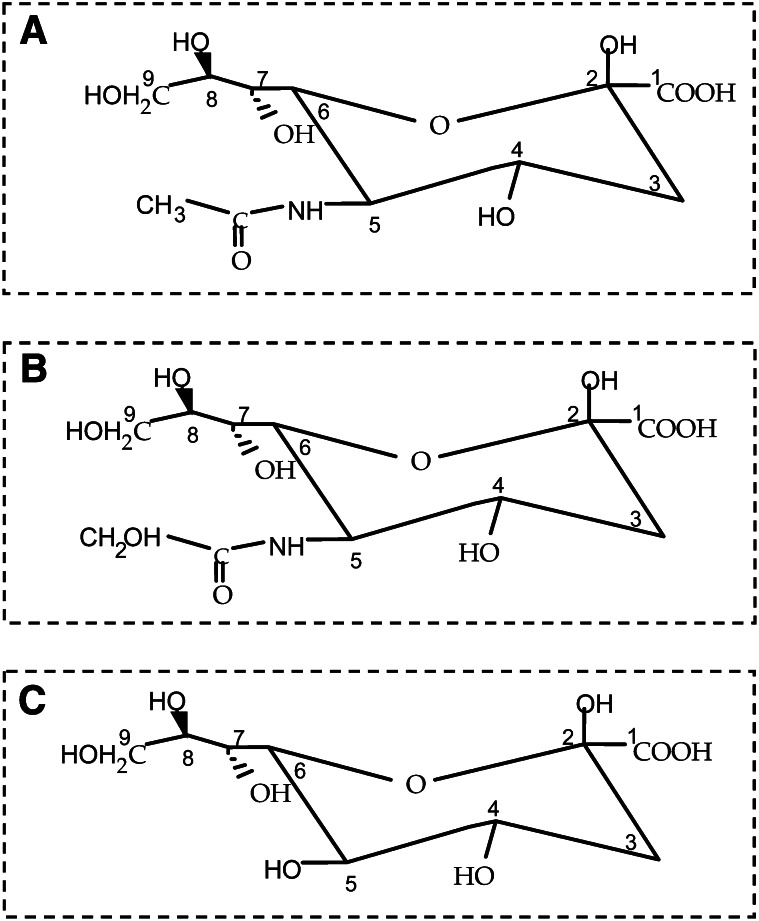
The Sia consists of a family of N- and O-substituted derivatives of the parent sugar, neuraminic acid, a 9-carbon sugar acid. While they are still increasing, there are now over 50 naturally occurring derivatives of Sia that have been identified (10). In humans, however, the predominant structure of Sia is Neu5Ac (N-acetylneuraminic acid) (Fig. 1), which is the key monomeric precursor of neural brain glycoproteins, including polySia (polySia), gangliosides, glycosaminoglycans, and mucins (11, 12). These sialoglycoconjugates are ubiquitously expressed throughout the human body. Hydroxylation of 1 of the 3 protons on the N-acetyl group of Neu5Ac at C5 gives rise to Neu5Gc (N-glycolylneuraminic acid) (Fig. 1), a Sia present on all nonhuman primate cells, including those of our closest relatives, the great apes (13). Neu5Gc is absent in healthy humans because of an exon deletion/frameshift mutation in the CMP-Neu5Ac hydroxylase gene responsible for the hydroxylation of Neu5Ac to form Neu5Gc (14). This mutation resulted in the total absence of Neu5Gc expression in all human tissues (14). Exogenous Neu5Gc can be metabolically incorporated into cultured human cells, however, via existing biochemical pathways (15). As a consequence, Neu5Gc is detected at low levels in human carcinomas, fetal tissue, and normal tissue types, including epithelium and endothelium of human specimens examined following surgery or autopsy (16). Neu5Gc in these tissues has now been shown to be derived from eating a diet rich in red meat and milk products (14–16). The different ratio of Neu5Ac:Neu5Gc in glycans is species and tissue specific and is specific for different body fluids (12). Another modification of the Sia family is the presence of an additional hydroxyl group instead of an acyl group at position C5 of the sugar, leading to KDN (2-keto-3-deoxy-nonulosonic acid, ketodeoxynonulosonic acid) (Fig. 1), the newest member of the family. KDN is found in species ranging from bacteria to vertebrates. Its abundant occurrence in animals was initially limited to lower vertebrates (17), e.g., fish eggs (caviar) (18), but is now known to be expressed at low levels in normal human RBC, where its concentration can be as low as 0.01 mol% of Neu5Ac (19). An elevated level of expression of KDN was found in fetal human RBC compared with adult RBC and in ovarian tumor tissues compared with normal controls (20). Thus, a bewildering array of sialylated glycoconjugates now accounts for the structural diversity of glycans that function in neural development, synaptic transmission, cognition and memory formation (21, 22), and immune function (23–25) and that serve as decoys for invading pathogens (26).
Figure 1.
Members of the Sia family. (A) Neu5Ac (present in neuroinvasive bacteria, human tissues, and foods). (B) Neu5Gc (present in trout eggs, some foods, and human tumors). (C) KDN (present in fish eggs and ovarian fluid, human fetal RBC, and human cancers. Reproduced with permission from (27). KDN, ketodeoxynonulosonic acid; Neu5Ac, N-acetylneuraminic acid; Neu5Gc, N-glycolylneuraminic acid; Sia, sialic acid.
PolySia is a linear homopolymer most commonly composed of the negatively charged α2–8-linked Neu5Ac residues (27). Neither poly-Neu5Gc nor poly-KDN chains have been reported in humans (28). The major membrane protein carrier of polySia in mammalian cells is NCAM (neural cell adhesion molecule). In addition to NCAM, other polysialylated protein included synaptic cell adhesion molecule 1 (29), the α subunit of the voltage-sensitive sodium channel (30), the integrin α5 subunit (31), the scavenger receptor CD36 (32), and neuropilin-2 (33). The ST8SiaII (polysialyltransferase II) and ST8SiaIV (polysialyltransferase IV) that synthesize polySia (34) are also modified with polySia when expressed behind strong promoters in in vitro studies. Expression of polySia-NCAM is developmentally regulated and polySia accounts for ∼30% of the molecular mass of the molecule in newborn rats. The level of polySia bound to NCAM in rats decreases to ∼10–14% at 6–8 d of age and then to only ∼4% in 28-d-old rats (35).
The “space-filling” polySia chains that are localized at the cell surface are postulated to restrict homophilic and heterophilic binding due to steric hindrance and negative charge repulsion between cells. The repulsion of adjacent cells expressing polySia is suggested to be caused by an expansion of the space-filling polySia chains that occupy greater steric space, because they imbibe large volumes of water (36). This prevents the binding of opposing cells expressing polySia-NCAM and other species on the surface of cells. It is for this reason that polySia-NCAM is considered an antiadhesive glycotope that interferes with both homophilic and heterophilic binding. PolySia confers unique properties that influence many cellular activities, including the regulation of cell migration, axon elongation, neurite outgrowth and neuronal pathfinding, regeneration, and synaptic formation (37–39). PolySia-NCAM is considered a key neuroplastic molecule enabling neuronal plasticity and is instrumental in the mechanism of memory formation (40). Recent studies have demonstrated a complex formation between polySia and several neurotrophic factors, including brain-derived neurotrophic factor, which plays a central role in synaptic plasticity and neurogenesis (41). This polySia brain-derived neurotrophic factor complex can upregulate growth or/and survival of neuroblastoma cells (42). PolySia-NCAM also regulates synaptic plasticity and learning by restraining the signaling through GluN2B-containing N-methyl D-aspartate receptors (43). An abnormally low level of polySia has been shown to be a major risk factor in the pathophysiology of schizophrenic brains (43, 44). Thus, polySia expression in human health and disease, and in tissue repair therapeutics, is an important area for future discovery.
Mammalian neuronal cell membranes have the highest concentration of Sia compared to other cell membrane throughout the body. The location and amount of Sia in different regions of the brain change during development (45). Gangliosides are glycosphingolipids containing a hydrophilic sialoligosaccharide head group and a hydrophobic ceramide chain consisting of one molecule of the amino alcohol, sphingosine, and one fatty acyl chain (46). Gangliosides comprise ∼6–10% of the total lipid mass of the human brain and ∼70% of the conjugated Sia. In the adult human brain, GM1, GD1a, GD1b, and GT1b comprise 80–90% of the total amount of gangliosides. The ganglioside concentration in human brain increases ~3-fold from wk 10 of gestation until the age of ∼5 y. The most rapid increase occurs around term, during the period of dendrite arborization, outgrowth of axons, and synaptogenesis (45). PolySia comprises a considerable proportion of the brain glycoprotein content, consisting of ∼30% in newborn rat brains (35). This level decreases to ∼10–14% at ∼6–8 d of age (35, 47), then decreases further to only 4% in 28-d-old rats (35). The polySia moiety on NCAM confers unique properties that influence many cellular processes, including cell migration, neurite outgrowth, branching, neuronal pathfinding, regeneration, and synaptic plasticity (37–39). The activity of the rate-limiting bifunctional enzyme, UDP-GlcNAc 2-epimerase (UDP-N-acetylglucosamine-2-epimerase) (25, 48), which is responsible for controlling the biosynthesis of Sia, is initially lower in rat pups and guinea pigs and maximum at postnatal d 15 (49). Thus, we postulated earlier that the infant may not have the full endogenous biosynthetic capacity to synthesize the Sia required to meet the demands for this sugar (50). Therefore, we further postulated that newborns could benefit from an exogenous source of Sia to meet the demands of the rapidly growing brain, particularly in preterm infants (27). This requirement for additional Sia is supported by the observation that the Sia concentration is abundant in early milk and declines during lactation (51). Additionally, the activity of neuraminidases, which cleaves Sia from sialoglycoconjugates, are high in the intestinal mucosal of rat pups, corresponding to the high concentration of Sia in the milk (50).
Sia concentration in human milk and infant formula
The Sia-glycoconjugates in human milk are species specific and therefore provide a natural source of structurally and biologically complex nutrients that are designed for the human infant. The level of Sia expressed in human breast milk glycoconjugates is influenced by a variety of genetic factors, geographic regions, and dietary intakes of mothers. Additionally, the variation in the different concentration of the sialyloligosaccharides in human milk is partially due to the use of different standardized analytical methods used for analyses. We demonstrated that breast milk in Australian women contained a large number of structurally diverse sialylated oligosaccharides that are not found in significant amounts in cow milk or infant formulas. For human milk at full term, ∼3.72 ± 0.15 mmol/L of total Sia was present in the colostrum (milk produced 1–6 d after birth) and 2.64 ± 0.14 mmol/L in transition milk (∼7–10 d after birth) and declined to 1.48 ± 0.07 and 0.73 ± 0.05 mmol/L at 1 and 3 mo after birth, respectively (51). Preterm human milk contains 13–23% more Sia than full-term milk during the first 3 mo of lactation (51). Other studies showed similar Sia concentrations and trends, the highest concentration being reported in colostrum, with a gradual decrease as lactation progresses, in women in Italy (52), Japan (53), Spain (54), and the US (55). Infant formulas based on bovine milk contain low amounts of Sia compared to human milk (51, 55, 56). Cow milk-based formulas designed for full-term infants, with a whey:casein ratio of 20:80, contain as little as 0.21 ± 0.01 mmol/L Sia. Preterm infant formulas contain 0.63 ± 0.12 mmol/L Sia, followed by follow-on formulas with 0.43 ± 0.03 mmol/L Sia (51). No single infant formula can replicate the dynamic nature of Sia in breast milk during the course of lactation. In human milk, most of the Sia is bound to free oligosaccharides (∼73.8%), followed by protein-bound (∼23.4%) and then free Sia- (∼2.8%) from mothers of full-term and preterm infants during the first 3 mo of lactation (51). In cow milk-based infant formulas, however, Sia is mostly bound to proteins (∼70%) and then free oligosaccharides (28%) and only 0.9% was found in the free form (51, 55). The total amount of ganglioside-bound Sia is ∼1% of the total Sia in human milk at levels of 9.51 ± 1.16 mg/L and 9.07 ± 1.15 mg/L in the colostrum and mature milk at 7–46 days postpartum, respectively (57), whereas the level in mature bovine milk is ∼3.98 ± 0.25 mg/L (57). The predominate ganglioside in human colostrum and mature bovine milk is GD3; however, in mature human milk, GM3 is the major ganglioside (57, 58).
Human milk contains extremely low levels of polySia on the glycoprotein CD36 (32). The amount of polySia detected was largely constant in milk from 7-d colostrum to milk at 6 mo (32). CD36 is a member of the B class of the scavenger receptor super family (32). Except for human milk, there is no report that polysialylated CD36 glycoproteins are expressed in milk from other species. The concentration of polySia on the CD36 glycoprotein was higher in human milk 1 mo after birth and then gradually declined during lactation (32). Although the possible functional importance of polySia on CD 36 in human milk is unknown, it may be important for neonatal development in terms of protection and nutrition because of the low Neu5Ac synthetic capacity and the high Neu5Ac requirement for neonatal brain development and cognition (55). These findings thus underscore again the important differences between human breast milk and bovine milk and also the need to further understand the functional importance of polysialylated CD36.
As noted, human breast milk is devoid of Neu5Gc, because Neu5Ac is the predominate Sia. However, many infant formulas are bovine milk based and contain Neu5Gc, representing ∼3–5% of the total level of Sia (16). Because of the immature immune system and rapidly growing tissues, particularly in the brain of newborns, bovine-derived Neu5Gc may more readily enter human infant neural cells and thus be incorporated into newly synthesized glycoconjugates (14). Presently, the potential impact or long-term effects of an increased uptake of Neu5Gc for neonate or infant development is not known. Also unknown is the potential consequence of this uptake on adult diseases, particularly as a risk factor for cancer and coronary artery disease. This suggests, therefore, the need for further, well-controlled human studies to better define the role of Neu5Gc in human health and disease. The detailed structural differences between human milk and bovine milk were summarized elsewhere [see review (59)].
Evidence that Sia is an important nutrient for infant development
We have demonstrated that the cortical tissue from human brain has 2–4 times more Sia than that of 7 other mammals, including our closest relative, the chimpanzee (60). Also, the concentration of brain Sia increased with age in several species, but there were no sex differences in human brain tissue. This finding confirms the ability to detect differences in brain Sia that correspond to maturation and evolutionary development (60). Importantly, we have also found that full-term and preterm exclusively breast-fed infants at 5 mo of age had nearly twice the amount of total salivary Sia compared with formula-fed infants (61, 62). The brain Sia concentration in breast-fed infants was significantly higher than that of formula-fed infants, as observed in babies who died from sudden infant death syndrome (63). The concentration of Sia in brain gangliosides significantly correlated with ganglioside ceramide, DHA, and total (n-3) fatty acids in breast-fed, but not in formula-fed, infants (63). This suggests that Sia and DHA may be functionally linked in the infant brain to act either together or synergistically to benefit early neurodevelopment and cognition (59, 63). Taken together, these findings suggest that an exogenous source of Sia in human milk may significantly contribute to greater sialylation of brain ganglioside, glycoprotein, body fluids, and tissue and may contribute to the observed neurological and intellectual advantages of breastfeeding over formula feeding that we reported earlier (27, 63). Of importance is the experimental evidence showing that there is only limited capacity for endogenous synthesis of Sia during early life when the demand for Sia is high. Infants, particularly premature infants who receive only artificial feeding, are less likely to receive adequate levels of Sia and are therefore more likely to be at greater risk of a neural deficit.
Sia supplementation and learning behavior in mammals
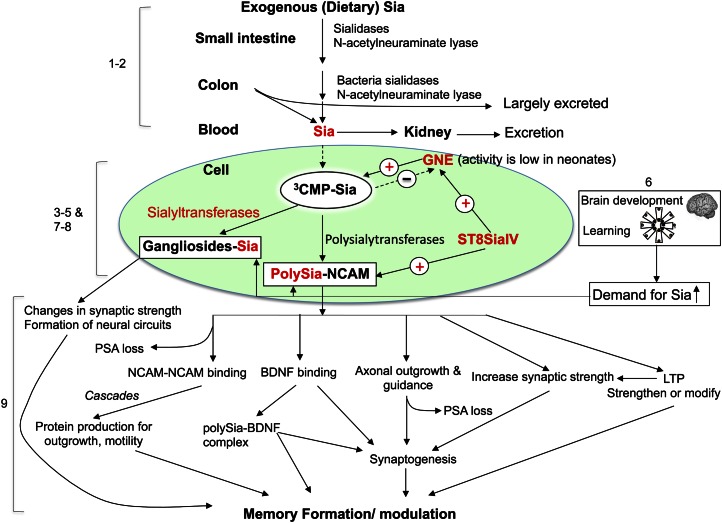
The effect of Sia supplementation on learning and memory behavior has been studied in rodents (64, 65) and was summarized earlier (27, 59). Based on the limitation of using rodents as an animal model for nutritional intervention study to assess the benefits on neurodevelopment and cognition, we developed a piglet model, because their brain structure and function more closely resemble that of human infants. This has allowed an in-depth analyses of learning and memory at various levels of development, including brain biochemistry and molecular genetics, that is not readily possible to carry out on human infants. For these studies, we supplemented 3-d-old newborn piglets with a diet rich in the protein-bound Sia designated casein-glycomacropeptide at the dose level of total Sia intake of 40 mg/(kg · d) (the control group; casein-glycomacropeptide–free diet), 85 mg/(kg · d) (low-dose level), 180 mg/(kg · d) (middle-dose level), and 240 mg/(kg · d) (high-dose level) for 5 wk (66). We used an easy and difficult visual cue in an 8-arm radial maze in the piglet model to assess the capability of learning and memory (66). We demonstrated there was a dose-response relationship between dietary Sia supplementation and cognitive function. Those piglets showing the fastest learning had been fed the higher doses of Sia and they made fewer mistakes in the maze than those fed lower levels of Sia. Concomitant with this finding, there was a corresponding dose-related increase in the amount of sialylated glycoproteins in the frontal cortex. Accompanying this finding, there was a 2- to 3-fold increase in mRNA levels in the hippocampus and liver for both Gne (UDP-GlcNAc 2-epimerases/N-acetylmannosamine kinase), a key enzyme for regulating Sia biosynthesis (25, 48), and in the polysialyltransferase gene, ST8SiaIV, which is 1 of the 2 eukaryotic genes required for the polysialylation of NCAM, a critical enzyme in neural development and brain plasticity (66–68). The increase in Sia from the diet corresponded with an increase in the expression of a ST8Sia in training piglets. We conclude from these findings that normal brain development and active learning increases the requirement for sialylated structures, including NCAM and gangliosides. Thus, the intracellular concentration of Sia appears to play a key role in regulating the polysialylation of NCAM (69). Our results also suggest that the increased levels of ST8Sia IV expression may also upregulate Sia synthesis in the developing brain, although the mechanism for this possibility remains obscure. Taken together, our findings suggest a molecular mechanism to explain how increased dietary levels of Sia correlate with increased learning performance and memory and also with an increase in Gne expression. These results are in accord with our hypothesis that both Gne and ST8SiaIV may function cooperatively to increase the synthesis of polySia on NCAM during normal neurodevelopment. A summary of the metabolic fate of how exogenous Sia and learning influences cellular functions during neurodevelopment and the mechanistic role of neuronal gangliosides and polySia-NCAM in memory formation is shown in Figure 2.
Figure 2.
Metabolic fate of how exogenous Sia and learning influences cellular function during neurodevelopment. Role and mechanism of neuronal gangliosides and polySia-NCAM in memory formation. (1) Exogenous (dietary) Sia in the food from milk oligosaccharides, glycoprotein, and gangliosides are digested and absorbed in the gastrointestinal system by bacterial and other neuraminidases (18, 27). (2) Much of the Sia is excreted in the stool and urine. (3) Sia is synthesized in the cell in the cytoplasm and transported into the nucleus where it is activated with CTP to form the activated nucleotide donor of Sia, CMP-Sia. CMP-Sia returns to the cytoplasm where it is then transported into the Golgi compartment and serves as the donor substrate of Sia for the sialyltransferases, including ST8Sia IV and ST8Sia II. CMP-Sia exerts negative feedback inhibition on GNE, the key bifunctional cytoplasmic enzyme that regulates the biosynthesis of Sia, thus limiting excess production of free Sia (69). GNE activity is low in neonates (49). (4) Exogenous sialylglycoconjugates can also be recycled from the extracellular fluid into lysosomes by receptor-mediated endocytosis. Lysosomal sialidases can cleave the Sia, which is then transported into the cytoplasm to be reutilized for the synthesis of CMP-Sia and, ultimately, new sialylglycoconjugates. (5) In the Golgi apparatus, the mono- and ST8Sia catalyze the transfer of Sia residues from the activated sugar nucleotide, CMP-Sia, to endogenous acceptors, including gangliosides and the NCAM. The intracellular concentration of Sia regulates the synthesis of polySia on NCAM (69). (6) Normal brain development and active learning increase the requirement for sialylated structures, including brain gangliosides and polySia-NCAM (45, 71,). (7) Upregulation of the level of ST8Sia IV mRNA significantly correlates with the level of GNE mRNA in the liver, hippocampus, and frontal cortex (66). This suggests that both GNE and ST8Sia IV may function in tandem to increase the synthesis of polySia on NCAM during times of high Sia demand, e.g., learning and brain growth. Under these conditions, the inhibitory feedback of CMP-Sia on GNE is minimized. (8) The mechanism whereby ST8Sia IV or other signaling molecules may upregulate GNE expression is not fully understood. It is also unclear if gangliosides may upregulate GNE expression during development. (9) The detailed molecular mechanisms underlying how neuronal gangliosides and polySia-NCAM may modulate memory formation, as shown, is under study. Figure modified from Wang et al. (27, 66). GNE, UDP-GlcNAc 2-epimerases/N-acetylmannosamine kinase; NCAM, neural cell adhesion molecule; polySia, polysialic acid; Sia, sialic acid; ST8Sia, polysialyltransferase.
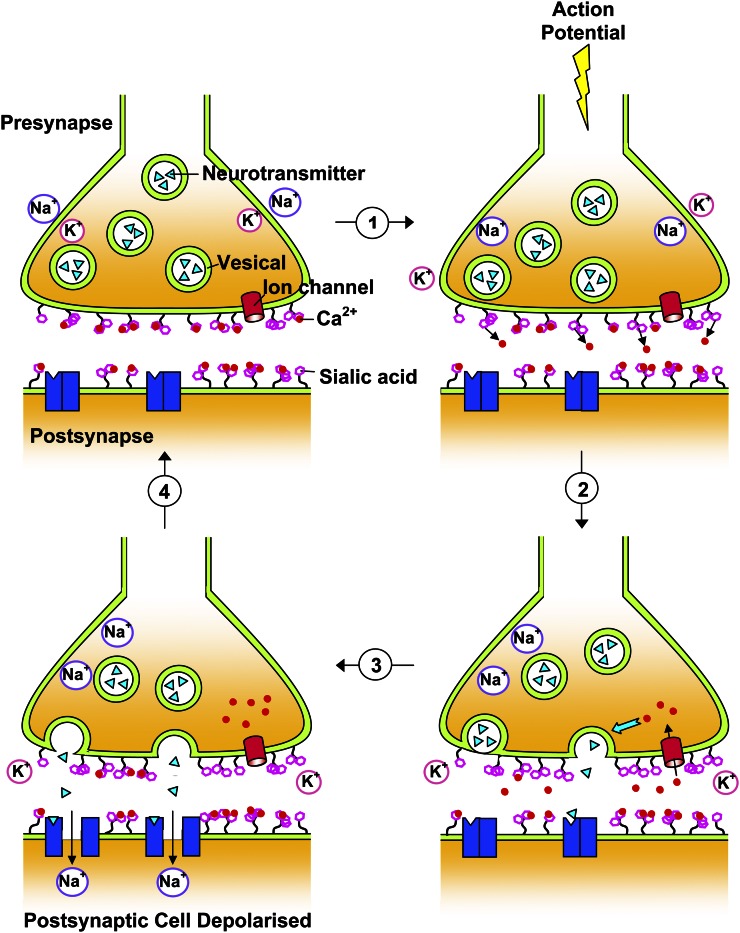
The role of gangliosides during learning and memory has been extensively examined in rodents and in our previous paper where we discussed possible mechanisms as to how neuronal gangliosides influence learning and memory (59). A long-held model of synaptic transmission is shown in Figure 3. This describes how terminal Sia residues on gangliosides at the synapse can modulate neurotransmission via interactions with calcium ions, which are important mediators in neuronal responses (22, 70).
Figure 3.
Sia on gangliosides and its role in neurotransmission. Figure modified from Karim and Wang (1) with kind permission from CAB Reviews. Sia residues on gangliosides around the synaptic cleft are thought to modulate Ca2+ levels via Ca2+-ganglioside interactions (1). During the resting potential, the presynaptic membrane is closed due to tight Ca2+-ganglioside associations with the aid of negative charges from terminally positioned Sia. When an action potential reaches the presynaptic membrane, the electric field strength and/or changes in ion concentration occur. This results in the rearrangement of gangliosides and release of Ca2+ from its binding sites, altering membrane permeability and leading to the influx of Ca2+ through ion channels into the pre-synapse (2). Increased Ca2+ initiates a number of intracellular responses and signal cascades and causes transmitter release (3). Neurotransmitters bind to specific receptors on the postsynaptic membrane, allowing the influx of sodium ions and resulting in a local depolarization of the post-synapse (4). The resting potential is restored as the transmitter degrades and Ca2+ is returned to the extracellular space by ganglioside-modulated Ca2+-ATPase, allowing the tight membrane Ca2+-ganglioside interaction to reform. Sia, sialic acid.
Conclusion
A detailed understanding of the molecular mechanisms underlying the nutritional significance of dietary Sia on neurodevelopment and cognitive function awaits further study. Although extensive hypothesis-based studies have demonstrated the important role of exogenous Sia on brain cognitive function and development, there are, to date, no reported randomized clinical trial studies in humans. In addition, a suitable animal model that more closely resembles the human infant, such as piglets, is required to examine the metabolic fate of human milk Sia and the molecular mechanisms of how dietary Sia effects the structure and function of the central nervous system and its impact on cognition and memory. Today, it is possible to experimentally examine the influence of nutrient supplementation on many parameters, including structural, molecular biology, biochemistry, genetics, learning and memory assessment, and psychological processes. Nevertheless, the currently available evidence strongly supports our original hypothesis that the Sia in human breast milk is an essential nutrient for early brain neurodevelopment and cognition.
Acknowledgments
The sole author had responsibility for all parts of the manuscript.
Footnotes
Published in a supplement to Advances in Nutrition. Presented at the conference “The First International Conference on Glycobiology of Human Milk Oligosaccharides” held in Copenhagen, Denmark, May 16–17, 2011. The conference was supported by Glycom A/S, Lyngby, Denmark. The supplement coordinators for this supplement were Clemens Kunz, University of Giessen, Germany, and Sharon M. Donovan, University of Illinois, Urbana, IL. Supplement Coordinator disclosures: Sharon Donovan has received human milk oligosaccharides from Glycom through their HMO biology donations program. Clemens Kunz has received human milk oligosaccharides from Glycom through their HMO biology donation program. The supplement is the responsibility of the Guest Editor to whom the Editor of Advances in Nutrition has delegated supervision of both technical conformity to the published regulations of Advances in Nutrition and general oversight of the scientific merit of each article. The Guest Editor for this supplement was Mark A. McGuire, University of Idaho. Guest Editor disclosure: Mark A. McGuire consults with Abbott Nutrition, a division of Abbott Laboratories, and provides professional opinions on matters related to human lactation and infant nutrition. Further, he collaborates with Dr. Lars Bode, University of California, San Diego, in research related to human milk oligosaccharides. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
Supported by a research grant from the National Health and Medical Research Council of the Commonwealth of Australia (ID: 302016).
Author disclosure: B. Wang, no conflicts of interest. Bing Wang is presently employed by Nestle Research Center, Beijing, China.
Abbreviations used: Gne, UDP-GlcNAc 2-epimerases/N-acetylmannosamine kinase; KDN, ketodeoxynonulosonic acid; Neu5Ac, N-acetylneuraminic acid; Neu5Gc, N-glycolyneuraminic acid; NCAM, neural cell adhesion molecule; polySia, polysialic acid; Sia, sialic acid; ST8Sia, polysialyltransferase; UDP-GlcNAc 2-epimerase, UDP-N-acetylglucosamine-2-epimerase.
Literature Cited
- 1.Karim M, Wang B. Is sialic acid in milk food for the brain? Perspect Agric Vet Sci Nutr Nat Resour. 2006;1:18–29 [Google Scholar]
- 2.Larroque B, Ancel PY, Marret S, Marchand L, André M, Arnaud C, Pierrat V, Rozé JC, Messer J, Thiriez G, et al. Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. Lancet. 2008;371:813–20 [DOI] [PubMed] [Google Scholar]
- 3.Marlow N, Wolke D, Bracewell M, Samara M. EpiCure Study Group. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352:9–19 [DOI] [PubMed] [Google Scholar]
- 4.Aylward GP. Neurodevelopmental outcomes of infants born prematurely. J Dev Behav Pediatr. 2005;26:427–40 [DOI] [PubMed] [Google Scholar]
- 5.Morgan BL. Nutritional requirements for normative development of the brain and behavior. Ann N Y Acad Sci. 1990;602:127–32 [DOI] [PubMed] [Google Scholar]
- 6.Uauy R, Mena P, Peirano P. Mechanisms for nutrient effects on brain development and cognition. Nestle Nutr Workshop Ser Clin Perform Programme. 2001;5:41–70, discussion 70–2 [DOI] [PubMed] [Google Scholar]
- 7.Brundtland GHBC. Global strategy for infant and young child feeding. Geneva: WHO Library Cataloguing-in-Publication Data; 2003. p. 1–30. [Google Scholar]
- 8.Uauy R, Peirano P. Breast is best: human milk is the optimal food for brain development. Am J Clin Nutr. 1999;70:433–4 [DOI] [PubMed] [Google Scholar]
- 9.Gibson RA. Long-chain polyunsaturated fatty acids and infant development. Lancet. 1999;354:1919–20 [DOI] [PubMed] [Google Scholar]
- 10.Varki A, Cummings R, Kesko J, Freeze H, Hart G, Marth J. Sialic acid. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1999. p. 195–209 [Google Scholar]
- 11.Rosenberg A. Biology of the sialic acids. New York: Plenum Press; 1995 [Google Scholar]
- 12.Schachter H, Montreuil J, Vliegenthart JFG. Glycoproteins II. Amsterdam, New York: Elsevier; 1997 [Google Scholar]
- 13.Muchmore EA, Diaz S, Varki A. A structural difference between the cell surfaces of humans and the great apes. Am J Phys Anthropol. 1998;107:187–98 [DOI] [PubMed] [Google Scholar]
- 14.Hedlund M, Tangvoranuntakul P, Takematsu H, Long JM, Housley GD, Kozutsumi Y, Suzuki A, Wynshaw-Boris A, Ryan AF, Gallo R, et al. N-glycolylneuraminic acid deficiency in mice: implications for human biology and evolution. Mol Cell Biol. 2007;27:4340–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bardor M, Nguyen DH, Diaz S, Varki A. Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. J Biol Chem. 2005;280:4228–37 [DOI] [PubMed] [Google Scholar]
- 16.Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki NM, Varki A. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci USA. 2003;100:12045–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue S, Kitajima K. KDN (deaminated neuraminic acid): dreamful past and exciting future of the newest member of the sialic acid family. Glycoconj J. 2006;23:277–90 [DOI] [PubMed] [Google Scholar]
- 18.Varki A. Diversity in the sialic acids. Glycobiology. 1992;2:25–40 Erratum in: Glycobiology 1992;2:following 168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue S, Sato C, Kitajima K. Extensive enrichment of N-glycolylneuraminic acid in extracellular sialoglycoproteins abundantly synthesized and secreted by human cancer cells. Glycobiology. 2010;20:752–62 [DOI] [PubMed] [Google Scholar]
- 20.Inoue S, Lin SL, Chang T, Wu SH, Yao CW, Chu TY, Troy FA II, Inoue Y. Identification of free deaminated sialic acid (2-keto-3-deoxy-D-glycero-D-galacto-nononic acid) in human red blood cells and its elevated expression in fetal cord red blood cells and ovarian cancer cells. J Biol Chem. 1998;273:27199–204 [DOI] [PubMed] [Google Scholar]
- 21.Becker CG, Artola A, Gerardy-Schahn R, Becker T, Welzl H, Schachner M. The polysialic acid modification of the neural cell adhesion molecule is involved in spatial learning and hippocampal long-term potentiation. J Neurosci Res. 1996;45:143–52 [DOI] [PubMed] [Google Scholar]
- 22.Rahmann H. Brain gangliosides and memory formation. Behav Brain Res. 1995;66:105–16 [DOI] [PubMed] [Google Scholar]
- 23.Ji YB, Ji CF, Zou X, Gao SY. [Study on the effects of two kinds of cactus polysaccharide on erythrocyte immune function of S180 mice]. Zhongguo Zhong Yao Za Zhi. 2005;30:690–3 [PubMed] [Google Scholar]
- 24.Drake PM, Nathan J, Stock C, Chang PV, Muench MO, Nakata D, Reader JR, Gip P, Golden KP, Weinhold B, et al. Polysialic acid, a glycan with highly restricted expression, is found on human and murine leukocytes and modulates immune responses. J Immunol. 2008;181:6850–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanner ME. The enzymes of sialic acid biosynthesis. Bioorg Chem. 2005;33:216–28 [DOI] [PubMed] [Google Scholar]
- 26.Radin NS. Preventing the binding of pathogens to the host by controlling sphingolipid metabolism. Microbes Infect. 2006;8:938–45 [DOI] [PubMed] [Google Scholar]
- 27.Wang B. Sialic acid is an essential nutrient for brain development and cognition. Annu Rev Nutr. 2009;29:177–222 [DOI] [PubMed] [Google Scholar]
- 28.Janas T. doi: 10.1016/j.bbamem.2011.08.036. Membrane oligo- and polysialic acids. Biochim Biophys Acta 2011;1808:2923–32. [DOI] [PubMed] [Google Scholar]
- 29.Galuska SP, Rollenhagen M, Kaup M, Kaup M, Eggers K, Oltmann-Norden I, Schiff M, Hartmann M, Weinhold B, Hildebrandt H, et al. Synaptic cell adhesion molecule SynCAM 1 is a target for polysialylation in postnatal mouse brain. Proc Natl Acad Sci USA. 2010;107:10250–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuber C, Lackie PM, Catterall WA, Roth J. Polysialic acid is associated with sodium channels and the neural cell adhesion molecule N-CAM in adult rat brain. J Biol Chem. 1992;267:9965–71 [PubMed] [Google Scholar]
- 31.Nadanaka S, Sato C, Kitajima K, Katagiri K, Irie S, Yamagata T. Occurrence of oligosialic acids on integrin alpha 5 subunit and their involvement in cell adhesion to fibronectin. J Biol Chem. 2001;276:33657–64 [DOI] [PubMed] [Google Scholar]
- 32.Yabe U, Sato C, Matsuda T, Kitajima K. Polysialic acid in human milk. CD36 is a new member of mammalian polysialic acid-containing glycoprotein. J Biol Chem. 2003;278:13875–80 [DOI] [PubMed] [Google Scholar]
- 33.Curreli S, Arany Z, Gerardy-Schahn R, Mann D, Stamatos NM. Polysialylated neuropilin-2 is expressed on the surface of human dendritic cells and modulates dendritic cell-T lymphocyte interactions. J Biol Chem. 2007;282:30346–56 [DOI] [PubMed] [Google Scholar]
- 34.Close BE, Colley KJ. In vivo autopolysialylation and localization of the polysialyltransferases PST and STX. J Biol Chem. 1998;273:34586–93 [DOI] [PubMed] [Google Scholar]
- 35.Margolis RK, Margolis RU. Distribution and characteristics of polysialosyl oligosaccharides in nervous tissue glycoproteins. Biochem Biophys Res Commun. 1983;116:889–94 [DOI] [PubMed] [Google Scholar]
- 36.Yang P, Major D, Rutishauser U. Role of charge and hydration in effects of polysialic acid on molecular interactions on and between cell membranes. J Biol Chem. 1994;269:23039–44 [PubMed] [Google Scholar]
- 37.Tang J, Rutishauser U, Landmesser L. Polysialic acid regulates growth cone behavior during sorting of motor axons in the plexus region. Neuron. 1994;13:405–14 [DOI] [PubMed] [Google Scholar]
- 38.Doherty P, Cohen J, Walsh FS. Neurite outgrowth in response to transfected N-CAM changes during development and is modulated by polysialic acid. Neuron. 1990;5:209–19 [DOI] [PubMed] [Google Scholar]
- 39.Muller D, Wang C, Skibo G, Toni N, Cremer H, Calaora V, Rougon G, Kiss JZ. PSA-NCAM is required for activity-induced synaptic plasticity. Neuron. 1996;17:413–22 [DOI] [PubMed] [Google Scholar]
- 40.Rutishauser U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat Rev Neurosci. 2008;9:26–35 [DOI] [PubMed] [Google Scholar]
- 41.Fernandes BS, Gama CS, Cereser KM, Yatham LN, Fries GR, Colpo G, de Lucena D, Kunz M, Gomes FA, Kapczinski F. Brain-derived neurotrophic factor as a state-marker of mood episodes in bipolar disorders: a systematic review and meta-regression analysis. J Psychiatr Res. 2011;45:995–1004 [DOI] [PubMed] [Google Scholar]
- 42.Kanato Y, Kitajima K, Sato C. Direct binding of polysialic acid to a brain-derived neurotrophic factor depends on the degree of polymerization. Glycobiology. 2008;18:1044–53 [DOI] [PubMed] [Google Scholar]
- 43.Kochlamazashvili G, Senkov O, Grebenyuk S, Robinson C, Xiao MF, Stummeyer K, Gerardy-Schahn R, Engel AK, Feig L, Semyanov A, et al. Neural cell adhesion molecule-associated polysialic acid regulates synaptic plasticity and learning by restraining the signaling through GluN2B-containing NMDA receptors. J Neurosci. 2010;30:4171–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barbeau D, Liang JJ, Robitalille Y, Quirion R, Srivastava LK. Decreased expression of the embryonic form of the neural cell adhesion molecule in schizophrenic brains. Proc Natl Acad Sci USA. 1995;92:2785–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Svennerholm L, Bostrom K, Fredman P, Mansson JE, Rosengren B, Rynmark BM. Human brain gangliosides: developmental changes from early fetal stage to advanced age. Biochim Biophys Acta. 1989;1005:109–17 [DOI] [PubMed] [Google Scholar]
- 46.Ando S. [Gangliosides in the nervous system] Tanpakushitsu Kakusan Koso. 1984;29:1146–59 [PubMed] [Google Scholar]
- 47.Finne J. Occurrence of unique polysialosyl carbohydrate units in glycoproteins of developing brain. J Biol Chem. 1982;257:11966–70 [PubMed] [Google Scholar]
- 48.Stäsche R, Hinderlich S, Weise C, Effertz K, Lucka L, Moormann P, Reutter W. A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. Molecular cloning and functional expression of UDP-N-acetyl-glucosamine 2-epimerase/N-acetylmannosamine kinase. J Biol Chem. 1997;272:24319–24 [DOI] [PubMed] [Google Scholar]
- 49.Gal B, Ruano MJ, Puente R, Garcia-Pardo LA, Rueda R, Gil A, Hueso P. Developmental changes in UDP-N-acetylglucosamine 2-epimerase activity of rat and guinea-pig liver. Comp Biochem Physiol B Biochem Mol Biol. 1997;118:13–5 [DOI] [PubMed] [Google Scholar]
- 50.Dickson JJ, Messer M. Intestinal neuraminidase activity of suckling rats and other mammals. Relationship to the sialic acid content of milk. Biochem J. 1978;170:407–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang B, Brand-Miller J, McVeagh P, Petocz P. Concentration and distribution of sialic acid in human milk and infant formulas. Am J Clin Nutr. 2001;74:510–5 [DOI] [PubMed] [Google Scholar]
- 52.Coppa GV, Pierani P, Zampini L, Carloni I, Carlucci A, Gabrielli O. Oligosaccharides in human milk during different phases of lactation. Acta Paediatr Suppl. 1999;88:89–94 [DOI] [PubMed] [Google Scholar]
- 53.Sumiyoshi W, Urashima T, Nakamura T, Arai I, Nagasawa T, Saito T, Tsumura N, Wang B, Brand Miller J, Watanabe Y, et al. Sialyl oligosaccharides in the milk of Japanese women: changes in concentration during the course of lactation. J Appl Glycosci. 2003;50:461–7 [Google Scholar]
- 54.Martín-Sosa S, Martin MJ, Garcia-Pardo LA, Hueso P. Distribution of sialic acids in the milk of spanish mothers of full term infants during lactation. J Pediatr Gastroenterol Nutr. 2004;39:499–503 [DOI] [PubMed] [Google Scholar]
- 55.Carlson SE. N-acetylneuraminic acid concentrations in human milk oligosaccharides and glycoproteins during lactation. Am J Clin Nutr. 1985;41:720–6 [DOI] [PubMed] [Google Scholar]
- 56.Sánchez-Diaz A, Ruano MJ, Lorente F, Hueso P. A critical analysis of total sialic acid and sialoglycoconjugate contents of bovine milk-based infant formulas. J Pediatr Gastroenterol Nutr. 1997;24:405–10 [DOI] [PubMed] [Google Scholar]
- 57.Pan XL, Izumi T. Variation of the ganglioside compositions of human milk, cow's milk and infant formulas. Early Hum Dev. 2000;57:25–31 [DOI] [PubMed] [Google Scholar]
- 58.Rueda R, Puente R, Hueso P, Maldonado J, Gil A. New data on content and distribution of gangliosides in human milk. Biol Chem Hoppe Seyler. 1995;376:723–7 [DOI] [PubMed] [Google Scholar]
- 59.Wang B, Brand-Miller J. The role and potential of sialic acid in human nutrition. Eur J Clin Nutr. 2003;57:1351–69 [DOI] [PubMed] [Google Scholar]
- 60.Wang B, Miller JB, McNeil Y, McVeagh P. Sialic acid concentration of brain gangliosides: variation among eight mammalian species. Comp Biochem Physiol A Mol Integr Physiol. 1998;119:435–9 [DOI] [PubMed] [Google Scholar]
- 61.Tram TH, Brand Miller JC, McNeil Y, McVeagh P. Sialic acid content of infant saliva: comparison of breast fed with formula fed infants. Arch Dis Child. 1997;77:315–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang B, Miller JB, Sun Y, Ahmad Z, McVeagh P, Petocz P. A longitudinal study of salivary sialic acid in preterm infants: Comparison of human milk-fed versus formula-fed infants. J Pediatr. 2001;138:914–6 [DOI] [PubMed] [Google Scholar]
- 63.Wang B, McVeagh P, Petocz P, Brand-Miller J. Brain ganglioside and glycoprotein sialic acid in breastfed compared with formula-fed infants. Am J Clin Nutr. 2003;78:1024–9 [DOI] [PubMed] [Google Scholar]
- 64.Morgan BL, Winick M. Effects of administration of N-acetylneuraminic acid (NANA) on brain NANA content and behavior. J Nutr. 1980;110:416–24 [DOI] [PubMed] [Google Scholar]
- 65.Morgan BL, Oppenheimer J, Winick M. Effects of essential fatty acid deficiency during late gestation on brain N-acetylneuraminic acid metabolism and behaviour in the progeny. Br J Nutr. 1981;46:223–30 [DOI] [PubMed] [Google Scholar]
- 66.Wang B, Yu B, Karim M, Hu H, Sun Y, McGreevy P, Petocz P, Held S, Brand-Miller J. Dietary sialic acid supplementation improves learning and memory in piglets. Am J Clin Nutr. 2007;85:561–9 [DOI] [PubMed] [Google Scholar]
- 67.Wang B, Hu H, Yu B. Molecular characterization of pig ST8Sia IV–a critical gene for the formation of neural cell adhesion molecule and its response to sialic acid supplement in piglets. Nutr Neurosci. 2006;9:147–54 [DOI] [PubMed] [Google Scholar]
- 68.Wang B, Hu H, Yu B, Troy FI. Molecular characterization of pig UDP-N acetlyglucosamine-2 epimerase/nacetylmannosamine kinase (GNE) gene: effect of dietary sialic acid supplementation on gene expression in piglets. Curr Top Nutraceutical Res. 2008:165–76 [Google Scholar]
- 69.Bork K, Reutter W, Gerardy-Schahn R, Horstkorte R. The intracellular concentration of sialic acid regulates the polysialylation of the neural cell adhesion molecule. FEBS Lett. 2005;579:5079–83 [DOI] [PubMed] [Google Scholar]
- 70.Rahmann H, Rosner H, Breer H. A functional model of sialo-glyco-macromolecules in synaptic transmission and memory formation. J Theor Biol. 1976;57:231–7 [DOI] [PubMed] [Google Scholar]
- 71.Savaki HE, Levis GM. Changes in rat brain gangliosides following active avoidance conditioning. Pharmacol Biochem Behav. 1977;7:7–12 [DOI] [PubMed] [Google Scholar]