Abstract
A 71 year old lady was treated for a squamous cell carcinoma of the oesophagus with neo-adjuvant chemotherapy followed by a two phase Ivor-Lewis oesophagectomy with two field lymphadenectomy. She presented four years later with life threatening bleeding from a fistula between the thoracic aorta and the gastric conduit, which was treated successfully with a thoracic aortic stent.
INTRODUCTION
Fistulation from aorta to gastric conduit after Ivor-Lewis oesophagectomy is a rare and life threatening condition. Reported cases have occurred early post operatively, with a mean post-operative day of presentation of 46 in a review of 23 cases. The majority of these patients presented with a herald bleed (93%) (1).
Various aetiologies have been hypothesised. Early post operative leakage from the suture line of the anastamosis, erosion of the gastric conduit by acidic gastric contents and reduced blood supply to the conduit have been suggested as potential causative factors (2,3). Thoracic lymphadenectomy, whereby the descending aorta is completely skeletonised, is believed to predispose to fistulation.
In this report we present a rare case of the successful treatment of an aorto enteric fistula developing four years after oesophagectomy.
CASE REPORT
A fit and well 71 year old woman presented with a history of reduced appetite and weight loss and was found to have a squamous cell carcinoma of the oesophagus at 30cm. Staging investigations suggested a T2N1 cancer with no haematogenous spread. The patient was offered neo-adjuvant chemotherapy followed by oesophagectomy. She received two cycles of Cisplatin and 5-Flurouracil. Following restaging an Ivor-Lewis oesophagectomy was performed with meticulous two field thoraco-abdominal lymphadenectomy. Histopathology confirmed a complete pathological response (Mandard regression grade 1) to neo-adjuvant therapy (T0N0 with 0/37 nodes positive). There were no immediate post operative complications and she was discharged day 16 post op. Over the subsequent three years she was troubled by recurrent dysphagia related to benign anastamotic strictures which required multiple dilatations.
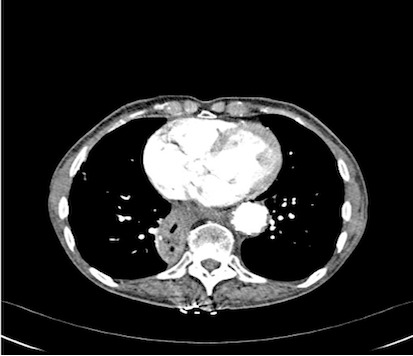
An endoscopy performed four years post-operatively demonstrated two atypical acute gastric ulcers in the distal stomach (figure 1), which were treated with a heat probe (figure 2). Two days later she presented as an emergency in hypovolaemic shock following a large volume haematemesis (haemoglobin of 40g/l). An urgent upper gastrointestinal endoscopy revealed a visible vessel at 35 cm forming part of an expansive mass within the gastric conduit. EUS demonstrated a heterogeneous mass indenting the gastric conduit, containing a strong Doppler signal (figure 3). An urgent computerised tomography (CT) angiogram revealed a Type-A thoracic aortic aneurysm with an enteric fistula (figure 4). The saccular aneurysm of the thoracic aorta was embedded in the gastric conduit. A 28x16cm Valiant® (Medtronic Ltd, Minneapolis, USA) stent was placed in the descending thoracic aorta with good occlusion of the aneurysm. The patient made an excellent recovery and was discharged on the seventh day post stenting.
Fig 1.
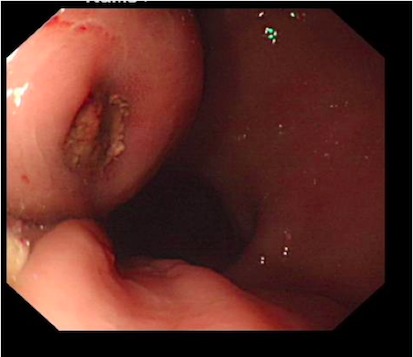
Acute gastric ulcer seen on endoscopy
Fig 2.
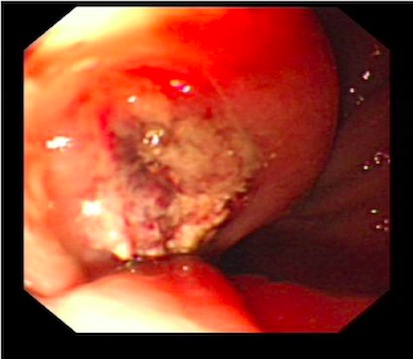
Acute gastric ulcer post treatment with gold probe
Fig 3.
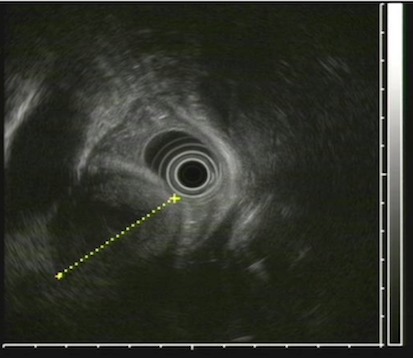
EUS examination showing thoracic aneurysm compressing esophagus
Fig 4.
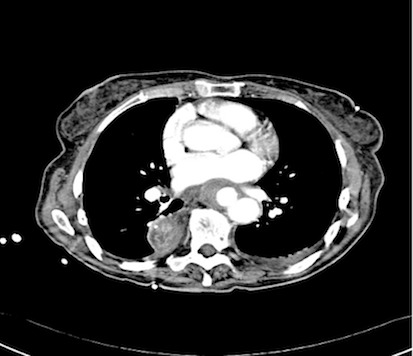
CT scan showing thoracic aortic aneurysm
Post operatively the patient developed a low grade stent infection requiring a prolonged course of antibiotics as an outpatient. Two years after insertion of the stent she remains well with no further gastrointestinal bleeding and follow up endoscopy has shown intact mucosa at the site of fistula. Computerised tomography scanning has demonstrated involution of the aneurysm sac and no endo-leak (figure 5).
Fig 5.
Stent in thoracic aortic aneurysm
DISCUSSION
Aorta-conduit or gastric fistulae are an uncommon complication following oesophagectomy. In this case report we have discussed a patient who developed an aorto-conduit fistula more than four years after an Ivor Lewis oesophagectomy.
In the few cases that have been reported in the literature oesophago-aortic fistulae have presented on average two to three weeks post operatively. Eighty two percent (82%) of cases reported presented between the second and sixth weeks postoperatively (1). The longest duration between oesophagectomy and presentation of bleed previously reported was fourteen months (1).
Aorta-conduit fistulae tend to present with a triad of symptoms, first described by Chiari-Strassburgh in 1914, of chest pain, sentinel haemorrhage and then finally exsanguination following a massive gastro-intestinal haemorrhage (5). In a review of 23 reported cases of aorto-conduit fistula 21 patients presented with a herald bleed and only one patient survived operative management of this complication. This presenting symptom may be the only sign prior to massive gastro-intestinal haemorrhage and death. Signs of gastro-intestinal bleeding in oesophagectomy patients should raise the suspicion of an aorto-conduit fistula. The latent period between herald bleed and exsanguination has an average duration of ten hours (1).
Proposed aetiology for this complication includes early post operative leakage from the suture line of the anastamosis causing an oesophago-pleural fistula and then an aorto-oesophageal fistula (2). This would seem an unlikely cause in the case we have described as four years had passed between the occurrence of the fistula and the original oesophagectomy.
Erosion of the gastric conduit by highly acidic gastric contents may also predispose to fistula formation (3). Gastric erosion and ulceration into both the aorta and pericardium from the intra-thoracic portion of the stomach has been reported post oesophagectomy. A reduced blood supply to the gastric conduit may predispose to gastric erosion. It is further proposed that chronic infection of these gastric ulcers may be a contributory factor. Pathological sections of aorta-oesophageal fistula have shown a granulomatous type inflammation suggestive of mycotic infection complicating ulceration (3). Indeed this may well have been the cause of the fistula in the case report we have described. An endoscopy performed two days prior to the presentation of the aorto-oesophageal fistula showed two acute atypical looking gastric ulcers.
REFERENCES
- 1.Molina-Navarro C, Hosking SW, Hayward SJ, Flowerdew ADS. Gastroaortic fistula as an early complication of esophagectomy. Ann Thorac Surg. 2001:72:1783–1788 [DOI] [PubMed] [Google Scholar]
- 2.Maguire WC, Mitchell N. Perforation of the aorta by acid gastric contents at the site of gastroesophagostomy. Surgery. 1947;22:842–4 [PubMed] [Google Scholar]
- 3.Brookes VS, Stafford JL. Peptic ulceration and perforation of the stomach after oesophagectomy. Thorax. 1952;7:167–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ullmann AS, Shier KJ, Horn RC. Aortoesophageal fistula: an unusual complication of esophago-gastrostomy following resection for carcinoma of the esophagus. Can Med Assoc J. 1961;85:27–31 [PMC free article] [PubMed] [Google Scholar]
- 5.Chiari-Strassburgh H. Injury of the esophagus with perforation of the aorta produced by a foreign body. Berl Klin Wochenschr. 1914:51:7–9 [Google Scholar]


