Abstract
We tested the hypothesis that racial differences in vitamin D levels are associated with racial disparities in insulin resistance between Blacks and Whites. Among 3,628 non-Hispanic Black and White adults in the National Health and Nutrition Examination Survey (NHANES) from 2001-2006, we examined the association between race and insulin resistance using the homeostasis assessment model for insulin resistance (HOMA-IR). We conducted analyses with and without serum 25-hydroxyvitamin D (25[OH] D). We adjusted for age, sex, educational level, body mass index, waist circumference, physical activity, alcohol intake, smoking, estimated glomerular filtration rate and urinary albumin/creatinine ratio. Blacks had a lower mean serum 25[OH] D level compared to Whites (14.6 (0.3) ng/ml versus 25.6 (0.4) ng/ml respectively; P < 0.0001). Blacks had a higher odds ratio (OR) for insulin resistance without controlling for serum 25[OH] D levels (OR 1.67; 95% confidence interval: 1.26, 2.20). The association was not significant (OR 1.28; 95% confidence interval: 0.90, 1.82) after accounting for serum 25[OH] D levels. The higher burden of insulin resistance in Blacks compared to Whites may be partially mediated by the disparity in serum 25[OH] D levels.
Keywords: Vitamin D, Insulin Resistance, African Americans, Cohort Studies, Minority Health
1. Introduction
Vitamin D inadequacy has been associated with insulin resistance (IR) [1-5] and is significantly more prevalent and severe in Blacks compared to Whites [6, 7]. Insulin resistance (IR) is associated with type 2 Diabetes Mellitus (DM)[8] and disproportionately affects Blacks more than Whites [9]. While vitamin D has been shown to influence skeletal health[10], some but not all, clinical trial data [4, 11] and cohort studies [2, 12] suggest that low vitamin D status is also associated with a higher prevalence of adverse extra-skeletal outcomes such as IR and cardiovascular diseases (CVD). For example, cross-sectional studies of the National Health and Nutrition Examination Survey (NHANES) suggest an inverse relationship between vitamin D status and cardiovascular risk factors [13, 14], CVD [15], and renal dysfunction [16].
The mechanisms by which vitamin D may impact insulin resistance remain controversial. It has been observed that chronic insulin resistance may be linked to deterioration of pancreatic β cell function secondary to high insulin secretory demands [17]. Improvement in pancreatic β cell function has been observed with vitamin D supplementation [18]. It may be that baseline pancreatic β cell function is a predictor for the potential benefits of vitamin D supplementation. In a post hoc analysis of a double-blind randomized placebo-controlled trail, supplementation with vitamin D and calcium of non-diabetic participants with impaired fasting glucose resulted in a significantly lesser increase in HOMA-IR over 3 years than in patients with normal fasting glucose [3].
We examined the hypothesis that racial differences in vitamin D levels, as reflected by the biomarker serum 25-hydroxyvitamin D (25[OH] D), are associated with racial disparities in the prevalence of IR between Blacks and Whites in the United States.
2. Methods and materials
2.1. Survey Overview
We examined data from the NHANES, 2001-2006. It is a large; federally-supported and administered cross-sectional survey performed at regular intervals that provide nationally representative estimates of health and disease in the U.S. population. Details regarding data collection in NHANES are available elsewhere [19].
2.2. Study participants
Our sample included 3,628 adult participants (20 years and older), for whom fasting plasma glucose (FPG), fasting serum insulin (FI) and serum 25[OH] D levels were available. We excluded participants who were taking anti-diabetes drugs or who had a positive urine pregnancy test result or who reported being pregnant at the time of the examination.
Race and Hispanic ethnicity were assessed by self-report and categorized as non-Hispanic White, non-Hispanic Black, Mexican American, or other race (other Hispanics, Asians, Native Americans, or participants who self-reported as more than one race/ethnic group). Given our focus on the Black-White disparity in IR, we confined our sample to non-Hispanic Blacks and non-Hispanic Whites.
Age at screening was grouped into categories of 20 to < 35, 35 to < 45, 45 to <55, 55 to < 65, and those ≥ 65 years old. Education was based on the highest-grade level completed including < high school, high school, and > high school. Smoking status was defined as never smoked, current smoker, and former smoker. Body mass index (BMI) was determined based on participants' weight (kg) and height (m) and categorized as < 20; 20 to < 25; 25 to < 30 and those ≥ 30 kg/m2. Waist circumference (cm) was assessed at the high point of the iliac crest. Physical activity was based on participant self-report compared to others the same age, and alcohol was based on mean daily intake (gm/day).
2.3. Biochemical measurements
Serum specimens were frozen to ≤ 70 C, shipped on dry ice and stored at ≤ 70 C. We used the homeostasis model assessment insulin resistance (HOMA-IR) model to assess IR, which was calculated using the formula: fasting insulin (uU/L) × fasting glucose (mmol/L])/22.5. IR was defined based on the weighted 75th percentile value as previously published [20].
Serum 25[OH] D was measured using a radioimmunoassay kit (DiaSorin, Stillwater, MN). Although 1,25-dihydroxyvitamin D is the biologically active form of vitamin D, serum 25[OH] D is considered the best indicator of vitamin D status in individuals without kidney disease [21]. We grouped serum 25[OH] D into quintiles based on the entire sample distribution of serum 25[OH] D levels given the potential for nonlinear effects [22]. For serum 25[OH] D levels, the quintiles were < 15.7 ng/ml, 15.7 ng/ml to < 20.7 ng/ml, 20.7 ng/ml to < 25.1 ng/ml, 25.1 ng/ml to < 31.0 ng/ml, and > 31.0 ng/ml.
FPG and FI levels were measured during the morning examination session only in available survey participants after fasting for at least 8 hours but less than 24 hours. FPG was measured enzymatically by the hexokinase method. FI was measured using radioimmunoassay with the double-antibody batch method (the Merocodia Insulin ELISA two-site immunoassay was used from 2003 to 2006). Measures of FPG and FI were adjusted for changes in laboratory methods between 2003 to 2006.
Markers of renal function were considered as possible confounders to our evaluation of the relationship between race and insulin resistance as relates to serum 25[OH] D levels. Low vitamin D status has been associated with albuminuria [23] and Blacks have been noted to be more likely to have albuminuria than Whites [24]. The untimed urinary albumin/creatinine ratio (ACR) was classified as normal if ACR was < 30 mg/g and abnormal if > 30 mg/g. Participants with estimated glomerular filtration rate (eGFR) < 60 were considered as having chronic kidney disease. The definition of ACR and chronic kidney disease was based on the criteria of the National Kidney Foundation [25].
2.4. Statistical analyses
Analyses were conducted with SUDAAN (version 10.01) and Stata (version 10.1, College Station, TX), adjusting for the complex survey design of NHANES to yield appropriate standard errors and population parameter estimates. We modeled the Black-White differences in log odds of IR adjusting for age, sex, educational level, body mass index, waist circumference, physical activity, alcohol intake, smoking, eGFR and ACR. We then compared the parameter estimates for Blacks from models that excluded (model 1) and included serum 25[OH] D (model 2) using the method of Clogg et al [26] as a test for the hypothesis that vitamin D partly mediates higher IR in Blacks compared to Whites. The percent attenuation was defined as 100 *(βModel 1 – β Model 2)/(β Model 1); where β is the parameter estimate for race.
3. Results
Of the 3,628 participants in our final sample, 946 met our criteria for IR. The prevalence of insulin resistance was higher in non-Hispanic Blacks than Whites (33.0% and 23.8%, respectively; P < 0.001) and men rather than women (28.3% and 23.8%, respectively; P < 0.0001) (not shown in table). The overall mean serum 25[OH] D level was 24.2 (0.4) ng/ml. Non-Hispanic Blacks had a significantly lower mean level of 14.6 (0.3) ng/ml than Whites (Table 1). There was a higher proportion of Blacks relative to Whites in the lower quintiles of serum 25[OH] D (Figure 1). Supplementary Table 1 shows additional weighted population characteristics stratified by presence of IR. Most in the sample were White (87.4%) and there were essentially an equal proportion of men and women (49.0 % and 51.0%, respectively). Forty-eight percent of the sample was younger than 45 years. Supplementary Table 2 demonstrates the consistent racial group differences in mean serum 25[OH] D levels.
Table 1. Population Characteristics Stratified by Race/Ethnicity: National Health and Nutrition Examination Survey (NHANES), 2001-2006a.
| Characteristics | Total Study Population (N=3628) % (n) | Non – Hispanic Whites (n=2690) % (n) | Non – Hispanic Blacks (n=938) % (n) | P-valueb | |
|---|---|---|---|---|---|
| Age (years) | < 0.0001 | ||||
| 20 –< 35 | 26.8 (848) | 25.4 (552) | 36.9 (296) | ||
| 35 – < 45 | 21.1 (618) | 21.0 (441) | 21.6 (177) | ||
| 45 – < 55 | 22.8 (672) | 22.9 (487) | 22.0 (185) | ||
| 55 – < 65 | 13.7 (533) | 14.3 (407) | 9.9 (126) | ||
| 65 + | 15.5 (957) | 16.4 (803) | 9.5 (154) | ||
| Sex | |||||
| Male | 49.0 (1865) | 49.5 (1396) | 46.1 (469) | 0.07 | |
| Female | 51.0 (1763) | 50.5 (1294) | 53.9 (469) | ||
| Education | < 0.0001 | ||||
| Less than high school | 12.9 (658) | 11.2 (404) | 25.0 (254) | ||
| High school | 27.1 (958) | 27.5 (733) | 24.1 (225) | ||
| More than high school | 60.0 (2012) | 61.3 (1553) | 50.9 (459) | ||
| Smoking Status | < 0.0001 | ||||
| Current | 25.8 (868) | 26.1 (642) | 24.2 (226) | ||
| Former | 25.7 (1019) | 27.4 (854) | 13.9 (165) | ||
| Never | 48.5 (1741) | 46.5 (1194) | 61.9 (547) | ||
| Body Mass Index (kg/m2) | < 0.0001 | ||||
| < 20 | 4.8 (185) | 4.8 (137) | 5.1 (48) | ||
| 20 - < 25 | 29.4 (1019) | 30.4 (809) | 22.8 (210) | ||
| 25 - < 30 | 34.3 (1265) | 35.0 (977) | 29.4 (288) | ||
| 30+ | 31.5 (1159) | 29.8 (767) | 42.7 (392) | ||
| Activity Levelc | 0.60 | ||||
| More Active | 37.2 (1474) | 36.9 (1093) | 38.9 (381) | ||
| About the same | 41.1 (1446) | 41.3 (1070) | 39.8 (376) | ||
| Less Active | 21.7 (708) | 21.8 (527) | 21.3 (181) | ||
| Mean (SE) | |||||
| Waist circumference (cm) | 97.2 (0.3) | 97.1 (0.4) | 97.4 (0.6) | 0.78 | |
| Alcohol (gm/day) | 4.5 (0.6) | 4.4 (0.5) | 5.1 (1.5) | 0.63 | |
| Serum 25[OH]D (ng/mL) | 24.2 (0.4) | 25.6 (0.4) | 14.6 (0.3) | < 0.0001 | |
| Systolic Blood Pressure (mmHg) | 121.9 (0.4) | 121.5 (0.4) | 124.7 (0.7) | < 0.0001 | |
| Diastolic Blood Pressure (mmHg) | 71.1 (0.3) | 70.9 (0.3) | 72.1 (0.5) | < 0.05 | |
Data presented as column percentages; means and percentages are weighted to the non-institutionalized US population. Data are shown as either % (n) or means (SE).
2-sided P-value for χ2 statistics between demographic and race or t-test for difference in means between non-Hispanic Blacks and Whites.
Activity level was based on participant self-report compared to others the same age.
Fig. 1.
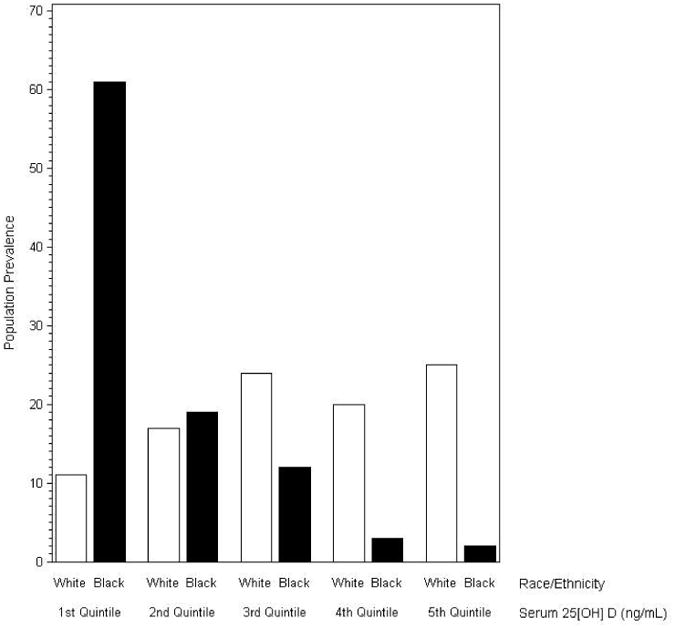
Prevalence of study participants (percent) in each quintile of serum 25[OH] D stratified by race/ethnicity. Serum 25[OH] D levels were grouped into quintiles based on the entire sample distribution. For serum 25[OH] D levels, the 1st quintile was < 15.7 ng/ml; 2nd quintile was 15.7 ng/ml to < 20.7 ng/ml; 3rd quintile was 20.7 ng/ml to < 25.1 ng/ml; 4th quintile was 25.1 ng/ml to < 31.0 ng/ml; and the 5th quintile was > 31.0 ng/ml.
Individuals in the lowest quintile of serum 25[OH] D levels were significantly more likely to have IR than those in the highest quintile (OR 1.65; 95% confidence interval (CI): 1.10, 2.47) (Table 2). The odds of IR was statistically significantly higher in Blacks compared to Whites (OR 1.67; 95% CI: 1.26, 2.20) in a logistic regression model that included age, sex, race, education, smoking status, BMI, waist circumference, physical activity, alcohol intake, eGFR and ACR (Table 2). When serum 25[OH] D levels were controlled for in the model, the association between IR and Black race was no longer statistically significant (OR 1.28; 95% CI: 0.90, 1.82) (Table 2). Notably, the reduction in the log odds of the race effect after adjusting for serum 25[OH] D levels was significant (0.27, 95% CI: 0.08, 0.47), confirming that there was a statistically significant reduction in the odds for Black race to be associated with IR.
Table 2. Multivariable-adjusted Odds Ratio for Insulin Resistance With and Without Controlling for Serum 25[OH]D levels.
| Participant Characteristics | Odds Ratioa | 95% CI | Odds Ratiob | 95% CI | |
|---|---|---|---|---|---|
| Race | |||||
| Non-Hispanic White | 1.00 | 1.00 | |||
| Non-Hispanic Black | 1.67 | 1.26, 2.20 | 1.28 | 0.90, 1.82 | |
| Age | |||||
| 20 – < 35 | 1.00 | 1.00 | |||
| 35 – < 45 | 0.98 | 0.73, 1.32 | 0.98 | 0.73, 1.33 | |
| 45 – < 55 | 0.94 | 0.72, 1.22 | 0.96 | 0.74, 1.25 | |
| 55 – < 65 | 0.88 | 0.61, 1.26 | 0.88 | 0.61, 1.27 | |
| 65 + | 1.07 | 0.76, 1.49 | 1.05 | 0.75, 1.46 | |
| Sex | |||||
| Male | 1.21 | 0.97, 1.50 | 1.28 | 1.03, 1.58 | |
| Female | 1.00 | 1.00 | |||
| Education | |||||
| Less than high school | 1.27 | 0.96, 1.67 | 1.27 | 0.95, 1.68 | |
| High school | 1.07 | 0.85, 1.34 | 1.05 | 0.84, 1.31 | |
| More than high school | 1.00 | 1.00 | |||
| Smoking Status | |||||
| Current | 0.80 | 0.60, 1.06 | 0.76 | 0.57, 1.01 | |
| Former | 0.97 | 0.74, 1.27 | 0.99 | 0.75, 1.31 | |
| Never | 1.00 | 1.00 | |||
| Body Mass Index | |||||
| < 20 | 1.00 | 1.00 | |||
| 20 - < 25 | 0.78 | 0.20, 2.96 | 0.79 | 0.21, 2.96 | |
| 25 - < 30 | 1.61 | 0.54, 4.80 | 1.60 | 0.54, 4.73 | |
| 30+ | 2.41 | 0.72, 8.03 | 2.40 | 0.72, 7.95 | |
| Waist circumference | 1.08 | 1.06, 1.09 | 1.08 | 1.06, 1.09 | |
| Activity Levelc | |||||
| More Active | 1.00 | 1.00 | |||
| About the same | 1.33 | 1.03, 1.72 | 1.32 | 1.03, 1.71 | |
| Less Active | 1.87 | 1.41, 2.50 | 1.75 | 1.29, 2.37 | |
| Serum 25[OH]D level quintiles | |||||
| 1st quintile (< 15.7 ng/ml) | 1.65 | 1.10, 2.47 | |||
| (< 15.7 ng/ml) | |||||
| 2nd quintile (15.7 ng/ml to < 20.7 ng/ml) | 1.63 | 1.17, 2.28 | |||
| (15.7 ng/ml to < 20.7 ng/ml) | |||||
| 3rd quintile | 0.89 | 0.65, 1.23 | |||
| (20.7 ng/ml to < 25.1 ng/ml) | |||||
| 4th quintile (25.1 ng/ml to < 31.0 ng/ml) | 0.96 | 0.64, 1.45 | |||
| (25.1 ng/ml to < 31.0 ng/ml) | |||||
| 5th quintile (> 31.0 ng/ml) | 1.00 | ||||
| (> 31.0 ng/ml) | |||||
| Estimated Glomerular Filtration Rate (eGFR) | |||||
| < 60 (indicates chronic kidney disease) | 1.26 | 0.81, 1.98 | 1.30 | 0.83, 2.05 | |
| ≥60 | 1.00 | 1.00 | |||
| Urine Albumin-to-Creatinine Ratio (mg/g) | |||||
| < 30 | 1.00 | 1.00 | |||
| ≥30 | 1.20 | 0.90, 1.59 | 1.11 | 0.84, 1.47 |
After adjustment for age, sex, educational level, body mass index, waist circumference, physical activity, alcohol intake, estimated glomerular filtration rate (< 60 vs. ≥ 60), urine albumin-to-creatinine ratio (< 30 mg/g vs. ≥ 30 mg/g) and smoking.
After adjustment for serum 25[OH] D levels, age, sex, educational level, body mass index, waist circumference, physical activity, alcohol intake, estimated glomerular filtration rate (< 60 vs. ≥ 60), urine albumin-to-creatinine ratio (< 30 mg/g vs. ≥ 30 mg/g) and smoking.
Activity level was based on participant self-report compared to others the same age.
While waist circumference was a significant predictor for IR in our analysis (OR 1.08; 95% CI: 1.06, 1.09), a higher BMI was not significant in predicting IR (OR 2.40; 95% CI: 0.72, 7.95) (Table 2). Less active individuals (OR 1.75; 95% CI: 1.29, 2.37) and men (OR 1.28; 95% CI: 1.03, 1.58) were more likely to have IR (Table 2).
4. Discussion
To our knowledge, the statistically significant attenuation of IR risk in Blacks compared to Whites after adjustment for serum 25 [OH] D levels has not been previously described in a large and nationally representative database. The combination of the findings of increased odds for IR in the lowest quintile of serum [OH] D compared to the highest quintile, and the higher proportion of Blacks relative to Whites in the lower quintiles of serum 25 [OH] D provides a viable explanation for our findings. A much smaller study of 50 women demonstrated that the lower whole-body insulin sensitivity index noted in Blacks, compared to Whites, was partially ameliorated by adjusting for serum 25 [OH] D[27]. Our findings are consistent with other studies that have demonstrated associations between racial differences in vitamin D levels and racial disparities in cardiovascular risk factors and mortality [15, 28, 29].
Our findings may seem to conflict with another analysis of the NHANES dataset (2001–2004), which revealed an inverse relationship between the diagnosis of diabetes and serum 25[OH] D quartiles only for non-Hispanic Whites and Mexican Americans, and not for Blacks [5]. In that analysis, Blacks had the lowest mean serum 25[OH] D levels of the three ethnic groups. These contradictory findings most likely reflect the different measures (diabetes versus IR) and the existence of a complex non-linear relationship between vitamin D status and insulin resistance that may vary between different racial/ethnic groups.
Adiposity is well documented to be associated with lower vitamin D levels [30-32] and higher rates of IR [33, 34]. The bioavailability of vitamin D may be diminished in obese patients because the fat soluble vitamin may be sequestered in adipose tissue to a greater extent than in non-obese patients [35]. An inverse relationship between vitamin D levels and adiposity has been described in both White [30, 36, 37] and Black populations [31, 32, 38]. Waist circumference was a significant predictor for IR in our analysis, but BMI was not, after controlling for the effects of both. Both waist circumference and BMI have previously been associated with IR [39] and serum 25[OH] D levels [30]. It remains debatable which measure of adiposity is the most relevant to insulin resistance for the different racial/ethnic groups [33]. Waist circumference was used in the definition of metabolic syndrome by the updated National Cholesterol Education Panel/American Heart Association criteria without any reference to BMI [34], however, the World Health Organization criteria include the option of using BMI in their assessment of the presence of metabolic syndrome [40]. The finding that a sedentary lifestyle increases the risk for IR (Table 2) has been demonstrated previously [41]. Men were also found to be more likely to have IR in our analysis which is a finding that has been described before [42] and has been hypothesized to be linked to the anti-diabetic action of estrogen [43]. Estrogen may influence fat distribution such that males tend to have greater visceral fat which lends itself to the development of insulin resistance[44].
The recent Institute of Medicine recommendations on the daily requirements for vitamin D [45] have generated debate in the scientific community in part because of their position on the cut-off points for adequate serum 25[OH] D levels. This is an issue of particular relevance to dark-skinned populations [46] as well as impoverished populations with poor dietary habits. The non-Hispanic Black population has been documented to have consistently lower mean serum 25[OH] D levels in relation to other racial/ethnic groups [16, 22, 31, 47-50]. For this reason, the Endocrine Society in their Clinical Practice Guidelines lists African Americans as high-risk candidates with a need for more vigilant serum 25[OH] D screening [51].
A limitation of the NHANES dataset is that it uses a proxy index of insulin resistance, the HOMA-IR, as opposed to the gold standard euglycemic clamp, which measures whole body insulin resistance. The HOMA-IR is based on fasting measures from peripheral venous punctures and is a better indicator of hepatic insulin sensitivity [50]. Studies have suggested that serum 25[OH] D may have different associations with insulin resistance depending on the technique used [50]. A bias towards the null hypothesis is more likely with the more crude HOMA-IR measure of insulin resistance. Another limitation is the cross-sectional nature of the NHANES dataset and therefore cause-effect relationships cannot be established from these results. It is evident that analyses involving vitamin D levels are subject to confounding because healthy behaviors such as outdoor activity in the sun and eating nutritious products will directly lead to higher vitamin D levels. In addition, the NHANES (2001–2006) dataset is limited by the absence of consistent seasonal data corresponding to when serum 25[OH] D levels were obtained [19]. Serum 25[OH] D levels are well known to vary by season (due to variation in UV exposure) [52].
In conclusion, the finding that controlling for racial differences in serum 25[OH] D levels attenuates the noted racial disparity in IR supports the hypothesis that Blacks may be at greater risk for insulin resistance by virtue of their tendency to have a lower vitamin D levels compared to Whites. The results from our study are preliminary and await confirmation through prospective and interventional designs.
Supplementary Material
Supplementary Table 1. Population Characteristics Stratified by Presence of Insulin Resistance: National Health and Nutrition Examination Survey (NHANES), 2001-2006a
aData presented as column percentages; means and percentages are weighted to the non-institutionalized US population. Data are shown as either % (n) or means (SE).
b2-sided P-value for χ2 statistics of association between demographic and insulin resistance or t-test for difference in means between insulin resistance category.
cActivity level was based on participant self-report compared to others the same age.
Supplementary Table 2. Mean Serum 25[OH] D (ng/ml) for Relevant Characteristics Stratified by Race/Ethnicity: National Health and Nutrition Examination Survey (NHANES), 2001-2006a
a2-sided P-value from F-test for analysis of variance for Vitamin D levels associated with demographic category.
b2-sided P-value for χ2 statistics between demographic and race or t-test for difference in means between non-Hispanic Blacks and Whites.
cActivity level was based on participant self-report compared to others the same age.
Acknowledgments
This work was supported by funding from the Agency for Healthcare Research and Quality T32 HS 000066; The National Heart Lung and Blood Institute R01 HL78566, R01 HL081066, R01 HL087301 and The National Institute of Minority Health and Health Disparities P60MD003421
Abbreviations
- 25[OH] D
25-hydroxyvitamin D
- BMI
Body mass index
- CVD
Cardiovascular diseases
- CI
Confidence interval
- DM
Diabetes Mellitus
- eGFR
Estimated Glomerular Filtration Rate
- FPG
Fasting plasma glucose
- FI
Fasting serum insulin
- HOMA-IR
Homeostasis assessment model for insulin resistance
- IR
Insulin resistance
- NHANES
National Health and Nutrition Examination Survey
- OR
Odds ratio
- ACR
Urinary Albumin/Creatinine Ratio
Footnotes
Conflict of Interest: None of the authors have any conflicts of interest associated with the work presented in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhao G, Ford ES, Li C. Associations of serum concentrations of 25-hydroxyvitamin D and parathyroid hormone with surrogate markers of insulin resistance among U.S. adults without physician-diagnosed diabetes: NHANES, 2003-2006. Diabetes Care. 2010;33:344–347. doi: 10.2337/dc09-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ. Baseline serum 25-hydroxy vitamin D is predictive of future glycemic status and insulin resistance: the Medical Research Council Ely Prospective Study 1990-2000. Diabetes. 2008;57:2619–2625. doi: 10.2337/db08-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980–986. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 4.von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient - a randomised, placebo-controlled trial. Br J Nutr. 2010;103:549–555. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- 5.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 6.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: Third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr. 2002;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 8.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006;119:S10–16. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haffner SM, D'Agostino R, Saad MF, Rewers M, Mykkanen L, Selby J, Howard G, Savage PJ, Hamman RF, Wagenknecht LE, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45:742–748. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 10.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337:670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 11.Tarcin O, Yavuz DG, Ozben B, Telli A, Ogunc AV, Yuksel M, Toprak A, Yazici D, Sancak S, Deyneli O, Akalin S. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009;94:4023–4030. doi: 10.1210/jc.2008-1212. [DOI] [PubMed] [Google Scholar]
- 12.Kilkkinen A, Knekt P, Aro A, Rissanen H, Marniemi J, Heliovaara M, Impivaara O, Reunanen A. Vitamin D status and the risk of cardiovascular disease death. Am J Epidemiol. 2009;170:1032–1039. doi: 10.1093/aje/kwp227. [DOI] [PubMed] [Google Scholar]
- 13.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Archives of Internal Medicine. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 14.Reis JP, von Muhlen D, Miller IER. Relation of 25-hydroxyvitamin D and parathyroid hormone levels with metabolic syndrome among US adults. European Journal of Endocrinology, Supplement 2008. 2008;159(151):141–148. doi: 10.1530/EJE-08-0072. [DOI] [PubMed] [Google Scholar]
- 15.Fiscella K, Franks P. Vitamin D, race, and cardiovascular mortality: findings from a national US sample. Ann Fam Med. 2010;8:11–18. doi: 10.1370/afm.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS. 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III) American Journal of Kidney Diseases. 2007;50:69–77. doi: 10.1053/j.ajkd.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes. 2002;51:2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 18.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 19.National Center for Health Statistics. [Accessed 10 January 2013];National Health and Nutrition Examination Survey. Available at: [ http://www.cdc.gov/nchs/nhanes.htm]
- 20.Zhao G, Ford ES, Li C. Associations of serum concentrations of 25- hydroxyvitamin D and parathyroid hormone with surrogate markers of insulin resistance among U.S. adults without physician-diagnosed diabetes: NHANES, 2003-2006. Diabetes Care. 2010;33:344–347. doi: 10.2337/dc09-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashraf A, Alvarez J, Saenz K, Gower B, McCormick K, Franklin F. Threshold for effects of vitamin D deficiency on glucose metabolism in obese female African-American adolescents. J Clin Endocrinol Metab. 2009;94:3200–3206. doi: 10.1210/jc.2009-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS. 25- Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2007;50:69–77. doi: 10.1053/j.ajkd.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Ogunniyi MO, Croft JB, Greenlund KJ, Giles WH, Mensah GA. Racial/ethnic differences in microalbuminuria among adults with prehypertension and hypertension: National Health and Nutrition Examination Survey (NHANES), 1999-2006. Am J Hypertens. 2010;23:859–864. doi: 10.1038/ajh.2010.77. [DOI] [PubMed] [Google Scholar]
- 25.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 26.Clogg CC, Petkova E, Haritou A. Statistical Methods for Comparing Regression Coefficients Between Models. American Journal of Sociology. 1995;100:1261–1293. [Google Scholar]
- 27.Alvarez JA, Ashraf AP, Hunter GR, Gower BA. Serum 25-hydroxyvitamin D and parathyroid hormone are independent determinants of whole-body insulin sensitivity in women and may contribute to lower insulin sensitivity in African Americans. Am J Clin Nutr. 2010;92:1344–1349. doi: 10.3945/ajcn.110.000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiscella KA, Winters PC, Ogedegbe G. Vitamin D and Racial Disparity in Albuminuria: NHANES 2001-2006. Am J Hypertens. 2011;24:1114–1120. doi: 10.1038/ajh.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reis JP, Michos ED, von Muhlen D, Miller ER., 3rd Differences in vitamin D status as a possible contributor to the racial disparity in peripheral arterial disease. Am J Clin Nutr. 2008;88:1469–1477. doi: 10.3945/ajcn.2008.26447. [DOI] [PubMed] [Google Scholar]
- 30.Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab. 2003;88:157–161. doi: 10.1210/jc.2002-020978. [DOI] [PubMed] [Google Scholar]
- 31.Young KA, Engelman CD, Langefeld CD, Hairston KG, Haffner SM, Bryer-Ash M, Norris JM. Association of plasma vitamin D levels with adiposity in Hispanic and African Americans. J Clin Endocrinol Metab. 2009;94:3306–3313. doi: 10.1210/jc.2009-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valina-Toth AL, Lai Z, Yoo W, Abou-Samra A, Gadegbeku CA, Flack JM. Relationship of vitamin D and parathyroid hormone with obesity and body composition in African Americans. Clin Endocrinol (Oxf) 2010;72:595–603. doi: 10.1111/j.1365-2265.2009.03676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 34.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of Metabolic Syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 35.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 36.Need AG, Morris HA, Horowitz M, Nordin C. Effects of skin thickness, age, body fat, and sunlight on serum 25-hydroxyvitamin D. Am J Clin Nutr. 1993;58:882–885. doi: 10.1093/ajcn/58.6.882. [DOI] [PubMed] [Google Scholar]
- 37.Snijder MB, van Dam RM, Visser M, Deeg DJ, Dekker JM, Bouter LM, Seidell JC, Lips P. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metab. 2005;90:4119–4123. doi: 10.1210/jc.2005-0216. [DOI] [PubMed] [Google Scholar]
- 38.Freedman BI, Wagenknecht LE, Hairston KG, Bowden DW, Carr JJ, Hightower RC, Gordon EJ, Xu J, Langefeld CD, Divers J. Vitamin D, adiposity, and calcified atherosclerotic plaque in African-Americans. J Clin Endocrinol Metab. 2010;95:1076–1083. doi: 10.1210/jc.2009-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanley AJ, Wagenknecht LE, Norris JM, Bryer-Ash M, Chen YI, Anderson AM, Bergman R, Haffner SM. Insulin resistance, beta cell dysfunction and visceral adiposity as predictors of incident diabetes: the Insulin Resistance Atherosclerosis Study (IRAS) Family study. Diabetologia. 2009;52:2079–2086. doi: 10.1007/s00125-009-1464-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 41.McGavock JM, Anderson TJ, Lewanczuk RZ. Sedentary lifestyle and antecedents of cardiovascular disease in young adults. Am J Hypertens. 2006;19:701–707. doi: 10.1016/j.amjhyper.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 42.Moller DE, Flier JS. Insulin resistance--mechanisms, syndromes, and implications. N Engl J Med. 1991;325:938–948. doi: 10.1056/NEJM199109263251307. [DOI] [PubMed] [Google Scholar]
- 43.Louet JF, LeMay C, Mauvais-Jarvis F. Antidiabetic actions of estrogen: insight from human and genetic mouse models. Curr Atheroscler Rep. 2004;6:180–185. doi: 10.1007/s11883-004-0030-9. [DOI] [PubMed] [Google Scholar]
- 44.Krotkiewski M, Bjorntorp P, Sjostrom L, Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest. 1983;72:1150–1162. doi: 10.1172/JCI111040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Institute of Medicine of the National Academies: Dietary Reference Intakes for Calcium and Vitamin D. The National Academies Press; 2011. [PubMed] [Google Scholar]
- 46.Armas LA, Dowell S, Akhter M, Duthuluru S, Huerter C, Hollis BW, Lund R, Heaney RP. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol. 2007;57:588–593. doi: 10.1016/j.jaad.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Scragg R, Holdaway I, Singh V, Metcalf P, Baker J, Dryson E. Serum 25- hydroxyvitamin D3 levels decreased in impaired glucose tolerance and diabetes mellitus. Diabetes Research & Clinical Practice. 1995;27:181–188. doi: 10.1016/0168-8227(95)01040-k. [DOI] [PubMed] [Google Scholar]
- 48.Reis JP, von Muhlen D, Miller ER, 3rd, Michos ED, Appel LJ. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics. 2009;124 doi: 10.1542/peds.2009-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Engelman CD, Fingerlin TE, Langefeld CD, Hicks PJ, Rich SS, Wagenknecht LE, Bowden DW, Norris JM. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. Journal of Clinical Endocrinology and Metabolism. 2008;93(9):3381–3388. doi: 10.1210/jc.2007-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alvarez JA, Ashraf AP, Hunter GR, Gower BA. Serum 25-hydroxyvitamin D and parathyroid hormone are independent determinants of whole-body insulin sensitivity in women and may contribute to lower insulin sensitivity in African Americans. American Journal of Clinical Nutrition. 2010;92:1344–1349. doi: 10.3945/ajcn.110.000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 52.Alemzadeh R, Kichler J, Babar G, Calhoun M. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism: Clinical & Experimental. 2008;57:183–191. doi: 10.1016/j.metabol.2007.08.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Population Characteristics Stratified by Presence of Insulin Resistance: National Health and Nutrition Examination Survey (NHANES), 2001-2006a
aData presented as column percentages; means and percentages are weighted to the non-institutionalized US population. Data are shown as either % (n) or means (SE).
b2-sided P-value for χ2 statistics of association between demographic and insulin resistance or t-test for difference in means between insulin resistance category.
cActivity level was based on participant self-report compared to others the same age.
Supplementary Table 2. Mean Serum 25[OH] D (ng/ml) for Relevant Characteristics Stratified by Race/Ethnicity: National Health and Nutrition Examination Survey (NHANES), 2001-2006a
a2-sided P-value from F-test for analysis of variance for Vitamin D levels associated with demographic category.
b2-sided P-value for χ2 statistics between demographic and race or t-test for difference in means between non-Hispanic Blacks and Whites.
cActivity level was based on participant self-report compared to others the same age.


