Abstract
Previous studies of inbred mouse strains have shown reinforcer-strain interactions that may potentially mask differences among strains in memory performance. The present research examined the effects of two qualitatively different reinforcers (heterogeneous mix of flavored pellets and sweetened-condensed milk) on responding maintained by fixed-ratio schedules of reinforcement in three inbred strains of mice (BALB/c, C57BL/6, & DBA/2). Responses rates for all strains were a bitonic (inverted U) function of the size of the fixed-ratio schedule and were generally higher when responding was maintained by milk. For the DBA/2 and C57BL/6 and to a lesser extent the BALB/c, milk primarily increased response rates at moderate fixed ratios, but not at the largest fixed ratios tested. A formal model of ratio-schedule performance, Mathematical Principles of Reinforcement (MPR), was applied to the response rate functions of individual mice. According to MPR, the differences in response rates maintained by pellets and milk were mostly due to changes in motoric processes as indicated by changes in the minimum response time (δ) produced by each reinforcer type and not specific activation (a), a model term that represents value and is correlated with reinforcer magnitude and the break point obtained under progressive ratio schedules. In addition, MPR also revealed that, although affected by reinforcer type, a parameter interpreted as the rate of saturation of working memory (λ), differed among the strains.
1. Introduction
The goal of much research in the behavioral neurosciences is to develop and validate preclinical models of restricted features of neuropsychiatric disorders (Chadman, Yang, & Crawley, 2009; Nestler & Hyman, 2010). A number of techniques are available to researchers that allow for the production of animal models expressing neurobiological markers of these illnesses (Monteggia, Carlezon, DiLeone, 2008; Fernando & Robbins, 2010; Markou, Chiamulera, Geyer, Tricklebank, & Steckler, 2009). Advances in molecular genetics have made it possible to produce mutant mice that model symptoms of human affective and psychiatric disorders (Grubb, Churchill, Bogue, 2004; Bućan & Abel, 2002; Cryan & Holmes, 2005). Behavioral research with genetically engineered mice aims to identify phenotypes related to the psychopathology of a neuropsychiatric disorder (Tarantino & Bućan, 2000; Seong, Seasholtz, & Burmeister, 2002). Mouse models of several prevalent disorders are now in existence and many reviews of the efforts of a number of laboratories to identify behavior phenotypes in these animals have appeared (Crawley, 1999; 2008; Sousa, Almeida, & Wotjak, 2006). Because the behavior phenotype of mutant mice is the product of both the targeted and background genes, a careful experimental analysis of the behavior of the background strain is critical to the interpretation of functional consequences of any mutation.
Crawley and colleagues (Crawley et al., 1997) provided a comprehensive overview and comparison of phenotyping studies conducted with several inbred mouse strains. Many reviews of behavior phenotypes of inbred mice have directed attention to issues of general health, however, researchers are also interested in making comparisons among inbred strains on more complex functions of the nervous system (i.e., learning and memory) to inform their choice of background (Hunsaker, 2012; Wehner & Silva, 1996). The prevailing tendency in this literature has been to make ordinal comparisons of performance of commonly used strains in learning and memory tasks. It is becoming increasing clear, however, that effects of strain on commonly employed measures of learning and memory may sometimes result from differential sensitivity to procedural variables (Cabib, Orsini, Le Moal, & Piazza, 2000; Crabbe, Wahlsten, & Dudek, 1999; Haluk & Wickman, 2010; Orsini, Buchini, Conversi, & Cabib, 2004). Common features of experimental protocols such as housing conditions, length of experimental sessions, and food restriction regimen have been shown to moderate strain differences.
The complex performances that are generated in the laboratory to model behavior seen in neuropsychiatric disorders require careful control over the quality and magnitude of the reinforcing consequences used to establish and maintain these performances. This is true of animal models of impulsive choice (Madden & Johnson, 2011), short-term memory (Brown & White, 2005; 2009), and behavioral flexibility or reversal learning (Chudasama & Robbins, 2006). Moreover, differences in reinforcer impact among strains may be mistaken for differences in the genetic contribution to a particular behavioral domain. For example, Youn et al., (2012) reported that the appearance of a difference in spatial memory among C57BL/6 and DBA/2 strains depended on the reinforcer for finding an escape cylinder in a modified Barnes maze. Specifically, when spatial search in the Barnes maze was maintained by negative reinforcement (i.e., escape from strong winds) the C57BL/6 mice located an escape cylinder faster than DBA/2 mice. When spatial search was maintained by positive reinforcement (opportunity to consume almond chips in the escape cylinder), however, the differences between the strains disappeared. Youn and colleagues also identified inconsistencies across prior reports of spatial memory among these strains that may have been confounded by similar procedural factors. Specifically, Youn and colleagues took note of variables that affect a strain’s response to stressful or novel environments as one determinant of the apparent inconsistencies in the literature (Ohl, Roedel, Binder, & Holsboer, 2003). Thus, prior studies likely found important differences among inbred strains, but may have attributed the difference to the incorrect mechanism(s).
Ratio schedules of reinforcement are an oft-used tool in behavioral pharmacology (Katz, 1990; Richardson & Roberts, 1996; Roberts & Richardson, 1993). Properties of responding on ratio schedules (e.g., Baron, Mikorski, & Schlund, 1992) have been employed to compare the efficacy of qualitatively different reinforcers (e.g., drugs with demonstrated abuse potential versus novel compounds (Griffiths, Brady, & Bigelow, 1981)), to assess the effects of neurotoxic lesions (Bezzina et al., 2008; Kheramin et al., 2005) and in investigations of the motoric/motivational effects of acute drug treatments (Mobini, Chiang, Ho, Bradshaw, & Szabadi, 2000; Zhang, Rickard, Asgari, Body, Bradshaw, & Szabadi, 2005) Because some measures of progressive ratio-schedule responding, such as the ratio at which responding ceases (‘break point’) and peak response rate, are sensitive to the nature of the progression employed and thus, are likely not an unambiguous index of reinforcer value (Killeen, Posadas-Sánchez, Borgå, & Thrailkill; 2009; Stafford & Branch, 1998), some research groups have applied formal models to ratio-schedule performance in an attempt to circumvent such interpretive difficulties (e.g., Bradshaw & Killeen, 2012).
Mathematical Principles of Reinforcement (MPR; Killeen, 1994; Killeen & Sitomer, 2003) is a general quantitative framework that predicts various measures of operant behavior on simple schedules of reinforcement. MPR posits three fundamental processes that underlie all schedule-controlled operant behavior: 1) presentation of an appetitive or reinforcing stimulus produces nonspecific activation of behavior; 2) the ceiling on the rate of a given behavior (e.g., lever pressing) is set by the minimum time required to emit an instance of that behavior; 3) arranging a contingency between behavior and a reinforcer causes certain responses to become coupled to the reinforcer. The strength of this association decreases as a function of events or time interposed between behavior and reinforcement. The equation for predicting response rate b on a fixed ratio schedule of reinforcement (Killeen & Sitomer, 2003) in which the every nth response is reinforced is
| (1) |
where specific activation a is a measure of reinforcer value, response time, δ, is the minimum time to complete a target response, and coupling, c, the degree of association between a target response class and reinforcer arranged by a schedule of reinforcement (Killeen, 1994; Killeen & Sitomer, 2003). The coupling coefficient, c, has been interpreted as the proportion of all behavior activated by the reinforcer that is measured by the target response (Killeen & Bizo, 1998).
The expression for c depends on the nature of the contingencies arranged by a schedule of reinforcement (see Killeen, 1994). For fixed-ratio schedules, c = 1 − e−λδn. Because ratio schedules require a fixed number of target responses, and these typically occur as an uninterrupted run, as the ratio requirement increases, more target responses be will coupled with the reinforcer. At low ratios, events other than the target response will become coupled to the reinforcer, but as the ratio requirement increases the number of reinforceable responses approaches a ceiling (c/δ). This ceiling represents a response count beyond which the influence of a reinforcer on non-target behavior is insignificant (Killeen & Sitomer, 2003). Coupling reaches an asymptote of 1.0, or saturates, with the event immediately preceding reinforcement, and therefore λ can be termed a saturation rate.
The rate parameter, λ, in the expression for coupling in ratio schedules captures the fact that a reinforcer has a diminishing impact on responses as they retreat into the past or, according to Killeen and colleagues, as quantifying “the rate of decay of response traces” (Killeen & Sitomer, 2003, p. 54). Thus as λ increases, the coupling to events more distal to reinforcement decreases. Coupling in the MPR framework serves the same role as eligibility traces in temporal difference (TD) learning algorithms (Sutton & Barto, 1998) to solve the temporal assignment of credit problem. In these models, responses or other events more distal from a reinforcer are eligible for less of its effect and other, competing, distal events may be strengthened. Eligibility traces in TD models may represent persistent neural activity observed in a number of cortical and subcortical areas in working memory and decision-making tasks (see Curtis & Lee, 2010). If it is assumed that a discrete number of events can be credited for reinforcement then, according to Killeen and colleagues, coupling is related to the construct of working memory capacity and λ is the rate at which saturation is approached (see Killeen, 2001; 2012).
Figure 1 illustrates the theoretical function for fixed ratio schedules given by MPR, along with changes in the shape of the function produced by changes in different parameters. The figure demonstrates the contribution of each constituent process to the shape of the response rate function. The saturation parameter λ dictates the position along the x-axis (fixed ratio) where the peak rate of responding will occur. The peak occurs when a value of the ratio schedule (i.e., number of target responses) is reached that exhausts the influence of the reinforcer. The minimum response time reflecting the biomechanics of the response device and the animal’s motor capabilities is δ; extrapolating to the y-axis gives the unconstrained maximum rate of target responding (1/δ). The x-axis intercept of the function, the breaking point, is given by the activation parameter and may be gainfully employed to construct a scale of reinforcer value (Reilly, 2003; Rickard, Body, Zhang, Bradshaw, & Szabadi, 2009).
Fig. 1.
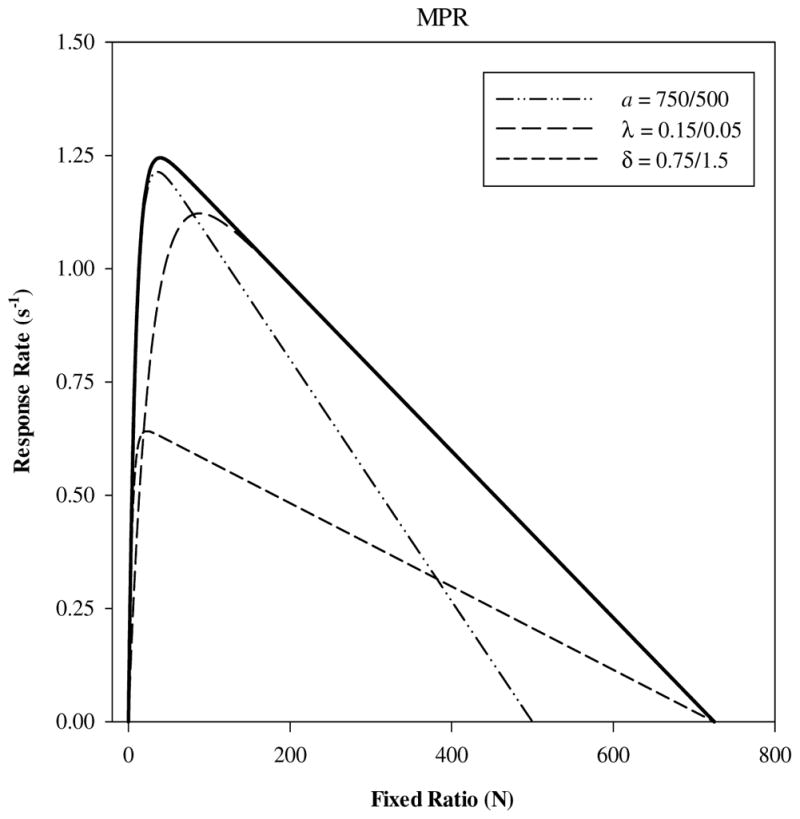
Predicted overall response rate (s−1) plotted as a function fixed ratio schedule by MPR (Eq. 1). The thick reference line represents predictions with a = 750, λ = 0.15, and δ = 0.75, respectively. Each dashed line indicates the changes in the shape of the predicted function given the change in one parameter as indicated at the right of the forward slash. The change in a = 750/500 (dot-dot-dash) corresponds to a less valued reinforcer that produces a lower break point and intercepts the x-axis at a smaller fixed ratio. The change in λ = 0.15/0.05 (long dash) corresponds to a decrease in the rate at which working memory saturates and a larger fixed ratio when the peak of the rate function is reached. The change in δ = 0.75/1.5 corresponds to an increase in the minimum response time and a decrease in the peak response rate from 1.3 to .66 responses s−1.
A number of studies have now confirmed the interpretive utility of MPR to consistently distinguish between manipulations that affect motivational (Reilly, 2003; Rickard, Body, Zhang, Bradshaw, & Szabadi, 2009) and motoric processes (Avila, et al, 2009, Stafford & Branch, 1998). A recent review by Bradshaw and Killeen (2012) establishes the power of the formal approach offered by MPR to illuminate the specific behavioral mechanisms affected by neurobiological interventions. The present study was designed to examine the determinants of responding of three inbred mouse strains reinforced with either flavored pellets or milk under fixed-ratio schedules. Application of MPR to the resulting response rate functions would clarify if the relative effectiveness of milk and sucrose pellet reinforcers in procedures reputed to assess executive function were due to differences in inherent value and/or the differential expression of motoric and mnemonic processes.
2. Methods
2.1. Subjects
Adult male BALB/c, C57BL/6n, and DBA/2 mice were purchased from Harlan Laboratories (Indianapolis, IN) at 6–8 weeks of age. Mice were housed (2 per cage) in clear polycarbonate cages with wire tops and woodchip bedding located in an AAALAC-accredited facility. A diagonal Plexiglas® barrier separated cage mates, who were always of the same strain, effectively creating single housing. The vivarium was temperature- and humidity-controlled and maintained on a 12-h light-dark cycle (lights on at 6:00 a.m.). Experimental sessions were conducted during the mice’s light period. Mice had free access to water when in their home cages. Mice were maintaining at a target weight of 24g by feeding a measured quantity of food following each experimental session (usually 2.5g). Groups consisted of 4–8 mice of a given strain for a total of 41 mice (8:7 (pellet: milk) BALB/c, 7:8 C57BL/6n, and 7:4 DBA/2). All mice had previous experience lever pressing for flavored reinforcers when the present experiment commenced. Experimental sessions were conducted daily, at approximately the same time with few exceptions.
2.2 Apparatus
Experimental sessions were conducted in 12 operant conditioning chambers (12.0″ L × 9.5″ W × 11.5″ H) manufactured by Med Associates (Med Associates Inc. St. Albans, VT, Model ENV-007) enclosed in sound-attenuating cabinets and modified to accommodate mice. The rear wall in each chamber was equipped with a non-retractable response lever (ENV-310W-X) and two Sonalert® tone generators located at the top of the chamber equidistant (L and R) from the centrally located houselight. The front wall of each chamber was equipped with two retractable response levers (ENV-312-2R). Only the left lever was used in the present experiment. The reinforcer delivery system was either a 20mg pellet dispenser (ENV-203M) or a liquid dipper system for mice (ENV-302W-SX). Reinforcers were single 20mg banana, chocolate, grain or sucrose/grain pellets (Purina) comprising a heterogeneous mix of flavors. Liquid reinforcers consisted of a 0.01 cc presentation of a 3:1 solution of water and sweetened condensed milk. In an adjacent room, a computer with Med Associates® IV programming and interface system controlled experimental events and collected data with a temporal resolution of 0.01 s.
2.2. Procedure
Because all mice had prior experience lever pressing, no preliminary training was required. Mice from each strain were divided into two groups defined by reinforcer type. In the first 3–4 sessions all mice earned pellet or milk deliveries according to a fixed ratio (FR) 1 schedule of reinforcement during 30-minute sessions. Next, mice lever pressed for milk or pellets under fixed-ratio requirements that increased across consecutive, daily sessions (Raslear, Bauman, Hursh, Shurtleff, & Simmons, 1988). Following FR 1 training, the fixed-ratio was increased each day in ascending order: FR 15, 45, 90, 180, 360 and 590. Following completion of the highest ratio, each mouse returned to FR 1 for two sessions, and then experienced the same progression again with the FR value increasing each session. Thus, the mice experienced all ratio values greater than FR 1 two times, on different days. Data presented are from the second determination.
2.3. Data Analysis
Analyses presented in figures and subjected to statistical analyses were taken from the second series of ratio requirements experienced by each subject. All statistical analyses were conducted using SYStat 11® software (Systat Software, Inc., Chicago, IL). All dependent measures were analyzed by two- (strain × reinforcer type) or 3-way (strain × reinforcer type × fixed-ratio value) analysis of variance (ANOVA), with fixed-ratio value treated as a repeated measure. The threshold for statistical significance for all tests was P < 0.05. When appropriate, follow up comparisons between strains or reinforcer type with strains were conducted using Tukey’s HSD for post hoc analyses.
2.3.1. Raw Data
2.3.1.1. Overall response rate and maximum response rate
Overall response rates were calculated as total lever presses divided by total session time (less reinforcer delivery time for milk). The peak of the response-rate function (see Fig. 5) for individual mice and taken as the maximum response rate. Maximum response rate was subjected to a square root transformation prior to statistical analyses because of unequal variances among groups.
Fig. 5.
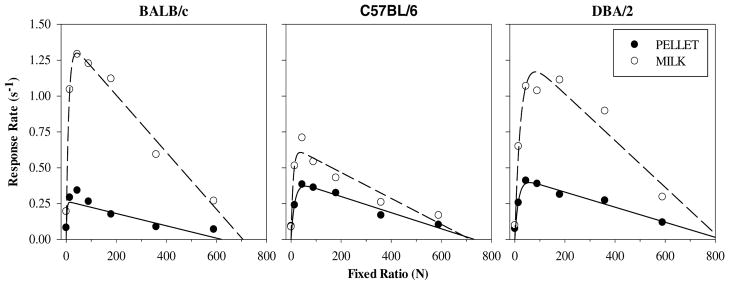
Response rate (s−1) plotted as a function of fixed ratio for each strain and reinforcer type. The dashed and solid curves are the predictions of MPR to the group data for milk and pellets, respectively.
2.3.1.2. Reinforcers earned and proportion FR1 reinforcers earned
The number of reinforcers earned at an FR1 schedule requirement is often considered a measure of unconstrained consumption (Hursh, Raslear, Bauman, Black, & Grunert, 1989). To assess the effects of increasing ratio requirements on reinforcers earned, we calculated the number of reinforcers earned at each ratio requirement relative to the number earned at FR1. We chose the proportion of FR1 reinforcers earned at FR15 for further analyses because, 1) response rate typically peaked at FR15, 2) the number of reinforcers earned generally decreased as a function of the increasing fixed ratio schedule, and 3) most published studies with inbred strains seldom employ greater schedule requirements,.
2.3.1.3. Quantitative Analysis
Eq. (1) was fitted to the overall response rate-function obtained for each mouse using least-squares regression. We used two approaches to fitting MPR to the present data. The first approach was a model-comparison approach (Burnham & Anderson, 2002) that uses Akaike weights to compare fits of MPR with different numbers of parameters allowed to vary across groups (e.g., Avila et al., 2009). The second, two-stage, approach was to fit MPR to the data of individual subjects and analyze the resulting parameter estimates at the group level with ANOVA. These two approaches to modeling the data yielded nearly identical results, however, the ANOVA identified one additional effect of reinforcer type on λ. We report only the two-stage analyses below.
3. Results
3.1. Raw Data
3.1.1. Cumulative Records
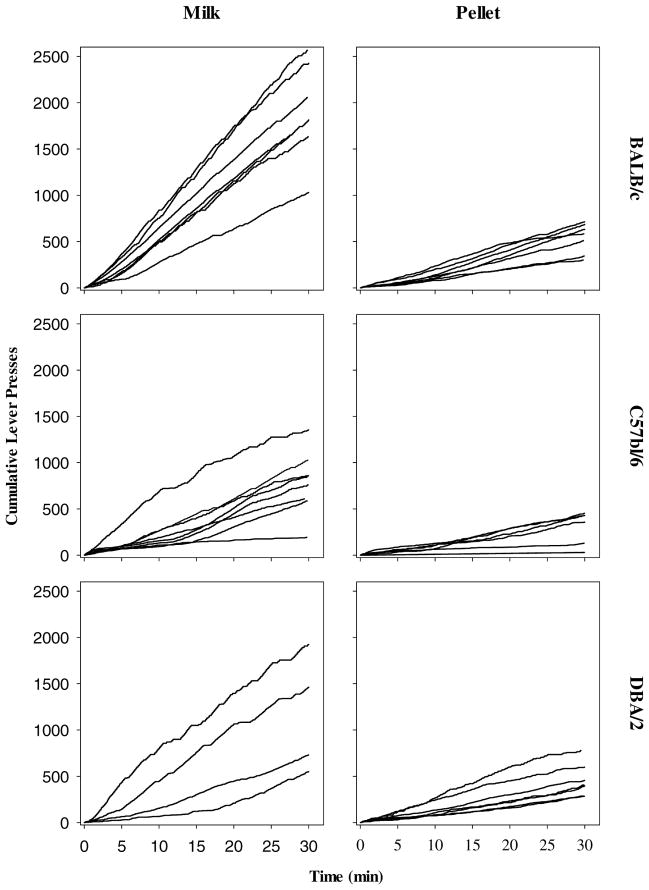
The major advantage of a cumulative record is the ability to detect visually changes in the moment-to-moment rate of a behavior and to identify similarities, or differences, across individuals in these patterns. Fig. 2 shows cumulative records of lever pressing from a single session for all mice in the study. The data were taken from a session in which mice responded for either milk or sucrose under an FR 15 schedule. This ratio was chosen because it was near the peak response rate for most mice and it was the ratio at which nearly all mice earned the greatest number of reinforcer deliveries, excluding reinforcers earned at FR 1. As can be seen in Fig. 1, milk supported more responding than pellets for all three strains, but the effect of reinforcer type was most evident for the BALB/c, intermediate for the C57BL/6, and less so for the DBA/2 mice. In addition, a strain-related difference in response rates occurred with milk but not pellet reinforcers. Also note from the cumulative records that responding occurred at a steady, consistent rate throughout the session; there was no evidence of satiation or fatigue, which would be evident as a negatively accelerated curve.
Fig. 2.
Cumulative records of lever pressing for milk (right) and sucrose pellets (left) for an entire session. Lever pressing was reinforced according to a FR 15 schedule. Rows correspond to strain.
3.1.2. Maximum response rate
Fig. 3 shows the square-root transformed maximum rate of lever pressing across all ratios for each mouse strain and reinforcer type. There was a significant strain × reinforcer type interaction (F(2,34) = 4.00, p = 0.027) and a main effect of reinforcer type (F(1,34) = 28.48, p < 0.001) but no significant effect of strain on maximum response rate (F(2,34) = 2.51, p = 0.09).
Fig. 3.
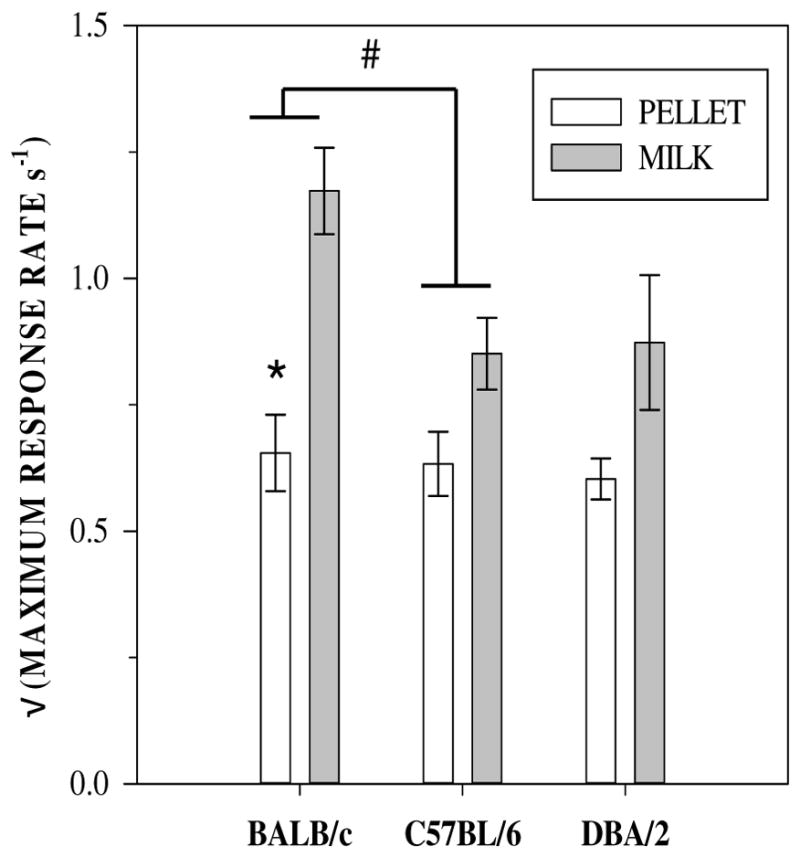
The (square-root transformed) maximum response rate (s−1) obtained for each strain of mice responding for milk or sucrose pellets.# * BALB/c mice responded at significantly higher maximum rates under milk compared to pellet reinforcers and at higher maximum rates than C57BL/6 mice.
The main effect of reinforcer type and the interaction between reinforcer type and strain were clarified by post hoc tests. BALB/c mice responded significantly more for milk than pellets (p < 0.01), but no significant effect of reinforcer type was seen for the other two strains. Maximum response rate was the same for all three strains for the pellet reinforcer but for the milk reinforcer. BALB/c mice responded at higher rates than the other two strains. Maximum response rate did not differ between the C57BL/6 and DBA/2 strains and the rate of responding for DBA/2 mice did not differ from that of the BALB/c mice (all p’s > 0.1).
3.1.3. Percent FR1 reinforcers earned
We compared the number of reinforcers delivered under the FR 1 schedule (‘unconstrained’ consumption) with the maximum number of reinforcers earned at ratios exceeding FR 1. The greatest number of reinforcers earned in a session when consumption was constrained by increasing costs (FR requirement) was under the FR 15 schedule for all but one mouse in the study. To examine constrained consumption relative to consumption under the FR1 we calculated the percent of reinforcers earned at FR 15 as a function of the number earned at FR 1. Table 1 shows the mean number of milk and pellet reinforcers earned under FR 1 for each strain.
Table 1.
Mean (+/− SEM) number of pellet or milk reinforcers earned under a FR 1 schedule for each strain. This unconstrained consumption is also the number of responses during the thirty-minute session. Reinforcers earned was the mean number across the two FR 1 sessions of exposure for each subject. BALB/c mice earned significantly more milk than pellet reinforcers (* p < 0.02) and more milk reinforcers than C57BL/6 mice (# p < 0.04).
| Strain
|
|||
|---|---|---|---|
| BALB/c | C57BL/6 | DBA/2 | |
| Pellet | 144.8 (13.4) *# | 166.4 (11.9) | 134.4 (17.1) |
| Milk | 240.7 (9.2) | 155.9 (9.0) | 175.0 (27.9) |
Reinforcer type (F(1,34) = 10.35, p = 0.003) and the interaction between reinforcer type and stain (F(2,34) = 6.13, p = 0.005) significantly affected the number of reinforcers earned when consumption was unconstrained (Table 1). Fig. 4 shows the percentage of FR 1 reinforcers earned at FR 15. The decrease in consumption due to increased cost (FR 15) was greater for sucrose pellets (F(1,34) = 18.35, p < 0.001). The main effect of strain and the strain by reinforcer type interaction on percent consumption were not significant. Note that a 15-fold increase in response cost reduced pellet consumption only 4–5 fold for pellets for all three strains, and 2–3 fold for milk. Thus, as cost increased so did response output, even as total reinforcers delivered decreased. The mice increased response output more, and maintained consumption levels closer to unconstrained consumption more for milk than for pellets.
Fig. 4.
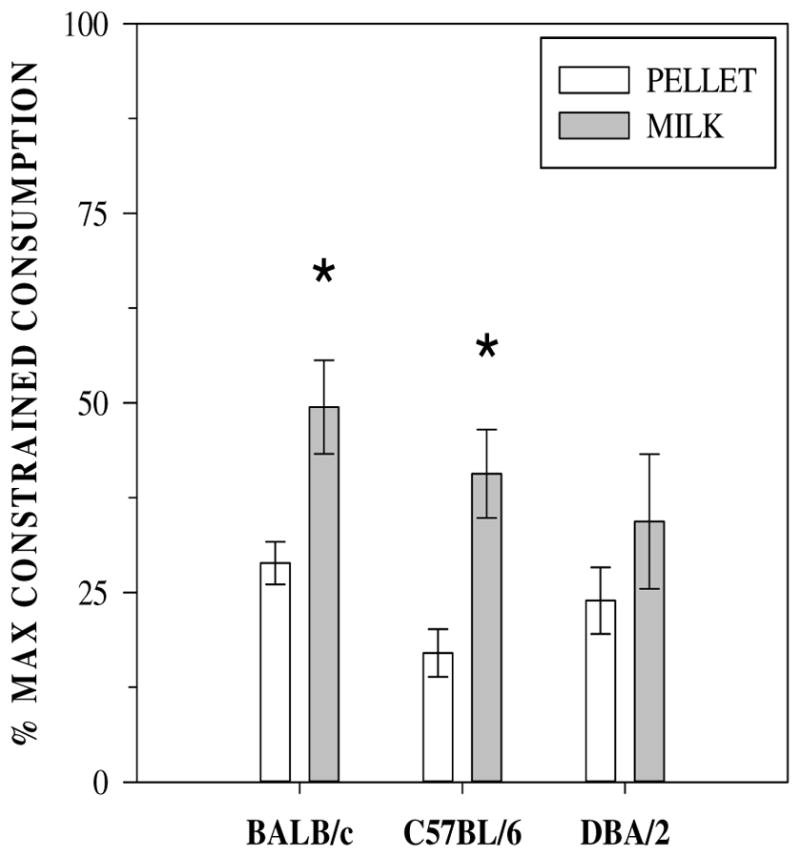
Percentage of reinforcers earned under FR 15 relative to the number earned under FR 1 (100*(R_FR15/R_FR1), where R = reinforcers earned). # The reduction in reinforcers earned was significantly less for BALB/c and C57BL/6 mice earning milk relative to sucrose pellets.
3.1.3. Overall response rate
Fig. 5 shows overall response rate as a function of fixed ratio schedule; the curves are the functions defined by Equation 1. Response rates tended to be lower for pellet-maintained responding than for milk-maintained responding. Repeated measures analysis of variance revealed a significant fixed ratio × strain × reinforcer type (F(12,204) = 2.714, p = 0.002) interaction. There were main effects of strain (F(2,34) = 3.626, p = 0.036), reinforcer type (F(1,34) = 23.98, p < 0.001), and fixed ratio (F(6,204) = 43.964, p = 0.001). There was a significant 2-way interactions between strain and reinforcer type (F(2,34) = 4.65, p = 0.016), fixed ratio and strain (F(12,204) = 2.994, p = 0.001) and fixed ratio × reinforcer type (F(6,204) = 11.237, p < 0.001).
3.2. Quantitative analysis: parameters of Eq. (1)
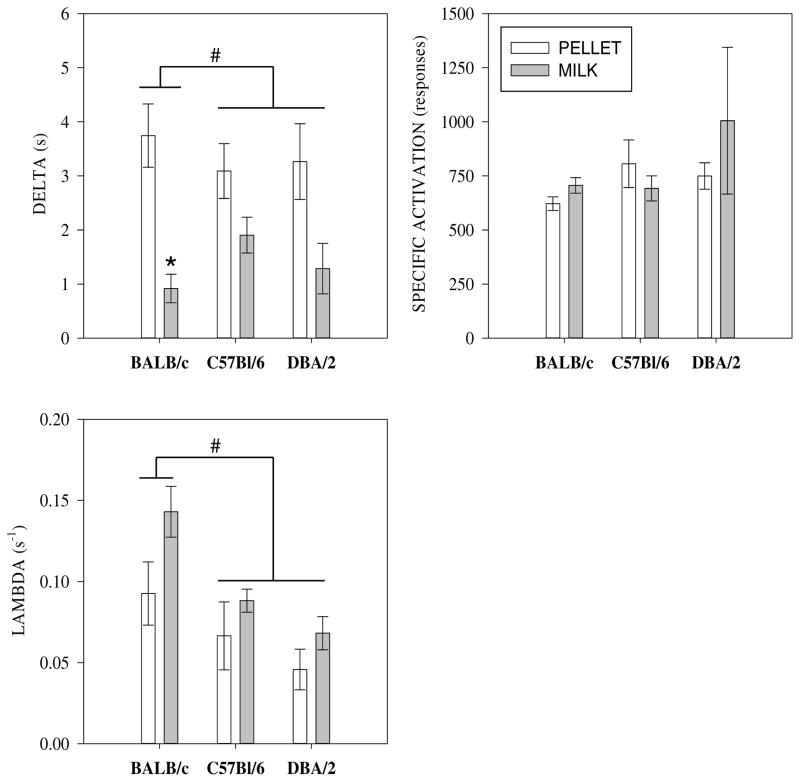
The MPR equation was fit to the response rate function of individual mice. Eq. (1) provided a good fit to the individual data accounting for on average 85% of the variance (MSE = 0.012). The values of the parameters derived from the fits to the data of individual mice are shown graphically for each group in Fig. 6.
Fig. 6.
Parameters obtained from fits of Eq. (1) to response rate functions of individual mice. (A) Delta (δ): # * BALB/c mice responded at significantly higher maximum rates under milk compared to pellet reinforcers and at higher maximum rates than C57BL/6 mice. (B) Specific Activation (a): Effects of strain, reinforcer type, and their interacton were all non-significant. (C) Saturation rate (λ): #* Rate of saturation is signficantly greater for BALB/c mice compared to C57BL/6 and DBA/2 mice.
Specific activation (a)
There were no effects of strain or reinforcer type on specific activation. The interaction between strain and reinforcer type on specific activation was also not statistically significant (all p’s > 0.15)
Response time (δ)
Prior to statistical analyses the reciprocal of the δ estimate for each mouse was square root transformed. Note that 1/δ is the estimate of maximum response rate possible according to Eq. (1). A regression was conducted on obtained maximum response rate versus that predicted according to Eq. (1). The regression results indicated that the estimated value of 1/δ explained 97.4% of the variance in obtained maximum response rate (β = 0.94, p < 0.001; R2 = 0.974, F(1,36) = 1355.85, p < 0.001). Thus, in addition to providing a good description of the entire response rate function for each mouse, MPR also provided a good prediction of the ratio at which maximal responding occurred. The effects of strain (F(2,32) = 5.77, p = 0.007) and reinforcer type (F(1,32) = 43.18, p < 0.001) on response time were significant. The interaction between strain and reinforcer type was also significant (F(2,32) = 8.69, p = 0.001). The nature of the significant interaction was clarified by post hoc tests. The effect of reinforcer type on response time appeared only for the BALB/c mice (p < 0.05). In addition, the response times of the BALB/c mice were shorter (i.e., response rate was higher) than that of the C57BL/6 and DBA/2 strains (p’s < 0.05).
Saturation rate (λ)
The effects of strain (F(2,34) = 6.87, p = 0.003) and reinforcer type (F(1,34) = 4.78, p = 0.035) on the rate at which the impact of reinforcement saturates were significant. The interaction between strain and reinforcer type was not significant. The larger λ indicates that milk delivery has a shorter temporal influence than sucrose delivery for all strains. The rate of saturation was also greater for BALB/c mice compared to the C57BL/6 and DBA/2 mice (p’s < 0.05). There was no difference in rate of saturation between the C57BL/6 and DBA/2 mice (p > 0.4).
4. Discussion
The present study was designed to evaluate the effectiveness of two qualitatively different reinforcers in maintaining fixed-ratio responding with genetically divergent mouse strains. The present study provided a direct and detailed assessment of each reinforcer by measuring the rate of lever pressing as a function of the increasing cost (fixed-ratio) of each reinforcer delivery. Parametric manipulation of the ratio-schedule requirement afforded the opportunity to draw precise conclusions regarding milk and sucrose pellet reinforcers. Specifically, we asked whether the greater effectiveness of milk as a reinforcer is the result of a more efficient response topography or a greater inherent value as compared to sucrose pellets.
A distinction between reinforcer efficacy and value may be useful in interpreting the present results (see Rowlett, 2000). The efficacy of a reinforcer refers to the maximum response rate produced by a reinforcer under a particular fixed-ratio schedule (Rowlett, 2000; Bizo, Kettle, & Killeen, 2001). The value of a reinforcer is reflected in the maximum number of responses a subject will make to gain access to a single presentation of that reinforcer. Inspection of response-rate functions over a range of fixed ratios (see Fig. 5) confirms that milk is a more efficacious reinforcer than sucrose pellets, in the sense that milk produced substantially higher peaks in the response rate functions. The two reinforcers did not differ, however, in the response rates maintained at the higher fixed ratios. MPR, therefore, predicts that the reinforcers would not produce consistently different break points (i.e., the extrapolated x intercept) under a progressive ratio schedule because the specific activation parameter for each reinforcer was approximately equal. It is for this reason that existing economic-based models that attempt to quantify the elasticity or essential value of each reinforcer type (e.g., Hursh & Silberberg, 2008) provide limited insight regarding potential mechanisms that contribute to the differences observed. MPR, a mechanistic approach (Killeen, 1995), implies a difference in efficacy between milk and sucrose pellets and this difference in efficacy is produced by the differential expression of motoric and mnemonic processes, but not in the inherent value of each consumable as a reinforcer of lever pressing.
The MPR parameter δ accounted for the difference in the maximum response rates (1/δ) supported by response-contingent delivery of milk and sucrose pellets. This parameter has frequently been taken as an index of the biomechanical limitations on responding imposed by the specific response device under study. For example, Bizo and Killeen (1997) found that δ varied in a predictable manner when, in different conditions, pigeons were required to peck keys or press treadles for milo. The finding, here, that δ is affected by reinforcer quality even though the response device is the same is not strictly predicted by MPR (Killeen & Sitomer, 2003). It should be noted that in the version of MPR used here, δ is a composite of the minimum time taken to make a single response and longer pauses in responding that occur, for example, following food delivery or if the animal frequently checks the dispenser for a pellet (see Bradshaw & Killeen, 2012). If there was more pausing with pellets than with milk then this might explain why the δ values obtained for pellets was longer.
A recent modification of MPR that separates post-reinforcement pausing from the minimum response time may have provided a better account of the relationship between δ and reinforcer type (see Bradshaw & Killeen, 2012), but we did not have the pauses available that would permit such an analysis. It is possible that our estimates of δ would have been shorter and constant across reinforcement types if pauses could have been isolated, thereby making a purer measure of response duration possible The interpretation of the composite δ used here is that it provides an index of the minimum interresponse time obtained under the particular contingencies of reinforcement examined.
Applied here, that interpretation might suggest that the termination of lever-pressing is more tightly controlled by milk than by pellet delivery. One important difference between liquid and pellet delivery systems is the extent to which delivery of each consumable competes with lever pressing (or other behavior). Delivery of liquid reinforcers is typically arranged according to a limited hold, that is, the opportunity to consume lasts only a few seconds (3 s here). When a ratio-schedule requirement is met, lever pressing is interrupted by milk delivery and mice must stop pressing (and do so rather quickly) in order to consume the solution. In contrast, a pellet remains in the food tray until the mouse consumes it, so the functional contingences on lever-release and disengagement from the response bout are less restrictive for pellets than for milk. Therefore, it is possible that milk differentially reinforces releasing the lever compared to sucrose pellets, producing shorter response durations and, hence, shorter response times. Perhaps the parameter, 1/δ, reflects the biomechanics of the response device as well as other implicit contingencies of reinforcement produced by the device. Thus, δ for milk is, in part, shorter because milk delivery is available for a limited period of time and is better discriminated.
Previous studies have found some effect of reinforcer quality or magnitude on response time, δ (as a composite), and saturation rate (λ). Killeen and Sitomer found a positive relationship between δ and λ among rats (Killeen & Sitomer, 2003) and revised MPR by putting δ in the expression for coupling. With this change, δ and λ became orthogonal to one another. This is the version used in Equation (1). Rickard et al., (2009) found an inverse relationship between δ and λ when the volume of a sucrose solution that reinforced rats’ lever pressing was manipulated across conditions under a progressive-ratio schedule. This is consistent with what is seen across groups in the present study. The present finding of an effect of reinforcer type on the maximum response rate possible is difficult to reconcile with the simpler interpretation of δ as a strict biomechanical limit within the MPR framework, but it should be noted that Herrnstein’s quantitative statement of response strength (Herrnstein, 1970; de Villiers & Herrnstein, 1976), the most prominent quantitative treatment of schedule-controlled behavior, has had the very same difficulties (Dallery & Soto, 2004; McDowell, 2005; 2012). Clearly, the mechanisms contributing to the obtained minimum response time require further investigation (Killeen & Sitomer, 2003, Bizo & Killeen, 1998).
Our finding and that of Rickard et al., (2009), of a tradeoff between the model parameters δ and λ (Fig. 6) suggest a probable dynamic interaction between two processes. Cumulative records of lever-pressing by mice in the current study (Fig. 2 & others not shown) consistently showed that responding was more ‘ragged’ (i.e., more pausing) when reinforced with pellets, particularly for the C57BL/6 and DBA/2 strains. The physical nature of the reinforcers (liquid vs. solid) likely played a role in this difference. In addition, delivery systems for liquid and pellet reinforcers may give rise to differences in the topography of lever pressing and the latency to consume each reinforcer. To the extent that mice consumed sucrose pellets moments after the lever press that produced them, these conditions are analogous to those arranged in studies of unsignaled, non-resetting delayed reinforcement (see Lattal, 2010). Therefore, the lower estimates of response time under sucrose pellets likely resulted from the effects of intermittent delayed reinforcement of lever pressing (or releasing), and greater coupling of non-target responses (Shahan & Lattal, 2005; Schaal, Shahan, Kovera, & Reilly, 1998).
The present findings are in keeping with those of Youn et al., (2012); that the observed phenotype of inbred mice is a result of genotype and specific motivational operations and/or qualitative properties of the reinforcer used in the particular study. Quantitative analyses with MPR showed that both motor and delay-of-reinforcement processes might be affected by reinforcer type (but see Calvert, Green, & Myerson, 2010). On a practical level, genotype-reinforcer interactions serve to guide researchers’ choice of protocol – milk is a particularly efficacious reinforcer for mice, especially when only a few responses are required to earn a single presentation. In discrete-trial procedures used to assess attentional (Steckler, 2001; Humby, Wilkinson, & Dawson, 2005) and memorial (Estapé & Steckler, 2001; 2002) function in inbred mouse strains, milk reinforcement may therefore optimize training protocols. Theoretically, the present findings suggest researchers take great care when interpreting effects of strain in phenotyping studies. For example, the efficacy of milk as a reinforcer is not due to its intrinsic value, which apparently does not differ among these strains, but to its engagement of motoric and memorial processes, which appears to differ among strains. Hence, the complexity of environment-genotype interactions that impede researchers’ attempts to determine the functional consequences of genetic differences among inbred mouse strains may be mitigated somewhat by identification of behavioral mechanisms and engaging formal theoretical models.
Finally, MPR suggests persistent differences in the coupling of reinforcers to target responses among the inbred strains studied here. If λ is large, then the influence of the reinforcer dissipates quickly, so it is coupled with fewer prior responses. Saturation rate was more rapid for BALB/c mice regardless of reinforcer type. Therefore, according to MPR, the delay of reinforcement gradient for BALB/c mice is steeper than that of C57BL/6 and DBA/2 mice (e.g. Killeen, 2011). Further support for this conclusion might be seen in species differences in temporal discounting. Previous studies have found differences between pigeons and rats in estimates of the rate of saturation: it is much larger for pigeons (cf., Killeen, Posadas-Sánchez, Borgå, & Thrailkill, 2009; Rickard, Body, Zhang, Bradshaw, & Szabadi, 2009). These findings are consistent with the steeper rates of discounting by pigeons compared to rats (Green, Myerson, Holt, Slevin, & Estle, 2004; Mazur & Biondi, 2009), suggesting that discount rate and λ may index similar behavioral mechanisms. The present findings, larger λ for BALB/c mice and comparable rates for C57BL/6 and DBA/2 mice, predict differences in the delay of reinforcement gradients for these strains. Remarkably, based on the few strain-comparison studies of delay discounting with mice, this prediction appears to be in accord with the available data (Helms, Reeves, & Mitchell, 2006; Otobe & Makino, 2004). That is, BALB/c mice appear to discount the value of delayed reinforcers at a higher rate than C57BL/6 and DBA/2 mice, who discount at comparable rates.
In conclusion, we have shown that common inbred mouse strains used as backgrounds for genetic mutations are sensitive to the specific reinforcer maintaining their behavior. The differences in reinforcer efficacy between milk and sucrose pellets may play an important role in the level of memorial performance observed with inbred strains. Thus, the presence or absence of strain differences in behavior phenotyping studies are likely to depend on the reinforcer and motivational operations employed to engage the animal in the procedure. In addition, the use of a formal quantitative model (MPR) provided a context to interpret the behavioral effects of differences in reinforcer efficacy. Use of a formal model aided the identification of behavioral mechanisms impacted by each reinforcer type and potential differences in these processes among inbred strains.
Supplementary Material
Highlights.
Three inbred mouse strains (BALB/c, C57BL/6, DBA/2) responded on fixed-ratio schedules of reinforcement.
The effects of two qualitatively different reinforcers on response rate were assessed.
Response rates were higher for milk compared to sucrose pellets on moderate ratios.
The responding of inbred mice is sensitive to reinforcer type.
Acknowledgments
The work was supported in part by NIH grant ES017448.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avila I, Reilly MP, Sanabria F, Posadas-Sánchez D, Chavez CL, Banerjee N, Killeen P, Castańeda E. Modeling operant behavior in the Parkinsonian rat. Behavioural Brain Research. 2009;198:298–305. doi: 10.1016/j.bbr.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron A, Mikorski J, Schlund M. Reinforcement magnitude and pausing on progressive-ratio schedules. Journal of the Experimental Analysis of Behavior. 1992;58:377– 388. doi: 10.1901/jeab.1992.58-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzina G, Body S, Cheung THC, Hampson CL, Deakin JFW, Anderson IM, et al. Effect of quinolinic acid-induced lesions of the nucleus accumbens core on performance on a progressive ratio schedule of reinforcement: Implications for inter- temporal choice. Psychopharmacology. 2008;197:339–350. doi: 10.1007/s00213-007-1036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizo LA, Killeen PR. Models of ratio schedule performance. Journal of Experimental Psychology: Animal Behavior Processes. 1997;23:351–367. doi: 10.1037//0097-7403.23.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizo LA, Kettle LC, Killeen PR. Rats don’t always respond faster for more food: the paradoxical incentive effect. Learning & Behavior. 2001;29:66–78. [Google Scholar]
- Bradshaw CM, Killeen PR. A theory of behaviour on progressive ratio schedules, with applications in behavioural pharmacology. Psychopharmacology. 2012;222:549– 564. doi: 10.1007/s00213-012-2771-4. [DOI] [PubMed] [Google Scholar]
- Brown GS, White KG. On the effects of signaling reinforcer probability and magnitude in delayed matching to sample. Journal of the Experimental Analysis of Behavior. 2005;83:119–128. doi: 10.1901/jeab.2005.94-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GS, White KG. Reinforcer probability, reinforcer magnitude, and the reinforcement context for remembering. Journal of Experimental Psychology: Animal Behavior Processes. 2009;35:238–249. doi: 10.1037/a0013864. [DOI] [PubMed] [Google Scholar]
- Bućan M, Abel T. The mouse: genetics meets behaviour. Nature Reviews Genetics. 2002;3:114–123. doi: 10.1038/nrg728. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodal inference: A practical information-theoretic approach. 2. New York: Springer-Verlag; 2002. [Google Scholar]
- Cabib S, Orsini C, Le Moal M, Piazza PV. Abolition and reversal of strain differences in behavioral responses to drugs of abuse after a brief experience. Science. 2000;289:463–465. doi: 10.1126/science.289.5478.463. [DOI] [PubMed] [Google Scholar]
- Calvert AL, Green L, Myerson J. Delay discounting of qualitatively different reinforcers in rats. Journal of the Experimental Analysis of Behavior. 2010;93:171–184. doi: 10.1901/jeab.2010.93-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadman KK, Yang M, Crawley JN. Criteria for validating mouse models of psychiatric diseases. American Journal of Medical Genetics. 2009;150B:1–11. doi: 10.1002/ajmg.b.30777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Robbins T. Functions of the frontostriatal systems in cognition: Comparative neuropsychopharmacological studies in rats, monkeys, and humans. Biological Psychiatry. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Homes A. The ascent of mouse: advances in modeling human depression and anxiety. Nature Reviews Drug Discovery. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Research. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57:809– 818. doi: 10.1016/j.neuron.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology. 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Lee D. Beyond working memory: the role of persistent activity in decision making. Trends in Cognitive Science. 2010;14:216–222. doi: 10.1016/j.tics.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Soto PL. Herrnstein’s hyperbolic matching equation and behavioral pharmacology: review and critique. Behavioural Pharmacology. 2004;15:443–459. doi: 10.1097/00008877-200411000-00001. [DOI] [PubMed] [Google Scholar]
- de Villiers PA, Herrnstein RJ. Toward a law of response strength. Psychological Bulletin. 1976;83:1131–1153. [Google Scholar]
- Estapé N, Steckler T. Effects of cholinergic manipulation on operant delayed non- matching to position performance in two inbred strains of mice. Behavioural Brain Research. 2001;121:39–55. doi: 10.1016/s0166-4328(00)00379-x. [DOI] [PubMed] [Google Scholar]
- Estapé N, Steckler T. Cholinergic blockade impairs performance in operant DNMTP in two inbred strains of mice. Pharmacology, Biochemistry, & Behavior. 2002;72:319– 334. doi: 10.1016/s0091-3057(01)00747-x. [DOI] [PubMed] [Google Scholar]
- Fernando ABP, Robbins TW. Animal models of neuropsychiatric disorders. Annual Review of Clinical Psychology. 2011;7:39–61. doi: 10.1146/annurev-clinpsy-032210-104454. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J, Holt DD, Slevin JR, Estle SJ. Discounting of delayed food rewards in pigeons and rats: is there a magnitude effect? Journal of the Experimental Analysis of Behavior. 2004;81:39–50. doi: 10.1901/jeab.2004.81-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Brady JV, Bigelow GE. Predicting the dependence liability of stimulant drugs. NIDA Research Monographs. 1981;37:182–196. [PubMed] [Google Scholar]
- Haluk DM, Wickman K. Evaluation of study design variables and their impact on food-maintained operant responding in mice. Behavioural Brain Research. 2010;207:394–401. doi: 10.1016/j.bbr.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Reeves JM, Mitchell SH. Impact of strain and d-amphetamine on impulsivity (delay discounting) in inbred mice. Psychopharmacology. 2006;188:144–151. doi: 10.1007/s00213-006-0478-0. [DOI] [PubMed] [Google Scholar]
- Herrnstein RJ. On the law of effect. Journal of the Experimental Analysis of Behavior. 1970;13:243–266. doi: 10.1901/jeab.1970.13-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humby T, Wilkinson L, Dawson G. Assaying aspects of attention and impulse control in mice using the 5-choice serial reaction time task. Current Protocols Neuroscience. 2005;Chapter 8(Unit 8 5H) doi: 10.1002/0471142301.ns0805hs31. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR. Comprehensive neurocognitive endophenotyping strategies for mouse models of genetic disorders. Progress in Neurobiology. 2012;96:220–241. doi: 10.1016/j.pneurobio.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Raslear TG, Bauman R, Black H, Grunert KG. The quantitative analysis of economic behavior with laboratory animals. In: Ölander F, editor. Understanding economic behaviour. Theory and decision library: Series A: Philosophy and methodology of the social sciences. New York, NY: Kluwer Academic/Plenum Publishers; 1989. pp. 393–407. [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychological Review. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Katz JL. Models of relative reinforcer efficacy of drugs and their predictive utility. Behavioural Pharmacology. 1990;1:283–301. doi: 10.1097/00008877-199000140-00003. [DOI] [PubMed] [Google Scholar]
- Kheramin S, Body S, Miranda Herrera F, Bradshaw CM, Szabadi E, Deakin JFW, et al. The effect of orbital prefrontal cortex lesions on performance on a progressive ratio schedule: Implications for models of inter-temporal choice. Behavioural Brain Research. 2005;156:145–152. doi: 10.1016/j.bbr.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Killeen PR. Mathematical principles of reinforcement. Behavioral and Brain Sciences. 1994;117:105–172. [Google Scholar]
- Killeen PR. Economics, ecologics, and mechanics: the dynamics of responding under conditions of varying motivation. Journal of the Experimental Analysis of Behavior, 1995. 1995;64:405–431. doi: 10.1901/jeab.1995.64-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen PR. Writing and overwriting short-term memory. Psychonomic Bulletin & Review. 2001;8:18–43. doi: 10.3758/bf03196137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen PR. Models of trace decay, eligibility for reinforcement, and delay of reinforcement gradients, from exponential to hyperboloid. Behavioural Processes. 2012;87:57–63. doi: 10.1016/j.beproc.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Killeen PR, Bizo LA. The mechanics of reinforcement. Psychonomic Bulletin & Review. 1998;5:221–238. [Google Scholar]
- Killeen PR, Sitomer MT. MPR. Behavioural Processes. 2003;62:49–64. doi: 10.1016/S0376-6357(03)00017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen PR, Posadas-Sánchez D, Borgå E, Thrailkill EA. Progressive ratio schedules of reinforcement. Journal of Experimental Psychology: Animal Behavior Processes. 2009;35:35–50. doi: 10.1037/a0012497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal KA. Delayed reinforcement of operant behavior. Journal of the Experimental Analysis of Behavior. 2010;93:129–139. doi: 10.1901/jeab.2010.93-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Johnson PS. A delay-discounting primer. In: Madden GJ, Bickel WK, editors. Impulsivity: The behavioral and neurological science of discounting. Washington, DC: American Psychological Association; 2011. pp. 11–38. [Google Scholar]
- Markou A, Chiamulera C, Geyer MA, Tricklebank Steckler T. Removing obstacles in neuroscience drug discovery: The future path for animal models. Neuropsychopharmacology Reviews. 2009;34:74–89. doi: 10.1038/npp.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE, Biondi DR. Delay-amount tradeoffs in choices by pigeons and rats: hyperbolic versus exponential discounting. Journal of the Experimental Analysis of Behavior. 2009;91:197–211. doi: 10.1901/jeab.2009.91-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JJ. On the classic and modern theories of matching. Journal of the Experimental Analysis of Behavior. 2005;84:111–127. doi: 10.1901/jeab.2005.59-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JJ. On the theoretical and empirical status of the matching law and matching theory. Psychological Bulletin. 2012 doi: 10.1037/a0019924. [DOI] [PubMed] [Google Scholar]
- Mobini S, Chiang T-J, Ho M-Y, Bradshaw CM, Szabadi E. Comparison of the effects of clozapine, haloperidol, chlorpromazine and d-amphetamine on performance on a time-constrained progressive-ratio schedule and locomotor behaviour in the rat. Psychopharmacology. 2000;152:47–54. doi: 10.1007/s002130000486. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Carlezon WA, DiLeone RJ. Functional genomics and models of mental illness. In: Charney DS, Nestler EJ, editors. The Neurobiology of Mental Illness. New York: Oxford Univ. Press; 2008. pp. 88–103. [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nature Neuroscience. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohl F, Roedel A, Binder E, Holsboer F. Impact of high and low anxiety on cognitive performance in a modified hole board test in C57Bl/6 and DBA/2 mice. European Journal of Neuroscience. 2003;17:128–136. doi: 10.1046/j.1460-9568.2003.02436.x. [DOI] [PubMed] [Google Scholar]
- Orsini C, Buchini F, Conversi D, Cabib S. Selective improvement of strain- dependent performances of cognitive tasks by food restriction. Neurobiology of Learning and Memory. 2004;81:96–99. doi: 10.1016/s1074-7427(03)00075-3. [DOI] [PubMed] [Google Scholar]
- Otobe T, Makino J. Impulsive choice in inbred strains of mice. Behavioural Processes. 2004;67:19–26. doi: 10.1016/j.beproc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Raslear TG, Bauman RA, Hursh SR, Shurtleff D, Simmons L. Rapid demand curves for behavioral economics. Learning & Behavior. 1988;16:330–339. [Google Scholar]
- Reilly MP. Extending mathematical principles of reinforcement into the domain of behavioral pharmacology. Behavioural Processes. 2003;62:75–88. doi: 10.1016/s0376-6357(03)00027-5. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self- administration studies in rats: a method to evaluate reinforcing efficacy. Journal of Neuroscience Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rickard JF, Body S, Zhang, Bradshaw CM, Szabadi E. Effect of reinforcer magnitude on performance maintained by progressive-ratio schedules. Journal of the Experimental Analysis of Behavior. 2009;91:75–87. doi: 10.1901/jeab.2009.91-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DCS, Richardson NR. Self-administration of psychomotor stimulants using progressive ratio schedules of reinforcement. Neuromethods. 1993;24:233–269. [Google Scholar]
- Rowlett JK. A labor-supply analysis of cocaine self-administration under progressive- ratio schedules: antecedents, methodologies, and perspectives. Psychopharmacology. 2000;153:1–16. doi: 10.1007/s002130000610. [DOI] [PubMed] [Google Scholar]
- Schaal DW, Shahan TA, Kovera CA, Reilly MP. Mechanisms underlying the effects of unsignaled delayed reinforcement on key pecking of pigeons under variable-interval schedules. Journal of the Experimental Analysis of Behavior. 1998;69:103–122. doi: 10.1901/jeab.1998.69-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong E, Seasholtz AF, Burmeister M. Mouse models of psychiatric disorders. Trends in Genetics. 2002;12:643–650. doi: 10.1016/s0168-9525(02)02807-x. [DOI] [PubMed] [Google Scholar]
- Shahan T, Lattal KA. Unsignaled delay of reinforcement, relative time, and resistance to change. Journal of the Experimental Analysis of Behavior. 2005;83:201–219. doi: 10.1901/jeab.2005.62-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D, Branch MN. Effects of step size and break-point criterion on progressive-ratio performance. Journal of the Experimental Analysis of Behavior. 1998;70:123–138. doi: 10.1901/jeab.1998.70-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology. 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Steckler T. Using signal detection methods for analysis of operant performance in mice. Behavioural Brain Research. 2001;125:237–248. doi: 10.1016/s0166-4328(01)00305-9. [DOI] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Reinforcement Learning: an introduction. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Sousa N, Almeida OFX, Wotjak CT. A hitchhiker’s guide to behavioral analysis in laboratory rodents. Genes, Brain & Behavior. 2006;5(S2):5–24. doi: 10.1111/j.1601-183X.2006.00228.x. [DOI] [PubMed] [Google Scholar]
- Tarantino L, Bućan M. Dissection of behavior and psychiatric disorders using the mouse as a model. Human Molecular Genetics. 2000;9:953–965. doi: 10.1093/hmg/9.6.953. [DOI] [PubMed] [Google Scholar]
- Wehner JM, Silva A. Importance of strain differences in evaluations of learning and memory processes in null mutants. Mental Retardation and Developmental Disabilities Research Reviews. 1996;2:243–248. [Google Scholar]
- Youn J, Ellenbroek BA, van Eck I, Roubos S, Verhage M, Stiedl O. Finding the right motivation: genotype-dependent differences in effective reinforcements for spatial learning. Behavioural Brain Research. 2012;226:397–403. doi: 10.1016/j.bbr.2011.09.034. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Rickard JF, Asgari K, Body S, Bradshaw CM, Szabadi E. Quantitative analysis of the effects of some “atypical” and “conventional” antipsychotics on progressive ratio schedule performance. Psychopharmacology. 2005;179:489–497. doi: 10.1007/s00213-004-2049-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.