Abstract
Background:
The etiological factors associated with prostate cancer (CaP) have not been completely understood as yet. Genetic predisposition and inflammation is fast emerging as risk factors for CaP is a key player in the innate immune response and plays role in immune- surveillance and inflammation. The present study was conducted to evaluate TLR-4 gene polymorphism in patients with CaP.
Material and Methods:
DNA was isolated from blood samples of 198 patients with CaP, 200 cases of Benign Prostatic Hyperplasia (BPH) and 119 controls. TLR-4 gene polymorphisms Asp299Gly and Thr399Ile were determined by Restriction Fragment Length Polymorphism (RFLP) technique using Nco1 and Hinf 1 restriction enzymes. All statistical calculations were performed using SPSS for windows, version 13 (SPSS Inc., Chicago, Illinois, USA)
Results:
A significantly high proportion of patients with CaP had AG genotype (16.6%) as compared to control (4.2%) [OR-4.4, 95% CI (1.57-13.26), P =0.0013] with respect to Asp299Gly single nucleotide polymorphism (SNP). AA genotype showed a protective effect towards CaP development [OR-0.39, 95% CI (0.18-0.83), P=0.007). A trend was observed towards development of BPH with respect to AG genotype (P=0.06). Thr399Ile SNP was not significantly different among the population groups studied.
Conclusions:
This finding highlights the genetic predispositions to CaP with respect to TLR-4 gene. Individuals with Asp299Gly polymorphism having AG genotype appear to have four fold higher risk for development of Prostate cancer
Keywords: Gene polymorphism, prostate cancer, toll like receptor-4, TLR-4
INTRODUCTION
Prostate cancer (CaP) is the most common noncutaneous malignancy worldwide, with a still unresolved etiology. Advancing age is a known risk factor. The population of India is now over a billion with an estimated 1.5 million cases of cancer diagnosed per year; among those cases, CaP accounts for 20/million.[1] Positive family history and high prevalence in certain ethnic groups are indicator of its genetic predisposition. Many gene polymorphisms such as those of metabolic pathway, hormone receptor,[2,3] and inflammation[4,5] have been implicated in CaP. A link between Toll-like receptor (TLR) polymorphism and CaP has also been suggested.[6,7]
TLR-4 is required for innate immunity to gram-negative bacteria. Lipopolysaccharide found on the cell wall of gram-negative bacteria acts as a ligand for TLR-4. Arbour et al[8] screened the entire coding region of TLR-4 using single-strand conformation polymorphism and direct sequencing. This led to the identification of single-nucleotide polymorphism (SNP), an A→G substitution at nucleotide position +896 resulting in an amino acid substitution Asp299Gly in the third exon of the TLR-4 gene. Asp299Gly was later shown to cosegregate with SNP Thr399Ile, a C→T substitution at nucleotide position 1196 also in the third exon of TLR-4. Both the SNPs are reported to have a positive correlation with the susceptibility to gram-negative sepsis, atherosclerosis, asthma, malaria, and Helicobacter pylori-induced gastric cancer and its precursors.[9–12] Recently, it was found that TLR-4 Thr399Ile substitution may be a risk factor for gastritis and precancerous lesions in North Indian population.[13] TLR-4 is associated with generation of inflammation and such responses have been found to be associated with CaP.[4,5] Moreover, sequence variants in TLR gene cluster (TLR6–TLR1–TLR10) and in TLR-4 are related to increased risk of CaP,[6,7] indicating a role of TLRs in its etiology. Thus, in this study an attempt was made to study the role of Asp299Gly and Thr399Ile SNPs as a risk factor for CaP in North Indian population.
MATERIALS AND METHODS
A total 198 histopathologically[14] confirmed cases of CaP and 200 patients with symptomatic benign prostatic hyperplasia (BPH) having normal digital rectal examination and serum prostate specific antigen (PSA) <4 ng/ml were included in the study. In all, 119 healthy males above 50 years of age with no symptoms of BPH, having normal size of prostate, and serum PSA level <4 ng/ml served as controls. Patients from the state of Punjab, Haryana, Himachal Pradesh and Western Uttar Pradesh attending Urology Clinic of the Institute were included into the study. Thus, the analysis was done on the available samples comprising various regions of North India.
Analysis of TLR-4 polymorphism
Preparation of genomic DNA
Genomic DNA was isolated from blood by the method described by Lahiri et al.[15] Briefly, 1 ml of salt buffer TKM (Tris EDTA, KCl, MgCl2) was added to 1 ml blood sample and then 100 μl of Triton X-100 was added to lyse the cells. After centrifugation (6000 rpm for 15 min), the pellet obtained was washed twice with 1 ml TKM buffer followed by the addition of 10% SDS, 200 μg/ml proteinase K, and incubated for 2 hours at 65°C and then it was treated with 150 μg/ml RNase at 37°C for 1 hour. DNA extraction was done with phenol-chloroform-isoamyl alcohol. DNA was precipitated with ethanol, and the pellet collected by centrifugation was washed twice with 70% ethanol. The pellet was air dried and dissolved in 100 μl of Tris EDTA (TE) buffer and stored at 4°C after DNA quantification for further use. DNA sample was diluted and quantified spectrophotometrically (UVICON spectrophotometer, Pharmacia) and absorbance was read at 260 and 280 nm. The concentration of DNA in solution was quantified using the equation:
DNA concentration (μg/ml) = A260 × 50 × dilution factor/1000.
One OD at A260 corresponds to 50 μg of DNA/ml. The purity of DNA was checked by calculating the ratio of OD at A260 to A280 and the integrity was assessed by gel electrophoresis on 0.8% agarose gel.
Polymerase chain reaction
Polymerase chain reaction (PCR) amplification of TLR-4 gene fragments was performed in Perkin Elmer thermocycler (USA) using gene-specific primers [Table 1]. Briefly, ~200 ng of DNA isolated from blood sample was used in the reaction as template; 25 μl of PCR mix contained 2.5 μl of 10× Taq polymerase buffer, 20 pM of each primer (forward and reverse), 200 μM of each dNTPs, and 3.0 U of Taq DNA polymerase (Sigma, USA). The amplification was done for 35 cycles: 30 s denaturation at 94°C, 30s annealing at 55°C, 30 s extension at 72°C, with 10 min final extension at 72°C. The amplified product was visualized under UV transilluminator on 2% agarose gel containing ethidium bromide (0.5 μg/ml). DNA markers were used for the detection of size of the amplified products.
Table 1.
Primer sequence and PCR condition for TLR-4 amplification

Restriction fragment length polymorphism
TLR-4 gene polymorphism was analyzed by restriction fragment length polymorphism (RFLP) technique.[16] In this technique, alteration of the forward primers was done to create Nco1and Hinf1 restriction sites in the mutant alleles and it allows distinction between wild-type and mutant TLR-4 alleles based on the presence of these restriction enzyme recognition sites. A portion of the resulting PCR product was digested for 5 hours with appropriate restriction enzymes (Nco1 for Asp299Gly and Hinf1 for Thr399Ile) and visualized on 2% agarose gel stained with ethidium bromide.
Statistical analysis
Genotype distribution and allele frequencies in different groups were compared by chi-square analysis. Pearson's correlation and linear regression analysis were carried out to determine the correlation between different parameters. A P value less than 0.05 was significant. All statistical calculations were performed using SPSS for windows, version 13 (SPSS Inc., Chicago, IL, USA).
RESULTS
PCR products for SNPs Asp299Gly and Thr399Ile showed fragments of 248 bp [Figure 1a] and 396 bp [Figure 1b], respectively. After restriction digestion of PCR products by Nco1 and Hinf1 restriction enzymes for RFLP, the products are shown in Figure 1c and d respectively. The presence of digested fragments of 219 and 367 bp] depicts the presence of SNPs. Single band indicates the presence of homozygous alleles (AA, GG, CC, and TT), and the twin band indicates the heterozygous alleles (AG and CT) with respect to the two SNPs.
Figure 1.
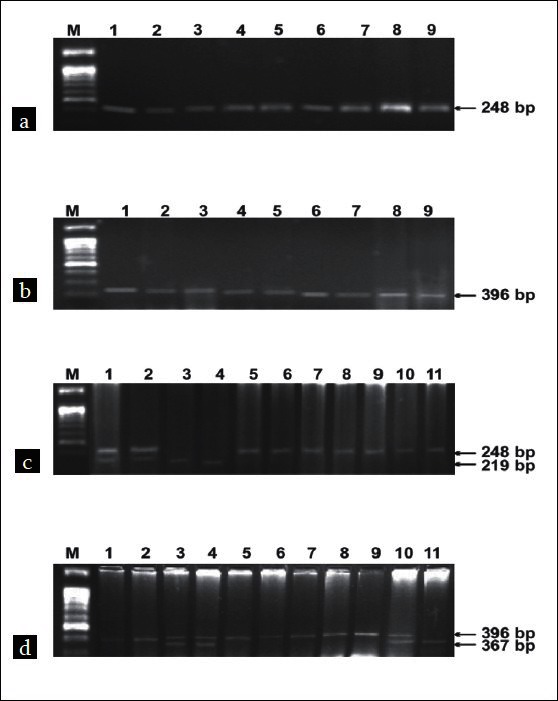
Agarose gel (2%) showing PCR amplification of TLR-4 (a, b) and restriction digestion of PCR products (c, d). Lanes M:Φ×BsuR1(HaeIII)[NEB]; 5-11 (c) and 1,2,6-9 (d): wild-type allele; 1,2 (c) and 3,4,10 (d) heterozygous alleles; 3,4 (c) and 11 (d): homozygous variant allele
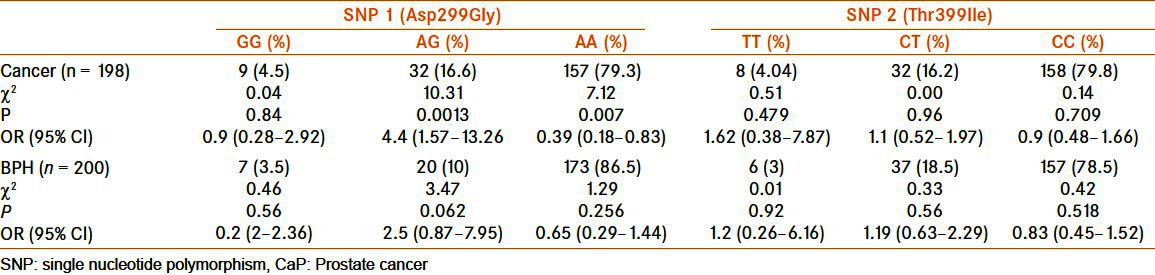
On comparing the frequency of genotypes with respect to Asp299Gly, it was found that AG had significantly high association [OR = 4.4, 95% CI (1.57–13.26)] and AA had significantly low association [OR = 0.39 95% CI (0.18–0.83)] with CaP [Table 2]. However, Thr399Ile SNP was not found to be associated with CaP risk [Table 2].
Table 2.
TLR-4 SNP in CaP and benign prostate hyperplasia as when compared with the control
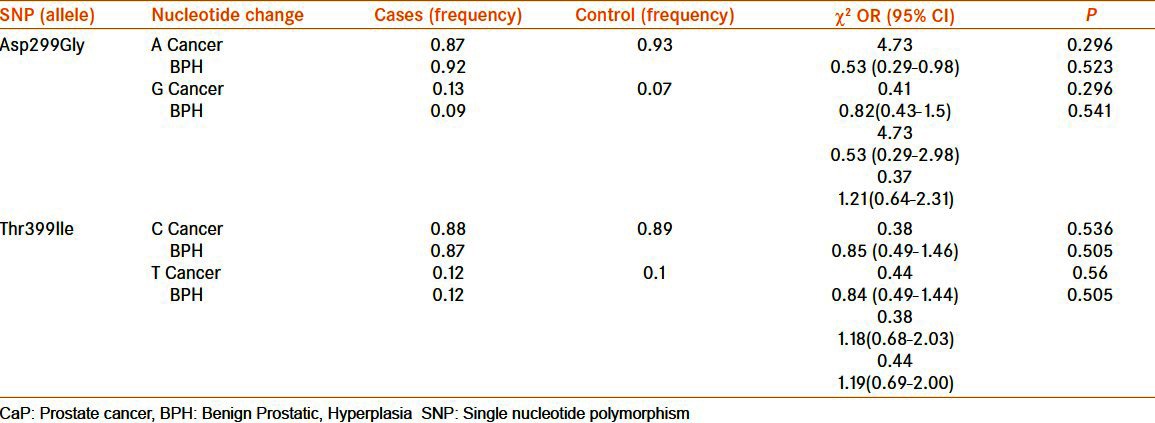
The allelic distribution pattern was calculated for various alleles, and in case of Asp299Gly polymorphism frequency of G allele was found to be more in CaP patients when compared with BPH and control, but the differences were not statistically significant [Table 3]. “A” allele was present at an almost equal level in all the three groups [Table 3]. Neither of the allele (C and T) frequency with respect to Thr399Ile was found to be significantly associated with either CaP or BPH [Table 3].
Table 3.
Allele frequency of the SNP in the TLR-4 gene in CaP, BPH, and control (frequency in patients with CaP and BPH was compared with respect to the control)
DISCUSSION
The incidence of CaP has been increasing worldwide due to increase in lifespan which is among the major risk factors. Familial clustering and varying prevalence of CaP according to ethnicity are the testimony to genetic basis of its causation.
In this study, an attempt was made to understand the genetic predisposition to CaP, with genetic variability in TLR-4 gene. TLR-4 is phylogenetically conserved receptor involved in the recognition of pathogen-associated molecular patterns and endogenous ligands and plays an important role in regulating inflammatory response and signaling the activation of adaptive immunity.[17] TLR-4 is expressed in the normal prostate gland. TLR-4 in prostate cells could act in synergy with innate immune cells and may contribute to an eventual inflammatory process, which in genetically prone individuals could promote carcinogenesis. Therefore, genetic variation of TLR-4 gene may play a role in determining susceptibility to human diseases which may have an inflammatory component, such as CaP and BPH.
TLR-4 SNPs exhibit ethnic variation, and there is rarity of Asp299Gly and Thr399Ile polymorphism in Asian ethnic group.[18–20] These SNPs are associated with innate immunity related diseases, such as chronic inflammatory diseases, atherosclerosis, and cancer.[10,11–13]
We examined the genetic diversity of TLR-4 by SNP analysis at two different positions Asp299Gly and Thr399Ile. It was tested whether genetic difference at this locus was present in CaP patients, BPH patients, and controls in Indian population. We observed that Asp299Gly SNP is associated with CaP risk. Zheng et al[21] in a Swedish population explored the relationship between TLR-4 sequence variations and risk of CaP. Among eight SNP studied, a sequence variant (11381G/C) in the 3′ untranslated region of TLR-4 was found to be associated with CaP risk. A few other association studies have reported the relationship between the risk of CaP and the polymorphism in the TLR-4 gene in European-ancestry populations.[6,7] In our study, G allele which was a result of nucleotide substitution was found to be present in high frequency in CaP patients when compared with BPH and control population. However, no such association was detected with Thr399Ile SNP. It was confirmed that failure to detect the association of Thr399Ile SNP with CaP was not due to insufficient sample size. Using EpiInfo power calculation software, power of our study is 80%. No significant association of CaP risk with the TLR-4 polymorphism was found when the comparison was made between two groups: patients with CaP and those with BPH along with the controls. This finding suggests that presence of these SNPs is not very different in the two major prostatic ailments.
It is interesting to note that the said SNPs are present in Indian population in contrast to other ethnic Asian populations. The strength of our study includes use of two independent case–control studies comprising two major ailments associated with prostate that is BPH which is nonmalignant and CaP which is malignant. A trend toward development of BPH was observed in population with Asp299Gly SNP, although the association was not found to be significant. In recent years, inflammation has gained much attention in the etiopathogenesis of cancer.[4,5,22] This functional polymorphism has been found to be associated with gastric cancer,[12,13] in which inflammation does play a role as an etiological factor. So, again this SNP might be a predisposing factor for CaP where inflammatory response has been observed.[4,5] As the presence of this SNP and its association with CaP was observed in our study population, future multidisciplinary research efforts will be needed to fully comprehend the risks and to assess its biologic mechanism that may shed some light on the etiology of this prevalent and age-related disease.
ACKNOWLEDGMENT
Informed consent was obtained from the patients. Human experimentation guidelines of the institutional review board were followed in the conduct of this research. Junior and senior research fellowship from Council for Scientific and Industrial Research (CSIR), New Delhi, is deeply acknowledged.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–6. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Sobti RC, Gupta L, Thakur H, Seth H, Seth A, Singh SK, et al. CyP17 gene polymorphism and its association in North Indian Prostate Cancer patients. Anticancer Res. 2009;29:1659–63. [PubMed] [Google Scholar]
- 3.Gupta L, Thakur H, Sobti RC, Seth A, Singh SK. Role of genetic polymorphism of estrogen receptor- alpha gene and risk of Prostate Cancer in North Indian population. Mol Cell Biochem. 2010;335:255–61. doi: 10.1007/s11010-009-0275-2. [DOI] [PubMed] [Google Scholar]
- 4.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: Implications for prostatic carcinogenesis. Am J Pathol. 1999;155:1985–92. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Marzo AM, Deweese TL, Platz EA, Meekar AK, Nakayama M, Epstein JI, et al. Pathological and molecular mechanisms of prostate carcinogenesis: Implications for diagnosis, detection, prevention, and treatment. J Cell Biochem. 2004;91:459–77. doi: 10.1002/jcb.10747. [DOI] [PubMed] [Google Scholar]
- 6.Chen YC, Giovannucci E, Lazarus R, Kraft P, Ketkar S, Hunter DJ. Sequence variants of Toll-like receptor 4 and susceptibility to prostate cancer. Cancer Res. 2005;65:11771–8. doi: 10.1158/0008-5472.CAN-05-2078. [DOI] [PubMed] [Google Scholar]
- 7.Sun J, Wiklund F, Zheng SL, Chang B, Balter K, Li L, et al. Sequence variants in Toll-like receptor gene cluster (TLR6-TLR1-TLR10) and prostate cancer risk. J Natl Cancer Inst. 2005;97:525–32. doi: 10.1093/jnci/dji070. [DOI] [PubMed] [Google Scholar]
- 8.Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–91. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 9.Lorenz E, Mira JP, Cornish KL, Arbour NC, Schwartz DA. A novel polymorphism in the toll like receptor 2 gene and its potential association with staphylococcal infection. Infect Immun. 2000;68:6398–401. doi: 10.1128/iai.68.11.6398-6401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiechl S, Lorenz E, Reindl M, Wiederman CJ, Oberhollenzer F, Bonora E, et al. Toll like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–92. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 11.Mockenhaupt FP, Cramer JP, Hamann L, Stegemann MS, Eckert J, Oh N, et al. Toll-like receptor (TLR) polymorphisms in African children: Common TLR-4 variants predispose to severe malaria. Proc Natl Acad Sci USA. 2006;103:177–82. doi: 10.1073/pnas.0506803102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santini D, Angeletti S, Ruzzo A, Dicuonzo G, Galluzos S, Vincenzi B, et al. Toll-like receptor- 4 Asp299Gly and Thr399Ile polymorphism in gastric cancer of intestinal and diffuse histotype. Clin Exp Immunol. 2008;154:360–4. doi: 10.1111/j.1365-2249.2008.03776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Achut BR, Ghishal UC, Moorchung N, Mittal B. Association of Toll like receptor-4 (Asp299Gly and Thr399Ile) gene polymorphism with gastritis and precancerous lesions. Human Immunol. 2007;68:901–7. doi: 10.1016/j.humimm.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974;111:58–64. doi: 10.1016/s0022-5347(17)59889-4. [DOI] [PubMed] [Google Scholar]
- 15.Lahiri DK, Bye S, Nurnberger JI, Jr, Hodes ME, Crisp M. A non organic and non enzymatic extraction method gives higher yield of genomic DNA from whole blood sample than do other methods tested. J Biochem Biophys Methods. 1992;25:193–205. doi: 10.1016/0165-022x(92)90014-2. [DOI] [PubMed] [Google Scholar]
- 16.Lorenz E, Frees KL, Schwartz DA. Determination of the TLR4 genotype using allele-specific PCR. Biotechniques. 2001;31:22–4. doi: 10.2144/01311bm01. [DOI] [PubMed] [Google Scholar]
- 17.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 18.Lin YC, Chang YM, Yu JM, Yen JH, Chang JC, Hu CJ. Toll-like receptor 4 gene C119A but no Asp299Gly polymorphism is associated with ischemic stroke among ethnic Chinese in Taiwan. Atherosclerosis. 2005;180:305–9. doi: 10.1016/j.atherosclerosis.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Nakada TA, Hirasawa H, Oda S, Shiga H, Matsuda K, Nakamura M, et al. Influence of toll-like receptor 4, CD14, tumor necrosis factor, and interleukine-10 gene polymorphisms on clinical outcome in Japanese critically ill patients. J Surg Res. 2005;129:322–8. doi: 10.1016/j.jss.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Ferwerda B, McCall BB, Verheijen K, Kullberg BJ, van der Ven AJ, Van der Meer JW, et al. Functional consequecces of Toll like receptor-4 polymorphisms. Mol Med. 2008;14:346–52. doi: 10.2119/2007-00135.Ferwerda. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng SL, Liu W, Dimitrov L, Sun J, Chang B, Loza B, et al. A comprehensive association study for genes in inflammation pathway provides support for their roles in prostate cancer risk in the CAPS study. Prostate. 2006;66:1556–64. doi: 10.1002/pros.20496. [DOI] [PubMed] [Google Scholar]
- 22.Macarthur M, Hold GL, El-Omar E. Inflammation and cancer. Role of Chronic inflammation and cytokine gene polymorphism in the pathogenesis of gastrointestinal malignancy. Am J Physiol Gastrointest Liver Physiol. 2004;286:G515–20. doi: 10.1152/ajpgi.00475.2003. [DOI] [PubMed] [Google Scholar]


