Abstract
The authors investigated associations of serum phospholipid n-3 and n-6 polyunsaturated fatty acids (PUFAs) and trans-fatty acids with prostate cancer risk, and whether myeloperoxidase G-463A (rs2333227) modified the associations in the Carotene and Retinol Efficacy Trial (CARET) (Seattle, Washington; Irvine, California; New Haven, Connecticut; San Francisco, California; Baltimore, Maryland; and Portland, Oregon, 1985–2003). Prerandomization sera were assayed for fatty acids among 641 men with incident prostate cancer (368 nonaggressive and 273 aggressive (stage III/IV or Gleason score ≥7)) and 1,398 controls. Overall, dihomo-γ-linolenic (quartiles 4 vs. 1: odds ratio (OR) = 0.66, 95% confidence interval (CI): 0.49, 0.95; Ptrend = 0.024) and docosatetraenoic (OR = 0.69, 95% CI: 0.46, 1.02; Ptrend = 0.011) acids were inversely associated with nonaggressive and aggressive prostate cancer risks, respectively. Among men with MPO GG, the genotype upregulating oxidative stress, quartiles 4 versus 1 eicosapentaenoic plus docosahexaenoic acids were suggestively associated with an increased risk of aggressive prostate cancer (OR = 1.66, 95% CI: 0.95, 2.92; Ptrend = 0.07). However, the association was the inverse among men with MPO GA/AA genotypes (Pinteraction = 0.011). Interactions were also observed for docosapentaenoic acid, total n-3 PUFAs, and arachidonic acid. MPO GA/AA vs. GG was associated with a 2-fold increase in aggressive prostate cancer risk among men with low (quartile 1) n-3 PUFAs. This study adds important evidence linking oxidative stress with prostate carcinogenesis.
Keywords: gene-environment interaction, myeloperoxidase, polyunsaturated fatty acids, prostate cancer, trans-fatty acids
Biological evidence supports a role for phospholipid fatty acid in prostate carcinogenesis (1–3). Among major types of fatty acids, polyunsaturated fatty acids (PUFAs), including the n-6 and n-3 PUFAs, are essential to cell membranes and inflammation signaling (4). n-6 PUFAs promote androgen-stimulated prostate cell growth, but long-chain and very-long-chain n-3 PUFAs, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), inhibit this pathway (2). n-3 PUFAs also have antiinflammatory, antiproliferative, and proapoptotic effects on prostate cancer cells (2). However, the multiple double bonds present in both n-6 and n-3 PUFAs attract reactive oxygen species or free radicals. This process is known as lipid peroxidation; that is, free radicals take electrons from the lipids in cell membranes, leading to membrane and DNA damage, which favors cancer development, including prostate cancer (5, 6).
An important determinant of lipid peroxidation is oxidative-stress regulatory enzymes, which metabolize free radicals and thus protect PUFAs from peroxidation (7, 8). Among the family of oxidative-stress regulatory enzymes, myeloperoxidase converts hydrogen peroxide (H2O2), the metabolite generated from superoxide dismutases, and chloride anion (Cl–) into hypochlorous acid (HOCl), a secondary, endogenous free radical peroxidizing PUFAs (9). Previous reports from the Carotene and Retinol Efficacy Trial (CARET) have linked MPO G-463A (rs2333227), a functional single nucleotide polymorphism of myeloperoxidase, to prostate cancer risk. The MPO A allele, conferring several folds less transcriptional activity than the G allele (10), is associated with a 60% reduced risk of aggressive prostate cancer (11). Also, MPO G-463A modifies the association of serum α-tocopherol, the body's primary fat-soluble antioxidant, with aggressive prostate cancer among CARET current smokers (12). Both n-3 and n-6 PUFAs are potentially prooxidative because of their double bonds, but their interaction with oxidative-stress regulatory enzymes has not been investigated in epidemiologic studies. The primary objective of this study was to examine the associations of serum phospholipid n-3 and n-6 PUFAs as well as trans-fatty acids and prostate cancer risk in CARET. We included trans-fatty acids because an increased prostate cancer risk was observed with higher levels of C18 trans-fatty acids in CARET (13), and the biological mechanisms may involve oxidative stress (14). We further investigated whether the MPO G-463A polymorphism modified the associations. We hypothesized that high percentages of PUFAs in the presence of the genotype producing high oxidative stress (GG) were associated with an increased risk of prostate cancer but not in the presence of the genotype producing low oxidative stress (GA/AA).
MATERIALS AND METHODS
CARET overview
CARET was a multicenter (Seattle, Washington; Irvine, California; New Haven, Connecticut; San Francisco, California; Baltimore, Maryland; and Portland, Oregon) randomized, double-blind placebo-controlled chemoprevention trial to test whether daily supplementation with 30 mg of β-carotene and 25,000 IU of retinyl palmitate would reduce the risk of lung cancer among 18,314 heavy smokers and asbestos-exposed workers. Details about the design and primary results of CARET have been published elsewhere (15). Briefly, eligible participants were men and women aged 50–69 years who were current or former smokers (within the previous 6 years) with a history of at least 20 pack-years of cigarette smoking (n = 14,254, 55.9% of whom were male) and men aged 45–69 years who were current or former smokers and exposed to asbestos in the workplace within the previous 15 years (n = 4,060). Recruitment began in 1985 and intervention was stopped in 1996; 94% of participants remained in active follow-up until 2005. The institutional review boards of the Fred Hutchinson Cancer Research Center and each of the 5 other participating institutions approved all procedures for the study, and participants provided written informed consent at recruitment and throughout the trial. For the analyses in this study, additional institutional review was obtained from the Roswell Park Cancer Institute.
Selection of cases and controls and endpoint ascertainment
We used a nested case-control study. At each CARET annual visit, as well as quarterly follow-up telephone calls, participants were asked to report if they had been diagnosed with any new cancers. All endpoints, including prostate cancer, were verified by the CARET Endpoints Review Committee. For the current study, participants with a previous report of prostate cancer were approached to request permission to obtain data on Gleason score and stage of disease at diagnosis through review of their medical records. For participants whose medical records were unavailable, the stage and Gleason score were obtained from pathology reports in the Western Washington Cancer Surveillance System, a part of the Surveillance, Epidemiology, and End Results registries. All medical and pathologic records were confirmed by one of the coauthors (G.E.G.). Case selection for this study was based on follow-up through 2003, by which time a total of 778 incident prostate cancer cases had been confirmed. After exclusion of 50 men with prior cancer history reported at the baseline visit and 19 without specimens available for laboratory analyses, 709 cases were eligible for this study (16). Eligible controls were men who were free of both prostate cancer and lung cancer at the time of selection (follow-up through 2003) and had available whole blood or extracted DNA. Biospecimens of lung cancer cases (the primary endpoint in CARET) were not provided for studies not investigating lung cancer. Cases and controls were frequency matched on age (5-year groups) and race/ethnicity, and controls were required to have follow-up time at least that of their matched case. The case:control ratios were 1:4 for blacks, wherever achievable, and 1:2 for other races. As a result, a total of 724 cases and 1,474 controls were selected (after reassigning 15 participants who were originally selected as controls and diagnosed subsequently with prostate cancer). Forty-five cases and 44 controls did not have data on serum phospholipid fatty acids because of insufficient specimens. In addition, 14 cases and 32 controls did not have complete baseline data on covariates. Staging information and Gleason scores were available for 87% and 93% of the cases, respectively (16). Consequently, 641 cases with known staging or Gleason score and 1,398 controls entered statistical analyses for the main associations of PUFAs and trans-fatty acids with prostate cancer. After exclusion of those without complete genotyping data, the analysis of interaction between genetic variation in MPO and those fatty acids was conducted in 458 cases and 1,369 controls. The missing genotyping data in the cases were mainly because whole blood collection was not initiated until 1994. For the whole blood collection, the overall rates of consent and completion were 70%.
Serum phospholipid fatty acid assay and MPO genotyping
Participants provided nonfasting blood specimens at their first CARET study center visit (prerandomization). Sera were stored in the CARET Coordinating Center specimen bank at −70°C until analysis. Total lipids were extracted by the method of Folch et al. (17), and phospholipids were separated from neutral lipids by one-dimensional thin-layer chromatography using 250-µm silica gel G plates and a 67.5:15:0.75 hexane:ether:acetic acid (0.005% butylated hydroxytoluene) development solvent. Samples of fatty acid methyl esters were prepared by direct transesterification using the method of Lepage and Roy (18). A gas chromatograph (model 5890B, series II; Hewlett-Packard, Avondale, Pennsylvania) equipped with a flame ionization detector, an automatic sampler (model 7673; Hewlett-Packard), and electronic pressure programming was used on samples dissolved in hexane. Fatty acid methyl esters were separated on a SP-2560 wall-coated open-tubular fused silica capillary column, 100 m × 0.25 mm inner-diameter, 0.20-µm film thickness (Supelco, Bellefonte, Pennsylvania). The carrier gas was helium. This method yielded 41 individual phospholipid fatty acids in total. Quantitative precision and identification were evaluated by using model mixtures of known fatty acid methyl esters and an established control pool. Interassay coefficients of variation were on the average 3.5% or lower for most of the fatty acids that were present at levels of 1% or higher. Individual fatty acids were expressed as a weight percentage of the total fatty acids.
The polymorphism in MPO G-463A (rs2333227) was selected for genotyping because this variant substantially influences the capacity for responding to oxidative stress (10). Genomic DNA was extracted with the use of QIAamp DNA blood Midi kits (Qiagen, Valencia, California). Genotyping was performed with high-throughput matrix-assisted laser desorption/ionizing time-of-flight mass spectrometry (Sequenom, San Diego, California) by BioServe Biotechnologies (Laurel, Maryland). Procedures and primers for polymerase chain reaction were previously reported (19). The genotype concordance was excellent among the 8% of randomly selected duplicates (k statistic: 0.95) with a <1% assay failure rate. The polymorphism was in Hardy-Weinberg equilibrium among the controls; thus, selection bias or genotyping error was unlikely.
Other data collection
Detailed information on demographic characteristics, smoking status, and personal and family health history was collected by a self-administered questionnaire at baseline. Current smokers were defined as those who smoked any cigarettes in the past month. The age of starting smoking and quitting smoking (for former smokers) and the average number of cigarettes smoked per day for the entire time of smoking were also assessed to calculate smoking pack-years. Height and weight were measured by trained staff using a standardized protocol at the baseline visit, and body mass index (weight (kg)/height (m)2) was calculated. Alcohol consumption was estimated from the CARET food frequency questionnaire, which was administered at baseline and then every 2 years during the trial. Alcohol intake values were averaged across all food frequency questionnaires completed prior to the prostate cancer diagnosis to best represent the long-term pattern of alcohol intake during follow-up.
Statistical analyses
Our analytical goals were to assess the association of individual fatty acids (main effect) and the joint association of individual fatty acids and the MPO G-463A genetic variation (effect modification) with prostate cancer risk. The percentages of individual n-3 and n-6 PUFAs as well as total trans C16 monounsaturated, C18 monounsaturated, and C18:2 diunsaturated fatty acids were categorized into quartiles on the basis of their distributions in the controls. Unconditional logistic regression was used to estimate odds ratios and 95% confidence intervals. Tests for linear trend across the quartiles were based on an ordinal variable corresponding to rank from the first to fourth quartiles. A covariate was included in multivariate models if a priori knowledge suggested that the variable was a confounder. The multivariate models included age at enrollment (continuous), race (white, black, or others), CARET randomization assignment (retinol plus β-carotene or placebo), family history of prostate cancer in first-degree relatives (yes or no), alcohol consumption (nondrinker, below median, or at or above median based on total alcohol amount in controls reporting use of alcohol where the median intake was 10 g/day), smoking status (current or former/never), smoking pack-years (<40, 40–59, or ≥60), and body mass index (continuous). Analyses were conducted for nonaggressive and aggressive prostate cancer separately, where aggressive prostate cancer was defined as clinical stage III or IV (extraprostatic extension or metastasis) tumors or with Gleason score ≥7 (11, 20). A secondary analysis was conducted to assess risks of 1) advanced stage prostate cancer, defined as stage III or IV (either clinical or pathological); 2) high-grade prostate cancer, defined as Gleason score ≥8; and 3) lethal prostate cancer, defined as metastatic tumor (clinical or pathological stage IV at diagnosis) or prostate cancer–specific death during follow-up through 2005 (21). To explore effect modification, the reference group for a given model was men in the lowest quartile of serum fatty acid percentages and with the MPO GG genotype. Participants with heterozygote alleles and homozygote A alleles were combined into 1 group since the 2 genotypes have the same transcriptional activity (10, 22). A cross-product term of the ordinal variable of fatty acid quartiles and the MPO genotypes was created; the interaction was based on likelihood ratio tests (1 df). All tests were 2-sided, and P < 0.05 was considered statistically significant. Statistical analyses were performed by using Stata, version 12, software (StataCorp LP, College Station, Texas).
RESULTS
Table 1 gives the characteristics of prostate cancer cases and controls in CARET. Compared with controls, higher proportions of cases had a family history of prostate cancer (P < 0.001) and high alcohol consumption (P = 0.010). Table 2 presents the 25th, 50th (or median), and 75th percentiles of serum n-3 and n-6 PUFAs and trans-fatty acids as the percentage of total phospholipid fatty acids in nonaggressive and aggressive prostate cancer cases and controls. Among controls, approximately 4% and 35% of total fatty acids were composed of n-3 and n-6 PUFAs, respectively. The largest components were linoleic acid (20.57%) followed by arachidonic acid (10.64%) among the n-6 PUFAs and DHA (2.56%) among the n-3 PUFAs.
Table 1.
Characteristics of Prostate Cancer Cases and Controls in the Carotene and Retinol Efficacy Trial, 1985–2003
| Characteristics | Cases |
Controls |
P Valuea | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | No.b | % | Mean (SD) | No.b | % | ||
| Total | 641 | 1,398 | |||||
| Age, years | |||||||
| Baseline | 60.4 (5.7) | 60.3 (5.8) | 0.56 | ||||
| Diagnosis | 66.9 (5.9) | N/A | |||||
| Race/ethnicity | |||||||
| White | 578 | 90.2 | 1,229 | 87.9 | 0.13 | ||
| African American | 39 | 6.1 | 121 | 8.7 | |||
| Other | 24 | 3.7 | 48 | 3.4 | |||
| Randomization | |||||||
| Intervention | 334 | 52.1 | 724 | 51.8 | 0.89 | ||
| Placebo | 307 | 47.9 | 674 | 48.2 | |||
| Family history of prostate cancer, yes | 42 | 6.6 | 46 | 3.3 | <0.001 | ||
| Smoking status | |||||||
| Current | 332 | 51.8 | 741 | 53.0 | 0.61 | ||
| Neverc/former | 309 | 48.2 | 657 | 47.0 | |||
| Smoking, pack-years | |||||||
| <40 | 238 | 37.1 | 530 | 37.9 | 0.85 | ||
| 40–59 | 216 | 33.7 | 477 | 34.1 | |||
| ≥60 | 187 | 29.2 | 391 | 28.0 | |||
| Alcohol intake | |||||||
| Nondrinkers | 145 | 22.6 | 341 | 24.4 | 0.010 | ||
| 1–9 g/day | 181 | 28.2 | 475 | 34.0 | |||
| ≥10 g/day | 262 | 40.9 | 472 | 33.8 | |||
| Unknown | 53 | 8.3 | 110 | 7.9 | |||
| Body mass indexd | |||||||
| <25.0 | 147 | 22.9 | 305 | 21.8 | 0.84 | ||
| 25.0–29.9 | 309 | 48.2 | 689 | 49.3 | |||
| ≥30.0 | 185 | 28.9 | 404 | 28.9 | |||
| Gleason score | |||||||
| <7 | 361 | 56.3 | N/A | ||||
| ≥7 | 258 | 40.3 | |||||
| Unknown | 22 | 3.4 | |||||
| Clinical stage | |||||||
| 0, I | 168 | 33.5 | N/A | ||||
| II | 280 | 55.8 | |||||
| III | 26 | 5.2 | |||||
| IV | 25 | 5.0 | |||||
| Unknown | 3 | 0.6 | |||||
| Year of diagnosise | N/A | ||||||
| 1986–1993 | 145 | 22.6 | |||||
| 1994–2003 | 496 | 77.4 | |||||
Abbreviations: CARET, Carotene and Retinol Efficacy Trial; N/A, nonapplicable; PSA, prostate-specific antigen; SD, standard deviation.
a The t-test for age at baseline and χ2 tests for the categorical variables were used.
b The numbers are numbers of participants and column percentages unless otherwise noted.
c Never smokers contributed a very small percentage (<2%). They were recruited in the CARET because of their occupational asbestos exposure.
d Body mass index: weight (kg)/height (m)2.
e 1994 approximates the advent of the PSA era.
Table 2.
Distributions of Serum Fatty Acid Composition as Total Phospholipids (%)a Shown as the 25th, 50th (Median), and 75th Percentiles in the Carotene and Retinol Efficacy Trial, 1985–2003
| Fatty Acids | Nonaggressive Prostate Cancer Cases, %b |
Aggressive Prostate Cancer Cases, %c |
Controls, %d |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 25th Percentile | Median | 75th Percentile | 25th Percentile | Median | 75th Percentile | 25th Percentile | Median | 75th Percentile | |
| n-3 PUFAs | |||||||||
| 18:3n-3 (α-linolenic) | 0.09 | 0.10 | 0.11 | 0.09 | 0.10 | 0.11 | 0.09 | 0.10 | 0.12 |
| 20:3n-3 (eicosatrienoic) | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 |
| 20:5n-3 (eicosapentaenoic) | 0.45 | 0.58 | 0.75 | 0.44 | 0.59 | 0.76 | 0.42 | 0.56 | 0.75 |
| 22:5n-3 (docosapentaenoic) | 0.71 | 0.80 | 0.90 | 0.71 | 0.81 | 0.91 | 0.71 | 0.81 | 0.91 |
| 22:6n-3 (docosahexaenoic) | 2.11 | 2.59 | 3.14 | 2.12 | 2.52 | 3.14 | 2.09 | 2.56 | 3.16 |
| Total n-3 | 3.52 | 4.08 | 4.72 | 3.53 | 4.08 | 4.81 | 3.53 | 4.05 | 4.76 |
| n-6 PUFAs | |||||||||
| 18:2n-6 (linoleic) | 18.76 | 20.55 | 22.29 | 18.95 | 20.75 | 22.44 | 18.83 | 20.57 | 22.32 |
| 18:3n-6 (γ-linolenic) | 0.05 | 0.07 | 0.09 | 0.05 | 0.07 | 0.09 | 0.05 | 0.07 | 0.09 |
| 20:2n-6 (eicosadienoic) | 0.30 | 0.33 | 0.37 | 0.31 | 0.34 | 0.38 | 0.31 | 0.34 | 0.38 |
| 20:3n-6 (dihomo-γ-linolenic) | 2.51 | 2.84 | 3.22 | 2.52 | 2.95 | 3.45 | 2.54 | 2.94 | 3.40 |
| 20:4n-6 (arachidonic) | 9.44 | 10.77 | 12.17 | 9.31 | 10.47 | 11.52 | 9.52 | 10.64 | 11.82 |
| 22:2n-6 (docosadienoic) | 0.02 | 0.03 | 0.04 | 0.03 | 0.03 | 0.04 | 0.02 | 0.03 | 0.04 |
| 22:4n-6 (docosatetraenoic) | 0.39 | 0.45 | 0.51 | 0.39 | 0.44 | 0.50 | 0.39 | 0.46 | 0.52 |
| Total n-6 | 34.11 | 35.10 | 36.58 | 33.82 | 35.33 | 36.39 | 34.07 | 35.26 | 36.41 |
| TFA 16:1 | 0.17 | 0.20 | 0.24 | 0.16 | 0.20 | 0.24 | 0.17 | 0.20 | 0.24 |
| TFA 18:1 | 1.20 | 1.63 | 2.07 | 1.18 | 1.59 | 2.12 | 1.20 | 1.59 | 2.11 |
| TFA 18:2 | 0.18 | 0.23 | 0.28 | 0.18 | 0.23 | 0.28 | 0.18 | 0.22 | 0.28 |
Abbreviations: PUFA, polyunsaturated fatty acid; TFA, trans-fatty acid.
a The summation of fatty acid shown in this table is not 100% because other groups (saturated and monounsaturated) of fatty acids are not listed.
b There were 368 cases with nonaggressive prostate cancer defined as stage 0–II tumors and Gleason score <7.
c There were 273 cases with aggressive prostate cancer defined as stage III/IV tumors or Gleason score ≥7.
d There were 1,398 controls.
In the main effect analysis, no significant association was observed for n-3 PUFAs (Tables 3 and 4) or for trans-fatty acids (Web Table 1 available at http://aje.oxfordjournals.org/), but 2 n-6 PUFAs were inversely associated with prostate cancer risk. Men with dihomo-γ-linolenic acid percentages in the fourth quartile were at 34% lower risk for nonaggressive prostate cancer, compared with those with the percentages in the first quartile (odds ratio (OR) = 0.66, 95% confidence interval (CI): 0.47, 0.95; Ptrend = 0.024) (Table 3). Docosatetraenoic acid was inversely associated with aggressive prostate cancer risk (for quartiles 4 vs. 1: OR = 0.69, 95% CI: 0.46, 1.02; Ptrend = 0.011) (Table 4).
Table 3.
Multivariable-adjusteda Association of Serum n-3 and n-6 Polyunsaturated Fatty Acids With Nonaggressive Prostate Cancerb Risk in the Carotene and Retinol Efficacy Trial, 1985–2003
| Fatty Acids | Quartile 1 |
Quartile 2 |
Quartile 3 |
Quartile 4 |
Ptrend | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | ||
| n-3 PUFAs | |||||||||||||||||
| α-Linolenic acid | 84 | 350 | 1.00 | Referent | 114 | 349 | 1.36 | 0.99, 1.88 | 88 | 349 | 1.06 | 0.75, 1.48 | 82 | 350 | 0.99 | 0.70, 1.41 | 0.59 |
| Eicosatrienoic acid | 85 | 350 | 1.00 | Referent | 88 | 349 | 1.07 | 0.77, 1.50 | 98 | 350 | 1.18 | 0.84, 1.64 | 96 | 349 | 1.15 | 0.83, 1.61 | 0.34 |
| Eicosapentaenoic acid | 77 | 350 | 1.00 | Referent | 97 | 349 | 1.24 | 0.89, 1.74 | 104 | 350 | 1.28 | 0.92, 1.79 | 90 | 349 | 1.07 | 0.75, 1.52 | 0.70 |
| Docosapentaenoic acid | 96 | 349 | 1.00 | Referent | 95 | 350 | 0.97 | 0.70, 1.33 | 88 | 349 | 0.88 | 0.63, 1.22 | 89 | 350 | 0.89 | 0.64, 1.24 | 0.41 |
| Docosahexaenoic acid | 88 | 349 | 1.00 | Referent | 93 | 350 | 1.05 | 0.75, 1.46 | 99 | 349 | 1.13 | 0.81, 1.58 | 88 | 350 | 1.00 | 0.70, 1.41 | 0.90 |
| EPA + DHA | 78 | 350 | 1.00 | Referent | 98 | 349 | 1.26 | 0.90, 1.77 | 102 | 350 | 1.31 | 0.93, 1.83 | 90 | 349 | 1.14 | 0.80, 1.63 | 0.45 |
| Total n-3 | 93 | 350 | 1.00 | Referent | 84 | 349 | 0.90 | 0.64, 1.25 | 103 | 349 | 1.09 | 0.79, 1.51 | 88 | 350 | 0.92 | 0.66, 1.30 | 0.95 |
| n-6 PUFAs | |||||||||||||||||
| Linoleic acid | 99 | 349 | 1.00 | Referent | 88 | 350 | 0.89 | 0.64, 1.23 | 90 | 350 | 0.91 | 0.65, 1.26 | 91 | 349 | 0.93 | 0.67, 1.30 | 0.72 |
| γ-Linolenic acid | 92 | 350 | 1.00 | Referent | 90 | 349 | 0.93 | 0.67, 1.29 | 91 | 349 | 0.89 | 0.64, 1.25 | 95 | 349 | 0.96 | 0.69, 1.34 | 0.78 |
| Eicosadienoic acid | 110 | 350 | 1.00 | Referent | 87 | 349 | 0.79 | 0.57, 1.09 | 80 | 350 | 0.71 | 0.51, 0.99 | 91 | 349 | 0.83 | 0.60, 1.14 | 0.18 |
| Dihomo-γ-linolenic acid | 101 | 349 | 1.00 | Referent | 104 | 350 | 1.01 | 0.74, 1.38 | 94 | 349 | 0.92 | 0.66, 1.27 | 69 | 350 | 0.66 | 0.47, 0.95 | 0.024 |
| Arachidonic acid | 99 | 350 | 1.00 | Referent | 76 | 349 | 0.78 | 0.56, 1.09 | 85 | 349 | 0.89 | 0.64, 1.24 | 108 | 350 | 1.16 | 0.84, 1.60 | 0.32 |
| Docosadienoic acid | 87 | 350 | 1.00 | Referent | 114 | 349 | 1.33 | 0.97, 1.83 | 79 | 349 | 0.92 | 0.66, 1.30 | 88 | 350 | 1.04 | 0.74, 1.46 | 0.61 |
| Docosatetraenoic acid | 95 | 350 | 1.00 | Referent | 98 | 349 | 1.03 | 0.74, 1.42 | 92 | 349 | 0.97 | 0.70, 1.34 | 83 | 350 | 0.89 | 0.63, 1.24 | 0.44 |
| Total n-6 | 89 | 350 | 1.00 | Referent | 105 | 349 | 1.21 | 0.88, 1.68 | 72 | 349 | 0.84 | 0.59, 1.19 | 102 | 350 | 1.21 | 0.87, 1.68 | 0.66 |
Abbreviations: CARET, Carotene and Retinol Efficacy Trial; CI, confidence interval; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; OR, odds ratio; PUFA, polyunsaturated fatty acid.
a Multivariate adjustment for age at enrollment (continuous), race (white, black, others), CARET randomization assignment (retinol plus β-carotene, placebo), family history of prostate cancer in first-degree relatives (yes, no), alcohol consumption (nondrinker, below median, at or above median, unknown), smoking status (current, former/never), smoking pack-years (<40, 40, <60, ≥60), and body mass index (continuous).
b Defined as stage 0–II tumors and Gleason score <7.
Table 4.
Multivariable-adjusteda Association of Serum n-3 and n-6 Polyunsaturated Fatty Acids With Aggressive Prostate Cancerb Risk in the Carotene and Retinol Efficacy Trial, 1985–2003
| Fatty Acids | Quartile 1 |
Quartile 2 |
Quartile 3 |
Quartile 4 |
Ptrend | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | ||
| n-3 PUFAs | |||||||||||||||||
| α-Linolenic acid | 65 | 350 | 1.00 | Referent | 87 | 349 | 1.33 | 0.93, 1.90 | 62 | 349 | 0.99 | 0.67, 1.45 | 59 | 350 | 0.93 | 0.62, 1.37 | 0.39 |
| Eicosatrienoic acid | 62 | 350 | 1.00 | Referent | 72 | 349 | 1.20 | 0.82, 1.74 | 65 | 350 | 1.07 | 0.73, 1.57 | 74 | 349 | 1.27 | 0.88, 1.85 | 0.31 |
| Eicosapentaenoic acid | 54 | 350 | 1.00 | Referent | 69 | 349 | 1.25 | 0.85, 1.85 | 77 | 350 | 1.32 | 0.90, 1.94 | 73 | 349 | 1.20 | 0.81, 1.79 | 0.38 |
| Docosapentaenoic acid | 71 | 349 | 1.00 | Referent | 69 | 350 | 0.94 | 0.65, 1.36 | 67 | 349 | 0.93 | 0.64, 1.35 | 66 | 350 | 0.91 | 0.63, 1.32 | 0.63 |
| Docosahexaenoic acid | 63 | 349 | 1.00 | Referent | 81 | 350 | 1.30 | 0.90, 1.87 | 62 | 349 | 1.05 | 0.71, 1.56 | 67 | 350 | 1.10 | 0.74, 1.63 | 0.92 |
| EPA + DHA | 65 | 350 | 1.00 | Referent | 62 | 349 | 0.97 | 0.66, 1.42 | 79 | 350 | 1.23 | 0.85, 1.78 | 67 | 349 | 1.05 | 0.71, 1.55 | 0.53 |
| Total n-3 | 66 | 350 | 1.00 | 69 | 349 | 1.07 | 0.74, 1.55 | 68 | 349 | 1.06 | 0.73, 1.55 | 70 | 350 | 1.07 | 0.73, 1.57 | 0.75 | |
| n-6 PUFAs | |||||||||||||||||
| Linoleic acid | 65 | 349 | 1.00 | Referent | 64 | 350 | 0.96 | 0.65, 1.40 | 71 | 350 | 1.07 | 0.73, 1.55 | 73 | 349 | 1.15 | 0.79, 1.70 | 0.38 |
| γ-Linolenic acid | 75 | 350 | 1.00 | Referent | 58 | 349 | 0.73 | 0.50, 1.07 | 71 | 349 | 0.85 | 0.59, 1.23 | 69 | 349 | 0.83 | 0.57, 1.20 | 0.48 |
| Eicosadienoic acid | 69 | 350 | 1.00 | Referent | 56 | 349 | 0.80 | 0.54, 1.18 | 81 | 350 | 1.19 | 0.83, 1.70 | 67 | 349 | 0.96 | 0.66, 1.40 | 0.63 |
| Dihomo-γ-linolenic acid | 70 | 349 | 1.00 | Referent | 65 | 350 | 0.92 | 0.63, 1.34 | 65 | 349 | 0.95 | 0.65, 1.38 | 73 | 350 | 1.03 | 0.71, 1.51 | 0.84 |
| Arachidonic acid | 73 | 350 | 1.00 | Referent | 72 | 349 | 1.02 | 0.71, 1.46 | 71 | 349 | 1.02 | 0.71, 1.46 | 57 | 350 | 0.83 | 0.56, 1.23 | 0.42 |
| Docosadienoic acid | 54 | 350 | 1.00 | Referent | 72 | 349 | 1.38 | 0.94, 2.03 | 73 | 349 | 1.39 | 0.94, 2.04 | 74 | 350 | 1.46 | 0.99, 2.14 | 0.08 |
| Docosatetraenoic acid | 76 | 350 | 1.00 | Referent | 91 | 349 | 1.22 | 0.86, 1.72 | 55 | 349 | 0.75 | 0.51, 1.10 | 51 | 350 | 0.69 | 0.46, 1.02 | 0.011 |
| Total n-6 | 77 | 350 | 1.00 | Referent | 52 | 349 | 0.69 | 0.47, 1.02 | 77 | 349 | 1.07 | 0.75, 1.53 | 67 | 350 | 0.95 | 0.66, 1.38 | 0.70 |
Abbreviations: CARET, Carotene and Retinol Efficacy Trial; CI, confidence interval; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; OR, odds ratio; PUFA, polyunsaturated fatty acid.
a Multivariate adjustment for age at enrollment (continuous), race (white, black, others), CARET randomization assignment (retinol plus β-carotene, placebo), family history of prostate cancer in first-degree relatives (yes, no), alcohol consumption (nondrinker, below median, at or above median, unknown), smoking status (current, former/never), smoking pack-years (<40, 40, <60, ≥60), and body mass index (continuous).
b Defined as stage III/IV tumors or Gleason score ≥7.
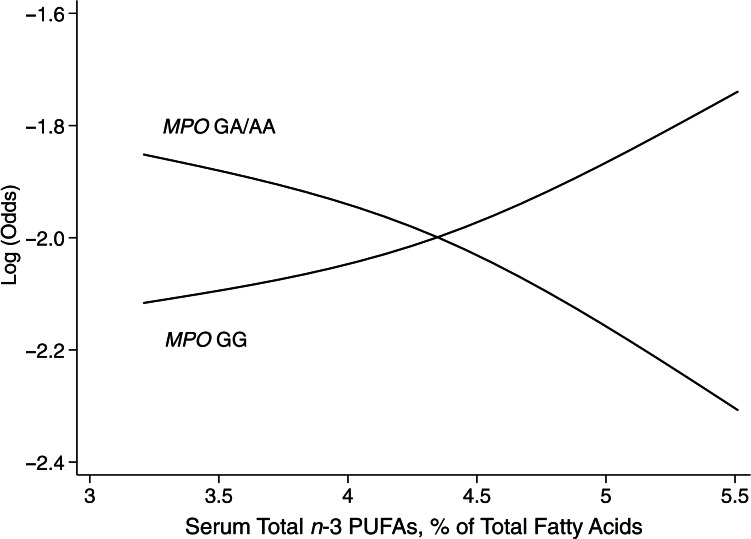
No effect modification of genetic variation in MPO G-463A on nonaggressive prostate cancer risk was observed for n-3 and n-6 PUFAs (Web Table 2) or on any prostate cancer risk for trans-fatty acids (Web Table 3). However, the polymorphism significantly modified the associations of several long-chain and very-long-chain n-3 and n-6 PUFAs with aggressive prostate cancer risk (Table 5). For n-3 PUFAs, the MPO GA/AA versus GG genotypes were associated with a nearly 2-fold increase in aggressive prostate cancer risk among men with low (quartile 1) EPA + DHA (OR = 1.97, 95% CI: 1.07, 3.63). Among men with the MPO GG genotypes, a positive, yet nonsignificant, association was observed between serum EPA + DHA percentages and aggressive prostate cancer risk. However, among men with the MPO GA/AA genotype, the direction of association was opposite. This risk difference by genetic variation in MPO was statistically significant (Pinteraction = 0.011). The effect modification of the MPO genotype was also observed for docosapentaenoic acid (DPA (22:5n-3); Pinteraction =0.013), DHA only (Pinteraction = 0.028), and total n-3 PUFAs (Pinteraction = 0.002) illustrated in Figure 1. For n-6 PUFAs, the effect modification was observed for arachidonic acid (Pinteraction = 0.036). In the secondary analysis (Web Table 4), the PUFA/MPO interactions remained significant for high-grade cancer. We observed a suggestive pattern that MPO modified the associations of EPA + DHA and total n-3 PUFAs with advanced stage prostate cancer risk, while there was no clear pattern of effect modification for lethal prostate cancer risk. In a sensitivity analysis, we additionally included serum α-linolenic acid, linoleic acid, and total trans-fatty acid percentages and α-tocopherol concentrations (all in quartiles) in the models because they are either metabolic precursors or correlated with individual PUFAs or lipid peroxidation (12, 23). The observed associations and effect modification remained unchanged (data not shown).
Table 5.
Joint Associationa of Serum n-3 and n-6 Polyunsaturated Fatty Acids and the MPO G-463A Polymorphism With Aggressive Prostate Cancerb in the Carotene and Retinol Efficacy Trial, 1985–2003
| Fatty Acids | MPOc Genotype | Quartile 1 |
Quartile 2 |
Quartile 3 |
Quartile 4 |
Ptrend | Pinteraction | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | ||||
| n-3 PUFAs | |||||||||||||||||||
| α-Linolenic acid | GG | 28 | 204 | 1.00 | Referent | 39 | 210 | 1.31 | 0.77, 2.22 | 27 | 189 | 1.08 | 0.61, 1.91 | 34 | 217 | 1.16 | 0.67, 2.01 | 0.96 | 0.54 |
| GA/AA | 19 | 138 | 1.01 | 0.54, 1.89 | 25 | 132 | 1.45 | 0.80, 2.61 | 19 | 156 | 0.92 | 0.49, 1.71 | 15 | 123 | 0.95 | 0.48, 1.87 | 0.80 | ||
| Eicosatrienoic acid | GG | 29 | 202 | 1.00 | Referent | 27 | 209 | 0.92 | 0.52, 1.62 | 33 | 197 | 1.18 | 0.69, 2.05 | 39 | 212 | 1.39 | 0.82, 2.37 | 0.16 | 0.88 |
| GA/AA | 14 | 144 | 0.68 | 0.35, 1.35 | 23 | 130 | 1.36 | 0.75, 2.48 | 23 | 145 | 1.18 | 0.65, 2.14 | 18 | 130 | 1.06 | 0.56, 2.01 | 0.21 | ||
| Eicosapentaenoic acid | GG | 25 | 216 | 1.00 | Referent | 31 | 203 | 1.25 | 0.71, 2.20 | 37 | 197 | 1.47 | 0.84, 2.55 | 35 | 204 | 1.25 | 0.71, 2.20 | 0.27 | 0.68 |
| GA/AA | 14 | 127 | 0.96 | 0.48, 1.93 | 23 | 144 | 1.39 | 0.75, 2.55 | 22 | 146 | 1.19 | 0.64, 2.22 | 19 | 132 | 1.11 | 0.58, 2.14 | 0.95 | ||
| Docosapentaenoic acid | GG | 31 | 227 | 1.00 | Referent | 26 | 191 | 0.96 | 0.55, 1.69 | 38 | 206 | 1.34 | 0.80, 2.25 | 33 | 196 | 1.21 | 0.71, 2.06 | 0.24 | 0.013 |
| GA/AA | 23 | 118 | 1.49 | 0.82, 2.70 | 26 | 150 | 1.28 | 0.73, 2.26 | 15 | 137 | 0.83 | 0.43, 1.60 | 14 | 144 | 0.72 | 0.37, 1.41 | 0.026 | ||
| Docosahexaenoic acid | GG | 25 | 227 | 1.00 | Referent | 40 | 196 | 1.88 | 1.09, 3.24 | 29 | 206 | 1.37 | 0.77, 2.45 | 34 | 191 | 1.64 | 0.93, 2.90 | 0.33 | 0.028 |
| GA/AA | 23 | 116 | 1.86 | 1.00, 3.44 | 24 | 146 | 1.55 | 0.84, 2.84 | 15 | 139 | 1.05 | 0.53, 2.08 | 16 | 148 | 1.09 | 0.55, 2.15 | 0.17 | ||
| EPA + DHA | GG | 25 | 230 | 1.00 | Referent | 30 | 199 | 1.39 | 0.79, 2.47 | 38 | 200 | 1.74 | 1.01, 3.02 | 35 | 191 | 1.66 | 0.95, 2.92 | 0.07 | 0.011 |
| GA/AA | 24 | 115 | 1.97 | 1.07, 3.63 | 19 | 144 | 1.24 | 0.65, 2.34 | 19 | 143 | 1.25 | 0.66, 2.38 | 16 | 147 | 1.06 | 0.54, 2.10 | 0.15 | ||
| Total n-3 | GG | 25 | 229 | 1.00 | Referent | 32 | 202 | 1.46 | 0.83, 2.56 | 35 | 201 | 1.64 | 0.94, 2.87 | 36 | 188 | 1.70 | 0.97, 2.98 | 0.07 | 0.002 |
| GA/AA | 28 | 118 | 2.21 | 1.23, 4.00 | 19 | 138 | 1.32 | 0.70, 2.51 | 14 | 142 | 0.92 | 0.46, 1.84 | 17 | 151 | 1.10 | 0.56, 2.14 | 0.033 | ||
| n-6 PUFAs | |||||||||||||||||||
| Linoleic acid | GG | 25 | 202 | 1.00 | Referent | 39 | 193 | 1.61 | 0.93, 2.78 | 32 | 197 | 1.31 | 0.74, 2.30 | 32 | 228 | 1.22 | 0.69, 2.16 | 0.96 | 0.09 |
| GA/AA | 18 | 139 | 1.14 | 0.59, 2.18 | 10 | 148 | 0.56 | 0.26, 1.20 | 23 | 146 | 1.27 | 0.69, 2.35 | 27 | 116 | 2.04 | 1.16, 3.74 | 0.017 | ||
| γ-Linolenic acid | GG | 35 | 221 | 1.00 | Referent | 26 | 191 | 0.78 | 0.45, 1.35 | 37 | 196 | 1.04 | 0.62, 1.74 | 30 | 211 | 0.78 | 0.46, 1.33 | 0.77 | 0.42 |
| GA/AA | 25 | 121 | 1.34 | 0.76, 2.36 | 17 | 151 | 0.70 | 0.37, 1.30 | 16 | 147 | 0.61 | 0.32, 1.16 | 20 | 130 | 0.86 | 0.47, 1.56 | 0.07 | ||
| Eicosadienoic acid | GG | 37 | 209 | 1.00 | Referent | 27 | 200 | 0.74 | 0.43, 1.27 | 38 | 194 | 1.17 | 0.71, 1.93 | 26 | 217 | 0.68 | 0.39, 1.17 | 0.29 | 0.09 |
| GA/AA | 14 | 134 | 0.62 | 0.32, 1.20 | 19 | 138 | 0.83 | 0.46, 1.52 | 21 | 151 | 0.80 | 0.45, 1.44 | 24 | 126 | 1.12 | 0.64, 1.99 | 0.06 | ||
| Dihomo-γ- linolenic acid | GG | 40 | 213 | 1.00 | Referent | 29 | 200 | 0.76 | 0.45, 1.28 | 31 | 207 | 0.80 | 0.48, 1.34 | 28 | 100 | 0.73 | 0.43, 1.25 | 0.33 | 0.30 |
| GA/AA | 17 | 131 | 0.70 | 0.38, 1.30 | 20 | 140 | 0.80 | 0.44, 1.43 | 18 | 132 | 0.74 | 0.40, 1.37 | 23 | 146 | 0.85 | 0.48, 1.51 | 0.66 | ||
| Arachidonic acid | GG | 30 | 207 | 1.00 | Referent | 34 | 220 | 1.11 | 0.65, 1.89 | 36 | 204 | 1.29 | 0.76, 2.19 | 28 | 189 | 1.03 | 0.59, 1.82 | 0.62 | 0.036 |
| GA/AA | 26 | 134 | 1.36 | 0.76, 2.41 | 21 | 124 | 1.24 | 0.68, 2.29 | 21 | 139 | 1.11 | 0.61, 2.03 | 10 | 152 | 0.49 | 0.23, 1.07 | 0.024 | ||
| Docosadienoic acid | GG | 24 | 202 | 1.00 | Referent | 33 | 207 | 1.38 | 0.78, 2.44 | 33 | 207 | 1.44 | 0.81, 2.54 | 38 | 204 | 1.69 | 0.97, 2.94 | 0.05 | 0.53 |
| GA/AA | 11 | 137 | 0.72 | 0.34, 1.53 | 19 | 135 | 1.29 | 0.68, 2.47 | 24 | 142 | 1.50 | 0.81, 2.77 | 24 | 135 | 1.67 | 0.90, 3.10 | 0.027 | ||
| Docosatetraenoic acid | GG | 37 | 208 | 1.00 | Referent | 44 | 197 | 1.31 | 0.81, 2.14 | 21 | 203 | 0.60 | 0.34, 1.07 | 26 | 212 | 0.70 | 0.41, 1.22 | 0.06 | 0.84 |
| GA/AA | 20 | 133 | 0.89 | 0.49, 1.61 | 29 | 142 | 1.19 | 0.69, 2.06 | 19 | 142 | 0.80 | 0.44, 1.46 | 10 | 132 | 0.46 | 0.22, 0.97 | 0.08 | ||
| Total n-6 | GG | 32 | 201 | 1.00 | Referent | 29 | 206 | 0.91 | 0.53, 1.57 | 38 | 198 | 1.28 | 0.76, 2.16 | 29 | 215 | 0.95 | 0.55, 1.64 | 0.97 | 0.74 |
| GA/AA | 21 | 138 | 1.02 | 0.56, 1.86 | 15 | 138 | 0.72 | 0.37, 1.39 | 21 | 145 | 1.02 | 0.56, 1.86 | 21 | 128 | 1.15 | 0.63, 2.11 | 0.49 | ||
Abbreviations: CARET, Carotene and Retinol Efficacy Trial; CI, confidence interval; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; OR, odds ratio; PUFA, polyunsaturated fatty acid.
a Multivariate adjustment for age at enrollment (continuous), race (white, black, others), CARET randomization assignment (retinol plus β-carotene, placebo), family history of prostate cancer in first-degree relatives (yes, no), alcohol consumption (nondrinker, below median, at or above median, unknown), smoking status (current, former/never), smoking pack-years (<40, 40, <60, ≥60), and body mass index (continuous).
b Defined as stage III/IV tumors or Gleason score ≥7.
c The myeloperoxidase gene, MPO.
Figure 1.
Predicted log(odds) of serum total n-3 polyunsaturated fatty acids (PUFAs) with aggressive prostate cancer by myeloperoxidase gene polymorphism (MPO G-463A) in the Carotene and Retinol Efficacy Trial, 1985–2003. Log(odds) were predicted from a logistic model containing a product term between the MPO genotype and serum total n-3 PUFAs as a continuous variable (Pinteraction = 0.028). The predicted log(odds) were fit by using a cubic spline with 4 knots (the midpoint of each quartile).
DISCUSSION
In this nested case-control study in CARET, the associations of serum EPA, DPA, DHA, total n-3 PUFAs, and arachidonic acid with aggressive prostate cancer were modified by the MPO G-463A polymorphism. We did not find any joint association of this polymorphism with serum trans-fatty acids. Our findings have important implications in the prevention of prostate cancer since EPA, DHA, and arachidonic acid are the most biologically relevant to signaling metabolic enzymes and inflammation (4). In addition, our data suggest that the effect modification is more relevant to aggressive prostate cancer compared with nonaggressive prostate cancer, as the former confers worse clinical outcome and should be the primary target of prostate cancer prevention. To our knowledge, this is the first study reporting the interaction between serum PUFAs and genetic variation in MPO. The interaction is consistent with previous analyses in CARET showing that high iron intake combined with the MPO GG genotype, both promoting oxidative stress, was associated with an increased risk of aggressive prostate cancer (11), and that high serum concentrations of α-tocopherol combined with the GA/AA genotypes, both lowering oxidative stress, decreased risk (12).
Two seemingly opposite mechanisms of oxidative stress and metabolic signaling of PUFAs potentially explain our findings. First, lipid peroxidation triggers myeloperoxidase located in neutrophils, monocytes, and some macrophages to generate endogenous free radicals (24) that damage prostate tissue (6, 7, 25). In addition, free radicals can directly react with PUFAs to form chlorohydrin, a substance that has higher polarity than the parent fatty acid and thus interrupts cell membrane structure, resulting in cell toxicity (9). Our analyses in CARET suggest that high MPO activity (the GG genotype), leading to high lipid peroxidation and free radical cellular concentrations, in conjunction with high percentages of serum n-3 PUFAs may increase prostate cancer risk. On the contrary, our joint effect model suggests that, under the condition of low percentages of total n-3 PUFAs, the genotype conferring low MPO activity (GA/AA) was associated with a 2-fold increase in aggressive prostate risk compared with the GG genotype. Studies have suggested that absence of oxidative stress and the protection of n-3 PUFA may lower cell apoptosis (26, 27). Nevertheless, since our study participants might already have an elevated level of oxidative stress due to their history of smoking (28), this observation warrants replication among nonsmokers.
Compared with n-3 PUFAs, n-6 PUFAs are more proinflammatory by influencing cell cycle regulatory genes and promoting cyclooxygenase and lipoxygenase syntheses (5, 10). Arachidonic acid signals prostaglandin E2, a major metabolite of cyclooxygenase-2 (29). In addition, after lipid peroxidation, n-6 PUFAs generate 4-hydroxy-2-nonenal, a cytotoxic aldehyde leading to DNA damage (5, 30). Epidemiologic findings of n-6 PUFAs in relation to prostate cancer are inconsistent (2, 23, 31, 32). For example, the Physicians' Health Study observed a positive association for dihomo-γ-linolenic acid in whole blood (23), while we found that dihomo-γ-linolenic acid and docosatetraenoic acid were inversely associated with nonaggressive and aggressive prostate cancer, respectively, Our observation has a biological rationale: Dihomo-γ-linolenic acid is a substrate for prostaglandin E1, which is more antiinflammatory than prostaglandin E2 (33). Also, docosatetraenoic acid is elongated from arachidonic acid; the replacement can lead to a lower level of inflammation. It is noted that the percentages of dihomo-γ-linolenic and docosatetraenoic acids in serum are relatively small and the number of comparisons has increased in order to estimate their associations. Thus, the significant findings should be interpreted with caution.
Our findings on interaction between n-6 PUFAs and MPO genotypes are parallel to those in the Alpha-Tocopherol Beta-Carotene Study. That study also recruited smokers and found that serum linoleic acid was inversely associated with prostate cancer risk only among men who received high-dose α-tocopherol supplements that lower oxidative stress and lipid peroxidation (for quartiles 4 vs. 1: OR = 0.17, 95% CI: 0.04, 0.68) (31). Our study found an inverse association of serum arachidonic acid with aggressive prostate cancer among men with the MPO GA/AA genotypes (related to low endogenous free radicals). These 2 observations suggest that the inflammatory response of n-6 PUFAs may depend on the oxidative stress level.
A major strength of our study is its nested case-control design, which measured fatty acids in serum collected on average 7 years prior to prostate cancer diagnosis. Also, a large number of cases enabled us to estimate risks for both nonaggressive and aggressive prostate cancer. The grade and stage of prostate cancer were confirmed by medical records or cancer registry files. Nevertheless, limitations to this study should be noted. First, it may not be appropriate to generalize our study findings to other populations because CARET participants were heavy smokers and/or had occupational asbestos exposure. Characteristics related to lipid peroxidation levels and expression of reactive oxygen species detoxifying enzymes in the study population may be different from those in other populations. Second, the long-term systematic or random variations of serum fatty acids may have biased our risk estimates toward the null since we measured them at one point in time. Third, we conducted statistical tests in individual components of n-3 and n-6 PUFAs, a strategy that may lead to an increase in type I error. However, since the hypothesis of PUFAs/MPO interaction was a priori and we found significant interactions in several n-3 and n-6 PUFAs with biological relevance, the probability of our findings due to chance alone was low (34). Finally, there is lack of consensus on defining aggressive prostate cancer. The Gleason pattern, for example, 4 + 3, was missing for most cases because of incomplete information on the pathology reports and medical records. We therefore used a Gleason score ≥8 to define “high-grade” prostate cancer and observed a consistent pattern of effect modification. However, we cannot make a clear conclusion for “advanced stage” or “lethal” prostate cancer because of the small numbers of patients with these tumors.
In conclusion, in this population of heavy smokers, the genetic variation in MPO G-463A is an important effect modifier of the association of n-3 and n-6 PUFAs, including EPA, DPA, DHA, and arachidonic acid, with aggressive prostate cancer. The longtime hypothesized beneficial and adverse effects of PUFAs on prostate cancer risk should be reevaluated, because they may depend on the activity of oxidative stress-regulatory enzymes.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, Washington (Ting-Yuan David Cheng, Irena B. King, Matt J. Barnett, Mark D. Thornquist, Gary E. Goodman, Marian L. Neuhouser); Department of Epidemiology, School of Public Health, University of Washington, Seattle, Washington (Ting-Yuan David Cheng, Marian L. Neuhouser); Department of Internal Medicine, School of Medicine, University of New Mexico, Albuquerque, New Mexico (Irena B. King); and Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, New York (Christine B. Ambrosone).
This work was supported in part by the National Cancer Institute at the National Institutes of Health (grants R01-CA-96789, U01-CA-63673, and N01-PC-35142).
The authors thank Dr. Alan Kristal for his critical comments on the earlier version of the manuscript.
Conflict of interest: none declared.
REFERENCES
- 1.Kolonel LN. Fat, meat, and prostate cancer. Epidemiol Rev. 2001;23(1):72–81. doi: 10.1093/oxfordjournals.epirev.a000798. [DOI] [PubMed] [Google Scholar]
- 2.Astorg P. Dietary n-6 and n-3 polyunsaturated fatty acids and prostate cancer risk: a review of epidemiological and experimental evidence. Cancer Causes Control. 2004;15(4):367–386. doi: 10.1023/B:CACO.0000027498.94238.a3. [DOI] [PubMed] [Google Scholar]
- 3.Rose DP. Effects of dietary fatty acids on breast and prostate cancers: evidence from in vitro experiments and animal studies. Am J Clin Nutr. 1997;66(6 suppl):1513S–1522S. doi: 10.1093/ajcn/66.6.1513S. [DOI] [PubMed] [Google Scholar]
- 4.De Caterina R. n-3 Fatty acids in cardiovascular disease. N Engl J Med. 2011;364(25):2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 5.Catala A. A synopsis of the process of lipid peroxidation since the discovery of the essential fatty acids. Biochem Biophys Res Commun. 2010;399(3):318–323. doi: 10.1016/j.bbrc.2010.07.087. [DOI] [PubMed] [Google Scholar]
- 6.Trzeciak AR, Nyaga SG, Jaruga P, et al. Cellular repair of oxidatively induced DNA base lesions is defective in prostate cancer cell lines, PC-3 and DU-145. Carcinogenesis. 2004;25(8):1359–1370. doi: 10.1093/carcin/bgh144. [DOI] [PubMed] [Google Scholar]
- 7.Khandrika L, Kumar B, Koul S, et al. Oxidative stress in prostate cancer. Cancer Lett. 2009;282(2):125–136. doi: 10.1016/j.canlet.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forsberg L, de Faire U, Morgenstern R. Oxidative stress, human genetic variation, and disease. Arch Biochem Biophys. 2001;389(1):84–93. doi: 10.1006/abbi.2001.2295. [DOI] [PubMed] [Google Scholar]
- 9.Spickett CM, Jerlich A, Panasenko OM, et al. The reactions of hypochlorous acid, the reactive oxygen species produced by myeloperoxidase, with lipids. Acta Biochim Pol. 2000;47(4):889–899. [PubMed] [Google Scholar]
- 10.Piedrafita FJ, Molander RB, Vansant G, et al. An Alu element in the myeloperoxidase promoter contains a composite SP1-thyroid hormone-retinoic acid response element. J Biol Chem. 1996;271(24):14412–14420. doi: 10.1074/jbc.271.24.14412. [DOI] [PubMed] [Google Scholar]
- 11.Choi JY, Neuhouser ML, Barnett MJ, et al. Iron intake, oxidative stress-related genes (MnSOD and MPO) and prostate cancer risk in CARET cohort. Carcinogenesis. 2008;29(5):964–970. doi: 10.1093/carcin/bgn056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng TY, Barnett MJ, Kristal AR, et al. Genetic variation in myeloperoxidase modifies the association of serum alpha-tocopherol with aggressive prostate cancer among current smokers. J Nutr. 2011;141(9):1731–1737. doi: 10.3945/jn.111.141713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King IB, Kristal AR, Schaffer S, et al. Serum trans-fatty acids are associated with risk of prostate cancer in β-Carotene and Retinol Efficacy Trial. Cancer Epidemiol Biomarkers Prev. 2005;14(4):988–992. doi: 10.1158/1055-9965.EPI-04-0517. [DOI] [PubMed] [Google Scholar]
- 14.Smith BK, Robinson LE, Nam R, et al. Trans-fatty acids and cancer: a mini-review. Br J Nutr. 2009;102(9):1254–1266. doi: 10.1017/S0007114509991437. [DOI] [PubMed] [Google Scholar]
- 15.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334(18):1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 16.Choi JY, Neuhouser ML, Barnett M, et al. Polymorphisms in oxidative stress-related genes are not associated with prostate cancer risk in heavy smokers. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1115–1120. doi: 10.1158/1055-9965.EPI-07-0040. [DOI] [PubMed] [Google Scholar]
- 17.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 18.Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986;27(1):114–120. [PubMed] [Google Scholar]
- 19.Ambrosone CB, Ahn J, Singh KK, et al. Polymorphisms in genes related to oxidative stress (MPO, MnSOD, CAT) and survival after treatment for breast cancer. Cancer Res. 2005;65(3):1105–1111. [PubMed] [Google Scholar]
- 20.Mikhak B, Hunter DJ, Spiegelman D, et al. Manganese superoxide dismutase (MnSOD) gene polymorphism, interactions with carotenoid levels and prostate cancer risk. Carcinogenesis. 2008;29(12):2335–2340. doi: 10.1093/carcin/bgn212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shui IM, Mucci LA, Kraft P, et al. Vitamin D-related genetic variation, plasma vitamin D, and risk of lethal prostate cancer: a prospective nested case-control study. J Natl Cancer Inst. 2012;104(9):690–699. doi: 10.1093/jnci/djs189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahn J, Gammon MD, Santella RM, et al. Myeloperoxidase genotype, fruit and vegetable consumption, and breast cancer risk. Cancer Res. 2004;64(20):7634–7639. doi: 10.1158/0008-5472.CAN-04-1843. [DOI] [PubMed] [Google Scholar]
- 23.Chavarro JE, Stampfer MJ, Li H, et al. A prospective study of polyunsaturated fatty acid levels in blood and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1364–1370. doi: 10.1158/1055-9965.EPI-06-1033. [DOI] [PubMed] [Google Scholar]
- 24.Sfanos KS, Wilson BA, De Marzo AM, et al. Acute inflammatory proteins constitute the organic matrix of prostatic corpora amylacea and calculi in men with prostate cancer. Proc Natl Acad Sci U S A. 2009;106(9):3443–3448. doi: 10.1073/pnas.0810473106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartsch H, Nair J, Owen RW. Dietary polyunsaturated fatty acids and cancers of the breast and colorectum: emerging evidence for their role as risk modifiers. Carcinogenesis. 1999;20(12):2209–2218. doi: 10.1093/carcin/20.12.2209. [DOI] [PubMed] [Google Scholar]
- 26.Fan YY, Ran Q, Toyokuni S, et al. Dietary fish oil promotes colonic apoptosis and mitochondrial proton leak in oxidatively stressed mice. Cancer Prev Res (Phila) 2011;4(8):1267–1274. doi: 10.1158/1940-6207.CAPR-10-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan YY, Zhan Y, Aukema HM, et al. Proapoptotic effects of dietary (n-3) fatty acids are enhanced in colonocytes of manganese-dependent superoxide dismutase knockout mice. J Nutr. 2009;139(7):1328–1332. doi: 10.3945/jn.109.106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrow JD, Frei B, Longmire AW, et al. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332(18):1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 29.Wortman P, Miyazaki Y, Kalupahana NS, et al. n3 and n6 polyunsaturated fatty acids differentially modulate prostaglandin E secretion but not markers of lipogenesis in adipocytes. Nutr Metab. 2009;6(5) doi: 10.1186/1743-7075-6-5. (doi: 10.1186/1743-7075-6-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pettazzoni P, Pizzimenti S, Toaldo C, et al. Induction of cell cycle arrest and DNA damage by the HDAC inhibitor panobinostat (LBH589) and the lipid peroxidation end product 4-hydroxynonenal in prostate cancer cells. Free Radic Biol Med. 2011;50(2):313–322. doi: 10.1016/j.freeradbiomed.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Mannisto S, Pietinen P, Virtanen MJ, et al. Fatty acids and risk of prostate cancer in a nested case-control study in male smokers. Cancer Epidemiol Biomarkers Prev. 2003;12(12):1422–1428. [PubMed] [Google Scholar]
- 32.Brasky TM, Till C, White E, et al. Serum phospholipid fatty acids and prostate cancer risk: results from the Prostate Cancer Prevention Trial. Am J Epidemiol. 2011;173(12):1429–1439. doi: 10.1093/aje/kwr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan YY, Chapkin RS. Importance of dietary γ-linolenic acid in human health and nutrition. J Nutr. 1998;128(9):1411–1414. doi: 10.1093/jn/128.9.1411. [DOI] [PubMed] [Google Scholar]
- 34.Wacholder S, Chanock S, Garcia-Closas M, et al. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Inst. 2004;96(6):434–442. doi: 10.1093/jnci/djh075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.