Abstract
The association of obesity with noncommunicable diseases, such as cardiovascular complications and diabetes, is considered a major threat to the management of health care worldwide. Epidemiological findings show that childhood obesity is rapidly rising in Western society, as well as in developing countries. This pandemic is not without consequences and can affect the risk of future cardiovascular disease in these children. Childhood obesity is associated with endothelial dysfunction, the first yet still reversible step towards atherosclerosis. Advanced research techniques have added further insight on how childhood obesity and associated comorbidities lead to endothelial dysfunction. Techniques used to measure endothelial function were further brought to perfection, and novel biomarkers, including endothelial progenitor cells, were discovered. The aim of this paper is to provide a critical overview on both in vivo as well as in vitro markers for endothelial integrity. Additionally, an in-depth description of the mechanisms that disrupt the delicate balance between endothelial damage and repair will be given. Finally, the effects of lifestyle interventions and pharmacotherapy on endothelial dysfunction will be reviewed.
1. Introduction
Society is faced with a new pandemic. Obesity and its associated non-communicable diseases, such as cardiovascular (CV) complications, diabetes [1], sleep apnea [2], and asthma, are a major threat to the management of health care worldwide. It may seem paradoxical but both childhood malnutrition in developing countries and the rapidly increasing prevalence of overweight and obesity in Western youth have a common denominator, low income [3].
Being obese as a child comes at a price. Childhood overweight and obesity increase the risk of obesity at adult age [4] and are associated with CV risk factors. A high BMI during childhood and adolescence has been associated with, respectively, premature death from disease and increased risk of coronary heart disease at adult age [5, 6]. Interestingly, however, in a recent meta-analysis of four studies, overweight and obese children who became nonobese in adult life were not different in terms of several risk parameters of cardiovascular disease to those patients who had never been obese [7]. This could be explained by the fact that other indicators of a healthy lifestyle, such a regular physical exercise, were not taken into account. During the past two decades, the concept of early vascular changes, which act as the primum movens for future CV complications, has been tested [8]. The development and refinement of technical tools that allow the in vivo evaluation of endothelial dysfunction, which is considered the earliest demonstrable feature of atherosclerosis, rapidly advanced pathophysiological insights. Recently, more fundamental research into disrupted bone-marrow-related endothelial repair mechanisms (i.e., endothelial progenitor cells), as well as the identification of novel markers of endothelial damage (i.e., endothelial microparticles), has created an entirely new line of research [9, 10]. These biomarkers hold promise in terms of designing effective strategies and to evaluate their effect in the combat against the devastating consequences of childhood obesity.
A long-lasting change of unhealthy lifestyles is fundamental in helping obese children and their parents to fight this disease. Therefore, a multidisciplinary approach to adopt physical activity and balanced calorie restriction into daily life is inevitable to counterbalance the attraction of highly processed food, motorized transportation, and TV-related sedentarism.
This paper will provide a critical overview on both in vivo as well as in vitro markers for endothelial integrity. As an introduction we will first describe the obesity-induced imbalance of endothelial repair and damage, resulting in early endothelial dysfunction. Currently available data on the effect of lifestyle changes as well as possible pharmacological interventions to counteract obesity-induced endothelial malfunction will be recapitulated. Although the focus of this review is on childhood obesity, it is important to stress that data from obese adults are preponderant and therefore will be incorporated in the text. Comparison of the available literature will expose differences and stresses the fact that the scientific community should invest more in childhood obesity research since, indeed, children are not small adults. In addition, the value of longitudinal studies, starting early in childhood, cannot be underestimated when it comes to exactly decipher the timing of pathophysiological events.
2. Endothelial Dysfunction
The endothelial cell-layer is located at the border between circulating blood and vascular smooth muscle cells (VSMC) [11]. In response to stimuli that indicate increased blood flow demand (e.g., increased shear stress), activation of the PhosphoInositol 3-Kinase (PI3K)/Akt pathway will lead to phosphorylation of endothelial Nitric Oxide Synthase (eNOS), which is necessary for its activation and the generation of Nitric Oxide (NO) (Figure 1) [12]. By fine-tuning vascular smooth muscle relaxation, NO is one of the main regulators of vascular tone [13, 14]. In addition, healthy endothelium is responsible for the maintenance of an atheroprotective environment: it prevents platelet aggregation, proliferation of VSMC, and adhesion and subsequent diapedesis of leukocytes through the vascular wall [15].
Figure 1.
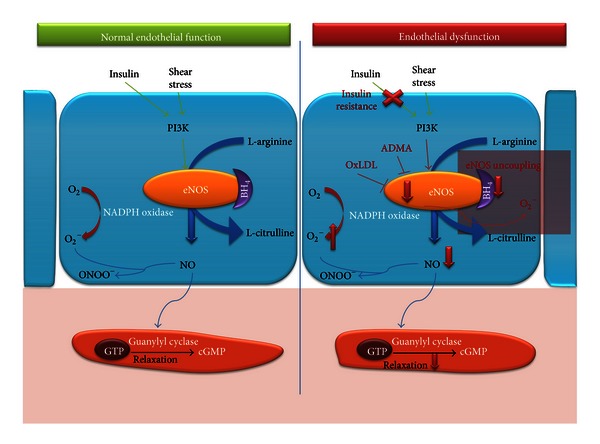
Normal endothelial function versus endothelial dysfunction. Schematic overview of nitric oxide (NO) production and relaxation of Vascular Smooth Muscle Cells (VSMC). In response to increased shear stress or as a result of insulin signaling, the phosphoinositol 3 kinase (PI3K)/akt pathway is activated leading to phosphorylation of endothelial Nitric Oxide Synthase (eNOS). eNOS, together with the necessary cofactor tetrahydrobiopterin (BH4), converts L-arginine to L-citrulline and NO. NO activates guanylyl cyclase, which induces smooth muscle relaxation, through increased production of cyclic Guanosine MonoPhosphate (cGMP). Superoxide reduced NO bioavailability by reacting with NO to form peroxynitrite (ONOO−), which has strong oxidant properties. Endothelial dysfunction in obese children is characterized by insulin resistance impairing insulin-mediated NO production and subsequent vasodilation. Furthermore, oxidized LDL and ADMA are inhibitors of eNOS activation. In the situation of diminished availability of BH4, eNOS becomes “uncoupled” and paradoxically leads to increased reactive oxygen species (ROS) generation, which also contributes to reduced bioavailability of NO and vasoconstriction. ADMA, Asymmetric DiMethylArginine; PI3K, PhosphatidylInositol 3-Kinase; BH4, tetrahydrobiopterin; eNOS, endothelial Nitric Oxide Synthase; O2 −, superoxide; ONOO−, peroxynitrite; GTP, Guanosine TriPhosphate; cGMP, cyclic Guanylyl MonoPhosphate; NADPH oxidase, Nicotinamide Adenine Dinucleotide Phosphate oxidase; OxLDL, Oxidized Low-Density Lipoprotein Cholesterol.
The common and straightforward definition of endothelial dysfunction is “an imbalance between vasodilating and vasoconstricting substances produced by (or acting on) endothelial cells” [16]. The close interaction between endothelial cells and their environment, however, highlights the process complexity. Indeed, due to its location within the vascular wall, the endothelium is directly exposed to damaging factors such as high blood pressure and elevated lipid levels, which are common in obese subjects. The notion that endothelial dysfunction is a necessary and first step towards atherosclerosis has led to a tremendous interest and research investment in an attempt to unravel underlying pathophysiological mechanisms [17].
Fortunately, endothelial dysfunction is not an irreversible process. Upon endothelial damage and ischemia, repair mechanisms are activated. The concept of endothelial homeostasis, which is the net result of endothelial damage and repair, has provided the basis for developing novel therapeutic options, as well as a fascinating new research domain of endothelial “rejuvenation.”
2.1. Factors Contributing to Endothelial Dysfunction in Obesity
Similar to adults, CV risk factors tend to cluster in obese children and include hypertension, high cholesterol and triglycerides, insulin resistance, a proinflammatory status, disturbances in adipocytokines, and physical inactivity [18]. As a result, assessing the specific contribution of one sole risk factor is extremely difficult. In the following, we will briefly cover recent insights into factors causing endothelial dysfunction in childhood obesity.
2.1.1. Hypertension
Hypertension is prevalent in obese children. Based on data gathered in the Avon Longitudinal Study of Parents and Children (ALSPAC; 7589 children aged 8.8–11.7 years), the odds ratio for hypertension was 10.7 (95% CI 7.2–15.9) for obese boys and 13.5 (95% CI 9.4–19.5) for obese girls [18], compared to children with normal weight. The interaction between endothelial dysfunction and hypertension is complex but recent data from longitudinal population studies do not support a bidirectional relationship.
In a cross-sectional and longitudinal study of 3500 adults, Shimbo et al. showed that hypertension is more prevalent in patients with low flow-mediated dilation (FMD) [19]. However, reverse findings could not be confirmed since impaired endothelial function was not predictive of incident hypertension. Similar findings were seen in The Cardiovascular Risk in Young Finns Study, where elevated systolic blood pressure in adolescent boys predicted impaired brachial endothelial function 21 years later in adulthood [20]. A recent analysis of data derived from the ALSPAC study [21] sheds light on the vascular consequences of obesity with time. In their study of 6,576 prepubertal children (aged 10 to 11 years; 80% normal weight, 16% overweight and 4% obese), obesity was associated with higher blood pressure, higher heart rate, and higher resting and hyperemic blood flow, all of which are compatible with a higher cardiac output state. Counterintuitively, these obese children had larger baseline brachial artery diameters, higher FMD, and lower arterial stiffness, despite an unfavorable metabolic profile. Although speculative, the authors propose an initial adaptive response, which ultimately, with longer exposure to obesity, will fail and culminate in vascular damage. Data derived from the Cardiovascular Risk in Young Finns Study support this view and demonstrate an adverse impact of CV risk factors including adiposity, high LDL cholesterol and high insulin level on the progression of intima-media thickness (IMT) in young adults aged 30 years [22]. Previous results from this longitudinal study provide further evidence for this hypothesis, since a correlation was found between CV risk factors and IMT only for participants with demonstrated endothelial dysfunction [23].
Obesity increases blood pressure through multiple mechanisms, which also affect endothelial function. These factors include increased activity of the renin-angiotensin aldosterone system (RAAS), as well as sympatho-activation. Angiotensin II, one of the main hormones in the RAAS system, also directly impairs NO production by affecting eNOS activity [30]. Increased sympathetic nervous activity leads to peripheral vasoconstriction and impairment of endothelial function [31]. Importantly, hypertension reduces bioavailability of NO and increases oxidative stress, due to increased Reactive Oxygen Species (ROS) generation and lower antioxidant capacity. In addition, Asymmetric DiMethyl Arginine (ADMA), a natural inhibitor of eNOS, has been found in higher concentrations in obese individuals [32].
2.1.2. Lipids
High levels of low-density lipoprotein (LDL) cholesterol and low levels of High-Density Lipoprotein (HDL)-cholesterol are well-known independent CV risk factors. Native LDL can impair endothelial function by decreasing NO bioavailability and eNOS activity. However, the effect is more pronounced when LDL is taken up by macrophages and oxidized to oxidized LDL [33]. HDL cholesterol reduces vascular tone by increasing NO bioavailability; it reduces the expression of adhesion molecules for leukocytes and increases endothelial integrity by upregulating endothelial cell migration and proliferation [34, 35].
Results from the PEP Family Heart Study in 3038 adolescents (12 to 18 years) have demonstrated that central obesity, defined as elevated waist circumference and/or waist-to-hip ratio, is an independent predictor of hypertension, fasting glucose, elevated triglycerides, LDL-cholesterol, non-HDL-cholesterol, triglyceride/HDL-cholesterol ratio, low HDL-cholesterol, and risk factor clustering [36]. Yet, despite having central obesity, both prevalence of an unfavorable lipid profile as well as absolute concentrations of lipids were less pronounced than those found in obese adults. For instance, the prevalence of increased triglyceride concentration was 5,8% and 11,2% in obese male and female children, respectively, but reached approximately 25% in a large cohort of Italian obese adults [37].
Recent research supports the hypothesis that the well-known beneficial effects of HDL, such as macrophage cholesterol efflux and endothelial NO stimulation, are highly heterogeneous and may be altered in patients with coronary artery disease as well as diabetes. Therefore, HDL dysfunction has to be taken into account when novel HDL-targeted therapeutic interventions, such as Cholesteryl Ester Transfer Protein (CETP) inhibitors, are introduced [38].
2.1.3. Physical (In)Activity
Physical activity is associated with a significant reduction in CV mortality in adult men and women [39–41], whereas physical inactivity predicts the development of overweight and obesity [42].
Using a questionnaire, physical activity in more than 6000 children between 11 and 19 years was investigated by De Bourdeaudhuij et al. Overweight children spent on average 5.27 ± 4.65 hours per week on physical activity versus 6.21 ± 5.07 hours per week for normal weight children. Significant differences were noted for vigorous and moderate physical activity as well, yet no differences in sedentary behavior were noted [43].
Similar to adults, endothelial function relates to fitness and physical activity in children, yet correlations are affected by age. In young children (5–10 years: mean age 8 years), physical activity was the strongest predictor of FMD in multivariate analysis [44]. Slightly older children (n = 145 (59 male, 86 female) with a mean age of 10.3 ± 0.03 years) demonstrated a significant correlation between FMD and the time spent at moderate and high intensity physical activity [45]. Correlation in that study with physical activity was strongest in the lowest tertile of endothelial function, suggesting that these children could benefit most from physical training. Endothelial function was also clearly related to fitness, objectively expressed as peak oxygen consumption during cardiopulmonary exercise testing. Based on a large study, involving 483 13-year-old adolescents, multivariate analysis pointed out that endothelial function correlated significantly to leisure time physical activity. This finding was only confirmed in boys, leading the authors to hypothesize that the overall lower physical activity seen in girls may explain these results [46]. A longitudinal study in children further demonstrated that an extensive increase in physical activity led to a significant improvement of FMD as well as less progression of intima-media thickness [47].
2.1.4. Adipokines
Normal adipose tissue consists of adipocytes, and furthermore macrophages, fibroblasts, endothelial cells, and preadipocytes are present in the so-called vascular-stromal fraction [48]. Although it has long been thought that storage and release of free fatty acids were its only functions, adipose tissue is currently seen as an important endocrine organ, regulating glucose and lipid metabolism and producing a vast amount of cytokines (adipocyte-derived cytokines or adipokines) and hormones [49, 50]. Obesity is characterized by hypertrophic adipose tissue. As an effect of hypercaloric intake, adipocytes enlarge and release more free fatty acids, which activate macrophages to produce Tumor Necrosis Factor-alpha (TNF-α). As a consequence, adipocyte expression of Intercellular Adhesion Molecule-1 (ICAM-1), InterLeukin (IL)-6, and Macrophage Chemo attractant Protein-1 (MCP-1) is enhanced, promoting diapedesis of monocytes from the circulation to adipose tissue. With the exception of adiponectin, adipocytokine levels rise with obesity. Growing adipose tissue poses higher demands in terms of oxygen and nutrient delivery. Despite vasculogenesis [51], local hypoxia induces several angiogenic factors, which further suppress adiponectin expression even more and upregulate leptin production. This “hypersecretion of proatherogenic, pro-inflammatory, and prodiabetic adipocytokines which is accompanied by a decreased production of adiponectin” is called adipose tissue dysfunction [52]. Of the adipokines, leptin and adiponectin have a proven direct influence on angiogenesis and the endothelium, and are therefore further discussed [53].
Leptin. Leptin is a cytokine, which is mainly synthesized by white adipose tissue and released into the circulation. Its physiological role is to suppress appetite and to increase energy expenditure via the hypothalamus. However, circulating levels of leptin contradictorily rise with increasing body fat percentage, while obese patients have no diminished appetite [54].
Leptin has several proangiogenic effects, including enhanced Akt-mediated eNOS phosphorylation [55] as well as stimulation of endothelial cell proliferation [56], which are expected to be beneficial in the setting of endothelial dysfunction in obese patients. However, leptin also exerts proatherogenic effects, including the induction of ROS, a pro-inflammatory vascular effect, and stimulation of the proliferative capacity of VSMC [57].
These insights have led to the concept of a selective leptin resistance for both central (appetite) and peripheral pro-angiogenic effects, without any changes in terms of pro-atherogenic stimuli. The inverse correlation between leptin and endothelial function, independent of the metabolic and inflammatory disturbances associated with obesity adds, further supports to this hypothesis [58].
Adiponectin. Adiponectin is a 30 kDa protein produced by adipocytes with anti-inflammatory, antiatherogenic, and insulin sensitizing effects. This adipokine plays a central role in lipid and energy metabolism. Contrary to all other adipokines, adiponectin concentrations are lower in obese. In their elegant paper, Torigoe et al. demonstrated that even in healthy young men, circulating concentrations of adiponectin determine endothelial function [59], through eNOS mRNA stabilization and eNOS phosphorylation [60]. Moreover, high-glucose level-induced generation of ROS by endothelial cells is suppressed by adiponectin [61]. Recent human data show that adiponectin stimulates the migratory capacity of circulating angiogenic cells [62], thereby supporting animal experiments that suggested angiogenesis-stimulating effects [63] and the promotion of neovascularization [64].
2.1.5. Inflammation
Obesity, and visceral obesity in particular, is associated with a low-grade pro-inflammatory status [65]. Upon activation, endothelial cells express adhesion molecules, which allow leukocytes to adhere and initiate a cascade of inflammatory reactions. Inflammatory cytokines produced by macrophages in adipose tissue (IL-18, TNF-α) further add to the expression of adhesion molecules. Not surprisingly inflammatory cytokines (e.g., C-Reactive Protein or CRP) are also associated with CV risk [66], and their concentration inversely correlates with endothelial function [67]. Another important mediator is IL-6 [68], which not only is a potent stimulator for the production of CRP, but also stimulates angiotensin II in VSMC and the associated production of ROS [69]. Besides reacting with NO, and thereby neutralizing its vasodilatory effect, ROSs also reduce eNOS activity and consequently NO production. Moreover, ROSs are able to directly inhibit eNOS and react with tetrahydrobiopterin (BH4), a necessary cofactor for eNOS activity [70]. In this situation, eNOS becomes “uncoupled” and paradoxically leads to increased ROS generation [71].
2.1.6. Insulin Resistance and Type 2 Diabetes Mellitus
Under physiological conditions, insulin is a potent vasodilator, via the stimulation of the PI3K/Akt pathway to augment NO production. However, in the setting of insulin resistance this reaction is absent [72]. Yet insulin is still able to activate the Extracellular signaling-Regulated Kinase (ERK1/2) pathway leading to production of EndoThelin 1 (ET-1) and thus vasoconstriction [73]. The same pathway also leads to expression of adhesion molecules like Vascular Cell Adhesion Molecule (VCAM)-1 on endothelial cells [74]. Endothelial dysfunction per se can even contribute to insulin resistance, since impaired microvascular vasodilation in skeletal muscle reduces delivery of insulin and glucose to skeletal muscle and thereby causes insulin resistance [75].
In type 2 diabetes mellitus, NO bioavailability is significantly diminished: a reduction in eNOS activity [76] as well as eNOS uncoupling [77] and increased generation of ROS have been demonstrated [78] and further deteriorate endothelial function. Hyperglycemia in diabetic patients leads to production of Advanced Glycation End products (AGE) after reacting with proteins, lipids, and nucleic acids. AGE can induce the expression of ROS via NF-kappa B activation and TNF-α [79]. Type 2 diabetes is additionally characterized by increased levels of ET-1 [80]. Interestingly, a recent double-blind-placebo controlled trial in 46 type II diabetes patients with microalbuminuria demonstrated that 4 weeks of treatment with an oral ET-1 receptor antagonist (bosentan) can repair endothelial function [81].
3. Clinical Assessment of Endothelial Dysfunction
The landmark study by Ludmer et al. [82] led to the concept of endothelial dysfunction as the primum movens of the atherosclerotic process. The consequences of an abnormal vasodilator response (i.e., impaired vasodilation and even paradoxical vasoconstriction of coronary arteries upon the administration of acetylcholine) have thereafter been extensively studied. Epicardial and microvascular coronary endothelial dysfunction predicts CV events in patients with and without coronary artery disease [83]. Obviously, acetylcholine infusion can be applied during coronary angiography, but the invasive character prohibits its use in healthy individuals and children. Since then, novel, less invasive techniques have been developed, which will be discussed in detail in the following paragraphs.
3.1. Flow-Mediated Dilation
In 1992, Celermajer et al. described a noninvasive technique that allows assessment of peripheral endothelial function [84]. Measurement of flow-mediated dilation (FMD) at the level of a large conduit artery, usually the brachial artery, has since then become the most applied technique [85]. Briefly, high-resolution ultrasound is used to measure the internal diameter of the brachial artery, from lumen-intima interface on the near and far vascular wall [86]. After assessing baseline diameter, the brachial artery is occluded during 5 minutes using a sphygmomanometer inflated to suprasystolic pressure. When the cuff is deflated; the increased flow causes endothelial-dependent dilation through raised shear stress [84]. It is this proportional response (i.e., related to baseline diameter) that is significantly impaired in the case of endothelial dysfunction.
Pathophysiological research confirms that this effect is mainly mediated by the release of NO by endothelial cells during the phase of hyperemia since it can be prevented by using N(G)-MonoMethyl-L-Arginine (L-NMMA), which is a selective inhibitor of eNOS. Importantly, NO dependence is influenced by location of the inflating cuff. Dilation upon hyperemia is completely blocked by infusion of L-NMMA when the cuff is placed distal to the measuring site, but only partially when the cuff is placed proximal to the echo probe [87]. The latter position of the probe creates an area of ischemia within the region of the probe, and therefore other vasoactive substances may play a role. Although in the past the predictive power of FMD was attributed to its NO dependency, a recent meta-analysis by Green et al. demonstrated that FMD measured after proximal occlusion is at least as predictive as measured after distal occlusion, although less NO dependent [88].
The assessment of FMD is still the most widely used method but it requires intensive training in order to achieve acceptable reproducibility. Besides technical factors, which are reviewed elsewhere, [86, 89], patient-related characteristics require a high level of standardization (Table 1). Measurements should be performed on the same time of day since FMD has a known diurnal variation [90]. A high fat meal [91] deteriorates endothelial function, and dietary components such as tea [92], Vitamin C [93], and chocolate [94] have short-term beneficial effects on endothelial function. Measurements should therefore always be performed in a fasting state before medication intake [95, 96]. Specifically for the pediatric setting, children frequently present with mild infections, which may impair endothelial function for up to 2 weeks [97]. Both mental stress [98] and room and skin temperature [99] influence endothelial function. It is therefore recommended to perform measurements in a dim-lighted and temperature-controlled (21–24°C) room and to carefully explain the procedure to studied subjects. Tobacco use should be avoided for 4 to 6 hours prior to measurements [100]. No guidelines are given regarding passive smoking, although this may be relevant for children, since childhood exposure to tobacco smoke leads to endothelial dysfunction in both children [101] and young adults [102]. Although we did not find evidence for a synergistic effect of smoking and childhood obesity on endothelial dysfunction, maternal smoking has been shown to correlate with several obesity-related risk factors in young children [103]. Gender matters in childhood since it influences endothelial function, with FMD being lower in boys [104]. Moreover, endothelial function differs during the menstrual cycle [105].
Table 1.
Patient-related factors influencing clinical assessment of endothelial function in children.
| Influencing factor | Solution |
|---|---|
| Time of day | Perform measurements between 8 and 12 am to rule out an effect of diurnal variation of endothelial function |
| Food | Perform measurement after an overnight fast |
| Medication | Ask patients to take their medication after the test |
| (Mild) infection | Postpone the test for >2 weeks |
| Active and passive smoking | Exclude smokers and/or record (parental) smoking habits |
| Gender | Check whether all groups are matched for sex |
| Age | Check whether all groups are matched for age and pubertal stage |
| Menstrual cycle | Girls in same phase of cycle or note the menstrual phase |
| Skin temperature | Allow sufficient patient acclimatization time; cover patients using a blanket |
| Mental stress | Avoid anxiety by providing patient information; perform the test in a quiet room |
Contrary to adults, children present more variability in time to peak dilation in response to hyperemia. While in adults peak dilation usually occurs 60 seconds after cuff release, in children maximal dilation has been described between 40 and 120 seconds after-occlusion [106]. This effect is age dependent, and time to peak tends to drop with increasing age [104], advocating against a fixed moment in time to record maximal endothelium-dependent dilation. Effects of age on maximal dilation are conflicting with Donald et al. demonstrating no effect of age on FMD in a very large population of children (n = 7557) [99] and Sarkola et al. mentioning a decrease in FMD, which they explained by an increasing baseline internal diameter of the brachial artery [104].
Although we found 2 articles describing the absence of endothelial dysfunction in obese children [21, 107], numerous research groups have reported on impaired endothelial-dependent vasodilation, assessed with FMD, in this population [108–122].
3.2. Peripheral Arterial Tonometry
Peripheral arterial tonometry was developed as a novel technique to overcome the disadvantages of user dependence of FMD. The only commercially available and validated apparatus (Endo-PAT 2000) involves finger probes to measure arterial pulse wave amplitudes at the fingertip.
Guidelines for FMD measurements are also implemented for Endo-PAT measurements, although the interference of several factors was investigated separately. Research confirmed the effect of diurnal variation [123] and the effect of dietary components such as omega-3 fatty acids [27], polyphenol rich olive oil [124], chocolate [125], and tea [126]. Furthermore, measurements were influenced by drugs [81] and mental stress [127].
The Endo-PAT probes are placed on one fingertip of both hands and are inflated to produce a subdiastolic counter pressure, in order to provide fixation and prevent venous pooling. Pressure differences secondary to dilating arterioles in the fingers are measured. The procedure is initiated with a 5-minute baseline assessment; then a manometer cuff is inflated to supra-systolic pressures and the brachial artery of the nondominant arm is occluded. After 5 minutes, the cuff is released and reactive hyperemia is observed during 5 minutes. The software provided calculates a Reactive Hyperemia Index (RHI) and is defined as the ratio of the mean Pulse Wave Amplitude (PWA) between 90 and 150 seconds after deflation divided by a preocclusion period during 210 seconds before occlusion. This ratio is then divided by the same ratio for the control arm and multiplied by a baseline correction factor. A “Framingham reactive hyperemia” is also calculated. For this ratio, the period used to assess the hyperemia is between 90 and 120 seconds after occlusion since this period most strongly correlated with CV risk factors [128]. The ratio is log transformed as data indicate that values are not normally distributed.
Since the probes can never be placed proximal to the occlusion site, provided by the cuff, there is an effect of local ischemia. In addition, the hyperemic response that is measured is not entirely caused by endothelial NO production since it is not fully abolished by L-NAME [129]. Other mediators that contribute to vasodilation include prostacyclin (PGI2), a derivative of arachidonic acid that is secreted by endothelial cells. In normal arteries, NO has inhibitory effects on PGI2 release [130], and therefore the effect of PGI2 on endothelial dilation can vary in diseases associated with reduced NO bioavailability. A third mediator responsible for endothelial dependent dilation is Endothelium-Derived Hyperpolarizing Factor (EDHF), which has a larger influence on arterial tone in smaller vessels [131]. Fourthly, sympathetic tone can also modulate endothelial function, and an inverse correlation between sympathetic nerve activity and RHI has been demonstrated in healthy subjects [132].
Feasibility and reproducibility of Endo-PAT in adults are excellent [133]. The technique also appears highly reproducible in adolescents and causes hardly any discomfort [134]. To our knowledge, only 6 studies have been published in which endothelial function measured with Endo-PAT in obese children is described, and only 3 of them compared obese to lean children (Table 2). Results have been conflicting with Landgraf et al. [29] and Mahmud et al. [24] describing a lower RHI in obese children versus lean controls, whereas Tryggestad et al. [28] reported no difference.
Table 2.
Overview of studies using Endo-PAT to measure endothelial function in obese children.
| Reference | Age (years) | Definition of obesity and overweight | Comorbidities | Parameter | Outcome |
|---|---|---|---|---|---|
| Mahmud et al. [24] | Obese: 13.4 ± 1.7; Lean: 14.0 ± 1.4 | Obesity = BMI > 95th percentile | All insulin resistant, based on the HOMA score | RHI | Mean RHI was significantly lower in obese adolescents compared with controls (1.51 ± 0.4 versus 2.06 ± 0.4) |
|
| |||||
| Metzig et al. [25] | 12.4 | Obesity = BMI > 95th percentile | 15% with hypertension, 15% with dyslipidemia, 9% with OSAS, and 15% with impaired glucose tolerance | RHI | No significant effect of glucose ingestion on RHI |
|
| |||||
| Kelly et al. [26] | 12.7 | Obesity = BMI > 95th percentile | ? | RHI | No significant effect of exenatide therapy on RHI |
|
| |||||
| Dangardt et al. [27] | 15.7 | ? | ? | Maximum dilation, area under the curve | Significant improvement of endothelial function after 3 months of omega-3 fatty acid supplementation |
|
| |||||
| Tryggestad et al. [28] | Obese: 13.9 ± 2.5; Lean: 13.3 ± 3.0 | Obesity = BMI > 95th percentile | ? | RHI | No significant difference between obese and normal weight children |
|
| |||||
| Landgraf et al. [29] | Obese: 11.8 ± 2.9; Lean: 12.9 ± 2.9 | Obesity = BMI > 97th percentile; overweight = BMI > 90th percentile | ? | RHI | Mean RHI was significantly lower in obese and overweight children compared with controls (1.28 ± 0.24 versus 1.96 ± 0.79) |
Importantly, the Endo-PAT measures microvascular endothelial function, whereas FMD assesses endothelial function at the level of larger conduit arteries. Therefore, discrepant results are conceivable. Although Endo-PAT and FMD correlate well in healthy subjects [135], in patients with chest pain [136] and with coronary artery disease [137] such comparisons between the two techniques in both healthy and obese children are still missing.
Pubertal development and its associated hormonal changes complicate the study of endothelial function in children. RHI increases during puberty in both genders [138] and correlates to changes in estradiol and dehydroepiandrosterone sulfate in peripheral blood [139]. Both hormones upregulate eNOS concentration and activity [140].
Chen et al. demonstrated that age influences timing of the peak response of endothelial-dependent dilation measured with PAT [127] and noted shorter time to peak after 3-year followup, similar to FMD. These authors therefore propose to analyze the entire hyperemic response curve, instead of focusing on a very specific postocclusion time interval. By calculating the area under the curve, the problem of variability regarding time to peak dilation may be circumvented.
4. Cell-Based Methods for Evaluating Endothelial Dysfunction
The cell-derived methods mentioned hereafter are considered as biomarkers for endothelial status justifying their discussion in this review. Traditional CV risk factors influence their numbers and function. Fundamental and translational research have demonstrated that these biomarkers are more than plain bystanders whose numbers drop or rise as a reflection of endothelial homeostasis, but rather appear to be active players in the process of endothelial damage and repair.
4.1. Endothelial Progenitor Cells
In 1997, Asahara et al, in a murine model of hind limb ischemia, described for the first time that CD34 (a stem cell marker) positive mononuclear cells, which were isolated from human peripheral blood, could selectively incorporate into new capillaries in areas of ischemic injury resulting into neovascularization of the affected limb [141]. These cells were termed Endothelial Progenitor Cells (EPC). Since then, numerous experiments have investigated the biological characteristics of EPC. It has become clear that the original term EPC actually covers 3 different cell types, denoted as Endothelial Cell Colony Forming Unit (CFU-EC), Circulating Angiogenic Cells (CAC), and “true” EPC. In this paper, only the latter 2 will be given further consideration, because great controversies still exist on the origin, the proliferative potential, and the differentiation capacity of CFU-EC [142].
True EPCs, alternatively called Endothelial Colony Forming Cells (ECFC), are released upon stimulation from bone marrow into the peripheral circulation and can incorporate into damaged endothelium (Figure 2) [143]. They are believed to be responsible for vasculogenesis and, as such, most closely fulfill the criteria for an Endothelial Progenitor Cell. EPCs appear in cultures of mononuclear cells after 7–21 days, have a cobblestone morphology, high proliferative capacity [144], and are able to form vessels in vivo [142]. Further analysis demonstrated that these cells express CD34 and Vascular Endothelial Growth Factor Receptor-2 (VEGFR-2) but not CD133 or CD45 (a pan-leukocytic marker) [145], even not at the mRNA level as was demonstrated by Case et al. [146].
Figure 2.
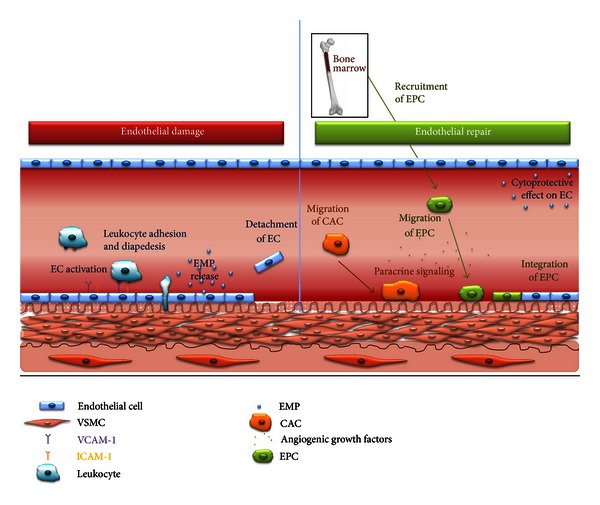
Mechanisms involved in endothelial damage and repair. Upon activation endothelial cells express adhesion molecules (i.e.; VCAM-1 and ICAM-1), which allow leukocytes to adhere, transmigrate, and initiate a cascade of inflammatory reactions and the release of EMP into the circulation. With significant endothelial damage, cells become senescent and are detached. This ultimately leads to the recruitment of CAC, monocyte-macrophage-derived cells that contribute to vascular repair by adhering to loci of endothelial damage, and producing angiogenic cytokines that induce the mobilization of EPC from the bone marrow. The angiogenic cytokines produced by CAC also serve as homing molecules with a chemotactic effect on EPC. As a consequence, EPC migrate to damaged endothelium and eventually integrate into the endothelial cell layer. Besides being released after endothelial activation, EMPs also contributes to endothelial homeostasis by cytoprotective effects on endothelial cells, including reduced apoptosis. EC, Endothelial Cell; VSMC, Vascular Smooth Muscle Cell; VCAM-1, Vascular Cell Adhesion Molecule 1; ICAM-1, InterCellular Adhesion Molecule 1; EMP, Endothelial MicroParticles; CAC, Circulating Angiogenic Cells; EPC, Endothelial Progenitor Cells.
The mechanism by which EPCs are released from the bone marrow is not fully understood but is the result of Matrix MetalloProteinase (MMP)-9 activation [147], in a NO-dependent mechanism [148]. As a consequence, reduced NO bioavailability not only leads to endothelial dysfunction, but also to the impaired recruitment of EPC to the loci of damaged endothelium as well, starting a vicious circle as described by Van Craenenbroeck and Conraads [149].
The technique most commonly used to quantify EPC is flow cytometry. Although this method is technically challenging due to very low numbers of circulating EPC, it is minimally invasive (blood sample via venipuncture) and therefore very convenient in children. Since there is no single, specific marker to identify EPC [150], a combination of markers is applied. Many different protocols have been developed between laboratories, with, however, poor intermethod agreement [151], which may have contributed to conflicting results in the literature. Schmidt-Lucke et al. introduced a modified International Society of Hematotherapy and Graft Engineering (ISHAGE) protocol for CD34+/KDR+ EPC enumeration gated on the basis of low SSC and low-to-bright CD45 fluorescence [152]. The authors concluded that it is in fact the CD45dim positive CD34+/KDR+ PC that correlate best with clinical characteristics of the studied patients with coronary artery disease. This protocol was presented to facilitate interlaboratory comparison and speed up EPC enumeration, yet technical recommendations for rare event analysis were not taken into account [153].
Since mature endothelial cells have limited regenerative capacity, EPC are necessary for endothelial repair. This notion is supported by a mathematical model of endothelial maintenance, which predicted a critical phase of endothelial cell defects that causes serious vascular damage. Such devastating consequences can be significantly delayed by incorporation of EPC [154]. Further in vivo evidence is provided by the fact that lower numbers of circulating EPC predict CV events and death in adults [155, 156] and by data showing that human EPCs are able to form fused vessels when implanted in immunodeficient mice [142].
Using flow cytometry it was demonstrated that EPC numbers correlate with endothelial function in CAD patients [157], in patients with type 1 diabetes [158], in young smokers [159], and in healthy subjects [160].
Müller-Ehmsen J et al. noted lower numbers of EPC in obese volunteers compared to healthy participants, while they observed a significant increase in EPC levels after weight loss [161]. Besides the direct effect of obesity, several other studies demonstrated reduced numbers of EPC in patients with obesity-related comorbidities such as hypertension [162, 163], hypercholesterolemia [164], and type II diabetes mellitus [165, 166].
Research in obese children has shown conflicting results. To our knowledge, there are only two papers comparing numbers of endothelial progenitor cells in obese children and lean controls. Unfortunately, each group used different protocols and different markers to detect these cells. Jung et al. were the first to investigate numbers of EPC in obese adolescents. They did not find significant differences in CD34+/KDR+/CD133+-cells, but did mention higher numbers of CD34-/KDR+CD133+-cells in overweight adolescents [167]. However, it has been shown that neither of these cells give rise to EPC in culture [146].
Arnold C et al. investigated whether numbers of circulating EPC related to physical fitness in obese children. Maximal oxygen uptake significantly correlated with CD34+ (r = 0.458) and CD133+/CD34+ cells (r = 0.456) in this population, yet the endothelial marker (KDR) was not assessed in this study [168].
In conclusion, studies comparing numbers of EPC between healthy and obese children that include CD45 as a marker and correlating their numbers to endothelial function in this specific population are not available and are eagerly awaited.
4.2. Circulating Angiogenic Cells
Circulating Angiogenic Cells (CAC) are grown from peripheral blood mononuclear cells after 4 to 7 days of culture in endothelial promoting media and fibronectin-coated plates. CACs have a low proliferative capacity and do not incorporate into endothelium, yet these cells restore damaged endothelium in a paracrine fashion by aiding in the recruitment and proliferation of, respectively, EPC and endothelial cells. CACs do not demonstrate an endothelial phenotype [169], but are hematopoietic cells, which closely resemble activated M2 monocytes [170]. Cell culture is the most widely used technique to determine their numbers and allows researchers to further explore their physiological activity. The migratory capacity towards Vascular Endothelial Growth Factor (VEGF) and Stromal cell-Derived Factor (SDF)-1α is commonly quantified using 7-day-old CAC cultures [171]. Both VEGF and SDF-1α recruit CAC to hypoxic tissues [172]. After an additional 3 days of culture, the supernatant can be collected and analyzed for protein secretion (such as VEGF) [173], and thus paracrine activity can be evaluated.
Numbers of CAC and their migratory capacity correlate with endothelial function [174]. Obese adults have lower numbers of CAC compared to healthy controls [175] and these cells display lower migratory capacity and reduced secretion of angiogenic growth factors, which can be reversed by weight loss [176]. However, results for obese children are still lacking.
NO is necessary for the migration of CAC towards VEGF [177], and therefore reduction in eNOS activity and reduced bioavailability of NO in obese patients could explain impaired migratory capacity of CAC in obese patients [178].
Leptin stimulates the migratory capacity of CAC, yet CACs of obese patients display a resistance against the leptin associated pro-angiogenic effect, which again is reversed by weight loss. Leptin resistance has been attributed to higher concentrations of Protein Tyrosine Phosphatase 1B (PTP-1B), a known inhibitor of the leptin signaling cascade. Higher levels of PTP-1B were seen in obese individuals, which returned to levels comparable to lean subjects after weight loss. Furthermore, blocking PTP-1B activity pharmacologically restored responsiveness to leptin in CAC of obese patients [176].
Adiponectin similarly enhances the migratory capacity of CAC towards SDF-1α [62]. This finding has been attributed to an upregulation of the receptor for SDF-1α, C-X-C Chemokine Receptor type 4 (CXCR-4) on CAC. Lower concentrations of adiponectin, seen in obese, could therefore further contribute to the functional deficit of CAC.
4.3. Endothelial MicroParticles
Endothelial MicroParticles (EMP) are small particles (100 nm to 1 μm) shed from the plasma membrane by endothelial cells upon damage, activation, or apoptosis [179]. These microparticles are covered with surface antigens from the parental endothelial cell, making quantification as well as identification of the underlying process for their generation possible using specific flow cytometry markers.
Initially, EMP were considered as indicators of endothelial disruption [180], but their role has been redefined; instead of being mere markers, they also appear to elicit physiological effects. On the one hand, EMP themselves can further compromise endothelial function [181], whereas, on the other hand, they exert beneficial effects on endothelial cell survival and even promote endothelial regeneration [182].
Interestingly, EMP also act as vectors carrying RNA (including microRNA), DNA, and proteins to target cells [183]. Several studies have shown that the content of EMP depends on the trigger by which their parental cells were stimulated [184, 185].
The exact mechanisms that regulate the release of EMP in vivo are not completely understood, but involve an end in membrane phospholipid asymmetry and the expression of phosphatidylserine on the outside of the cell membrane, membrane budding, and eventually microparticle release.
EMPs are widely identified by their constitutive expression of CD31 (platelet endothelial cell adhesion molecule), but not CD42b. The absence of the CD42b (platelet-specific glycoprotein Ib marker) marker is used to avoid potential contamination with platelet microparticles that have the same size range as EMP. EMPs are to be detected in platelet poor plasma that was subjected to sequential centrifugation. In fact, careful attention has to be paid to all preanalytical and analytical steps, as variables in both phases may affect accurate microparticle enumeration and could be an important source of variability, interlaboratory discrepancies, and artifacts [186, 187].
Numbers of EMP are elevated in obese women and inversely correlate with endothelial function [188]. Van Ierssel et al. noted about 100 to 400 EMP per μL blood in healthy volunteers [189], yet numbers may vary depending on methods used for sample preparation and analysis.
Data on EMP in children are scarce. Siklar et al. [190] provided indirect evidence that EMP numbers are higher in obese versus healthy children. By using a commercially available test (STA-PROCOAG-PPL Kit (Diagnostica Stago SAS, France)), they measured the procoagulant activity of phospholipids through assembly of the prothrombinase complex. Healthy children had a significantly longer microparticle release time, which was presumably due to a lower number of EMPs. Furthermore, Gündüz et al. showed that circulating vascular endothelial cadherin (CD144)+EMP are higher in obese and overweight children than in lean controls [191], but unfortunately, endothelial function was not assessed in this study. Further research is necessary to investigate the potential role of EMP as a biomarker for endothelial damage in obese children.
5. Pharmacological and Nonpharmacological Interventions to Restore Endothelial Damage
5.1. Weight Loss
The ultimate goal of therapy remains improvement of long-term physical health through permanent healthy lifestyle habits. Although weight maintenance is advised for younger children and a decline in BMI is achieved through growth, weight loss remains the cornerstone of therapy for obesity in adolescents [192].
Weight loss leads to an improvement of CV risk factors associated with endothelial dysfunction in childhood obesity [193, 194]. Because many studies have focused on the effect of multidisciplinary treatment approaches, little is known about the effect of weight loss alone on endothelial function in obese children. Kaufman et al. [195] studied the effect of a 5-month dietary intervention with a goal of a 5 to 8% decrease in total body mass in 15 overweight children. The study group consisted of six boys and nine girls (aged 11.4 ± 0.5 years). After the intervention, a significant decrease in weight, body fat percentage, and BMI was observed, with a trend towards improvement of endothelial function.
Woo et al. [196] compared the effects of weight loss alone to a combined treatment arm of diet and 6 weeks of exercise training in 82 overweight children, 9 to 12 years of age. In this study, an improvement of endothelial function was seen in both groups, albeit more pronounced in the diet plus exercise group (P = 0.01).
5.2. Exercise
Exercise training appears more effective than weight loss alone to improve endothelial function in obese children. As little as eight weeks of exercise training consisting of three 1-hour sessions of circuit training each week led to a significant improvement in endothelial function, even without weight loss in a randomized cross-over study involving 19 obese adolescents [197]. Two other studies, one involving endurance training and another applying aerobic interval training, confirmed the effect of exercise alone on endothelial function [198, 199].
In addition to the effect on factors leading to endothelial dysfunction [200], exercise training can more directly affect endothelial function through its effect on endothelial shear stress. Translational research using arterial biopsies of adults demonstrated that regular exercise leads to an upregulated expression of eNOS mRNA and higher eNOS protein and increased eNOS -phosphorylation levels in endothelial cells [201].
On top of increasing NO synthesis, exercise decreases production of ROS by reducing Nicotinamide Adenine Dinucleotide Phosphate (NAD(P)H) oxidase activity [202] and by enhancement of antioxidant capacity [203]. In addition, elevated shear stress, as seen with regular exercise training, can increase levels of BH4 [204] and thereby reduces eNOS uncoupling.
Exercise training has a significant effect on endothelial repair mechanisms, including both EPC and CAC. In adults with metabolic syndrome, 8 weeks of exercise training led to a significant increase in repair capacity of CAC [205]. These findings were confirmed by transplantation of human EPC into nude mice with defined carotid endothelial injury. In adults, a multitreatment approach including physical activity restored functional impairment of CAC [176]. In children, a program consisting of 12 weeks of exercise training increased the percentages of CD34+, CD133+ and CD34+/CD133+ cells and reduced carotid IMT [206]. In addition, 1 year of exercise training led to a significant rise in CD45low/CD34+/KDR+ EPC [207].
5.3. Pharmacological Interventions
Studies on the effect of pharmacological therapy in reversing endothelial dysfunction in children are very scarce. Therefore, potential candidates investigated in obese adults will be briefly discussed.
Since the withdrawal of both rimonabant and sibutramine, due the increased risk of psychiatric adverse events [208] and increased CV risk [209], respectively, orlistat a reversible blocker of lipase, is the only drug still available to aid weight loss. A recent meta-analysis of available data in children points out that the drug leads to 5 kg weight loss and 5 cm reduction in weight circumference after at least 6 months of therapy compared with placebo, but no improvement in the lipid profile nor insulin level was observed [210]. An open-label 3-month trial in adults consisting of a calorie-restricted diet and 120 mg of orlistat could not demonstrate a significant improvement in FMD [211].
Metformin is approved in many countries to treat insulin resistance in obese children. Although 3 months of metformin ingestion significantly improved both endothelial function and insulin resistance in adults with metabolic syndrome [212], adding metformin to a structured lifestyle intervention did not reverse insulin resistance in children [213].
5.4. Dietary Components
Dietary components have recently gained significant interest because of their potential benefit on endothelial function and presumed prevention of CV disease.
Adding vitamins C (500 mg/d) and E (400 IU/d) for 6 weeks to a program of diet and exercise further improved endothelial function in hyperlipidemic children [93]. Dangardt et al. were able to show that 3 months of omega-3 fatty acid supplementation improved vascular function in obese adolescents [27].
Although results from several randomized controlled trials with dietary components are quite promising, effects need to be confirmed in larger population-based studies.
5.5. Future Research Needs
As mentioned earlier in the paper, children are not just small adults and the speed by which physiological changes occur during puberty is tremendous. Puberty modulates endothelial function, and this improvement seems to be mediated through hormonal changes. The underlying mechanisms are not yet completely understood and deserve further investigation, as it may reveal key factors capable of ameliorating endothelial function later in life.
Further observation and perfectioning of flow cytometry and culture techniques have made EPC enumeration even more reliable. Yet studies on childhood and adolescent obesity that have adequately implemented these optimized strategies are lacking. In addition, the relationship of EPC and EMP with endothelial function also needs to be fully addressed in obese and overweight children.
And last but not least, whereas pharmacological interventions in obese children have been largely disappointing, inclusion of specific dietary components has shown to be highly effective in improving endothelial function in the short term. Further research, however, is needed to confirm whether these beneficial effects still remain at long-term followup.
6. Conclusions
Recent research has demonstrated the relevance of obesity-induced endothelial dysfunction, both in adults and in children. Fundamental and translational studies have led to significant understanding of cellular and molecular alterations held responsible for endothelial disruption. Unfortunately, research has until now mainly focused on the progression of atherosclerosis in obese adults, who have been exposed to the consequences of endothelial dysfunction during many years. Despite technical difficulties and ethical concerns, the investigation of endothelial function in obese children is a necessary step to further examine the initiation and progression of endothelial dysfunction, which is crucial to the development of treatment strategies.
Use of novel circulating markers can further unravel the delicate balance between endothelial damage and repair that causes endothelial dysfunction in obese subjects.
Health care management should aim to avoid the catastrophic rise in CV morbidity and mortality, which will accompany obese children into adulthood. Therefore, multi-disciplinary prevention programs need to be set up and tested for their clinical effect. In order to speed up advances achieved in this domain, clinical research will largely depend on the effect of such interventions on the so-called surrogate endpoints, such as the correction of endothelial function.
Acknowledgments
This work was supported by an interdisciplinary doctoral grant from the University Research Fund (BOF-ID) of the University of Antwerp to V. M. Conraads, J. Ramet, D. K. Vissers, and L. Bruyndonckx. V. M. Conraads is a senior clinical investigator supported by the Fund for Scientific Research (FWO), Flanders (Belgium). The authors kindly thank Dr. Ilse Van Brussel (Laboratory of Physiopharmacology, Faculty of Medicine and Health Sciences, Antwerp University, Belgium) for providing images of endothelial and smooth muscle cells to prepare Figure 2.
References
- 1.Andersson C, van Gaal L, Caterson ID, et al. Relationship between HbA1c levels and risk of cardiovascular adverse outcomes and all-cause mortality in overweight and obese cardiovascular high-risk women and men with type 2 diabetes. Diabetologia. 2012;55(9):2348–2355. doi: 10.1007/s00125-012-2584-3. [DOI] [PubMed] [Google Scholar]
- 2.Verhulst SL, Schrauwen N, Haentjens D, et al. Sleep-disordered breathing and the metabolic syndrome in overweight and obese children and adolescents. Journal of Pediatrics. 2007;150(6):608–612. doi: 10.1016/j.jpeds.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 3.Sharma A, Grummer-Strawn LM, Dalenius K, et al. Obesity prevalence among low-income, preschool-aged children—United States, 1998–2008. The Journal of the American Medical Association. 2010;303(1):28–30. [Google Scholar]
- 4.Srinivasan SR, Bao W, Wattigney WA, Berenson GS. Adolescent overweight is associated with adult overweight and related multiple cardiovascular risk factors: the Bogalusa heart study. Metabolism. 1996;45(2):235–240. doi: 10.1016/s0026-0495(96)90060-8. [DOI] [PubMed] [Google Scholar]
- 5.Tirosh A, Shai I, Afek A, et al. Adolescent BMI trajectory and risk of diabetes versus coronary disease. The New England Journal of Medicine. 2011;364(14):1315–1325. doi: 10.1056/NEJMoa1006992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. The New England Journal of Medicine. 2010;362(6):485–493. doi: 10.1056/NEJMoa0904130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. The New England Journal of Medicine. 2011;365(20):1876–1885. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- 8.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111(3):363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 9.Asahara T, Kawamoto A, Masuda H. Concise review: circulating endothelial progenitor cells for vascular medicine. Stem Cells. 2011;29(11):1650–1655. doi: 10.1002/stem.745. [DOI] [PubMed] [Google Scholar]
- 10.Viera AJ, Mooberry M, Key NS. Microparticles in cardiovascular disease pathophysiology and outcomes. Journal of the American Society of Hypertension. 2012;6(4):243–252. doi: 10.1016/j.jash.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 12.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399(6736):601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 13.Flammer AJ, Lüscher TF. Human endothelial dysfunction: EDRFs. Pflügers Archiv: European Journal of Physiology. 2010;459(6):1005–1013. doi: 10.1007/s00424-010-0822-4. [DOI] [PubMed] [Google Scholar]
- 14.Virdis A, Ghiadoni L, Taddei S. Human endothelial dysfunction: EDCFs. Pflügers Archiv: European Journal of Physiology. 2010;459(6):1015–1023. doi: 10.1007/s00424-009-0783-7. [DOI] [PubMed] [Google Scholar]
- 15.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. The New England Journal of Medicine. 2005;352(16):1685–1626. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 16.Deanfield J, Donald A, Ferri C, et al. Endothelial function and dysfunction—part I: methodological issues for assessment in the different vascular beds: a statement by the working group on endothelin and endothelial factors of the European society of hypertension. Journal of Hypertension. 2005;23(1):7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Vanhoutte PM. Endothelial dysfunction—the first step toward coronary arteriosclerosis. Circulation Journal. 2009;73(4):595–601. doi: 10.1253/circj.cj-08-1169. [DOI] [PubMed] [Google Scholar]
- 18.Falaschetti E, Hingorani AD, Jones A, et al. Adiposity and cardiovascular risk factors in a large contemporary population of pre-pubertal children. European Heart Journal. 2010;31(24):3063–3072. doi: 10.1093/eurheartj/ehq355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimbo D, Muntner P, Mann D, et al. Endothelial dysfunction and the risk of hypertension: the multi-ethnic study of atherosclerosis. Hypertension. 2010;55(5):1210–1216. doi: 10.1161/HYPERTENSIONAHA.109.143123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juonala M, Viikari JSA, Rönnemaa T, Helenius H, Taittonen L, Raitakari OT. Elevated blood pressure in adolescent boys predicts endothelial dysfunction: the cardiovascular risk in young finns study. Hypertension. 2006;48(3):424–430. doi: 10.1161/01.HYP.0000237666.78217.47. [DOI] [PubMed] [Google Scholar]
- 21.Charakida M, Jones A, Falaschetti E, et al. Childhood obesity and vascular phenotypes: a population study. Journal of the American College of Cardiology. 2012;60(25):2643–2650. doi: 10.1016/j.jacc.2012.08.1017. [DOI] [PubMed] [Google Scholar]
- 22.Koskinen J, Kähönen M, Viikari JSA, et al. Conventional cardiovascular risk factors and metabolic syndrome in predicting carotid intima-media thickness progression in young adults: the cardiovascular risk in young finns study. Circulation. 2009;120(3):229–236. doi: 10.1161/CIRCULATIONAHA.108.845065. [DOI] [PubMed] [Google Scholar]
- 23.Juonala M, Viikari JSA, Laitinen T, et al. Interrelations between brachial endothelial function and carotid intima-media thickness in young adults: the cardiovascular risk in young finns study. Circulation. 2004;110(18):2918–2923. doi: 10.1161/01.CIR.0000147540.88559.00. [DOI] [PubMed] [Google Scholar]
- 24.Mahmud FH, Hill DJ, Cuerden MS, Clarson CL. Impaired vascular function in obese adolescents with insulin resistance. Journal of Pediatrics. 2009;155(5):678–682. doi: 10.1016/j.jpeds.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 25.Metzig AM, Schwarzenberg SJ, Fox CK, Deering MM, Nathan BM, Kelly AS. Postprandial endothelial function, inflammation, and oxidative stress in obese children and adolescents. Obesity. 2011;19(6):1279–1283. doi: 10.1038/oby.2010.318. [DOI] [PubMed] [Google Scholar]
- 26.Kelly AS, Metzig AM, Rudser KD, et al. Exenatide as a weight-loss therapy in extreme pediatric obesity: a randomized, controlled pilot study. Obesity. 2012;20(2):364–370. doi: 10.1038/oby.2011.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dangardt F, Osika W, Chen Y, et al. Omega-3 fatty acid supplementation improves vascular function and reduces inflammation in obese adolescents. Atherosclerosis. 2010;212(2):580–585. doi: 10.1016/j.atherosclerosis.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 28.Tryggestad JB, Thompson DM, Copeland KC, Short KR. Obese children have higher arterial elasticity without a difference in endothelial function: the role of body composition. Obesity. 2012;20(1):165–171. doi: 10.1038/oby.2011.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landgraf K, Friebe D, Ullrich T, et al. Chemerin as a mediator between obesity and vascular inflammation in children. Journal of Clinical Endocrinology and Metabolism. 2012;97(4):E556–E564. doi: 10.1210/jc.2011-2937. [DOI] [PubMed] [Google Scholar]
- 30.Loot AE, Schreiber JG, Fisslthaler B, Fleming I. Angiotensin II impairs endothelial function via tyrosine phosphorylation of the endothelial nitric oxide synthase. Journal of Experimental Medicine. 2009;206(13):2889–2896. doi: 10.1084/jem.20090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hijmering ML, Stroes ESG, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. Journal of the American College of Cardiology. 2002;39(4):683–688. doi: 10.1016/s0735-1097(01)01786-7. [DOI] [PubMed] [Google Scholar]
- 32.Eid HMA, Arnesen H, Hjerkinn EM, Lyberg T, Seljeflot I. Relationship between obesity, smoking, and the endogenous nitric oxide synthase inhibitor, asymmetric dimethylarginine. Metabolism. 2004;53(12):1574–1579. doi: 10.1016/j.metabol.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 33.Steinberg D, Witztum JL. Is the oxidative modification hypothesis relevant to human atherosclerosis? Do the antioxidant trials conducted to date refute the hypothesis? Circulation. 2002;105(17):2107–2111. doi: 10.1161/01.cir.0000014762.06201.06. [DOI] [PubMed] [Google Scholar]
- 34.Toth PP. Activation of intracellular signaling systems by high-density lipoproteins. Journal of Clinical Lipidology. 2010;4(5):376–381. doi: 10.1016/j.jacl.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Nofer JR, Kehrel B, Fobker M, Levkau B, Assmann G, Eckardstein AV. HDL and arteriosclerosis: beyond reverse cholesterol transport. Atherosclerosis. 2002;161(1):1–16. doi: 10.1016/s0021-9150(01)00651-7. [DOI] [PubMed] [Google Scholar]
- 36.Schwandt P, Bertsch T, Haas GM. Anthropometric screening for silent cardiovascular risk factors in adolescents: the PEP family heart study. Atherosclerosis. 2010;211(2):667–671. doi: 10.1016/j.atherosclerosis.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 37.Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Prevalence of uncomplicated obesity in an Italian obese population. Obesity Research. 2005;13(6):1116–1122. doi: 10.1038/oby.2005.130. [DOI] [PubMed] [Google Scholar]
- 38.Landmesser U. High density lipoprotein—should we raise it? Current Vascular Pharmacology. 2012;10(6):718–719. doi: 10.2174/157016112803520710. [DOI] [PubMed] [Google Scholar]
- 39.Paffenbarger RS, Jr., Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. The New England Journal of Medicine. 1993;328(8):538–545. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 40.Blair SN, Kohl HW, Barlow CE, Paffenbarger RS, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality: a prospective study of healthy and unhealthy men. The Journal of the American Medical Association. 1995;273(14):1093–1098. [PubMed] [Google Scholar]
- 41.Nocon M, Hiemann T, Müller-Riemenschneider F, Thalau F, Roll S, Willich SN. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. European Journal of Cardiovascular Prevention and Rehabilitation. 2008;15(3):239–246. doi: 10.1097/HJR.0b013e3282f55e09. [DOI] [PubMed] [Google Scholar]
- 42.Trost SG, Kerr LM, Ward DS, Pate RR. Physical activity and determinants of physical activity in obese and non-obese children. International Journal of Obesity. 2001;25(6):822–829. doi: 10.1038/sj.ijo.0801621. [DOI] [PubMed] [Google Scholar]
- 43.de Bourdeaudhuij I, Lefevre J, Deforche B, Wijndaele K, Matton L, Philippaerts R. Physical activity and psychosocial correlates in normal weight and overweight 11 to 19 year olds. Obesity Research. 2005;13(6):1097–1105. doi: 10.1038/oby.2005.128. [DOI] [PubMed] [Google Scholar]
- 44.Abbott RA, Harkness MA, Davies PSW. Correlation of habitual physical activity levels with flow-mediated dilation of the brachial artery in 5–10 year old children. Atherosclerosis. 2002;160(1):233–239. doi: 10.1016/s0021-9150(01)00566-4. [DOI] [PubMed] [Google Scholar]
- 45.Hopkins ND, Stratton G, Tinken TM, et al. Relationships between measures of fitness, physical activity, body composition and vascular function in children. Atherosclerosis. 2009;204(1):244–249. doi: 10.1016/j.atherosclerosis.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Pahkala K, Heinonen OJ, Lagström H, et al. Vascular endothelial function and leisure-time physical activity in adolescents. Circulation. 2008;118(23):2353–2359. doi: 10.1161/CIRCULATIONAHA.108.791988. [DOI] [PubMed] [Google Scholar]
- 47.Pahkala K, Heinonen OJ, Simell O, et al. Association of physical activity with vascular endothelial function and intima-media thickness. Circulation. 2011;124(18):1956–1963. doi: 10.1161/CIRCULATIONAHA.111.043851. [DOI] [PubMed] [Google Scholar]
- 48.Otto TC, Lane MD. Adipose development: from stem cell to adipocyte. Critical Reviews in Biochemistry and Molecular Biology. 2005;40(4):229–242. doi: 10.1080/10409230591008189. [DOI] [PubMed] [Google Scholar]
- 49.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. Journal of Clinical Endocrinology and Metabolism. 2004;89(6):2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 50.van Gaal LF, Mertens IL, de Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 51.Lijnen HR. Angiogenesis and obesity. Cardiovascular Research. 2008;78(2):286–293. doi: 10.1093/cvr/cvm007. [DOI] [PubMed] [Google Scholar]
- 52.Hajer GR, van Haeften TW, Visseren FLJ. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. European Heart Journal. 2008;29(24):2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 53.Christiaens V, Lijnen HR. Angiogenesis and development of adipose tissue. Molecular and Cellular Endocrinology. 2010;318(1-2):2–9. doi: 10.1016/j.mce.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 54.Friedman JM. The function of leptin in nutrition, weight, and physiology. Nutrition Reviews. 2002;60(10, part 2):S1–S14. doi: 10.1301/002966402320634878. [DOI] [PubMed] [Google Scholar]
- 55.Vecchione C, Maffei A, Colella S, et al. Leptin effect on endothelial nitric oxide is mediated through Akt-endothelial nitric oxide synthase phosphorylation pathway. Diabetes. 2002;51(1):168–173. doi: 10.2337/diabetes.51.1.168. [DOI] [PubMed] [Google Scholar]
- 56.Park HY, Kwon HM, Lim HJ, et al. Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Experimental and Molecular Medicine. 2001;33(2):95–102. doi: 10.1038/emm.2001.17. [DOI] [PubMed] [Google Scholar]
- 57.Singh M, Bedi US, Singh PP, Arora R, Khosla S. Leptin and the clinical cardiovascular risk. International journal of cardiology. 2010;140(3):266–271. doi: 10.1016/j.ijcard.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 58.Singhal A, Farooqi S, Cole TJ, et al. Influence of leptin on arterial distensibility: a novel link between obesity and cardiovascular disease? Circulation. 2002;106(15):1919–1924. doi: 10.1161/01.cir.0000033219.24717.52. [DOI] [PubMed] [Google Scholar]
- 59.Torigoe M, Matsui H, Ogawa Y, et al. Impact of the high-molecular-weight form of adiponectin on endothelial function in healthy young men. Clinical Endocrinology. 2007;67(2):276–281. doi: 10.1111/j.1365-2265.2007.02876.x. [DOI] [PubMed] [Google Scholar]
- 60.Wang ZV, Scherer PE. Adiponectin, cardiovascular function, and hypertension. Hypertension. 2008;51(1):8–14. doi: 10.1161/HYPERTENSIONAHA.107.099424. [DOI] [PubMed] [Google Scholar]
- 61.Ouedraogo R, Wu X, Xu SQ, et al. Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: evidence for involvement of a cAMP signaling pathway. Diabetes. 2006;55(6):1840–1846. doi: 10.2337/db05-1174. [DOI] [PubMed] [Google Scholar]
- 62.Adams V, Höllriegel R, Beck EB, et al. Adiponectin promotes the migration of circulating angiogenic cells through p38-mediated induction of the CXCR4 receptor. International Journal of Cardiology. 2012 doi: 10.1016/j.ijcard.2012.05.056. [DOI] [PubMed] [Google Scholar]
- 63.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of AMP-activated protein kinase signaling. The Journal of Biological Chemistry. 2004;279(27):28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 64.Eren P, Camus S, Matrone G, et al. Adiponectinemia controls pro-angiogenic cell therapy. Stem Cells. 2009;27(11):2712–2721. doi: 10.1002/stem.219. [DOI] [PubMed] [Google Scholar]
- 65.Faber DR, van der Graaf Y, Westerink J, Visseren FLJ. Increased visceral adipose tissue mass is associated with increased C-reactive protein in patients with manifest vascular diseases. Atherosclerosis. 2010;212(1):274–280. doi: 10.1016/j.atherosclerosis.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 66.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. The New England Journal of Medicine. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 67.Fichtlscherer S, Rosenberger G, Walter DH, Breuer S, Dimmeler S, Zeiher AM. Elevated C-reactive protein levels and impaired endothelial vasoreactivity in patients with coronary artery disease. Circulation. 2000;102(9):1000–1006. doi: 10.1161/01.cir.102.9.1000. [DOI] [PubMed] [Google Scholar]
- 68.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148(2):209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 69.Wassmann S, Stumpf M, Strehlow K, et al. Interleukin-6 induces oxidative stress and endothehal dysfunction by overexpression of the angiotensin II type 1 receptor. Circulation Research. 2004;94(4):534–541. doi: 10.1161/01.RES.0000115557.25127.8D. [DOI] [PubMed] [Google Scholar]
- 70.d’Uscio LV, Milstien S, Richardson D, Smith L, Katusic ZS. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circulation Research. 2003;92(1):88–95. doi: 10.1161/01.res.0000049166.33035.62. [DOI] [PubMed] [Google Scholar]
- 71.Landmesser U, Dikalov S, Price SR, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. Journal of Clinical Investigation. 2003;111(8):1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang ZY, Lin YW, Clemont A, et al. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. Journal of Clinical Investigation. 1999;104(4):447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Potenza MA, Marasciulo FL, Chieppa DM, et al. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. The American Journal of Physiology. 2005;289(2):H813–H822. doi: 10.1152/ajpheart.00092.2005. [DOI] [PubMed] [Google Scholar]
- 74.Montagnani M, Golovchenko I, Kim I, et al. Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. The Journal of Biological Chemistry. 2002;277(3):1794–1799. doi: 10.1074/jbc.M103728200. [DOI] [PubMed] [Google Scholar]
- 75.Kim JA, Montagnani M, Kwang KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113(15):1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 76.Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNFα and vascular oxidative stress. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29(8):1164–1171. doi: 10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guzik TJ, Mussa S, Gastaldi D, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105(14):1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 78.Cheang WS, Wong WT, Tian XY, et al. Endothelial nitric oxide synthase enhancer reduces oxidative stress and restores endothelial function in db/db mice. Cardiovascular Research. 2011;92(2):267–275. doi: 10.1093/cvr/cvr233. [DOI] [PubMed] [Google Scholar]
- 79.Morita M, Yano S, Yamaguchi T, Sugimoto T. Advanced glycation end products-induced reactive oxygen species generation is partly through NF-κB activation in human aortic endothelial cells. Journal of Diabetes and its Complications. 2013;27(1):11–15. doi: 10.1016/j.jdiacomp.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 80.Gogg S, Smith U, Jansson PA. Increased MAPK activation and impaired insulin signaling in subcutaneous microvascular endothelial cells in type 2 diabetes: the role of endothelin-1. Diabetes. 2009;58(10):2238–2245. doi: 10.2337/db08-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rafnsson A, Böhm F, Settergren M, Gonon A, Brismar K, Pernow J. The endothelin receptor antagonist bosentan improves peripheral endothelial function in patients with type 2 diabetes mellitus and microalbuminuria: a randomised trial. Diabetologia. 2012;55(3):600–607. doi: 10.1007/s00125-011-2415-y. [DOI] [PubMed] [Google Scholar]
- 82.Ludmer PL, Selwyn AP, Shook TL. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. The New England Journal of Medicine. 1986;315(17):1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 83.Halcox JPJ, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106(6):653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 84.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. The Lancet. 1992;340(8828):1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 85.Flammer AJ, Anderson T, Celermajer DS, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126(6):753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thijssen DHJ, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. The American Journal of Physiology. 2011;300(1):H2–H12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Doshi SN, Naka KK, Payne N, et al. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clinical Science. 2001;101(6):629–635. [PubMed] [Google Scholar]
- 88.Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011;57(3):363–369. doi: 10.1161/HYPERTENSIONAHA.110.167015. [DOI] [PubMed] [Google Scholar]
- 89.Charakida M, Masi S, Lüscher TF, Kastelein JJP, Deanfield JE. Assessment of atherosclerosis: the role of flow-mediated dilatation. European Heart Journal. 2010;31(23):2854–2861. doi: 10.1093/eurheartj/ehq340. [DOI] [PubMed] [Google Scholar]
- 90.Otto ME, Svatikova A, de Mattos Barretto RB, et al. Early morning attenuation of endothelial function in healthy humans. Circulation. 2004;109(21):2507–2510. doi: 10.1161/01.CIR.0000128207.26863.C4. [DOI] [PubMed] [Google Scholar]
- 91.Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. The American Journal of Cardiology. 1997;79(3):350–354. doi: 10.1016/s0002-9149(96)00760-6. [DOI] [PubMed] [Google Scholar]
- 92.Alexopoulos N, Vlachopoulos C, Aznaouridis K, et al. The acute effect of green tea consumption on endothelial function in healthy individuals. European Journal of Cardiovascular Prevention and Rehabilitation. 2008;15(3):300–305. doi: 10.1097/HJR.0b013e3282f4832f. [DOI] [PubMed] [Google Scholar]
- 93.Engler MM, Engler MB, Malloy MJ, et al. Antioxidant vitamins C and E improve endothelial function in children with hyperlipidemia: endothelial assessment of risk from lipids in youth (EARLY) trial. Circulation. 2003;108(9):1059–1063. doi: 10.1161/01.CIR.0000086345.09861.A0. [DOI] [PubMed] [Google Scholar]
- 94.Engler MB, Engler MM, Chen CY, et al. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. Journal of the American College of Nutrition. 2004;23(3):197–204. doi: 10.1080/07315724.2004.10719361. [DOI] [PubMed] [Google Scholar]
- 95.Harris RA, Nishiyama SK, Wray DW, Tedjasaputra V, Bailey DM, Richardson RS. The effect of oral antioxidants on brachial artery flow-mediated dilation following 5 and 10 min of ischemia. European Journal of Applied Physiology. 2009;107(4):445–453. doi: 10.1007/s00421-009-1147-x. [DOI] [PubMed] [Google Scholar]
- 96.Magen E, Viskoper JR, Mishal J, Priluk R, London D, Yosefy C. Effects of low-dose aspirin on blood pressure and endothelial function of treated hypertensive hypercholesterolaemic subjects. Journal of Human Hypertension. 2005;19(9):667–673. doi: 10.1038/sj.jhh.1001910. [DOI] [PubMed] [Google Scholar]
- 97.Charakida M, Donald AE, Terese M, et al. Endothelial dysfunction in childhood infection. Circulation. 2005;111(13):1660–1665. doi: 10.1161/01.CIR.0000160365.18879.1C. [DOI] [PubMed] [Google Scholar]
- 98.Ghiadoni L, Donald AE, Cropley M, et al. Mental stress induces transient endothelial dysfunction in humans. Circulation. 2000;102(20):2473–2478. doi: 10.1161/01.cir.102.20.2473. [DOI] [PubMed] [Google Scholar]
- 99.Donald AE, Charakida M, Falaschetti E, et al. Determinants of vascular phenotype in a large childhood population: the avon longitudinal study of parents and children (ALSPAC) European Heart Journal. 2010;31(12):1502–1510. doi: 10.1093/eurheartj/ehq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Celermajer DS, Sorensen KE, Georgakopoulos D, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88(5 I):2149–2155. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 101.Kallio K, Jokinen E, Raitakari OT, et al. Tobacco smoke exposure is associated with attenuated endothelial function in 11-year-old healthy children. Circulation. 2007;115(25):3205–3212. doi: 10.1161/CIRCULATIONAHA.106.674804. [DOI] [PubMed] [Google Scholar]
- 102.Juonala M, Magnussen CG, Venn A, et al. Parental smoking in childhood and brachial artery flow-mediated dilatation in young adults: the cardiovascular risk in young finns study and the childhood determinants of adult health study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(4):1024–1031. doi: 10.1161/ATVBAHA.111.243261. [DOI] [PubMed] [Google Scholar]
- 103.Peterson MD, Liu D, IglayReger HB, Saltarelli WA, Visich PS, Gordon PM. Principal component analysis reveals gender-specific predictors of cardiometabolic risk in 6th graders. Cardiovascular Diabetology. 2012;11(1, article 146) doi: 10.1186/1475-2840-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sarkola T, Manlhiot C, Slorach C, et al. Evolution of the arterial structure and function from infancy to adolescence is related to anthropometric and blood pressure changes. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(10):2516–2524. doi: 10.1161/ATVBAHA.112.252114. [DOI] [PubMed] [Google Scholar]
- 105.Hashimoto M, Akishita M, Eto M, et al. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation. 1995;92(12):3431–3435. doi: 10.1161/01.cir.92.12.3431. [DOI] [PubMed] [Google Scholar]
- 106.Järvisalo MJ, Rönnemaa T, Volanen I, et al. Brachial artery dilatation responses in healthy children and adolescents. The American Journal of Physiology. 2002;282(1 51-1):H87–H92. doi: 10.1152/ajpheart.2002.282.1.H87. [DOI] [PubMed] [Google Scholar]
- 107.Naylor LH, Green DJ, Jones TW, et al. Endothelial function and carotid intima-medial thickness in adolescents with type 2 diabetes mellitus. Journal of Pediatrics. 2011;159(6):971–974. doi: 10.1016/j.jpeds.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 108.Bhattacharjee R, Alotaibi WH, Kheirandish-Gozal L, Capdevila OS, Gozal D. Endothelial dysfunction in obese non-hypertensive children without evidence of sleep disordered breathing. BMC Pediatrics. 2010;10(article 8) doi: 10.1186/1471-2431-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bhattacharjee R, Kim J, Alotaibi WH, Kheirandish-Gozal L, Capdevila OS, Gozal D. Endothelial dysfunction in children without hypertension: potential contributions of obesity and obstructive sleep apnea. Chest. 2012;141(3):682–691. doi: 10.1378/chest.11-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Caballero AE, Bousquet-Santos R, Robles-Osorio L, et al. Overweight latino children and adolescents have marked endothelial dysfunction and subclinical vascular inflammation in association with excess body fat and insulin resistance. Diabetes Care. 2008;31(3):576–582. doi: 10.2337/dc07-1540. [DOI] [PubMed] [Google Scholar]
- 111.Ciccone MM, Miniello V, Marchioli R, et al. Morphological and functional vascular changes induced by childhood obesity. European Journal of Cardiovascular Prevention and Rehabilitation. 2011;18(6):831–835. doi: 10.1177/1741826711398180. [DOI] [PubMed] [Google Scholar]
- 112.Farpour-Lambert NJ, Aggoun Y, Marchand LM, Martin XE, Herrmann FR, Beghetti M. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. Journal of the American College of Cardiology. 2009;54(25):2396–2406. doi: 10.1016/j.jacc.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 113.Kapiotis S, Holzer G, Schaller G, et al. A proinflammatory state is detectable in obese children and is accompanied by functional and morphological vascular changes. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26(11):2541–2546. doi: 10.1161/01.ATV.0000245795.08139.70. [DOI] [PubMed] [Google Scholar]
- 114.Karpoff L, Vinet A, Schuster I, et al. Abnormal vascular reactivity at rest and exercise in obese boys. European Journal of Clinical Investigation. 2009;39(2):94–102. doi: 10.1111/j.1365-2362.2008.02068.x. [DOI] [PubMed] [Google Scholar]
- 115.Meyer AA, Kundt G, Steiner M, Schuff-Werner P, Kienast W. Impaired flow-mediated vasodilation, carotid artery intima-media thickening, and elevated endothelial plasma markers in obese children: the impact of cardiovascular risk factors. Pediatrics. 2006;117(5):1560–1567. doi: 10.1542/peds.2005-2140. [DOI] [PubMed] [Google Scholar]
- 116.Mimoun E, Aggoun Y, Pousset M, et al. Association of arterial stiffness and endothelial dysfunction with metabolic syndrome in obese children. Journal of Pediatrics. 2008;153(1):65.e1–70.e1. doi: 10.1016/j.jpeds.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 117.Peña AS, Belobrajdic DP, Wiltshire E, Gent R, Hirte C, Couper J. Adiponectin relates to smooth muscle function and folate in obese children. International Journal of Pediatric Obesity. 2010;5(2):185–191. doi: 10.3109/17477160903111748. [DOI] [PubMed] [Google Scholar]
- 118.Peña AS, Wiltshire E, MacKenzie K, et al. Vascular endothelial and smooth muscle function relates to body mass index and glucose in obese and nonobese children. Journal of Clinical Endocrinology and Metabolism. 2006;91(11):4467–4471. doi: 10.1210/jc.2006-0863. [DOI] [PubMed] [Google Scholar]
- 119.Tounian P, Aggoun Y, Dubern B, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. The Lancet. 2001;358(9291):1400–1404. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- 120.Woo KS, Chook P, Yu CW, et al. Overweight in children is associated with arterial endothelial dysfunction and intima-media thickening. International Journal of Obesity. 2004;28(7):852–857. doi: 10.1038/sj.ijo.0802539. [DOI] [PubMed] [Google Scholar]
- 121.Yilmazer MM, Tavli V, Carti OU, et al. Cardiovascular risk factors and noninvasive assessment of arterial structure and function in obese Turkish children. European Journal of Pediatrics. 2010;169(10):1241–1248. doi: 10.1007/s00431-010-1216-5. [DOI] [PubMed] [Google Scholar]
- 122.Zhu W, Huang X, He J, Li M, Neubauer H. Arterial intima-media thickening and endothelial dysfunction in obese Chinese children. European Journal of Pediatrics. 2005;164(6):337–344. doi: 10.1007/s00431-005-1642-y. [DOI] [PubMed] [Google Scholar]
- 123.Liu J, Wang J, Jin Y, Roethig HJ, Unverdorben M. Variability of peripheral arterial tonometry in the measurement of endothelial function in healthy men. Clinical Cardiology. 2009;32(12):700–704. doi: 10.1002/clc.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Widmer RJ, Freund MA, Flammer AJ, et al. Beneficial effects of polyphenol-rich olive oil in patients with early atherosclerosis. European Journal of Nutrition. 2012 doi: 10.1007/s00394-012-0433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nogueira LDP, Knibel MP, Torres MRSG, Nogueira Neto JF, Sanjuliani AF. Consumption of high-polyphenol dark chocolate improves endothelial function in individuals with stage 1 hypertension and excess body weight. International Journal of Hypertension. 2012;2012:9 pages. doi: 10.1155/2012/147321.147321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Miller RJ, Jackson KG, Dadd T, et al. The impact of the catechol-O-methyltransferase genotype on vascular function and blood pressure after acute green tea ingestion. Molecular Nutrition and Food Research. 2012;56(6):966–975. doi: 10.1002/mnfr.201100726. [DOI] [PubMed] [Google Scholar]
- 127.Chen Y, Dangardt F, Osika W, Berggren K, Gronowitz E, Friberg P. Age- and sex-related differences in vascular function and vascular response to mental stress. Longitudinal and cross-sectional studies in a cohort of healthy children and adolescents. Atherosclerosis. 2012;220(1):269–274. doi: 10.1016/j.atherosclerosis.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 128.Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham heart study. Circulation. 2008;117(19):2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. Journal of Applied Physiology. 2006;101(2):545–548. doi: 10.1152/japplphysiol.01285.2005. [DOI] [PubMed] [Google Scholar]
- 130.Osanai T, Fujita N, Fujiwara N, et al. Cross talk of shear-induced production of prostacyclin and nitric oxide in endothelial cells. The American Journal of Physiology. 2000;278(1):H233–H238. doi: 10.1152/ajpheart.2000.278.1.H233. [DOI] [PubMed] [Google Scholar]
- 131.Shimokawa H, Yasutake H, Fujii K, et al. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. Journal of Cardiovascular Pharmacology. 1996;28(5):703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 132.Sverrisdóttir YB, Jansson LM, Hägg U, Gan LM. Muscle sympathetic nerve activity is related to a surrogate marker of endothelial function in healthy individuals. PLoS ONE. 2010;5(2) doi: 10.1371/journal.pone.0009257.Article numbere9257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McCrea CE, Skulas-Ray AC, Chow M, West SG. Test-retest reliability of pulse amplitude tonometry measures of vascular endothelial function: implications for clinical trial design. Vascular Medicine. 2012;17(1):29–36. doi: 10.1177/1358863X11433188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tierney ESS, Newburger JW, Gauvreau K, et al. Endothelial pulse amplitude testing: feasibility and reproducibility in adolescents. Journal of Pediatrics. 2009;154(6):901–905. doi: 10.1016/j.jpeds.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 135.Dhindsa M, Sommerlad SM, DeVan AE, et al. Interrelationships among noninvasive measures of postischemic macro- and microvascular reactivity. Journal of Applied Physiology. 2008;105(2):427–432. doi: 10.1152/japplphysiol.90431.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. The American Heart Journal. 2003;146(1):168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 137.Onkelinx S, Cornelissen V, Goetschalckx K, Thomaes T, Verhamme P, Vanhees L. Reproducibility of different methods to measure the endothelial function. Vascular Medicine. 2012;17(2):79–84. doi: 10.1177/1358863X12436708. [DOI] [PubMed] [Google Scholar]
- 138.Radtke T, Khattab K, Eser P, Kriemler S, Saner H, Wilhelm M. Puberty and microvascular function in healthy children and adolescents. Journal of Pediatrics. 2012;161(5):887–891. doi: 10.1016/j.jpeds.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 139.Bhangoo A, Sinha S, Rosenbaum M, Shelov S, Ten S. Endothelial function as measured by peripheral arterial tonometry increases during pubertal advancement. Hormone Research in Paediatrics. 2011;76(4):226–233. doi: 10.1159/000328455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Duckles SP, Miller VM. Hormonal modulation of endothelial NO production. Pflügers Archiv: European Journal of Physiology. 2010;459(6):841–851. doi: 10.1007/s00424-010-0797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–966. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 142.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109(5):1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wassmann S, Werner N, Czech T, Nickenig G. Improvement of endothelial function by systemic transfusion of vascular progenitor cells. Circulation Research. 2006;99(8):E74–E83. doi: 10.1161/01.RES.0000246095.90247.d4. [DOI] [PubMed] [Google Scholar]
- 144.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104(9):2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 145.Timmermans F, van Hauwermeiren F, de Smedt M, et al. Endothelial outgrowth cells are not derived from CD133+ Cells or CD45+ hematopoietic precursors. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(7):1572–1579. doi: 10.1161/ATVBAHA.107.144972. [DOI] [PubMed] [Google Scholar]
- 146.Case J, Mead LE, Bessler WK, et al. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Experimental Hematology. 2007;35:1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 147.Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of Kit-ligand. Cell. 2002;109(5):625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Aicher A, Heeschen C, Mildner-Rihm C, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nature Medicine. 2003;9(11):1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 149.van Craenenbroeck EM, Conraads VM. Endothelial progenitor cells in vascular health: focus on lifestyle. Microvascular Research. 2010;79(3):184–192. doi: 10.1016/j.mvr.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 150.Yoder MC. Defining human endothelial progenitor cells. Journal of Thrombosis and Haemostasis. 2009;7(1):49–52. doi: 10.1111/j.1538-7836.2009.03407.x. [DOI] [PubMed] [Google Scholar]
- 151.van Craenenbroeck EMF, Conraads VMA, van Bockstaele DR, et al. Quantification of circulating endothelial progenitor cells: a methodological comparison of six flow cytometric approaches. Journal of Immunological Methods. 2008;332(1-2):31–40. doi: 10.1016/j.jim.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 152.Schmidt-Lucke C, Fichtlscherer S, Aicher A, et al. Quantification of circulating endothelial progenitor cells using the modified ISHAGE Protocol. PLoS ONE. 2010;5(11) doi: 10.1371/journal.pone.0013790.e13790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.van Craenenbroeck EM, van Craenenbroeck AH, van Ierssel S, et al. Quantification of circulating CD34+/KDR+/CD45dim endothelial progenitor cells: analytical considerations. International Journal of Cardiology. 2012 doi: 10.1016/j.ijcard.2012.10.047. [DOI] [PubMed] [Google Scholar]
- 154.den Buijs JO, Musters M, Verrips T, Post JA, Braam B, van Riel N. Mathematical modeling of vascular endothelial layer maintenance: the role of endothelial cell division, progenitor cell homing, and telomere shortening. The American Journal of Physiology. 2004;287(6):H2651–H2658. doi: 10.1152/ajpheart.00332.2004. [DOI] [PubMed] [Google Scholar]
- 155.Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. The New England Journal of Medicine. 2005;353(10):999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 156.Schmidt-Lucke C, Rössig L, Fichtlscherer S, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111(22):2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 157.Werner N, Wassmann S, Ahlers P, et al. Endothelial progenitor cells correlate with endothelial function in patients with coronary artery disease. Basic Research in Cardiology. 2007;102(6):565–571. doi: 10.1007/s00395-007-0680-1. [DOI] [PubMed] [Google Scholar]
- 158.Sibal L, Aldibbiat A, Agarwal SC, et al. Circulating endothelial progenitor cells, endothelial function, carotid intima-media thickness and circulating markers of endothelial dysfunction in people with type 1 diabetes without macrovascular disease or microalbuminuria. Diabetologia. 2009;52(8):1464–1473. doi: 10.1007/s00125-009-1401-0. [DOI] [PubMed] [Google Scholar]
- 159.Kim W, Myung HJ, Suk HC, et al. Effect of green tea consumption on endothelial function and circulating endothelial progenitor cells in chronic smokers. Circulation Journal. 2006;70(8):1052–1057. doi: 10.1253/circj.70.1052. [DOI] [PubMed] [Google Scholar]
- 160.Murphy C, Kanaganayagam GS, Jiang B, et al. Vascular dysfunction and reduced circulating endothelial progenitor cells in young healthy UK South Asian men. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(4):936–942. doi: 10.1161/01.ATV.0000258788.11372.d0. [DOI] [PubMed] [Google Scholar]
- 161.Müller-Ehmsen J, Braun D, Schneider T, et al. Decreased number of circulating progenitor cells in obesity: beneficial effects of weight reduction. European Heart Journal. 2008;29(12):1560–1568. doi: 10.1093/eurheartj/ehn213. [DOI] [PubMed] [Google Scholar]
- 162.Pirro M, Schillaci G, Menecali C, et al. Reduced number of circulating endothelial progenitors and HOXA9 expression in CD34+ cells of hypertensive patients. Journal of Hypertension. 2007;25(10):2093–2099. doi: 10.1097/HJH.0b013e32828e506d. [DOI] [PubMed] [Google Scholar]
- 163.Oliveras A, Soler MJ, Martínez-Estrada OM, et al. Endothelial progenitor cells are reduced in refractory hypertension. Journal of Human Hypertension. 2008;22(3):183–190. doi: 10.1038/sj.jhh.1002304. [DOI] [PubMed] [Google Scholar]
- 164.Rossi F, Bertone C, Montanile F, et al. HDL cholesterol is a strong determinant of endothelial progenitor cells in hypercholesterolemic subjects. Microvascular Research. 2010;80(2):274–279. doi: 10.1016/j.mvr.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 165.Fadini GP, Miorin M, Facco M, et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. Journal of the American College of Cardiology. 2005;45(9):1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 166.Egan CG, Lavery R, Caporali F, et al. Generalised reduction of putative endothelial progenitors and CXCR4-positive peripheral blood cells in type 2 diabetes. Diabetologia. 2008;51(7):1296–1305. doi: 10.1007/s00125-008-0939-6. [DOI] [PubMed] [Google Scholar]
- 167.Jung C, Fischer N, Fritzenwanger M, et al. Endothelial progenitor cells in adolescents: impact of overweight, age, smoking, sport and cytokines in younger age. Clinical Research in Cardiology. 2009;98(3):179–188. doi: 10.1007/s00392-008-0739-5. [DOI] [PubMed] [Google Scholar]
- 168.Arnold C, Wenta D, Mller-Ehmsen J, Sreeram N, Graf C. Progenitor cell number is correlated to physical performance in obese children and young adolescents. Cardiology in the Young. 2010;20(4):381–386. doi: 10.1017/S1047951109990278. [DOI] [PubMed] [Google Scholar]
- 169.Medina RJ, O’Neill CL, Sweeney M, et al. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Medical Genomics. 2010;3(1, article 18) doi: 10.1186/1755-8794-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Medina RJ, O'Neill CL, O'Doherty TM, et al. Myeloid angiogenic cells act as alternative M2 macrophages and modulate angiogenesis through interleukin-8. Molecular Medicine. 2011;(9-10):1045–1055. doi: 10.2119/molmed.2011.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.van Craenenbroeck EM, Hoymans VY, Beckers PJ, et al. Exercise training improves function of circulating angiogenic cells in patients with chronic heart failure. Basic Research in Cardiology. 2010;105(5):665–676. doi: 10.1007/s00395-010-0105-4. [DOI] [PubMed] [Google Scholar]
- 172.Hoenig MR, Bianchi C, Sellke FW. Hypoxia inducible factor-1α, endothelial progenitor cells, monocytes cardiovascular risk, wound healing, cobalt and hydralazine: a unifying hypothesis. Current Drug Targets. 2008;9(5):422–435. doi: 10.2174/138945008784221215. [DOI] [PubMed] [Google Scholar]
- 173.van Craenenbroeck EM, Beckers PJ, Possemiers NM, et al. Exercise acutely reverses dysfunction of circulating angiogenic cells in chronic heart failure. European Heart Journal. 2010;31(15):1924–1934. doi: 10.1093/eurheartj/ehq058. [DOI] [PubMed] [Google Scholar]
- 174.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. Journal of the American College of Cardiology. 2005;45(9):1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 175.MacEneaney OJ, Kushner EJ, van Guilder GP, Greiner JJ, Stauffer BL, DeSouza CA. Endothelial progenitor cell number and colony-forming capacity in overweight and obese adults. International Journal of Obesity. 2009;33(2):219–225. doi: 10.1038/ijo.2008.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Heida NM, Müller JP, Cheng IF, et al. Effects of obesity and weight loss on the functional properties of early outgrowth endothelial progenitor cells. Journal of the American College of Cardiology. 2010;55(4):357–367. doi: 10.1016/j.jacc.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 177.Heiss C, Schanz A, Amabile N, et al. Nitric oxide synthase expression and functional response to nitric oxide are both important modulators of circulating angiogenic cell response to angiogenic stimuli. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(11):2212–2218. doi: 10.1161/ATVBAHA.110.211581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Williams IL, Wheatcroft S, Shah AM, Kearney MT. Obesity, atherosclerosis and the vascular endothelium: mechanisms of reduced nitric oxide bioavailability in obese humans. International Journal of Obesity. 2002;26(6):754–764. doi: 10.1038/sj.ijo.0801995. [DOI] [PubMed] [Google Scholar]
- 179.Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(1):27–33. doi: 10.1161/ATVBAHA.110.218123. [DOI] [PubMed] [Google Scholar]
- 180.Boulanger CM, Amabile N, Tedgui A. Circulating microparticles: a potential prognostic marker for atherosclerotic vascular disease. Hypertension. 2006;48(2):180–186. doi: 10.1161/01.HYP.0000231507.00962.b5. [DOI] [PubMed] [Google Scholar]
- 181.Brodsky SV, Zhang F, Nasjletti A, Goligorsky MS. Endothelium-derived microparticles impair endothelial function in vitro. The American Journal of Physiology. 2004;286(5):H1910–H1915. doi: 10.1152/ajpheart.01172.2003. [DOI] [PubMed] [Google Scholar]
- 182.Leroyer AS, Ebrahimian TG, Cochain C, et al. Microparticles from ischemic muscle promotes postnatal vasculogenesis. Circulation. 2009;119(21):2808–2817. doi: 10.1161/CIRCULATIONAHA.108.816710. [DOI] [PubMed] [Google Scholar]
- 183.Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circulation Research. 2010;107(9):1047–1057. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- 184.Weerheim AM, Kolb AM, Sturk A, Nieuwland R. Phospholipid composition of cell-derived microparticles determined by one-dimensional high-performance thin-layer chromatography. Analytical Biochemistry. 2002;302(2):191–198. doi: 10.1006/abio.2001.5552. [DOI] [PubMed] [Google Scholar]
- 185.Huber J, Vales A, Mitulovic G, et al. Oxidized membrane vesicles and blebs from apoptotic cells contain biologically active oxidized phospholipids that induce monocyte-endothelial interactions. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22(1):101–107. doi: 10.1161/hq0102.101525. [DOI] [PubMed] [Google Scholar]
- 186.Lacroix R, Judicone C, Poncelet P, et al. Impact of pre-analytical parameters on the measurement of circulating microparticles: towards standardization of protocol. Journal of Thrombosis and Haemostasis. 2012;10(3):437–446. doi: 10.1111/j.1538-7836.2011.04610.x. [DOI] [PubMed] [Google Scholar]
- 187.van Ierssel SH, van Craenenbroeck EM, Conraads VM, et al. Flow cytometric detection of endothelial microparticles (EMP): effects of centrifugation and storage alter with the phenotype studied. Thrombosis Research. 2010;125(4):332–339. doi: 10.1016/j.thromres.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 188.Esposito K, Ciotola M, Schisano B, et al. Endothelial microparticles correlate with endothelial dysfunction in obese women. Journal of Clinical Endocrinology and Metabolism. 2006;91(9):3676–3679. doi: 10.1210/jc.2006-0851. [DOI] [PubMed] [Google Scholar]
- 189.van Ierssel SH, Hoymans VY, van Craenenbroeck EM, et al. Endothelial microparticles (EMP) for the assessment of endothelial function: an in vitro and in vivo study on possible interference of plasma lipids. PLoS ONE. 2012;7(2) doi: 10.1371/journal.pone.0031496.e31496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Siklar Z, Öçal G, Berberoğlu M, et al. Evaluation of hypercoagulability in obese children with thrombin generation test and microparticle release: effect of metabolic parameters. Clinical and Applied Thrombosis/Hemostasis. 2011;17(6):585–589. doi: 10.1177/1076029611404216. [DOI] [PubMed] [Google Scholar]
- 191.Gündüz Z, Dursun I, Tülpar S, et al. Increased endothelial microparticles in obese and overweight children. Journal of Pediatric Endocrinology and Metabolism. 2012;25(11-12):1111–1117. doi: 10.1515/jpem-2012-0194. [DOI] [PubMed] [Google Scholar]
- 192.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(supplement 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 193.Sondike SB, Copperman N, Jacobson MS. Effects of a low-carbohydrate diet on weight loss and cardiovascular risk factors in overweight adolescents. Journal of Pediatrics. 2003;142(3):253–258. doi: 10.1067/mpd.2003.4. [DOI] [PubMed] [Google Scholar]
- 194.Reinehr T, Andler W. Changes in the atherogenic risk factor profile according to degree of weight loss. Archives of Disease in Childhood. 2004;89(5):419–422. doi: 10.1136/adc.2003.028803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kaufman CL, Kaiser DR, Kelly AS, Dengel JL, Steinberger J, Dengel DR. Diet revision in overweight children: effect on autonomic and vascular function. Clinical Autonomic Research. 2008;18(2):105–108. doi: 10.1007/s10286-008-0466-z. [DOI] [PubMed] [Google Scholar]
- 196.Woo KS, Chook P, Yu CW, et al. Effects of diet and exercise on obesity-related vascular dysfunction in children. Circulation. 2004;109(16):1981–1986. doi: 10.1161/01.CIR.0000126599.47470.BE. [DOI] [PubMed] [Google Scholar]
- 197.Watts K, Beye P, Siafarikas A, et al. Exercise training normalizes vascular dysfunction and improves central adiposity in obese adolescents. Journal of the American College of Cardiology. 2004;43(10):1823–1827. doi: 10.1016/j.jacc.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 198.Meyer AA, Kundt G, Lenschow U, Schuff-Werner P, Kienast W. Improvement of early vascular changes and cardiovascular risk factors in obese children after a six-month exercise program. Journal of the American College of Cardiology. 2006;48(9):1865–1870. doi: 10.1016/j.jacc.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 199.Tjønna AE, Stølen TO, Bye A, et al. Aerobic interval training reduces cardiovascular risk factors more than a multitreatment approach in overweight adolescents. Clinical Science. 2009;116(4):317–326. doi: 10.1042/CS20080249. [DOI] [PubMed] [Google Scholar]
- 200.Maggio ABR, Aggoun Y, Martin XE, Marchand LM, Beghetti M, Farpour-Lambert NJ. Long-term follow-up of cardiovascular risk factors after exercise training in obese children. International Journal of Pediatric Obesity. 2011;6(2):e603–e610. doi: 10.3109/17477166.2010.530665. [DOI] [PubMed] [Google Scholar]
- 201.Hambrecht R, Adams V, Erbs S, et al. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107(25):3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 202.Adams V, Linke A, Kränkel N, et al. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation. 2005;111(5):555–562. doi: 10.1161/01.CIR.0000154560.88933.7E. [DOI] [PubMed] [Google Scholar]
- 203.Ferrari GSL, Ferrari CKB. Exercise modulation of total antioxidant capacity (TAC): towards a molecular signature of healthy aging. Frontiers in Life Science. 2011;5(3-4):81–90. [Google Scholar]
- 204.Widder JD, Chen W, Li L, et al. Regulation of tetrahydrobiopterin biosynthesis by shear stress. Circulation Research. 2007;101(8):830–838. doi: 10.1161/CIRCRESAHA.107.153809. [DOI] [PubMed] [Google Scholar]
- 205.Sonnenschein K, Horváth T, Mueller M, et al. Exercise training improves in vivo endothelial repair capacity of early endothelial progenitor cells in subjects with metabolic syndrome. European Journal of Cardiovascular Prevention and Rehabilitation. 2011;18(3):406–414. doi: 10.1177/1741826710389373. [DOI] [PubMed] [Google Scholar]
- 206.Park JH, Miyashita M, Kwon YC, et al. A 12-week after-school physical activity programme improves endothelial cell function in overweight and obese children: a randomised controlled study. BMC Pediatrics. 2012;12(article 111) doi: 10.1186/1471-2431-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Walther C, Gaede L, Adams V, et al. Effect of increased exercise in school children on physical fitness and endothelial progenitor cells: a prospective randomized trial. Circulation. 2009;120(22):2251–2259. doi: 10.1161/CIRCULATIONAHA.109.865808. [DOI] [PubMed] [Google Scholar]
- 208.Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. The Lancet. 2007;370(9600):1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- 209.James WPT, Caterson ID, Coutinho W, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. The New England Journal of Medicine. 2010;363(10):905–917. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- 210.Czernichow S, Lee CMY, Barzi F, et al. Efficacy of weight loss drugs on obesity and cardiovascular risk factors in obese adolescents: a meta-analysis of randomized controlled trials. Obesity Reviews. 2010;11(2):150–158. doi: 10.1111/j.1467-789X.2009.00620.x. [DOI] [PubMed] [Google Scholar]
- 211.Brook RD, Bard RL, Glazewski L, et al. Effect of short-term weight loss on the metabolic syndrome and conduit vascular endothelial function in overweight adults. The American Journal of Cardiology. 2004;93(8):1012–1016. doi: 10.1016/j.amjcard.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 212.Vitale C, Mercuro G, Cornoldi A, Fini M, Volterrani M, Rosano GMC. Metformin improves endothelial function in patients with metabolic syndrome. Journal of Internal Medicine. 2005;258(3):250–256. doi: 10.1111/j.1365-2796.2005.01531.x. [DOI] [PubMed] [Google Scholar]
- 213.Clarson CL, Mahmud FH, Baker JE, et al. Metformin in combination with structured lifestyle intervention improved body mass index in obese adolescents, but did not improve insulin resistance. Endocrine. 2009;36(1):141–146. doi: 10.1007/s12020-009-9196-9. [DOI] [PubMed] [Google Scholar]


