Abstract
Vitamin D has become increasingly recognized in the literature for its extra-skeletal roles, including an effect on inflammation and the immune response to infection. Our goal was to describe the role of vitamin D in the immune response and implications for the risk of influenza infection in humans. In this review, we first consider literature that provides molecular and genetic support to the idea that vitamin D is related to the adaptive and innate immune responses to influenza infection in vitro and in animal models. We then discuss observational studies and randomized controlled trials of vitamin D supplementation in humans. Finally, we consider some of the knowledge gaps surrounding vitamin D and immune response that must be filled.
Introduction
Vitamin D is a fat-soluble vitamin, unique in that it is primarily produced in the skin during sun exposure rather than absorbed from the diet (1). Vitamin D has long been known to play a role in the skeletal system and calcium homeostasis; vitamin D deficiency is known to be a cause of rickets and osteoporosis (2). Recently, cells of the immune system have been found to possess vitamin D receptors (VDR)3 and are capable of metabolizing the active form of vitamin D [also known as calcitriol, 1,25-dihydroxyvitamin D, or 1,25(OH)2D] (1), suggesting that this nutrient may be an important factor in the immune response to infection (3). For example, activated T- and B-cells can convert the inactive form of vitamin D [also known as 25-hydroxyvitamin D, or 25(OH)D] to 1,25(OH)2D in human cells in vitro (4); locally produced 1,25(OH)2D then acts on immune cells in an autocrine or paracrine fashion. VDR have also been identified on peripheral blood mononuclear cells (PBMC) in human cells in vitro (5), lending support for a potential role of vitamin D in the regulation of the immune system and infectious diseases (6).
Further investigation has revealed that vitamin D plays important roles in signaling during both the adaptive and innate immune response to viral and bacterial infection (3, 7). 25(OH)D can be converted to 1,25(OH)2D in human respiratory epithelium cells in vitro (8), and 1,25(OH)2D and 25(OH)D have both been implicated in the immune response to several types of respiratory infections, including respiratory syncytial virus and tuberculosis, in vitro (8–10). Studies have also found associations between either 25(OH)D or 1,25(OH)2D deficiency and clinical illnesses, including influenza (11–14), tuberculosis (15–19), respiratory syncytial virus (9, 20), and other respiratory illnesses (21–24), in observational studies. An estimate provided by data from the NHANES states that more than one-half of U.S. adults have 25(OH)D <30 μg/L (23), a status defined as vitamin D insufficient (1). However, an Institute of Medicine report suggests that vitamin D deficiency be defined as 25(OH)D <10 μg/L (25), a condition shared by only 2% of Americans (23). No matter the cutoff, 25(OH)D levels have been found to be significantly lower among children with respiratory illnesses, older adults, women, and individuals with darker skin pigmentation (26–29).
In this review, we will describe evidence for the role of vitamin D in modulating the adaptive and innate immune response. We will then consider how those aspects of the immune system respond to influenza infection. We will also consider observational studies and randomized controlled trials of vitamin D supplementation in humans and the concurrent seasonality of poor vitamin D status and increased risk of influenza infection. Finally, we will note the problems in accurately assessing vitamin D and consider the next steps for assessing vitamin D in the context of respiratory illness.
Current status of knowledge
Vitamin D and adaptive immune response to infection
The adaptive immune response to infection is complex and multi-faceted and involves a diverse population of cell types and other factors such as cytokines, chemokines, enzymes, and hormones (3, 30, 31) (Fig. 1). Additionally, the immune response to an infectious insult is not static; characteristics of the response (measured by serum cytokines in human adults) change from the initial period of antigenic stimulation to the later stage of disease clearance (32). Vitamin D may therefore affect one component of an immune system response but not other components, meaning that the net effect of vitamin D on immune function and clinical illness is difficult to characterize. This also suggests that evidence of vitamin D’s role in the immune system in vitro may not apply to vitamin D’s role in vivo. Nevertheless, vitamin D [in particular, 1,25(OH)2D] has been found to act on specific cell parameters of the adaptive immune response, most notably T- and B-cells (Fig. 1A).
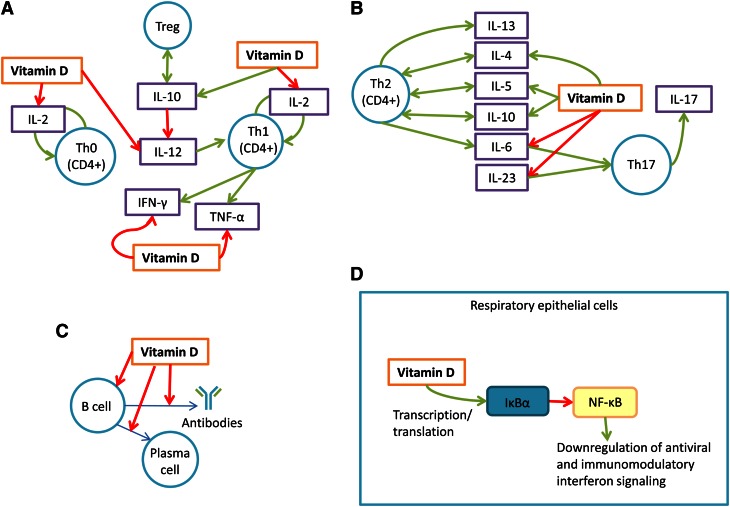
Figure 1.
Vitamin D and its various actions in the immune system. (A) Vitamin D inhibits the production and proliferation of Th1 and Th0 cells by inhibiting IL-2, IFNγ, and TNFα vitamin D promotes the production of Treg cells by facilitating production of IL-10. (B) Vitamin D promotes a Th2-mediated immune response profile by promoting IL-4, IL-5, and IL-10. Vitamin D inhibits a Th17-mediated immune response profile (and thus inhibits IL-17) by inhibiting IL-6 and IL-23. (C) Vitamin D inhibits the production of B-cells, the differentiation of B-cells into plasma cells, and the production of antibodies by B-cells. (D) Vitamin D promotes nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, α in respiratory epithelial cells, which inhibits NF-κB, in turn promoting antiviral and immunomodulatory interferon signaling. Th, T helper cell; Treg, T regulatory cell.
The T-cell profile shift
Vitamin D [espcially 1,25(OH)2D] is widely acknowledged to shift the T-cell response profile from a T helper cell (Th) 1- to a Th2-mediated response by inhibiting cells of the Th1 profile (3, 31, 33-43) in vitro in mouse fibroblasts, pancreatic islets, cultured splenocytes, and host serum; and in vivo in mouse dendritic cells. Vitamin D also promotes cells of the Th2 profile (31, 39, 41, 42, 44, 45) in mouse fibroblasts (Fig. 1A). This bias is thought to reduce inflammation and promote an immunosuppressive state (31). 1,25(OH)2D inhibits the proliferation of Th1 helper cells in mouse lymphocytes and human T-cell clones in vitro in part by inhibiting IFNγ and IL-2, cytokines that promote Th1 production and recruitment and macrophage production (3, 46–48). 1,25(OH)2D also reduces IL-12 (another cytokine that promotes Th1 production and recruitment) in vitro in human PBMC (15); it is thought to accomplish this by downregulating molecules in human dendritic cells, which produce IL-12 (3, 15, 47, 49), and by promoting IL-10, a cytokine thought to inhibit production of IL-12 (39, 50, 51). 1,25(OH)2D suppresses an additional Th1-mediated cytokine, TNFα, in vitro in human monocytes, further pushing immune response to a Th2 profile (52, 53). 1,25(OH)2D has also been found to suppress the production and recruitment of Th17 cells in mice in vivo by downregulating Th17-mediated cytokines IL-23 and IL-6 (3, 50) (Fig. 1B). In contrast, 1,25(OH)2D has been shown to upregulate IL-4, IL-5, and IL-10 in mouse lymphocytes in vitro; these cytokines promote a Th2 response profile (3, 46). IL-10 also promotes the proliferation of T regulatory (Treg) cells in mouse colon cells in vivo; these Treg cells inhibit the Th1 response profile (3, 50) (Fig. 1A).
Despite overwhelming in vitro evidence that 1,25(OH)2D biases the immune system response toward a Th2-dominated profile, it has been shown to repress both Th1 and Th2 response profiles in human cord blood cells in vitro (54). This suggests that the effects of 1,25(OH)2D on Th helper cell selection are more complex in vivo and in differing molecular and cellular environments (44). A study on the immune response to allergy stimulus in mice in vivo suggested that vitamin D supplementation [100 ng 1,25(OH)2D injection] given after the initial period of sensitization prevented high levels of eosinophils associated with reduced local inflammatory response in bronchoalveolar lavage fluid and lung tissue. However, constant vitamin D supplementation [100 ng 1,25(OH)2D injection every other day during the study] did not protect against a high eosinophil count in mice respiratory epithelia (38). The proposal that the effect of vitamin D is time-sensitive is further bolstered by a study that showed that low vitamin D [measured by serum 25-hydroxy-(ergocalciferol + cholecalciferol] was common among patients with tuberculosis (35.6 μg/L compared with 37.2 μg/L in patients without tuberculosis; P < 0.05) but was not associated with the initial, acute-phase response to infection (measured by levels of α1-antichymotrypsin, an acute-phase protein) (19). A 3-mo prospective, randomized controlled trial investigating whether vitamin D has an effect on cytokine levels in humans showed that supplementation of 2000 IU cholecalciferol in ambulatory adults does not have a significant effect on the association between levels of serum 25(OH)D and IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, IFNγ, and TNFα, although this study did not take into account infection status and there was no difference between 25(OH)D levels pre- and postsupplementation in the group randomized to receive cholecalciferol (55).
In contrast, another study found that many of the cytokines inhibited by 1,25(OH)2D in humans (IL-2, IL-12, IFNγ, IL-6, TNFα, IL-17, and IL-23) are produced in significantly greater quantities in individuals with pandemic H1N1 influenza compared with healthy controls; some cytokines that are promoted by 1,25(OH)2D (IL-5, IL-10) are also produced in significantly greater quantities in those individuals (3, 56) (measured by serum cytokine levels) (Fig. 1A,B). Early secretion of Th1- and Th17-mediated cytokines was also found to be significantly increased in individuals infected with severe novel H1N1 influenza (37), suggesting that serum 25(OH)D may play a role in inflammatory response during influenza infection, even if it does not affect levels of inflammatory cytokines in a noninfectious state.
B-cell differentiation
1,25(OH)2D inhibits proliferation and promotes apoptosis of activated human B-cells in vitro, although the initial production of B-cells remains unaffected (4). 1,25(OH)2D is thought to thereby inhibit the differentiation of B-cells into plasma cells (4) (Fig. 1C). B-cell deficiency in mice and ferrets has been associated with decreased heterologous immunity between seasonal and pandemic H1N1 influenzas (57) and memory B-cells against influenza infection have persisted for >5 mo after infection in lung epithelial cells in vitro (58). Although there is relatively little evidence linking influenza infection to B-cell levels in humans, a small study involving 15 children aged 2–14 y with pandemic or seasonal H1N1 influenza showed that a significantly higher percentage of B-cells were found in children infected with any strain of influenza, compared with controls (59).
Vitamin D and innate immune response
Inflammation and cell signaling.
Both the active form of vitamin D [1,25(OH)2D] and a fluorescent vitamin D analogue have been found to decrease proinflammatory chemokine production during infection in vitro in human respiratory epithelial cells (for the active form of vitamin D) and mouse pancreatic islets (for the vitamin D analogue) (9, 35). Additionally, 1,25(OH)2D has been found to downregulate proinflammatory cytokines such as IL-1, IL-6, IL-8, and TNFα in many different cell types in vitro (60). Significantly higher serum levels of IL-6, IL-8, IL-17, and TNFα were found in patients hospitalized with pandemic H1N1 influenza in a case control study (37) and have elsewhere been implicated in influenza infection (32, 56).
In human lymphocytes in vitro, the antiinflammatory effect of vitamin D is carried out in part through inhibition of NF-κB (61) (Fig. 1C). NF-κB is a protein complex that is associated with the transcription of inflammatory proteins during infection (62), including cytokines and chemokines, acute phase proteins, adhesion molecules, and inducible effector enzymes (63). 1,25(OH)2D induces nuclear factor of κ light polypeptide gene enhancer in B-cells inhibitor, α, the inhibitor of NF-κB, in human airway epithelial cells in vitro during infection with respiratory syncytial virus (9). NF-κB itself has been found to modulate T-cell response profiles in various types of mouse cells (64) and to downregulate antiviral and immunomodulatory interferon signaling involved against influenza infection in mouse embryonic fibroblasts (65, 66). 1,25(OH)2D inhibits NF-κB in vitro in human promyelotic leukemia HL-60 cells during the early infection period (67) (Fig. 1D) but promotes it later on (67).
Monocytes and differentiation.
1,25(OH)2D has been shown to promote the differentiation of monocytes into macrophages in both mouse and human cells in vitro (47) and suppress the differentiation of human monocytes into dendritic cells (49) (Fig. 2). 1,25(OH)2D (10 nmol/L) has also been found to induce a tolerogenic state in human myeloid (but not plasmacytoid) dendritic cells in vitro (68) and has an effect on the trafficking and translocation of differentiated but immature dendritic cells in mice (69). Mouse dendritic cells exposed to 1,25(OH)2D in vivo for 24 h were able to retain antigen-presenting abilities and avoid sequestration in lymph nodes. However, mouse dendritic cells that were differentiated in the constant presence of 1,25(OH)2D in vitro did not retain these capabilities (69), a result that supports the idea that immune system involvement of active vitamin D is modulated by infection time frame as well as the type of immune cell involved. In older adults, loss of dendritic cell function (including reduction in dendritic cell-mediated cytokines, such as TNFα) is associated with poor influenza vaccine response (70) and impaired response to influenza infection as a result of decreased induction of dendritic cell-stimulated CD8+ T-cells (71). Notably, infection with influenza A virus induced human blood monocytes to rapidly differentiate into mature dendritic cells in vitro (72). However, it has been found that plasmacytoid dendritic cells, which are unaffected by 1,25(OH)2D, may be responsible for dendritic cell-mediated protection to influenza infection in mice (73).
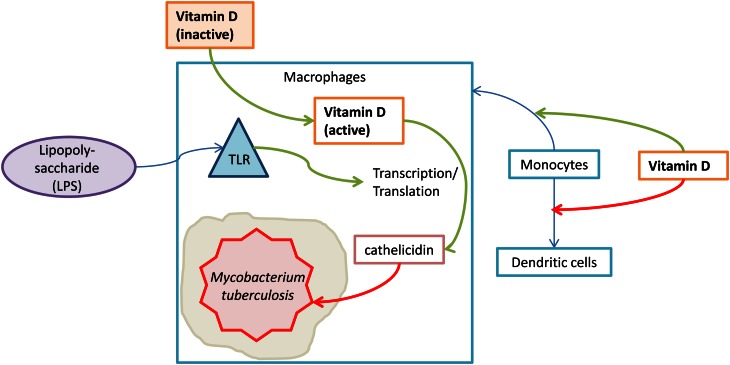
Figure 2.
Vitamin D activation and mycobacterial immune response. Vitamin D inhibits the differentiation of monocytes into dendritic cells and promotes the differentiation of monocytes into macrophages. When toll-like receptors are activated by circulating LPS, they promote vitamin D-mediated transcription of cathelicidin, an antimicrobial peptide that kills Mycobacterium tuberculosis.
1,25(OH)2D is also thought to suppress IFNγ-mediated activation of macrophages through inhibition of the Th1 response profile (3); the deactivation of IFNγ-activated macrophages is contingent upon a functional VDR in vitro in mouse macrophages (74). However, macrophages are capable of responding to and producing 1,25(OH)2D in human alveolar macrophages in vitro (75), and 1,25(OH)2D increases the production of cathelicidin in human macrophages in vitro by increasing expression of the VDR (76).
Antimicrobial properties.
1,25(OH)2D is associated with increased bactericidal activity in human PBMC (15) and the innate antibacterial response in human trophoblasts in vitro (77). VDR expression is upregulated by the activation of toll-like receptors in human macrophages and trophoblasts; this upregulation leads to the transcription of cathelicidin, which kills intracellular Mycobacterium tuberculosis (42, 77) (Fig. 2). This finding has been corroborated by in vivo evidence: vitamin D supplementation (a single oral mega-dose of 100,000 IU ergocalciferol) has been found to improve antimycobacterial immunity in humans (78). Because cathelicidin’s main mechanism of action is the destruction of envelope proteins of foreign agents, it may also be implicated in the destruction of influenza virus, which possesses an envelope protein (2, 79).
Additionally, 1,25(OH)2D has been found to upregulate human β-defensin 2 in a variety of human cells (80); human β-defensin 2 is thought to act as a chemoattractant for monocytes during viral infection (7). 1,25(OH)2D also induces hydrogen peroxide production in human monocytes in vitro (81). However, serum 25(OH)D was not associated with levels of serum cathelicidin or β-defensin-2 in patients with community-acquired pneumonia (82).
In total, the literature describing vitamin D’s role in the adaptive and innate immune systems suggests that vitamin D is involved in reducing inflammation during infection. Although 1,25(OH)2D suppresses the response of Th1 cells and proinflammatory cytokines, it promotes antimycobacterial factors such as cathelicidin and human β-defensin 2. Additionally, the literature suggests that vitamin D’s role in modulating immune system response to infection is not constant over time and changes according to the host and state of infection; 25(OH)D deficiency has been shown to be related to tuberculosis but not the acute phase of infection in humans (19): 1,25(OH)2D has been shown to have a positive effect on infection only when given after the initial phase of infection in mice (38); 1,25(OH)2D has been shown to affect NF-κB in different ways at different time points of infection in human leukemia cells (67) and prevents mouse dendritic cells from presenting antigens and differentiating only at certain time points (69).
Vitamin D and respiratory diseases in humans.
Due to the complexity of adaptive and innate immune responses to antigenic stimulation, it is difficult to pinpoint the overall effect of vitamin D during infection. This complexity sometimes results in different results in in vitro compared with in vivo studies. In several observational studies, lower 25(OH)D serum levels have been associated with increased risk of respiratory infection in adults (21, 23, 83, 84), children (26, 85, 86), and infants (20). A robust, dose-response association was found between lower levels of 25(OH)D and increased risk of upper respiratory infection in large, population-based studies in the United States (23) and Great Britain (21). 25(OH)D deficiency has also been connected to increased severity of acute lower respiratory infection in children (27, 86) and mortality from pneumonia in adults (82). Furthermore, children with VDR gene polymorphisms (specifically, the FokI ff or TaqI polymorphisms) are more likely to have acute lower respiratory infections (87). The FokI ff polymorphism results in a downregulation of the vitamin D target gene, CYP24A1, which codes for an enzyme that degrades 1,25(OH)2D (88); the TaqI polymorphism is associated with lower VDR protein levels (89).
However, randomized controlled trials supplementing vitamin D have yielded mixed results; a summary of these results can be found in Table 1. No significant effect was found for 1,25(OH)2D supplementation as an adjuvant to increase the efficacy of an influenza vaccine in the general adult population (90) or in HIV-positive (91) adults, and supplementation with oral cholecalciferol did not significantly reduce the risk of infection in an elderly population (92). General vitamin D supplementation (median daily dose of 2000 IU/d, or 50 μg/d) was not associated with improved serologic response to an influenza vaccine (measured by a ≥1:40 hemagglutinin antibody inhibition titer ratio or 4-fold increase in hemagglutinin antibody inhibition titer at 3-mo postvaccination) in prostate cancer patients (13). However, the baseline 25(OH)D serum concentration in the same population was associated with an improved response to influenza vaccine (P = 0.045) (13). Supplementation with cholecalciferol (1200 IU/d for 4 mo) was also found to be associated with a reduced risk of seasonal influenza A in Japanese schoolchildren (P = 0.04) (12), but the study did not measure serum concentrations of 25(OH)D or serum antibody concentrations to influenza. An additional study found a lower rate of upper respiratory infection and influenza in participants taking a cholecalciferol supplement (2000 IU or 50 μg/d), but this analysis was based on self-reported illness (14) and the results were not reproduced (93). A systematic review of randomized controlled trials supplementing vitamin D for the prevention or treatment of infectious disease found that the strongest evidence for the effectiveness of vitamin D in prevention or treatment of infectious diseases is for the reduction of risk of acute respiratory illness and influenza (45).
Table 1.
Randomized controlled trials investigating the association between vitamin D supplementation and increased risk of respiratory illness as an outcome
| Author(s) | Year | Sample size and participants | Location | Dose and duration of supplementation | Outcome measure | Strength of association |
| Jorde et al. (93) | 2011 | n = 569 adults 32–84 y old | Norway, Austria, USA, Scotland, Denmark, and Belgium | 1111–6800 IU/d cholecalciferol for at least 12 wk during influenza season | Risk of influenza-like illness, measured by self-report | RR of influenza-like illness1: 0.88 (95%CI: 0.58–1.32) Median increased duration of ILI2: 3 d (P < 0.01) |
| Urashima et al. (12) | 2010 | n = 334 school-children | Japan | One 200-IU tablet cholecalciferol 3 times/d for 120 d | PCR-confirmed influenza A | RR of infection1: 0.58 (95%CI: 0.34–0.99) |
| Li-Ng et al. (22) | 2009 | n = 162 adults 18–80 y old | USA | 2000 IU cholecalciferol/ d for 12 wk | Risk of URTI | RR of URTI1: 0.96 (95%CI: 0.75–1.23) |
| Wejse et al. (100) | 2009 | n = 281 adults with TB | Guinea-Bissau | 100,000 IU of cholecalciferol at inclusion and again at 5 and 8 mo after TB treatment | Clinical severity and mortality of tuberculosis | RR of mortality1: 1.19 (95%CI: 0.58–1.95) |
| Aloia and Li-Ng (14) | 2007 | n = 208 postmenopausal African American women | USA | 800 IU cholecalciferol/d for 2 y, then increased to 2000 IU/d for an additional year (3 y total) | Risk of self-reported cold or influenza | RR of cold or influenza1: 0.31 (95%CI: 0.15–0.65) |
| Avenell et al. (92) | 2007 | n = 3444 adults ≥70 y old | England and Scotland | 800 IU/d cholecalciferol, 1000 mg calcium, both, or placebo, for 24–62 mo | Risk of any infection, measured by self-report | Relative odds of any infection1: 0.90 (95%CI: 0.76–1.07) |
| Martineau et al. (78) | 2007 | n = 131 adults >17 y old | England | Single dose of 100,000 IU ergocalciferol at beginning of study | Immunity to TB mycobacteria measured by BCG-lux luminescence ratio at 24 h postinfection | Relative increase in immunity3: 20.4% (95%CI: 1–25%) |
| Nursyam et al. (17) | 2006 | n = 67 TB patients 15–59 y old with moderately advanced TB lesions | Indonesia | 10,000 IU vitamin D (unspecified)/d | Rate of sputum conversion | Relative odds of conversion4: 1.32 (95%CI: 1.09–1.60) |
| Morcos et al. (18) | 1998 | n = 24 children with TB, 1–13 y old | Egypt | 1000 IU cholecalciferol/d for the length of tuberculosis treatment | Concentration of serum vitamin D in supplemented vs. unsupplemented groups | Difference in vitamin D (pg/mL) between supplemented and unsupplemented groups was not significant (data not shown) |
| Rehman (102) | 1994 | n = 47 children 3–12 y old | India | 60,000 IU vitamin D (unspecified)/wk for 6 wk, plus 650 mg calcium/d | Frequency of any infection in children | No difference in frequency seen between supplemented and control groups (data not shown) |
RR refers to the risk of illness while being supplemented with vitamin D compared with being supplemented with placebo. TB, tuberculous; URTI, upper respiratory tract infection.
“Relative increase in duration” refers to the extra number of days that participants in the placebo group reported experiencing ILI symptoms, compared with the number of days that supplemented participants reported; -value is a chi-square value comparing the 2 proportions.
“Relative increase” refers to the increase in immunity in vitamin D-supplemented compared with placebo-supplemented participants.
“Relative conversion” refers to the conversion proportion of vitamin D-supplemented compared with conversion proportion of placebo-supplemented participants.
Influenza and vitamin D: seasonality.
Edgar Hope-Simpson (94) was the first to make the argument that the seasonality of influenza hinges upon times of less sunlight; since then, several researchers have corroborated the finding that influenza is more prevalent in the winter during times of less sunlight and therefore less available vitamin D (40, 95, 96). Healthy volunteers inoculated with live attenuated influenza virus in northern latitudes of Russia during different seasons of the year were 8 times more likely to have influenza infection during the winter than summer (97). However, another study recently found that model simulations of vitamin D seasonal fluctuation are not able to consistently reproduce observed seasonal patterns in influenza; the authors concluded that seasonal variation in vitamin D is unlikely to be the main factor affecting the seasonality of influenza (98). Because there are other important environmental factors that could affect virulence of influenza that are synchronous with periods of less sunlight (e.g., colder temperatures and lower humidity), it may be difficult to separate these factors in an analysis of influenza and vitamin D seasonality.
Problems in assessing vitamin D.
Lee (99) has pointed out several problems in assessing vitamin D. First, a consensus has not yet been reached on what level of vitamin D is considered “deficient” (1), and studies with different definitions of deficiency may not have comparable results. Additionally, there is no consensus on biologically relevant doses of vitamin D for use in randomized controlled trials; studies cited in this paper supplemented vitamin D in a broad range of forms, from 200 IU cholecalciferol 3 times/d (12 to 2000 IU/d (22), and including one-time supplements of cholecalciferol in 100,000-IU doses (78, 100). Second, concentrations of 25(OH)D obtained from serum vary depending on the method of assay and reproducibility is poor (99, 101). Third, concentrations of 25(OH)D are subject to change depending on levels of binding proteins and rapid fluid shifts, factors of concern in critically ill patients (99).
In 8 of 10 supplementation studies cited in this review that looked at respiratory illness as an outcome, serum vitamin D pre- and postsupplementation was not reported (12, 14, 17, 22, 92, 93, 100, 102). Lack of vitamin D measurement in these studies makes interpretation of the results more difficult, because there is no way to demonstrate that participants in the supplementation group had significantly higher serum vitamin D than participants in the control group. In such studies, determining the biological relevance of a particular dose of vitamin D is also difficult. One study that supplemented vitamin D and measured vitamin D serum status postsupplementation found no significant difference in respiratory illness between the supplementation and control groups (18); one study found a significant difference in the level of mean 25(OH)D after a single mega-dose of cholecalciferol (78).
Limitations of current literature.
Evidence from in vitro, in vivo animal, and in vivo human studies has been accumulating over the past decade, suggesting that vitamin D may influence the risk of respiratory infections, including influenza. However, much work remains to be done: there must be a focus on the application of laboratory and animal findings to human populations, because it has already been observed that the results of in vitro studies are not always replicated in vivo and the results of animal studies are not always replicated in humans. It is important to note that several of the studies listed here were done in mouse models. Although mouse models are accepted for studying influenza infection, they are not ideal; mice do not possess a vitamin D response element for the production of cathelicidin (3) and other differences in cell signaling have been observed between mouse and human cells (42, 103). However, a “humanized” mouse model is currently being developed that may prove useful in studying this mechanism more closely (39).
Several of the studies on vitamin D and respiratory illness cited here were conducted in settings where other micronutrient and macronutrient deficiencies are common (16, 19, 85, 104, 105). It is possible that other nutrient deficiencies act as effect modifiers or confounders for respiratory illness outcomes in such settings. Additionally, other infectious diseases with a higher prevalence in these settings than in other settings (e.g., malaria, multi-drug–resistant tuberculosis, and HIV/AIDS) may affect the outcome measurement. It is therefore important to keep in mind that such different settings limit generalizability of results.
The difference between the role of vitamin D during the acute initial infection state and after the initial infection state must be considered in future studies, because it is possible that the effects of vitamin D on the immune system change over time and between immune cell types. It is also important to differentiate between the effect of vitamin D status on influenza incidence and influenza severity, taking into special consideration the possibility that the serum 25(OH)D levels of critically ill patients may be subject to change more rapidly than in healthy individuals. Furthermore, a distinction must be made between “deficient” and “suboptimal” vitamin D status. Analyses considering not only vitamin D deficiency but the continuous range of serum vitamin D values will add to the literature. Finally, rigorous methodology, including a standardized serum vitamin D assay, consistent reporting of specific types of vitamin D [e.g., 1,25(OH)2D as opposed to 25(OH)D] and other measures of immune response in addition to hemagglutinin antibody inhibition titers, such as cell-mediated immunity, are needed. PCR confirmation of influenza diagnosis is the gold standard for measuring outcome; if other methods are used, these methods should be clearly reported.
Conclusion
The evidence for an association between vitamin D and risk of influenza infection exists, albeit mainly in in vitro and animal studies describing the role of 1,25(OH)2D in innate and adaptive immunity. Observational human studies of 25(OH)D deficiency and randomized controlled trials supplementing various forms of vitamin D have yielded mixed but promising results. More rigorous research studies with large populations and outcome measures including 25(OH)D serostatus postsupplementation are needed to further elucidate the possible relationships between vitamin D and risk of influenza infection. The establishment of a clear link between vitamin D status and influenza infection has broad implications for influenza research, especially in groups that are likely to have low vitamin D levels, as well as the formulation of policy regarding vitamin D supplementation.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Supported by funding from the Marshfield Clinic Research Foundation in Marshfield, WI.
Author disclosures: M. Sundaram and L. Coleman, no conflicts of interest.
Abbreviations used: 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D; PBMC, peripheral blood mononuclear cell; Th, T helper cell; Treg, T regulatory cell; VDR, vitamin D receptor.
Literature Cited
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81 [DOI] [PubMed] [Google Scholar]
- 2.Schwalfenberg GK. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol Nutr Food Res. 2011;55:96–108 [DOI] [PubMed] [Google Scholar]
- 3.Bikle DD. Vitamin D and the immune system: role in protection against bacterial infection. Curr Opin Nephrol Hypertens. 2008;17:348–52 [DOI] [PubMed] [Google Scholar]
- 4.Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179(1634–1647):1634 [DOI] [PubMed] [Google Scholar]
- 5.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-Dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–3 [DOI] [PubMed] [Google Scholar]
- 6.Chung M, Balk EM, Brendel M, Ip S, Lau J, Lee J, Lichtenstein A, Patel K, Raman G, Tatsioni A, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 2009;183:1–420 [PMC free article] [PubMed] [Google Scholar]
- 7.Beard JA, Bearden A, Striker R. Vitamin D and the anti-viral state. J Clin Virol. 2011;50:194–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181:7090–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansdottir S, Monick MM, Lovan N, Powers L, Gerke A, Hunninghake GW. Vitamin D decreases respiratory syncytial virus induction of NF-kB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol. 2010;184:965–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, et al. Toll-like receptor triggering on a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3 [DOI] [PubMed] [Google Scholar]
- 11.Young GA, Underdahl NR, Carpenter LE. Vitamin D intake and susceptibility of mice to experimental swine influenza virus infection. Proc Soc Exp Biol Med. 1949;72:695–7 [DOI] [PubMed] [Google Scholar]
- 12.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–60 [DOI] [PubMed] [Google Scholar]
- 13.Chadha MK, Fakih M, Muindi J, Tian L, Mashtare T, Johnson CS, Trump D. Effect of 25-hydroxyvitamin D status on serological response to influenza vaccine in prostate cancer patients. Prostate. 2011;71:368–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aloia JF, Li-Ng M. Re: Epidemic influenza and vitamin D. Epidemiol Infect. 2007;135:1095–6; author reply 7–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vidyarani M, Selvaraj P, Jawahar MS, Narayanan PR. 1,25 dihydroxyvitamin D3 modulated cytokine response in pulmonary tuberculosis. Cytokine. 2007;40:128–34 [DOI] [PubMed] [Google Scholar]
- 16.Tostmann A, Wielders JPM, Kibiki GS, Verhoef H, Boeree MJ. van der Ven AJ. Serum 25-hydroxy-vitamin D3 concentrations increase during tuberculosis treatment in Tanzania. Int J Tuberc Lung Dis. 2010;14:1147–52 [PubMed] [Google Scholar]
- 17.Nursyam EW, Amin Z, Rumende CM. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculosis lesion. Acta Med Indones. 2006;38:3–5 [PubMed] [Google Scholar]
- 18.Morcos MM, Gabr AA, Samuel S, Kamel M, Baz ME, Beshry ME, Michail RR. Vitamin D administration to tuberculous children and its value. Boll Chim Farm. 1998;137:157–64 [PubMed] [Google Scholar]
- 19.Friis H, Range H, Pedersen ML, Mølgaard C, Changalucha J, Krarup H, Magnussen P, Søborg C. Andersen AsB. Hypovitaminosis D is common among pulmonary tuberculosis patients in Tanzania but is not explained by the acute phase response. J Nutr. 2008;138:2474–80 [DOI] [PubMed] [Google Scholar]
- 20.Belderbos ME, Houben ML, Wilbrink B, Lentjes E, Bloemen EM, Kimpen JLL, Rovers M, Bont L. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics. 2011;127:e1513–20 [DOI] [PubMed] [Google Scholar]
- 21.Berry DJ, Hesketh K, Power C, Hypponen E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br J Nutr. 2011;106:1433–40 [DOI] [PubMed] [Google Scholar]
- 22.Li-Ng M, Aloia JF, Pollack S, Cunha BA, Mikhail M, Yeh J, Berbari N. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect. 2009;137:1396–404 [DOI] [PubMed] [Google Scholar]
- 23.Ginde AA, Mansbach JM, Carlos A, Camargo J. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the third national health and nutrition examination survey. Arch Intern Med. 2009;169:384–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the Third National Health and Nutrition Examination Survey. Chest. 2005;128:3792–8 [DOI] [PubMed] [Google Scholar]
- 25.Ross AC, Taylor CL, Yaktine AL, Valle HBD. Dietary Reference Intakes for calcium and vitamin D. Washington, DC: Institute of Medicine of the National Academies; . 2011 [PubMed] [Google Scholar]
- 26.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58:563–7 [DOI] [PubMed] [Google Scholar]
- 27.McNally JD, Leis K, Matheson LA, Karuananyake C, Sankaran K, Rosenberg AM. Vitamin D deficiency in young children with severe acute lower respiratory infection. Pediatr Pulmonol. 2009;44:981–8 [DOI] [PubMed] [Google Scholar]
- 28.Oliveri B, Plantalech L, Bagur A, Wittich AC, Rovai G, Pusiol E, Giovanelli JL, Ponce G, Nieva A, Chaperon A, et al. High prevalence of vitamin D insufficiency in healthy elderly people living at home in Argentina. Eur J Clin Nutr. 2004;58(337–342):337 [DOI] [PubMed] [Google Scholar]
- 29.Cashman KD, Wallace JM, Horigan G, Hill TR, Barnes MS, Lucey AJ, Bonham MP, Taylor N, Duffy EM, Seamans K, et al. Estimation of the dietary requirement for vitamin D in free-living adults ≥ 64 y of age. Am J Clin Nutr. 2009;89:1366–74 [DOI] [PubMed] [Google Scholar]
- 30.Cameron MJ, Kelvin DJ. Cytokines, chemokines and their receptors. Madame Curie Bioscience Database. Austin (TX): Landes Bioscience; 2000 [Google Scholar]
- 31.Hughes DA, Norton R. Vitamin D and respiratory health. Clin Exp Immunol. 2009;158:20–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee N, Wong CK, Chan PK, Chan MC, Wong RY, Lun SW, Ngai KL, Lui GC, Wong BC, Lee SK, et al. Cytokine response patterns in severe pandemic 2009 H1N1 and seasonal influenza among hospitalized adults. PLoS ONE. 2011;6:e26050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cantorna MT, Yu S, Bruce D. The paradoxical effects of vitamin D on type 1 mediated immunity. Mol Aspects Med. 2008;29:369–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80 Suppl:S1717–20 [DOI] [PubMed] [Google Scholar]
- 35.Giarratana N, Penna G, Amuchastegui S, Mariani R, Daniel KC, Adorini L. A vitamin D analog down-regulates proinflammatory chemokine production by pancreatic islets inhibiting T cell recruitment and type 1 diabetes development. J Immunol. 2004;173:2280–7 [DOI] [PubMed] [Google Scholar]
- 36.Ginanjar E. Sumariyono, Setiati S, Setiyohadi B. Vitamin D and autoimmune disease. Acta Med Indones. 2007;39:133–41 [PubMed] [Google Scholar]
- 37.Bermejo-Martin JF, Ortiz de Lejarazu R, Pumarola T, Rello J, Almansa R, Ramirez P, Martin-Loeches I, Varillas D, Gallegos MC, Seron C, et al. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care. 2009;13:R201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matheu V, Bäck O, Mondoc E, Issazadeh-Navikas S. Dual effects of vitamin D-induced alteration of Th1/Th2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J Allergy Clin Immunol. 2003;112:585–92 [DOI] [PubMed] [Google Scholar]
- 39.Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4:1151–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Juzeniene A, Ma L-W, Kwitniewski M, Polev GA, Lagunova Z, Dahlback A, Moan J. The seasonality of pandemic and non-pandemic influenzas: the roles of solar radiation and vitamin D. Int J Infect Dis. 2010;14:e1099–105 [DOI] [PubMed] [Google Scholar]
- 41.Sun J, Kong J, Duan Y, Szeto FL, Liao A, Madara JL, Li YC. Increased NF-kB activity in fibroblasts lacking the vitamin D receptor. Am J Physiol Endocrinol Metab. 2006;291:E315–22 [DOI] [PubMed] [Google Scholar]
- 42.White JH. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infect Immun. 2008;76:3837–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Griffin MD, Kumar R. Effects of 1alpha,25(OH)2D3 and its analogs on dendritic cell function. J Cell Biochem. 2003;88:323–6 [DOI] [PubMed] [Google Scholar]
- 44.Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin North Am. 2010;39:365–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamshchikov AV, Desai NS, Blumberg HM, Ziegler TR, Tangpricha V. Vitamin D for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocr Pract. 2009;15:438–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daynes RA, Enioutina EY, Butler S, Mu H-H, McGee ZA, Araneo BA. Induction of common mucosal immunity by hormonally immunomodulated peripheral immunization. Infect Immun. 1996;64:1100–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest. 2005;35:290–304 [DOI] [PubMed] [Google Scholar]
- 48.Lemire JM, Archer DC, Beck L, Spiegelberg HL. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: Preferential inhibition of Th1 functions. J Nutr. 1995;125:S1704–8 [DOI] [PubMed] [Google Scholar]
- 49.Lyakh LA, Sanford M, Chekol S, Young HA, Roberts AB. TGF-beta and vitamin D3 utilize distinct pathways to suppress IL-12 production and modulate rapid differentiation of human monocytes into CD83+ dendritic cells. J Immunol. 2005;174:2061–70 [DOI] [PubMed] [Google Scholar]
- 50.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324:23–33 [DOI] [PubMed] [Google Scholar]
- 51.Adorini L, Penna G. Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists. Hum Immunol. 2009;70:345–52 [DOI] [PubMed] [Google Scholar]
- 52.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–96 [DOI] [PubMed] [Google Scholar]
- 53.Almerighi C, Sinistro A, Cavazza A, Ciaprini C, Rocchi G, Bergamini A. 1Alpha,25-dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine. 2009;45:190–7 [DOI] [PubMed] [Google Scholar]
- 54.Pichler J, Gerstmayr M, Szepfalusi Z, Urbanek R, Peterlik M, Willheim M. 1-alpha,25(OH)2D3 inhibits not only Th1 but also Th2 differentiation in human cord blood T cells. Pediatr Res. 2002;52:12–8 [DOI] [PubMed] [Google Scholar]
- 55.Yusupov E, Li-Ng M, Pollack S, Yeh JK, Mikhail M, Aloia JF. Vitamin D and serum cytokines in a randomized clinical trial. Int J Endocrinol. 2010;2010:305054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu X, Zhang X, Zhao B, Wang J, Zhu Z, Teng Z, Shao J, Shen J, Gao Y, Yuan Z, et al. Intensive cytokine induction in pandemic H1N1 influenza virus infection accompanied by robust production of IL-10 and IL-6. PLoS ONE. 2011;6:e28680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fang Y, Banner D, Kelvin AA, Huang SSH, Paige CJ, Corfe SA, Kane KP, Bleackley RC, Rowe T, Leon AJ, et al. Seasonal H1N1 influenza virus infection induces cross-protective pandemic H1N1 virus immunity through a CD8-independent, B cell-dependent mechanism. J Virol. 2012;86:2229–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Onodera T, Takahashi Y, Yokoi Y, Ato M, Kodama Y, Hachimura S, Kurosaki T, Kobayashi K. Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus infection. Proc Natl Acad Sci USA. 2012;109:2485–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frisullo G, Iorio R, Plantone D, Nociti V, Patanella AK, Marti A, Palermo C, Valentini P, Mariotti P, Batocchi AP. Involvement of type I immune responses in swine-origin H1N1 influenza virus infection. Hum Immunol. 2011;72:632–5 [DOI] [PubMed] [Google Scholar]
- 60.Sun J. Vitamin D and mucosal immune function. Curr Opin Gastroenterol. 2010;26:591–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu X-P, Bellido T, Manolagas SC. Down-regulation of NF-kB protein levels in activated human lymphocytes by 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA. 1995;92:10990–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haddad JJ, Abdel-Karim NE. NF-kB cellular and molecular regulatory mechanisms and pathways: therapeutic pattern or pseudoregulation? Cell Immunol. 2011;271:5–14 [DOI] [PubMed] [Google Scholar]
- 63.Ghosh S, Karin M. Missing pieces in the NF-kB puzzle. Cell. 2002;109:S81–96 [DOI] [PubMed] [Google Scholar]
- 64.Zhu M, Fu Y. The complicated role of NF-kappaB in T-cell selection. Cell Mol Immunol. 2010;7:89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei L, Sandbulte MR, Thomas PG, Webby RJ, Homayouni R, Pfeffer LM. NF-kB negatively regulates interferon-induced gene expression and anti-influenza activity. J Biol Chem. 2006;281:11678–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pfeffer LM. The role of nuclear factor kappaB in the interferon response. J Interferon Cytokine Res. 2011;31:553–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tse AK-W, Wan C-K, Shen X-L, Zhu G-Y, Cheung H-Y, Yang M, Fong W-F. 1,25-dihydroxyvitamin D3 induces biphasic NF-kB responses during HL-60 leukemia cells differentiation through protein induction and PI3K/Akt-dependent phosphorylation/degradation of IkB. Exp Cell Res. 2007;313:1722–34 [DOI] [PubMed] [Google Scholar]
- 68.Penna G, Amuchastegui S, Giarratana N, Daniel KC, Vulcano M, Sozzani S, Adorini L. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J Immunol. 2007;178:145–53 [DOI] [PubMed] [Google Scholar]
- 69.Enioutina EY, Bareyan D, Daynes RA. Vitamin D3-mediated alterations to myeloid dendritic cell trafficking in vivo expand the scope of their antigen presenting properties. Vaccine. 2007;25:1236–49 [DOI] [PubMed] [Google Scholar]
- 70.Panda A, Qian F, Mohanty S, van Duin D, Newman FK, Zhang L, Chen S, Towle V, Belshe RB, Fikrig E, et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010;184:2518–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu WM, Nahar TER, Jacobi RHJ, Gijzen K. Beek JV, Hak E, Jonges M, Boog CJ, van der Zeijst BA, Soethout EC. Impaired production of TNF-alpha by dendritic cells of older adults leads to a lower CD8+ T cell response against influenza. Vaccine. 2012;30:1659–66 [DOI] [PubMed] [Google Scholar]
- 72.Hou W, Gibbs JS, Lu X, Brooke CB, Roy D, Modlin RL, Bennink JR, Yewdell JW. Viral infection triggers rapid differentiation of human blood monocytes into dendritic cells. Blood. 2012;119:3128–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaminski MM, Ohnemus A, Cornitescu M, Staeheli P. Plasmacytoid dendritic cells and TLR7-dependent signaling promote efficient protection of mice against highly virulent influenza A virus. J Gen Virol. 2012;93:555–9 [DOI] [PubMed] [Google Scholar]
- 74.Helming L, Bose J, Ehrchen J, Schiebe S, Frahm T, Geffers R, Probst-Kepper M, Balling R, Lengeling A. 1alpha,25-Dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation. Blood. 2005;106:4351–8 [DOI] [PubMed] [Google Scholar]
- 75.Adams JS, Sharma OP, Gacad MA, Singer FR. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest. 1983;72:1856–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–3 [DOI] [PubMed] [Google Scholar]
- 77.Liu N, Kaplan AT, Low J, Nguyen L, Liu GY, Equils O, Hewison M. Vitamin D induces innate antibacterial responses in human trophoblasts via an intracrine pathway. Biol Reprod. 2009;80:398–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martineau AR, Wilkinson RJ, Wilkinson KA, Newton SM, Kampmann B, Hall BM, Packe GE, Davidson RN, Eldridge SM, Maunsell ZJ, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176:208–13 [DOI] [PubMed] [Google Scholar]
- 79.Golec M. Cathelicidin LL-37: LPS-neutralizing, pleiotropic peptide. Ann Agric Environ Med. 2007;14:1–4 [PubMed] [Google Scholar]
- 80.Wang T-T, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–12 [DOI] [PubMed] [Google Scholar]
- 81.Cohen MS, Mesler DE, Snipes RG, Gray TK. 1,25-dihydroxyvitamin D3 activates secretion of hydrogen peroxide by human monocytes. J Immunol. 1986;136:1049–53 [PubMed] [Google Scholar]
- 82.Leow L, Simpson T, Cursons R, Karalus N, Hancox RJ. Vitamin D, innate immunity and outcomes in community acquired pneumonia. Respirology. 2011;16:611–6 [DOI] [PubMed] [Google Scholar]
- 83.Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin D and the incidence of acute viral respiratory tract infections in healthy adults. PLoS ONE. 2010;5:e11088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Laaksi I, Ruohola J-P, Tuohimaa P, Auvinen A, Haataja R, Ylikomi HPT. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86:714–7 [DOI] [PubMed] [Google Scholar]
- 85.Roth DE, Shah R, Black RE, Baqui AH. Vitamin D status and acute lower respiratory infection in early childhood in Sylhet, Bangladesh. Acta Paediatr. 2010;99:389–93 [DOI] [PubMed] [Google Scholar]
- 86.Inamo Y, Hasegawa M, Saito K, Hayashi R, Ishikawa T, Yoshino Y, Hashimoto K, Fuchigami T. Serum vitamin D concentrations and associated severity of acute lower respiratory tract infections in Japanese hospitalized children. Pediatr Int. 2011;53:199–201 [DOI] [PubMed] [Google Scholar]
- 87.Roth DE, Jones AB, Prosser C, Robinson JL, Vohra S. Vitamin D receptor polymorphisms and the risk of acute lower respiratory tract infection in early childhood. J Infect Dis. 2008;197:676–80 [DOI] [PubMed] [Google Scholar]
- 88.Alimirah F, Peng X, Murillo G, Mehta RG. Functional significance of vitamin D receptor FokI polymorphism in human breast cancer cells. PLoS ONE. 2011;6:e16024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ogunkolade B-W, Boucher BJ, Prahl JM, Bustin SA, Burrin JM, Noonan K, North BV, Mannan N, McDermott MF, DeLuca HF, et al. Vitamin D receptor (VDR) mRNA and VDR protein levels in relation to vitamin D status, insulin secretory capacity, and VDR genotype in Bangladeshi Asians. Diabetes. 2002;51:2294–300 [DOI] [PubMed] [Google Scholar]
- 90.Kriesel JD, Spruance J. Calcitriol (1,25-dihydroxy-vitamin D3) coadministered with influenza vaccine does not enhance humoral immunity in human volunteers. Vaccine. 1999;17:1883–8 [DOI] [PubMed] [Google Scholar]
- 91.Cooper C, Thorne A. Vitamin D supplementation does not increase immunogenicity of seasonal influenza vaccine in HIV-infected adults. HIV Clin Trials. 2011;12:275–6 [DOI] [PubMed] [Google Scholar]
- 92.Avenell A, Cook JA, MacLennan GS, MacPherson GC. Vitamin D supplementation to prevent infections: a sub-study of a randomized placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438). Age Ageing. 2007;36:574–7 [DOI] [PubMed] [Google Scholar]
- 93.Jorde R, Witham M, Janssens W, Rolighed L, Borchhardt K, de Boer IH, Grimnes G, Hutchinson MS. Vitamin D supplementation did not prevent influenza-like illness as diagnosed retrospectively by questionnaires in subjects participating in randomized clinical trials. Scand J Infect Dis. 2012;44:126–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hope-Simpson RE. The role of season in the epidemiology of influenza. J Hyg (Lond). 1981;86:35–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hayes DP. Influenza pandemics, solar activity cycles, and vitamin D. Med Hypotheses. 2010;74:831–4 [DOI] [PubMed] [Google Scholar]
- 96.Moan J, Dahlback A, Ma L, Juzeniene A. Influenza, solar radiation and vitamin D. Dermatoendocrinol. 2009;1:307–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shadrin AS, Marinich IG, Taros LY. Experimental and epidemiological estimation of seasonal and climato-geographical features of non-specific resistance of the organism to influenza. J Hyg Epidemiol Microbiol Immunol. 1977;21:155–61 [PubMed] [Google Scholar]
- 98.Shaman J, Jeon CY, Giovannucci E, Lipsitch M. Shortcomings of vitamin D-based model simulations of seasonal influenza. PLoS ONE. 2011;6:e20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee P. Vitamin D metabolism and deficiency in critical illness. Best Pract Res Clin Endocrinol Metab. 2011;25:769–81 [DOI] [PubMed] [Google Scholar]
- 100.Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, Andersen PL, Glerup H, Sodemann M. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2009;179:843–50 [DOI] [PubMed] [Google Scholar]
- 101.Roth HJ, Schmidt-Gayk H, Weber H, Niederau C. Accuracy and clinical implications of seven 25-hydroxyvitamin D methods compared with liquid chromatography-tandem mass spectrometry as a reference. Ann Clin Biochem. 2008;45:153–9 [DOI] [PubMed] [Google Scholar]
- 102.Rehman PKM. Sub-clinical rickets and recurrent infection. J Trop Pediatr. 1994;40:58. [DOI] [PubMed] [Google Scholar]
- 103.Hewison M. Vitamin D and the intracrinology of innate immunity. Mol Cell Endocrinol. 2010;321:103–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wahed MA, Islam MA, Khondakar P, Haque MA. Effect of micronutrients and duration of hospital stay in childhood pneumonia. Mymensingh Med J. 2008;17 Suppl 2:S77–83 [PubMed] [Google Scholar]
- 105.Zaman K, Baqui AH, Yunus M, Sack RB, Chowdhury HR, Black RE. Malnutrition, cell-mediated immune deficiency and acute upper respiratory infections in rural Bangladeshi children. Acta Paediatr. 1997;86:923–7 [DOI] [PubMed] [Google Scholar]