Abstract
Childhood stunting is an important and intractable public health problem that underlies ∼20% of deaths among children aged <5 y in developing countries. Environmental enteropathy (EE), a subclinical condition of the small intestine characterized by reduced absorptive capacity and increased intestinal permeability, is almost universal among children in developing countries and may mediate stunting. However, the etiology of EE is poorly understood. Mycotoxins are metabolites of fungi that frequently contaminate the staple foods of children living in developing countries. We review evidence from human and animal studies that exposure to mycotoxins, particularly aflatoxin (AF), fumonisin (FUM), and deoxynivaenol (DON), may impair child growth. Although these toxins have distinct actions, they all mediate intestinal damage through: 1) inhibition of protein synthesis (AF, DON); 2) an increase in systemic proinflammatory cytokines (DON); and 3) inhibition of ceramide synthase (FUM). The intestinal pathology that arises from mycotoxin exposure is very similar to that of EE. We propose that future studies should address the role of mycotoxins in the pathogenesis of EE and evaluate interventions to limit mycotoxin exposure and reduce childhood stunting.
Introduction
Child stunting, severe wasting, and intrauterine growth restriction together are responsible for more than 2 million deaths and 20% of disability-adjusted life-years among children <5 y of age (1). Child stunting has primarily been treated as a nutritional problem, with interventions focused on micro- and macronutrient solutions. However, a recent review of dietary interventions showed that the most successful of these studies achieved only a 0.7 increase in height-for-age Z-score, which is about one-third of the average growth deficit among children in Africa and Asia (2). In response, research is currently focused on investigating new pathways or mechanisms that will better explain the pathogenesis of stunting. Recent evidence, reviewed here, suggests several mechanisms by which mycotoxins, specifically aflatoxin (AF)4, fumonisin (FUM), and deoxynivalenol (DON), may share a downstream pathway for impaired growth by targeting the intestinal tract and inducing environmental enteropathy (EE).
EE, a subclinical condition characterized by villous atrophy, crypt hyperplasia, increased small intestinal permeability, inflammatory cell infiltrate, and modest malabsorption, is highly prevalent in the developing world (3). Rural Gambian infants between 3 and 15 mo of age were found to have intestinal barrier dysfunction 75% of the time and the presence and severity of the enteropathy (measured by lactulose:mannitol urinary excretion ratio, which is an indicator of intestinal permeability) could explain 43% of the long-term linear growth faltering (4). A subsequent study by the same researchers showed that enteropathy was associated with chronic immune stimulation, which was inversely correlated with growth during infancy (5). Increased intestinal permeability may allow the translocation of luminal antigens and microbial products than can stimulate a systemic inflammatory response. There are therefore 2 main pathways by which EE may cause growth retardation: malabsorption of nutrients in the small intestine and chronic systemic immune activation.
Although the cause of EE is unknown, it is almost universal in settings of poor environmental sanitation and it has been proposed that EE arises due to chronic exposure to feco-oral bacteria. However, in a study of 200 children in Benin, West Africa, nearly every child was exposed to AF, a toxin that commonly contaminates maize and groundnuts. Two observational studies have found that high exposure to AF is associated with growth retardation (6, 7). Children in developing countries are therefore potentially exposed to high levels of AF in areas where EE is common.
AF are one of a group of mycotoxins, secondary metabolites produced by microfungi that are capable of causing disease and death in humans and animals. There are 300–400 compounds that are currently classified as mycotoxins, with ∼12 recognized to be important to animal and human health (8). Two families of mycotoxins are particularly important in the context of child health in sub-Saharan Africa, because they are highly prevalent in the food chain and may have substantial negative health consequences. Aspergillus mold strains can produce AF and ochratoxin and Fusarium mold strains can produce FUM, DON, and zearalenone. Both molds are particularly common in maize and groundnuts, which constitute a major portion of the diet in many developing countries.
AF are the most well-known group of mycotoxins and have been extensively studied because of their role in liver cancer. Major types of AF include B1, B2, G1, and G2 as well as M1 and M2, which are metabolites present in human and animal milk. AF B1 is the most toxic of the AF compounds and is most often the subject of AF research. AF is fat soluble and can be measured in blood as an AF-albumin adduct, in urine (AF M1) as an AF-guanine adduct, and in breast milk as AF M1. Although there are few human data, animal studies provide evidence that chronic AF exposure retards growth and interferes with micronutrient absorption and utilization. The possible mechanisms suggested are: 1) gastrointestinal cytotoxicity resulting in increased diarrhea and abdominal pain; 2) interference with carbohydrate metabolism and fatty acid and phospholipid synthesis; 3) exacerbation of existing diseases that have been shown to retard growth; and 4) interference with nutrient availability and utilization (8).
FUM B is the most potent of the FUM toxins and may cause decreased expression of local proinflammatory cytokines and disruption of sphingolipid metabolism. FUM B is water soluble and can be measured in urine (9). FUM B has been associated with renal tumors (10), self-reported abdominal pain and diarrhea (11), esophageal cancer (12, 13), increased risk for neural tube defects (14), and retarded growth (15, 16). A recent study found a positive relationship between maize consumption and HIV transmission in Africa and suggested that 67% of the variation among HIV transmission in African countries was explained by variations in maize consumption between nations (17). It was speculated that FUM exposure may underlie this finding because of its action in increasing cell permeability and disrupting sphingolipid metabolism.
DON was given the name vomatoxin because of its ability to induce vomiting in pigs. DON is partially water soluble and exposure can be assessed by the urinary biomarker DON glucuronide (18). To date, there have been few epidemiological studies in humans that have assessed exposure using DON glucuronide. DON has been associated with diarrhea and vomiting (19), impaired gastric emptying and gut mobility (20), reduced weight gain (21), and impaired immune function in humans (22).
This review focuses on recent advances in evidence that suggests convergent pathways by which mycotoxins may cause stunting in children. Although exposure to AF has been documented in many countries in Africa, Asia, and the Middle East (23), studies reporting exposure to FUM and DON are limited due to the recent discovery of relevant biomarkers, and studies focusing on child growth are relatively recent and have been limited in scope to Africa. This review is not an exhaustive synthesis of the mycotoxin literature; rather, each section will present key studies that provide information to inform our understanding of the biological mechanisms by which food chain mycotoxin exposure may lead to growth retardation in children.
Current status of knowledge
Food chain mycotoxin exposure and growth
AF and birth weight.
In a recent review focusing on the reproductive health effects of AF, Shuaib et al. (24) concluded that there was no consensus on findings regarding the relationship between AF and birth weight. The wide variation in methodology and findings made it difficult to assess outcomes across studies. The review included only 5 studies assessing birth weight and all were cross-sectional. In 4 of the studies, authors reported a negative correlation between birth weight and maternal or infant AF levels, but in 2 studies, this relationship was only found in female infants (25–28). In the fifth study, there was no correlation between the presence of AF and birth weight (29).
The authors of these studies suggested various potential mechanisms underlying adverse birth outcomes and impaired growth. Turner et al. (25) suggested that intestinal malabsorption leads to zinc deficiency, which then causes growth faltering and immune deficiency. In addition, they suggested that AF exposure could cause enterocyte damage and increased intestinal permeability. Abdulrazzaq et al. (27) suggested that AF exposure inhibits the synthesis of proteins, enzymes, and clotting factors and impairs glucose metabolism, fatty acid synthesis, and phospholipid synthesis.
AF and stunting.
There are 2 key studies focusing on the relationship between AF exposure and growth retardation in children, both undertaken by the same research group in West Africa. Gong et al. (7) found serum AF-alb adducts were associated with stunting in children and demonstrated a clear dose-response relationship with height-for-age and weight-for-age Z-scores. The same group subsequently conducted a longitudinal study and found that the highest quartile of AF-albumin adduct was associated with a 1.7-cm mean height reduction compared with the lowest quartile (6). Given the intractable nature of stunting, these findings have stimulated discussion in the public health field and propelled a surge of research interest into further characterizing this potential relationship. The authors acknowledged that AF exposure may be a proxy for poor diet quality and suggested that future research must address this potential confounder. However, there are 3 biologically plausible pathways through which AF may affect growth: zinc deficiency, inhibition of protein synthesis leading to impaired metabolism, and enterocyte damage ultimately leading to systemic immune activation.
FUM and stunting.
A biomarker for FUM has only recently been validated (9) and no studies to date have investigated the relationship between FUM in urine and stunting. Dilkin et al. (15) found that pigs fed FUM alone or FUM and AF combined had a decrease in food consumption and body weight. There was an interactive effect of the 2 toxins, which indicates that the combination of AF and FUM may have a greater impact on growth than either alone. This finding is particularly interesting, because grain is often contaminated with multiple mycotoxins and it is common for AF and FUM to coexist in the same food source (30). Kimanya et al. (31) found that children with FUM intakes greater than the provisional maximum tolerable daily intake (PMTDI) were significantly shorter and lighter than children with FUM intakes less than the PMTDI. The major causal pathways suggested are decreased food intake and the inhibition of sphingolipid metabolism, which could lead to the degradation of the epithelial barrier and stimulation of an inflammatory immune response. It has been shown that FUM inhibits ceramide synthase, which inhibits sphingolipid metabolism (32). Complex sphingolipids are integral to cell membrane integrity and disturbance in this biosynthetic pathway could affect intestinal epithelial cell viability and proliferation, modify cytokine production, and modulate intestinal barrier function.
DON and stunting.
The effects of DON exposure on growth in children have not yet been studied, because a biomarker for DON was only recently discovered (33). Nonetheless, it is likely that DON has a negative effect on growth because of decreased food intake and reduced weight gain that has been observed in animal studies. The PMTDI for DON was established based on an observed reduction in weight gain in rodents (10). Rotter et al. (34) found that pigs fed grain contaminated with DON had a 20% lower feed intake and a 13% lower weight gain than the control group and suggested that DON induces feed refusal in pigs. In a recent study, Amuzie and Pestka (21) found that DON intake in mice induced a decrease in circulating levels of insulin-like growth factor (IGF)-1 (IGF-1), an important mediator of the growth hormone axis, and hepatic IGF acid-labile subunit (IGF-ALS), which forms a complex with circulating IGF-1. These data therefore suggest a direct mechanism by which mycotoxin ingestion may reduce linear growth, which requires further investigation.
Mycotoxins and gut health
We have discussed 4 main pathways by which AF, FUM, and DON may impair growth: 1) reduced nutrient absorption; 2) inhibition of protein synthesis; 3) inhibition of sphingolipid synthesis; and 4) food refusal. Three of these proposed pathways cause damage to the intestinal tract, leading to reduced absorptive capacity or impaired intestinal barrier function. The following section will review the current literature, focusing on the effects of mycotoxins on the intestine to further elucidate the possible relationship between mycotoxin exposure and growth.
AF and gut health.
Although several researchers have proposed that AF exposure may have negative effects on gut health, there is very little research on this hypothetical pathway. In one study in Ghana, researchers found a correlation between AF exposure and history of vomiting and abdominal swelling (35). The only studies in which a specific effect of AF on the gut has been examined were conducted in chickens. Yunus et al. (36) found that the small intestines of chickens that were exposed to AF weighed less compared with intestine of unexposed chickens, suggesting a decrease in absorptive capacity. Applegate et al. (37) found that chickens exposed to AF had reduced crypt depth but not villus length, which increased the villus:crypt ratio, indicating reduced intestinal absorptive capacity. The authors suggested that these physical alterations of the small intestine could cause zinc deficiency. In addition, they suggested that AF affects gut health and growth by inhibition of protein synthesis and not apoptosis of intestinal cells directly from AF exposure.
FUM and gut health.
Key studies in animal models and in vitro cell lines have investigated the impact of FUM on gut health. Devriendt et al. (38) found that pigs exposed to FUM that are infected with E. coli had higher and more prolonged shedding of E. coli, suggesting that FUM exposure may increase the duration of infection. Mice injected with FUM had an increase in sphingolipid bases, suggesting that FUM inhibits ceramide synthase, interrupts sphingolipid metabolism, and impairs membrane formation (39). Bouhet et al. (40) found that FUM exposure in intestinal porcine epithelial cells led to a decrease in the transepithelial electric resistance (a marker of increased epithelial permeability). In a follow-up study (41), FUM-exposed pigs showed a downregulation of local IL-8 measured in the intestine, suggesting that FUM affects mucosal immunity, which may increase the risk of enteric infection. Taken together, FUM may cause increased intestinal permeability due to disruptions in sphingolipid metabolism and increased risk of infection due to altered mucosal immunity.
DON and gut health.
No studies in humans have investigated the relationship between DON and gut health, but studies in poultry and several in vitro studies using cell lines have explored the potential causal pathway by which DON impairs growth. Two studies recently reported that poultry exposed to DON have a dose-/time-dependent decrease in the density of the small intestine due to decreases in villus height and circumference, which may result in decreased nutrient absorption (42, 43). Studies conducted in Human Colon Carcinoma cells-2 and intestinal porcine epithelial cells-1 found that DON exposure impairs protein synthesis and affects the expression of claudin-4, a protein critical to the proper functioning of the tight junctions that regulate intestinal permeability (44, 45). These authors linked impairment in protein synthesis directly to increased intestinal permeability and suggested that translocation of luminal antigens may stimulate a systemic immune response that negatively affects growth. Additionally, using human epithelial intestinal cell line HT-29-D4 as an in vitro model, Maresca et al. (46) found that DON impaired sugar and protein uptake by modulating the activity of sodium-glucose transport protein 1 (SGLT1), fructose transporter GLUT5, and L-serine transporters. They suggested that low levels of DON may cause inhibition of the intestinal SGLT1 transporter, resulting in a decrease of D-glucose–associated water absorption and increased water content of the intestinal lumen. High levels of DON may lead to inhibition of SGLT1 and local destruction of the epithelial barrier, resulting in inflammatory diarrhea. Severe and persistent diarrhea can lead to dehydration, loss of appetite, and impaired intake of key nutrients such as copper and zinc (47). The authors suggest that DON acts by inhibiting protein biosynthesis in HT-29-D4 cells.
Overall, DON inhibits protein synthesis, leading to: 1) decreased circulating IGF-1 and IGF-ALS; 2) reduced expression of claudin-4, which increases intestinal permeability and enables systemic immune activation; and 3) inhibition of SGLT1, GLUT5, and L-serine transporters, which may cause diarrhea and/or increased intestinal permeability.
Food chain mycotoxin exposure, gut health, and impaired growth: a conceptual framework
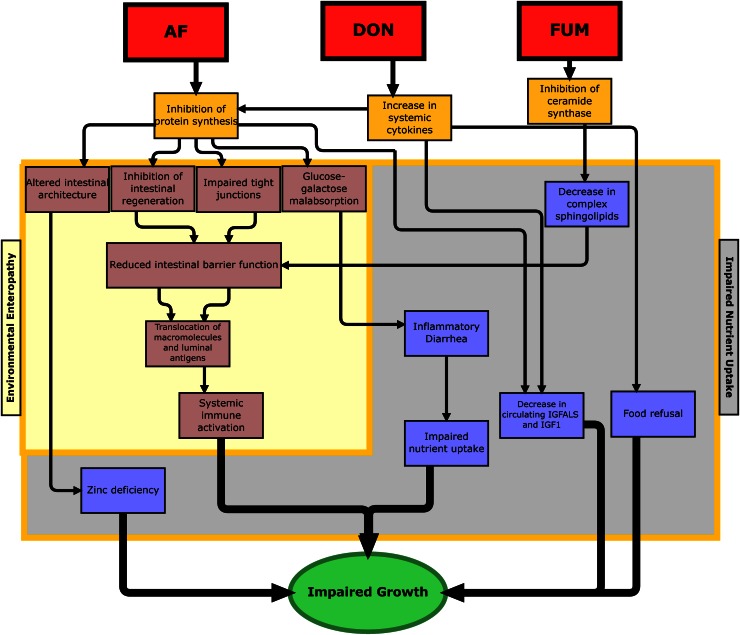
Although the mycotoxins described here have distinct actions, we propose that the intestine is a major target for the toxic effects of all 3, resulting in impaired nutrient uptake and intestinal pathology that resembles EE (Fig. 1). Persistent diarrhea results in impaired nutrient uptake (47). Altered intestinal architecture and EE can result in a loss of enzymes, leading to malabsorption of a variety of nutrients; most notably, EE has been associated with zinc deficiency (48). AF, FUM, and DON may therefore share a convergent pathway in which mucosal damage can lead to impaired nutrient absorption and/or increased intestinal permeability, pathology that resembles the changes seen in EE.
Figure 1.
A conceptual framework for the effect of mycotoxin exposure on growth retardation. The proposed pathways are: 1) inhibition of protein synthesis (AF, DON); 2) increase in systemic cytokines (DON); and 3) inhibition of ceramide synthase (FUM). Inhibition of protein synthesis can result in physical alterations to the intestine, leading to malabsorption of nutrients and impaired intestinal barrier function, similar to the pathology in EE. Increases in systemic cytokines can lead to impaired hepatic protein synthesis and reduced production of IGF-ALS and IGF-1. DON inhibits protein synthesis, which affects several important proteins, including claudin-4, SGLT1, GLUT5, L-serine transporters, IGF-1, and IGF-ALS. Claudin-4 is important in the proper functioning of tight junctions, and reduced expression of claudin-4 increases intestinal permeability. Reduced expression of SGLT1, GLUT5, and L-serine transporters leading to glucose-galactose malabsorption and impaired reabsorption of water in the colon may cause diarrhea, which could affect intestinal permeability as well as the uptake of key nutrients such as copper and zinc. IGF-1 mediates the effects of growth hormone, which is required for linear growth; the ALS forms a ternary complex with IGF-1 and its major binding protein (IGFBP3). FUM-induced inhibition of ceramide synthase affects sphingolipid metabolism, which compromises the cellular wall and may also lead to increased intestinal permeability directly or by inhibiting regeneration of the epithelial barrier. AF, aflatoxin; DON, deoxynivalenol; FUM, fumonisin; GLUT 5, fructose transporter; IGF, insulin-like growth factor; IGF-ALS, insulin-like growth factor acid labile subunit.
Conclusions
Historically, interventions to improve child growth have focused on macro- and micronutrient supplementation. Given the relatively modest impact of these approaches, nutrition and public health researchers have refocused their attention on EE because of its association with growth retardation in children. EE has been most commonly attributed to focally derived bacteria found in high concentrations in disadvantaged settings, where sanitation, hygienic practices, and drinking water treatment practices are frequently poor. However, there are multiple overlapping causes of enteropathies in developing countries (49), and the role of mycotoxins in mediating enteropathy has received little attention to date.
The downstream pathways described here are remarkably similar to the proposed causal pathways of EE (3, 47, 50) (Fig. 1). Our framework therefore presents a strong rationale for exploring the role of mycotoxins in the pathogenesis of EE. Human epidemiological research should include biomarkers assessing AF, FUM, and DON exposure and intermediary biological mechanisms presented in Figure 1 as well as a rigorous assessment of dietary diversity and sanitation and hygiene practices. Recent advances in biomarker development now permit epidemiological research on AF, FUM, and DON, which are often found in the same foods. Preventing mycotoxin exposure would require a set of interventions that focus on the whole food production chain, including: planting disease-resistant crops, bio-control agents, cultural planting practices, and harvesting, storing, and preparation practices (51). Because the cause of childhood growth faltering is likely to be multifactorial, future studies would benefit from collaborations between different research communities that have previously been quite separate, including both animal and human fields of nutrition, gastroenterology, toxicology and agronomy.
Acknowledgments
The authors thank Kathy Rasmussen for her help in editing the paper. All authors read and approved the final manuscript.
Footnotes
Author disclosures: L. E. Smith, R. J. Stoltzfus, and A. Prendergast, no conflicts of interest.
Abbreviations used: AF, aflatoxin; AF M1, urinary metabolite of aflatoxin; DON, deoxynivalenol; EE, environmental enteropathy; FUM, fumonisin; GLUT 5, fructose transporter; IGF, insulin-like growth factor; IGF-ALS, insulin-like growth factor acid-labile subunit; PMTDI, provisional maximum tolerable daily intake.
Literature Cited
- 1.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. Maternal. Maternal and child undernutrition 1: maternal and child undernutrition. Global and regional exposures and health consequences. Lancet. 2008;371:243–60 [DOI] [PubMed] [Google Scholar]
- 2.Dewey KG, Adu-Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern Child Nutr. 2008;4:24–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKay S, Gaudier E, Campbell DI, Prentice AM, Albers R. Environmental enteropathy: new targets for nutritional interventions. Int Health. 2010;2:172–80 [DOI] [PubMed] [Google Scholar]
- 4.Lunn PG, Northropclewes CA, Downes RM. Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet. 1991;338:907–10 [DOI] [PubMed] [Google Scholar]
- 5.Campbell DI, Elia M, Lunn PG. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J Nutr. 2003;133:1332–8 [DOI] [PubMed] [Google Scholar]
- 6.Gong Y, Hounsa A, Egal S, Turner PC, Sutcliffe AE, Hall AJ, Cardwell K, Wild CP. Postweaning exposure to aflatoxin results in impaired child growth: a longitudinal study in Benin, West Africa. Environ Health Perspect. 2004;112:1334–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gong YY, Cardwell K, Hounsa A, Egal S, Turner PC, Hall AJ, Wild CP. Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: cross sectional study. BMJ. 2002;325:20–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev. 2003;16:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong YY, Torres-Sanchez L, Lopez-Carrillo L, Peng JH, Sutcliffe AE, White KL, Humpf HU, Turner PC, Wild CP. Association between tortilla consumption and human urinary fumonisin b1 levels in a Mexican population. Cancer Epidemiol Biomarkers Prev. 2008;17:688–94 [DOI] [PubMed] [Google Scholar]
- 10. FAO, WHO. Joint FAO/WHO expert committee on food additives: fifty-sixth meeting. Geneva: WHO; 2001.
- 11.Bhat RV, Shetty PH, Rao PA, Rao VS. A foodborne disease outbreak due to the consumption of moldy sorghum and maize containing fumonisin mycotoxins. J Toxicol Clin Toxicol. 1997;35:249–55 [DOI] [PubMed] [Google Scholar]
- 12.Sydenham EW, Thiel PG, Marasas WFO, Shephard GS, Vanschalkwyk DJ, Koch KR. Natural occurrence of some Fusarium mycotoxins in corn from low and high esophageal cancer prevalence areas of the Transkei, Southern Africa. J Agric Food Chem. 1990;38:1900–3 [Google Scholar]
- 13.Chu FS, Li GY. Simultaneous occurrence of fumonisin b-1 and other mycotoxins in moldy corn collected from the Peoples-Republic-of-China in regions with high incidences of esophageal cancer. Appl Environ Microbiol. 1994;60:847–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Missmer SA, Suarez L, Felkner M, Wang E, Merrill AH, Rothman KJ, Hendricks KA. Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Environ Health Perspect. 2006;114:237–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dilkin P, Zorzete P, Mallmann CA, Gomes JDF, Utiyama CE, Oetting LL, Correa B. Toxicological effects of chronic low doses of aflatoxin b-1 and fumonisin b-1-containing fusarium mondiforme culture material in weaned piglets. Food Chem Toxicol. 2003;41:1345–53 [DOI] [PubMed] [Google Scholar]
- 16.Oswald IP, Desautels C, Laffitte J, Fournout S, Peres SY, Odin M, Le Bars P, Le Bars J, Fairbrother JM. Mycotoxin fumonisin b-1 increases intestinal colonization by pathogenic Escherichia coli in pigs. Appl Environ Microbiol. 2003;69:5870–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams JH, Grubb JA, Davis JW, Wang JS, Jolly PE, Ankrah NA, Ellis WO, Afriyie-Gyawu E, Johnson NM, Robinson AG, et al. HIV and hepatocellular and esophageal carcinomas related to consumption of mycotoxin-prone foods in Sub-Saharan Africa. Am J Clin Nutr. 2010;92:154–60 [DOI] [PubMed] [Google Scholar]
- 18.Meky FA, Turner P, Ashcroft A. Development of a urinary biomarker of human exposure to deoxynivanol. Food Chem Toxicol. 2003;41:265–73 [DOI] [PubMed] [Google Scholar]
- 19.Bhat RV, Ramakrishna Y, Beedu SR, Munshi KL. Outbreak of trichothecene mycotoxicosis associated with consumption of mold-damaged wheat products in Kashmir Valley, India. Lancet. 1989;1:35–7 [DOI] [PubMed] [Google Scholar]
- 20.Fioramonti J, Dupuy C, Dupuy J, Bueno L. The mycotoxin, deoxynivalenol, delays gastric-emptying through serotonin-3 receptors in rodents. J Pharmacol Exp Ther. 1993;266:1255–60 [PubMed] [Google Scholar]
- 21.Amuzie CJ, Pestka JJ. Suppression of insulin-like growth factor acid-labile subunit expression-a novel mechanism for deoxynivalenol-induced growth retardation. Toxicol Sci. 2010;113:412–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pestka JJ, Smolinski AT. Deoxynivalenol: toxicology and potential effects on humans. J Toxicol Environ Health B Crit Rev. 2005;8:39–69 [DOI] [PubMed] [Google Scholar]
- 23.Khlangwiset P, Shephard GS, Wu F. Aflatoxins and growth impairment: a review. Crit Rev Toxicol. 2011;41:740–55 [DOI] [PubMed] [Google Scholar]
- 24.Shuaib FMB, Ehiri J, Abdullahi A, Williams JH, Jolly PE. Reproductive health effects of aflatoxins: a review of the literature. Reprod Toxicol. 2010;29:262–70 [DOI] [PubMed] [Google Scholar]
- 25.Turner PC, Collinson AC, Cheung YB, Gong YY, Hall AJ, Prentice AM, Wild CP. Aflatoxin exposure in utero causes growth faltering in Gambian infants. Int J Epidemiol. 2007;36:1119–25 [DOI] [PubMed] [Google Scholar]
- 26.Shuaib FMB, Jolly PE, Ehiri JE, Yatich N, Jiang Y, Funkhouser E, Person SD, Wilson C, Ellis WO, Wang JS, et al. Association between birth outcomes and aflatoxin b-1 biomarker blood levels in pregnant women in Kumasi, Ghana. Trop Med Int Health. 2010;15:160–7 [DOI] [PubMed] [Google Scholar]
- 27.Abdulrazzaq YM, Osman N, Ibrahim A. Fetal exposure to aflatoxins in the United Arab Emirates. Ann Trop Paediatr. 2002;22:3–9 [DOI] [PubMed] [Google Scholar]
- 28.Abdulrazzaq YM, Osman N, Yousif ZM, Trad O. Morbidity in neonates of mothers who have ingested aflatoxins. Ann Trop Paediatr. 2004;24:145–51 [DOI] [PubMed] [Google Scholar]
- 29.Maxwell SM, Familusi JB, Sodeinde O, Chan MCK, Hendrickse RG. Detection of naphthols and aflatoxins in Nigerian cord-blood. Ann Trop Paediatr. 1994;14:3–5 [DOI] [PubMed] [Google Scholar]
- 30.Kimanya ME, De Meulenaer B, Tiisekwa B, Ndomondo-Sigonda M, Devlieghere F, Van Camp J, Kolsteren P. Co-occurrence of fumonisins with aflatoxins in home-stored maize for human consumption in rural villages of Tanzania. Food Addit Contams Part A Chem Anal Control Expo Risk Assess. 2008;25:1353–64 [DOI] [PubMed] [Google Scholar]
- 31.Kimanya ME, De Meulenaer B, Roberfroid D, Lachat C, Kolsteren P. Fumonisin exposure through maize in complementary foods is inversely associated with linear growth of infants in Tanzania. Mol Nutr Food Res. 2010;54:1659–67 [DOI] [PubMed] [Google Scholar]
- 32.Bouhet S, Oswald IP. The intestine as a possible target for fumonisin toxicity. Mol Nutr Food Res. 2007;51:925–31 [DOI] [PubMed] [Google Scholar]
- 33.Pestka JJ. Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch Toxicol. 2010;84:663–79 [DOI] [PubMed] [Google Scholar]
- 34.Rotter BA, Thompson BK, Lessard M. Effects of deoxynivalenol-contaminated diet on performance and blood parameters in growing swine. Can J Anim Sci. 1995;75:297–302 [Google Scholar]
- 35.Jolly PE, Jiang Y, Ellis WO, Awuah RT, Appawu J, Nnedu O, Stiles JK, Wang J-S, Adjei O, Joly CM, et al. Association between aflatoxin exposure and health characteristics, liver function, hepatitis and malaria infections in Ghanaians. J Nutr Environ Med (Abingdon). 2007;16:242–57 [Google Scholar]
- 36.Yunus AW, Ghareeb K, Abd-El-Fattah AAM, Twaruzek M, Boehm J. Gross intestinal adaptations in relation to broiler performance during chronic aflatoxin exposure. Poult Sci. 2011;90:1683–9 [DOI] [PubMed] [Google Scholar]
- 37.Applegate TJ, Schatzmayr G, Pricket K, Troche C, Jiang Z. Effect of aflatoxin culture on intestinal function and nutrient loss in laying hens. Poult Sci. 2009;88:1235–41 [DOI] [PubMed] [Google Scholar]
- 38.Devriendt B, Gallois M, Verdonck F, Wache Y, Bimczok D, Oswald IP, Goddeeris BM, Cox E. The food contaminant fumonisin b1 reduces the maturation of porcine cd11r1+ intestinal antigen presenting cells and antigen-specific immune responses, leading to a prolonged intestinal etec infection. Vet Res. 2009;40:40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Enongene EN, Sharma RP, Bhandari N, Voss KA, Riley RT. Disruption of sphingolipid metabolism in small intestines, liver and kidney of mice dosed subcutaneously with fumonisin b-1. Food Chem Toxicol. 2000;38:793–9 [DOI] [PubMed] [Google Scholar]
- 40.Bouhet S, Hourcade E, Loiseau N, Fikry A, Martinez S, Roselli M, Galtier P, Mengheri E, Oswald IP. The mycotoxin fumonisin b-1 alters the proliferation and the barrier function of porcine intestinal epithelial cells. Toxicol Sci. 2004;77:165–71 [DOI] [PubMed] [Google Scholar]
- 41.Bouhet S, Le Dorze E, Peres S, Fairbrother JM, Oswald IP. Mycotoxin fumonisin b-1 selectively down-regulates the basal IL-8 expression in pig intestine: In vivo and in vitro studies. Food Chem Toxicol. 2006;44:1768–73 [DOI] [PubMed] [Google Scholar]
- 42.Yunus AW, Blajet-Kosicka A, Kosicki R, Khan MZ, Rehman H, Bohm J. Deoxynivalenol as a contaminant of broiler feed: Intestinal development, absorptive functionality, and metabolism of the mycotoxin. Poult Sci. 2012;91:852–61 [DOI] [PubMed] [Google Scholar]
- 43.Awad WA, Bohm J, Razzazi-Fazeli E, Zentek J. Effects of feeding deoxynivalenol contaminated wheat on growth performance, organ weights and histological parameters of the intestine of broiler chickens. J Anim Physiol Anim Nutr (Berl). 2006;90:32–7 [DOI] [PubMed] [Google Scholar]
- 44.Van de Walle JV, Sergent T, Piront N, Toussaint O, Schneider Y-J, Larondelle Y. Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicol Appl Pharmacol. 2010;245:291–8 [DOI] [PubMed] [Google Scholar]
- 45.Pinton P, Braicu C, Nougayrede J-P, Laffitte J, Taranu I, Oswald IP. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through a mitogen-activated protein kinase-dependent mechanism. J Nutr. 2010;140:1956–62 [DOI] [PubMed] [Google Scholar]
- 46.Maresca M, Mahfoud R, Garmy N, Fantini J. The mycotoxin deoxynivalenol affects nutrient absorption in human intestinal epithelial cells. J Nutr. 2002;132:2723–31 [DOI] [PubMed] [Google Scholar]
- 47.Dewey KG, Mayers DR. Early child growth: how do nutrition and infection interact? Matern Child Nutr. 2011;7:129–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manary MJ, Abrams SA, Griffin IJ, Quimper MM, Shulman RJ, Hamzo MG, Chen ZS, Maleta K. Perturbed zinc homeostasis in rural 3–5-y-old Malawian children is associated with abnormalities in intestinal permeability attributed to tropical enteropathy. Pediatr Res. 2010;67:671–5 [DOI] [PubMed] [Google Scholar]
- 49.Prendergast A, Kelly P. Enteropathies in the developing world: Neglected effects on global health. Am J Trop Med Hyg. 2012;86:756–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Humphrey JH. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet. 2009;374:1032–5 [DOI] [PubMed] [Google Scholar]
- 51.Waliyar F, Kumar PL, Traore A, Ntare BR, Diarra B, Kodio O, Bi C. Pre- and postharvest management of aflatoxin contamination in peanuts. In: Leslie JF, et al. ed. Mycotoxins: Detection methods, management, public health and agricultural trade. Cambridge, MA: CABI, . 2008:209–218.