Abstract
The number of persons who relocate to regions of high altitude for work, pleasure, sport, or residence increases every year. It is known that the reduced supply of oxygen (O2) induced by acute or chronic increases in altitude stimulates the body to adapt to new metabolic challenges imposed by hypoxia. Sleep can suffer partial fragmentation because of the exposure to high altitudes, and these changes have been described as one of the responsible factors for the many consequences at high altitudes. We conducted a review of the literature during the period from 1987 to 2012. This work explored the relationships among inflammation, hypoxia and sleep in the period of adaptation and examined a novel mechanism that might explain the harmful effects of altitude on sleep, involving increased Interleukin-1 beta (IL-1β), Interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) production from several tissues and cells, such as leukocytes and cells from skeletal muscle and brain.
1. Background
In recent years, the interest in activities carried out at high altitudes has grown. Millions of people travel to regions of high altitudes (i.e., above 2500 m) for tourism, sport, work, or permanent residence. However, living in high altitudes can lead to hypoxia. The effects of exposure to hypobaric hypoxia, which is present at high altitude, are dependent on the length of time spent at high altitude and the altitude reached.rt Because O2 is required for the maintenance of vital functions, blood oxygenation can affect several physiological functions. Exposure to hypobaric hypoxia can result in extreme conditions, such as acute mountain sickness (AMS), high altitude pulmonary edema (HAPE), and high altitude cerebral edema (HACE), as well as other conditions, such as headache, nausea, vomiting, and gastrointestinal alterations [1–5].
Alterations in cardiovascular and respiratory functions promoted by altitude have been previously described. More recently, attention has focused on neurobiological functions, including sleep, cognition, and humor [6, 7]. Thus, this review discusses the effects of hypoxia stimulated by high altitude on sleep, with an emphasis on neuroimmunoendocrine interactions.
2. Methods
For this study, we conducted a systematic and integrative review of the literature, using source articles indexed by the ISI database, PubMED and MEDLINE by searching for books that addressed specific aspects related to altitude/hypoxia, cytokines, and sleep during the period from 1987 to 2012.
The keywords searched were “cytokines and hypoxia,” “cytokines and altitude,” “inflammation and hypoxia,” “inflammation and altitude,” “sleep and hypoxia,” “sleep and altitude,” “sleep and cytokines,” and “sleep and inflammation.” These descriptors were used in a Boolean-specific basis to obtain various arrangements thought to maximize both the coverage and quality of the search. No restrictions were made regarding age or gender.
3. Altitude
The principal characteristic of exposure to high altitudes is the fact that there is an inverse correlation between altitude and the partial pressure of O2. Therefore, at high altitudes, the body tries to adapt by generating many responses, including changes in skeletal muscle and in the endocrine and nervous systems [8].
Although the barometric pressure decreases with increasing altitude, the gas composition does not change until above the 1200 m level. Although the percentage of ambient oxygen is maintained at 20.93%, the increase in altitude decreases the O2 partial pressure in expired air. This decrease promotes a partial impairment in the support of O2, resulting in less oxygen transported by hemoglobin and consequently less O2 available for tissues. In fact, all tissues that need O2 for energy production are affected by hypoxia, and each tissue response depends on several factors, including the O2 demand by the tissue, the time of exposure, and the individual's characteristics [9].
The classical response induced by high altitude includes respiratory and cardiovascular changes that are initiated within minutes after the person reaches the altitude [10]. In fact, there is an inverse correlation between increases in altitude and hemoglobin saturation. In addition, the number of hemoglobin molecules begins to increase, even at altitudes as low as 500 m. At the same time, alterations in hyperventilation occur at rest and during acute physical exercise. The heart rate increases in a manner similar to the increase seen in cardiac output, which attempts to compensate by decreasing the partial pressure of carbon dioxide in the arterial blood (PaCO2); however, these alterations are not sufficient to affect the oxygen consumption (VO2) decrease and aerobic energy production. As a result, remaining at high altitudes might result in fatigue and a significant decrease in the capacity to work and physically perform, especially aerobic and endurance exercise. In addition, it is possible to have an increase in blood pressure due to an increase of norepinephrine levels because of the impact of stimulated activities of rest and exercise [11, 12].
High altitude (above 3000 m) is a powerful stressor. Being at these altitudes can modify metabolic and physiological functions, and the body then tries to reestablish the homeostasis that was altered by hypoxia [13]. Several studies have shown that acute or chronic exposure to altitudes between 2500 and 5000 m results in sympathoadrenal responses that are exacerbated by metabolic alterations to other systems [13], including the immune system [12, 14].
Under these conditions, it is possible to produce a rapid adrenaline hormonal response and a transient increase in plasma cortisol concentrations [15, 16].
Altitude-induced hypoxia can also stimulate the release of other hormones involved in the recovery of homeostasis. One of those hormones is erythropoietin (EPO). Humans exhibit increased EPO concentrations two hours after exposure to high altitudes [17]. EPO is fundamentally important to the organization of the physiological response to altitude and can modulate the expression of many proteins. Increases in EPO and hemoglobin are essential for acclimatization and the maintenance of the O2 supply to tissues.
It has been demonstrated that acute exposure to elevated altitudes can result in changes to several immunological parameters [12, 18]. Hypoxia for even a few hours is sufficient to induce significant changes in neutrophil and lymphocyte numbers, which are mainly characterized by reductions in cluster of differentiation (CD), cell numbers, and cellular proliferation [19]. Several studies have shown that acute hypoxia results in an increase in natural killer cells (NK cells) numbers and activity [20].
Studies have shown that lymphocytes and phagocytes present some signs of adaptation if the hypobaric stimulus is chronic, due to alterations in the production and release of substances such as cytokines and antibodies [21]. Other studies have shown that immunity mediated by T lymphocytes can be stopped by exposure to elevated altitudes [12, 22]. Facco et al. [21] confirmed that exposure to elevated altitudes can alter the number and cellular function and suggested that new studies be carried out to evaluate the expression of cytokines by T lymphocytes, particularly to determine the maintenance of the T helper cells (Th1/Th2) response.
It was suggested that remaining at an altitude of 4000 m above sea level was associated with increased plasma concentrations of IL-6 and Interleukin-1 receptor antagonist (IL-1ra). Furthermore, C-reactive protein (CRP) increases are associated with the development of pulmonary edema [23]. Numerous stressful events are associated with increases in cytokine release and disturbances in the pro/anti-inflammatory cytokine ratio [24]. Hypoxia alone seems to have a decisive role; however, the mechanisms responsible for the induction of cytokines under hypoxic conditions are not clear. Exposure to elevated attitudes can cause cellular damage due to increased oxidative stress and altered cytokine release; in turn, these cytokines participate in the recovery from cellular damage [25, 26].
4. Altitude and Inflammation
The exposure to hypoxia promotes several transcription factors, including nuclear factor-κB (NF-κB), which plays a central role in stimulating the proinflammatory cytokines TNF-α and IL-6 [27]. Similarly, several studies with rodents and humans have shown that effects-induced hypoxia can cause inflammation, including increase in transvascular leakage and oxidative stress with increased NF-κB expression in lungs followed by significant increase in proinflammatory cytokines IL-1, IL–6, and TNF-α [28–30].
A decrease in plasma cytokine concentration or the treatment with appropriate antagonists promotes partial reversion of the symptoms and illnesses, including cardiovascular disease, obesity, insulin resistance, and depression [31, 32]. Therefore, we suggest that sleep disturbances due to high altitudes could also be caused by increases in proinflammatory cytokines from several cells, such as skeletal muscle and immune cells, in association with the capillary leakage or repeated wakening aspects of AMS, which usually occur concurrently with the hyperopic phase of periodic breathing.
Hojman et al. [33] observed that augments the acute inflammatory effect in humans. In this study, the authors demonstrated that when EPO was given prior to a bolus injection of endotoxin, the levels of TNF-α and IL-6 were enhanced by 5- and 40-fold, respectively, whereas the endotoxin-induced increase in Interleukin-10 (IL-10) was not influenced by EPO. This interaction between EPO and inflammation may corroborate with sleep disruptions found at high altitudes.
However, Hojman et al. [34] used animal experiments to show that when EPO was expressed at supraphysiological levels, there were substantial metabolic effects, including protection against diet-induced obesity and normalization of glucose sensitivity, associated with a shift towards increased fat metabolism in the muscles.
Unfortunately, only limited information from well-controlled laboratory and field studies is available on this topic. Relatively, little is known about the influence of altitude on the interaction of cytokines and sleep. The significant effects (pro- and anti-inflammatory) of EPO in acute and chronic high altitudes should be investigated further. Thus, the sleep complains due to high altitudes could also be caused by increases in proinflammatory cytokines from several cells, such as skeletal muscle and immune cells, in association with the capillary leakage or repeated wakening aspects of AMS, which usually occur concurrently with the hyperopic phase of periodic breathing. This interaction between EPO and inflammation may corroborate with sleep disruptions found at high altitudes.
5. Cytokines
Cytokines are proteins produced and released by different cells, for example: leukocytes, muscle cells, and neurons. These proteins can act in a pleiotropic way or in synergy with other substances and can modulate the production of other cytokines [35]. Cytokines function in the regulation of metabolism by influencing hormone secretion, regulating the TH1/TH2 immune responses, and inducing inflammatory responses; in the nervous system, they influence complex neuronal actions and modulate thermoregulation, food intake, and neurobiological patterns [35, 36] during sleep.
Interleukin-1 (IL-1) increased in plasma concentration may cause fever, sickness behavior, increased heart rate, increased blood flow in many vascular beds, and increased sympathetic tone; changes in carbohydrate, fat, and protein metabolism also occur [24, 35]. The effects of IL-1 can be reversed by treatment with IL-1ra, an antagonist of IL-1, which functions to prevent IL-1 binding to its specific receptors [35].
The TNF-α is mainly produced by macrophages and neutrophils, but other cells, such as lymphocytes, NK cells, endothelial cells and neural cells, might also have the capacity to produce it [24]. TNF-α production occurs in response to a wide variety of stimuli, including infections and stimulation by other cytokines or mitogens [37]. TNF-α is a potent pleiotropic cytokine due to its ability to activate multiple signal transduction pathways and induce or suppress the expression of a number of genes. In addition, it has potent endogenous pyrogenic properties and may promote changes in the body's physiological temperature [38]. Moreover, tissues that present marked cachexia show high TNF-α activity, as observed in catabolic conditions, such as cancer and systemic inflammatory diseases [24].
The Interleukin-6 (IL-6) plays a significant role in regulating the pro-inflammatory response [24]. However, due to its capacity to stimulate the hypothalamus-pituitary-adrenal axis to produce cortisol and anti-inflammatory cytokines, such as interleukin-4 (IL-4) and it also has anti-inflammatory properties [24].
The Interleukin-10 (IL-10) has multiple biological activities and affects many different cell types. These include monocytes/macrophages, T cells, B cells, NK cells, neutrophils, endothelial cells, and peripheral blood mononuclear cells (PBMCs). IL-10 also acts in the regulation of inflammation because it is produced by adipose and muscle tissues, which are important to the pro/anti-inflammatory ratio in conditions such as physical exercise, obesity, and inflammatory diseases [39, 40].
Cytokines can penetrate the blood-brain barrier (BBB) and act indirectly on the brain by stimulating the production of chemical second messengers that carry information to targets such as NF-κB and adenosine [41, 42] as shown in Figure 1. The hypothesis that cytokines could influence the functions of the nervous system (NS) is based on observations that treatment with cytokines, such as Interferon-γ (INF-γ), promotes neuroendocrine alterations, and other studies show that there are receptors for these cytokines in many areas of the brain [38, 43, 44]. Additional studies have shown that an increase in proinflammatory cytokine concentrations promotes a decrease in the transendothelial electrical resistance and an increase in the permeability of the BBB [45]. Finally, it is possible that cytokines can be produced within the brain itself in response to neuronal activity [35].
Figure 1.
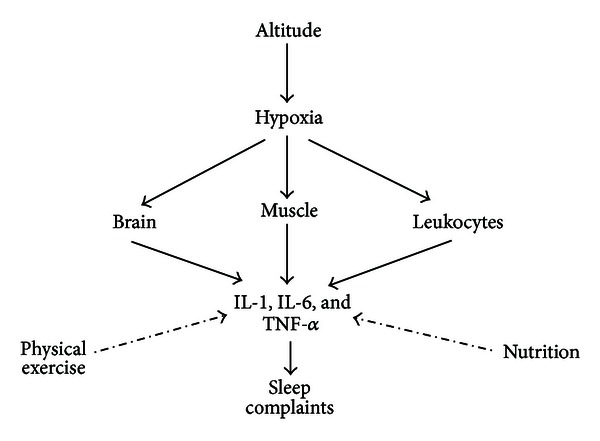
Solid line indicates stimulation; dotted line indicates inhibition.
More recently, several studies have shown the existence of an afferent neural pathway by which inflammation in the peritoneal cavity might influence the brain [46]. Subdiaphragmatic transection of the vagus produces reduction of fever, poor sleep, nocturnal excretion of norepinephrine, and hypothalamic production of IL-1 induced by lipopolysaccharides (LPS) in the peritoneal cavity [47], thereby validating this hypothesis. These alterations are not due to a reduction in the circulating levels of cytokines or to the attenuation of the inflammatory response induced by lipopolysaccharide (LPS) but rather to a defective translation of cytokines in the brain [48].
High altitudes are potent stressors known to alter physiological and metabolic functions in the search for mechanisms to try to restore homeostasis by hypoxia disbalance. The acute or chronic exposure to altitudes between 2500 and 5000 m stimulates in a response sympathoadrenal leading to numerous other metabolic changes that, in turn, could affect several physiological systems including the production of cytokines and worsen the quality of sleep [49–51].
Currently, a strong relationship between sleep and immune process has been shown. The proinflammatory cytokines, including IL-1, IL-6, and TNF-α, are known as sleep-regulatory cytokines. However, sleep-promoting properties are also possessed by several other immune and proinflammatory cellular classes. Many studies reporting these relationships are focused on the perspective of low-grade inflammation associated with significant sleep alterations and on the perspective of immune dysregulation associated with several primary sleep disorders [52].
6. Altitude and Sleep
Sleep is a functional state that includes a complex combination of physiological and behavioral processes. It has some characteristic manifestations, such as a cyclic pattern, relative immobility, and an increase in the response threshold to external stimuli [53]. Sleep is very important, as it is evident from studies of acute or chronic sleep deprivation and sleep disorders; these impairments promote several alterations, including a marked increase in the production of stress hormones, including catecholamines and cortisol, a reduction in cognitive capacity, and a reduction in the state of alertness, among others [54].
Sleep can be divided into two phases: the nonrapid eye movement (nREM) phase, in which the electroencephalogram (EEG) records a synchronized tracing, and the rapid eye movement (REM) sleep phase, in which the electroencephalogram records signals similar to those in the wake period that are associated with the rapid eye movements [55, 56].
Two hypotheses attempt to explain the mechanisms involved in sleep regulation, and it is possible that these hypotheses are not mutually exclusive and could happen simultaneously. One hypothesis describes the role of circadian rhythms, while the other is related to the homeostatic effects of sleep [55].
The biochemical mechanisms that control sleep are very complex because sleep modulation is dependent on several factors, including carbon dioxide (CO2) concentrations, as well as potassium, free radical, nitric oxide, hormone, and adenosine levels [57]. Proinflammatory cytokines play an important role in sleep regulation [58]. Some cytokines have an antisomnogenic action by decreasing prosomnogenic cytokine production, while others cytokines have the opposite effect [59].
Most of the existing studies on sleep and altitude were carried out in the field. There have also been studies carried out in normobaric hypoxic rooms that simulate conditions of high altitude [60]. High altitude has frequently been associated with sensations of suffocation when awakening from sleep. In fact, several studies showed that hypoxia directly acts on the architecture and quality of sleep in humans and rodents; these effects include increases in Stage I, decreases in REM sleep, lesions in cerebral regions that control sleep, and increases in the sensations of sleep deprivation and sleep fragmentation [61–63].
In fact, around 60% of persons subjected to altitudes of 3500 m or higher experience various sleep complaints. Recurring wakefulness is the most common characteristic due to the decreased O2 saturation, which leads to sleep fragmentation [45, 64, 65]. In addition, hypoxia can cause poor sleep quality due to slight reductions in delta sleep, relative reductions in REM sleep, and agitation during the night [63]; however, overall total sleep time (TST) is not reduced. Therefore, the reduced subjective sleep quality is due to a higher arousal frequency. Despite previous studies suggesting that the impairment of sleep persists even after a season of acclimatization [64, 65], partial recovery of the damage during sleep can occur after spending some days at high altitude [26]. This finding has been shown in animal studies in which several days were spent in hypoxic conditions but not after a sudden ascent.
7. Altitude, Sleep, and Cytokines
To date, the effects of altitude on the architecture and quality of sleep are not well known [66]. Studies in rodents and humans suggest that prolonged exposure to hypoxia can alter circadian rhythms by reducing the amplitude of circadian oscillations and by possibly leading to changes in several variables, such as activity, hunger, metabolic rate, and the dark and light cycle [60, 67, 68]. The modification of melatonin and neurotransmitter release, metabolism in peripheral tissues, and modulation of several hormones and cytokines that participate in sleep regulation and gene expression responsible for the functions of the biological clock are also affected [69–71]. In part, this alteration on the sleep leads to upregulation of proinflammatory cytokines in response at high altitude.
The relationship between sleep and cytokines was first established through observations that sleep deprivation increases INF-γ production. To date, the roles of several growth factors, including epidermal growth factor, fibroblast growth factor, nerve growth factor, brain-derived neurotrophic factor, granulocyte-macrophage colony-stimulating factor and insulin-like growth factor-1 (IGF-1), have also been investigated for their roles in sleep modulation [59]. However, this review focuses on the pro- and anti-inflammatory cytokines IL-1β, IL-6, IL-10, and TNF-α.
8. Hypoxia, Physical Exercise, Cytokines and Sleep
In relation to physical exercise in hypoxia, few and contradictory studies evaluated the effect of exercise on condition of hypoxia on the production of cytokines [72]. The exercise performed under hypoxic conditions/high altitude represents an additional stress condition in relation to the exercise performed at sea level [73]. Even when the exercise intensity is relative, that is, taking into account that the maximum VO2 and performance decreases as the altitude increases [73, 74]. So many factors should be taken into account when discussing the interaction hypoxia and cytokines. The increase in altitude or the extent of hypoxia is a primary factor that influences the level of variation in physiological and biochemical parameters which can modulate the immune response mediated by exercise [12]. Collectively, analyzing the results of the previously published works, one can speculate that there is a threshold elevation that should be followed to achieve the benefits associated with living or training at altitude with the least possible damage [12].
The concentration of cytokines, notably IL-6 and inflammatory markers such as the acute phase proteins CRP has its increased concentrations in response to a session with moderate exercise intensity of 50% VO2 Max at an altitude of 4300 m over the same exercise at sea level [75]. However, in this study, the authors evaluated the effects of varying intensities of exercise in normoxic and hypoxic environments at equivalently 3100 m on immune regulation and metabolic responses and showed that during prolonged physical exercise at 40 and 60% of VO2 Max this doesnot seem to dramatically alter the response of the selected immune system including IL-1 or TNF-α and metabolic markers. Exercise training that uses acute hypoxic environments does not adversely affect immune regulation system status and may be beneficial for those individuals looking to increase endurance performance [76].
One way to partially reverse the effects of hypoxia on sleep patterns can be by performing moderate exercise, taking into account that in normoxic condition physical exercise beside improving sleep also modulates the memory, attention, and mood state [77].
Physical exercise has been considered as the best strategy to prevent and treat chronic inflammatory diseases of low grade [78, 79], such as those generated by sleep disorders. Regular physical training is able to increase the production of anti-inflammatory cytokines and decrease the concentrations of circulating proinflammatory cytokines and can improve the quality of sleep.
9. Conclusions
The relationships among inflammation, hypoxia, and sleep are discussed in the present study; we conclude that hypoxia induced by elevated altitudes in the adaptation period results in a disturbance in the balance of homeostasis and affects several physiological systems. Consequently, severe changes in sleep architecture and sleep quality may occur. These changes might be mediated by increases in plasma concentrations of IL-1, IL-6, and TNF-α and possibly through the stimulation of EPO.
Acknowledgments
All authors are grateful to the Associação Fundo de Incentivo a Psicofarmacologia (AFIP), Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq), Centro Multidisciplinar em Sonolência e Acidentes (CEMSA), Centros de Pesquisa, Expansão e Difusão do Instituto do Sono CEPID/SONO, Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP), CEPID no. 98/143003-3, Universidade Federal de São Paulo (UNIFESP), and the Centro de Estudos em Psicobiologia e Exercício (CEPE).
References
- 1.Pollard AJ, Murdoch DR, Bartsch P. Children in the mountains. British Medical Journal. 1998;316(7135):874–875. doi: 10.1136/bmj.316.7135.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hackett PH, Roach RC. High-altitude illness. New England Journal of Medicine. 2001;345(2):107–114. doi: 10.1056/NEJM200107123450206. [DOI] [PubMed] [Google Scholar]
- 3.Debudaj A, Bobiński R. The pathophysiology of acute mountain sickness. Polski Merkuriusz Lekarski. 2010;28(168):478–481. [PubMed] [Google Scholar]
- 4.Luks AM, McIntosh SE, Grissom CK, et al. Wilderness medical society consensus guidelines for the prevention and treatment of acute altitude illness. Wilderness and Environmental Medicine. 2010;21(2):146–155. doi: 10.1016/j.wem.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Imray C, Wright A, Subudhi A, Roach R. Acute mountain sickness: pathophysiology, prevention, and treatment. Progress in Cardiovascular Diseases. 2010;52(6):467–484. doi: 10.1016/j.pcad.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 6.De Aquino Lemos V, Antunes HK, Santos RV, Lira FS, Tufik S, Mello MT. High altitude exposure impairs sleep patterns, moos and cognitive functions. Psychophysiology. 2012;49(9):1298–1306. doi: 10.1111/j.1469-8986.2012.01411.x. [DOI] [PubMed] [Google Scholar]
- 7.Lemos VA, Antunes HKM, Dos Santos RVT, Prado JMDS, Tufik S, De Mello MT. Effects of exposure to altitude on neuropsychology aspects: a literature review. Revista Brasileira de Psiquiatria. 2010;32(1):70–76. doi: 10.1590/s1516-44462009005000013. [DOI] [PubMed] [Google Scholar]
- 8.Virués-Ortega GJ, Garrido EC, Javierre KC, Kloezeman KC. Human behaviour and development under high-altitude conditions. Developmental Science. 2006;9(4):400–410. doi: 10.1111/j.1467-7687.2006.00505.x. [DOI] [PubMed] [Google Scholar]
- 9.Gore CJ, Clark SA, Saunders PU. Nonhematological mechanisms of improved sea-level performance after hypoxic exposure. Medicine and Science in Sports and Exercise. 2007;39(9):1600–1609. doi: 10.1249/mss.0b013e3180de49d3. [DOI] [PubMed] [Google Scholar]
- 10.Napoli AM, Milzman DP, Damergis JA, Machan J. Physiologic affects of altitude on recreational climbers. American Journal of Emergency Medicine. 2009;27(9):1081–1084. doi: 10.1016/j.ajem.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 11.Koller A. Exercise-induced increases in cardiac troponins and prothrombotic markers. Medicine and Science in Sports and Exercise. 2003;35(3):444–448. doi: 10.1249/01.MSS.0000053736.51903.0E. [DOI] [PubMed] [Google Scholar]
- 12.Mazzeo RS. Altitude, exercise and immune function. Exercise Immunology Review. 2005;11:6–16. [PubMed] [Google Scholar]
- 13.Mazzeo RS, Child A, Butterfield GE, et al. Sympathoadrenal responses to submaximal exercise in women after acclimatization to 4,300 meters. Metabolism. 2000;49(8):1036–1042. doi: 10.1053/meta.2000.7706. [DOI] [PubMed] [Google Scholar]
- 14.Mazzeo RS. Physiological responses to exercise at altitude: an update. Sports Medicine. 2008;38(1):1–8. doi: 10.2165/00007256-200838010-00001. [DOI] [PubMed] [Google Scholar]
- 15.Barnholt KE, Hoffman AR, Rock PB, et al. Endocrine responses to acute and chronic high-altitude exposure (4,300 meters): modulating effects of caloric restriction. American Journal of Physiology-Endocrinology and Metabolism. 2006;290(6):E1078–E1088. doi: 10.1152/ajpendo.00449.2005. [DOI] [PubMed] [Google Scholar]
- 16.Niess AM, Fehrenbach E, Strobel G, et al. Evaluation of stress responses to interval training at low and moderate altitudes. Medicine and Science in Sports and Exercise. 2003;35(2):263–269. doi: 10.1249/01.MSS.0000048834.68889.81. [DOI] [PubMed] [Google Scholar]
- 17.Blegen M, Cheatham C, Caine-Bish N, Woolverton C, Marcinkiewicz J, Glickman E. The immunological and metabolic responses to exercise of varying intensities in normoxic and hypoxic environments. Journal of Strength and Conditioning Research. 2008;22(5):1638–1644. doi: 10.1519/JSC.0b013e318181fdfd. [DOI] [PubMed] [Google Scholar]
- 18.Thake CD, Mian T, Garnham AW, Mian R. Leukocyte counts and neutrophil activity during 4 h of hypocapnic hypoxia equivalent to 4000 m. Aviation Space and Environmental Medicine. 2004;75(9):811–817. [PubMed] [Google Scholar]
- 19.Pedersen BK, Steensberg A. Exercise and hypoxia: effects on leukocytes and interleukin-6-shared mechanisms? Medicine and Science in Sports and Exercise. 2002;34(12):2004–2012. doi: 10.1097/00005768-200212000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Caldwell CC, Kojima H, Lukashev D, et al. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. Journal of Immunology. 2001;167(11):6140–6149. doi: 10.4049/jimmunol.167.11.6140. [DOI] [PubMed] [Google Scholar]
- 21.Facco M, Zilli C, Siviero M, et al. Modulation of immune response by the acute and chronic exposure to high altitude. Medicine and Science in Sports and Exercise. 2005;37(5):768–774. doi: 10.1249/01.mss.0000162688.54089.ce. [DOI] [PubMed] [Google Scholar]
- 22.Hartmann G, Tschöp M, Fischer R, et al. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine. 2000;12(3):246–252. doi: 10.1006/cyto.1999.0533. [DOI] [PubMed] [Google Scholar]
- 23.Dosek A, Ohno H, Acs Z, Taylor AW, Radak Z. High altitude and oxidative stress. Respiratory Physiology and Neurobiology. 2007;158(2-3):128–131. doi: 10.1016/j.resp.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Moldoveanu AI, Shephard RJ, Shek PN. The cytokine response to physical activity and training. Sports Medicine. 2001;31(2):115–144. doi: 10.2165/00007256-200131020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lahiri S, Di Giulio C, Roy A. Lessons from chronic intermittent and sustained hypoxia at high altitudes. Respiratory Physiology and Neurobiology. 2002;130(3):223–233. doi: 10.1016/s0034-5687(01)00343-7. [DOI] [PubMed] [Google Scholar]
- 26.Mortola JP, Seifert EL. Hypoxic depression of circadian rhythms in adult rats. Journal of Applied Physiology. 2000;88(2):365–368. doi: 10.1152/jappl.2000.88.2.365. [DOI] [PubMed] [Google Scholar]
- 27.Taylor CT. Interdependent roles for hypoxia inducible factor and nuclear factor-kappaB in hypoxic inflammation. Journal of Physiology. 2008;1(586, part 17):4055–4059. doi: 10.1113/jphysiol.2008.157669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazzeo RS. Altitude, exercise and immune function. Exercise Immunology Review. 2005;11(6):p. 16. [PubMed] [Google Scholar]
- 29.De Gonzalo-Calvo D, Neitzert K, Fernández M, et al. Differential inflammatory responses in aging and disease: TNF-alpha and IL-6 as possible biomarkers. Free Radical Biology & Medicine. 2010;149(5):733–737. doi: 10.1016/j.freeradbiomed.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Sarada SKS, Veeramohan PH, Mathew T, Saumya S, Chitharanjan M. NIfedipine inhibits hypoxia induced transvascular leakage through down regulation of NFκB. Respiratory Physiology & Neurobiology. 2012;183(1):26–34. doi: 10.1016/j.resp.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Barbarroja N, Lopez-Pedrera C, Garrido-Sanchez L, et al. Progression from high insulin resistance to type 2 diabetes does not entail additional visceral adipose tissue inflammation. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0048155.e48155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valassi E, Klibanski A, Biller BMK, Misra M. Adipokines and cardiovascular risk in Cushing's syndrome. Neuroendocrinology. 2012;95(3):187–206. doi: 10.1159/000330416. [DOI] [PubMed] [Google Scholar]
- 33.Hojman P, Taudorf S, Lundby C, Pedersen BK. Erythropoietin augments the cytokine response to acute endotoxin-induced inflammation in humans. Cytokine. 2009;45(3):154–157. doi: 10.1016/j.cyto.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Hojman P, Brolin C, Gissel H, et al. Erythropoietin over-expression protects against diet-induced obesity in mice through increased fat oxidation in muscles. PLoS ONE. 2009;4(6) doi: 10.1371/journal.pone.0005894.e5894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiological Reviews. 1999;79(1):1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Li HY, Ericsson A, Sawchenko PE. Distinct mechanisms underlie activation of hypothalamic neurosecretory neurons and their medullary catecholaminergic afferents in categorically different stress paradigms. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(6):2359–2364. doi: 10.1073/pnas.93.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beyaert R, Fiers W. Molecular mechanisms of tumor necrosis factor-induced cytotoxicity: what we do understand and what we do not. FEBS Letters. 1994;340(1-2):9–16. doi: 10.1016/0014-5793(94)80163-0. [DOI] [PubMed] [Google Scholar]
- 38.Baes M, Allaerts W, Denef C. Evidence for functional communication between folliculo-stellate cells and hormone-secreting cells in perifused anterior pituitary cell aggregates. Endocrinology. 1987;120(2):685–691. doi: 10.1210/endo-120-2-685. [DOI] [PubMed] [Google Scholar]
- 39.Lira FS, Rosa JC, Zanchi NE, et al. Regulation of inflammation in the adipose tissue in cancer cachexia: effect of exercise. Cell Biochemistry and Function. 2009;27(2):71–75. doi: 10.1002/cbf.1540. [DOI] [PubMed] [Google Scholar]
- 40.Rosa Neto JC, Lira FS, Oyama LM, et al. Exhaustive exercise causes an anti-inflammatory effect in skeletal muscle and a pro-inflammatory effect in adipose tissue in rats. European Journal of Applied Physiology. 2009;106(5):697–704. doi: 10.1007/s00421-009-1070-1. [DOI] [PubMed] [Google Scholar]
- 41.Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Progress in Neurobiology. 2004;73(6):379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Chen D, Buchanan GF, Ding JM, Hannibal J, Gillette MU. Pituitary adenylyl cyclase-activating peptide: a pivotal modulator of glutamatergic regulation of the suprachiasmatic circadian clock. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(23):13468–13473. doi: 10.1073/pnas.96.23.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aubry JM, Turnbull AV, Pozzoli G, Rivier C, Vale W. Endotoxin decreases corticotropin-releasing factor receptor 1 messenger ribonucleic acid levels in the rat pituitary. Endocrinology. 1997;138(4):1621–1626. doi: 10.1210/endo.138.4.5050. [DOI] [PubMed] [Google Scholar]
- 44.Bernhagen J, Calandra T, Mitchell RA, et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365(6448):756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 45.De Vries HE, Blom-Roosemalen MCM, Van Oosten M, et al. The influence of cytokines on the integrity of the blood-brain barrier in vitro. Journal of Neuroimmunology. 1996;64(1):37–43. doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 46.Watkins LR, Wiertelak EP, Goehler LE, et al. Neurocircuitry of illness-induced hyperalgesia. Brain Research. 1994;639(2):283–299. doi: 10.1016/0006-8993(94)91742-6. [DOI] [PubMed] [Google Scholar]
- 47.Opp MR, Toth LA. Somnogenic and pyrogenic effects of interleukin-1β and lipopolysaccharide in intact and vagotomized rats. Life Sciences. 1998;62(10):923–936. doi: 10.1016/s0024-3205(98)00010-1. [DOI] [PubMed] [Google Scholar]
- 48.Layé S, Bluthe RM, Kent S, et al. Subdiaphragmatic vagotomy blocks induction of IL-1β mRNA in mice brain in response to peripheral LPS. American Journal of Physiology. 1995;268(5):R1327–R1331. doi: 10.1152/ajpregu.1995.268.5.R1327. [DOI] [PubMed] [Google Scholar]
- 49.Kryger HM, Roth T, Dement WC. Principles and Prectice of Sleep Medicine. New York, NY, USA: Elsevier; 2005. [Google Scholar]
- 50.Rodway GW, Huffman LA, Sanders MH. High-altitude-related disorders-part I: pathophysiology, differential diagnosis, and treatment. Heart and Lung. 2003;32(6):353–359. doi: 10.1016/j.hrtlng.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Pedersen BK. The diseasome of physical inactivity and the role of myokines in muscle-fat cross talk. Journal of Physiology. 2009;587(23):5559–5568. doi: 10.1113/jphysiol.2009.179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gamaldo CE, Shaikh AK, McArthur JC. The sleep-immunity relationship. Neurologic Clinics. 2012;30(4):1313–1343. doi: 10.1016/j.ncl.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Nicolau MC, Akaârir M, Gamundí A, González J, Rial RV. Why we sleep: the evolutionary pathway to the mammalian sleep. Progress in Neurobiology. 2000;62(4):379–406. doi: 10.1016/s0301-0082(00)00013-7. [DOI] [PubMed] [Google Scholar]
- 54.Kalonia H, Bishnoi M, Kumar A. Possible mechanism involved in sleep deprivation-induced memory dysfunction. Methods and Findings in Experimental and Clinical Pharmacology. 2008;30(7):529–535. doi: 10.1358/mf.2008.30.7.1186074. [DOI] [PubMed] [Google Scholar]
- 55.Krueger JM, Fang J, Hansen MK, Zhang J, Obál F. Humoral regulation of sleep. News in Physiological Sciences. 1998;13(4):189–194. doi: 10.1152/physiologyonline.1998.13.4.189. [DOI] [PubMed] [Google Scholar]
- 56.Iber C, Ancoli-Israel S, Chesson A, Quan SF. For The American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, Ill, USA: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 57.Wilson MH, Newman S, Imray CH. The cerebral effects of ascent to high altitudes. The Lancet Neurology. 2009;8(2):175–191. doi: 10.1016/S1474-4422(09)70014-6. [DOI] [PubMed] [Google Scholar]
- 58.Krueger JM. The role of cytokines in sleep regulation. Current Pharmaceutical Design. 2008;14(32):3408–3416. doi: 10.2174/138161208786549281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krueger JM, Takahashi S, Kapas L, et al. Cytokines in sleep regulation. Advances in Neuroimmunology. 1995;5(2):171–188. doi: 10.1016/0960-5428(95)00007-o. [DOI] [PubMed] [Google Scholar]
- 60.Veasey SC, Davis CW, Fenik P, et al. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27(2):194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- 61.Hamrahi H, Stephenson R, Mahamed S, Liao KS, Horner RL. Physiological and genomic consequences of intermittent hypoxia: selected contribution: regulation of sleep-wake states in response to intermittent hypoxic stimuli applied only in sleep. Journal of Applied Physiology. 2001;90(6):2490–2501. doi: 10.1152/jappl.2001.90.6.2490. [DOI] [PubMed] [Google Scholar]
- 62.Jafarian S, Gorouhi F, Taghva A, Lotfi J. High-altitude sleep disturbance: results of the Groningen sleep quality questionnaire survey. Sleep Medicine. 2008;9(4):446–449. doi: 10.1016/j.sleep.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 63.Morrell MJ, McRobbie DW, Quest RA, Cummin AR, Ghiassi R, Corfield DR. Changes in brain morphology associated with obstructive sleep apnea. Sleep Medicine. 2003;4(5):451–454. doi: 10.1016/s1389-9457(03)00159-x. [DOI] [PubMed] [Google Scholar]
- 64.Buguet A, Cespuglio R, Radomski MW. Sleep and stress in man: an approach through exercise and exposure to extreme environments. Canadian Journal of Physiology and Pharmacology. 1998;76(5):553–561. doi: 10.1139/cjpp-76-5-553. [DOI] [PubMed] [Google Scholar]
- 65.Burgess KR, Johnson P, Edwards N, Cooper J. Acute mountain sickness is associated with sleep desaturation at high altitude. Respirology. 2004;9(4):485–492. doi: 10.1111/j.1440-1843.2004.00625.x. [DOI] [PubMed] [Google Scholar]
- 66.Johnson PL, Edwards N, Burgess KR, Sullivan CE. Sleep architecture changes during a trek from 1400 to 5000 m in the Nepal Himalaya. Journal of Sleep Research. 2010;19(1):148–156. doi: 10.1111/j.1365-2869.2009.00745.x. [DOI] [PubMed] [Google Scholar]
- 67.Bosco G, Ionadi A, Panico S, et al. Effects of hypoxia on the circadian patterns in men. High Altitude Medicine & Biology. 2003;4(3):305–318. doi: 10.1089/152702903769192269. [DOI] [PubMed] [Google Scholar]
- 68.Kwarecki K, Krawczyk J. Comparison of the circadian rhythm in cell proliferation in corneal epithelium of male rats studied under normal and hypobaric (hypoxic) conditions. Chronobiology International. 1989;6(3):217–222. doi: 10.3109/07420528909056921. [DOI] [PubMed] [Google Scholar]
- 69.Chilov D, Hofer T, Bauer C, Wenger RH, Gassmann M. Hypoxia affects expression of circadian genes PER1 and CLOCK in mouse brain. FASEB Journal. 2001;15(14):2613–2622. doi: 10.1096/fj.01-0092com. [DOI] [PubMed] [Google Scholar]
- 70.Greenberg H, Ye X, Wilson D, Htoo AK, Hendersen T, Liu SF. Chronic intermittent hypoxia activates nuclear factor-κB in cardiovascular tissues in vivo. Biochemical and Biophysical Research Communications. 2006;343(2):591–596. doi: 10.1016/j.bbrc.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 71.Gómez-González B, Domínguez-Salazar E, Hurtado-Alvarado G, et al. Role of sleep in the regulation of the immune system and the pituitary hormones. Annals of the New York Academy of Sciences. 2012;1261:97–106. doi: 10.1111/j.1749-6632.2012.06616.x. [DOI] [PubMed] [Google Scholar]
- 72.Kiratli PO, Demir AU, Volkan-Salanci B, Demir B, Sahin A. Cerebral blood flow and cognitive function in obstructive sleep apnea syndrome. Hellenic Journal of Nuclear Medicine. 2010;13(2):138–143. [PubMed] [Google Scholar]
- 73.Reite M, Jackson D, Cahoon RL, Weil JV. Sleep physiology at high altitude. Electroencephalography and Clinical Neurophysiology. 1975;38(5):463–471. doi: 10.1016/0013-4694(75)90188-1. [DOI] [PubMed] [Google Scholar]
- 74.Amann M, Kayser B. Nervous system function during exercise in hypoxia. High Altitude Medicine & Biology. 2009;10(2):149–164. doi: 10.1089/ham.2008.1105. [DOI] [PubMed] [Google Scholar]
- 75.Hagobian TA, Jacobs KA, Subudhi AW, et al. Cytokine response at high altitude: effects of exercise and antioxidants at 4300 m. Medicine and Science in Sports and Exercise. 2006;38(2):276–285. doi: 10.1249/01.mss.0000188577.63910.51. [DOI] [PubMed] [Google Scholar]
- 76.Blegen M, Cheatham C, Caine-Bish N, Woolverton C, Marcinkiewicz J, Glickman E. The immunological and metabolic responses to exercise of varying intensities in normoxic and hypoxic environments. Journal of Strength and Conditioning Research. 2008;22(5):1638–1644. doi: 10.1519/JSC.0b013e318181fdfd. [DOI] [PubMed] [Google Scholar]
- 77.Lane AM, Terry PC, Stevens MJ, Barney S, Dinsdale SL. Mood responses to athletic performance in extreme environments. Journal of Sports Sciences. 2004;22(10):886–897. doi: 10.1080/02640410400005875. [DOI] [PubMed] [Google Scholar]
- 78.Walsh NP, Gleeson M, Shephard RJ, et al. Position statement. Part one: immune function and exercise. Exercise Immunology Review. 2011;17:6–63. [PubMed] [Google Scholar]
- 79.Shukitt BL, Banderet LE. Mood states at 1600 and 4300 meters terrestrial altitude. Aviation Space and Environmental Medicine. 1988;59(6):530–532. [PubMed] [Google Scholar]


