Abstract
Rapamycin (RAPA) is an immunosuppressive drug that prevents and treats graft versus host disease (GVHD) after allogeneic hematopoeitic cell transplant (HCT). One possible mechanism for its efficacy is induction of tolerance, through increased number or enhanced survival of regulatory T cells. In our experiments, B10.D2 bone marrow and splenocytes were injected into lethally irradiated BALB/cJ recipients. The mice received intraperitoneal injections of either RAPA or vehicle control on days 1–28. There was a significant survival advantage in RAPA treated mice. Evaluation of the skin biopsies demonstrated a dense cellular infiltrate in RAPA treated mice. Further characterization of these cells revealed a higher percentage of regulatory T cells characterized by FoxP3 positive cells in high dose RAPA treated mice as compared to controls on day 30. This effect appears to be dose dependant. When peripheral blood analysis for FoxP3 positive cells was done, there was no significant difference observed in the RAPA treated mice as compared to control mice. These data demonstrates a novel mechanism of rapamycin in GVHD, accumulation of regulatory T cells in the GvHD target tissue: the skin.
Introduction
RAPA is a macrolide antibiotic produced by streptomyces hygroscopicus and is also a potent immunosuppressant. RAPA is used extensively in solid organ transplant, and has an emerging role in GVHD 1–7. RAPA prevents GVHD in several murine models, although the mechanism is not clear. It is well established that RAPA suppresses proliferation of conventional CD4+ T cells (Tconv) via blocking mammalian target of RAPA (mTOR). Further studies demonstrate that T cells activated in the presence of RAPA may have a more tolerogenic phenotype 8,9. There are emerging data suggesting that RAPA helps induce tolerance by conditioning dendritic cells to preferentially activate suppressive T cell subsets 10. Finally, RAPA appears to cause an increase in regulatory T cells (Tregs), or at least provide selective survival advantage 11–14.
In studies done in our lab and others, RAPA prevented mortality from GVHD in murine models 1, 4. On histopathology, it was observed that there was a dense infiltrate of inflammatory cells, later analysis indicated that a majority of these cells were CD4+4. When the splenocytes were analyzed at a later date they were found to have suppressive properties in mixed lymphocyte cultures.
Tregs are a subset of T cells characterized by CD4+CD25hi cells that also express forkhead transcription factor FoxP3. They suppress Tconv and help promote tolerance. Several laboratories have demonstrated that giving donor Tregs can suppress GVHD in murine models 15–17. Some studies demonstrate in humans with acute GVHD that there is a decrease in the number of Tregs in the peripheral blood and the affected tissue 18–20. It is unclear where in the immunologic reaction Tregs play a role, in the peripheral blood, lymph nodes or affected tissues.
Our aim in is the current study was to further explore the nature of the cellular infiltrate in the mice that clinically don’t have significant GVHD. Both ear biopsies and peripheral blood were analyzed for Tregs. The data demonstrates a novel mechanism of rapamycin in GVHD, accumulation of regulatory T cells in the GvHD target tissue: the skin.
Materials and Methods
Animals
B10.D2/nSnJ (H2d, Mls-2b, Mls-3b) and BALB/cJ (H2d, Mls-2a, Mls-3a) were purchased from the Jackson Laboratory (Bar Harbor, ME). For the in vivo studies, only female mice were used. The mice were kept in a specific pathogen-free facility throughout the study. All animal protocols were approved by our IACUC.
GVHD Model
10 × 106 B10/D2 bone marrow cells and 100 × 106 B10.D2 splenocytes were injected in 0.5 mL plain RPMI 1640 via tail vein to lethally irradiated (8.5 Gy) BALB/cJ recipients. Both recipient and donor mice were 12–14 weeks at time of the experiments. The mice were monitored daily for weight and mortality. In experiment 1: mice were sacrificed at days 14, 28 and 42 and organs harvested for histology. In experiment 2: mice were monitored for 60 days and had ear biopsies and blood draws on days 14, 28 and 42. Additionally, paraffinized ear biopsies from experiments done previously in the laboratory4 were also used.
Drug and treatment
RAPA was purchased from LC Laboratories (Woburn, MA) in a pure powder form. It was prepared fresh daily in carboxymethylcellulose 0.2% (CMC) and thoroughly homogenized prior to injection. The mice were given 3–5 mg/kg of RAPA or CMC vehicle control daily via IP injection on days 1–28 following transplantation. The volume given was 0.01 ml/g. Doses of greater than 5 mg/kg resulted in increased toxicity and mortality.
Histology and Immunohistochemistry
We obtained ear biopsies from the mice given the ease of procurement, and the findings are consistent with skin biopsies (data not shown).
Tissue was obtained, placed in 10% formalin, and paraffin embedded. Tissues from previous experiments were in paraffin and processed. Samples were then stained with hematoxyln-eosin (H&E) or immunohistochemistry (IHC) for intracellular FoxP3. IHC was done on 5 micron slices obtained from paraffin blocks. The M.O.M. Immunodetection Kit from Vector Laboratories (Cat# PK-2200) was used. The methods were fully described by Nakmura et al. 21 Briefly, the slides were placed in steam bath for 40 minutes for antigen retrieval. Then, after several blocking steps, they were incubated overnight with 1:10 mouse anti -mouse/human/rat - FoxP3 IgG. The slides were washed then incubated with anti- mouse IgG conjugated to biotin substrate. The slides were then developed with fluorescein avidin DCS, and subsequently developed.
The cells were counted using Leica microscope. For the H&E stained slides to assess cellularity of the tissues, a total of 10 hpf were counted, or the maximum possible depending on the quality of the slide. For the IHC slides, 1000 nucleated cells were assessed, or as many as possible given quality of staining.
Images of skin and liver sections were acquired with an AxioCam MRc digital camera mounted on an Axiovert 200 inverted microscope (Carl Zeiss Microimaging, Thornwood, NY). A-Plan 10x/0.25 and LD-Plan NEOFLUAR 20X/0.4 objectives were used. Images were recorded using AxioVision Rel. 4.5 software (Carl Zeiss Microimaging).
FACS analysis
Cells were stained with a mouse Treg staining kit (Biolegend, San Diego, CA Cat# 320018) as described by Bamias et al.22 Briefly, peripheral blood (50 μL) was incubated with anti-CD4 APC (clone RM4–5), anti-CD25 PE (clone PC61) at room temperature for 15 minutes. The cells were then rinsed with FACS buffer, followed by treatment with 1x FACS lysing solution (Becton Dickinson, San Jose, CA). Cells were then permeablized with Biolegend fix/perm solution × 30 minutes. Then the cells were incubated with anti-FoxP3 conjugated to alexa-fluor 488 (Clone 150D) or isotype control for 20 minutes at room temperature. The cells were washed with FACS buffer, and analyzed on FACS Canto machine, using FACS Diva software.
Statistical analysis
Group comparisons were done with student t-test.
Results
Rapamycin prevents GVHD
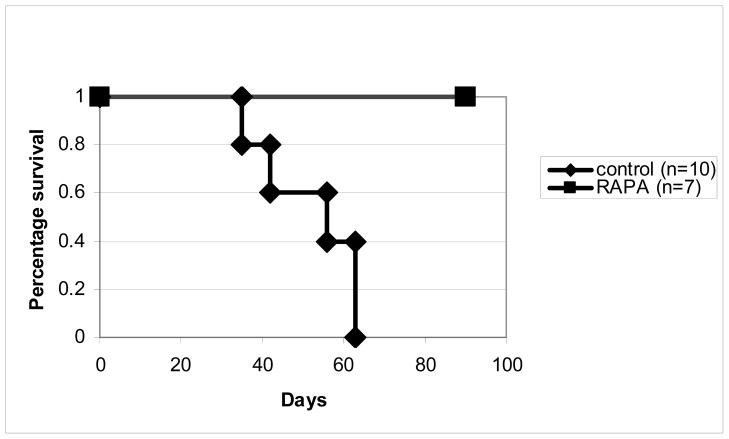
Irradiated BALB/c recipients of B10.D2 bone marrow and splenocytes were treated with RAPA at a dose of 3–5 mg/kg for the first 4 weeks after transplantation. Consistent with previous experiments4, mice treated with RAPA (n=7) did not develop GVHD and all RAPA-treated mice survived more than 90 days (Figure 1–2). By contrast, all mice treated with CMC (n=10) developed severe GVHD and all of them died within 70 days after transplantation with a median survival day of 55 days (Figure 1–2). Similar to our published data4, histological analysis demonstrated that the mice treated with RAPA were free of acute and chronic GVHD in the skin, liver, and intestine (data not shown). These mice were used for the subsequent mechanistic studies.
Figure 1. Survival curve of mice treated with RAPA or vehicle control.
BALB/cJ mice were lethally irradiated, and given BM and splenocytes from B10.D2 mice. The mice were monitored over a 3 month period for mortality.
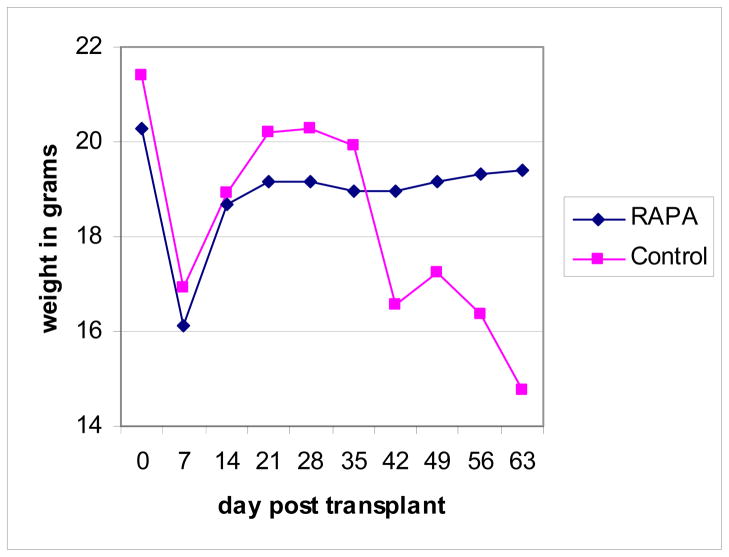
Figure 2. Weight of mice in grams following transplant.
Following transplant, mice were weighed twice weekly.
Histologic changes
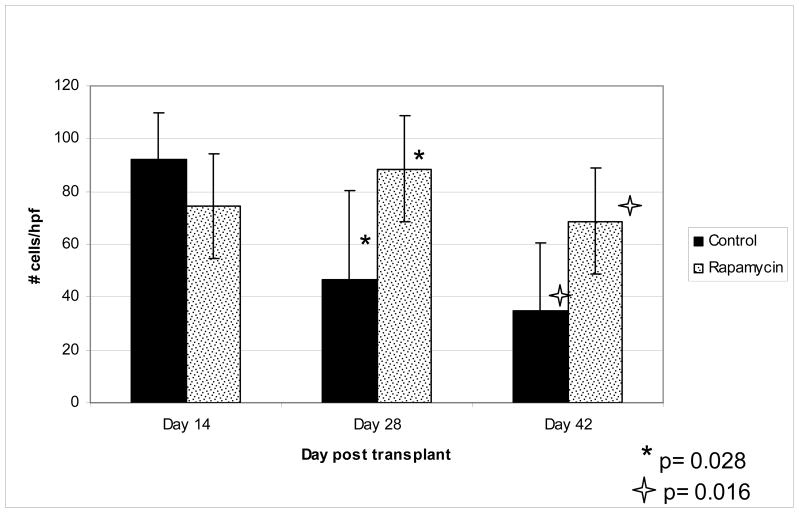
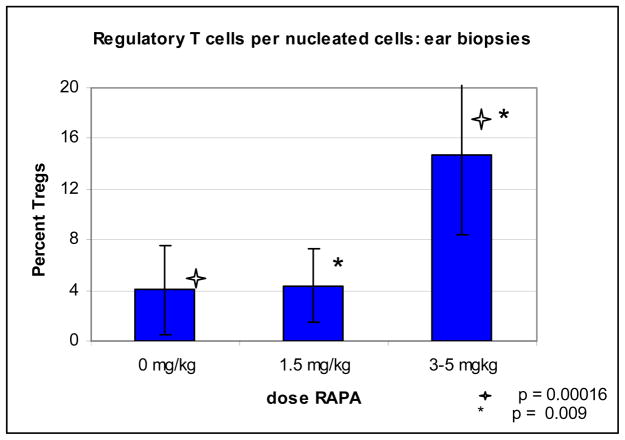
As seen in previous experiments 4, there was a significant cellular infiltrate noted in the ear skin of the RAPA-treated mice as compared to control mice. Analysis of H&E slides demonstrated significantly more cells/high power fields on mice treated with RAPA as compared to control on day 28 and 42 (Figure 3). On day 28 and 42, control mice had an significantly fewer cells per high powered field, as compared to RAPA mice, 46 vs 89 (p = 0.028) and 36 vs 68 (p = 0.016) respectively. There was no significant difference on day 14. Evaluation of IHC staining done on the ear biopsies demonstrated increase in percentage of FoxP3 positive cells per infiltrating nucleated cells. This was demonstrated over three separate experiments (figure 4 & 5), and appears to be dose dependant. In mice treated with control vehicle and low dose RAPA (1.5 mg/kg) there was a low percentage, however in high dose of RAPA (3–5 mg/kg) the percentage increased to 14% of FoxP3 positive cells per total nucleated cells, which was statistically significant (p <0.01).
Figure 3. Infiltrating nucleated cells per high power field.
H&E stained biopsies were evaluated for cellular infiltrate. Shown is infiltrating cells per high powered field. 10 hpf or maximum possible was counted and average calculated. In control group the following number of slides were analyzed: Day 14, n=5; day 28, n=7; day 42, n=6. In RAPA group the following number of slides were analyzed: Day 14, n=6; day 28, n=6; day 42, n=6.
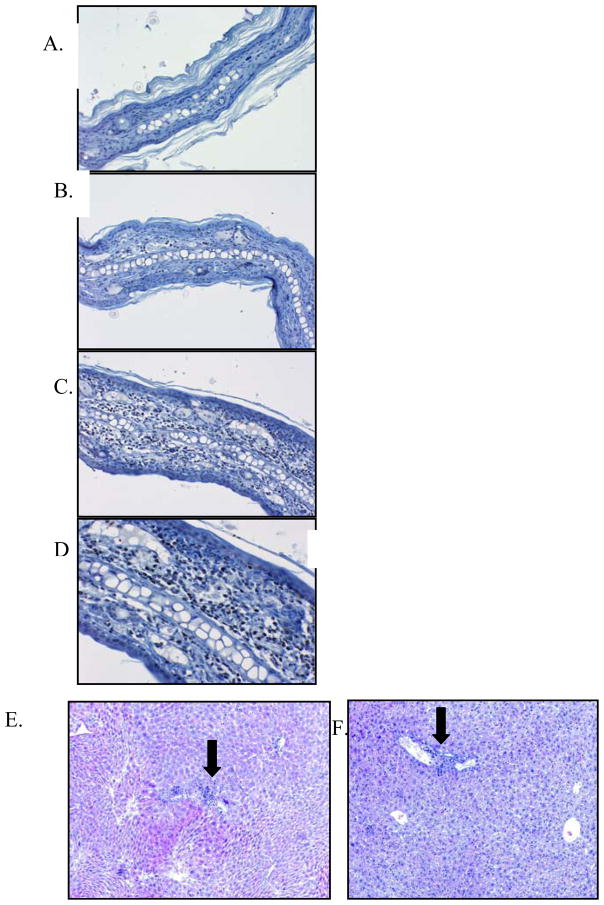
Figure 4. Histopathology of ear biopsies and liver biopsies.
(A) IHC for FoxP3 Vehicle control 10x. (B) IHC for FoxP3 RAPA 1.5 mg/kg (C) IHC for FoxP3. RAPA 3–5 mg/kg 10x (D) IHC for FoxP3 RAPA 3–5 mg/kg 20x. H&E liver biopsy: (H) Vehicle control treated mouse10x. (I) RAPA treated mouse 10x. These are representative samples.
Figure 5. Percent FoxP3 positive cells per total infiltrating nucleated cells.
Immunohistochemistry staining was done on the ear biopsies from day 30 for FoxP3. A percentage of FoxP3 positive cells per total nucleated infiltrating cells were analyzed. 1000 cells, or maximum possible cells were counted to determine percentage. Above graph represents slides obtained in three separate experiments, in 0 mg/kg group n = 10; in 1.5 mg/kg group, n=4; in 3–5 mg/kg group, n=11.
To determine whether Tregs were present in other target organs of GVHD, we further analyzed liver and gut. In the liver, a cellular infiltrate was noted in the control mice, in addition to destruction of the structures in the portal triad. There was minimal cellular infiltration noted in the liver of RAPA treated mice, and no compromise of the structures was noted on day 30 (figure 4). There was no evidence of Tregs in any of the liver specimens (data not shown). In the gut, there was more inflammation in the control mice as compared to the RAPA treated mice, however, this was difficult to quantify and Tregs were present in both treated and untreated mice (data not shown).
Peripheral Blood
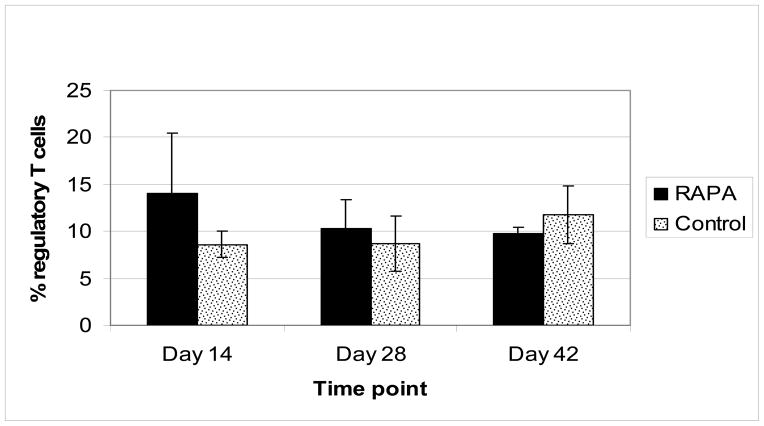
In order to determine whether RAPA expanded Tregs systemically, FoxP3+ cells were quantified in peripheral blood. As shown in Figure 6, analysis of peripheral blood did not show any significant difference in FoxP3 positive cells on days 14, 28 or 42 in the RAPA treated vs control mice, suggesting that RAPA does not affect Treg numbers systemically.
Figure 6. Percent FoxP3 positive cells per CD4+ cells in peripheral blood.
Peripheral blood was obtained on day 14, 28 and 42 and analyzed via FACS analysis. Above represents FoxP3 positive cells as a percentage of total CD4+ cells. No significant difference was observed between groups. In control group the following number samples were analyzed: Day 14, n=5; day 28, n=7; day 42, n=6. In RAPA group the following number of samples were analyzed: Day 14, n=6; day 28, n=6; day 42, n=6.
Discussion
These experiments confirm that RAPA can prevent mortality from GVHD, as observed in previous experiments 1,4. We also demonstrate that mice treated with GVHD that a densely cellular infiltrate that contains a high percentage of FoxP3 positive cells is present in the clinically unaffected tissues. These findings suggest that abrogation of clinical disease is mediated at least in part by Tregs. It is notable that an increase in Tregs is not appreciated in the peripheral blood at the time points measured.
The immunomodulatory effects of RAPA have been an area of active interest for a number of years. Initial studies by Blazar et al 1 10 years ago demonstrated RAPA improved survival in mice undergoing transplantation, however, the mechanism remains a subject of debate. One possible mechanism is the suppression of Tconv proliferation, mediated by RAPA binding to FK binding protein and inhibition of mTOR. This is mediated through an IL-2 independent pathway.
Another potential mechanism is through induction of Tregs. Several investigators have demonstrated the ability to expand Tregs by culturing them in the presence of RAPA 11,23. Zeiser et al further explored this phenomenon and demonstrated that RAPA promoted Treg expansion and proliferation in vivo with luciferase imaging 24. Tregs appear to induce the STAT5 pathway in the presence of RAPA which promotes proliferation 13. Furthermore, RAPA may exert immodulatory effects through dendritic cells. Turnquist et al demonstrated that RAPA conditioned dendritic cells were poor stimulators of effector T cells, but enriched Tregs 10. This may be secondary to effects on maturation 25, antigen uptake 26, or survival 27.
It is intriguing that Tregs were present in the skin, however not increased in the peripheral blood, liver or gut. Luciferase imaging data suggests that Tregs are primed in the lymph nodes, then home to organs to prevent inflammation 28, and this current study confirmed the presence of FoxP3+ cells in one affected tissue, the skin. The fraction of Tregs in the peripheral blood in each group is equal, consistent with previous studies 13,28. This phenomenon may reflect the fact that Tregs proliferate in the lymph nodes prior to entering the target tissues 28. In our experiments, the presence of Tregs in the ear biopsies was very clear. However, although it appears that there is no increased accumulation of Tregs in the gut or liver, this needs to be confirmed on further analysis as the sample size was small and could be subject to sampling error.
One question remains unanswered: how does RAPA prevent mortality in this model? Although we clearly demonstrate presence of Tregs in the ear biopsies, skin GVHD, though well described in this model, is not the cause of mortality. Presumably, there are other mechanisms that result in improved survival. The most likely cause of mortality in this model is GVHD of the gut. The gut biopsies obtained were unrevealing, though notably there were Tregs in both the RAPA treated and control mice. We also might have missed areas with increased Tregs due to sampling error. However, we know that even at baseline, Tregs are present in intestinal tissue 29. In the initial experiments using RAPA to prevent GVHD in this mouse model, the splenocytes were rich in T cells that were suppressive in mixed lymphocyte reactions, which may in fact be Tregs4. This would suggest Tregs present in the lymphatic system may suppress Tconv function prior to their entrance into the affected tissues.
This study indicates that at least part of the mechanism of GVHD suppression provided by RAPA is via induction of Tregs, and these cells act within the affected tissues, mainly the skin. Our study demonstrates the presence of Tregs in tissues known to be affected by GVHD in untreated mice. The presence of a dense lymphocytic infiltrate without tissue injury indicates that at least in the skin, their presence prevents action of Tconv. However, based on previous data demonstrating cells of a suppressive nature populate the spleen4 indicates that they may also exert their effect on effector cells in the lymphatic tissue. Further studies will be necessary to better understand this phenomenon and how this can be exploited in a clinical trial.
Acknowledgments
Many thanks to Zouwei Su, PhD, for his help in preparation of histopathology.
Footnotes
This data was presented in abstract form at ASBMT, February 17, 2008.
The authors of this paper have no conflicts of interest to declare.
Contribution: J.M.P., N.J.C. and B.J.C. designed experiments, analyzed data and wrote the paper. J.M.P. and D.O. performed experiments. N.D.L. assisted with initial pathology analysis.
References
- 1.Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Vallera DA. Rapamycin Inhibits the Generation of Graft-Versus-Host Disease- and Graft-Versus-Leukemia-Causing T Cells by Interfering with the Production of Th1 or Th1 Cytotoxic Cytokines. Journal of Immunology. 1998:5355–5365. [PubMed] [Google Scholar]
- 2.Couriel DR, Saliba R, Escalon MP, Hsu Y, Ghosh S, Ippoliti C, et al. Sirolimus in combination with tacrolimus and corticosteroids for the treatment of resistant chronic graft-versus-host disease. British Journal of Haematology. 2005:409–417. doi: 10.1111/j.1365-2141.2005.05616.x. [DOI] [PubMed] [Google Scholar]
- 3.Antin JH, Cutler C. Sirolimus for GVHD prophylaxis in allogeneic stem cell transplantation. Bone Marrow Transplantation. 2004;34:471–476. doi: 10.1038/sj.bmt.1704604. [DOI] [PubMed] [Google Scholar]
- 4.Chen BJ, Morris R, Chao NJ. Graft-Versus-Host Disease Prevention by Rapamycin: Cellular Mechanisms. Biology of Blood and Marrow Transplantation. 2000;6:529–536. doi: 10.1016/s1083-8791(00)70062-0. [DOI] [PubMed] [Google Scholar]
- 5.Noris M, Casiraghi F, Todeschini M, Cravedi P, Cugini D, Monteferrante G, et al. Regulatory T Cells and T Cell Depletion: Role of Immunosuppressive Drugs. Journal American Society of Nephrology. 2007:1007–1018. doi: 10.1681/ASN.2006101143. [DOI] [PubMed] [Google Scholar]
- 6.Antin JH, Kim HT, Cutler C, Ho VT, Lee SJ, Miklos DB, et al. Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood. 2003:1601–1605. doi: 10.1182/blood-2003-02-0489. [DOI] [PubMed] [Google Scholar]
- 7.Cutler C, Li S, Ho VT, Koreth J, Alyea E, Soiffer RJ, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007:3108–3114. doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valmori D, Tosello V, Souleimanian NE, Godefroy E, Scotto L, Wang Y, et al. Rapamycin-Mediated Enrichment of T Cells with Regulatory Activity in Stimulated CD4+ T Cell Cultures Is Not Due to the Selective Expansion of Naturally Occurring Regulatory T Cells but to the Induction of Regulatory Functions in Conventional CD4+ T Cells. Journal of Immunology. 2006:944–949. doi: 10.4049/jimmunol.177.2.944. [DOI] [PubMed] [Google Scholar]
- 9.Powell JD, Lerner CG, Schwartz RH. Inhibition of Cell Cycle Progression by Rapamycin Induces T Cell Clonal Anergy Even in the Presence of Costimulation. Journal of Immunology. 1999:2775–2784. [PubMed] [Google Scholar]
- 10.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-Conditioned Dendritic Cells Are Poor Stimulators of Allogeneic CD4+ T Cells, but Enrich for Antigen-Specific Foxp3+ T Regulatory Cells and Promote Organ Transplant Tolerance. Journal of Immunology. 2007:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 11.Battaglia M, Stabilini A, Roncarolo M-G. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 12.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo M-G. Rapamycin Promotes Expansion of Functional CD4+CD25+FOXP3+ Regulatory T Cells of Both Healthy Subjects and Type 1 Diabetic Patients. Journal of Immunology. 2006:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 13.Zeiser R, Leveson-Gower DB, Zambricki EA, Kambham N, Beilhack A, Loh J, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008:453–462. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective Survival of Naturally Occurring Human CD4+CD25+Foxp3+ Regulatory T Cells Cultured with Rapamycin. Journal of Immunology. 2007:320–329. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- 15.Ermann J, Hoffmann P, Edinger M, Dutt S, Blankenberg FG, Higgins JP, et al. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005:2220–2226. doi: 10.1182/blood-2004-05-2044. [DOI] [PubMed] [Google Scholar]
- 16.Wysocki CA, Jiang Q, Panoskaltsis-Mortari A, Taylor PA, McKinnon KP, Su L, et al. Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood. 2005:3300–3307. doi: 10.1182/blood-2005-04-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4+CD25+ Regulatory T Cells Suppress Lethal Acute Graft-Versus-Host Disease after Allogeneic Bone Marrow Transplantation. Journal of Experimental Medicine. 2002:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rieger K, Loddenkemper C, Maul J, Fietz T, Wolff D, Terpe H, et al. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood. 2006:1717–1723. doi: 10.1182/blood-2005-06-2529. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen VH, Zeiser R, Negrin RS. Role of Naturally Arising Regulatory T Cells in Hematopoietic Cell Transplantation. Biology of Blood and Marrow Transplantation. 2006;12(10):995–1009. doi: 10.1016/j.bbmt.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Schneider M, Munder M, Karakhanova S, Ho AD, Goerner M. The initial phase of graft-versus-host disease is associated with a decrease of CD4+CD25+ regulatory T cells in the peripheral blood of patients after allogeneic stem cell transplantation. Clinical Laboratory Hematology. 2006:382–390. doi: 10.1111/j.1365-2257.2006.00825.x. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura T, Shima T, Saeki A, Hidaka T, Nakashima A, Takikawa O, et al. Expression of indoleamine 2, 3-dioxygenase and the recruitment of Foxp3-expressing regulatory T cells in the development and progression of uterine cervical cancer. Cancer Science. 2007;98(6):874–881. doi: 10.1111/j.1349-7006.2007.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bamias G, Okazawa A, Rivera-Nieves J, Arseneau KO, De La Rue SA, Pizarro TT, et al. Commensal Bacteria Exacerbate Intestinal Inflammation but Are Not Essential for the Development of Murine Ileitis. J Immunol. 2007;178(3):1809–1818. doi: 10.4049/jimmunol.178.3.1809. [DOI] [PubMed] [Google Scholar]
- 23.Keever-Taylor CA, Browning M, Johnson B, Truitt R, Bredeson C, Behn B, et al. Rapamycin enriches for CD4(+) CD25(+) CD27(+) Foxp3(+) regulatory T cells in ex vivo-expanded CD25-enriched products from healthy donors and patients with multiple sclerosis. Cytotherapy. 2007;9:144–157. doi: 10.1080/14653240601145223. [DOI] [PubMed] [Google Scholar]
- 24.Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J, et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006:390–399. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hackstein H, Taner T, Zahorchak AF, Morelli AE, Logar AJ, Gessner A, et al. Rapamycin inhibits IL-4--induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101(11):4457–4463. doi: 10.1182/blood-2002-11-3370. [DOI] [PubMed] [Google Scholar]
- 26.Hackstein H, Taner T, Logar AJ, Thomson AW. Rapamycin inhibits macropinocytosis and mannose receptor-mediated endocytosis by bone marrow-derived dendritic cells. Blood. 2002;100(3):1084–1087. doi: 10.1182/blood.v100.3.1084. [DOI] [PubMed] [Google Scholar]
- 27.Woltman AM, van der Kooij SW, Coffer PJ, Offringa R, Daha MR, van Kooten C. Rapamycin specifically interferes with GM-CSF signaling in human dendritic cells, leading to apoptosis via increased p27KIP1 expression. Blood. 2003;101(4):1439–1445. doi: 10.1182/blood-2002-06-1688. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen VH, Zeiser R, da Silva DL, Chang DS, Beilhack A, Contag CH, et al. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood. 2007:2649–2656. doi: 10.1182/blood-2006-08-044529. [DOI] [PubMed] [Google Scholar]
- 29.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A Metabolites Induce Gut-Homing FoxP3+ Regulatory T Cells. Journal of Immunology. 2007:3724–3733. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]