Abstract
As a group, people with the sex chromosome aneuploidy 49,XXXXY have characteristic physical and cognitive/behavioral tendencies, although there is high individual variation. In this study we use magnetic resonance imaging (MRI) to examine brain morphometry in 14 youth with 49,XXXXY compared to 42 age-matched healthy controls. Total brain size was significantly smaller (t = 9.0, p < .001), and rates of brain abnormalities such as colpocephaly, plagiocephaly, periventricular cysts, and minor craniofacial abnormalities were significantly increased. White matter lesions were identified in 50% of subjects, supporting the inclusion of 49,XXXXY in the differential diagnosis of small multifocal white matter lesions. Further evidence of abnormal development of white matter was provided by the smaller cross sectional area of the corpus callosum. These results suggest that increased dosage of genes on the X chromosome has adverse effects on white matter development.
Keywords: Sex chromosome aneuploidy, MRI, Children, Adolescents, X chromosome
Highlights
► Total brain size was significantly smaller. ► Rates of brain abnormalities were significantly increased. ► Smaller cross sectional area of the corpus callosum.
1. Introduction
Approximately 1 in 400 people are born with a sex chromosome combination other than XX or XY (Nielsen and Wohlert, 1990; Ratcliffe, 1994). Most sex chromosome variations (SCVs) are from the addition of a single X or Y (i.e., XXY, XXX, or XYY). However, more than one supernumerary chromosome may occur; in approximately 1 of 85,000 to 100,000 live male births, boys have 3 extra X chromosomes (49,XXXXY) (Kleczkowska et al., 1988; Linden et al., 1995).
The first reports of the effects of additional X chromosomes in males came from a series of large-scale prospective population-based studies in the United States and Europe during the 1970s. These groups of studies karyotyped over 200,000 live births, and then mapped the longitudinal physical, cognitive, and emotional development of the SCV individuals into adulthood (Nielsen and Wohlert, 1990; Ratcliffe et al., 1979; Robinson et al., 1983, 1990; Tennes et al., 1975).
Largely due to the rarity of 49,XXXXY, early prospective studies of the 1970s were confined to case studies. However, the findings were fairly consistent and indicated that 49,XXXXY is generally associated with severe developmental delays, such as learning and intellectual disabilities as well as speech and motor delays, and physical manifestations, most frequently affecting the skeletal, cardiac, and genital systems. Unlike other males with SCV such as Klinefelter syndrome, who are typically above average in height, 49,XXXXY patients often have decreased stature (Gropman et al., 2010; Linden et al., 1995; Ottesen et al., 2010; Visootsak et al., 2007). For a comprehensive review of the clinical phenotype, see Tartaglia et al. (2011).
Information about the impact of three extra X chromosomes on cognitive development is sparse. Linden et al. (1995) reported on 3 cases of 49,XXXXY identified as a part of the Denver prospective SCV study and indicated that these individuals had IQ scores that fell into the intellectually disabled range. More recent studies have utilized larger referred samples than the early prospective studies and have relied on parent report of adaptive function (e.g., Visootsak et al. (2007)). Together, these studies have indicated that both intellectual (Linden et al., 1995) and adaptive functioning skills (Visootsak et al., 2007) often fall into the intellectually disabled range. However, a recent study by Gropman et al. (2010) that utilized a nonverbal intelligence test (in lieu of a traditional IQ test that has both verbal and nonverbal components) reported relatively preserved nonverbal intelligence, although language skills still showed significant impairment (as assessed by standardized language testing).
There is even less information available about how 49,XXXXY affects brain anatomy. Head size tends to be smaller than average, suggesting that brain volumes are also likely to be smaller (Linden et al., 1995; Visootsak et al., 2007). Brain imaging studies to date have also been largely limited to case reports.
Linden et al. (1995) reported computerized tomography (CT) results from three 49,XXXXY subjects. The first was a 21-year-old male with an IQ estimated at 39, little speech, and a history of a seizure disorder, who was found to have diffuse atrophy of the cerebrum and cerebellum on CT performed at the age of 12. The second was a 27-year-old male with an IQ of 56 and was found to have mildly prominent ventricles. The third was a 15-year-old male with an IQ of 40 whose head size was at the 25th percentile, but whose CT was otherwise read as normal (Linden et al., 1995).
An MRI of a 12-year-old 49,XXXXY male demonstrated unilateral mild left colpocephaly and mild cerebral atrophy (Galasso et al., 2003). A 3-year-old male was found to have prominent ventricles, mild cerebral atrophy, and thin corpus callosum (Haeusler et al., 1992). A 1-year-old male with 49,XXXXY had extensive isolated and confluent lesions in the subcortical white matter; these lesions were hyperintense on T2-weighted and FLAIR images. This child also had prominent ventricles and thin corpus callosum (García-Cazorla et al., 2004). Most recently, MRI results from a 30-month-old male demonstrated regional periventricular white matter abnormalities with a posterior predominance that also involved the spinothalamic tracts; a thin corpus callosum was also demonstrated (Tabarki et al., 2012).
The largest 49,XXXXY imaging case series to date included three individuals studied at different ages (Hoffman et al., 2008). One subject who underwent MRI for significant developmental delay was scanned at 14 months of age and again at 20 months. Both exams were significant for cerebral atrophy, prominent lateral ventricles and third ventricle, and thin corpus callosum. There was patchy and confluent abnormal signal in the periventricular white matter, predominantly in the parietal and frontal lobes. The second subject was scanned at 7 years of age. He had mild colpocephaly, thin corpus callosum, and several foci of abnormal signal in the periventricular and subcortical white matter. The third case was an adult of 39 years, who underwent MRI following new onset of generalized tonic–clonic seizures. Findings included atrophy involving both the cerebrum and cerebellum, mild colpocephaly, and thin corpus callosum. Numerous focal lesions were present in the periventricular, deep, and subcortical white matter, many more than would be expected for an individual of his age.
The case reports above include the description of 6 patients who had MRI studies, which provide a much more detailed evaluation of the white matter than does CT. The abnormalities described in these patients with 49,XXXXY syndrome predominantly relate to white matter: there were discrete white matter lesions of both the focal and confluent/regional types; a thin corpus callosum reflects a generalized paucity of white matter in the cerebrum; and colpocephaly reflects selective underdevelopment of white matter in the posterior portions of the cerebrum. Atrophy of the cerebrum and/or cerebellum can also result from a white matter abnormality. All six of these patients had at least 2 of these types of abnormalities, and 1 had all of the above.
Previous studies in other sex chromosome variations also found an association of additional X or Y chromosomes with an increased risk of focal lesions in the white matter. In a sample of 20 males with XXY or XYY, 6 showed one or more focal white matter lesion, while no lesions were found in 26 healthy matched control subjects (Warwick et al., 1999). Among 5 children with SCV (one each with 47,XXX, 48,XXYY, and 49,XXXXY and two with 47,XXY), all of them had focal white matter lesions (García-Cazorla et al., 2004).
Based on these previously reported neuroanatomical findings, we hypothesized that we would find a similar pattern of abnormalities affecting white matter in this largest 49,XXXXY case series to date, and we sought to further characterize the frequency of these findings.
2. Materials and methods
2.1. Participants
49,XXXXY males were recruited from throughout the USA with the help of Neurodevelopmental Diagnostic Center for Young Children and a parent advocacy group, Klinefelter Syndrome and Associates (KS&A). Parents of 49,XXXXY subjects were interviewed by telephone and asked to report their child's health, developmental, and educational history. Children with severe head injuries or other conditions that might have affected gross brain development were not accepted into the study. Several 49,XXXXY males participated in the protocol but are not presented in this report because they were either too anxious to scan (n = 3), were unable to lie still in the scanner (n = 3) which produced excessive motion artifact, or had a ventriculo-peritoneal shunt to treat hydrocephalus (n = 1), resulting in 14 patients who met the inclusion criteria for this report. Four of these 49,XXXXY patients have a history of seizures, 2 of whom had experienced absence seizures and 2 had febrile seizures. No obvious brain abnormalities identified on the MRIs were common to these four patients.
The diagnosis of 49,XXXXY was confirmed with karyotype testing on all subjects. High resolution G-band karyotyping was performed on phytohemagglutinin-stimulated patient peripheral blood cultures. A minimum of 50 metaphases were analyzed, and 3 karyotypes per patient were produced (all karyotyping was performed by Quest Diagnostics or the Cytogenetics Laboratory, Department of Obstetrics and Gynecology, Georgetown University Hospital). Subjects were included on the basis of a 49,XXXXY karyotype and not on the presence of specific clinical features. The group consisted of nonmosaic 49,XXXXY males ranging in age from 5.1 to 17.2 years (Table 1). The ethnic composition of the group was 11 white, 1 Hispanic, 1 African-American, and 1 biracial. Nine subjects were right-handed, 3 were left-handed, and 2 were mixed-handed. None of the boys were born prematurely; they all had gestational ages of 37–42 weeks (gestational age was not reported for 1 of these boys). None of the boys were diagnosed in utero through amniocentesis; they were all diagnosed postnatally (range 0–60 months, mean 11.2 months).
Table 1.
Demographics; cognitive and behavioral results.
| 49,XXXXY |
46,XY |
|||||
|---|---|---|---|---|---|---|
| Mean (SD) | N | Mean (SD) | N | t | P | |
| Age, y | 11.6 (4.7) | 14 | 11.6 (4.5) | 42 | 0.1 | .961 |
| Height, in | 56.8 (9.9) | 14 | 58.7 (10.1) | 41 | 0.6 | .529 |
| Weight, lb | 107.6 (71.5) | 14 | 107.5 (49.8) | 42 | 0.0 | .995 |
| Tanner stage | 2.0 (1.3) | 10 | 2.7 (1.4) | 38 | 1.5 | .161 |
| SES | 56.8 (23.9) | 14 | 51.7 (21.3) | 42 | 0.7 | .484 |
| Full scale IQ | 60.9 (9.9) | 8 | 114.9 (12.2) | 41 | 13.5 | < .001a |
| Verbal IQ | 63.6 (11.4) | 8 | 113.7 (14.6) | 39 | 10.8 | < .001a |
| Performance IQ | 63.3 (9.0) | 8 | 112.3 (12.3) | 39 | 13.1 | < .001a |
| ABAS composite GAC | 61.9 (14.3) | 8 | 111.4 (8.6) | 11 | 8.7 | < .001a |
| PPVT standard score | 65.0 (19.9) | 9 | ||||
| PPVT age equivalent, y | 5.2 (2.2) | 10 | ||||
| CBCL total problems | 63.4 (7.7) | 12 | 40.3 (8.7) | 32 | 8.5 | < .001a |
| CBCL internalizing | 60.7 (7.7) | 12 | 42.3 (7.6) | 32 | 7.1 | < .001a |
| CBCL anxious/depressed | 57.3 (5.4) | 12 | 50.8 (2.2) | 32 | 4.1 | .001a |
| CBCL withdrawn/depressed | 59.5 (6.8) | 12 | 51.0 (2.3) | 32 | 4.2 | .001a |
| CBCL somatic complaints | 62.5 (8.3) | 12 | 51.9 (4.2) | 32 | 4.2 | .001a |
| CBCL externalizing | 59.8 (10.6) | 12 | 43.3 (8.1) | 32 | 4.9 | < .001a |
| CBCL rule-breaking behavior | 58.7 (7.1) | 12 | 51.4 (3.0) | 32 | 3.4 | .005a |
| CBCL aggressive behavior | 62.2 (9.9) | 12 | 51.2 (3.3) | 32 | 3.8 | .003a |
| CBCL social problems | 66.3 (7.2) | 11 | 50.1 (2.6) | 31 | 6.9 | < .001a |
| CBCL thought problems | 61.8 (10.0) | 12 | 50.6 (2.0) | 32 | 3.8 | .003a |
| CBCL attention problems | 59.7 (5.6) | 12 | 50.8 (1.9) | 32 | 5.4 | < .001a |
All cognitive and behavioral t-tests survived Bonferroni adjustment except rule-breaking behavior.
Statistically significant.
Forty-two healthy XY males ranging in age from 5.2 to 17.8 years were selected as controls from an ongoing longitudinal brain imaging project being conducted by the Child Psychiatry Branch of the National Institute of Mental Health (NIMH) (Giedd et al., 1999). These controls were matched to the fourteen 49,XXXXY boys in the sample by age and SES. They were a subset of healthy volunteers recruited from the community through the National Institutes of Health (NIH) Normal Volunteer Office, newspaper advertisements, and outreach to schools in the Washington, DC area. The ethnic composition of this group was 39 white, 2 African-American, and 1 biracial. Thirty-seven controls were right-handed, 1 was left-handed, and 3 were mixed-handed (handedness was missing for one control subject). None of the XY boys was born prematurely; they all had gestational ages of 37–43 weeks (gestational age was not reported for 7 of these boys).
Healthy controls were screened to confirm normative development via an initial telephone interview with the parents. Exclusion criteria included psychiatric diagnosis in the subject or a first-degree relative and head injury or other conditions that might have affected gross brain development. Furthermore, those participants who had ever required special services in school, taken psychiatric medications, or received mental health treatment were excluded.
During their visit to the NIH, subjects underwent physical, psychiatric, and neurocognitive assessment. IQ scores were obtained using the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999) for 41 of the healthy controls and 8 of the 49,XXXXY subjects. The Peabody Picture Vocabulary Test — 4 (PPVT-4) (Dunn and Dunn, 2007) is a norm-referenced measure of receptive vocabulary that was administered to 10 of the 49,XXXXY subjects with limited verbal ability. Pubertal maturation was quantified using Tanner stages, as assessed by a questionnaire given to the parents (Petersen et al., 1988). Developmental history of subjects, including delayed milestones, language delay, or use of special education services, as well as presence of psychiatric syndromes were assessed by a doctoral level clinician through a semi-structured clinical interview with the participant and a parent.
For all study participants, handedness was assessed using the Physical And Neurological Examination for Soft Signs (PANESS) (Denckla, 1985). In this examination, the individual is asked to write his name, and then demonstrate how he performs 11 other activities (e.g., throwing a ball, using a saw, etc.). If the child writes with his right hand and performs 10 or all of the remaining 11 items with his right hand (or right hand first, indicating preference), he is categorized as right-handed. Conversely, if he writes with his left hand and performs 10 or all of the remaining 11 items with his left hand (or left hand first), he is categorized as left-handed. All other outcomes result in categorization as mixed-handed. For the five males with 49,XXXXY who could not complete the PANESS, writing hand was used to indicate handedness. Socioeconomic condition was measured using the Hollingshead scale (Hollingshead, 1975).
The Adaptive Behavior Assessment System (ABAS) is a parent-completed questionnaire to provide comprehensive, norm-referenced assessment of adaptive skills for children (Harrison and Oakland, 2003). Ten scaled scores are determined from which a General Adaptive Composite (GAC) is calculated. The parent rating version of the Child Behavior Checklist (Achenbach and Ruffle, 2000) was used to assess behavioral and emotional problems and competencies.
We obtained verbal or written assent from the child and written consent from the parents for their participation in the study. The National Institute of Mental Health Institutional Review Board approved the protocol.
2.2. MRI studies
All images were acquired on the same General Electric 1.5-T Signa scanner (Waukesha, WI), which was located at the NIH clinical center in Bethesda, Maryland. The following parameters were utilized for both patients (scanned between 2001 and 2011) and healthy volunteers (scanned between 1992 and 2011). A sagittal T1-weighted spin-echo sequence was acquired with 5 mm thickness and 1.5 mm gap (FOV = 300 mm, acquisition matrix 256 × 128, TR = 400 ms, TE = 14 ms). A 3-dimensional spoiled-gradient recalled-echo sequence in the steady-state sequence, designed to optimize discrimination between gray matter, white matter, and cerebrospinal fluid (CSF), was used to acquire 124 contiguous 1.5-mm-thick slices in the axial plane (TE = 5 ms; TR = 24 ms; flip angle: 45°; acquisition matrix = 256 × 192; number of excitations: 1; field of view: 240 mm; acquisition time: 9 min, 52 s). A dual-echo fast-spin-echo imaging sequence (generating proton-density weighted and T2-weighted images) was acquired to complete the clinical evaluation (effective thickness is 7.25 mm (6 mm plus 1.25 mm gap); effective TE = 11.88 and 83.16 ms; TR = 2400 ms; FOV = 240 mm; acquisition matrix = 256 × 192).
Patients were not sedated for the exams (due to lack of justification for incurring the additional risk); this resulted in moderate to severe motion artifacts in five of the cases (36%). This rate is higher than for the healthy controls in this sample (11.9%) and for a large group of 126 healthy male volunteers (11.9%) reported previously (Blumenthal et al., 2002). Thus, further analysis of the images was limited to methods that are relatively more tolerant of motion artifacts.
2.3. Qualitative MRI review procedures
The clinical MRI studies underwent qualitative review by a neuroradiologist (EB). Potential anatomical abnormalities of the brain and skull were assessed and recorded; assessment included shape and thickness of the skull, prominence and shape of the ventricles, position and size of the cerebellar tonsils, conformation of the corpus callosum, asymmetry, atrophy, abnormalities of neural migration, presence of cysts, and presence of white matter lesions. White matter lesions were counted, and classified as falling into a predominantly periventricular or subcortical pattern.
2.4. Quantitative MRI Procedures: Automated Pipeline
MRI data was processed using an automated image method called CIVET that was developed at the Montreal Neurological Institute. The native SPGR MRI scans were registered into standardized stereotaxic space using a linear transformation (Collins et al., 1994) and corrected for non-uniformity artifacts (Sled et al., 1998). The registered and corrected volumes were segmented into white matter, gray matter, and CSF using a neural net classifier (Zijdenbos et al., 2002). Total brain volume included volumes of gray matter, white matter, and the lateral ventricles. The regions which have been validated by comparison with other methods are the following: gray and white matter volumes of the total cerebrum and lateral ventricles (Collins et al., 1995).
2.5. Quantitative MRI procedures: hand tracing & quantification of the corpus callosum
The axial images were manually rotated into a standardized space using MIPAV's (Medical Image Processing, Analysis and Visualization, version 4.3.1; http://mipav.cit.nih.gov/) protractor alignment tool. In the axial plane, the posterior and anterior points of the longitudinal fissure were brought into vertical alignment such that the angle of deviation between the points was zero. In the sagittal plane, the deviation angle between the anterior-most and posterior-most points of the CC was set to zero. Similarly, in the coronal plane, the deviation angle between the medial–posterior pons and the superior-most point of the longitudinal fissure was set to zero. Scans for two of the patients exhibited motion artifact too severe for manual tracing and were discarded from these analyses.
Corpus callosum (CC) area was hand traced using five slices — the midsagittal and 4 parasagittal slices (two on either side of midline). CC area measures can vary substantially with only slight changes in the angle of the chosen midsagittal slice, rater error, or even as a result of within-scanner measurement drift (Takao et al., 2011). In order to limit our measurement error, we used four additional paired parasagittal slices to increase the overall number of pixels sampled thereby increasing our power to detect between-group differences (Wade et al., in press). All tracing was performed using MIPAV by a single trained rater (BW) with high intra-rater (both ICC > 0.95) reliability. We used the Hofer–Frahm guidelines (Hofer and Frahm, 2006) to partition the CC into five functionally distinct subregions across each slice included in the schemes, using an automated in-house MATLAB program (see Fig. 1).
Fig. 1.
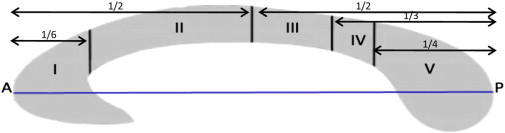
Illustration of Hofer–Frahm scheme subdivision proportions relative to the anterior–posterior line of the corpus callosum. Region I: prefrontal; Region II: premotor and supplementary motor; Region III: motor; Region IV: sensory; Region V: parietal, temporal, and occipital. A, anterior; P, posterior (Hofer and Frahm, 2006).
The Hofer–Frahm guidelines group WM bundles that traverse the CC into five vertically divided partitions along the anterior–posterior length of the callosum where ‘vertical’ is defined as being perpendicular to the A–P axis. Region I contains fibers thought to project primarily to the prefrontal cortex. Region II is comprised of fibers thought to project to the premotor and supplementary motor areas. Region III fibers are thought to project to the primary motor cortex, Region IV to the sensory cortices and Region V to the parietal, temporal and occipital cortex (Hofer and Frahm, 2006).
2.6. Statistical analysis
Demographic, cognitive, behavioral, and brain volume differences between groups were assessed with independent samples t-tests. We performed outlier analysis on all of the brain morphometric data, and no extreme outliers (defined as 3 or more SD above the mean) were identified. Two-tailed significance levels were employed with alpha = 0.05.
3. Results
The 49,XXXXY and control groups were not significantly different with regards to age, height, weight, Tanner stage, or SES (Table 1). Consistent with prior reports of 49,XXXXY being associated with intellectual disability, the mean IQ and adaptive function composite scores were 60.9 and 61.9, respectively (i.e., 3 to 4 standard deviations below the mean scores in the control group). Receptive language abilities, as measured by the PPVT, were similarly low, with a mean of 65. The PPVT also provides an age-equivalent score, which approximates the absolute number of vocabulary items identified correctly by an average child of a particular age. The PPVT age equivalent for the 49,XXXXY males was 5.2, less than half of their chronological age. Internalizing complaints and externalizing behaviors, as well as social, thought, and attention problems were significantly greater in the 49,XXXXY males, as reported on the CBCL.
3.1. Quantitative brain volumes
As shown in Table 2, total brain volume was 20% smaller in subjects with 49,XXXXY as compared to the normal controls. This is in contrast to approximately 7% reduction seen in youth with XXY (Giedd et al., 2007).
Table 2.
Brain volumes.
| 49,XXXXY |
46,XY |
||||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | N | Mean (SD) | N | t | P | d | |
| Total brain volume, ml | 1117.4 (98.6) | 14 | 1402.0 (112.4) | 42 | 9.0 | < .001a | 2.5 |
| Corpus callosum region I, area | 452.7 (50.8) | 12 | 517.8 (81.7) | 42 | 3.4 | .002a | 0.8 |
| Corpus callosum region II, area | 382.1 (55.0) | 12 | 444.6 (74.5) | 42 | 3.2 | .004a | 0.8 |
| Corpus callosum region III, area | 133.1 (25.5) | 12 | 178.9 (36.1) | 42 | 5.0 | < .001a | 1.3 |
| Corpus callosum region IV, area | 65.0 (21.2) | 12 | 91.8 (24.6) | 42 | 3.7 | .001a | 1.1 |
| Corpus callosum region V, area | 494.0 (72.7) | 12 | 573.8 (94.5) | 42 | 3.1 | .005a | 0.8 |
All t-tests survived Bonferroni adjustment. Large effect sizes (> 0.8) were found for all comparisons using Cohen's d.
Statistically significant.
Furthermore, hand tracings of CC regions I, II, and V were 13–14% smaller and CC region III and IV were 26% and 29% smaller, respectively, in subjects with 49,XXXXY. The combined cross sectional area of the five CC regions were subjected a 2 × 5 mixed model ANOVA with one between-subjects factor (group) and one within-subjects factor (CC region: 1–5). Results revealed a main effect of group (F[1,52] = 11.61, p = .001), but no region by group interaction (F[4,208] = 1.91, p = .11), indicating that the CC of the 49, XXXXY group was smaller overall but these findings were not regionally-specific. After controlling for the smaller TBV among the 49,XXXXY males, there was no main effect of group (F[1,51] = 0.05, p = .82) and no region by group interaction (F[4,204] = 1.68, p = .16).
3.2. Qualitative MRI assessment
Table 3 summarizes the rates of clinical findings for the two groups. These were organized into five categories, described below.
Table 3.
Clinical MRI observations.
| 49,XXXXY (N = 14) |
46,XY (N = 42) |
|||||
|---|---|---|---|---|---|---|
| Count | % | Count | % | χ2 | P | |
| Skull deformity | ||||||
| Craniofacial disproportion | 2 | 14 | 0 | 0 | 6.2 | .013a |
| Plagiocephaly | 4 | 29 | 1 | 2 | 8.9 | .003a,b |
| Dolichocephaly | 1 | 7 | 0 | 0 | 3.1 | .081 |
| Thickening of the calvarium | 1 | 7 | 0 | 0 | 3.1 | .081 |
| Focal WM signal abnormalities | ||||||
| Exclusively periventricular | 1 | 7 | 0 | 0 | 3.1 | .081 |
| Periventricular > subcortical | 1 | 7 | 0 | 0 | 3.1 | .081 |
| Subcortical > periventricular | 3 | 21 | 0 | 0 | 9.5 | .002a,b |
| Exclusively subcortical | 2 | 14 | 1 | 2 | 2.9 | .087 |
| Colpocephaly | 9 | 64 | 8 | 19 | 10.2 | .001a,b |
| Prominent ventricles | 4 | 29 | 5 | 12 | 2.2 | .141 |
| Periventricular cyst(s) | 3 | 21 | 1 | 2 | 5.7 | .017a |
Statistically significant.
Survived Bonferroni adjustment.
3.2.1. Skull deformities
As summarized in Table 3, six of the patients had no skull deformity, while two (14%) showed craniofacial disproportion, four (29%) had plagiocephaly (two of which were mild; see Fig. 2 panels A and E), one (7%) had mild dolichocephaly, and one (7%) had mild thickening of the calvarium.
Fig. 2.
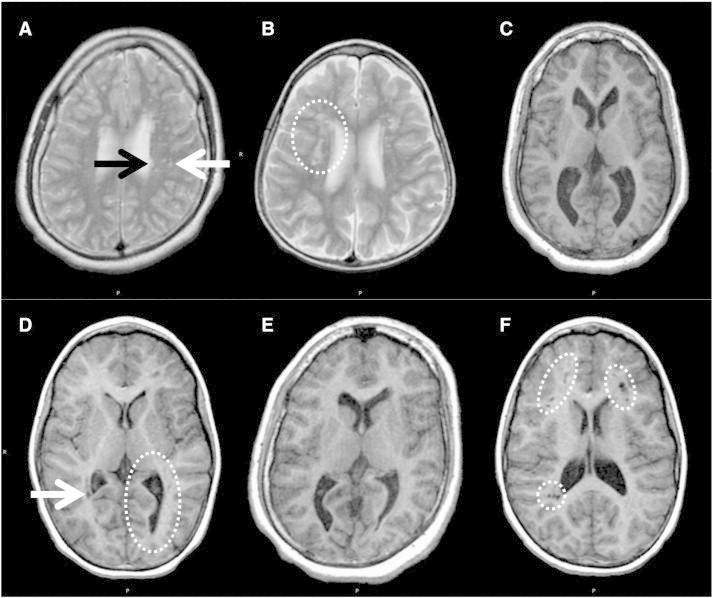
Examples of MRI abnormalities in the brains of 49,XXXXY patients. A) Focal white matter abnormalities, subcortical location (white arrow) and periventricular location (black arrow). This patient also has plagiocephaly. White matter lesions occurred in 7 of 14 patients, but were rare in the normal controls (one lesion was found in one normal control). Subcortical location was more common than periventricular location. More lesions were located on the left than on the right. The number of lesions was highly variable, ranging from 7 to more than 50. B) Confluent white matter abnormalities (circle). This finding was present in 1 patient. C) Prominent ventricles and colpocephaly. Ventricles were prominent in 4 patients. All had only a mild degree of prominence; the case shown was the most prominent. Colpocephaly among the patients ranged from minimal to moderate; none were severe and none were associated with any degree of agenesis of the corpus callosum. D) Periventricular cyst (arrow) and unilateral colpocephaly (circle). E) Colpocephaly and plagiocephaly. F) Unusually large, clustered perivascular spaces (circles). 3 patients demonstrated this unusual pattern of perivascular spaces, which was not seen at all in the normal controls.
Forty-one of the controls had no skull deformity and one (2%) had mild plagiocephaly.
3.2.2. Focal WM Signal abnormalities
Seven patients (50%) had focal white matter signal abnormalities (see Fig. 2A). Subcortical location was more common than periventricular location; specifically, 1 (7%) had exclusively periventricular lesions, 1 (7%) had more periventricular than subcortical, 3 (21%) had more subcortical than periventricular, and 2 (14%) had exclusively subcortical lesions. The number of lesions was highly variable, ranging from 7 to more than 50. The majority of lesions were in the frontal lobes (an average of 4 on the left and 1 on the right), and the next most common location was the parietal lobes (an average of 2 on the left and 2 on the right); very few lesions were found in the occipital or temporal lobes. One patient had many confluent lesions (precluding an exact count) in the frontal and occipital lobes, and is not included in the averages given above (see Fig. 2B). Seven of the patients had prominent perivascular spaces; these are a non-pathological normal variant, but their presence can create ambiguity as to the exact number of white matter lesions, since prominent perivascular spaces can look very much like small white matter lesions on low-resolution thick-slice images (such as were used in this study), a problem that is further compounded by motion artifacts. Three of the seven patients having prominent perivascular spaces demonstrated an unusual pattern of clustered perivascular spaces (see Fig. 2F).
One control subject (2%) had a single focal white matter lesion that was located in the subcortical white matter of the right frontal lobe. Twenty-two of the controls had prominent perivascular spaces, without any clustering.
The corpus callosum (CC) was completely formed in all patients and control subjects. However, the splenium of the corpus callosum was small (by qualitative evaluation) in 2 patients, and 4 of the patients showed thinning of the corpus callosum (2 minimal and 2 mild, again by qualitative evaluation).
3.2.3. Colpocephaly and ventricular abnormalities
Colpocephaly was operationalized as disproportionate enlargement of the occipital horns, with the remaining ventricular system maintaining normal size and configuration, and was observed in nine patients (64%; ranging from minimal to moderate; see Fig. 2 panels C, D, and E). The pattern was predominantly asymmetrical with preferential involvement of the left side. Independent of colpocephaly, 4 patients (29%) had abnormal prominence of part or all of the ventricular system (see Fig. 2C), and 1 had a small fourth ventricle. One patient had a small cavum septum pellucidum and small cavum vergae, which is generally considered to be a normal variant. Periventricular cysts were present in three patients (21%; see Fig. 2D).
Colpocephaly was observed in 8 control subjects (19%), four had minimal to mild colpocephaly on the left side and another four had minimal bilateral colpocephaly. Independent of colpocephaly, 5 control subjects (12%) had abnormal prominence of part or all of the ventricular system, 2 had asymmetrical lateral ventricles, 2 had a small fourth ventricle, and one had a large fourth ventricle. Two control subjects had a small cavum septum pellucidum. Two periventricular cysts were present in one control subject (2%).
3.2.4. Other clinical observations
None of the patients showed generalized cerebral or cerebellar atrophy, megacisterna magna, or arachnoid cysts. However, two patients had asymmetry of the cerebellar hemispheres (one had a slightly small left hemisphere, while the other had a slightly small right hemisphere). Size and position of the cerebellar tonsils were within normal range for all of these patients. We looked for Dandy–Walker malformation and hypoplasia of the inferior vermis because they are found in a variety of genetic disorders but we did not find either of these conditions in any of the patients. No clear heterotopia was found, although motion artifacts in some of the patients were prominent enough to potentially obscure subtle cases.
None of the control subjects showed generalized cerebral or cerebellar atrophy, however one showed megacisterna magna and two had arachnoid cysts. Size and position of the cerebellar tonsils were within normal range with the following exceptions: mild Dandy–Walker variant and cerebellar tonsil ectopia were each observed in one control subject, while Chiari I malformation was observed in 2 controls (one with and one without crowding). No heterotopia was found.
As seen in Table 3, chi-square analyses were performed comparing the rates of these abnormalities between the patients and healthy controls. The males with 49,XXXXY showed increased rates of craniofacial disproportion (χ2 = 6.2, p = .013), plagiocephaly (χ2 = 8.9, p = .003), colpocephaly (χ2 = 10.2, p = .001), and periventricular cysts (χ2 = 5.7, p = .017) in contrast to the healthy controls in this sample. Furthermore, in terms of locations of focal WM signal abnormalities, subcortical location was more common than periventricular location (χ2 = 9.5, p = .002). These results survived Bonferroni adjustment except for craniofacial disproportion and periventricular cysts.
4. Discussion
This study presented the first quantitative comparison of brain morphology in a large sample of 49,XXXXY compared with healthy controls. An overall reduction in brain volume was evident for males with 49,XXXXY along with significantly higher rates of white matter abnormalities identified through qualitative inspection by a neuroradiologist. As can be seen in this study, white matter lesions are rare among healthy children and are generally found only in advanced age among healthy adults (Kirkpatrick and Hayman, 1987). However, the rate of white matter lesions, especially subcortical lesions, among the patients with 49,XXXXY was high enough to suggest considering 49,XXXXY as part of the differential for small multifocal white matter lesions. The etiology of the white matter lesions is not evident from the imaging. Their presence implies some kind of permanent injury to the white matter. The 49,XXXXY patients also had a higher rate of minor skull deformities and thinning of the corpus callosum, both of which are almost non-existent in the healthy controls.
Mahon et al. (2010) discuss the correlation between decreased cognitive functions and the presence and severity of white matter lesions in healthy elderly adults and young bipolar patients. This same relationship may exist in the 49,XXXXY patients as a result of disruption of the axons or demyelination.
In analyses of the corpus callosum, we found a general reduction in size in the 49,XXXXY group. However, the group by region interaction did not reach statistical significance. Given the sample size of the 49,XXXXY group, this may be due to limited power. A qualitative examination of the reductions in the corpus callosum revealed that regions 3 and 4 were most strongly affected in the 49,XXXXY sample (though these differences failed to reach statistical significance when the group by section interaction was evaluated using a mixed model ANOVA). These regions are thought to be primarily associated with motor and sensory function, respectively. Interestingly, this fits with aspects of the clinical phenotype associated with 49,XXXXY. Specifically, these patients are reported to have motor difficulties including delayed motor milestones acquisition and oral motor dyspraxia along with tactile hypersensitivity (e.g., tags on shirts, specific fabrics) and other forms of sensory dysfunction (Gropman et al., 2010).
Although a limitation of the present study is a possible ascertainment bias in this self-referred sample, the clinical characteristics of this sample were largely similar to what had been reported from the previous studies, including decreased IQ and increased internalizing complaints and externalizing behaviors compared to controls.
Another limitation is that, because we started the study in 1992 and were obligated to keep the scan technique as consistent as possible throughout the study, the images we used are no longer state-of-the-art. This does not change conclusions regarding brain volumes and structural anomalies. Conclusions involving white matter lesions are quantitatively (but not qualitatively) affected. That is, we may have under-counted the number of lesions in the patients, but this does not change the finding that white matter lesions are much more common in the patients than in the healthy volunteers. The contrast and resolution of the images were not ideal for excluding heterotopia, which would be better assessed using current state-of-the-art images in a future study.
Furthermore, because the study was conducted over a period of 20 years, there is missing cognitive-behavioral data for patients and healthy volunteers who participated after the addition of several instruments (most notably, ABAS, CBCL, and PPVT). In addition, we were not able to administer the WASI to six of the patients who exhibited limited verbal ability, resulting in additional missing data. However, the PPVT was introduced to the battery to serve as a receptive vocabulary estimate of IQ. We recognize that there are differences between the samples for individual instruments and, therefore, inferences should be made with caution.
This study reports both qualitative and quantitative differences between a large sample of 49,XXXXY males and their healthy controls in terms of white matter lesions and thinning of the corpus callosum, which may be related to their clinical phenotype and their behavioral and cognitive abilities.
4.1. Conclusions
This study presented the first quantitative comparison of brain morphology in a large sample of 49,XXXXY compared with healthy controls. The results of the current investigation indicate that the neuroanatomical phenotype associated with 49,XXXXY syndrome is characterized by brain volume reductions overall and increased rates of brain abnormalities such as colpocephaly, plagiocephaly, periventricular cysts, and minor craniofacial abnormalities. White matter lesions were identified in 50% of subjects, supporting the inclusion of 49,XXXXY in the differential diagnosis of small multifocal white matter lesions.
The mild colpocephaly among the 49,XXXXY patients was predominantly on the left side, consistent with Graham et al. (1988) who investigated intellectual deficits of XXY patients and summarized them as functions that tend to lateralize to the left side (especially receptive language deficits that are typically processed in the left planum temporale). Colpocephaly is the result of limited development of the adjacent white matter.
Further evidence of abnormal development of white matter was provided by the smaller cross sectional area of the corpus callosum. These results suggest that increased dosage of genes on the X chromosome has significant adverse effects on white matter development, which would benefit from methods able to capture microstructural features of white matter such as diffusion tensor imaging.
Acknowledgments
The Intramural Research Program of the NIMH supported this research.
We thank the families who participated in this research, and, for assisting us in the recruitment of participants, we thank Klinefelter Syndrome and Associates (genetic.org) and Neurodevelopmental Diagnostic Center for Young Children (ndcforyoungchildren.com).
We also thank Michelle Williams, RT, the MRI technologist who assisted us in the acquisition of the images.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement: No conflicts declared.
References
- Achenbach T.M., Ruffle T.M. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatrics in Review. 2000;21(8):265–271. doi: 10.1542/pir.21-8-265. [DOI] [PubMed] [Google Scholar]
- Blumenthal J.D., Zijdenbos A., Molloy E., Giedd J.N. Motion artifact in magnetic resonance imaging: implications for automated analysis. NeuroImage. 2002;16(1):89–92. doi: 10.1006/nimg.2002.1076. [DOI] [PubMed] [Google Scholar]
- Collins D.L., Neelin P., Peters T.M., Evans A.C. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. Journal of Computer Assisted Tomography. 1994;18(2):192–205. [PubMed] [Google Scholar]
- Collins D.L., Holmes C.J., Peters T.M., Evans A.C. Automatic 3-D model-based neuroanatomical segmentation. Human Brain Mapping. 1995;3:190–208. [Google Scholar]
- Denckla M.B. MB, revised physical and neurological examination for subtle signs. Psychopharmacology Bulletin. 1985;21:773–800. [PubMed] [Google Scholar]
- Dunn L.M., Dunn D.M. fourth ed. Pearson; Minneapolis: 2007. Peabody Picture Vocabulary Test. [Google Scholar]
- Galasso C., Arpino C., Fabbri F., Curatolo P. Neurologic aspects of 49, XXXXY syndrome. Journal of Child Neurology. 2003;18(7):501–504. doi: 10.1177/08830738030180071001. [DOI] [PubMed] [Google Scholar]
- García-Cazorla A., Sans A., Baquero M., García-Bargo M.D., Arellano M., Poo P., Gean E., Campistol J. White matter alterations associated with chromosomal disorders. Developmental Medicine and Child Neurology. 2004;46(3):148–153. doi: 10.1017/s0012162204000271. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Clasen L.S., Wallace G.L., Lenroot R.K., Lerch J.P., Wells E.M., Blumenthal J.D., Nelson J.E., Tossell J.W., Stayer C., Evans A.C., Samango-Sprouse C.A. XXY (Klinefelter syndrome): a pediatric quantitative brain magnetic resonance imaging case-control study. Pediatrics. 2007;119(1):e232–e240. doi: 10.1542/peds.2005-2969. [DOI] [PubMed] [Google Scholar]
- Graham J.M., Bashir A.S., Stark R.E. Oral and written language abilities of XXY boys: implications for anticipatory guidance. Pediatrics. 1988;81:795–806. [PubMed] [Google Scholar]
- Gropman A.L., Rogol A., Fennoy I., Sadeghin T., Sinn S., Jameson R., Mitchell F., Clabaugh J., Lutz-Armstrong M., Samango-Sprouse C.A. Clinical variability and novel neurodevelopmental findings in 49, XXXXY syndrome. American Journal of Medical Genetics. Part A. 2010;152A(6):1523–1530. doi: 10.1002/ajmg.a.33307. [DOI] [PubMed] [Google Scholar]
- Haeusler G., Frisch H., Guchev Z., Hadziselimovic F., Neuhold A., Vormittag W. Hypoplasia of the corpus callosum and growth hormone deficiency in the XXXXY syndrome. American Journal of Medical Genetics. 1992;44(2):230–232. doi: 10.1002/ajmg.1320440221. [DOI] [PubMed] [Google Scholar]
- Harrison P.L., Oakland T. Psychological Corp., Harcourt Assessment Company; San Antonio: 2003. Manual of the Adaptive Behaviour Assessment System II. [Google Scholar]
- Hofer S., Frahm J. Topography of the human corpus callosum revisited — comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage. 2006;32(3):989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Hoffman T.L., Vossough A., Ficicioglu C., Visootsak J. Brain magnetic resonance imaging findings in 49, XXXXY syndrome. Pediatric Neurology. 2008;38(6):450–453. doi: 10.1016/j.pediatrneurol.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A.B. Yale University Department of Sociology; New Haven: 1975. Four Factor Index of Social Status. [Google Scholar]
- Kirkpatrick J.B., Hayman L.A. White-matter lesions in MR imaging of clinically healthy brains of elderly subjects: possible pathologic basis. Radiology. 1987;162(2):509–511. doi: 10.1148/radiology.162.2.3797666. [DOI] [PubMed] [Google Scholar]
- Kleczkowska A., Fryns J.P., Van den Berghe H. X-chromosome polysomy in the male. The Leuven experience 1966–1987. Human Genetics. 1988;80(1):16–22. doi: 10.1007/BF00451449. [DOI] [PubMed] [Google Scholar]
- Linden M., Bender B., Robinson A. Sex chromosome tetrasomy and pentasomy. Pediatrics. 1995;96:672–682. [PubMed] [Google Scholar]
- Mahon K., Burdick K.E., Szeszko P.R. A role for white matter abnormalities in the pathophysiology of bipolar disorder. Neuroscience and Biobehavioral Reviews. 2010;34(4):533–554. doi: 10.1016/j.neubiorev.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J., Wohlert M. Sex chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Birth Defects Original Article Series. 1990;26(4):209–223. [PubMed] [Google Scholar]
- Ottesen A.M., Aksglaede L., Garn I., Tartaglia N., Tassone F., Gravholt C.H., Bojesen A., Sorensen K., Jorgensen N., Rajpert-De Meyts E. Increased number of sex chromosomes affects height in a nonlinear fashion: a study of 305 patients with sex chromosome aneuploidy. American Journal of Medical Genetics. Part A. 2010;152A(5):1206–1212. doi: 10.1002/ajmg.a.33334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A.C., Crockett L., Richards M., Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Ratcliffe S.G. The psychological and psychiatric consequences of sex chromosome abnormalities in children, based on population studies. In: Poustka F., editor. Basic Approaches to Genetic and Molecularbiological Developmental Psychiatry. Quintessenz; Berlin: 1994. pp. 99–122. [Google Scholar]
- Ratcliffe S.G., Axworthy D., Ginsborg A. The Edinburgh study of growth and development in children with sex chromosome abnormalities. Birth Defects Original Article Series. 1979;15(1):243–260. [PubMed] [Google Scholar]
- Robinson A., Bender B., Borelli J., Puck M., Salbenblatt J. Sex chromosomal anomalies: prospective studies in children. Behavior Genetics. 1983;13(4):321–329. doi: 10.1007/BF01065770. [DOI] [PubMed] [Google Scholar]
- Robinson A., Bender B.G., Linden M.G. Summary of clinical findings in children and young adults with sex chromosome anomalies. Birth Defects Original Article Series. 1990;26(4):225–228. [PubMed] [Google Scholar]
- Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Tabarki B., Al Shafi S., Al Adwani N., Al Shahwan S. Further magnetic resonance imaging (MRI) brain delineation of 49, XXXXY syndrome. Journal of Child Neurology. 2012;27(5):650–653. doi: 10.1177/0883073811424797. [DOI] [PubMed] [Google Scholar]
- Takao H., Hayashi N., Ohtomo K. Effect of scanner in longitudinal studies of brain volume changes. Journal of Magnetic Resonance Imaging. 2011;34(2):438–444. doi: 10.1002/jmri.22636. [DOI] [PubMed] [Google Scholar]
- Tartaglia N., Ayari N., Howell S., D'Epagnier C., Zeitler P. 48, XXYY, 48, XXXY and 49, XXXXY syndromes: not just variants of Klinefelter syndrome. Acta Paediatrica. 2011;100(6):851–860. doi: 10.1111/j.1651-2227.2011.02235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennes K., Puck M., Bryant K., Frankenburg W., Robinson A. A developmental study of girls with trisomy X. American Journal of Human Genetics. 1975;27(1):71–80. [PMC free article] [PubMed] [Google Scholar]
- Visootsak J., Rosner B., Dykens E., Tartaglia N., Graham J.M., Jr. Behavioral phenotype of sex chromosome aneuploidies: 48, XXYY, 48, XXXY, and 49, XXXXY. American Journal of Medical Genetics. Part A. 2007;143A(11):1198–1203. doi: 10.1002/ajmg.a.31746. [DOI] [PubMed] [Google Scholar]
- Wade, B.S.C., Stockman, M., McLaughlin, M.J., Raznahan, A., Lalonde, F., Giedd, J.N., in press. Improved corpus callosum area measurements by analysis of adjacent parasagittal slices. Psychiatry Research: Neuroimaging. [DOI] [PMC free article] [PubMed]
- Warwick M.M., Doody G.A., Lawrie S., Kestelman J.N., Best J., Johnstone E.C. Volumetric magnetic resonance imaging study of the brain in subjects with sex chromosome aneuploidies. Journal of Neurology, Neurosurgery & Psychiatry. 1999;66(5):628–632. doi: 10.1136/jnnp.66.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio: 1999. Wechsler Abbreviated Scale of Intelligence (WASI) Manual. [Google Scholar]
- Zijdenbos A.P., Forghani R., Evans A.C. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Transactions on Medical Imaging. 2002;21(10):1280–1291. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]


