Abstract
Rheumatic heart disease is still a major cause of mitral valve dysfunction in developing countries. We present our early results of rheumatic mitral valve repair.
From August 2009 through July 2011, 60 patients (24 male and 36 female) with rheumatic disease underwent mitral repair. The mean age was 51.1 ± 13.8 years (range, 16–77 yr). Forty-nine patients were in New York Heart Association functional class III or IV. Repair procedures included chordal and papillary muscle splitting, secondary chordal division, mitral ring annuloplasty (n=58), commissurotomy (n=36), chordal replacement (n=9), posterior leaflet extension (n=4), annular decalcification (n=2), and quadrangular resection (n=2). Secondary procedures included tricuspid ring annuloplasty, left atrial ablation, obliteration of left atrial appendage, aortic valve replacement, and left atrial reduction.
The early (30-d) mortality rate was 1.7%. The mean follow-up time was 14.9 ± 5 months (range, 4–26 mo). Follow-up echocardiography revealed trivial or no mitral regurgitation (MR) in 35.5% and mild (1+) MR in 49.1% of patients. Only 1 patient presented with severe (3+) MR. The mean MR grade decreased from 3.2 ± 0.9 to 0.3 ± 0.4 postoperatively (P=0.001). Left ventricular end-diastolic diameter and left atrial diameter significantly decreased postoperatively (P=0.006 and P=0.001, respectively). The mean gradient over the mitral valve decreased significantly from 11 ± 5.9 mmHg to 3.5 ± 1.8 mmHg (P=0.001).
Because current techniques of mitral repair can effectively correct valve dysfunction in most patients with rheumatic disease, the number of repair procedures should be increased in developing countries to prevent complications of mechanical valve placement.
Key words: Chordae tendineae/surgery, follow-up studies, mitral valve insufficiency/surgery, mitral valve prolapse/surgery, mitral valve stenosis/surgery, rheumatic heart disease/surgery, retrospective studies, treatment outcome
Rheumatic heart disease remains a major health problem in developing countries. It is the most severe sequela of rheumatic fever and occurs in approximately 30% of patients with rheumatic fever.1 Rheumatic heart disease presents with varying degrees of pancarditis and associated valve dysfunction. Involvement of the mitral leaflets can cause mitral regurgitation (MR) or stenosis and eventually can lead to heart failure. Mitral repair is therefore recommended before left ventricular (LV) dysfunction develops.2–7 In developing countries, mitral valve replacement is usually preferred in cases of rheumatic disease. However, mitral repair yields low perioperative mortality rates, preservation of LV function, avoidance of long-term anticoagulation therapy, decreased thromboembolic complications, low risk of native-valve endocarditis, and long-term freedom from reoperation.2,3
Surgical repair is an acceptable way of converting a diseased valve to a functional valve, and it improves postoperative morbidity and survival. Although mitral repair is successful in up to 95% of patients with degenerative valves, repair procedures are feasible in only about 75% of patients with rheumatic valve disease.2,8 This difference is attributable to the varying degrees of inflammation on rheumatic valves, which can present with extensive fibrosis and calcification of the leaflet's free margin and with chordal fusion. Moreover, occasional fibrosis and calcification of the papillary muscle, commissures, and annulus can hamper the success of valve repair. Various repair techniques—including commissurotomy, shaving of diseased leaflets, chordal division or transfer, neochordal replacement with GORE-TEX® sutures, leaflet augmentation with pericardial patching, and splitting of papillary muscle—can be used in rheumatic valve repair. To enhance coaptation, an annuloplasty ring generally accompanies valve repair. Alternatively, aggressive excision of the diseased leaflet's tissue and of the supporting fused subvalvular apparatus might be preferable, in order to remove all valvular tissue that has been affected by rheumatic disease.4 All these procedures aim to improve valve mobility and leaflet coaptation.
In this report of a retrospective single-center study, we present our early experience in rheumatic mitral valve repair, and we review the literature with regard to clinical indications, different repair techniques, and outcomes of rheumatic mitral valve repair.
Patients and Methods
The ethical committee of our institution approved the study, and written informed consent was obtained from all patients for the retrospective analysis. From August 2009 through July 2011, 90 patients with rheumatic valve disease were referred to our clinic for operation. Twenty-eight of the 90 underwent mechanical valve replacement, without our ever intending to perform repair, due to severe fibrosis of the mitral valve and its subvalvular apparatus. The decisions to attempt mitral repair in the remaining 62 patients were made after examination by means of intraoperative transesophageal echocardiography (TEE) and intraoperative mitral valve inspection and exploration. Mitral repair was successful in 60 of the 62 patients (96.8%) in whom we attempted repair. Mitral repair was unsuccessful in 2 patients due to restricted mobility of the leaflets; these 2 cases were converted intraoperatively to valve replacement surgery. Overall, 66.7% of patients (60/90) presenting with rheumatic disease underwent mitral valve repair. In addition, during the same period, balloon mitral valvuloplasty was performed for mitral stenosis in 39 patients in our institution.
All preoperative, intraoperative, and postoperative data were collected from our surgical records and the hospital's medical network. The data focused on preoperative and postoperative LV functions, the presentation of the mitral valve disease, the types of repair techniques, and postoperative complications.
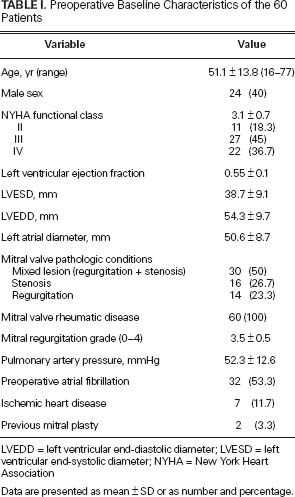
Preoperative patient characteristics appear in Table I. The mean age of the 60 patients was 51.1 ± 13.8 years (range, 16–77 yr). The mean New York Heart Association (NYHA) functional class of the 60 patients was 3.1 ± 0.7. Two patients (3.3%) had experienced previous mitral valve repair. Mitral valve presentations included combined regurgitation and stenosis in 30 patients (50%), isolated stenosis in 16 patients (26.7%) and isolated regurgitation with a mean gradient of less than 5 mmHg in 14 patients (23.3%). In the patients with isolated mitral stenosis, balloon mitral valvotomy or closed mitral commissurotomy was not performed due to concomitant severe tricuspid regurgitation (n=7), aortic valve disease (n=4), left atrial (LA) thrombus (n=3), annular calcification (n=1), or annular dilation (n=1). Mitral regurgitation was grade 3 in 21 patients (35%) and grade 4 in 23 patients (38.3%). Atrial fibrillation was documented in 32 patients (53.3%).
TABLE I. Preoperative Baseline Characteristics of the 60 Patients
Surgical Technique
Operations were performed through median sternotomy with cardiopulmonary bypass (CPB). Myocardial protection was achieved via the administration, antegrade and retrograde in combination, of blood cardioplegic solution. A superior transseptal incision (Guiraudon) was the routine approach to mitral valve exploration and to the achievement of optimal surgical exposure during repair. Leaflet repair techniques were performed in accordance with principles originally reported by Carpentier9 and Duran and colleagues,10 but several modifications provided a larger coaptation area that both increased valve surface area and improved leaflet mobility. Commissurotomy, splitting of the papillary muscles or chordae, and resection of secondary chordae were frequently performed as initial maneuvers. Leaflet resection and chordal replacement with GORE-TEX® sutures (W.L. Gore & Associates, Inc.; Flagstaff, Ariz) were performed if the state of the mitral disease warranted that. Posterior leaflet extension with pericardial patching was performed in 4 patients who had retracted and immobile mitral leaflets. We made an incision over the leaflet parallel to the mitral annulus, preserving the free edge of the leaflet with the chordae attached. Then we sutured to the gap a patch of bovine pericardium of appropriate size. The height of the posterior leaflet then became 1 to 1.5 cm. After valve repair, the anterior leaflet occupied three quarters of the mitral valve orifice, and the posterior leaflet occupied one quarter. Decalcification of calcified leaflets or annulus and removal of thickened valve tissue were performed, if required. In the mitral position, a flexible annuloplasty ring (St. Jude Medical, Inc.; St. Paul, Minn) was generally used in the presence of grade 3 to 4 MR or residual regurgitation after mitral commissurotomy. The size of the ring was selected in accordance with the commissural and anteroposterior measurements of the anterior mitral leaflet. After the completion of repair, mitral valve competence was tested by injecting cold saline solution into the LV cavity. Transesophageal echocardiography was used to evaluate the result intraoperatively.
In some patients with atrial fibrillation (AF) (paroxysmal or chronic), left atrial ablation was performed by means of a Cardioblate® unipolar radiofrequency pen with a saline-irrigated tip (Medtronic, Inc.; Minneapolis, Minn).11 Briefly, this pen was used to create intra-atrial ablation lines. Lines of electric isolation surrounded the 4 pulmonary vein ostia. Another line connected the mitral annulus with the line isolating the pulmonary veins, starting in the P3 region toward the right inferior pulmonary vein. Internal obliteration of the LA appendage, and LA size reduction, were performed in patients with AF to prevent postoperative thromboembolic events and to increase the efficacy of ablation. Obliteration of the appendage was performed by closing the appendage from the inside with a double running suture. Left atrial size reduction—performed if the LA diameter was more than 50 mm on preoperative echocardiography—was done by plicating the area between the left and right pulmonary arteries in a linear fashion, with Prolene suture. However, these procedures were not performed in the presence of calcification within the LA. If the diameter of the tricuspid valve annulus was above 40 mm on transthoracic echocardiography (TTE) preoperatively, tricuspid ring annuloplasty (St. Jude Medical) was performed.
Operative Data
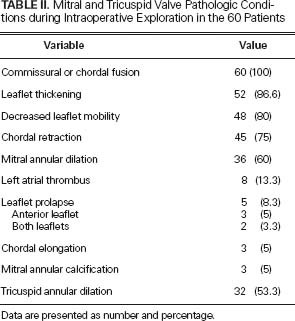
Mitral and tricuspid valve conditions encountered during intraoperative exploration are presented in Table II. Commissural fusion, thickened leaflets, decreased leaflet mobility, and mitral annular dilation were the most common such conditions. Leaflet prolapse, elongation or rupture of chordae, and annular calcification were also observed. Tricuspid annular dilation was present in 32 patients (53.3%).
TABLE II. Mitral and Tricuspid Valve Pathologic Conditions during Intraoperative Exploration in the 60 Patients
Echocardiographic Evaluation
All patients were evaluated preoperatively with TTE and intraoperatively with TEE. Echocardiographic evaluation encompassed the mitral annulus, leaflet thickness and mobility, commissural and chordal fusion, calcification, regurgitation jets, thickness of the chordae tendineae, LA thrombus, and other valvular lesions. Intraoperative TEE was used in all cases, both to determine the mechanism of mitral valve dysfunction before surgical incision and to judge the quality of the repair after CPB. All patients underwent TTE before hospital discharge and at follow-up visits. Echocardiographic evaluation of the patients during the study period was performed by means of the Vivid® S5 (GE Healthcare; Wausheka, Wisc) ultrasonography system.
Follow-Up
All patients underwent TTE before hospital discharge. Echocardiographic findings were recorded into the computerized database of the hospital. During follow-up, patients were contacted directly and were individually requested to make an appointment with the primary surgeon and referring cardiologist to evaluate mitral valve status. All TTE during follow-up visits was performed at our institution.
Clinical data recorded during the follow-up period included death after surgery, postoperative myocardial infarction, AF, re-exploration for bleeding, pleural effusion requiring drainage, prolonged need for an inotropic agent (≥24 hr), low cardiac output, MR recurrence, reoperation, thromboembolism, and postoperative endocarditis. All patients were maintained on anticoagulation with warfarin sodium for 3 months after surgery, and permanently if they had AF.
Statistical Analysis
Statistical data were expressed as mean ± SD, and categorical variables were reported as number (and %). Comparison of variables was done using Student's t test. All data were analyzed by using the statistical software SPSS version 16.0 for Windows (IBM Corporation; Armonk, NY). A P value of less than 0.05 was considered statistically significant.
Results
There was no operative death. All patients had mild, trivial, or no MR on intraoperative TEE examination after repair. In most patients, repair procedures were completed without leaflet or tissue resection. Mitral ring annuloplasty using a flexible ring was performed in 58 patients (96.7%), and the mean ring size was 30.5 ± 2.1 mm. Isolated mitral commissurotomy without ring annuloplasty was performed in only 2 patients (3.3%). Mitral commissurotomy and repair of the subvalvular apparatus were generally performed. Additional procedures included obliteration of the LA appendage in 19 patients, LA size reduction in 10, and LA thrombectomy in 8 patients. Sixteen neochordae were replaced in 9 patients (15%): 9 chordae were implanted in the anterior leaflet and 7 in the posterior leaflet. Posterior leaflet extension with bovine pericardial patch was performed in 4 patients with rheumatic disease. Annular decalcification and quadrangular resection were performed in 2 patients.
Secondary procedures frequently included tricuspid ring annuloplasty in 29 patients (48.3%), followed by LA radiofrequency ablation in 20 patients (33.3%), aortic valve replacement with mechanical valves in 16 patients (26.7%), coronary artery bypass grafting (CABG) in 2 patients (3.3%), atrial septal defect closure in 1 patient (1.7%), and left anterior descending coronary artery–right atrial fistula closure in 1 patient (1.2%). The mean tricuspid ring size in 29 patients was 33 ± 0.9 mm. The mean CPB and aortic cross-clamp times were 174.2 ± 62.7 min and 129.1 ± 44 min, respectively.
Postoperative Outcomes
The mean follow-up time was 14.9 ± 5 postoperative months (range, 4–26 mo). Only 1 patient (1.2%) died. This patient was a 67-year-old woman who presented with mixed (ischemic and rheumatic) MR, an ejection fraction of 0.45, and NYHA functional class IV. The patient underwent mitral repair and concomitant CABG, but she died on postoperative day 29 due to low cardiac output.
The mean mechanical ventilation time was 10.2 ± 5.9 hours (median, 8 hr). The lengths of stay in the intensive care unit and hospital were 3 ± 7.6 days (median, 1 d) and 11 ± 7.7 days (median, 8 d), respectively. Prolonged delivery of inotropic agents (>24 hr) was needed in 8 patients. Acute renal failure (creatinine level, >1.5 mg/dL) developed in 2 patients, 1 of whom needed hemodialysis. Twenty patients underwent radiofrequency ablation, 14 of whom were in sinus rhythm during the follow-up visits. New-onset AF developed in only 2 of 28 patients (7.1%) who had presented with sinus rhythm preoperatively. Pacemaker implantation was performed in 1 patient (1.2%) with postoperative sinus node dysfunction. Other postoperative morbidities included bleeding (that required exploration) in 4 patients, pleural effusion in 4, superficial wound infection in 1, mediastinitis in 1, and cardiac tamponade in 1. There was no procedure-related postoperative morbidity such as thromboembolism, endocarditis, or reoperation.
Echocardiographic Follow-Up
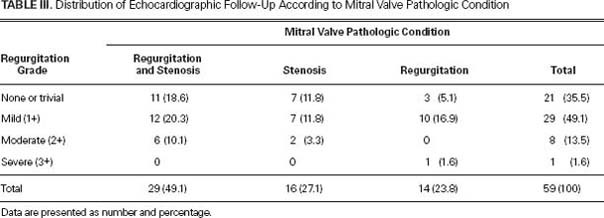
Echocardiographic follow-up results appear in Table III. At hospital discharge, there was no residual moderate-to-severe MR. At the follow-up visits of the 59 surviving patients, 35.5% presented with trivial or no MR, 49.1% presented with mild (1+) MR, and 13.5% presented with moderate (2+) MR. The mean MR grade decreased postoperatively from 3.2 ± 0.9 to 0.3 ± 0.4 (P=0.001). Most patients with mixed lesions and isolated MR or stenosis presented with less than 2+ MR during follow-up visits. Moderate (2+) MR was diagnosed in 6 patients with mixed lesions and in 2 patients with isolated mitral stenosis. Only 1 patient (1.7%) with isolated MR presented with residual 3+ MR. This patient rejected reoperation and was treated medically.
TABLE III. Distribution of Echocardiographic Follow-Up According to Mitral Valve Pathologic Condition
During the follow-up period, the mean LV ejection fraction decreased from 0.55 ± 0.1 to 0.53 ± 0.1 (95% confidence interval [CI], 0.3–4.7, P <0.023). The mean LV end-diastolic diameter decreased significantly from 54.3 ± 9.8 mm to 51.4 ± 7.9 mm (95% CI, 0.8–4.8, P=0.006). The mean LV end-systolic diameter decreased from 38.4 ± 9.1 mm to 37 ± 7.8 mm (95% CI, 0.04–2.87, P=0.05). The mean LA diameter showed a significant decrease postoperatively (P=0.001). The mean pulmonary artery pressure decreased from 52.3 ± 12.6 mmHg to 37.9 ± 6.1 mmHg postoperatively (95% CI, 10.4–18.3, P=0.001). The mean mitral valve area increased from 1.4 ± 0.5 cm2 to 2 ± 0.4 cm2 postoperatively (95% CI, −0.8 to 0.2, P=0.001). The mean gradient over the mitral valve decreased significantly from 11 ± 5.9 mmHg to 3.5 ± 1.8 mmHg postoperatively (95% CI, 2.1–6.9, P=0.001).
Discussion
Although the prevalence of rheumatic disease and its valvular complications has decreased in Europe and the United States during the last few decades, rheumatic valve disease is still an extremely important cause of mitral valve dysfunction in developing countries. It is associated with substantial morbidity and a tremendous health-care expense. These days, valve repair in cases of degenerative mitral valve disease is known to be superior to replacement in operative mortality rates, functional outcomes, and long-term survival.2,3 Although surgical repair of rheumatic mitral valve disease is technically more demanding and has a higher potential failure rate compared with repair of degenerative disease, many surgeons agree that mitral valve repair should be preferred to replacement for primary correction.12–14 However, the fibrotic process—involving the mitral valve and calcification of the mitral leaflets or annulus—can diminish the likelihood of repair in rheumatic disease, even in experienced hands. In the presence of less leaflet and subvalvular fibrosis, mitral repair can be the initial procedure of choice in rheumatic disease.
Preoperative and intraoperative echocardiographic examinations, as well as intraoperative inspection of the mitral valve, help to predict the necessity of mitral valve repair. The presence of minimal subvalvular lesions and pliable leaflets increases the likelihood that mitral repair will be needed. During the study period, 66.6% of patients with rheumatic disease underwent mitral valve repair in our preliminary series. In the literature, it has been noted that repair procedures can be feasible in about 75% of patients with rheumatic valve disease.2,8 In our experience, the intention to perform mitral repair was realized in 96.8% of patients (60/62) with rheumatic disease. Many patients with rheumatic mitral valve disease have important stenotic and regurgitant components owing to commissural fusion. In our series, 50% of patients presented with mixed stenotic and regurgitant lesions. Mitral repair can be challenging in such cases, because they require complex repair techniques. Moreover, 26.7% of patients presented with isolated mitral stenosis, and neither balloon mitral valvotomy nor closed mitral commissurotomy was performed in these patients—due to concomitant severe tricuspid regurgitation, aortic valve disease, LA thrombus, annular calcification, and annular dilation. These patients underwent surgical repair.
Rheumatic mitral stenosis that is not associated with severe chordal fusion or shortening, or with calcification, may be treated with mitral commissurotomy.2 In our patients, commissurotomy, when necessary, was first performed, and then the leaflets were explored for further procedures. Elongation or retraction of the chordal and papillary structures, thickening of the leaflets, or calcification of any valve component decreases the success of isolated commissurotomy. Annular dilation in mixed mitral lesions can lead to severe MR after commissurotomy. Therefore, rheumatic repair procedures, including commissurotomy, can be completed with ring annuloplasty to improve leaflet coaptation. As mentioned by Carpentier in the discussion section of Yau's article,5 the main factors predicting reoperation in Yau's rheumatic series were annular dilation without the use of a ring on the initial operation (16%), the predominance of stenotic lesions (16%), leaflet retraction (32%), and leaflet prolapse (32%). In our series, mitral ring annuloplasty was performed in almost all patients who needed commissurotomy.
The use of prosthetic-ring annuloplasty has become a standard technique in mitral valve repair, because of its proven short- and long-term efficacy.15,16 Ring annuloplasty reduces the incidence of both mitral and tricuspid valve reoperations in rheumatic patients.6 In our experience, flexible annuloplasty rings were used to improve leaflet coaptation and to reduce the incidence of recurrent MR. We used flexible rings in different procedures, including quadrangular resection and the augmentation of a posterior leaflet with a pericardial patch. In general, large rings (for example, 31 to 32 mm in men and 29 to 31 mm in women) were used. At the present time, there is no consensus on which type of annuloplasty ring—flexible, semi-rigid or rigid—is best in reducing MR, but larger rings are recommended in treating rheumatic mitral disease.15,16 The rigid rings can have some problems, such as systolic anterior motion, which obstructs the outflow tract of the left ventricle. The mitral valve is dynamic throughout the cardiac cycle (decreasing its annular circumference and area in systole). This variable geometry can be preserved with the use of a flexible ring. In association with flexible-ring implantation, both early LV function and diastolic flow through the mitral valve during exercise have improved, according to reports in the literature.17,18
Chordal replacement has been introduced to repair mitral prolapse and other complex valve conditions, including rheumatic lesions.19–22 The use of artificial chordae is indicated whenever adequate native chordae are not available. This procedure can be necessary in the presence of chordal fusion, retracted papillary muscles, chordal rupture, and annular dilation. All these presentations of rheumatic disease can decrease the availability of suitable native chordae (those exhibiting proper mobility and adequate length). Although satisfactory results have been obtained with the transfer of secondary cords to the leaflet margins, these procedures can be complicated and technically demanding when applied to a fibrotic rheumatic leaflet.12 Chordal replacement with PTFE sutures has been reported to be effective, with a freedom from reoperation rate of 82% to 94% at 10 years.19,20 Nevertheless, the long-term results of chordal replacement in rheumatic disease are not clear.
In our series, 10% of our patients needed chordal replacement of the anterior leaflet with PTFE, and 5% needed such replacement for the posterior leaflet. These sutures were placed between the tip of the papillary muscle and the edge of the mitral leaflet. The “reference-point method” was used as the guideline for the adjustment of the length of the artificial chordae.9 The level of the zone of opposition was adjusted in accordance with the level of the non-prolapsing leaflet. Non-elongated chordae were pulled upward by a hook. The level was exposed and then PTFE sutures were affixed. This technique was also used in some patients who had MR due to leaflet prolapse associated with ruptured chordae tendineae. There was no mild or moderate MR on intraoperative and postoperative echocardiographic examinations.
In rheumatic valve disease, progressive fibrosis of the leaflets and subvalvular apparatus can lead to the restriction of leaflets and failure of coaptation. It has been reported that posterior leaflet retraction is a frequent event, occurring in almost 60% of cases.12 This can be associated with prolapse of the anterior leaflet due to the elongation of chordae or papillary muscle. In order to improve leaflet coaptation, augmentation of the retracted leaflets is recommended.18,21,22 This technique can be beneficial in the presence of a calcified or rigid posterior leaflet. The use of autologous or heterologous patch material, rather than diseased leaflet tissue left in situ, might prevent new episodes of rheumatic inflammation that affect valve function.12 In our series, posterior leaflet augmentation was performed in 4 patients, with a favorable outcome.
An enlarged LA in mitral valve disease affects LV function and decreases the likelihood of return to sinus rhythm after radiofrequency ablation.11 The volume of a severely enlarged LA can be reduced to improve atrial flow and LV function, as well as to decrease the risk of thrombus formation. Left atrial volume reduction also improves the outcomes of the modified maze procedure to correct permanent AF during concomitant mitral surgery.11,23 In this experience, reduction of the LA size was performed in 16.7% of patients—those who had a LA diameter of more than 50 mm on preoperative echocardiography. Technically, a helicoidal plication of the LA was performed in this experience, without removing atrial tissue. Plication of the atrium started at the origin of the right lower pulmonary vein, progressed under the posterior mitral annulus, and ended at the ostia of the left pulmonary veins. This procedure was combined with internal obliteration of the LA appendage to increase the efficacy of volume reduction and to eliminate the risk of thrombus formation.
This study examined the early follow-up of mitral repair in a group of patients with rheumatic mitral valve dysfunction. Ours was a consecutive series of patients of whom 81.7% (49/60) were in NYHA functional class III or IV and 50% had mixed mitral lesions (stenosis and regurgitation). During follow-up visits, clinical outcome was satisfactory in almost all cases. Echocardiographic examinations at 14.9 ± 5 months revealed trivial or no MR in 35.5% of the patients, and mild (1+) MR in 49.2%. The mean mitral valve area significantly increased from 1.4 ± 0.5 cm2 to 2 ± 0.4 cm2 postoperatively. Previously, Kim and colleagues7 reported that, during a mean echocardiographic follow-up duration of 66 ± 38.6 months, 16.7% of 115 patients with rheumatic mitral repair showed significant MR (>grade 2) or moderate mitral stenosis (mitral valve area, 1.2–1.4 cm2), but no severe stenosis (mitral valve area, <1 cm2). These investigators also stated that most reoperations for residual or recurrent MR occurred within the first 6 months. After that time, the number of reoperations decreased.7 In regard to results, we would predict that our results of mitral repair in 60 patients with a relatively short follow-up will be satisfactory in longer follow-up. But in truth our very early follow-up results are insufficient to enable conclusions on the success of mitral valve repair—particularly in rheumatic valve disease, which is often progressive. Nonetheless, our early follow-up results have encouraged us to continue to follow this cohort and to present longer-term outcomes in a subsequent report.
In conclusion, rheumatic valve repair appears to be possible in most patients with rheumatic mitral valve dysfunction. Current techniques can effectively correct valve dysfunction and yield favorable outcomes. We believe that the number of mitral repair procedures should be increased in developing countries, to prevent the sequelae that too often are associated with mechanical prosthesis implantation.
Footnotes
Address for reprints: Ihsan Bakir, MD, Department of Cardiovascular Surgery, Istanbul Mehmet Akif Ersoy Thoracic and Cardiovascular Surgery Training & Research Hospital, Istanbul Caddesi, Bezirganbahce mevkii, Kucukcekmece, 34303 Istanbul, Turkey
E-mail: ihsanbak@yahoo.com
References
- 1.Rheumatic fever and rheumatic heart disease. World Health Organ Tech Rep Ser 2004;923:1–122, back cover. [PubMed]
- 2.American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease); Society of Cardiovascular Anesthesiologists; Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons [published erratum appears in J Am Coll Cardiol 2007;49(9):1014]. J Am Coll Cardiol 2006;48(3):e1–148. [DOI] [PubMed]
- 3.DiBardino DJ, ElBardissi AW, McClure RS, Razo-Vasquez OA, Kelly NE, Cohn LH. Four decades of experience with mitral valve repair: analysis of differential indications, technical evolution, and long-term outcome. J Thorac Cardiovasc Surg 2010;139(1):76–84. [DOI] [PubMed]
- 4.El Oumeiri B, Boodhwani M, Glineur D, De Kerchove L, Poncelet A, Astarci P, et al. Extending the scope of mitral valve repair in rheumatic disease. Ann Thorac Surg 2009;87(6): 1735–40. [DOI] [PubMed]
- 5.Yau TM, El-Ghoneimi YA, Armstrong S, Ivanov J, David TE. Mitral valve repair and replacement for rheumatic disease. J Thorac Cardiovasc Surg 2000;119(1):53–60. [DOI] [PubMed]
- 6.Bernal JM, Ponton A, Diaz B, Llorca J, Garcia I, Sarralde JA, et al. Combined mitral and tricuspid valve repair in rheumatic valve disease: fewer reoperations with prosthetic ring annuloplasty. Circulation 2010;121(17):1934–40. [DOI] [PubMed]
- 7.Kim JB, Kim HJ, Moon DH, Jung SH, Choo SJ, Chung CH, et al. Long-term outcomes after surgery for rheumatic mitral valve disease: valve repair versus mechanical valve replacement. Eur J Cardiothorac Surg 2010;37(5):1039–46. [DOI] [PubMed]
- 8.Choudhary SK, Talwar S, Dubey B, Chopra A, Saxena A, Kumar AS. Mitral valve repair in a predominantly rheumatic population. Long-term results. Tex Heart Inst J 2001;28(1):8–15. [PMC free article] [PubMed]
- 9.Carpentier A. Cardiac valve surgery–the “French correction”. J Thorac Cardiovasc Surg 1983;86(3):323–37. [PubMed]
- 10.Duran CG, Revuelta JM, Gaite L, Alonso C, Fleitas MG. Stability of mitral reconstructive surgery at 10–12 years for predominantly rheumatic valvular disease. Circulation 1988;78 (3 Pt 2):I91–6. [PubMed]
- 11.Bakir I, Casselman FP, Brugada P, Geelen P, Wellens F, Degrieck I, et al. Current strategies in the surgical treatment of atrial fibrillation: review of the literature and Onze Lieve Vrouw Clinic's strategy. Ann Thorac Surg 2007;83(1):331–40. [DOI] [PubMed]
- 12.Suri RM, Schaff HV, Dearani JA, Sundt TM 3rd, Daly RC, Mullany CJ, et al. Survival advantage and improved durability of mitral repair for leaflet prolapse subsets in the current era. Ann Thorac Surg 2006;82(3):819–26. [DOI] [PubMed]
- 13.Chauvaud S, Fuzellier JF, Berrebi A, Deloche A, Fabiani JN, Carpentier A. Long-term (29 years) results of reconstructive surgery in rheumatic mitral valve insufficiency. Circulation 2001;104(12 Suppl 1):I12–5. [DOI] [PubMed]
- 14.Duran CM, Gometza B, Saad E. Valve repair in rheumatic mitral disease: an unsolved problem. J Card Surg 1994;9(2 Suppl):282–5. [DOI] [PubMed]
- 15.Antunes MJ, Magalhaes MP, Colsen PR, Kinsley RH. Valvuloplasty for rheumatic mitral valve disease. A surgical challenge. J Thorac Cardiovasc Surg 1987;94(1):44–56. [PubMed]
- 16.Silberman S, Klutstein MW, Sabag T, Oren A, Fink D, Merin O, Bitran D. Repair of ischemic mitral regurgitation: comparison between flexible and rigid annuloplasty rings. Ann Thorac Surg 2009;87(6):1721–7. [DOI] [PubMed]
- 17.David TE, Komeda M, Pollick C, Burns RJ. Mitral valve annuloplasty: the effect of the type on left ventricular function. Ann Thorac Surg 1989;47(4):524–8. [DOI] [PubMed]
- 18.Okada Y, Shomura T, Yamaura Y, Yoshikawa J. Comparison of the Carpentier and Duran prosthetic rings used in mitral reconstruction. Ann Thorac Surg 1995;59(3):658–63. [DOI] [PubMed]
- 19.David TE, Omran A, Armstrong S, Sun Z, Ivanov J. Long-term results of mitral valve repair for myxomatous disease with and without chordal replacement with expanded polytetrafluoroethylene sutures. J Thorac Cardiovasc Surg 1998;115(6):1279–86. [DOI] [PubMed]
- 20.Kobayashi J, Sasako Y, Bando K, Minatoya K, Niwaya K, Kitamura S. Ten-year experience of chordal replacement with expanded polytetrafluoroethylene in mitral valve repair. Circulation 2000;102(19 Suppl 3):III30–4. [DOI] [PubMed]
- 21.Gupta A, Gharde P, Kumar AS. Anterior mitral leaflet length: predictor for mitral valve repair in a rheumatic population. Ann Thorac Surg 2010;90(6):1930–3. [DOI] [PubMed]
- 22.Acar C, de Ibarra JS, Lansac E. Anterior leaflet augmentation with autologous pericardium for mitral repair in rheumatic valve insufficiency. J Heart Valve Dis 2004;13(5):741–6. [PubMed]
- 23.Badhwar V, Rovin JD, Davenport G, Pruitt JC, Lazzara RR, Ebra G, Dworkin GH. Left atrial reduction enhances outcomes of modified maze procedure for permanent atrial fibrillation during concomitant mitral surgery. Ann Thorac Surg 2006;82(5):1758–64. [DOI] [PubMed]


