Abstract
Objective To assess the overall cost effectiveness of the NHS breast screening programme, based on findings of the Independent UK Panel on Breast Cancer Screening and taking into account the uncertainty of associated estimates of benefits, harms, and costs.
Design A life table model comparing data from two cohorts.
Setting United Kingdom’s health service.
Participants and interventions 364 500 women aged 50 years—the population of 50 year old women in England and Wales who would be eligible for screening—were followed up for 35 years without screening, compared with a similar cohort who had regular mammographic screening between ages 50 and 70 years and were then followed for another 15 years.
Main outcome measures Between the cohorts, we compared the number of breast cancer diagnoses, number of deaths from breast cancer, number of deaths from other causes, person years of survival adjusted for health quality, and person years of survival with breast cancer. We also calculated the costs of treating primary and end stage breast cancer, and the costs of screening. Probabilistic sensitivity analysis explored the effect of uncertainty in key input parameters on the model outputs.
Results Under the base case scenario (using input parameters derived from the Independent Panel Review), there were 1521 fewer deaths from breast cancer and 2722 overdiagnosed breast cancers. Discounting future costs and benefits at a rate of 3.5% resulted in an additional 6907 person years of survival in the screened cohort, at a cost of 40 946 additional years of survival after a diagnosis of breast cancer. Screening was associated with 2040 additional quality adjusted life years (QALYs) at an additional cost of £42.5m (€49.8m; $64.7m) in total or £20 800 per QALY gained. The gain in person time survival over 35 years was 9.2 days per person and 2.7 quality adjusted days per person screened. Probabilistic sensitivity analysis showed that this incremental cost effectiveness ratio varied widely across a range of plausible scenarios. Screening was cost effective at a threshold of £20 000 per QALY gained in 2260 (45%) scenarios, but in 588 (12%) scenarios, screening was associated with a reduction in QALYs.
Conclusion The NHS breast screening programme is only moderately likely to be cost effective at a standard threshold. However, there is substantial uncertainty in the model parameter estimates, and further primary research will be needed for cost effectiveness studies to provide definitive data to inform policy.
Introduction
The United Kingdom’s health service established a breast screening programme in 1988, following the publication of the Forrest Report in 1987.1 Since then, additional data from the randomised trials on which the report was based and results from other trials and observational studies have become available. The availability of additional data has fuelled a continuing debate about the relative harms and benefits of breast screening, and several reviews of the evidence have been published in the past 10 years by various stakeholders, including the International Agency for Research on Cancer,2 United States Preventive Services Task Force,3 4 Canadian Taskforce on Preventive Healthcare,5 and Nordic Cochrane Centre.6 As a result of the continuing controversy, Michael Marmot was asked to chair an independent panel to review the evidence for the benefits and harms of breast cancer screening in the UK. The full report and a summary of the panel’s findings were published in 2012.7
The panel undertook a meta-analysis of 11 randomised trials of breast screening mammography, and reported a relative risk of breast cancer mortality for women invited to screening compared with controls of 0.80 (95% confidence interval 0.73 to 0.89). The key harm considered by the panel was that of overdiagnosis—defined as the diagnosis of a breast cancer as a result of screening that would not otherwise have been detected in the woman’s lifetime. The excess incidence of breast cancer associated with screening was estimated to be 11% (95% confidence interval 9% to 12%) when expressed as a proportion of cancers diagnosed in the invited group in the long term, and 19% (15% to 23%) when expressed as a proportion of the cancers diagnosed during the active screening period. The panel concluded that screening reduces deaths from breast cancer but at a cost of overdiagnosis and overtreatment. The panel estimated that for every 10 000 women in the UK aged 50 years invited to screening for the next 20 years, 43 deaths from breast cancer would be prevented and 129 patients with breast cancer, invasive and non-invasive, would be overdiagnosed. Of about 307 000 women aged 50-52 years who are invited to begin screening every year, over 3000 would have an overdiagnosis of breast cancer in the next 20 years. However, the panel acknowledged that these estimates were based on the results from studies with many limitations and whose relevance to screening programmes in the present day can be questioned.
Key recommendations of the panel were that information should be made available in a transparent and objective way to women invited to breast screening so that they can make informed decisions, and that the overall cost effectiveness of the UK breast screening programme needed to be reassessed. The aim of this study was to assess the overall cost effectiveness of the NHS breast screening programme, based on the findings of the panel and taking into account the uncertainty of the estimated benefits, harms, and costs.
Methods
We used a life table approach to model two cohorts of healthy, 50 year old women followed up for 35 years. Each cohort comprised 364 500 women, which is the 2009 population of 50 year old women in England and Wales who would be eligible for screening, according to the Office for National Statistics.8 One cohort received no screening, and the other cohort was offered breast screening mammography at age 50 and every three years thereafter until the age of 70. Subsequent analyses assumed that 75% of eligible women took up the offer of screening. A life table was constructed for each cohort based on predicted age specific incidence of breast cancer, breast cancer specific mortality, and mortality from other causes (web appendix).
In addition to age specific incidence and mortality, we used six key parameters to inform the life table for each cohort of women (the value of each parameter used for the base case scenario is in brackets):
Relative risk of breast cancer mortality associated with regular mammographic screening (0.8)
Relative risk of death from non-breast cancer, in women diagnosed with breast cancer (1.06)
Relative overdiagnosis of breast cancer related to screening (1.19)
Weight for health related quality of life for a 50 year old woman without breast cancer (0.85)
Annual decline in health related quality of life (0.0043)
Relative reduction in quality of life associated with living after a diagnosis of breast cancer (0.9).
Key outputs from the life table model were: number of breast cancer diagnoses, number of deaths from breast cancer, number of deaths from other causes, person years of survival adjusted for health quality, and person years of survival with breast cancer. These outputs were compared for the cohort of women offered screening with 75% uptake and the cohort of unscreened women. The number of overdiagnoses associated with screening was defined as the difference in the number of patients diagnosed with breast cancer over a time horizon of 35 years in the screened and unscreened cohorts. Quality adjusted person years of survival were calculated as follows: the person years of survival without breast cancer (adjusted for the age specific, quality of life weight) plus the person years of survival with breast cancer (adjusted for the quality of life weight associated with living after a diagnosis of breast cancer).
We used four cost parameters to generate the cost effectiveness of the screening programme (that is, the programme’s cost per quality adjusted life year (QALY) gained):
Cost of the screening programme for the cohort of women followed for 35 years (£4.8m for each year screened)
Absolute cost of treating one overdiagnosis of breast cancer (£1800)
Relative cost of treating a clinically detected patient with breast cancer compared with the cost if it had been detected earlier by screening (1.1)
Cost of treating advanced metastatic breast cancer. This parameter is important because screening reduces the number of breast cancer deaths; thus, the costs associated with managing end stage disease will decrease (£20 000).
The estimated overall cost of the screening programme was obtained from an estimate published by the NHS breast screening programme. Costs of treating primary and metastatic breast cancer were taken from NHS treatment reference costs and from the National Institute for Health and Care Excellence (NICE). All future costs and benefits were discounted at a rate of 3.5%. The model’s primary output was the incremental cost effectiveness ratio of screening, calculated as the difference in the costs of the screening programme and treating breast cancer between the screened and unscreened cohorts, divided by the difference in the total QALYs between the two cohorts. To account for the uncertainty in the estimated input parameters, we did a probabilistic sensitivity analysis. This analysis involved recalculating the model after sampling independently each parameter from an underlying distribution that reflects the degree of uncertainty in the parameter estimate (web appendix, web fig 1). We then recalculated the model 5000 times for each of six scenarios for the effect of screening on breast cancer incidence used to construct the life tables. The model was generated using Stata/SE 12.1.
Results
Table 1 shows the key outputs under the base case scenario for the screened and unscreened cohorts. There were 1521 fewer deaths from breast cancer and 2722 overdiagnosed breast cancers in the screened cohort than in the unscreened cohort. Discounting at 3.5% resulted in 6907 added person years of survival in the screened cohort, at a cost of 40 946 additional years of survival after a diagnosis of breast cancer. Screening was associated with 2040 additional QALYs at an additional cost of £42.5m (€49.8m; $64.7m)—an incremental cost effectiveness ratio of £20 800 per QALY gained. Table 1 also shows the equivalent results comparing a screened cohort of 10 000 women with 100% uptake of screening with an unscreened cohort. In this scenario, screening reduced breast cancer deaths by 55 at the cost of 102 patients with overdiagnoses and 27 deaths from other causes. The gain in person time survival over 35 years was 9.2 days per person and 2.7 quality adjusted days per person screened.
Table 1.
Comparison of outcomes between screened and unscreened cohorts, under different screening conditions
| Screened cohort | Unscreened cohort | Difference (interquartile range)† | |
|---|---|---|---|
| Population n=364 500, screening uptake 75% | |||
| Breast cancer cases | 29 111 | 26 389 | 2722 (2153 to 2829) |
| Breast cancer deaths | 8742 | 10 263 | −1521 (−1075 to −1600) |
| Deaths from other causes | 208 449 | 207 720 | 729 (546 to 784) |
| Deaths from all causes | 217 192 | 217 983 | −792 (−525 to −823) |
| Person years of survival* | 6 630 068 | 6 623 161 | 6907 (4798 to 7328) |
| Person years of survival after diagnosis of breast cancer* | 179 847 | 138 901 | 40 946 (36 194 to 43 710) |
| Quality adjusted life years* | 5 330 702 | 5 328 662 | 2040 (847 to 2974) |
| Cost (£m)* | 179 | 136 | 42.5 (36.8 to 49.9) |
| Population n=10 000, screening uptake 100% | |||
| Breast cancer cases | 825 | 723 | 102 (79 to 103) |
| Breast cancer deaths | 226 | 281 | −55 (−39 to −59) |
| Deaths from other causes | 5733 | 5691 | 27 (20 to 29) |
| Death from all causes | 5960 | 5972 | −29 (−19 to −30) |
| Person years of survival* | 182 208 | 181 456 | 253 (176 to 268) |
| Person years of survival after diagnosis of breast cancer* | 5316 | 3805 | 1498 (1324 to 1599) |
| Quality adjusted life years* | 146 467 | 145 991 | 75 (31 to 109) |
| Cost (£m)* | 5.3 | 3.7 | 1.6 (1.3 to 1.8) |
*Discounted at 3.5% per year.
†Interquartile range for outputs from probabilistic sensitivity analysis.
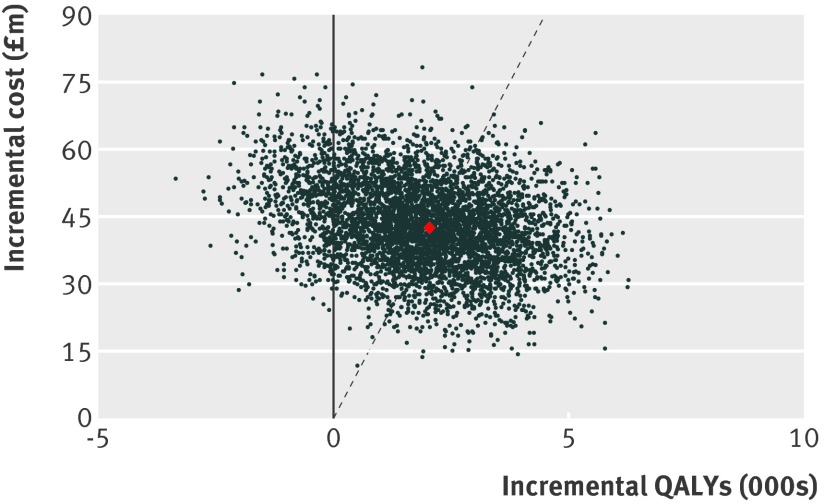
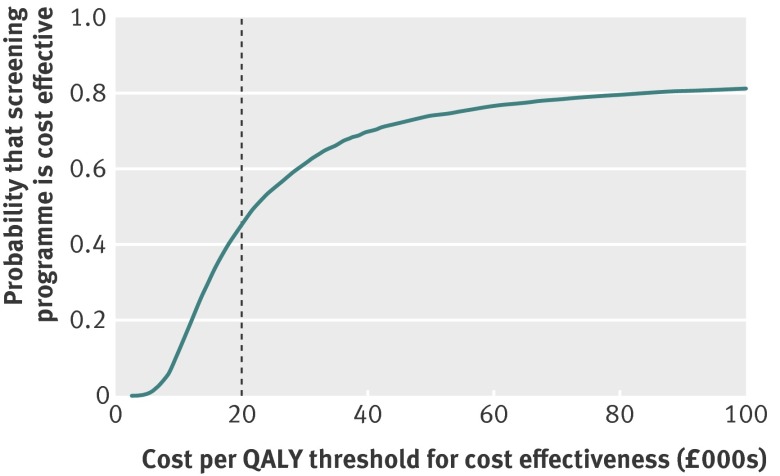
Web figure 2 shows the effect of all modelled uncertainties on the primary output of incremental cost per QALY associated with the screening programme, and web figure 3 shows the distribution of the other outputs of the model. Figure 1 shows the incremental cost of screening against the change in QALYs for each of the 5000 model runs under the base case scenario for the effect of screening on breast cancer incidence. Overall, in 588 (12%) model runs, the screening programme was associated with a reduction in QALYs. In an additional 2152 (43%) runs of the model, the cost per QALY exceeded the £20 000 threshold commonly used by the National Institute for Health and Care Excellence (NICE) to determine whether an intervention should be funded through the NHS. The probability that the breast screening programme is cost effective compared with no screening was 45% (2260 scenarios) at a threshold of £20 000 per QALY. The cost per QALY exceeded £30 000 for 1944 model runs (39%) and exceeded £100 000 for 933 runs of the model (19%). Figure 2 shows the cost effectiveness acceptability curve: the probability of screening being cost effective at different thresholds for the incremental cost effectiveness ratio when all uncertainty is considered.
Fig 1 Incremental cost of screening against effectiveness of screening (gain in QALYs) in the 5000 runs of the probabilistic sensitivity analysis, under the assumption that screening advances diagnosis by five years during screening and results in a reduction of 10% in incidence when screening stops. Red point=base case scenario for all the input parameters. Points to the right of the dashed line=models with an incremental cost effectiveness ratio better than £20 000 per QALY
Fig 2 Cost effectiveness acceptability curve showing probability of the screening programme being cost effective by threshold for cost effectiveness, based on 5000 runs of the probabilistic sensitivity analysis and under the assumption that screening advances diagnosis by five years during screening and results in a reduction of 10% in incidence when screening stops
Web figures 4 and 5 show the effect of independently varying each of the six input parameters in the life table on the number of QALYs gained and total costs. Web figure 6 shows the effect of varying each of the four cost parameters on the incremental cost effectiveness ratio. The web figures show that the estimated reduction in deaths from breast cancer associated with screening had the biggest effect on the number of QALYs gained. This effect is further exemplified in figure 1; in model scenarios where the relative risk of death from breast cancer was higher than 0.85, screening was unlikely to be cost effective at the £20 000 cost per QALY threshold, whereas for scenarios where the relative risk of death from breast cancer was lower than 0.8, screening was highly likely to be cost effective. Small changes in the effect of screening on the relative risk of death from breast cancer had large effects on the incremental cost effectiveness ratio. The weight in health related quality of life associated with a diagnosis of breast cancer, the relative overdiagnosis associated with screening, and the cost associated with the screening programme were also important parameters. Deaths from non-breast cancer causes in women diagnosed with breast cancer had a small effect on cost effectiveness, and the cost of treating overdiagnosed or metastatic breast cancer had a limited effect.
In constructing the life tables, we assumed that breast cancer screening advanced the diagnosis of breast cancer by five years on average until regular screening stopped, and would then be associated with a 10% reduction in incidence. We reran the models and the probabilistic sensitivity analysis under five alternative scenarios, with screening advancing diagnosis by three, five, or seven years and with a 10% or 20% reduction in incidence after screening. Table 2 shows the probability of screening being cost effective at different cost effectiveness thresholds under the six different scenarios for the effect of screening on breast cancer incidence. The more breast screening advanced the diagnosis of breast cancer, the greater the incremental cost effectiveness ratio. This was because the early diagnosis of breast cancer detected by screening was associated with an increase in the number of person years lived with a breast cancer diagnosis without an additional mortality benefit. The timing of the diagnosis of a screen detected cancer did not affect the benefit of reduced breast cancer deaths associated with screening used in the model. A greater reduction in the incidence of breast cancer after the cessation of screening was associated with a reduction in the incremental cost effectiveness ratio, because it would result in fewer breast cancers being diagnosed.
Table 2.
Distribution of incremental cost effectiveness ratios (ICERs) for 5000 model runs under six scenarios of the effect of screening on breast cancer incidence
| Advance in cancer diagnosis with breast screening (no of years) | Reduction in cancer incidence after breast screening stops (%) | Distribution (%) of model runs by ICER threshold | ICER base case scenario (cost (£) per QALY) | ||
|---|---|---|---|---|---|
| <£20 000 per QALY | £20 000-29 999 per QALY | ≥£30 000 per QALY† | |||
| 7 | 10 | 37 | 15 | 48 | 27 650 |
| 7 | 20 | 39 | 16 | 45 | 25 020 |
| 5* | 10* | 45 | 16 | 39 | 20 800 |
| 5 | 20 | 50 | 16 | 34 | 19 210 |
| 3 | 10 | 56 | 16 | 28 | 16 700 |
| 3 | 20 | 59 | 15 | 26 | 15 590 |
*Base case scenario.
†Includes model runs where screening was associated with a reduction in QALYs.
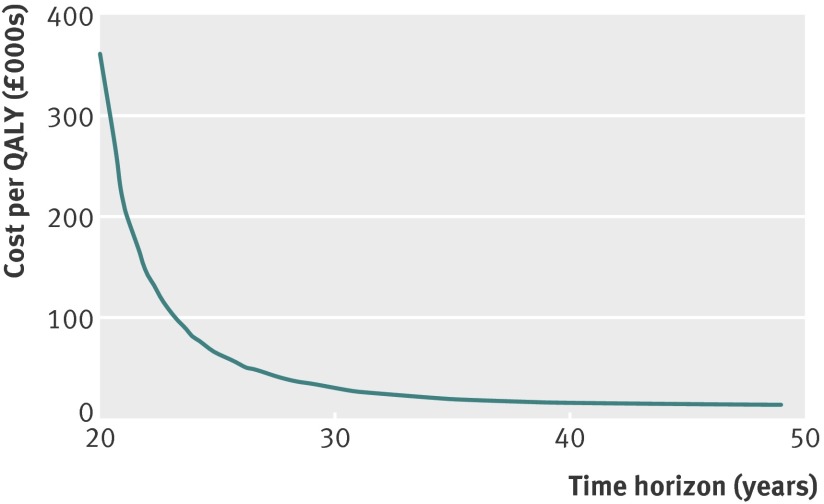
We used a time horizon of 35 years. However, this assumes that at the end of follow-up, there would be no additional gains in survival or NHS costs—a time horizon of 48 years would be needed to follow the cohort until 99% have died. Figure 3 shows the incremental cost effectiveness ratio under the base case scenario for time horizons from 20 to 50 years. Cost effectiveness improved considerably for time horizons from 20 to 30 years before flattening out. There is substantial uncertainty around most of the model parameters—even over a relatively short time horizon of 15 years—and this uncertainty becomes extremely large as the cohort is modelled into late age. For example, the long term effects of screening on breast cancer incidence and mortality, of radiotherapy, and of treatment related morbidity on health related, quality of life in older people could differ substantially from the short term effects. Thus, it seemed reasonable to use a time horizon of 35 years.
Fig 3 Incremental cost effectiveness ratio (£ per QALY) of the base case scenario, by time horizon
Discussion
The absolute benefits of breast cancer screening, based on estimates of the benefits and harms reported by the Independent UK Panel on Breast Cancer Screening,7 are modest, with 67 added QALYs per 10 000 women screened for 20 years and followed up for another 10 years. Under this scenario, the cost effectiveness of the NHS breast screening programme represents marginal value, being higher than the threshold of £20 000 per QALY as used by NICE. However, as recognised by the panel, there were considerable uncertainties in the estimates of the benefits and harms as well as in the underlying costs. We investigated the effect of these uncertainties by conducting a probabilistic sensitivity analysis that has shown that modest differences in combinations of these parameters can result in large changes, resulting in cost effectiveness estimates ranging from a reduction in QALYs associated with a screening programme to the screening programme representing excellent value for money. Screening was cost effective at the £20 000 per QALY gained threshold in 45% of scenarios.
The key input parameter affecting the cost effectiveness was the relative risk of death from breast cancer; small differences in this parameter made substantial differences to the cost effectiveness. If the relative risk was set at 0.85 as estimated by the Cochrane review,6 leaving all other inputs the same as the base case scenario, the incremental cost effectiveness ratio increased to £70 007 per QALY. Of 3231 scenarios where this parameter was greater than 0.8, screening was cost effective at £20 000 per QALY gained in fewer than 801 (25%). The estimate in incremental cost effectiveness ratio was also sensitive to the weight of long term quality of life associated with a diagnosis of breast cancer. However, little evidence is available on which to estimate this parameter, and high quality studies of this parameter are urgently needed.
Uncertainty in the proportion of patients with overdiagnoses and the cost of the screening programme was also important. Estimates of the proportion of patients with overdiagnoses have varied enormously, from fewer than one in 109 to one in three of all diagnoses of breast cancer.10 The true cost of the NHS breast screening programme is difficult to estimate, owing to its complexity and the fact that it is not commissioned as one centrally organised service. It was possible to attempt to synthesise the cost of the screening programme from unit costs. However, this would have added substantial complexity to the model and each of these unit costs and their contribution to the overall cost of screening would have themselves been associated with uncertainties. We therefore chose to use the estimated cost of screening provided by the NHS breast screening programme and to model the uncertainty associated with this cost as part of the probabilistic sensitivity analysis. The outputs were moderately sensitive to this cost. Screening was cost effective at £20 000 per QALY in 940 (38%) of 2488 scenarios where the annual cost of the screening programme was greater than £96m.
Strengths and limitations of study
One limitation of the model was the lack of current data for age specific incidence of breast cancer in an unscreened population, because the current incidence data reflected a population that had a population breast screening programme for 20 years. However, the incidence of breast cancer in a population in which 75% of women was predicted by the model is close to the observed incidence for 2009 (web figure 7). Furthermore, the Independent Panel estimated that 43 deaths from breast cancer would be prevented by screening a cohort of 10 000 women from ages 50 to 70 years with another 10 years’ follow-up. The same number of deaths was estimated by the model used as the base case scenario for these analyses, suggesting that the model was reasonably robust. We have assumed that the input parameters used in the sensitivity analysis were uncorrelated. While there is no reason to believe that this was not true for the parameters used in the construction of the life tables, it is possible that the uncertainties around the treatment costs were correlated. However, there are no data on which to estimate such a correlation and, in view of the relative unimportance of these parameters on the outcomes, any correlation would have a limited effect on the findings.
Comparison with other studies
There have been no published estimates of cost effectiveness of the NHS breast screening programme compared with no screening since the publication of the Forrest report,1 which used a life table model and estimated that screening would cost £3309 per QALY gained. This value is equivalent to £8094 today, assuming an annual inflation of 3.5%. However, the calculations underlying the cost effectiveness estimate did not take into account the adverse consequences of overdiagnosis, and used an implausible estimate for the reduction in breast cancer mortality of about 50% for the first 10 years of screening. Several other studies have evaluated the incremental cost effectiveness of alternative screening strategies, including studies comparing the existing screening programme to age extension,11 12 shortening the screening interval,12 using two view mammography,13 14 and digital mammography.15 All these studies used decision modelling (Markov chain) or microsimulation methods to simulate disease development and progression. A study by Madan and colleagues estimated that the age extension would cost £27 400 per QALY gained, with a 29% probability of cost effectiveness at a threshold of £20 000 per QALY.11
Conclusions and policy implications
The probabilistic sensitivity analysis illustrates the fact that estimating cost effectiveness to provide definitive evidence to aid policy decision making is particularly difficult for an intervention with small effects that apply over long time scales where there is uncertainty in those effects. In conclusion, there is only a moderate probability of a breast screening programme being cost effective at the standard NICE threshold of £20 000 per QALY gained. Risk based screening could improve the balance of benefit to harm of breast cancer screening, but such a strategy would need further evaluation of cost effectiveness. The cost effectiveness estimates were particularly sensitive to the values used for the reduction in deaths from breast cancer and for overdiagnosis, and these are parameters for which there is little evidence from randomised trials of modern digital mammography coupled with modern surgery, radiotherapy, and adjuvant chemotherapy. If the NHS breast screening programme is to be implemented on a solid evidence base, further randomised trials are urgently required. Because the infrastructure of the NHS breast screening is already in place, such a trial is likely to represent good value for money compared with the cost of the programme.
What is already known on this topic
According to the Independent UK Panel on Breast Cancer Screening, breast cancer screening by mammography reduces breast cancer mortality at a cost of overdiagnosis and overtreatment
There are uncertainties in the estimates of the benefits and harms of breast cancer screening
What this study adds
Using input parameters from the panel’s review—regular breast screening of women aged 50-70 years—the gain in quality adjusted life years over a time horizon of 35 years was modest
At the standard NICE threshold of £20 000 per QALY, the probability that the NHS breast screening programme is cost effective was 45%
Contributors: PDPP conceived the study, developed the initial model, and drafted the manuscript. NP, DB, HSB, and DF provided critical input into the model development and critical editorial comment on the manuscript. All authors reviewed and approved the submitted manuscript.
Funding: This study received no specific funding.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; NP is a Cancer Research UK clinician scientist fellow; no other financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: The Stata code used to construct the life table model and the probabilistic sensitivity analyses is available on request from the corresponding author.
Cite this as: BMJ 2013;346:f2618
Web Extra. Extra material supplied by the author
Web appendix: Supplementary information
References
- 1.Forrest APM. Breast cancer screening: report to the health ministers of England, Wales, Scotland, and Northern Ireland by a working group. Department of Health and Social Security, 1987.
- 2.International Agency for Research on Cancer. Handbook of cancer prevention: breast cancer screening, volume 7. IARC, 2002.
- 3.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med 2009;151:727-37, W237-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolf SH. The 2009 breast cancer screening recommendations of the US Preventive Services Task Force. JAMA 2010;303:162-3. [DOI] [PubMed] [Google Scholar]
- 5.Tonelli M, Gorber SC, Joffres M, Dickinson J, Singh H, Lewin G, et al. Recommendations on screening for breast cancer in average-risk women aged 40-74 years. CMAJ 2011;183:1991-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev 2011;1:CD001877. [DOI] [PubMed] [Google Scholar]
- 7.Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet 2012;380:1778-86. [DOI] [PubMed] [Google Scholar]
- 8.Office of National Statistics. Reference table: population estimates for England and Wales, mid-2002 to mid-2010 revised (national). 2012. www.ons.gov.uk/ons/taxonomy/index.html?nscl=Population#tab-data-tables.
- 9.Puliti D, Miccinesi G, Zappa M, Manneschi G, Crocetti E, Paci E. Balancing harms and benefits of service mammography screening programs: a cohort study. Breast Cancer Res 2012;14:R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jorgensen KJ, Gotzsche PC. Overdiagnosis in publicly organised mammography screening programmes: systematic review of incidence trends. BMJ 2009;339:b2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madan J, Rawdin A, Stevenson M, Tappenden P. A rapid-response economic evaluation of the UK NHS Cancer Reform Strategy breast cancer screening program extension via a plausible bounds approach. Value Health 2010;13:215-21. [DOI] [PubMed] [Google Scholar]
- 12.Boer R, de Koning H, Threlfall A, Warmerdam P, Street A, Friedman E, et al. Cost effectiveness of shortening screening interval or extending age range of NHS breast screening programme: computer simulation study. BMJ 1998;317:376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryan S, Brown J, Warren R. Mammography screening: an incremental cost effectiveness analysis of two view versus one view procedures in London. J Epidemiol Community Health 1995;49:70-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston K, Brown J. Two view mammography at incident screens: cost effectiveness analysis of policy options. BMJ 1999;319:1097-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole JA, Lawinski CP, Clinch PJ, Emerton DP, Mackenzie A. Cost-effectiveness of full field digital mammography and computed radiography versus film/screen imaging for mammography. Centre for Evidence Based Purchasing, 2008.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary information