Spindles are oscillations dominating EEG activity during stage 2 of sleep, and they are also present during slow-wave sleep (SWS). Multiple data point to their thalamic origin and their modulation by cortical activities, in particular by the slow oscillation. Detailed analyses of spindles have revealed the presence of both slow (9-12 Hz) and fast (12-15 Hz) spindles, with different topographical distributions over the scalp. In humans, fast spindles dominate centroparietal regions, while slow spindles dominate at frontal areas during SWS.1,2
In this issue of SLEEP, Ayoub and colleagues3 demonstrate that in sleeping subjects, a reduction in the efficacy of Na+ channels reduced fast spindles in central and parietal cortices, but enhanced slow spindles in frontal areas and slow oscillation over investigated regions. By contrast, a reduction in the efficacy of Ca2+ channels (primarily T-type) did not affect slow oscillation or slow spindles, but it decreased fast spindles power in central regions, in particular during SWS. These results point to differences in mechanisms generating slow and fast spindles, and they raise a question relative to whether all spindles have the same mechanism of generation.
The “classical” model of spindle generation has stood for many years. In cats anesthetized with barbiturates, spindle activity of 8-12 Hz dominates over frontal areas and over the suprasylvian gyrus. Electrical stimulation of intralaminar thalamic nuclei induced augmenting responses similar in appearance to spindles. These findings suggested a possible role of the thalamus in initiating of spindle activities.4 Independent of the type of anesthesia, after a full decortication of cats, spindle activity persists in the thalamus.5,6 Within the thalamus, spindles are generated as follows: low-threshold Ca2+-dependent spike burst (LTS) in reticular thalamic nucleus induces IPSPs in thalamocortical neurons, which in turn generate rebound LTS that drives the next Ca2+ spike in the reticular thalamic nucleus.7 Importantly, isolated reticular thalamic nucleus can generate oscillations with spindle frequencies,8 because at hyperpolarized voltages, intra-reticular nucleus inhibition has a depolarizing action that is sufficient to drive LTS.9 Cortical neurons are synchronously excited by thalamocortical neurons that generate cortical field potential spindles. The cortical network is not just passively reflecting thalamic spindles. Rather, it drives spindle onset,10 particularly during slow oscillation,11 and it also effectively contributes to the spindle termination.10,12
There are many inconsistencies between the “classical” model of spindle generation and recent results. (a) The classical model does not explain the different frequencies of fast and slow spindles. It could be explained by different dynamics in small ensembles of thalamic neurons,13 but exact data on these different dynamics are missing. (b1) Global slow waves occur most conspicuously in early sleep, while global spindles occur prominently during late sleep.14 (b2) In relation to global slow waves, slow spindles occur primarily at a transition toward silent states, while fast spindles occur mainly at the onset of active states.1,2 This suggests that only fast spindles fit in the “classical” description as being heavily controlled by cortical activities. (c) Evidence from EEG and MEG recordings15 led to a hypothesis that EEG spindles are generated by matrix (nonspecific) thalamic nuclei, but MEG spindles are generated by core (specific) thalamic pathways.16 (d) Optogenetic stimulation of the reticular thalamic nucleus may trigger cortical spindle activities, which are not detected in corresponding thalamic sites.17 (e) Finally, in this issue of SLEEP, Ayoub et al. reported that Na+ and Ca2+ antagonists differently influence fast and slow spindles.3 Only fast spindles were reduced after administration of a Ca2+ channel blocker, pointing to a “classical” LTS-dependent mechanism of their generation.
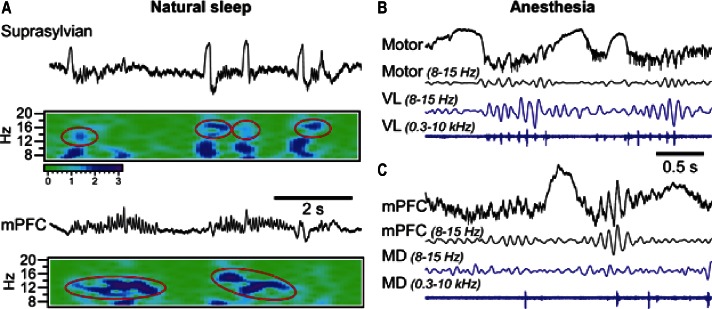
The inconsistencies between the classical model and recent results on spindle control have raised an important question—do all spindles, in particular slow spindles, require an active thalamic contribution? Figure 1A shows a segment of local field potential recordings from a sleeping cat. In agreement with properties of “classical” spindles, within the suprasylvian gyrus short fast spindles (14-16 Hz) were faithfully following slow waves, while simultaneously recorded activities in medial prefrontal cortex generated slower spindles (12-14 Hz) that did not interact with the slow oscillation. In the case of slow spindles, calculating their distribution in relation to global slow waves1,2 would suggest their occurrence at the end of an active cortical state and even during a silent state. Figures 1B and 1C show separately recorded spindles from a cat anesthetized with ketamine-xylazine with addition of propofol. In agreement with the classical mechanism of generation, field potential spindles in the motor cortex occurred simultaneously with thalamic ones, and phased-locked multiunit firing of thalamocortical neurons occurred during both depicted spindles recorded from the corresponding ventro-lateral (VL) nucleus (Figure 1B). By contrast, spindles in the medial prefrontal cortex were not accompanied with vigorous field potential oscillations and phase-locked neuronal firing within the corresponding medio-dorsal (MD) nucleus of the thalamus.
Figure 1.
Spindle activities during sleep and anesthesia (see text for details).
If projecting thalamic nuclei are not involved in the generation of slow frontal spindles, what would be their origin? Slow spindles could potentially be generated within nonspecific (matrix) thalamic nuclei; however, given the wide cortical projections from these nuclei, one should expect synchronous spindles appearance over wide cortical areas, which is not demonstrated yet. Another option is that a set of intracortical mechanisms might be responsible for the generation of slow spindles. The cortical origin of sleep slow oscillation is well accepted. The study by Ayoub et al.3 in this issue demonstrates that a reduction in the efficacy of Na+ channels enhances both slow oscillation and slow spindles. Although exact cellular mechanisms of this phenomenon are not clear, this finding suggests that similar cellular mechanisms or structures might be responsible for the generation of both sleep slow oscillation and sleep slow spindles. Future studies could shed light on the possibilities of the intracortical origin of slow spindles.
CITATION
Timofeev I; Chauvette S. The spindles: are they still thalamic? SLEEP 2013;36(6):825-826.
DISCLOSURE STATEMENT
The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Supported by CIHR, NSERC, and NIH (1R01-NS060870 and 1R01-NS059740).
REFERENCES
- 1.Andrillon T, Nir Y, Staba RJ, et al. Sleep spindles in humans: insights from intracranial EEG and unit recordings. J Neurosci. 2011;31:17821–34. doi: 10.1523/JNEUROSCI.2604-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mölle M, Bergmann TO, Marshall L, Born J. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep. 2011;34:1411–21. doi: 10.5665/SLEEP.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayoub A, Aumann D, Hörschelmann A, et al. Differential effects on fast and slow spindle activity, and the sleep slow oscillation in humans with carbamazepine and flunarizine to antagonize voltage-dependent Na+ and Ca2+ channel activity. Sleep. 2013;36:905–11. doi: 10.5665/sleep.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dempsey EW, Morison RS. The reproduction of rhythmically recurrent cortical potentials after localized thalamic stimulation. Am J Physiol. 1942;135:293–300. [Google Scholar]
- 5.Morison RS, Bassett DL. Electrical activity of the thalamus and basal ganglia in decorticate cats. J Neurophysiol. 1945;8:309–14. [Google Scholar]
- 6.Timofeev I, Steriade M. Low-frequency rhythms in the thalamus of intact-cortex and decorticated cats. J Neurophysiol. 1996;76:4152–68. doi: 10.1152/jn.1996.76.6.4152. [DOI] [PubMed] [Google Scholar]
- 7.Bal T, von Krosigk M, McCormick DA. Role of the ferret perigeniculate nucleus in the generation of synchronized oscillations in vitro. J Physiol. 1995;483:665–85. doi: 10.1113/jphysiol.1995.sp020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steriade M, Domich L, Oakson G, Deschenes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol. 1987;57:260–73. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- 9.Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Self-sustained rhythmic activity in the thalamic reticular nucleus mediated by depolarizing GABAA receptor potentials. Nat Neurosci. 1999;2:168–74. doi: 10.1038/5729. [DOI] [PubMed] [Google Scholar]
- 10.Timofeev I, Bazhenov M, Sejnowski T, Steriade M. Contribution of intrinsic and synaptic factors in the desynchronization of thalamic oscillatory activity. Thalamus Relat Syst. 2001;1:53–69. [Google Scholar]
- 11.Contreras D, Steriade M. Spindle oscillation in cats: the role of corticothalamic feedback in a thalamically generated rhythm. J Physiol. 1996;490:159–79. doi: 10.1113/jphysiol.1996.sp021133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonjean M, Baker T, Lemieux M, Timofeev I, Sejnowski T, Bazhenov M. Corticothalamic feedback controls sleep spindle duration in vivo. J Neurosci. 2011;31:9124–34. doi: 10.1523/JNEUROSCI.0077-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen P, Andersson SA, Lømo T. Nature of thalamo-cortical relations during spontaneous barbiturate spindle activity. J Physiol. 1967;192:283–307. doi: 10.1113/jphysiol.1967.sp008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nir Y, Staba RJ, Andrillon T, et al. Regional slow waves and spindles in human sleep. Neuron. 2011;70:153–69. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dehghani N, Cash SS, Chen CC, et al. Divergent cortical generators of MEG and EEG during human sleep spindles suggested by distributed source modeling. PLoS One. 2010;5:e11454. doi: 10.1371/journal.pone.0011454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonjean M, Baker T, Bazhenov M, Cash S, Halgren E, Sejnowski T. Interactions between core and matrix thalamocortical projections in human sleep spindle synchronization. J Neurosci. 2012;32:5250–63. doi: 10.1523/JNEUROSCI.6141-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halassa MM, Siegle JH, Ritt JT, Ting JT, Feng G, Moore CI. Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nat Neurosci. 2011;14:1118–20. doi: 10.1038/nn.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]