Abstract
Study Objectives:
To identify the factual morbidity and mortality of narcolepsy in a controlled design.
Setting:
National Patient Registry.
Patients:
All national diagnosed patients (757) with health information at least 3 years prior to and after diagnose of narcolepsy.
Controls:
Randomly selected four citizens (3,013) matched for age, sex, and socioeconomic status from the Danish Civil Registration System Statistics.
Results:
Increased morbidity prior to narcolepsy diagnosis included (odds ratio, 95% confidence interval):- diseases of the endocrine, nutritional, and metabolic systems (2.10, 1.32-3.33); nervous system (5.27, 3.65-7.60); musculoskeletal system (1.59, 1.23-2.05); and other abnormal symptoms and laboratory findings (1.66, 1.25-2.22). After the diagnosis, narcolepsy patients experienced diseases of the endocrine, nutritional, and metabolic (2.31, 1.51-3.54), nervous (9.19, 6.80-12.41), musculoskeletal (1.70, 1.28-2.26), eye (1.67, 1.03-2.71), and respiratory systems (1.84, 1.21-2.81). Specific diagnoses were diabetes (2.4, 1,2-4.7, P < 0.01), obesity (13.4, 3.1-57.6, P < 0.001), sleep apnea (19.2, 7.7-48.3, P < 0.001), other sleep disorders (78.5, 11.8-523.3, P < 0.001), chronic obstructive pulmonary disease (2.8, 1.4-5.8, P < 0.01), lower back pain (2.5, 1.4-4.2, P < 0.001), arthrosis/arthritis (2.5, 1.3-4.8, P < 0.01), observation of neurological diseases (3.5, 1.9-6.5, P < 0.001), observation of other diseases (1.7, 1.2-2.5, P < 0.01), and rehabilitation (5.0, 1.5-16.5, P < 0.005). There was a trend towards greater mortality in narcolepsy (P = 0.07).
Conclusions:
Patients with narcolepsy present higher morbidity several years prior to diagnose and even higher thereafter. The mortality rate due to narcolepsy was slightly but not significantly higher.
Citation:
Jennum P; Ibsen R; Knudsen S; Kjellberg J. Comorbidity and mortality of narcolepsy: a controlled retro- and prospective national study. SLEEP 2013;36(6):835-840.
Keywords: Narcolepsy, sleep apnea, morbidity, mortality
INTRODUCTION
Narcolepsy with cataplexy (NC) or without cataplexy (NwC) is a relatively common neurological sleep disorder found in one of 2,000 individuals. It is characterized by instability of sleep-wake regulation (daytime sleepiness, short sleep latency, disrupted nocturnal sleep with awakenings) and instability of REM sleep regulation and motor tonus regulation (hypnagogic hallucinations, sleep paralysis, cataplexy, and REM sleep behavior disorder).1,2 The main aspect of the pathophysiology is loss of the sleep-wake and motor tonus-regulating hypocretinergic neurons in the hypothalamus,3,4 resulting in low or undetectable cerebrospinal fluid hypocretin-1 (hcrt-1) levels in the majority of NC patients, but in only a small proportion of NwC patients.5,6 Hypocretin neuron loss most probably has an autoimmune cause, mainly based on the very close association of specific HLA-types.7,8 The pathophysiology of NC/NwC patients with normal hcrt-1 levels is less clear.
We have previously shown a strong link between narcolepsy and health care usage, employment, and economy.9 Furthermore, existing studies suggest that narcolepsy is often unrecognized and has a delayed diagnosis.10,11 It is associated with reduced quality of life12 and increased patient and societal burdens.9,13–16 However, our knowledge of long-term morbidity and the mortality consequences of narcolepsy is limited. We used the Danish National Patient Registry [NPR] to evaluate the factual mortality and morbidity before and after a narcolepsy diagnosis, including both NC and NwC.
METHODS
Subjects
In Denmark, all in-hospital and ambulatory contacts are obligatory recorded in the National Patient Registry (NPR) with respect to the time of contact and with information about primary and secondary diagnoses. The NPR is controlled and owned by the Danish State and includes administrative information, diagnoses, and diagnostic (e.g., polysomnography [PSG], multiple sleep latency test [MSLT]) and treatment procedures using several international classification systems, including the International Classification of Disorders (ICD-10). The NPR is a time-based national database containing data from all inpatient and outpatient contacts, so the data are representative of all patients in Denmark who have received a diagnosis of narcolepsy, irrespective of other diagnoses. As data are available from the entire observation period, patients can be traced retrospectively and prospectively relative to the time of their diagnosis. Furthermore, all contacts in the primary sector and medication use are recorded in the databases of the National Health Security and the Danish Medicine Agency, respectively. There is a small risk of underestimating the number of patients with narcolepsy, as patient contacts but not the specific diagnoses are registered in the primary sector. Specific clinical information, such as age of narcolepsy onset, delay to diagnosis time, body mass index (BMI), laboratory assessments, and results from PSG and MSLT, was not considered in this study, since these data are incomplete in the NPR.
Using the NPR, we identified all patients who were diagnosed with narcolepsy between 1997 and 2009. For the narcolepsy diagnoses, we used the International Classification of Disease (ICD-9) codes (G474) in Denmark, which are assigned after patient evaluation in each hospital (e.g., based on PSG, MSLT, lumbar puncture) or on the basis of clinical information. It is possible to record the presence of cataplexy or secondary narcolepsy, but we regard this information as inaccurate because it is recorded by the different clinics. Using data from Denmark's Civil Registration System Statistics, we then randomly selected matched control subjects with same age, sex, and marital status as the patients, but without a central hyper-somnia diagnosis (essential hypersomnia or narcolepsy). Parity of socioeconomic status (SES) was achieved by selecting controls from each patient's individual county. A control-to-patient ratio of 4:1 was used to reduce variation among controls. Data from patients and control subjects who could not be identified in the Coherent Social Statistics database were excluded from the sample. All of the observations in both groups were successfully matched. The patients and control subjects were followed from their year of diagnosis until 2009. To ensure sufficient observation time, our analysis included only patients who were represented in the data at least one year before the diagnosis date; for this reason, the number of cases in the population were reduced from 816 to 757.
Evaluation of Morbidity before, and Morbidity/Mortality after a Narcolepsy Diagnosis
Information before and after the narcolepsy diagnosis was extracted from the database for the years 1998-2009. Morbidity data were extracted as primary and secondary diagnoses and further classified into main disease groups, in accordance with the World Health Organization (WHO) ICD-10 criteria. A conditional logit model was developed, yielding outputs expressed as odds ratios (ORs) with 95% confidence intervals (CIs). First, a conditional logit was estimated for the main diagnoses. A conditional logit was then estimated for diagnoses occurring in ≥ 1% of patients or controls. Diagnostic codes were grouped manually and reported on the basis of their statistical significance (P < 0.05) and relevance. Additionally, mortality data were extracted from patients and controls.
Ethics
The study was approved by the Danish Data Protection Agency. Data handling was anonymous, so individual and ethical approval was not mandatory.
Statistical Analysis
Statistical analyses were done with SAS 9.1.3 (SAS, Inc., Cary, NC, USA). These took the form of conditional logit models. In the first of these, the dependent variable was the binary variable for case-control groups, and the independent variables were dummies for the 21 diagnosis groups. A second analysis included dummies for ICD-10 diagnosis occurring in > 1% of individuals in the case or control group. ICD-10 diagnostic groups with ≤ 1% of the sample were combined in the main diagnosis groups. Only estimates for the ICD-10 diagnosis are reported in the results for the second analysis, but the dummies for the main groups (including only the remaining diagnosis) were included in the regression. A patient could belong to more than one diagnostic group or ICD-10 diagnosis in the 3 years before the diagnosis.
Not all patients had the full 3-year observation period before diagnosis, since during the first 3 years of the period, the patient could only have data for 1 or 2 previous years. However, since this was also the case for the control group we included the data for these shorter periods in our analysis.
The results are presented as ORs with their associated 95% CI's and P-values. Extreme values were manually validated, and no errors were identified.
RESULTS
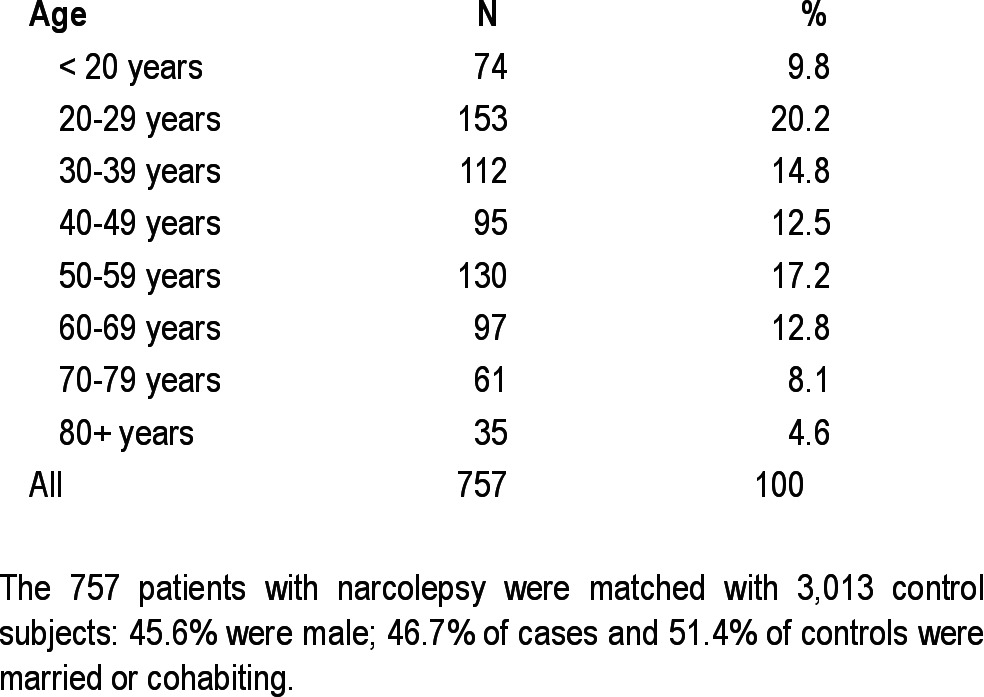
The age distribution of the 757 patients and 3,013 control subjects is shown in Table 1.
Table 1.
Age distribution
Comorbidity Before Narcolepsy Diagnosis
Patients with narcolepsy presented higher morbidity at least 3 years before their diagnosis (Table 2). The most common contacts with the health system for narcolepsy were classified into 4 main groups: endocrine, nutritional and metabolic system; nervous system; musculoskeletal system; other abnormal symptoms and laboratory findings (Table 1).
Table 2.
Morbidities three years before a diagnosis of narcolepsy, by major disease groups
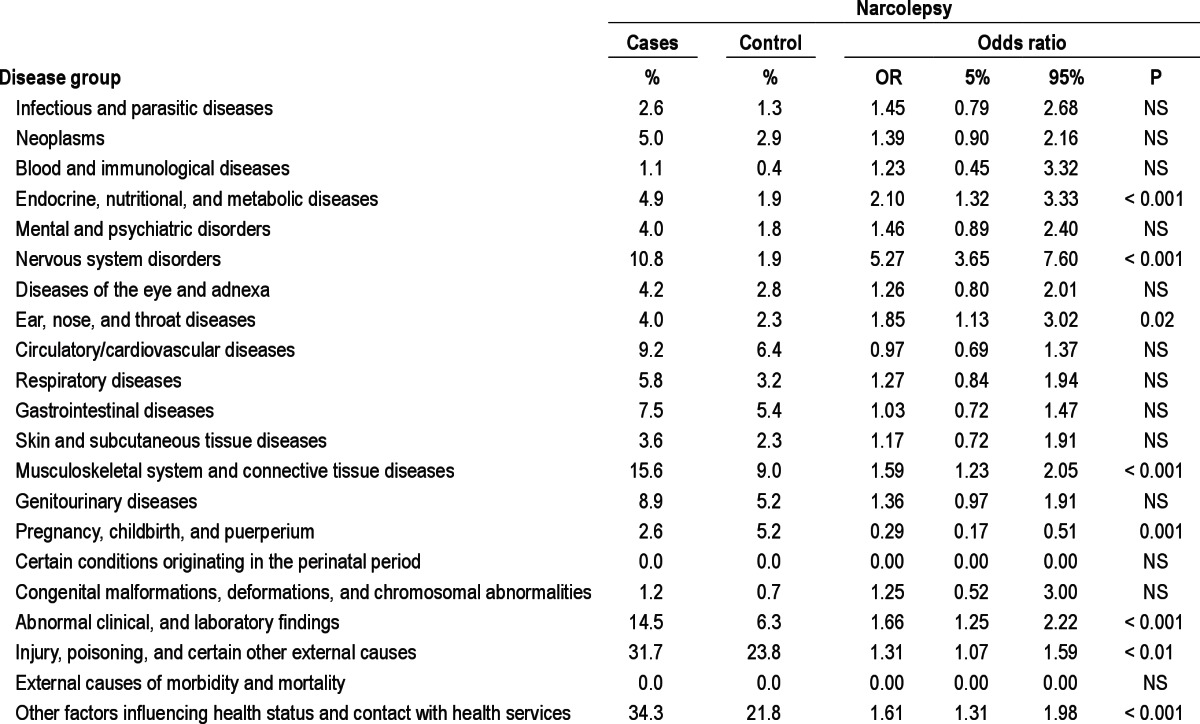
The most common significant diagnoses in the sub-analyses before the narcolepsy diagnosis were sleep apnea (44.5, 13.1-151.3, P < 0.001), central hypersomnias or other sleep disorders (32, 3.9-263.6, P < 0.001), lower back pain (1.9, 1.2-3.2, P < 0.001), induced abortion (2.6, 1.2-5.7, P < 0.05), injuries to the head (1.5, 1.1-2.1, P < 0.05) or to the trunk/extremities (1.3, 1.1-1.7, P < 0.05), observation of neurological diseases (8.5, 4.7-15.3, P < 0.001), observation of other diseases (3.6, 2.1-6.2, P < 0.001), other laboratory abnormalities (6.7, 1.6-28.9, P < 0.01), and control evaluations (2.7, 1.2-6.1, P < 0.05). Epilepsy and diabetes were common before diagnosis (2.2, 0.9-5.7, NS, and 1.3, 0.6-3.0, NS, respectively).
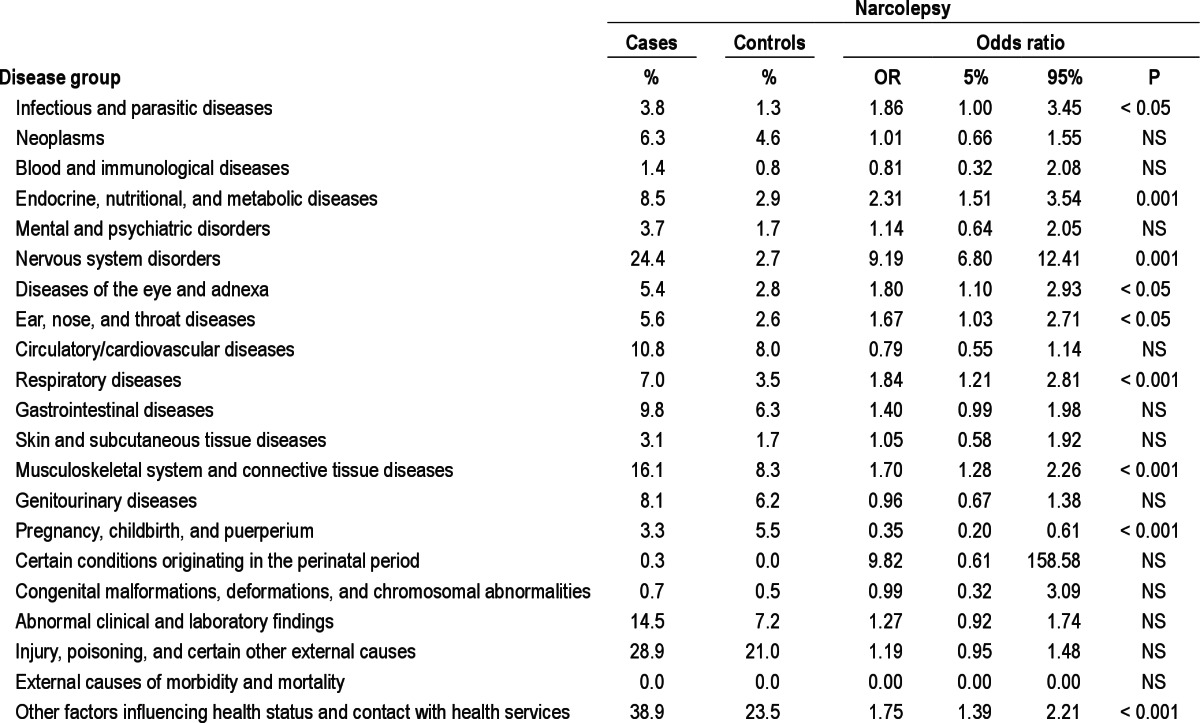
Comorbidity and Mortality after Narcolepsy Diagnosis
After diagnosis, narcolepsy was most commonly associated with diseases of the endocrine, nutritional and metabolic system, the nervous system, the eye and adnexa, the respiratory system, and the musculoskeletal system. The most common diagnoses in the sub-analyses were sleep apnea and obesity (Table 3). The most significant diagnoses were diabetes (2.4, 1.2-4.7, P < 0.01), obesity (13.4, 3.1-57.6, P < 0.001), sleep apnea (19.2, 7.7-48.3, P < 0.001), sleep disorders other than narcolepsy (78.5, 11.8-523.3, P < 0.001), chronic obstructive pulmonary disease (2.8, 1.4-5.8, P < 0.01), lower back pain (2.5, 1.4-4.2, P < 0.001), arthrosis/arthritis (2.5, 1.3-4.8, P < 0.01), observation of neurological diseases (3.5, 1.9-6.5, P < 0.001), observation of other diseases (1.7, 1.2-2.5, P < 0.01), and re habilitation (5.0, 1.5-16.5, P < 0.005).
Table 3.
Morbidities three years after a diagnosis of narcolepsy, by major disease groups
Moreover, 12-year survival showed a trend towards increased mortality in the narcolepsy group (0.83, 0.78-0.87) compared with controls (0.86, 0.84-0.88) (hazard ratio = 0.80, P = 0.07; Table 3 and Figure 1).
Figure 1.
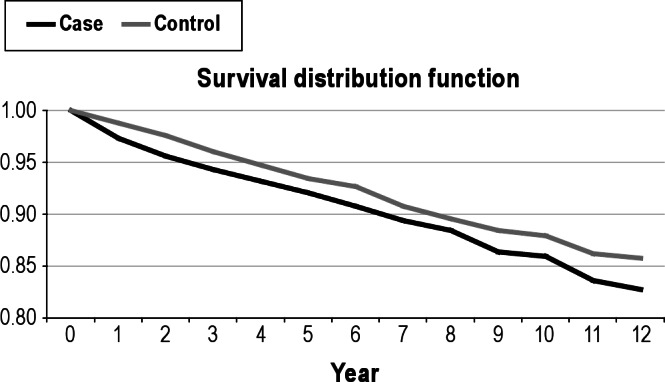
Twelve-year survival of narcolepsy patients and controls shows nonsignificantly higher mortality in the narcolepsy group (0.83, 0.78-0.87) compared with the controls (0.86, 0.84-0.88) (hazard ratio = 0.80, P = 0.07).
DISCUSSION
This national case/control study shows that narcolepsy patients register a wide range of comorbidity diagnoses before their narcolepsy diagnosis. Comorbidities were represented mainly by endocrine disorders and neurological and musculo-skeletal diseases. With respect to specific diagnoses, the most frequent were sleep apnea/central hypersomnias, as well as unspecific contacts with the health care system and use of laboratory investigations. We also detected a higher incidence of pain, injuries to the head and trunk, and induced abortions.
After a diagnosis of narcolepsy, we found two general patterns: (1) the use of the health care system increased further, and (2) the number of comorbidity diagnoses increased: endocrine (diabetes, obesity), sleep-related conditions (sleep apnea, other sleep disorders), nervous system, and musculoskeletal diseases (pain, arthritis) continued to be associated with the narcolepsy diagnosis, but there were additional associations with diseases of the eye and respiratory system (including chronic obstructive pulmonary disease).
Interestingly, the prevalence of common differential diagnoses to narcolepsy—major psychiatric disorders or epilepsy—were not significantly elevated either before or after a narcolepsy diagnosis.
Finally, the overall mortality was slightly but not significantly higher among narcolepsy patients than in the controls.
The current study was performed to evaluate potential (known and unidentified) comorbidities before and after a narcolepsy diagnosis had been established. On the basis of case or case-series reports, narcolepsy has previously been associated with the presence of sleep apnea, periodic leg movements, and overweight,17–26 but systematic controlled studies are scarce. In particular, psychiatric diagnoses and epilepsy are suspected to be common misdiagnoses before a correct narcolepsy diagnosis is established.10
Consistent with this, we also detected a very high incidence of sleep apnea/central hypersomnia before and after diagnosis, and of possible metabolic syndrome (obesity and diabetes [NS]) after diagnosis. Obesity has been consistently reported in narcolepsy,27 which has partly been linked to hypocretin deficiency28 but not verified in other studies.17 The causal effect of obesity and metabolic diseases/diabetes in narcolepsy have not been identified, though a relation to glucose response has been suggested.29 Recently, hypocretin deficiency has also been linked to reduced reward, feed seeking behavior and spontaneous physical activity.30 Sleep apnea may occur more often among narcoleptic patients due to overweight, although the occurrence may also be partially explained by a selection bias arising from the greater availability of non-PSG screening before diagnosis and the incidental detection of sleep apnea on PSG after diagnosis in the same population.
The incidence of musculoskeletal pain was increased as compared to controls. Pain records and perceptions have been speculated to be linked to sleep quantity and possibly depression in patients with narcolepsy.31 Hypocretinergic neurons modulate nociception.32 Likewise, it is interesting that injuries were significantly more frequent before, but not after diagnosis, suggesting that undiagnosed/untreated cataplexy could be present at that time.
In contrast with some previous studies, we found the incidence of psychiatric diagnosis to be slightly but not significantly higher among narcoleptic patients compared with controls. However, in accordance with previous studies of psychiatric comorbidity in narcolepsy,33,34 the present study points to the fact that although narcoleptic patients may suffer from mood problems or symptoms that may mimic primary psychiatric diseases, the patients were not diagnosed with major psychiatric disorders when in contact with the secondary health sector. The overlapping sleep related diagnostic criteria of depression and narcolepsy, and possibly also definition overlap between hypnagogic hallucinations and psychosis can explain some of the past disagreement on psychiatric comorbidity in narcolepsy populations.
Likewise, an epilepsy diagnosis was only slightly and not significantly more frequent before diagnosis, a result that differs from those of an earlier study suggesting that narcolepsy may be misdiagnosed as one of a range of epileptic disorders.10 We also detected that nervous system disorders were significantly common both before and after a diagnosis of narcolepsy. Prior to—but not after—diagnosis, this could partly be explained by that clinicians are unaware of the specific diagnosis and just conclude an overall observation diagnosis. After diagnosis, the cause is more unclear, as no specific neurological disorder was sufficiently prevalent (1%) to be selected in the subanalyses. In particular, an interesting relation between narcolepsy and Parkinson disease has previously been suggested.35,36 We have also previously reported on increased REM sleep behavior disorder in narcolepsy—a phenomenon originally regarded as part of the synucleinopathies.2 Hypocretin levels have been found lower in advanced Parkinson disease, which may support potential narcoleptic subtypes and presence of cataplexy.37 Furthermore in case reports, development of Parkinson disease has been observed in narcoleptic patients.38,39 However, in the current study design, it should be noted that small number of patients with disease onset early in life makes it difficult to detect late-occurring degenerative disorders such as Parkinsonian syndromes.2
We found that induced abortion was more frequent than in controls, both before and after diagnosis. We have previously shown that social effects and morbidity can be identified up to eight years before a diagnosis of narcolepsy.40 We believe this is because narcolepsy is often diagnosed at a time when the disease has already affected the patient's social functioning and morbidity. Hence, the increased abortion rates might reflect patients' consequent lower ability than controls to cope with pregnancy and child care.
This study has several limitations: it is based on hospital and clinic reports to the NPR; the diagnostic accuracy depends on each clinic's presentation and reporting of the diagnosis and comorbidities; confounder variables (e.g., BMI and results of laboratory investigations) were not recorded; symptoms and clinical evaluation results (e.g., PSG, MSLT) were not considered, so we cannot relate the findings to disease severity. Although NwC and NC are classified separately, we did not evaluate these two conditions separately in the current study, as this would require the validation of these records from the other departments. We did not exclude diseases, especially narcolepsy or other sleep disorders among the controls, but narcolepsy is so rare that very few subjects would be expected to be present in the control group. We included all ages at the time of first diagnosis, but no information was available about the first symptom.
The strength of the NPR is that it is a national database containing all identified patients; it is time-locked (all reports must be associated with the patient contacts), and it includes a substantial follow-up time. With the current identified subjects, the number of patients identified is approximately one-fifth to one-sixth of the expected number of patients in Denmark.
In conclusion, the current study shows that patients with narcolepsy have significant morbidity at least three years before a diagnosis of narcolepsy. Morbidities following such a diagnosis include diseases of the endocrine, nutritional, metabolic, nervous, respiratory, and musculoskeletal systems, of the eye and adnexa, and diffuse central nervous symptoms like hypersomnia. The most common diagnoses were sleep apnea, diabetes, obesity, and musculoskeletal symptoms associated with pain; but notably, there was no significantly greater incidence of diagnoses of psychiatric conditions or epilepsy. Although not significant, narcolepsy showed a trend towards an overall greater mortality compared to controls.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by an unrestricted grant from the Center for Healthy Aging, Faculty of Health Sciences, University of Copenhagen. Poul Jennum is the principal investigator, is the main author, and did interpretation of the data. Jakob Kjellberg and Rikke Ibsen performed the statistical analysis and interpretation of the data. All authors contributed to and have approved the final manuscript.
REFERENCES
- 1.Ferri R, Miano S, Bruni O, et al. NREM sleep alterations in narcolepsy/ cataplexy. Clin Neurophysiol. 2005;116:2675–84. doi: 10.1016/j.clinph.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Knudsen S, Gammeltoft S, Jennum PJ. Rapid eye movement sleep behaviour disorder in patients with narcolepsy is associated with hypocretin-1 deficiency. Brain. 2010 Feb;133(Pt 2):568–79. doi: 10.1093/brain/awp320. [DOI] [PubMed] [Google Scholar]
- 3.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 4.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypo-cretin neurons in human narcolepsy. Neuron. 2000;27:469–74. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hyper-somnias. Arch Neurol. 2002;59:1553–62. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 6.Knudsen S, Jennum PJ, Alving J, Sheikh SP, Gammeltoft S. Validation of the ICSD-2 criteria for CSF hypocretin-1 measurements in the diagnosis of narcolepsy in the Danish population. Sleep. 2010;33:169–76. doi: 10.1093/sleep/33.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kornum BR, Kawashima M, Faraco J, et al. Common variants in P2RY11 are associated with narcolepsy. Nat Genet. 2011;43:66–71. doi: 10.1038/ng.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hor H, Kutalik Z, Dauvilliers Y, et al. Genome-wide association study identifies new HLA class II haplotypes strongly protective against narcolepsy. Nat Genet. 2010;42:786–9. doi: 10.1038/ng.647. [DOI] [PubMed] [Google Scholar]
- 9.Jennum P, Knudsen S, Kjellberg J. The economic consequences of narcolepsy. J Clin Sleep Med. 2009;15;5:240–5. [PMC free article] [PubMed] [Google Scholar]
- 10.Kryger MH, Walid R, Manfreda J. Diagnoses received by narcolepsy patients in the year prior to diagnosis by a sleep specialist. Sleep. 2002;25:36–41. doi: 10.1093/sleep/25.1.36. [DOI] [PubMed] [Google Scholar]
- 11.Morrish E, King MA, Smith IE, Shneerson JM. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med. 2004;5:37–41. doi: 10.1016/j.sleep.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Beusterien KM, Rogers AE, Walsleben JA, et al. Health-related quality of life effects of modafinil for treatment of narcolepsy. Sleep. 1999;22:757–65. doi: 10.1093/sleep/22.6.757. [DOI] [PubMed] [Google Scholar]
- 13.Dodel R, Peter H, Walbert T, et al. The socioeconomic impact of narcolepsy. Sleep. 2004;27:1123–8. doi: 10.1093/sleep/27.6.1123. [DOI] [PubMed] [Google Scholar]
- 14.Broughton WA, Broughton RJ. Psychosocial impact of narcolepsy. Sleep. 1994;17(8 Suppl):S45–S49. doi: 10.1093/sleep/17.suppl_8.s45. [DOI] [PubMed] [Google Scholar]
- 15.Broughton R, Ghanem Q, Hishikawa Y, Sugita Y, Nevsimalova S, Roth R. [Socioeconomic consequences of narcolepsy. Comparative study of 3 populations in various countries] Cesk Neurol Neurochir. 1987;50:42–6. [PubMed] [Google Scholar]
- 16.Broughton RJ, Guberman A, Roberts J. Comparison of the psychosocial effects of epilepsy and narcolepsy/cataplexy: a controlled study. Epilepsia. 1984;25:423–33. doi: 10.1111/j.1528-1157.1984.tb03438.x. [DOI] [PubMed] [Google Scholar]
- 17.Heier MS, Jansson TS, Gautvik KM. Cerebrospinal fluid hypocretin 1 deficiency, overweight, and metabolic dysregulation in patients with narcolepsy. J Clin Sleep Med. 2011;7:653–8. doi: 10.5664/jcsm.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonka K, Kemlink D, Buskova J, et al. Obesity accompanies narcolepsy with cataplexy but not narcolepsy without cataplexy. Neuro Endocrinol Lett. 2010;31:631–4. [PubMed] [Google Scholar]
- 19.Peacock J, Benca RM. Narcolepsy: clinical features, co-morbidities & treatment. Indian J Med Res. 2010;131:338–49. [PubMed] [Google Scholar]
- 20.Kotagal S. Hypersomnia in children: interface with psychiatric disorders. Child Adolesc Psychiatr Clin N Am. 2009;18:967–77. doi: 10.1016/j.chc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Melamed Y, Daliahu Y, Paleacu D. Narcolepsy and psychotic states--a case report. Isr J Psychiatry Relat Sci. 2009;46:70–3. [PubMed] [Google Scholar]
- 22.BaHammam A. Prevalence and impact of periodic leg movements in narcolepsy patients. J Sleep Res. 2009;18:142. doi: 10.1111/j.1365-2869.2008.00684.x. [DOI] [PubMed] [Google Scholar]
- 23.Dauvilliers Y, Paquereau J, Bastuji H, Drouot X, Weil JS, Viot-Blanc V. Psychological health in central hypersomnias: the French Harmony study. J Neurol Neurosurg Psychiatry. 2009;80:636–41. doi: 10.1136/jnnp.2008.161588. [DOI] [PubMed] [Google Scholar]
- 24.Douglass AB. Narcolepsy: differential diagnosis or etiology in some cases of bipolar disorder and schizophrenia? CNS Spectr. 2003;8:120–6. doi: 10.1017/s1092852900018344. [DOI] [PubMed] [Google Scholar]
- 25.Jara CO, Popp R, Zulley J, Hajak G, Geisler P. Determinants of depressive symptoms in narcoleptic patients with and without cataplexy. J Nerv Ment Dis. 2011;199:329–34. doi: 10.1097/NMD.0b013e3182174fd3. [DOI] [PubMed] [Google Scholar]
- 26.Sansa G, Iranzo A, Santamaria J. Obstructive sleep apnea in narcolepsy. Sleep Med. 2010;11:93–5. doi: 10.1016/j.sleep.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Dahmen N, Bierbrauer J, Kasten M. Increased prevalence of obesity in narcoleptic patients and relatives. Eur Arch Psychiatry Clin Neurosci. 2001;251:85–9. doi: 10.1007/s004060170057. [DOI] [PubMed] [Google Scholar]
- 28.Kok SW, Overeem S, Visscher TL, et al. Hypocretin deficiency in narcoleptic humans is associated with abdominal obesity. Obes Res. 2003;11:1147–54. doi: 10.1038/oby.2003.156. [DOI] [PubMed] [Google Scholar]
- 29.Burdakov D, Alexopoulos H. Metabolic state signalling through central hypocretin/orexin neurons. J Cell Mol Med. 2005;9:795–803. doi: 10.1111/j.1582-4934.2005.tb00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plaza-Zabala A, Maldonado R, Berrendero F. The hypocretin/orexin system: implications for drug reward and relapse. Mol Neurobiol. 2012;45:424–39. doi: 10.1007/s12035-012-8255-z. [DOI] [PubMed] [Google Scholar]
- 31.Dauvilliers Y, Bayard S, Shneerson JM, Plazzi G, Myers AJ, Garcia-Borreguero D. High pain frequency in narcolepsy with cataplexy. Sleep Med. 2011;12:572–7. doi: 10.1016/j.sleep.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Bartsch T, Levy MJ, Knight YE, Goadsby PJ. Differential modulation of nociceptive dural input to [hypocretin] orexin A and B receptor activation in the posterior hypothalamic area. Pain. 2004;109:367–78. doi: 10.1016/j.pain.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Mayer G, Kesper K, Peter H, Ploch T, Leinweber T, Peter JH. [Comorbidity in narcoleptic patients] Dtsch Med Wochenschr. 2002;127:1942–6. doi: 10.1055/s-2002-34207. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien LM. The neurocognitive effects of sleep disruption in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2009;18:813–23. doi: 10.1016/j.chc.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Arnulf I, Leu S, Oudiette D. Abnormal sleep and sleepiness in Parkinson's disease. Curr Opin Neurol. 2008;21:472–7. doi: 10.1097/WCO.0b013e328305044d. [DOI] [PubMed] [Google Scholar]
- 36.Baumann C, Ferini-Strambi L, Waldvogel D, Werth E, Bassetti CL. Parkinsonism with excessive daytime sleepiness--a narcolepsy-like disorder? J Neurol. 2005;252:139–45. doi: 10.1007/s00415-005-0614-5. [DOI] [PubMed] [Google Scholar]
- 37.Haq IZ, Naidu Y, Reddy P, Chaudhuri KR. Narcolepsy in Parkinson's disease. Expert Rev Neurother. 2010;10:879–84. doi: 10.1586/ern.10.56. [DOI] [PubMed] [Google Scholar]
- 38.Christine CW, Marks WJ, Jr., Ostrem JL. Development of Parkinson's disease in patients with narcolepsy. J Neural Transm. 2012;119:697–9. doi: 10.1007/s00702-011-0761-z. [DOI] [PubMed] [Google Scholar]
- 39.Economou NT, Manconi M, Ghika J, Raimondi M, Bassetti CL. Development of Parkinson and Alzheimer diseases in two cases of narcolepsycataplexy. Eur Neurol. 2012;67:48–50. doi: 10.1159/000334733. [DOI] [PubMed] [Google Scholar]
- 40.Jennum P, Ibsen R, Petersen ER, Knudsen S, Kjellberg J. Health, social, and economic consequences of narcolepsy: a controlled national study evaluating the societal effect on patients and their partners. Sleep Med. 2012;13:1086–93. doi: 10.1016/j.sleep.2012.06.006. [DOI] [PubMed] [Google Scholar]


