Abstract
Study Objectives:
The aim of the study was to determine whether apolipoprotein E epsilon 4 genotype (APOE4) modifies the association of sleep disordered breathing (SDB) with cognitive function in a middle-aged population.
Design:
Cross-sectional analysis of a community-dwelling cohort.
Settings:
Sleep laboratory at the Clinical Research Unit of the University of Wisconsin Hospitals and Clinics.
Participants:
There were 755 adults from the Wisconsin Sleep Cohort who provided a total of 1,843 polysomnography and cognitive evaluations (most participants were assessed multiple times at approximately 4-y intervals); 56% males, average age 53.9 years (range 30-81 years).
Interventions:
None.
Measurement and Results:
In-laboratory overnight polysomnography was used to assess SDB. Cognition was evaluated by a battery of six neurocognitive tests assessing memory and learning, attention, executive function, and psychomotor efficiency. The APOE4 genotype (ε3/ε4 or ε4/ ε4) was identified in 200 participants. Data were analyzed using linear mixed-effects models, accounting for multiple observations per participant. Cognitive test scores were regressed on SDB categories (AHI < 5, 5 ≤ AHI < 15, AHI ≥ 15); APOE4 and their interaction; and age, education, sex, and body mass index. There was no statistically significant association between SDB and cognitive performance among APOE4-negative individuals. However, in APOE4-positive individuals, those with AHI ≥ 15 had significantly worse performance on the Auditory Verbal Learning Test and the Controlled Oral Word Association Test.
Conclusions:
In APOE4-positive individuals, moderate to severe sleep disordered breathing (AHI ≥ 15) was associated with poorer performance on cognitive tests that require both memory and executive function engagement.
Citation:
Nikodemova M; Finn L; Mignot E; Salzieder N; Peppard PE. Association of sleep disordered breathing and cognitive deficit in APOE ε4 carriers. SLEEP 2013;36(6):873-880.
Keywords: APOE4, cognitive deficit, sleep apnea
INTRODUCTION
Obstructive sleep apnea, the most common form of sleep disordered breathing (SDB), affects almost 17% of the US adult population.1 Whereas associations of SDB with increased morbidity and mortality related to hypertension, cardiovascular diseases, stroke, and daytime sleepiness are well documented in the literature,2–11 the relationship between SDB and cognition is less understood. Several studies suggest that psychomotor vigilance and executive function are the most affected cognitive domains,12–15 but others found only weak or no association between SDB and cognitive functions.16–19 These inconsistent findings likely reflect significant differences in methodologic approach used in these studies including selection of participants (clinical versus population based, age of the participants), sample size, and considerable variation in tests to assess cognition.
Studies by O'Hara et al.20 and Spira et al.21 showed that in an older population (age 70 y or older), SDB was associated with greater cognitive impairment in apolipoprotein E ε4 (APOE4) carriers than in noncarriers, thus providing early evidence that some subpopulations may be more vulnerable to negative effects of SDB on the central nervous system (CNS). This finding was further supported by findings that SDB is associated with an increased risk for neurocognitive dysfunction in children with APOE4.22
APOE4 is a major genetic risk factor for the late-onset form of Alzheimer disease (after age 60 years)23,24 and Alzheimer disease is the most common form of dementia in older populations, characterized by a progressive decline in memory and other cognitive functions.23,25 The prevalence of SDB is higher in patients with Alzheimer disease; some studies estimate that 70% of patients with Alzheimer disease have SDB and 30% of these have moderate or severe disease with apnea-hypopnea index (AHI) ≥ 15.26,27
Although there is some evidence of an association between SDB and poorer cognition in APOE4 carriers in either older populations or in young children, such an association is less evident in middle-aged populations. A clinical study by Cosentino et al.28 showed significant memory impairment in 60- to 70-year-old patients with AHI ≥ 15 and APOE4 genotype; however, large population-based studies investigating the presence of an APOE4 and SDB interaction on cognition are lacking. Therefore, the aim of this study was to investigate how APOE4 genotype may modulate the association between SDB and cognition in middle-aged adults participating in the ongoing Wisconsin Sleep Cohort Study (WSCS).
METHODS
Participants and Data Collection
The WSCS was approved by the University of Wisconsin Health Sciences Institutional Review Board. Written informed consent was obtained from all participants. A subcohort of 755 participants from the WSCS was evaluated in this study. The WSCS, established in 1988, is a prospective epidemiologic study of the natural history, causes, and consequences of SDB in community-dwelling adults.29 The WSCS was formed from a random selection of 30- to 60-y-old employees of Wisconsin state agencies who responded to a mailed survey on sleep characteristics and other factors between 1989 and 1993. The response rate to the initial survey was 71%. A stratified sample (n = 2,884) of survey respondents was invited to an overnight baseline polysomnography study. There were 1,545 participants who completed a baseline study (a 53% response rate; the primary stated reason for nonparticipation was the inconvenience of sleeping overnight in the sleep laboratory).
Overnight polysomnography studies were performed at the Clinical Research Unit of the University of Wisconsin Hospitals and Clinics. Protocols included body habitus measurements,6 a neurocognitive test battery, and questionnaires on lifestyle, health, and medication, followed by overnight polysomnography. All participants had a baseline study between 1988 and 2000. After their baseline studies, participants were invited approximately every 4 y for follow-up polysomnography and cognitive assessments. Most participants have had multiple studies, and, to efficiently use all available data, up to five studies per participant were used; models (described in the following paragraphs) statistically accounted for multiple observations per participant. Blood samples for genetic analysis were initiated in 1993 and so are not available for all participants. For this analysis, participants must have had genetic data and at least one study that provided both polysomnography and cognitive data. Also, by design, cognitive testing was not performed on all WSCS study participants at all study visits (starting in 2008, however, every overnight study has included a cognitive evaluation). Figure 1 depicts the number of participants with varying numbers of sleep studies; the number of studies with available genetic and cognitive assessment data; study participation refusals; and the number of participants not (yet) invited for follow-up studies. Most participants have had two or more studies, yielding a total of 1,843 studies from 755 participants with genetic, polysomnography, and cognitive data. Not all participants have had the maximum possible number of studies because not all participants have yet had the opportunity to be invited for multiple repeat studies.
Figure 1.
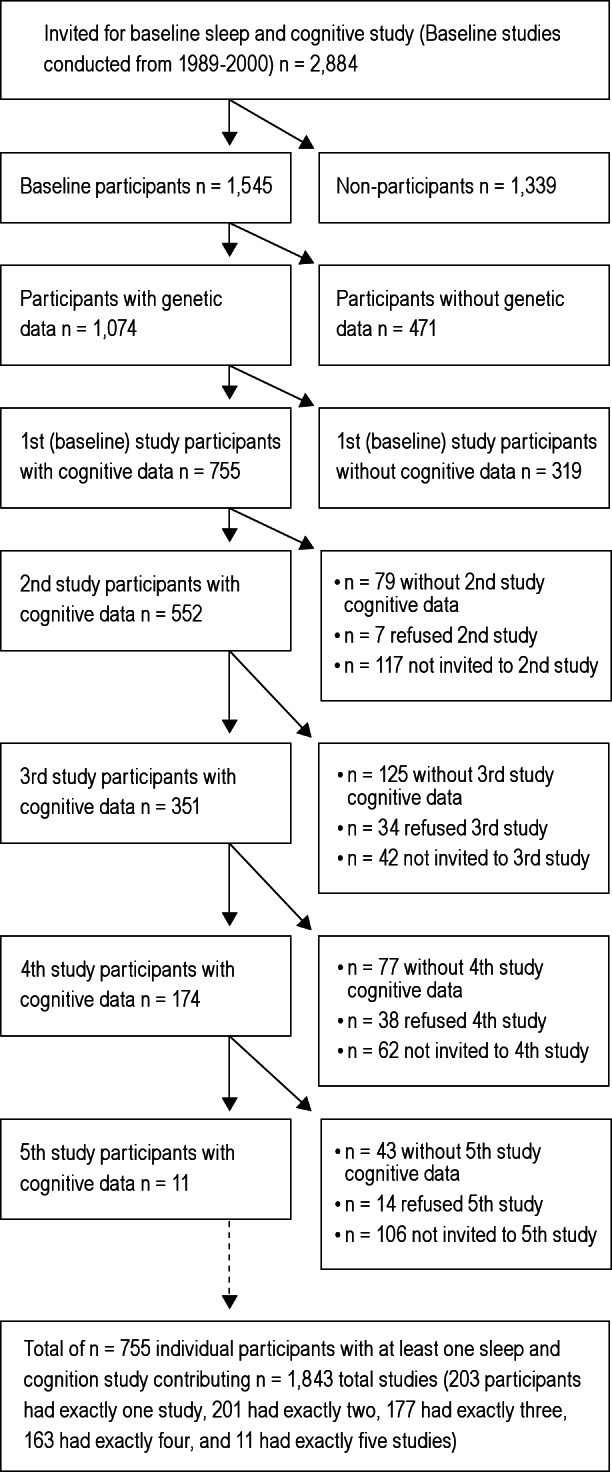
Sample sizes of Wisconsin Sleep Cohort Study participants with one or more (up to five) overnight polysomnography studies at approximately 4-y intervals. Participants who refused baseline or follow-up studies and participants without genetic (APOE genotype) or cognitive data are also indicated. There were 755 participants who provided a total of 1,843 studies with requisite genetic, polysomnography, and cognitive data.
APOE Genotype and Neurocognitive Test Battery
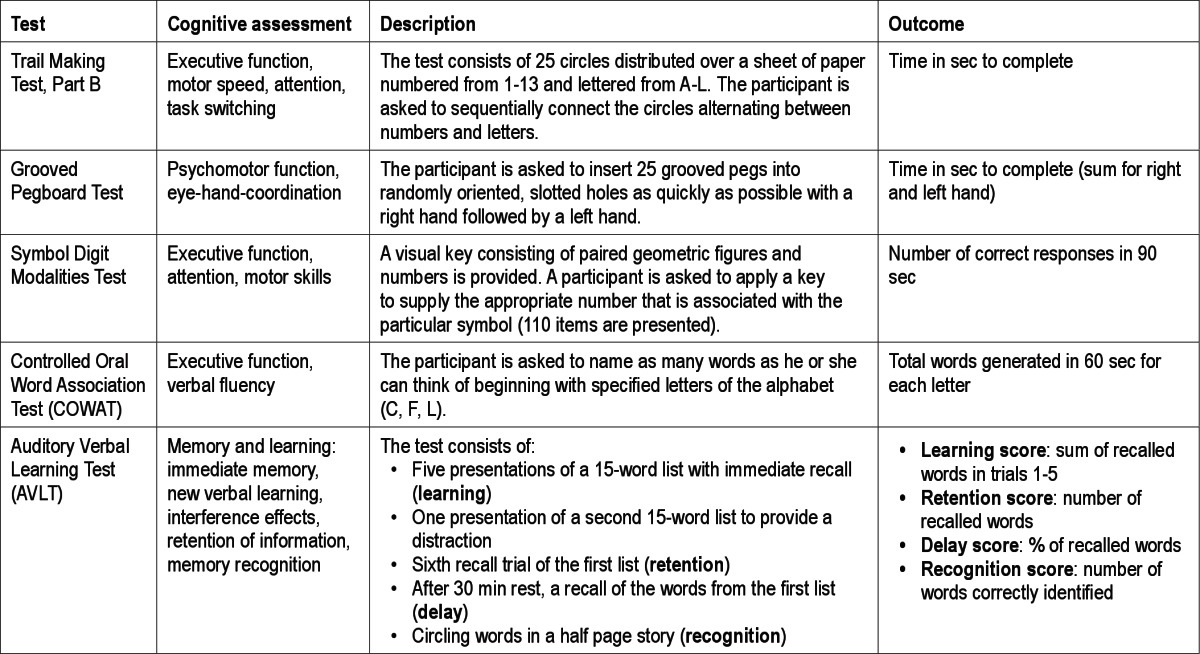
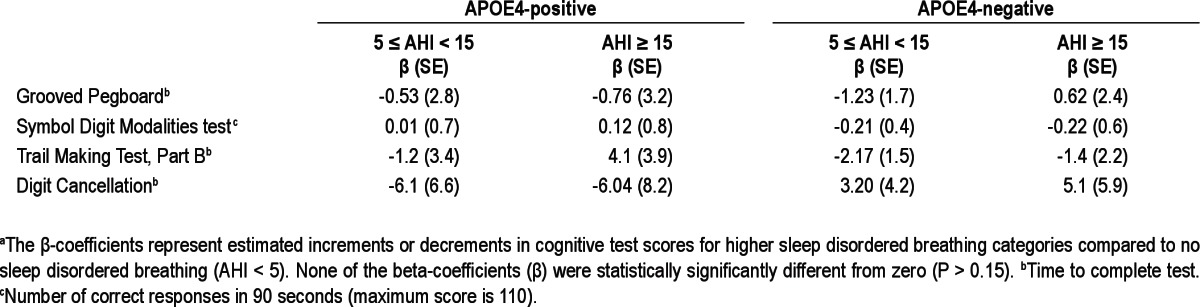
APOE genotype was identified by polymerase chain reaction-restriction fragment length method as described previously.30,31 Neurocognitive tests used in the WSCS were described in detail previously.32 The test battery included the Rey Auditory Verbal Learning Test (AVLT), Controlled Oral Word Association Test (COWAT), Grooved Pegboard Test, Trail Making Test-part B, Digit Cancelation Test, and Symbol Digit Modalities Test. Tests were administered by a trained technician on the night of the polysomnography study. The duration of testing is approximately 45 min. Individual tests are described in Table 1.
Table 1.
Description of neurocognitive test battery
Polysomnograph Recordings
All participants underwent overnight in-laboratory polysomnography as described in detail previously.6,29,30 The se verity of SDB was parameterized as the AHI, the number of apnea+hypopnea events per hour of sleep). Apnea was defined as a cessation of breathing for at least 10 seconds; hypopnea was defined as a discernible reduction in the airflow and ≥ 4% reduction in oxyhemoglobin saturation. AHI < 5 events per hour was classified as no SDB, 5 ≤ AHI < 15 as mild, and AHI ≥ 15 as moderate to severe SDB. Those undergoing continuous positive airway pressure (CPAP) treatment were included in moderate to severe SDB category.
Covariates
Data on medical history, medication use (prescription and over-the-counter), age, CPAP treatment, year of education, exercise (hours per week of planned exercise), smoking (current and past cigarette packs per week), alcohol consumption (usual weekly number of cans/bottles of beer, glasses of wine, mixed drinks or shots of liquor), consumption of caffeinated beverages (usual number of cups of coffee or tea with caffeine or cans of cola or other soft drinks with caffeine consumed in a typical day), subjective sleepiness (by the Epworth Sleepiness Scale score) and other covariates were obtained by interview and questionnaires during overnight polysomnography laboratory study.
Statistical Analysis
Data were analyzed with SAS software (SAS Institute Inc, Cary, NC). To maximize study power, linear mixed-effects models estimated weighted averages of the cross-sectional and within-subject associations of SDB and cognitive test scores while accounting for intrasubject correlation due to the use of multiple studies from the majority of participants.33 Par ticipants with the APOE ε3/ε4 and ε4/ε4 (“APOE4”) genotype were considered as having a “high risk” genotype. The primary predictors were SDB categories (AHI < 5, 5 ≤ AHI < 15, AHI ≥ 15), APOE4 status, and their interactions. Examined covariates included sex, age, education, body mass index (BMI), alcohol consumption (drinks/week), current smoking, exercise (hours/week), classes of prescription medication use, caffeine consumption, hypertension, percentage of sleep < 90% blood oxygen saturation, and sleepiness. Of these covariates, those for which we observed evidence of confounding the SDB-cognitive function association were retained in final models; these were sex, age, education, and BMI. Two-sided P < 0.05 was taken to indicate statistical significance for both main effect and interaction terms. As indicated in Table 1, the six examined cognitive tests were designed to assess varying cognitive domains, and thus our primary analyses do not adjust for multiple testing; we view each cognitive test as a separate subinvestigation. Nevertheless, as a supplemental analysis, we applied a conservative Bonferroni-adjusted α level to the hypothesis tests examining SDB-cognition associations identified as significant at the 0.05 level. Specifically, we divided 0.05 by nine (corresponding to the six cognitive tests, one of which—the AVLT—had four examined subscores) to arrive at an adjusted α level of 0.006. In the Results section, we describe both Bonferroni-adjusted and -unadjusted hypothesis tests only for those tests that were significant at the unadjusted level.
RESULTS
Demographic, health, and lifestyle characteristics of the participants (mean age 54 y, range 30-81 y) are described in Table 2. AHI < 5 events per hour (no SDB) was found in 62% of studies, mild SDB (5 ≤ AHI < 15) in 22% and 16% of studies had moderate to severe SDB (AHI ≥ 15). Participants with moderate to severe sleep apnea were older (on average by 4.5 y compared to participants without SDB), more overweight (mean BMI = 36.6 compared to 28.9 in the group without SDB), predominantly males, and had lower education compared to participants without SDB. High-risk APOE4 genotype (APOE ε3/ε4 or ε4/ε4) was identified in 200 participants who provided 500 sleep and cognition studies. Only three participants (totaling six studies) had ε4/ε4 genotype. APOE4 status prevalences did not differ significantly across SDB categories.
Table 2.
Characteristics of the sample by sleep disordered breathing categories; sample sizes correspond to numbers of polysomnography studies; 755 participants provided 1,843 total studies
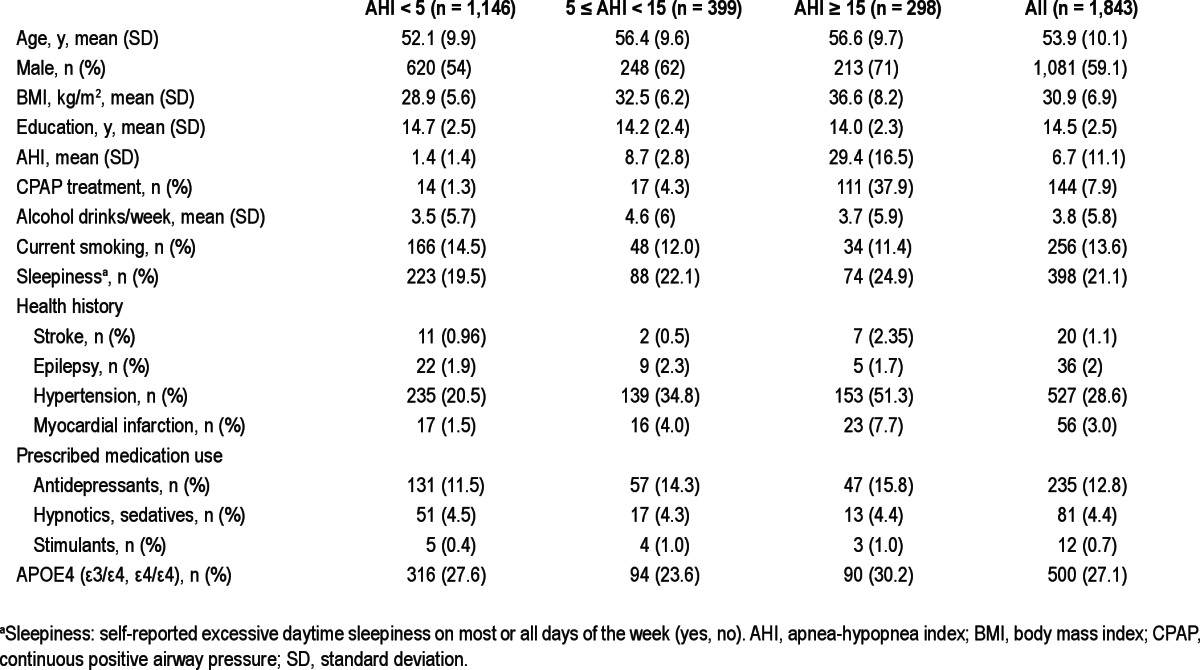
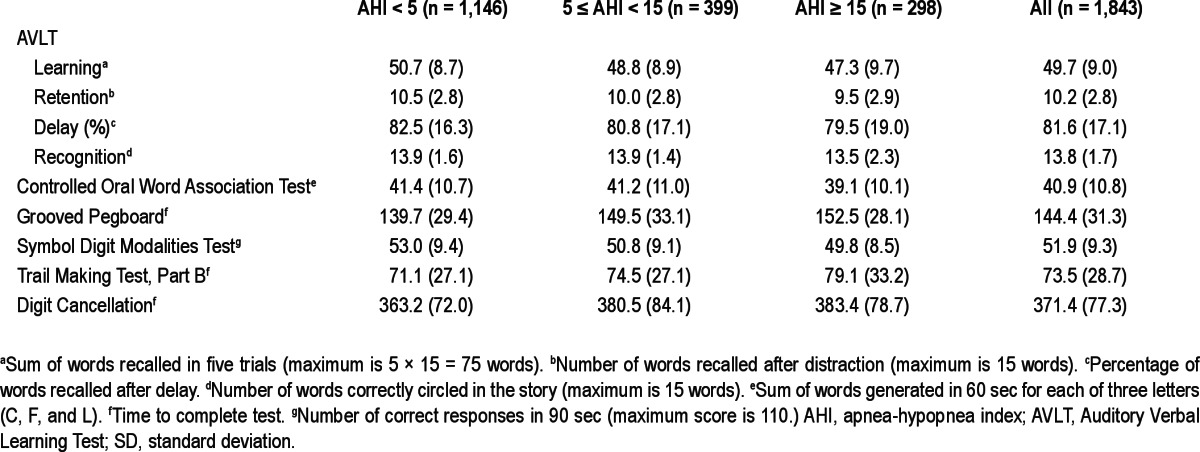
Table 3 provides the unadjusted mean neurocognitive test scores stratified by SDB categories. Participants with AHI < 5 events per hour had consistently better unadjusted scores on all tests compared to those with mild or moderate to severe SDB.
Table 3.
Unadjusted mean cognitive test scores by sleep disordered breating categories (mean, SD); sample sizes correspond to numbers of polysomnography studies; 755 participants provided 1,843 total studies
Because we hypothesized that there are different associations of SDB and cognition for participants with and without APOE4, we tested SDB-APOE4 interaction terms in regression models predicting neurocognitive test scores. We found signifi-cant interactions for AVLT recognition and COWAT (P < 0.02). We therefore stratified further analyses of cognitive outcomes by APOE4 status. We did not find any significant associations of SDB with the Grooved Pegboard, Symbol Digit Modalities, Trail Making, and Digit Cancellation test scores, regardless of APOE4 status (Table 4).
Table 4.
Mixed-effect linear regression model for neurocognitive scores (dependent variable) stratified by APOE4, adjusted for age, sex, education, and body mass indexa
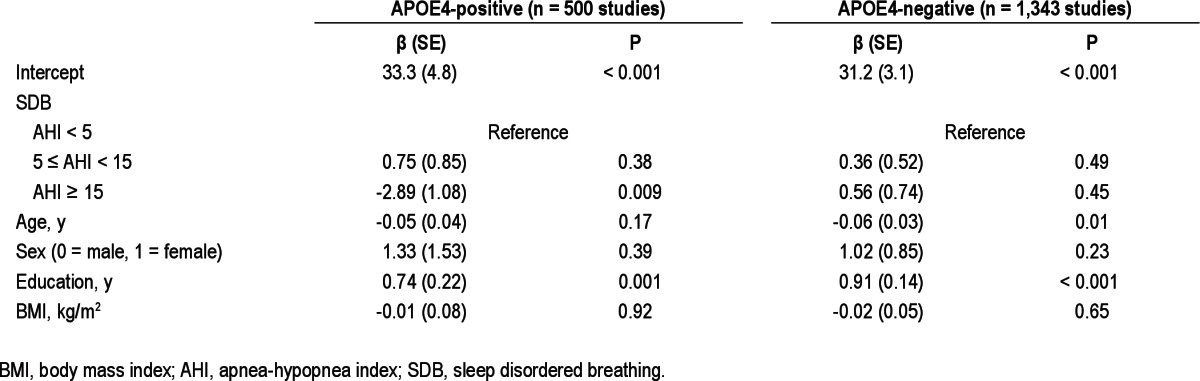
However, the regression analyses of neurocognitive test scores stratified by APOE4 indicated that moderate to severe SBD (AHI ≥ 15) was significantly associated with poorer performance on the AVLT recognition test (P < 0.001, significant at both α = 0.05 and Bonferroni-adjusted α level = 0.006) and COWAT test (P = 0.009, significant at α = 0.05, but not at the Bonferroni-adjusted α level = 0.006) in APOE4-postive, but not in APOE4-negative participants, independent of age, sex, education, and BMI. Final regression models for AVLT recognition (Table 5) and COWAT (Table 6) stratified by APOE4 genotype are provided. Education was positively and statistically significantly associated with the performance on both tests in all strata whereas age had a negative effect on test scores. Self-reported sleepiness or Epworth Sleepiness Score did not have a substantial effect on SDB regression coefficients. Based on the models, the adjusted mean (standard error [SE]) AVLT recognition test scores in APOE4-positive participants were: 13.9 (0.1) for AHI < 5, 13.7 (0.2) for 5 ≤ AHI < 15, and 12.9 (0.2) for AHI ≥ 15. The calculated adjusted mean (SE) COWAT scores were: 41.2 (0.8) for AHI < 5, 42.0 (0.9) for 5 ≤ AHI < 15, and 38.4 (1.1) for AHI ≥ 15.
Table 5.
Mixed-effect linear regression model results for sleep disordered breathing category and covariates predicting AVLT recognition score stratified by APOE4 genotype
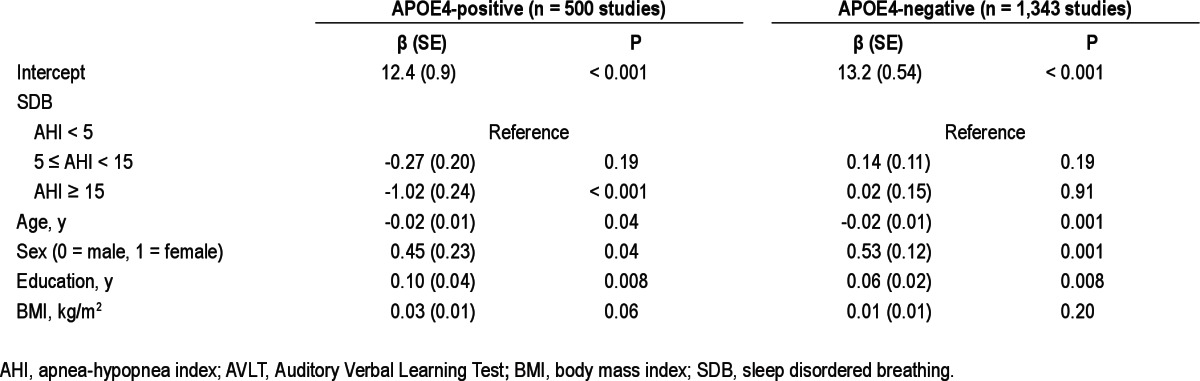
Table 6.
Mixed-effect linear regression model results for sleep disordered breathing category and covariates predicting Controlled Oral Word Association Test score stratified by APOE4 genotype
We also evaluated a number of covariates—medical conditions (e.g., stroke) and medications (e.g., stimulants) described in Table 1—that could potentially affect performance on neurocognitive test battery. After adjusting for AHI, APOE4 status, age, sex, education, and BMI, we found no additional effect of these variables on cognitive scores.
DISCUSSION
In a nonclinical community-dwelling sample, we found SDB to be associated with cognitive deficit only in APOE4 carriers. This decreased performance was seen in participants with moderate to severe SDB (AHI ≥ 15) and in cognitive tests combining memory and executive function assessment. Based on the regression coefficients for moderate to severe SDB in our regression model (Table 5), we estimated that among APOE4 carriers, moderate to severe SDB (compared to no SDB) was associated with a one-word decrement on the AVLT recognition test. This decrement represents 60% of a standard deviation unit of the AVLT recognition score in our sample and is equivalent to the decrement in AVLT recognition score associated with approximately 10 fewer years of education predicted from the same model. Similarly, we estimated that among APOE4 carriers, moderate to severe SDB was associated with a deficit of approximately three words on the COWAT test. This three-word decrement represents 27% of a standard deviation unit of the COWAT score in our sample and is equivalent to the decrement in COWAT score associated with approximately 3.9 fewer years of education predicted from the model presented in Table 6. The SDB-APOE4 association was independent of lifestyle variables such as smoking, alcohol and caffeinated beverage consumption, and the use of sedatives or antidepressant medications. The association was also unrelated to self-reported daytime sleepiness, whether measured with a single-item sleepiness question or by the Epworth Sleepiness Scale.
The AVLT and COWAT are often used to assist in diagnosing neurologic impairment in a variety of CNS disorders such as Alzheimer disease, Parkinson disease, schizophrenia, and vascular dementia.34–36 Recently, these two tests were used to differentiate the diagnosis of Alzheimer disease and vascular dementia.34 Patients with Alzheimer disease performed worse on the AVLT recognition test, whereas those with vascular dementia performed worse on COWAT.34 Those results suggest that potential mechanisms of cognitive deficit in APOE4-positive participants with severe SDB may be complex and multiple pathways need to be considered.
In a battery of six neurocognitive tests spanning major cognitive domains, we did not find a significant association with SDB regardless of APOE4 status in four tests (mainly related to executive function, attention, and psychomotor efficiency), suggesting that SDB may have a small effect on overall neuro-cognitive performance and that older age, male sex, and lower education associations with SDB severity are more likely to be responsible for poorer unadjusted neurocognitive scores in participants with AHI ≥ 15 reported in Table 3. Our finding of the lack of broad and strong associations between SDB and cognitive measurements is similar to that of the Sleep Heart Health Study16 and Autonomic Nervous System Activity, Aging and Sleep Apnea/Hypopnea (SYNAPSE) study.17 However, it is notable that in our sample, relatively few participants had severe SDB, and so we were unable to carefully examine associations of cognition and SDB at a severity level often seen in tertiary sleep clinic patients.
Mechanisms underlying the association of sleep apnea and cognition are poorly understood. Some authors suggest that daytime sleepiness, sleep fragmentation, and hypoxemia might contribute to neurocognitive decline associated with SDB.12,18,37,38 In our study, addition of self-reported sleepiness or Epworth Sleepiness Scale score did not have any effect on SDB-cognitive function associations or other regression coefficients in the model, suggesting that the association of sleep apnea and cognition in APOE4-positive participants was independent from sleepiness. This might be because sleepiness is not highly specific to SDB but is possibly a consequence of other health conditions or lifestyle circumstances. We found that the proportion of people reporting sleepiness without SDB (19.5%) was not substantially less than in those with moderate to severe SDB (24.9%). There was also only a modest difference in mean in Epworth Sleepiness Scale scores between these two groups: 8.3 (SD 3.9) for people without SDB and 9.6 (SD 4.2) for people with AHI ≥ 15. However, one factor that is rarely, if ever, considered in studies on the association between SDB and cognition is duration of the disease. SDB may affect the CNS in many ways, whether it is through hypoxia, oxidative stress, sleep fragmentation, cerebrovascular changes, sympathetic nerve activation, etc., and it is likely that with longer duration of disease, CNS injury accumulates that may at some point lead to irreversible damage. Unfortunately, disease duration cannot be known for participants who entered the study with SDB (a minority in which SDB had been diagnosed previously).
Another potential mechanism whereby sleep apnea may affect cognition is brain injury caused by radical oxygen species. Murine models of chronic intermittent hypoxia that mimic respiratory disturbances associated with SDB showed that reoxygenation following a hypoxia event cause oxidative stress, neuroinflammation, and organelle injury in the CNS, leading to neurobehavioral impairment.39,40 These observations suggest that the AHI may better reflect episodic hypoxia-reoxygenation events in SDB than other parameters such as time spent under 90% of blood oxygen saturation. We and others did not find an association between oxygen desaturation and cognitive scores.17,20,37,41 However, there are reports of larger cognitive impairment in those SDB patients experiencing more severe hypoxia.15,16,18,21
Several studies showed that APOE ε4 allele is associated with increased CNS vulnerability to many insults23,42 that may include SDB. CPAP treatment of patients with Alzheimer disease and SDB significantly improved cognitive scores, sleep, and mood and reduced daytime sleepiness,43–45 suggesting that SDB might contribute to cognitive decline in patients with Alzheimer disease. We found memory impairment in APOE4-positive patients with moderate to severe SBD. It is not clear why other cognitive domains were unassociated. However, subtle problems with memory are often exhibited long before clinical diagnosis of Alzheimer disease; therefore, it is possible that SDB may precipitate these effects in APOE4 carriers. Insight into the possible mechanism whereby the SDB and APOE4 interaction can affect cognition was provided by a recent study by Kheirandish et al.46 in an animal model. Kheirandish et al. showed that cognitive impairment induced by chronic intermittent hypoxia in APOE-deficient mice (mimicking dysfunctional APOE4) was accompanied by increased oxidative damage and inflammation in hippocampus. However, more studies are needed to elicit exact mechanisms by which SDB facilitates cognitive deficit in APOE4-positive persons.
Strengths of this study include a relatively large sample of middle-aged to older adults, the use of the gold standard for assessment of SDB by overnight in-laboratory polysomnography, and a validated neurocognitive test battery for cognitive assessment. Thus, both predictors and outcomes were measured objectively and in a standard way, minimizing (but not eliminating) measurement error. In the analysis we accounted for known and many potential confounders of SDB and cognition such as age, education, BMI, and sex. In addition, we examined a number of other factors (e.g., health history and habits, medications) as potential confounders. However, the study has a few important limitations. First, this is a cross-sectional analysis and thus we could not determine, for example, how long SDB must be present for the interaction with APOE4 on cognition to occur. A pure (intrasubject cognitive decline) longitudinal analysis of WSCS data will require several more years of follow-up data collection to accrue sufficient study power to examine within-subject reductions in cognitive function in relation to SDB and APOE status. Second, selection bias may be a concern. The participants of the study were recruited from Wisconsin state agencies and at the baseline assessment all were employed; it is possible that people with more profound cognitive problems and severe SDB were less likely to be employed and be part of the study. Third, not all participants in the cohort consented to both genetic testing and neurocognitive assessment. Poorer cognitive performance of those not consenting may have made it more difficult to accurately measure associations between SDB and cognition.
In summary, this study suggests that moderate to severe SDB in combination with the APOE4 genotype is associated with poorer performance on some neurocognitive tests with memory and executive function components. This is not surprising considering that the first subtle signs of Alzheimer disease may occur several years or decades before diagnosis. Thus SDB may be a significant risk factor that could aggravate cognitive impairment in people with high risk for Alzheimer disease. Whereas there is currently no treatment or cure for Alzheimer disease, SDB can be well managed by adherent CPAP treatment. Our study, together with the evidence that APOE4 and SDB interaction on cognitive function may also exist in young children, suggest that APOE4 carriers may be more vulnerable to SDB effects on the CNS.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Mignot has received or is receiving research support from GSK, Jazz Pharmaceuticals, Actelion, NovoNordisk and Cephalon. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful for the advice and comments of Karen Cruickshanks, PhD; Gordon Mitchell, PhD; Terry Young, PhD; and the technical expertise of K. Mae Hla, MD, MHS; Hyon Kim, PhD; Diane Austin; Andrea Peterson; Stephanie Hall; Jodi Barnet; Kathryn Cacic, DNP, APNP; Linda Evans; Kathy Stanback; Kathryn Pluff; Amanda Rasmuson; Robin Stubbs; and Mary Sundstrom. This work was supported by the National Heart, Lung, and Blood Institute (R01HL62252), National Institute of Aging (1R01AG036838), National Institute of Neurological Disorders and Stroke (3P50NS023724), and the National Center for Research Resources (1UL1RR025011) at the National Institutes of Health.
REFERENCES
- 1.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: Eighteen-year follow-up of the Wisconsin Sleep Cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Punjabi NM, Newman AB, Young TB, Resnick HE, Sanders MH. Sleep-disordered breathing and cardiovascular disease: an outcome-based definition of hypopneas. Am J Respir Crit Care Med. 2008;177:1150–5. doi: 10.1164/rccm.200712-1884OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: An American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118:1080–111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 5.Hla KM, Young T, Finn LA, Peppard PE, Kinsey TJ, Ende D. Electrocardiographically indicated cardiovascular disease in sleep-disordered breathing. Sleep Breath. 2008;12:251–8. doi: 10.1007/s11325-007-0168-0. [DOI] [PubMed] [Google Scholar]
- 6.Peppard PE, Ward NR, Morrell MJ. The impact of obesity on oxygen de-saturation during sleep-disordered breathing. Am J Respir Crit Care Med. 2009;180:788–93. doi: 10.1164/rccm.200905-0773OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 8.Arzt M, Young T, Peppard PE, et al. Dissociation of obstructive sleep apnea from hypersomnolence and obesity in patients with stroke. Stroke. 2010;41:e129–34. doi: 10.1161/STROKEAHA.109.566463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–51. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munoz R, Duran-Cantolla J, Martinez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37:2317–21. doi: 10.1161/01.STR.0000236560.15735.0f. [DOI] [PubMed] [Google Scholar]
- 11.Kim H, Young T. Subjective daytime sleepiness: dimensions and correlates in the general population. Sleep. 2005;28:625–34. doi: 10.1093/sleep/28.5.625. [DOI] [PubMed] [Google Scholar]
- 12.Engleman H, Joffe D. Neuropsychological function in obstructive sleep apnoea. Sleep Med Rev. 1999;3:59–78. doi: 10.1016/s1087-0792(99)90014-x. [DOI] [PubMed] [Google Scholar]
- 13.Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsycho-logical effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 14.Kim H, Dinges DF, Young T. Sleep-disordered breathing and psychomotor vigilance in a community-based sample. Sleep. 2007;30:1309–16. doi: 10.1093/sleep/30.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams N, Strauss M, Schluchter M, Redline S. Relation of measures of sleep-disordered breathing to neuropsychological functioning. Am J Respir Crit Care Med. 2001;163:1626–31. doi: 10.1164/ajrccm.163.7.2004014. [DOI] [PubMed] [Google Scholar]
- 16.Quan SF, Wright R, Baldwin CM, et al. Obstructive sleep apnea-hypopnea and neurocognitive functioning in the Sleep Heart Health Study. Sleep Med. 2006;7:498–507. doi: 10.1016/j.sleep.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Sforza E, Roche F, Thomas-Anterion C, et al. Cognitive function and sleep related breathing disorders in a healthy elderly population: the SYNAPSE study. Sleep. 2010;33:515–21. doi: 10.1093/sleep/33.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quan SF, Chan CS, Dement WC, et al. The association between obstructive sleep apnea and neurocognitive performance--the apnea positive pressure long-term efficacy study (APPLES) Sleep. 2011;34:303–14B. doi: 10.1093/sleep/34.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–9. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Hara R, Schroder CM, Kraemer HC, et al. Nocturnal sleep apnea/hypopnea is associated with lower memory performance in APOE epsilon4 carriers. Neurology. 2005;65:642–4. doi: 10.1212/01.wnl.0000173055.75950.bf. [DOI] [PubMed] [Google Scholar]
- 21.Spira AP, Blackwell T, Stone KL, et al. Sleep-disordered breathing and cognition in older women. J Am Geriatr Soc. 2008;56:45–50. doi: 10.1111/j.1532-5415.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 22.Gozal D, Capdevila OS, Kheirandish-Gozal L, Crabtree VM. APOE epsilon 4 allele, cognitive dysfunction, and obstructive sleep apnea in children. Neurology. 2007;69:243–9. doi: 10.1212/01.wnl.0000265818.88703.83. [DOI] [PubMed] [Google Scholar]
- 23.Strittmatter WJ, Roses AD. Apolipoprotein e and Alzheimer's disease. Annu Rev Neurosci. 1996;19:53–77. doi: 10.1146/annurev.ne.19.030196.000413. [DOI] [PubMed] [Google Scholar]
- 24.Raber J, Huang Y, Ashford JW. APOE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging. 2004;25:641–50. doi: 10.1016/j.neurobiolaging.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer's disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ancoli-Israel S, Klauber MR, Butters N, Parker L, Kripke DF. Dementia in institutionalized elderly: relation to sleep apnea. J Am Geriatr Soc. 1991;39:258–63. doi: 10.1111/j.1532-5415.1991.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 27.Gehrman PR, Martin JL, Shochat T, Nolan S, Corey-Bloom J, Ancoli-Israel S. Sleep-disordered breathing and agitation in institutionalized adults with Alzheimer disease. Am J Geriatr Psychiatry. 2003;11:426–33. [PubMed] [Google Scholar]
- 28.Cosentino FI, Bosco P, Drago V, et al. The APOE epsilon4 allele increases the risk of impaired spatial working memory in obstructive sleep apnea. Sleep Med. 2008;9:831–9. doi: 10.1016/j.sleep.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occur-rence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 30.Kadotani H, Kadotani T, Young T, et al. Association between apolipo-protein E epsilon4 and sleep-disordered breathing in adults. JAMA. 2001;285:2888–90. doi: 10.1001/jama.285.22.2888. [DOI] [PubMed] [Google Scholar]
- 31.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with hhai. J Lipid Res. 1990;31:545–8. [PubMed] [Google Scholar]
- 32.Kim HC, Young T, Matthews CG, Weber SM, Woodward AR, Palta M. Sleep-disordered breathing and neuropsychological deficits. A population-based study. Am J Respir Crit Care Med. 1997;156:1813–9. doi: 10.1164/ajrccm.156.6.9610026. [DOI] [PubMed] [Google Scholar]
- 33.Palta M. Quantitative methods in population health: extensions of ordinary regression. New Jersey: John Wiley & Sons, Inc; 2003. [Google Scholar]
- 34.Tierney MC, Black SE, Szalai JP, et al. Recognition memory and verbal fluency differentiate probable Alzheimer disease from subcortical ischemic vascular dementia. Arch Neurol. 2001;58:1654–9. doi: 10.1001/archneur.58.10.1654. [DOI] [PubMed] [Google Scholar]
- 35.Zhou A, Jia J. Different cognitive profiles between mild cognitive impairment due to cerebral small vessel disease and mild cognitive impairment of Alzheimer's disease origin. J Int Neuropsychol Soc. 2009;15:898–905. doi: 10.1017/S1355617709990816. [DOI] [PubMed] [Google Scholar]
- 36.Schoenberg MR, Dawson KA, Duff K, Patton D, Scott JG, Adams RL. Test performance and classification statistics for the Rey Auditory Verbal Learning Test in selected clinical samples. Arch Clin Neuropsychol. 2006;21:693–703. doi: 10.1016/j.acn.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Cohen-Zion M, Stepnowsky C, Marler, Shochat T, Kripke DF, Ancoli-Israel S. Changes in cognitive function associated with sleep disordered breathing in older people. J Am Geriatr Soc. 2001;49:1622–7. doi: 10.1046/j.1532-5415.2001.t01-1-49270.x. [DOI] [PubMed] [Google Scholar]
- 38.Lim DC, Veasey SC. Neural injury in sleep apnea. Curr Neurol Neurosci Rep. 2010;10:47–52. doi: 10.1007/s11910-009-0078-6. [DOI] [PubMed] [Google Scholar]
- 39.Veasey S. Insight from animal models into the cognitive consequences of adult sleep-disordered breathing. ILAR J. 2009;50:307–11. doi: 10.1093/ilar.50.3.307. [DOI] [PubMed] [Google Scholar]
- 40.Nair D, Dayyat EA, Zhang SX, Wang Y, Gozal D. Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of sleep apnea. PLoS One. 2011;6:e19847. doi: 10.1371/journal.pone.0019847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hrubos-Strom H, Nordhus IH, Einvik G, et al. Obstructive sleep apnea, verbal memory, and executive function in a community-based high-risk population identified by the Berlin Questionnaire Akershus Sleep Apnea Project. Sleep Breath. 2012;16:223–31. doi: 10.1007/s11325-011-0493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bliwise DL. Sleep apnea, APOE4 and Alzheimer's disease 20 years and counting? J Psychosom Res. 2002;53:539–46. doi: 10.1016/s0022-3999(02)00436-1. [DOI] [PubMed] [Google Scholar]
- 43.Cooke JR, Ayalon L, Palmer BW, et al. Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer's disease and obstructive sleep apnea: a preliminary study. J Clin Sleep Med. 2009;5:305–9. [PMC free article] [PubMed] [Google Scholar]
- 44.Ancoli-Israel S, Palmer BW, Cooke JR, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer's disease: a randomized controlled study. J Am Geriatr Soc. 2008;56:2076–81. doi: 10.1111/j.1532-5415.2008.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chong MS, Ayalon L, Marler M, et al. Continuous positive airway pressure reduces subjective daytime sleepiness in patients with mild to moderate Alzheimer's disease with sleep disordered breathing. J Am Geriatr Soc. 2006;54:777–81. doi: 10.1111/j.1532-5415.2006.00694.x. [DOI] [PubMed] [Google Scholar]
- 46.Kheirandish L, Row BW, Li RC, Brittian KR, Gozal D. Apolipoprotein E-deficient mice exhibit increased vulnerability to intermittent hypoxia-induced spatial learning deficits. Sleep. 2005;28:1412–7. doi: 10.1093/sleep/28.11.1412. [DOI] [PubMed] [Google Scholar]


