Abstract
Study Objectives:
Impaired sleep patterns are known to be associated with many chronic conditions and ultimately they may lead to permanent work incapacity. Less is known about the associations between sleep patterns and cause-specific disability pensions, such as low back diagnoses, or whether familial factors (genetics and family environment) can affect the associations. The objective of this study was to investigate sleep patterns as predictors of disability pension due to low back diagnoses with a 23-year follow-up.
Design and Setting:
A prospective cohort study with comprehensive mailed questionnaires about sleep patterns, e.g., quality and length of sleep in 1975 and 1981. Follow-up from the national disability pension register data until 2004.
Interventions:
Not applicable.
Participants:
There were 18,979 individuals (7,722 complete twin pairs) born before 1958.
Measurements and Results:
Cox proportional hazards regression was used to calculate hazard ratios (HR) with 95% confidence intervals (95% CI). Disability pension due to low back diagnoses had been granted to 467 individuals during the follow-up. Sleeping moderately well (HR 1.25; 95% CI 1.02, 1.53), or fairly poorly/poorly (HR 2.05; 95% CI 1.53, 2.73) at baseline predicted a significantly higher risk for disability pension. Stable patterns of sleeping either fairly well (HR 1.29; 95% CI 1.01, 1.64), or stably fairly poorly/poorly (HR 2.29; 95% CI 1.49, 3.52) between 1975 and 1981 were associated with a higher risk as compared to a stable pattern of sleeping well. Furthermore, a decrease in quality of sleep from 1975 to 1981 was associated (HR 1.34; 95% CI 1.03, 1.76) with an increased risk of disability pension.
Conclusions:
Sleep quality and changes in sleep quality appear to be early predictors for disability pension due to low back diagnoses independently from other confounding factors.
Citation:
Ropponen A; Silventoinen K; Hublin C; Svedberg P; Koskenvuo M; Kaprio J. Sleep patterns as predictors for disability pension due to low back diagnoses: a 23-year longitudinal study of Finnish twins. SLEEP 2013;36(6):891-897.
Keywords: Sleep, sick leave, disability pension, low back diagnoses
INTRODUCTION
Both impaired sleep and disability pension (DP) are associated with high costs for society, such as loss of productivity and need for medication.1,2 Ultimately impaired sleep patterns, such as too short or too long sleep length, or poor sleep quality, have been shown to have a predictive role for mortality.3–5 Fur thermore, impaired sleep is known to be associated with many chronic disorders such as musculoskeletal disorders including rheumatoid arthritis and low back diagnoses (LBD),6–8 which is the main diagnosis group for DP, as well as with chronic (low back) pain.9,10 In recent years, an increasing number of studies have detected an association between disturbed sleep patterns and DP.11–17 Because chronic disease or illness always precedes DP, it is interesting to study the specific diagnoses for DP in association with disturbed sleep patterns.
In this respect, self-reported disturbances in sleep patterns were shown to be associated with an increased risk of all-cause and cause-specific work disability (i.e., musculoskeletal or mental diagnoses) in a 3-y follow-up of municipal workers18 and in a 10-year follow-up of 40-60-y-old employees in Finland.14,16 A study of industrial workers also indicated that impaired sleep could predict hospitalization related to LBD in a 28-y follow-up study.19 Because impaired sleep and medical comorbidities coexist, the early detection of sleep disturbances could potentially help to prevent the serious consequences of these comorbidities. Currently it is not known whether impaired sleep exists prior to the onset of these comorbidities, worsens their progress, or if the sleep impairments are consequences of the pain due to disorders such as LBD.19,20 Moreover, most of the studies examining the association between impaired sleep patterns and permanent work disability (DP) so far have had follow-up times of 10 y or less,11,12,14,16,18 and large studies with long follow-up times appear to be lacking.
However, simple associations between sleep patterns and DP risk are not direct evidence of causality because it could be postulated that familial factors (including genetics and shared family environment such as social background) could also modify their association. Genetic factors are known to affect the risk of DP due to LBD,21–23 and insomnia-related symptoms also have shown a moderate degree of heritability, 35-45%.4,24–26 Hence, shared genetic influences may underlie the association between sleep patterns and DP due to LBD. Therefore, it is interesting to investigate this association in a setting that can control for confounding familial influence (genetics and family environment) through examining twin pairs who are discordant for DP. In the twin setting, co-twins differing in their sleep patterns provide an opportunity to study the underlying causal pathways in these associations. If familial factors are of importance, then no association should be present between sleep patterns and DP within discordant twin pairs. Instead, if factors specific to each individual are more important, then discordant twin pairs will show similar associations as in analyses between all individuals. However, there is little information available about impaired sleep patterns with long follow-ups for DP due to LBD; as far as we are aware there is no study that has taken into account familial confounding in assessing these associations. The aim of this study was to investigate sleep patterns as predictors for DP due to LBD in a 23-y follow-up study.
METHODS
Sample
In the Finnish Twin Cohort study, the baseline questionnaire including comprehensive questions on sociodemographic, work, health, lifestyle and psychosocial factors, and sleep patterns was mailed in 1975 to all same-sex Finnish twin pairs born before 1958 and in whom both co-twins were alive (response rate 89%).27,28 The follow-up questionnaire was mailed in 1981 to all these twins irrespective of whether they had responded to the baseline questionnaire (response rate 84%). The current analysis consisted of twin individuals responding to both questionnaires with information on sleep length and sleep quality, not retired from work before January 1, 1982, and residing in Finland in 1981. The study sample was 18,979 twins (53% women) including 2,547 monozygotic (MZ) and 5,175 dizygotic (DZ) complete twin pairs, as well as 3,535 twin individuals without his or her co-twin.
Disability Pension
Information on DP was obtained from the official Finnish pension registers.21 The eighth, ninth, and 10th revisions of International Classification of Diseases (ICD-8, ICD-9, and ICD-10; ICD-10 codes M40-M54 and the corresponding codes in ICD-8 and ICD-9) were used for LBD encoding by the Finnish insurance institutions. Information on mortality and migration was derived from the Population Register Centre of Finland to assess censoring. The unique personal identification codes of all Finnish residents were used for record linkage. The follow-up time was from the date of the 1981 questionnaire to the date when DP was awarded, or until the person began to receive an old age pension, or until the date of death/emigration, or until December 31, 2004, whichever occurred first.
Risk Factors
The risk factors in this study included sleep patterns reported both from the baseline questionnaire 1975 and from the follow-up questionnaire 1981. Sleep quality in 1975 and 1981 was categorized as sleeping well, fairly well, or fairly poorly/poorly.3 The stability of sleep quality between 1975 and 1981 was assessed using combinations of categories (three alternatives both in 1975 and 1981 giving nine subgroups in each sleep quality level). The stability of sleep quality was further combined into five classes reflecting stability or changes in 1975 to 1981: stable well (i.e., sleeping well was reported in 1975 and 1981), stable fairly well (i.e., sleeping fairly well was reported both in 1975 and 1981), stable fairly poor or poor (sleeping fairly poorly or poorly was reported both in 1975 and 1981), reduced quality (any change from 1975 to a lesser quality of sleep in 1981), and increased quality (any change from 1975 to a better quality in 1981). Sleep length in 1975 and 1981 was categorized into three classes: short (< 7 h), average (7-8 h), and long (> 8 h).3 Classes of stability of sleep length between 1975 and 1981 were formed as for sleep quality, but then categorized into three classes — stable, reduced and increased sleep length — between 1975 and 1981.
Background Factors
The following background factors from the 1981 follow-up questionnaire were included in this study: age, sex, education (nine categories by year of education, high school graduation equals 12 year; converted into year of education), marital status (never married, widowed, separated, married, newly married, and cohabiting), and musculoskeletal pain assessed with the question of having pain in the low back, neck, or shoulder area that had affected work capacity in recent year (i.e., immediately before the time of the questionnaire, yes versus no). The responses to these three items were used to calculate a summary pain score (0-3 locations), which was dichotomized into “no pain” versus “any pain: one to three pain locations”. Life satisfaction was measured with a four-item scale on levels of interest, happiness, easiness, and loneliness of life29 and analyzed in a continuous range from 4-20; with a low score representing the most satisfied. Body mass index (BMI, kg/m2), smoking status (never smoked, occasional, former, and current smokers), and alcohol consumption (based on self-reported average quantities of beer, wine, and spirits consumed30) grouped into four categories of abstainers, light, moderate, and heavy users according to the sex-specific criteria of the National Institute on Alcohol Abuse and Alcoholism31 were also included. Furthermore, monthly frequency, mean duration, and mean intensity of leisure-time physical activity were used to compute metabolic equivalent (MET) values.32 The diurnal type was determined by a question according to the Diurnal Type Scale.33,34 The an swer was categorized into four classes: clearly a morning type, somewhat a morning type, somewhat an evening type, clearly an evening type.34 Type of work was a trichotomous variable, i.e., day work, shift work, and evening and night work. The use of hypnotic agents and/or tranquilizers was subdivided into three categories: no use of hypnotic agents or tranquilizers, infrequent use (1-59 days per y of either medication), and frequent use (60 or more days per y of either medication).3 Because there were some missing data for both in hypnotic agents and tranquilizers, those with missing data were coded as a missing category to be included in the analyses. Occupational socioeconomic status was included from the 1975 questionnaire because such data were not available in 1981 (upper and lower white-collar workers, skilled and unskilled blue-collar workers, farmers, and others including military conscripts, students, and those not otherwise classified).
Statistical Analysis
Descriptive statistics were used for reporting background factors including the chi square (÷2) test, t-test, and Fisher exact test as appropriate. Cox proportional hazards models with the follow-up time in days, and DP due to LBD as the outcome variable were used to compute hazard ratios (HR) with 95% confidence intervals (CI). Due to the dependent nature of the sample, i.e., twin pairs, all the analyses were clustered through pair identity to adjust the standard errors for a lack of statistical independence within pairs. Furthermore, we adjusted all analyses for age as a continuous variable. In addition, the analyses of the whole cohort were analyzed by stratification with sex to provide men and women with their own baseline hazards to control for the effects of sex. The ‘log-log’ curves for the categories of risk factors were used to test the proportional hazards assumption graphically, and all curves were observed to be acceptably parallel.
First, we analyzed HR separately for each sleep pattern. Second, we adjusted the models for all of the background factors. Education, socioeconomic status, BMI, musculoskeletal pain locations, and smoking status have been shown to be significant predictors for DP due to LBD in an earlier study of partially the same cohort,35 although all of the background factors may also influence sleep patterns.
Third, twin pairs discordant for DP due to LBD were investigated. Conditional Cox proportional hazards models were estimated by analyzing the follow-up time to DP in relation to the follow-up time of the co-twin, in the situation where one twin had a DP due to LBD during the follow-up but his or her twin had not been granted DP due to LBD. The conditional Cox proportional hazards models were performed with stratification by twin pairs, allowing each twin pair to have his or her own baseline hazard to control for the effects of potentially confounding familial factors. Therefore, actual associations found between sleep patterns and DP would not be explained by familial factors shared by the twins in a pair. If the association between sleep patterns and DP were to be because of family background, then the association should be present only between twin pairs; in other words, the association should exist in the analyses of the whole cohort but not within discordant co-twins reared together. Alternatively, if the association was due to a common genetic trait, then the association should be present within DZ twin pairs (sharing on average 50% of their segregating genes), but not within MZ twin pairs (sharing 100% of gene sequence). Furthermore, if the association were to be independent from familial effects (mainly due to nonfamilial environmental factors), then it should be found within both MZ and DZ twin pairs. This would include all environmental factors unique to twin individuals as well as direct causal associations between sleep patterns and DP risk. The number of discordant pairs for DP due to LBD was 83 MZ pairs (46 male and 37 female) and 238 DZ pairs (125 male, and 113 female). The data analyses were performed using the Stata statistical software, version 12.1 (Stata Corporation, College Station, TX, USA).
RESULTS
Over the average 18 y (standard deviation for mean 7 y) of follow-up, 467 individuals were granted DP due to LBD; 44% of them were women. At baseline, those destined to have future DP due to LBD were more often morning type (Table 1), not sleeping well (i.e., moderately well, fairly poorly, or poorly), and had a short sleep length in comparison with those not granted DP during follow-up (Table 2).
Table 1.
Means and percentages of baseline (1981) background factors for individuals with disability pension due to low back diagnoses and no disability pension during the follow-up among Finnish twins
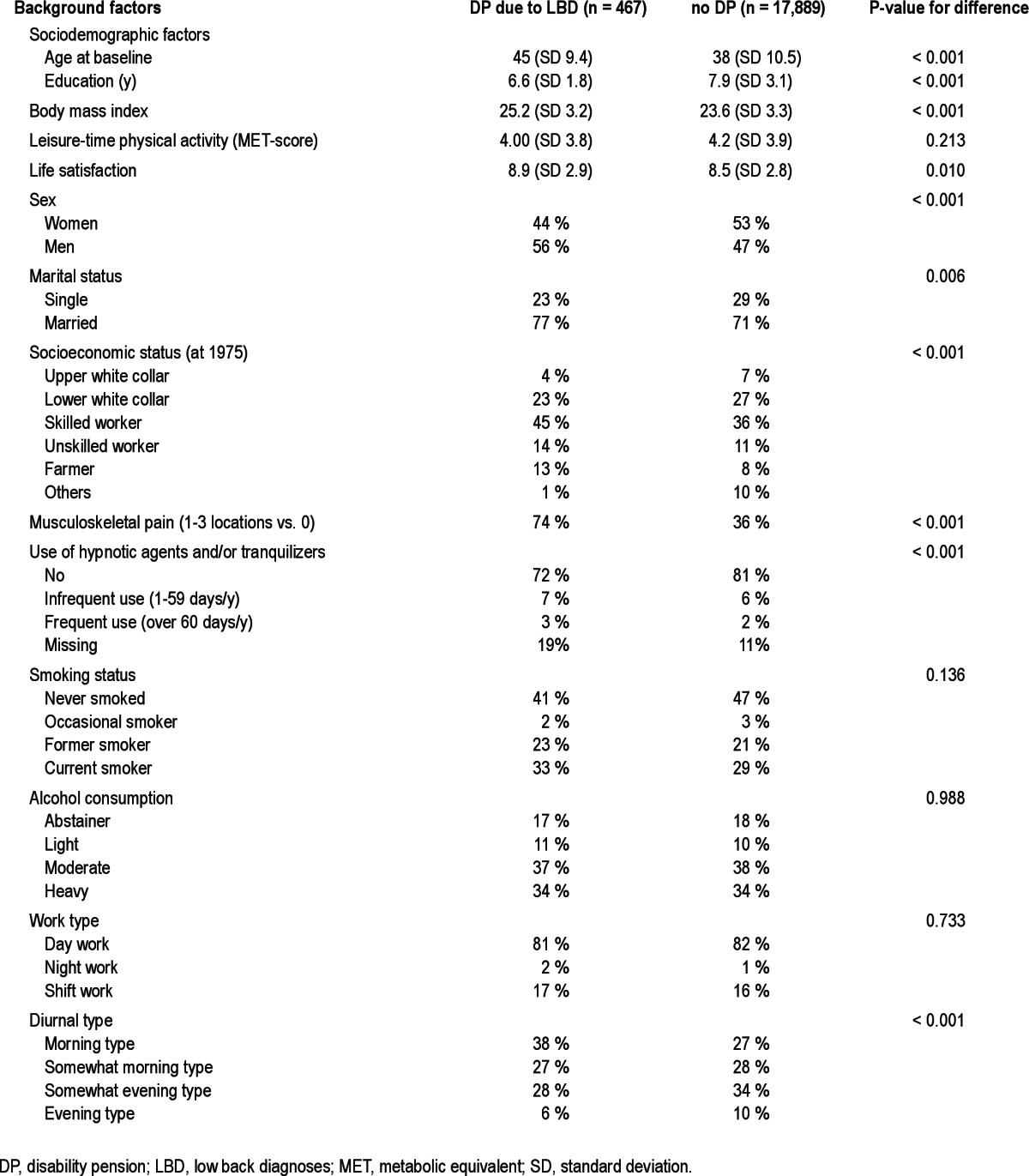
Table 2.
Percentages of sleep patterns for individuals with disability pension (DP) due to low back diagnoses and in those without DP as well as Cox proportional hazard ratios with 95% confidence intervals (CI) for DP due to LBD during the follow-up
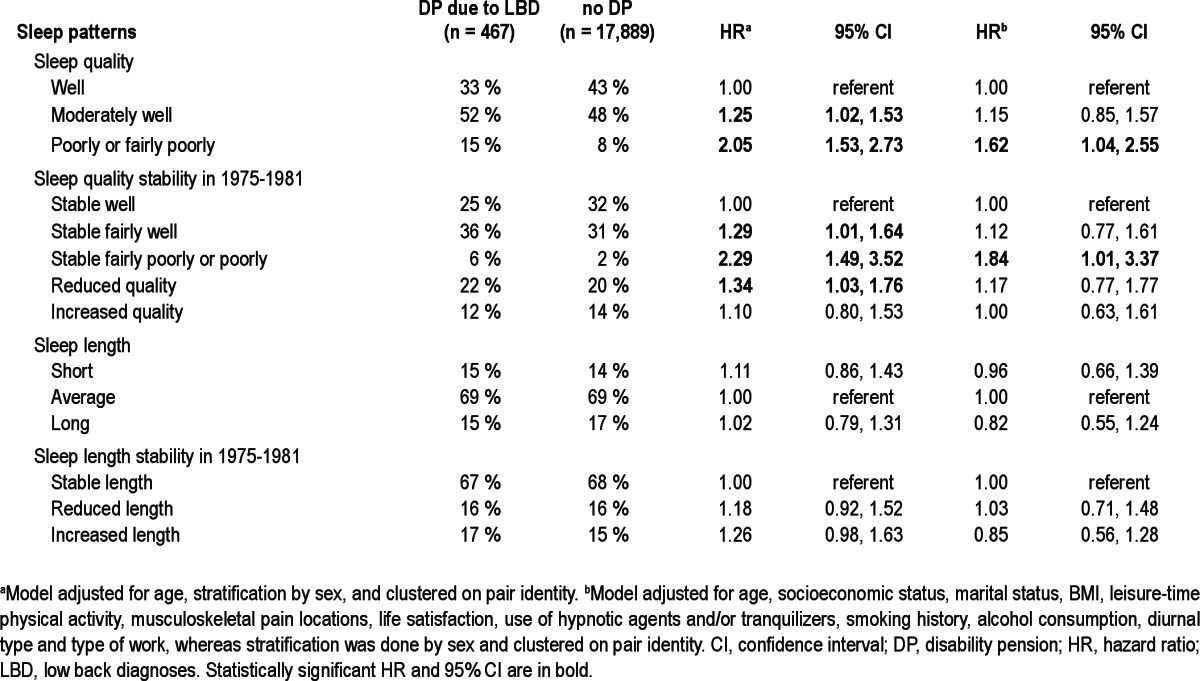
Sleeping moderately well, or fairly poorly or poorly at baseline predicted a significantly higher risk for DP due to LBD. In addition, those individuals sleeping stable fairly well, or stable fairly poorly or poorly between 1975 and 1981 had a significantly increased risk as did those whose quality of sleep decreased from 1975 to 1981 (Table 2). These associations of sleeping fairly poorly or poorly at baseline and those who were poor sleepers throughout 1975-1981 remained significant in the fully adjusted model accounting for socioeconomic status, marital status, BMI, leisure-time physical activity, musculoskeletal pain locations, life satisfaction, use of hypnotic agents and/or tranquilizers, smoking status, alcohol consumption, diurnal type, and type of work (Table 2). The statistical significance of the associations attenuated clearly when familial confounding was taken into account in the analyses of discordant twin pairs. However, the magnitude and direction of associations remained the same (Table 3), and thus the lack of significance was primarily due to the lower statistical power in these analyses.
Table 3.
Conditional Cox proportional hazard ratios with 95% confidence intervals for disability pension due to low back diagnoses during follow-up

DISCUSSION
This over 20-y follow-up of 18,979 twin individuals provides a unique opportunity to investigate the association between sleep patterns and DP due to LBD because it allows control for familial confounding. To the best of our knowledge, no such study has been conducted before. Furthermore, this study is one of the first to investigate how stability or change of sleep length and quality over 6 years of follow-up affects the likelihood of DP due to LBD. The results indicate that not only fairly poor or poor sleep quality at one time point, but also that having stable poor quality of sleep (as measured at two time points 6 years apart) early in the life course are significant predictors for future DP due to LBD. In addition, with respect to the actual sleep parameters, sleep quality was the strongest predictor for future DP due to LBD; sleep length was not associated with the risk of DP. The associations between sleeping fairly poorly or poorly at baseline and having stable poor sleep between 1975-1981 and DP due to LBD were not dependent on the extensive list of background factors including socioeconomic status, marital status, BMI, leisure-time physical activity, musculoskeletal pain locations, life satisfaction, use of hypnotic agents and/or tranquilizers, smoking status, alcohol consumption, diurnal type, and type of work. However, we did identify some indications that these associations between sleep quality and DP due to LBD were also independent of the family background because the risk estimates in the twins were approximately at the same magnitude and direction. However, this finding needs to be viewed with caution due to the low number of discordant pairs.
The long follow-up time of this study, 23 y, adds to the existing epidemiologic knowledge of sleep patterns and DP that have been mainly based on follow-ups of 10 y or less.11,12,14,16,18 Furthermore, these previous studies have been limited to one single assessment of sleep patterns, and thus have not been able to investigate changes in sleep quality or length. In addition, this study may be one of the first reports of cause-specific DP and sleep patterns adding to the previous results based on either DP in general or on more broad diagnostic groups.11–17
We were able to evaluate the complex role of comorbidities and sleep because we accounted for the baseline musculoskeletal pain in our analyses, but this did not seem to play any role in the associations between sleep patterns and DP due to LBD. This finding could be interpreted to mean that at least for DP due to LBD, impaired sleep quality and musculoskeletal pain evaluated at the same time point over 20 y earlier may coexist without confounding the association between sleep quality and DP due to LBD. This adds some pieces to the puzzle of whether impaired sleep exists prior to the onset of back disorders, worsens the progress of these disorders, or if impaired sleep is a consequence of pain associated with those disorders.19,20 Hence, it can be postulated that the early detection of sleep disturbances would potentially help to prevent permanent work incapacity due to LBD.
We found that the association between sleep patterns and DP due to LBD probably is independent of familial factors (including genetics and shared family environment such as social background), i.e., sleep patterns seem to have a direct effect on risk of DP. Although we had a relatively large sample (almost 19,000 individuals), we lacked enough discordant twin pairs to confirm this finding with sufficient statistical confidence. However, based on the previous twin studies, both DP due to LBD21–23 and sleep patterns4,24–26 were known to possess relatively strong genetic components. Therefore, some shared genetic influences could be expected to underlie the association between sleep patterns and DP due to LBD. In future studies, even larger sample sizes, richer family structures, measured genotypes, and alternative statistical models would be needed to explore the association between sleep patterns and cause-specific DP and the importance of familial background in these associations.
This study had several strengths: its long prospective follow-up from 1981 to 2004 with detailed survey data from two time points separated by a relatively long time period (6 y), enabling measurement of stability or changes in sleep patterns. Furthermore, we had access to high-quality register data about DP including diagnoses and date awarded in this large cohort. In addition, the use of the co-twin control design made it possible to include familial confounding. This design provided us a powerful tool to analyze discordant twins, i.e., twins who within a twin pair differed in DP and sleep patterns, by the optimal matching of cases and controls as they were same-sexed twin pairs. However, partially related to the longitudinal study design, which inevitably resulted in some missing data, the final sample was reduced to 18,979 individuals. Consequently, this resulted in some lack of power, i.e., we were not able to analyze all the available sleep patterns between 1975 and 1981.3 Furthermore, the number of discordant twin pairs was rather low and therefore no solid conclusions on familial confounding could be drawn. However, we may assume that the used variables of sleep patterns, particularly for the sleep quality, provided valuable information on stability and change over time and this has not been evaluated before for diagnosis-specific DP such as DP due to LBD. Regarding sleep patterns, a limitation is that our questionnaire was not specifically validated for the sleep questions. However, previous studies with the same questionnaire have clearly indicated that both sleep length and quality have strong genetic component suggesting stability of sleep patterns,3,4,24–26 sleep length has predictive value for mortality,36 and poor sleep quality has been associated with life dissatisfaction.24 We also lacked data on specific insomnia-related questions, hence limiting our data to self-reported sleep quality. In addition, for the sleep time, we did not have data to assess less than 6 h of sleep, although this study focused on the normal variation of sleep and not insomnia. That may partially explain why no association with sleep length and DP due to LBD was found, suggesting that a larger sample size with detailed data of sleep length at the short end would be needed for further elaboration of this association. However, it can be assumed that due to these limitations, the associations may have been weakening and thus our results show the lowest level of the effect size when the real effect size is likely to be stronger.
CONCLUSION
Sleep quality and changes in sleep quality seem to be early predictors for DP due to LBD. These associations of sleep quality are independent from many other mediating factors, but further studies will be needed to confirm the influence of family background in these associations.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by the Social Insurance Institution, Finland for the TwinKela-project, and by the Academy of Finland Center of Excellence in Complex Disease Genetics (grant numbers: 213506, 129680) for the Finnish Twin Cohort studies. Dr. Ropponen is supported by an Academy of Finland researcher grant (# 122080); Dr. Svedberg by grants from the Swedish Council for Working Life and Social Research (2007-0830) and the Centre for Health Care Science, Karolinska Institutet.
REFERENCES
- 1.Wade AG. The societal costs of insomnia. Neuropsychiatr Dis Treat. 2011;7:1–18. doi: 10.2147/NDT.S15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.OECD. Organisation for Economic Co-operation and Development. High-Level Forum. Sickness, disability and work: keeping on track in the economic downturn - background paper. 2009. Available on January 4 th 2013 at http://www.oecd.org/employment/employmentpoliciesand-data/42699911.pdf.
- 3.Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep and mortality: a population-based 22-year follow-up study. Sleep. 2007;30:1245–53. doi: 10.1093/sleep/30.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hublin C, Partinen M, Koskenvuo M, Kaprio J. Heritability and mortality risk of insomnia-related symptoms: a genetic epidemiologic study in a population-based twin cohort. Sleep. 2011;34:957–64. doi: 10.5665/SLEEP.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kronholm E, Laatikainen T, Peltonen M, Sippola R, Partonen T. Self-reported sleep duration, all-cause mortality, cardiovascular mortality and morbidity in Finland. Sleep Med. 2011;12:215–21. doi: 10.1016/j.sleep.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Marin R, Cyhan T, Miklos W. Sleep disturbance in patients with chronic low back pain. Am J Phys Med Rehabil. 2006;85:430–5. doi: 10.1097/01.phm.0000214259.06380.79. [DOI] [PubMed] [Google Scholar]
- 7.Abad VC, Sarinas PS, Guilleminault C. Sleep and rheumatologic disorders. Sleep Med Rev. 2008;12:211–28. doi: 10.1016/j.smrv.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Gore M, Sadosky A, Stacey BR, Tai KS, Leslie D. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine (Phila Pa 1976) 2012;37:E668–77. doi: 10.1097/BRS.0b013e318241e5de. [DOI] [PubMed] [Google Scholar]
- 9.Dikeos D, Georgantopoulos G. Medical comorbidity of sleep disorders. Curr Opin Psychiat. 2011;24:346–54. doi: 10.1097/YCO.0b013e3283473375. [DOI] [PubMed] [Google Scholar]
- 10.Kelly GA, Blake C, Power CK, O'keeffe D, Fullen BM. The association between chronic low back pain and sleep: a systematic review. Clin J Pain. 2011;27:169–81. doi: 10.1097/AJP.0b013e3181f3bdd5. [DOI] [PubMed] [Google Scholar]
- 11.Sivertsen B, Overland S, Neckelmann D, et al. The long-term effect of insomnia on work disability. Am J Epidemiol. 2006;163:1018–24. doi: 10.1093/aje/kwj145. [DOI] [PubMed] [Google Scholar]
- 12.Sivertsen B, Ã~verland S, Bjorvatn B, Mæland JG, Mykletun A. Does insomnia predict sick leave?: The Hordaland Health Study. J Psychosom Res. 2009;66:67–74. doi: 10.1016/j.jpsychores.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Overland S, Glozier N, Sivertsen B, et al. A comparison of insomnia and depression as predictors of disability pension: the HUNT Study. Sleep. 2008;31:875–80. doi: 10.1093/sleep/31.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lallukka T, Haaramo P, Lahelma E, Rahkonen O. Sleep problems and disability retirement: a register-based followup study. Am J Epidemiol. 2011;173:871–81. doi: 10.1093/aje/kwq462. [DOI] [PubMed] [Google Scholar]
- 15.Rahkonen O, Lallukka T, Kronholm E, Vahtera J, Lahelma E, Laaksonen M. Sleep problems and sickness absence among middle-aged employees. Scand J Work Environ Health. 2011;38:47–55. doi: 10.5271/sjweh.3186. [DOI] [PubMed] [Google Scholar]
- 16.Haaramo P, Rahkonen O, Lahelma E, Lallukka T. The joint association of sleep duration and insomnia symptoms with disability retirement - a longitudinal, register-linked study. Scand J Work Environ Health. 2012;38:427–35. doi: 10.5271/sjweh.3269. [DOI] [PubMed] [Google Scholar]
- 17.Rahkonen O, Lallukka T, Kronholm E, Vahtera J, Lahelma E, Laaksonen M. Sleep problems and sickness absence among middle-aged employees. Scand J Work Environ Health. 2012;38:47–55. doi: 10.5271/sjweh.3186. [DOI] [PubMed] [Google Scholar]
- 18.Salo P, Oksanen T, Sivertsen B, et al. Sleep disturbances as a predictor of cause-specific work disability and delayed return to work. Sleep. 2010;33:1323–31. doi: 10.1093/sleep/33.10.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaila-Kangas L, Kivimäki M, Härmä M, et al. Sleep distrurbances as predictors of hospitalization for back disorders - a 28-year follow-up of industrial employees. Spine. 2006;31:51–6. doi: 10.1097/01.brs.0000193902.45315.e5. [DOI] [PubMed] [Google Scholar]
- 20.Davis JA, Robinson RL, Le TK, Xie J. Incidence and impact of pain conditions and comorbid illnesses. J Pain Res. 2011;4:331–45. doi: 10.2147/JPR.S24170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harkonmäki K, Silventoinen K, Levälahti E, et al. The genetic liability to disability retirement: a 30-year follow-up study of 24,000 Finnish twins. PLoS ONE. 2008;3:e3402. doi: 10.1371/journal.pone.0003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartvigsen J, Nielsen J, Kyvik KO, et al. Heritability of spinal pain and consequences of spinal pain: a comprehensive genetic epidemiologic analysis using a population-based sample of 15,328 twins ages 20-71 years. Arthritis Rheum. 2009;61:1343–51. doi: 10.1002/art.24607. [DOI] [PubMed] [Google Scholar]
- 23.Narusyte J, Ropponen A, Silventoinen K, et al. Genetic liability to disability pension in women and men: a prospective population-based twin study. PLoS ONE. 2011;6:e23143. doi: 10.1371/journal.pone.0023143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paunio T, Korhonen T, Hublin C, et al. Longitudinal study on poor sleep and life dissatisfaction in a nationwide cohort of twins. Am J Epidemiol. 2009;169:206–13. doi: 10.1093/aje/kwn305. [DOI] [PubMed] [Google Scholar]
- 25.Tafti M. Genetic aspects of normal and disturbed sleep. Sleep Med. 2009;10:S17–21. doi: 10.1016/j.sleep.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Lam S, Li SX, et al. Insomnia, sleep quality, pain, and somatic symptoms: Sex differences and shared genetic components. Pain. 2012;153:666–73. doi: 10.1016/j.pain.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Hammar N, Kaprio J, Hagstrom U, Alfredsson L, Koskenvuo M, Hammar T. Migration and mortality: a 20 year follow up of Finnish twin pairs with migrant co-twins in Sweden. J Epidemiol Community Health. 2002;56:362–6. doi: 10.1136/jech.56.5.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaprio J, Koskenvuo M. Genetic and environmental factors in complex diseases: the older Finnish twin cohort. Twin Research. 2002;5:358–65. doi: 10.1375/136905202320906093. [DOI] [PubMed] [Google Scholar]
- 29.Allardt E. About Dimension of Welfare: An Explanatory Analysis of the Comparative Scandinavian Survey: University of Helsinki Research Group of Comparative Sociology. Research Report 1. 1973 [Google Scholar]
- 30.Romanov K, Rose RJ, Kaprio J, Koskenvuo M, Langinvainio H, Sarna S. Self-reported alcohol use: a longitudinal study of 12,994 adults. Alcohol Alcohol Suppl. 1987;S1:619–23. [PubMed] [Google Scholar]
- 31.Järvenpää T, Rinne JO, Koskenvuo M, Räihä I, Kaprio J. Binge drinking in midlife and dementia risk. Epidemiol. 2005;16:766–71. doi: 10.1097/01.ede.0000181307.30826.6c. [DOI] [PubMed] [Google Scholar]
- 32.Kujala UM, Kaprio J, Sarna S, Koskenvuo M. Relationship of leisure-time physical activity and mortality: the Finnish twin cohort. JAMA. 1998;279:440–4. doi: 10.1001/jama.279.6.440. [DOI] [PubMed] [Google Scholar]
- 33.Torsvall L, Akerstedt T. A diurnal type scale. Construction, consistency and validation in shift work. Scand J Work Environ Health. 1980;6:283–90. doi: 10.5271/sjweh.2608. [DOI] [PubMed] [Google Scholar]
- 34.Koskenvuo M, Hublin C, Partinen M, Heikkila K, Kaprio J. Heritability of diurnal type: a nationwide study of 8753 adult twin pairs. J Sleep Res. 2007;16:156–62. doi: 10.1111/j.1365-2869.2007.00580.x. [DOI] [PubMed] [Google Scholar]
- 35.Pietikäinen S, Silventoinen K, Svedberg P, et al. Health-related and sociodemographic risk factors for disability pension due to low back disorders: a 30-year prospective Finnish twin cohort study. J Occup Environ Med. 2011;53:488–96. doi: 10.1097/JOM.0b013e31821576dd. [DOI] [PubMed] [Google Scholar]
- 36.Partinen M, Kaprio J, Koskenvuo M, Putkonen P, Langinvainio H. Genetic and environmental determination of human sleep. Sleep. 1983;6:179–85. doi: 10.1093/sleep/6.3.179. [DOI] [PubMed] [Google Scholar]


