Abstract
Study Objectives:
Sleep spindles play an important functional role in sleep-dependent memory consolidation. They are a hallmark of non-rapid eye movement (NREM) sleep and are grouped by the sleep slow oscillation. Spindles are not a unitary phenomenon but are differentiated by oscillatory frequency and topography. Yet, it is still a matter of debate whether these differences relate to different generating mechanisms. As corticothalamic networks are known to be involved in the generation of spindles and the slow oscillation, with Ca2+ and Na+ conductances playing crucial roles, we employed the actions of carbamazepine and flunarizine to reduce the efficacy of Na+ and Ca2+ channels, respectively, for probing in healthy human subjects mechanisms of corticothalamocortical excitability.
Design:
For each pharmacologic substance a within-design study was conducted on 2 experimental nights in young, healthy adults.
Measurements and Results:
Results indicate differential effects for slow frontocortical (approximately 10 Hz) and fast centroparietal (approximately 14 Hz) spindles. Carbamazepine enhanced slow frontal spindle activity conjointly with an increment in slow oscillation power (approximately 0.75 Hz) during deep NREM sleep. In contrast, fast centroparietal spindle activity (approximately 14 Hz) was decreased by carbamazepine. Flunarizine also decreased fast-spindle electroencephalogram power, but affected neither slow frontal spindle nor slow oscillation frequency bands.
Conclusions:
Our findings indicate a differential pharmacologic response of the two types of sleep spindles and underscore a close linkage of the generating mechanisms underlying the sleep slow oscillation and the slow frontal sleep spindles for the signal transmission processes manipulated in the current study.
Citation:
Ayoub A; Aumann D; Hörschelmann A; Kouchekmanesch A; Paul P; Born J; Marshall L. Differential effects on fast and slow spindle activity, and the sleep slow oscillation in humans with carbamazepine and flunarizine to antagonize voltage-dependent Na+ and Ca2+ channel activity. SLEEP 2013;36(6):905-911.
Keywords: EEG, ion channels, sleep spindles, slow oscillations, theta
INTRODUCTION
The sleep spindle oscillations of non-rapid eye movement (NREM) sleep have been closely associated with synaptic plasticity and at the systems level with a wide range of cognitive functions.1–4 The presence of at least two types of sleep spindles differing distinctly in oscillatory frequency, topography, and time-evolution during nocturnal sleep has been revealed not only in humans5–10 but also in animals.11,12 Electroencephalogram (EEG) slow spindles (9-12 Hz) are expressed strongest over frontal cortical areas whereas the fast spindles (12-15 Hz) reveal a widespread centroparietal distribution. In humans, centroparietal spindles show the typical waxing and waning dynamics mainly during NREM Stage 2 sleep, but fast-spindle power remains elevated during slow wave sleep (SWS). Frontal spindle power reaches maximum levels during SWS, together with slow oscillation power (0.5-1 Hz).10,13 Sleep spindles arise within the corticothalamic network,14,15 whereby functional magnetic resonance imaging and EEG analyses in humans have suggested for the fast and slow spindles not only different underlying neocortical regions but also in part different thalamic regions and contributions.6,16–18 The cortex is relevant for synchronizing spindle generation, contributing to thalamocortical spindle initiation and termination.19 Yet, investigations of spindle-generating mechanisms have largely ignored possible differences between fast and slow types of spindles.20
The sleep slow oscillation (< 1 Hz) is another distinguishing rhythm of NREM sleep. Generated primarily within neocortical networks, the sleep slow oscillation synchronizes neuronal activity into generalized up-and-down states not only in the neo-cortex but also in other brain structures.15,21 Thus, by providing a global temporal frame for coordinating electrophysiologic events between brain structures, including thalamocortical spindles and hippocampal ripples, slow oscillations facilitate processes of memory consolidation.
Interestingly, the synchrony with the slow oscillation cycle differs in phase for fast and slow spindles,10 suggesting that the underlying neocortical regulation of excitability affects the two types of spindles in different ways. In the current study, we compared in humans changes in the expression of the dominant NREM sleep rhythms after preferentially reducing the efficacy of voltage-dependent Na+ channels (by carbamazepine) and Ca2+ channels (by flunarizine), i.e., channels implicated in both the regulation of cortical excitability and the generation of spindles and slow oscillations.22–25 We find differential effects for slow and fast spindles, especially after carbamazepine, thereby arguing toward the existence of separate generator mechanisms for these spindle types.
METHODS
Study Participants
Participants were healthy nonsmoking young adults with regular sleep/wake rhythms. They were to refrain from napping and caffeine and alcohol consumption 24 hours prior to recording. Participants with increased liver enzyme values were excluded from the carbamazepine study (gamma-glutamyltransferase > 18 U/L, aspartate aminotransferase > 50 U/L, and alanine aminotransferase > 50 U/L). Participants were habituated to sleep in the laboratory under experimental conditions by an adaptation night prior to the experiments proper. Both studies were approved by the local ethics committee and all participants signed an informed consent prior to participation.
Design and Procedures
Two experiments were performed, each according to a placebo-controlled double-blind crossover design, in which carbamazepine (200 mg, Tegretol, Novartis Pharma, Germany) versus placebo, and flunarizine (10 mg Sibelium, Janssen-Cilag, Germany) versus placebo were tested after oral administration before sleep in 13 participants (carbamazepine study: 10 men, 3 women, age 25.18 ± 0.25 years) and 15 participants (flunarizine study: 8 men, 7 women, age 25.93 ± 1.18 years), respectively.
Carbamazepine is primarily an antagonist of voltage-dependent Na+ channels, which exhibits stronger inhibitory effects at more depolarized membrane potential levels, and preferentially inhibits high-frequency firing.26–29 Of pharmacologically employed T-type Ca2+-current antagonists, flunarizine was shown to reveal the strongest efficiency.30 However, flunarizine also reveals a broad-spectrum profile of effects, and is more efficacious at increased extracellular potassium concentrations resulting from heightened spontaneous activity.31,32 Carbamaze-pine (and placebo) capsules were administered at 21:00 on the experimental night and 24 hours before (2 × 200 mg), to minimize side effects. Flunarizine (and placebo) was administered as a single dose at 21:00 before sleep. At the respective doses peak plasma concentrations can be obtained approximately 2-4 hours after administration, which for flunarizine is closely comparable to those obtained at steady-state,33–35 and within this time period prominent effects within the central nervous system have been measured.36,37 Lights were turned off at 22:30 to allow for sleep until 06:00. The two sessions (verum or placebo) were separated by at least 10 days for each individual.
Recordings and Analyses
During the nights the EEG was recorded from midline electrode locations (Fz, Cz, Pz, referenced to a mastoid electrode, with the ground electrode on the forehead). Additional recordings from lateral sites (F3, F4, C3, C4, P3, P4) did not add essential information and will not be reported here. Also, the vertical and horizontal electrooculogram (EOG) and submental electromyogram (EMG) were recorded. Signals were amplified by a Toennies DC/AC amplifier (Jaeger, Germany, EEG, EOG: 0-35 Hz; EMG: 1-80 Hz, amplification 500 μV/V), and digitized with 16-bit precision at 250 Hz using a CED 1401 Plus (Cambridge Electronics Design, UK); channel offsets were subsequently set to zero (finite impulse response [FIR] filter, time constant = 1 sec; f = 0.159 Hz).
Analyses concentrated on the first 180 min of the sleep period because this contains most of SWS, which was the focus of this study. (In addition, study participants were in a restrained position in order to reduce movement artifacts for a measurement not reported here). Recordings were first analyzed according to standard polysomnographic criteria.38 Periods with movement time, movement arousals, and visually detected artifacts were excluded from further analysis. EEG power spectra were then computed separately for NREM Stage 2 sleep and SWS using the fast Fourier transform on nonoverlapping blocks of artifact-free EEG with a resolution of 4,096 data points (approximately 16.38 sec, frequency resolution of 0.061 Hz). On each data block a Hanning window was applied to taper start and end of the block. Spike 2 software (Cambridge Electronics Design, UK) was used for EEG data analysis. EEG power was calculated in the following bands: slow oscillation 0.5-1.0 Hz, delta 1.0-4.0 Hz, theta 5.0-9.0 Hz, slow spindle 8.0-12.0 Hz, fast spindle 12.0-16.0 Hz, and beta 16.0-25.0 Hz. Frequency bands were further subdivided to specify the exact frequency focus of effects. Discrete spindles within Stage 2 sleep and SWS were detected using an automatic algorithm10,39 implemented in Matlab R2008a (MathWorks, Natick, MA, USA). A band-pass FIR filter was applied to identify fast spindles (12.0-15.0 Hz) at Cz and the slow spindles (8.0-12.0 Hz) at Fz. Subsequently, the averaged root mean square of the signal was computed with a moving window of 0.2 sec. A fixed threshold was chosen per subject (carbamazepine study, slow spindles: 8.7 ± 1.2 μV, fast spindles: 4.1 ± 0.3 μV and flunarizine study, slow spindles: 8.9 ± 1.1 μV; fast spindles: 5.1 ± 0.4 μV) such that the average number of spindles detected per 30 sec approximated two in the placebo condition. A spindle was identified when the root mean square signal exceeded the threshold for a time period of 0.5-3.0 sec. Spindle density, number of detected spindles per 30 sec, spindle length, and amplitude were determined.
To further characterize the observed strong effects of carbamazepine on the slow oscillation frequency band, supplementary analyses were conducted to investigate the morphology of the slow oscillation. For this purpose, slow oscillations were detected automatically from Fz during SWS using a custom-made algorithm.39 In brief, data were first low-pass filtered using a FIR filter of 2 Hz, and zero crossings spaced 0.8-2.0 sec apart were detected. Amplitude thresholds for slow oscillation selection were set per subject such that the individual half-wave amplitudes were greater than two thirds of the mean half-wave amplitude in the placebo condition, which resulted in average thresholds of -45.3 ± 2.91 μV and 39.96 ± 2.53 μV, for the negative and positive half-waves, respectively. The pairs of negative and succeeding positive slow oscillation half-waves were subjected to amplitude and slope analysis. Besides half-wave amplitudes, slopes of the half-waves were computed. The times between the zero-to-zero crossings were used to assess the duration of the half-waves.
Data from two subjects in the carbamazepine study were excluded from analyses due to technical difficulties during recordings or loss of EEG data. Statistical analyses were based on analyses of variance (ANOVA) performed separately for both experiments, with the repeated measures factors Treatment (verum versus placebo) and Topography (Fz, Cz, Pz). In the case that in the ANOVA sphericity was violated, degrees of freedom were adjusted using the Greenhouse-Geisser correction. For post hoc comparisons Wilcoxon signed-rank tests were used because not all data were normally distributed (according to Kolmogorov-Smirnov tests).
RESULTS
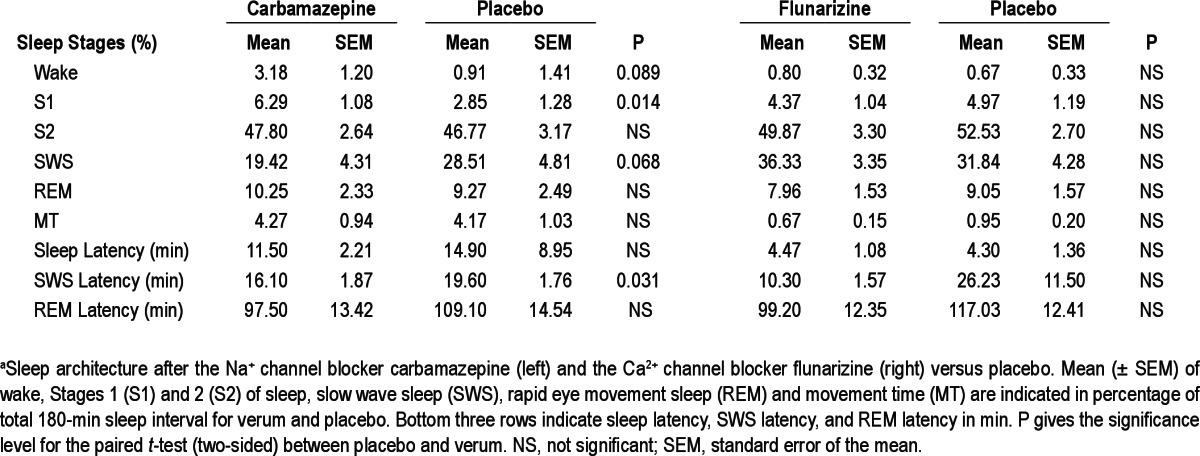
Table 1 reveals the amount of time spent in the different sleep stages as well as latencies for sleep onset, SWS, and rapid eye movement sleep. Following the Na+ channel antagonist carbamazepine, SWS latency was significantly reduced while total time spent in Stage 1 sleep across the 180-min sleep period was increased (P < 0.05). A mean reduction in time spent in SWS following carbamazepine as well as a slight increment in wakefulness failed to reach significance. Sleep architecture was not consistently altered after the Ca2+ channel antagonist flunarizine. In the group receiving flunarizine the data of one subject were excluded from SWS analyses because this stage was greatly reduced.
Table 1.
Sleep architecturea
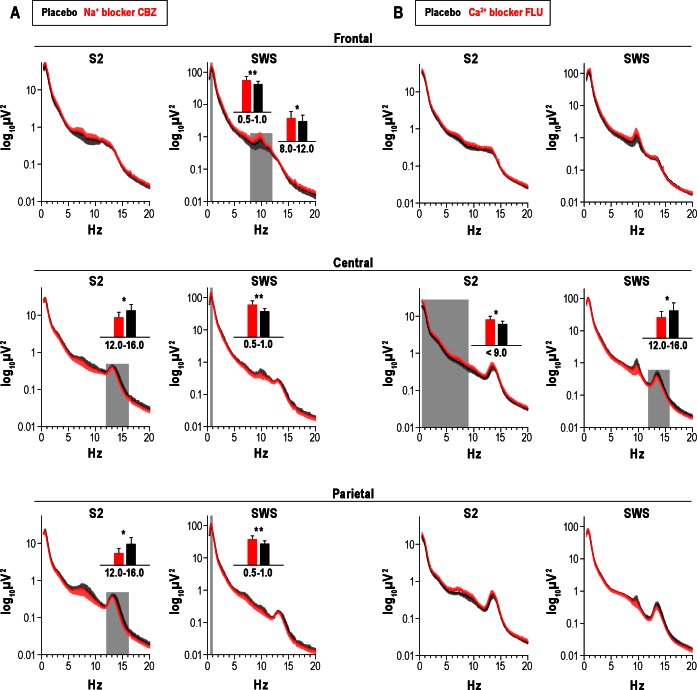
Analyses of EEG frequency bands in both experiments revealed the characteristic topographical distribution of power in the fast- and slow-spindle bands as well as in the slow oscillation band during Stage 2 sleep and SWS. Fast-spindle power dominated centroparietally (P < 0.01 for Topography main effect), whereas slow-spindle power showed a maximum at Fz with this effect restricted to SWS (P < 0.05, for Topography × Stage). Slow oscillation and delta power were greater during SWS than in Stage 2 sleep (P < 0.05) and decreased from anterior to posterior locations independent from sleep stage (P < 0.01).
Reduction in Na+ channels efficacy by carbamazepine suppressed fast-spindle activity at Cz and Pz during Stage 2 sleep (F(2,20) = 7.75, P < 0.01 for Topography main effect, P < 0.05 for pairwise comparisons at each electrode site, Figure 1A). This suppression in fast-spindle power during Stage 2 sleep accompanied a decrease in count, density, and amplitude of fast spindles at Cz (count: 263 ± 18 versus 336 ± 17, P < 0.05; density: 1.6 ± 0.1/sec versus 2.0 ± 0.0/sec, P < 0.01; amplitude: 19.5 ± 1.4 μV versus 20.0 ± 1.4 μV, P < 0.05; respectively). Importantly, opposite to the effect on fast spindles, the Na+ channel antagonist enhanced slow-spindle power at Fz during SWS (F(2,20) = 7.66, P < 0.05, for Topography main effect, P < 0.05 for pairwise comparison at Fz), which also coincided with an increase in slow-spindle count during SWS (226 ± 38 versus 118 ± 23, P < 0.05). Concurrently, carbamaze-pine distinctly increased slow oscillation power during SWS (F(1,10) = 15.17, P < 0.01, for Treatment main effect) with this effect being significant at all three midline sites (P < 0.05). As revealed in supplementary analyses, the positive (depolarizing) half-wave amplitude was significantly increased by carbamaze-pine (80.01 ± 4.73 μV versus 77.1 ± 4.5 μV, P < 0.05), and carbamazepine increased the steepness of the positive-half-wave downward slope (300.4 ± 20.6 μV/s versus 286.9 ± 20.9 μV/s Figure 2), P < 0.005).
Figure 1.
Mean (± standard error of the mean) power spectra for Stage 2 sleep (S2) and slow wave sleep (SWS) after the Na+ channel blocker carbamazepine, Na+ blocker CBZ (A) and the Ca2+ channel blocker flunarizine, Ca2+ blocker FLU (B). Frontal, central, and parietal correspond to the midline electroencephalographic recording sites Fz, Cz, and Pz. Gray areas indicate frequency bands revealing differences between verum and placebo in paired t-tests. Inserted bar charts give power on linear scale for significantly different frequency bands (*P < 0.05, **P < 0.01).
Figure 2.
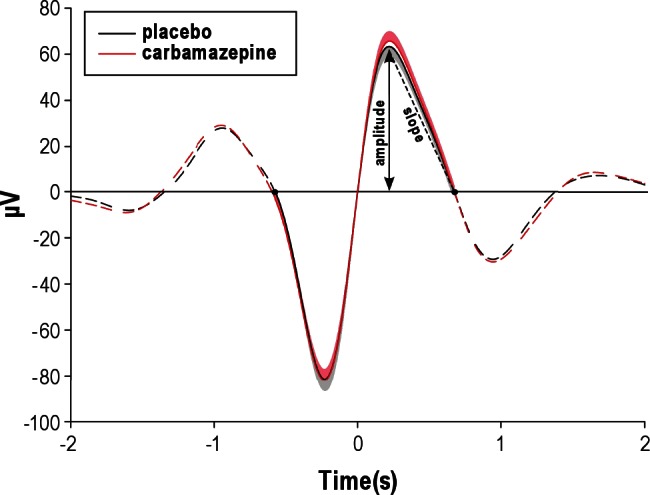
Mean (± standard error of the mean) time course of selected successive slow oscillation negative half-waves and positive half-waves time locked to the zero-crossing between negative and positive half-waves. The peak of the positive half-wave and the positive-half-wave downward slope are indicated. Black dots along the line at 0 μV demarcate beginning and end of the selected successive half-waves. Dashed lines represent the means of all previous and subsequent electroencephalographic activity.
Flunarizine administration revealed a response in the EEG frequency bands that differed from that after carbamazepine and overall appeared to be somewhat weaker (Figure 1B). Compared with placebo, the Ca2+ channel antagonist affected neither slow oscillation nor slow-spindle activity but modulated instead fast-spindle activity. Like carbamazepine, the Ca2+ channel antagonist decreased fast-spindle power, in particular during SWS at Cz (P < 0.05, F(2,26) = 13.41, P < 0.01, for Topography), and this effect was paralleled by a decrease in average amplitude (23.9 ± 2.0 μV versus 24.7 ± 2.2 μV, P < 0.05) and a tendency to reduce length (0.74 ± 0.02 s versus 0.77 ± 0.02 s, P = 0.09) of fast spindles at Cz during SWS. Interestingly, flunarizine also increased power of slow EEG frequencies below 9 Hz in Cz during Stage 2 sleep (P < 0.05, for pairwise comparisons with the slow oscillation, delta, and theta bands).
DISCUSSION
In humans a distinction is readily made between two types of sleep spindles primarily based on their frequency and topographic distribution. However, the differential underlying mechanisms are rarely discussed. The data presented here enable a pharmacologic distinction between the fast centro-parietal sleep spindles most pronounced in Stage 2 sleep and slow frontal sleep spindles most evident during SWS, and point to contrasting generating mechanism contributing to the two kinds of spindles.
Comodulation of Slow Spindles and Slow Oscillatory Activity But Not of Fast Spindles
The increase in slow frontal spindle EEG power and slow spindle count conjointly with an increase in slow oscillation power during SWS following the Na+ channel antagonist carbamazepine is most intriguing in light of recent findings that slow frontal spindle activity is associated in time with the downward negative phase of the slow oscillation.10 Typically, the more distinguishable centroparietal fast spindles are investigated in temporal relation to the slow oscillation, revealing a grouping effect around the up-state of the slow oscillation.10,15,39 Here, however, fast-spindle power during lighter Stage 2 sleep even decreased after carbamazepine. These opposite effects of reduced Na+ channel efficacy by carbamazepine on slow- and fast-spindle activity during SWS are reflected not only in EEG power, but also in the spindle count. An inverse dependence of slow and fast spindles on changes in membrane excitability is already indicated by the occurrence of the two spindle types at opposing phases of the slow oscillation.10
The transition into the slow oscillation up-state relies on the voltage-dependent persistent sodium current, which becomes activated through depolarization by miniature excitatory post-synaptic potentials (EPSPs) during the hyperpolarized slow oscillation phase. For the maintenance of cortical network activity during the depolarizing up-state, Na+ action potentials and also activation of the persistent Na+ channel are relevant.40 Carbamazepine reduces cortical neuronal excitability primarily by a voltage-dependent inhibition of the persistent Na+ current, and also slows the recovery rate from inactivation of the fast transient sodium current.27,28,41 Thus, at a first glance, the observed enhancement in slow oscillatory EEG power appears to be contrary to the expected effect of the Na+ channel antagonist. However, the effects could well be a consequence of a stronger pronunciation of the (hyperpolarizing) down state after carbamazepine. Our finding that the positive half-wave downward slope is stronger (steeper) after carbamazepine than placebo could be taken to support this notion, and also to suggest that mainly cortical activity reflected in the downward hyperpolarizing slow oscillation phase contributed to the enhanced EEG power in the slow oscillation band. The failure of the negative half- wave increment per se to reach significance in this context may reflect that network hyperpolarization reached saturation. Further corroboration for the concept that carbamazepine leads to a more pronounced up-to-down state component of the slow oscillation comes from the increased expression of slow spindles, which appear mostly on the downward phase of the slow oscillation.10 The increments in both slope and amplitude of the slow oscillation may result from a secondary inhibitory action of carbamzepine on activity-dependent potassium currents.42,43 Reduction in efficacy of Na+ channels by carbamazepine has been linked, e.g., via Na+ dependent potassium currents, to reduced potassium outflow that has been revealed to enhance both the recruitment of activity in a local network leading to a steeper down-to-up state slope as well as increments of the hyperpolarizing downward slope.44,45 At the low dosage a strong effect of carbamzepine on gamma-aminobutyric acid release is rather unlikely, although not to be completely excluded. Whether the pharmacologically induced steeper slopes of the slow oscillation and the conjoint increment in slow-spindle activity are associated with any increment in neuronal plasticity remains to be investigated.46–49
The decremental effect of carbamazepine on fast-spindle power and density in Stage 2 sleep could reflect a direct effect on thalamic neurons, which uphold a voltage-dependent Na+ conductance. Pharmacologic antagonism of the Na+ current that supports the postinhibitory depolarization leads in thalamocortical cells to a sustained hyperpolarization and to a loss of the rhythmic rebound effect in the spindle frequency range.27,50–52 The reduction in fast-spindle activity after carbamazepine may, however, also be a consequence of a decrease in cortical excitability and subsequently reduced corticothalamic input in thalamic spindle generator mechanisms. Computational models and in vivo data have shown that spindle initiation and termination, as well as spatially coherent thalamic spindle activity, are dependent on corticothalamic input; even the mid-phase of a spindle can become enhanced by corticothalamic feedback.19,53,54 Thus, if corticothalamic input decreases spindle initiation and maintenance likely decrease, as was found here for the fast centroparietal spindles.
The decrease in power and amplitude of fast spindles fits well with the ability of flunarizine to reduce the efficacy of voltage-dependent Ca2+ channels. Calcium conductances, in particular the T-type Ca2+ channel, play an essential role in thalamic spindle generation and maintenance.22,55–57 The slow frontal spindles remained, however, unaffected by flunarizine. This differential effect on the two spindle types suggests again a distinctive difference in the generating networks of the fast and slow spindles.
A reduction in spindle power in Cav3.1 (alpha1G) and Cav3.3 knockout mice with no reported effect on EEG slow oscillatory power is also seemingly in line with the suppression of thalamocortical fast- spindle power yet no apparent change in delta or slow oscillatory power, as found in the current study during SWS after flunarizine administration.58,59 Correspondence between the different spindle subtypes in humans and rodents have, however, not been systematically investigated. In contrast to the absence of changes in power of slower EEG rhythms by flunarizine in SWS Stage 2 sleep revealed an increment in slow wave activity. This may reflect the typical reciprocal relationship between fast- spindle power and slow wave activity attributed essentially to rhythmogenesis within the thalamocortical system and that can even become increasingly pronounced by pharmacologic intervention.13 The increment in theta power in parallel to that of slow wave activity is, however, an unexpected finding. The modulation of Ca2+ channel efficiency by flunarizine may be linked to the recently reported relevance of T channels within the thalamocortical circuit in the genesis of alpha and theta rhythms56,60 and/or to the ability of flunarizine to also modulate neurotransmitter activity.31,61,62
In summary, although the precise contribution(s) of membrane conductances to the oscillatory EEG networks during the brain states investigated here cannot be made, several important overall conclusions can yet be drawn: first, that sleep slow oscillatory and frontal slow spindles were pharmacologically comodulated. A comodulation was not found between the slow oscillatory and fast centroparietal EEG power. Second, the effects of flunarizine on EEG theta activity during Stage 2 sleep may be mediated by a third mode of thalamocortical firing. A refinement of these findings using more selective drugs also in animal models is suggested.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Dr. Michael A. Rogawski for advice regarding pharmacology and greatly acknowledge the work of Anja Niepelt and Britta Kannengiesser for help in preprocessing data. The studies were supported by grants from the German Research Foundation (DFG, SFB 654 “Plasticity and Sleep” and GSC 235/1, Graduate School for Computing in Medicine and Life Sciences) and the Federal Ministry of Education and Research (BMBF). The work was performed at the Department of Neuroendocrinology, University of Lübeck, Germany.
Footnotes
A commentary on this article appears in this issue on page 825.
REFERENCES
- 1.Timofeev I, Grenier F, Bazhenov M, Houweling AR, Sejnowski TJ, Steriade M. Short- and medium-term plasticity associated with augmenting responses in cortical slabs and spindles in intact cortex of cats in vivo. J Physiol. 2002;542:583–98. doi: 10.1113/jphysiol.2001.013479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schabus M, Gruber G, Parapatics S, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27:1479–85. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- 3.Fogel SM, Smith CT. Learning-dependent changes in sleep spindles and Stage 2 sleep. J Sleep Res. 2006;15:250–5. doi: 10.1111/j.1365-2869.2006.00522.x. [DOI] [PubMed] [Google Scholar]
- 4.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 5.Gibbs FA, Gibbs EL. Atlas of Electroencephalography. Cambridge: Addison-Wesley Press; 1950. [Google Scholar]
- 6.Caderas M, Niedermeyer E, Uematsu S, Long DM, Nastalski J. Sleep spindles recorded from deep cerebral structures in man. Clin Electroencephalogr. 1982;13:216–25. doi: 10.1177/155005948201300402. [DOI] [PubMed] [Google Scholar]
- 7.Werth E, Achermann P, Dijk DJ, Borbely AA. Spindle frequency activity in the sleep EEG: individual differences and topographic distribution. Electroencephalogr Clin Neurophysiol. 1997;103:535–42. doi: 10.1016/s0013-4694(97)00070-9. [DOI] [PubMed] [Google Scholar]
- 8.Knoblauch V, Krauchi K, Renz C, Wirz-Justice A, Cajochen C. Homeo-static control of slow-wave and spindle frequency activity during human sleep: effect of differential sleep pressure and brain topography. Cereb Cortex. 2002;12:1092–100. doi: 10.1093/cercor/12.10.1092. [DOI] [PubMed] [Google Scholar]
- 9.Mölle M, Marshall L, Gais S, Born J. Learning increases human electroencephalographic coherence during subsequent slow sleep oscillations. Proc Natl Acad Sci U S A. 2004;101:13963–8. doi: 10.1073/pnas.0402820101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mölle M, Bergmann TO, Marshall L, Born J. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep. 2011;34:1411–21. doi: 10.5665/SLEEP.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terrier G, Gottesmann CL. Study of cortical spindles during sleep in the rat. Brain Res Bull. 1978;3:701–6. doi: 10.1016/0361-9230(78)90021-7. [DOI] [PubMed] [Google Scholar]
- 12.Gandolfo G, Glin L, Gottesmann C. Study of sleep spindles in the rat: a new improvement. Acta Neurobiol Exp (Wars) 1985;45:151–62. [PubMed] [Google Scholar]
- 13.De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7:423–40. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- 14.Contreras D, Destexhe A, Sejnowski TJ, Steriade M. Control of spatio-temporal coherence of a thalamic oscillation by corticothalamic feedback. Science. 1996;274:771–4. doi: 10.1126/science.274.5288.771. [DOI] [PubMed] [Google Scholar]
- 15.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 16.Anderer P, Klosch G, Gruber G, et al. Low-resolution brain electromagnetic tomography revealed simultaneously active frontal and parietal sleep spindle sources in the human cortex. Neuroscience. 2001;103:581–92. doi: 10.1016/s0306-4522(01)00028-8. [DOI] [PubMed] [Google Scholar]
- 17.Doran MS. The dynamic topography of individual sleep spindles. Sleep Research Online. 2003;5:133–9. [Google Scholar]
- 18.Schabus M, ng-Vu TT, Albouy G, et al. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2007;104:13164–9. doi: 10.1073/pnas.0703084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonjean M, Baker T, Lemieux M, Timofeev I, Sejnowski T, Bazhenov M. Corticothalamic feedback controls sleep spindle duration in vivo. J Neurosci. 2011;31:9124–34. doi: 10.1523/JNEUROSCI.0077-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dehghani N, Cash SS, Halgren E. Topographical frequency dynamics within EEG and MEG sleep spindles. Clin Neurophysiol. 2011;122:229–35. doi: 10.1016/j.clinph.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn Sci. 2007;11:442–50. doi: 10.1016/j.tics.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Model of thalamo-cortical slow-wave sleep oscillations and transitions to activated States. J Neurosci. 2002;22:8691–704. doi: 10.1523/JNEUROSCI.22-19-08691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steriade M, Timofeev I. Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron. 2003;37:563–76. doi: 10.1016/s0896-6273(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 24.Destexhe A, Hughes SW, Rudolph M, Crunelli V. Are corticothalamic ‘up’ states fragments of wakefulness? Trends Neurosci. 2007;30:334–42. doi: 10.1016/j.tins.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crunelli V, Hughes SW. The slow (< 1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat Neurosci. 2010;13:9–17. doi: 10.1038/nn.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo CC, Chen RS, Lu L, Chen RC. Carbamazepine inhibition of neuronal Na+ currents: quantitative distinction from phenytoin and possible therapeutic implications. Mol Pharmacol. 1997;51:1077–83. doi: 10.1124/mol.51.6.1077. [DOI] [PubMed] [Google Scholar]
- 27.Landmark CJ. Targets for antiepileptic drugs in the synapse. Med Sci Monit. 2007;13:RA1–7. [PubMed] [Google Scholar]
- 28.Sun GC, Werkman TR, Battefeld A, Clare JJ, Wadman WJ. Carbamaze-pine and topiramate modulation of transient and persistent sodium currents studied in HEK293 cells expressing the Na(v)1.3 alpha-subunit. Epilepsia. 2007;48:774–82. doi: 10.1111/j.1528-1167.2007.01001.x. [DOI] [PubMed] [Google Scholar]
- 29.Porter RJ, Dhir A, Macdonald RL, Rogawski MA. Mechanisms of action of antiseizure drugs. In: Stefan H, Theodore W H, editors. Handbook of clinical neurology, 108. Epilepsy Part II: Treatment. Elsevier Science and Technology; 2012. in press. [DOI] [PubMed] [Google Scholar]
- 30.Peters T, Wilffert B, Vanhoutte PM, van Zwieten PA. Calcium channels in the brain as targets for the calcium-channel modulators used in the treatment of neurological disorders. J Cardiovasc Pharmacol. 1991;18:S1–5. [PubMed] [Google Scholar]
- 31.Golumbek PT, Rho JM, Spain WJ, van Brederode JF. Effects of flunarizine on spontaneous synaptic currents in rat neocortex. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:176–82. doi: 10.1007/s00210-004-0968-8. [DOI] [PubMed] [Google Scholar]
- 32.Ye Q, Yan LY, Xue LJ, et al. Flunarizine blocks voltage-gated Na(+) and Ca(2+) currents in cultured rat cortical neurons: A possible locus of action in the prevention of migraine. Neurosci Lett. 2011;487:394–9. doi: 10.1016/j.neulet.2010.10.064. [DOI] [PubMed] [Google Scholar]
- 33.Géradin AP, Abadie FV, Campestrini JA, Theobald W. Pharmacokinetics of carbamazepine in normal humans after single and repeated oral doses. J Pharmacokinet Biopharm. 1976;4:521–35. doi: 10.1007/BF01064556. [DOI] [PubMed] [Google Scholar]
- 34.Pynnönen S. Pharmacokinetics of carbamazepine in man: a review. Ther Drug Monit. 1979;1:409–31. doi: 10.1097/00007691-197901030-00014. [DOI] [PubMed] [Google Scholar]
- 35.Holmes B, Brogden RN, Heel RC, Speight TM, Avery GS. Flunarizine. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use. Drugs. 1984;27:6–44. doi: 10.2165/00003495-198427010-00002. [DOI] [PubMed] [Google Scholar]
- 36.Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996;40:367–78. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- 37.Nitsche MA, Fricke K, Henschke U, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington: NIH Publ. 204, US Government Printing Office; 1968. [Google Scholar]
- 39.Mölle M, Marshall L, Gais S, Born J. Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. J Neurosci. 2002;22:10941–7. doi: 10.1523/JNEUROSCI.22-24-10941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Timofeev I, Grenier F, Steriade M. Impact of intrinsic properties and synaptic factors on the activity of neocortical networks in vivo. J Physiol Paris. 2000;94:343–55. doi: 10.1016/s0928-4257(00)01097-4. [DOI] [PubMed] [Google Scholar]
- 41.Macdonald RL, Kelly KM. Antiepileptic drug mechanisms of action. Epilepsia. 1993;34:S1–8. doi: 10.1111/j.1528-1157.1993.tb05918.x. [DOI] [PubMed] [Google Scholar]
- 42.Dreixler JC, Bian J, Cao Y, Roberts MT, Roizen JD, Houamed KM. Block of rat brain recombinant SK channels by tricyclic antidepressants and related compounds. Eur J Pharmacol. 2000;401:1–7. doi: 10.1016/s0014-2999(00)00401-5. [DOI] [PubMed] [Google Scholar]
- 43.Ambrosio AF, Soares-Da-Silva P, Carvalho CM, Carvalho AP. Mechanisms of action of carbamazepine and its derivatives, oxcarbazepine, BIA 2-093, and BIA 2-024. Neurochem Res. 2002;27:121–30. doi: 10.1023/a:1014814924965. [DOI] [PubMed] [Google Scholar]
- 44.Compte A, Sanchez-Vives MV, McCormick DA, Wang XJ. Cellular and network mechanisms of slow oscillatory activity (< 1 Hz) and wave propagations in a cortical network model. J Neurophysiol. 2003;89:2707–25. doi: 10.1152/jn.00845.2002. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez-Vives MV, Mattia M, Compte A, et al. Inhibitory modulation of cortical up states. J Neurophysiol. 2010;104:1314–24. doi: 10.1152/jn.00178.2010. [DOI] [PubMed] [Google Scholar]
- 46.Jokeit H, Okujava M, Woermann FG. Carbamazepine reduces memory induced activation of mesial temporal lobe structures: a pharmacological fMRI-study. BMC Neurol. 2001;1:6. doi: 10.1186/1471-2377-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bernardi RB, Barros HM. Carbamazepine enhances discriminative memory in a rat model of epilepsy. Epilepsia. 2004;45:1443–7. doi: 10.1111/j.0013-9580.2004.52403.x. [DOI] [PubMed] [Google Scholar]
- 48.Li Q, Jin CL, Xu LS, et al. Histidine enhances carbamazepine action against seizures and improves spatial memory deficits induced by chronic transauricular kindling in rats. Acta Pharmacol Sin. 2005;26:1297–302. doi: 10.1111/j.1745-7254.2005.00220.x. [DOI] [PubMed] [Google Scholar]
- 49.Esser SK, Hill SL, Tononi G. Sleep homeostasis and cortical synchronization: I. Modeling the effects of synaptic strength on sleep slow waves. Sleep. 2007;30:1617–30. doi: 10.1093/sleep/30.12.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–85. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 51.McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 52.Steriade M, McCarley RW. Brain control of wakefulness and sleep. New York: Plenum Publishers; 2005. [Google Scholar]
- 53.Contreras D, Destexhe A, Sejnowski TJ, Steriade M. Spatiotemporal patterns of spindle oscillations in cortex and thalamus. J Neurosci. 1997;17:1179–96. doi: 10.1523/JNEUROSCI.17-03-01179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Destexhe A, Contreras D, Steriade M. Mechanisms underlying the synchronizing action of corticothalamic feedback through inhibition of thalamic relay cells. J Neurophysiol. 1998;79:999–1016. doi: 10.1152/jn.1998.79.2.999. [DOI] [PubMed] [Google Scholar]
- 55.Huguenard JR, McCormick DA. Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends Neurosci. 2007;30:350–6. doi: 10.1016/j.tins.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 56.Crunelli V, Cope DW, Hughes SW. Thalamic T-type Ca2+ channels and NREM sleep. Cell Calcium. 2006;40:175–90. doi: 10.1016/j.ceca.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blethyn KL, Hughes SW, Toth TI, Cope DW, Crunelli V. Neuronal basis of the slow (< 1 Hz) oscillation in neurons of the nucleus reticularis thalami in vitro. J Neurosci. 2006;26:2474–86. doi: 10.1523/JNEUROSCI.3607-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee J, Kim D, Shin HS. Lack of delta waves and sleep disturbances during non-rapid eye movement sleep in mice lacking alpha1G-subunit of T-type calcium channels. Proc Natl Acad Sci U S A. 2004;101:18195–9. doi: 10.1073/pnas.0408089101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Astori S, Wimmer RD, Prosser HM, et al. The CaV3.3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc Natl Acad Sci U S A. 2011;108:13823–8. doi: 10.1073/pnas.1105115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hughes SW, Lorincz M, Cope DW, et al. Synchronized oscillations at alpha and theta frequencies in the lateral geniculate nucleus. Neuron. 2004;42:253–68. doi: 10.1016/s0896-6273(04)00191-6. [DOI] [PubMed] [Google Scholar]
- 61.Zakir Hossain SM, Shinohara H, Kitano H. Drug assessment based on detection of L-glutamate released from C6 glioma cells using an enzyme-luminescence method. Anal Chem. 2008;80:3762–8. doi: 10.1021/ac702392p. [DOI] [PubMed] [Google Scholar]
- 62.Ferrari A, Spaccapelo L, Pinetti D, Tacchi R, Bertolini A. Effective prophylactic treatments of migraine lower plasma glutamate levels. Cephalalgia. 2009;29:423–9. doi: 10.1111/j.1468-2982.2008.01749.x. [DOI] [PubMed] [Google Scholar]