Abstract
Background:
Obstructive sleep apnea (OSA) is a common health problem in children and increases the risk of cardiovascular disease (CVD). Triggering receptor expressed on myeloid cells-1 (TREM-1) plays an important role in innate immunity and amplifies inflammatory responses. Pentraxin-3 is predominantly released from macrophages and vascular endothelial cells, plays an important role in atherogenesis, and has emerged as a biomarker of CVD risk. Thus, we hypothesized that plasma TREM-1 and pentraxin-3 levels would be elevated in children with OSA.
Methods:
One hundred six children (mean age: 8.3 ± 1.6 y) were included after they underwent overnight polysomnographic evaluation and a fasting blood sample was drawn the morning after the sleep study. Endothelial function was assessed with a modified hyperemic test after cuff-induced occlusion of the brachial artery. Plasma TREM-1 and pentraxin-3 levels were assayed using commercial enzyme-linked immunosorbent assay kits. Circulating microparticles (MPs) were assessed using flow cytometry after staining with cell-specific antibodies.
Results:
Children with OSA had significantly higher TREM-1 and pentraxin-3 levels (versus controls: P < 0.01, P < 0.05, respectively). Plasma TREM-1 was significantly correlated with both body mass index (BMI)-z score and the obstructive apnea-hypopnea index (AHI) in univariate models. Pentraxin-3 levels were inversely correlated with BMI-z score (r = -0.245, P < 0.01), and positively associated with endothelial MPs and platelet MPs (r = 0.230, P < 0.01 and r = 0.302, P < 0.01). Both plasma TREM-1 and pentraxin-3 levels were independently associated with AHI in multivariate models after controlling for age, sex, race, and BMI-z score (P < 0.001 for TREM-1 and P < 0.001 for pentraxin-3). However, no significant associations emerged between TREM-1, pentraxin-3, and endothelial function.
Conclusions:
Plasma TREM-1 and pentraxin-3 levels are elevated in pediatric OSA, and may play a role in modulating the degree of systemic inflammation. The short-term and long-term significance of elevated TREM-1 and pentraxin-3 in OSA-induced end-organ morbidity remains to be defined.
Citation:
Kim J; Gozal D; Bhattacharjee R; Kheirandish-Gozal L. TREM-1 and pentraxin-3 plasma levels and their association with obstructive sleep apnea, obesity, and endothelial function in children. SLEEP 2013;36(6):923-931.
Keywords: Endothelial function, inflammation, obesity, obstructive sleep apnea, pentraxin-3, triggering receptor expressed on myeloid cells-1
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by repeated events of partial and complete upper airway obstruction during sleep that result in disruption of normal ventilation, hypoxemia, and sleep fragmentation. In recent years, OSA has emerged as a chronic low-grade inflammatory disease,1 and increasing evidence supports a pathophysiologic link between pediatric OSA and cardiovascular diseases (CVD) such as hypertension, endothelial dysfunction, and atherosclerosis.1–4
Triggering receptor expressed on myeloid cells-1 (TREM) is a transmembrane glycoprotein that is expressed in neutrophils and in subsets of monocytes and macrophages, i.e., the main effector cells of the innate immune response.5,6 TREM-1 not only plays an important role in innate immunity but also functions to amplify inflammatory responses, particularly in vascular beds.7 Recently Hermus and colleagues8 revealed that soluble TREM-1 (sTREM-1) levels are increased in patients with coronary artery disease, suggesting that TREM-1 might be a potential biomarker that reflects carotid plaque instability.
Pentraxin-3 is an evolutionally conserved and acute phase inflammatory glycoprotein that belongs to the same family of the now well-established cardiovascular biomarker C-reactive protein (CRP).9,10 Recent studies have shown that pentraxin-3, which is predominantly released from macrophages and vascular endothelial cells, is considered to as a good reporter of processes underlying atherogenesis, and could also provide a better predictive biomarker of CVD when compared with CRP.10,11 Kasai et al. have recently reported that pentraxin-3 levels are elevated in adult patients with moderate to severe OSA, and that pentraxin-3 levels were also significantly associated with the cardio-ankle vascular index, suggesting that pentraxin-3 concentrations may reflect early vascular damage associated with OSA.12 However, no studies on either TREM-1 or pentraxin-3 have been performed in children with OSA, despite the now well-established evidence that this condition increases the risk for hypertension and is strongly associated with endothelial dysfunction.13
Not surprisingly, there is growing interest in the identification of biomarkers that can serve as early reliable detectors of CVD risk, under the assumption that timely interventions could reduce the CVD risk. Based on aforementioned considerations, we hypothesized that plasma TREM-1 and pentraxin-3 levels would be elevated in pediatric OSA and be associated with previously reported cardiovascular risk factors in such children.
METHODS
Patients
The study was approved by the University of Louisville (protocol #474.99) and by the University of Chicago (protocol #10-708A) Human Research Committees, and informed consent was obtained from the legal caregiver of each participant. Inclusion criteria were the presence of OSA according to polysomnographic criteria and age between 5 and 10 y. Furthermore, age-, sex-, and ethnicity-matched healthy nonsnoring children without OSA who underwent overnight polysomnography were also invited to participate in the study. Children were excluded if they had known diabetes or prediabetes, any defined genetic abnormality or underlying systemic disease, or if they were within acute infectious processes. The diagnosis of children with mild and moderate to severe OSA was defined by the presence of an obstructive apnea-hypopnea index (AHI) ≥ 1/h of total sleep time and AHI ≥ 5/h of total sleep time, respectively. Control children had AHI < 1/h of total sleep time.
Anthropometry
Children were weighed in a calibrated scale to the nearest 0.1 kg, and height (to 0.1 cm) was measured with a stadiometer (Holtain, Crymych, UK). Body mass index (BMI) was calculated and BMI-z score was computed using Centers for Disease Control and Prevention (CDC) 2000 growth standards (www.cdc.gov/growthcharts) and online software (www.cdc.gov/epiinfo). A BMI-z score > 1.65 (> 95th percentile) was considered as fulfilling obese criteria.
Overnight Polysomnographic Evaluation
Overnight polysomnography was conducted and scored as previously described.14–16 Central, obstructive, and mixed apneic events were counted. Obstructive apnea was defined as the absence of airflow with continued chest wall and abdominal movement for duration of at least two breaths. Hypopneas were defined as a decrease in oronasal flow of ≥ 50% with a corresponding decrease in oxyhemoglobin saturation measured by pulse oximetry (SpO2) of ≥ 4% or more and/or an arousal. The obstructive AHI was defined as the number of obstructive apneas and hypopneas per hour of total sleep time. Arousals were identified as defined by the American Sleep Disorders Association Task Force report.17,18
Endothelial Function Tests
Endothelial function was assessed using a modified hyperemic test after cuff-induced occlusion of the radial and ulnar arteries by placing the cuff over the wrist as previously described.13,19 In brief, a laser Doppler sensor (Perimed AB, Periflux 5000 System integrated with the PF 5050 pressure unit, Järfälla, Sweden) was applied over the volar aspect of the hand at the second-finger distal metacarpal surface, and the hand was gently immobilized. Once cutaneous blood flow over the area was stable, the pressure within an inflatable cuff placed distal to the elbow and connected to a computer-controlled manometer was raised to 160-180 mm Hg for 60 sec, during which blood flow was reduced to undetectable levels. To enable consistent deflation times, the cuff was deflated under computer control and hyperemic responses were assessed. Time to peak blood flow following relief of occlusion was considered representative of the postocclusion hyperemic response.20
Circulating Microparticles in Plasma
Circulating microparticles (MPs) were assessed using standard methods as previously described.21 In brief, flow cytometry (FACS) analysis was performed using settings at logarithmic gain where thresholds were lowered to 200, and forward scatter (FSC) and side scatter (SSC) gates were drawn to include events smaller than 1 μm. Endothelial MPs were defined as CD31+ or CD62E+ after gating for the CD42b- population, because CD31 occurs on both platelet MPs and endothelial MPs but CD42b occurs only on platelets. Endothelial progenitor MPs were also defined as CD34+ and CD309+ (vascular endothelial growth factor receptor-2) population. Moreover, CD45+, CD11b+, and CD41a+ were used to identify leukocyte MPs and platelet MPs. Data were acquired on a FACS Canto II cytometer using the FACS Diva 5.5 software (BD Biosciences, San Jose, CA). The results were analyzed by FlowJo software (Tree Star, San Carlos, CA).
Lipid Profile and Plasma High-Sensitivity CRP, Myeloid-Related-Protein 8/14, TREM-1, and Pentraxin-3 Levels
Fasting blood samples were drawn by venipuncture in the morning after the sleep study. Blood samples were immediately centrifuged and frozen at -80°C until assays were performed. Plasma myeloid-related protein (MRP) 8/14, TREM-1, and pentraxin-3 levels were measured using commercial enzyme-linked immunosorbent assay kits (ALPCO Diagnostics, Salem, NH for MRP 8/14 and R&D systems, Minneapolis, MN for TREM-1 and pentraxin-3). MRP 8/14, TREM-1, and pentraxin-3 assays have sensitivities of 0.4 μg/mL, 3.88 pg/mL, and 0.07 ng/mL, respectively. The interassay and intra-assay coefficients of variability for MRP 8/14 were 6.4% and 4.8%. For TREM-1 and pentraxin-3, the assays had an intra-assay coefficient of variability of 5.8% and 6.6%, and an interassay coefficient of variability of 8.2% and 7.3%. High-sensitivity CRP (hsCRP) was measured within 2-3 h after collection using the Flex reagent cartridge (Date Behring, Newark, DE), which is based on a particle-enhanced turbidimetric immunoassay technique. Serum levels of lipids, including total cholesterol, high-density lipoprotein (HDL) cholesterol, calculated low-density lipoprotein (LDL) cholesterol, and triglycerides, were also assessed with a Flex reagent cartridge.
Statistical Analysis
Data were expressed by mean ± standard deviation. Signifi-cant differences within groups were analyzed using analysis of variance for continuous variables and chi-square tests for categorical variables. Bonferroni corrections were applied for multiple comparisons. If the data were not normally distributed, data were logarithmically transformed. Pearson correlation analyses were performed to examine the association between four inflammatory markers (hsCRP, MRP 8/14, TREM-1, and pentraxin-3) and other related variables. Multiple stepwise regression analyses were then conducted while treating TREM-1 and pentraxin-3 as dependent variables in relation to AHI and covariates. Statistical analyses were performed using SPSS software (version 17.0; SPPS Inc., Chicago, IL). All P values reported are two-tailed with statistical significance P < 0.05.
RESULTS
General Characteristics of the Study Population
One hundred six children were included in this study. The demographic, polysomnographic, and biochemical characteristics of the cohort are shown in Tables 1 and 2. Mean age, sex, and ethnic distribution were similar across the three OSA severity groups (P > 0.05). Mean AHI of children with mild OSA and those with moderate to severe OSA was 2.04 and 15.5, respectively. The levels of four inflammatory markers according to severity of OSA are shown in Table 1. hsCRP and MRP 8/14 levels showed significant differences in agreement with our previous reports.1,22 Log TREM-1 and log pentraxin-3 also revealed significant differences according to the categorical severity of OSA (Table 1, moderate to severe OSA versus mild OSA versus control for log TREM-1: 2.09 ± 0.04 versus 2.00 ± 0.03 versus 2.00 ± 0.04, P < 0.01; moderate to severe OSA versus mild OSA versus control for log pentraxin-3, -0.10 ± 0.25 versus -0.28 ± 0.22 versus -0.34 ± 0.22, P < 0.05).
Table 1.
Characteristics of children with obstructive sleep apnea and healthy control patients
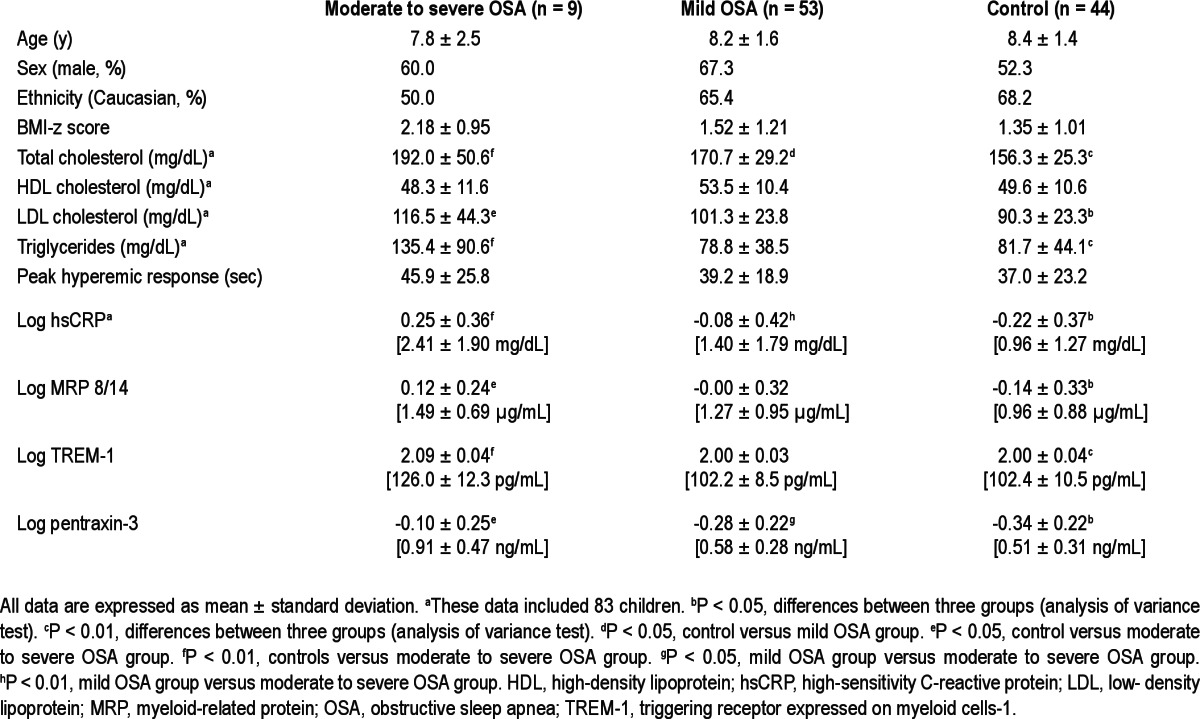
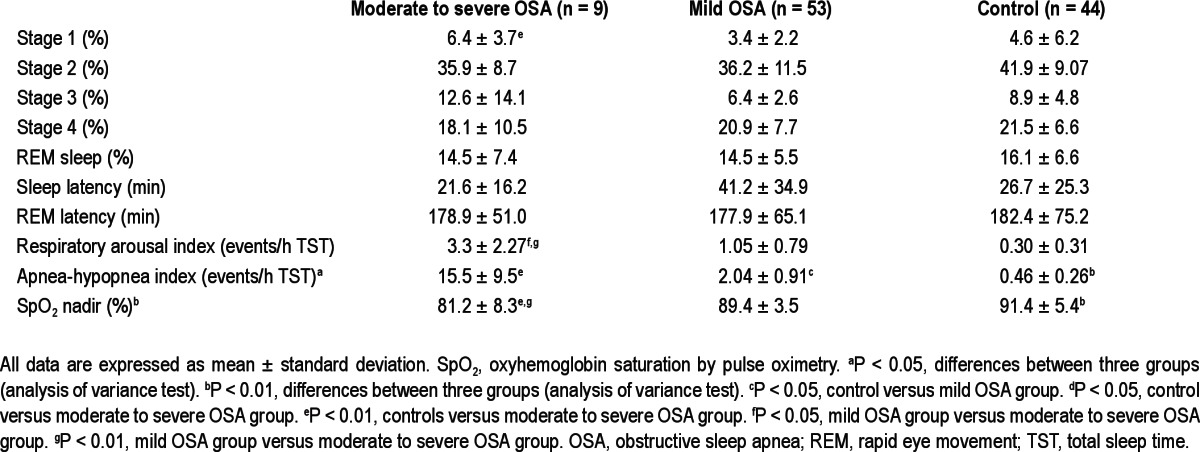
Table 2.
Polysomnographic data of children with obstructive sleep apnea and healthy control patients
TREM-1 and Pentraxin-3 in Children with OSA
Log TREM-1 and log pentraxin-3 levels were stratified according to the severity of OSA and the presence or absence of obesity. Mean age, sex, and ethnic distribution were not significantly different across the various OSA severity-based groups in the presence or absence of obesity (P > 0.05). No differences in mean BMI-z scores emerged based on OSA severity among the obese children (moderate to severe OSA versus mild OSA versus control: 2.55 ± 0.53 versus 2.37 ± 0.28 versus 2.2 ± 0.28, P = 0.058) and among the nonobese children (moderate to severe OSA versus mild OSA versus control: 0.59 ± 0.28 versus 0.37 ± 1.03 versus 0.56 ± 0.73, P > 0.05). As shown in Figures 1 and 2, increases of log TREM-1 and log pentraxin-3 levels emerged based on AHI categories regardless of obesity. Moreover, children with moderate to severe OSA had the highest log TREM-1 levels compared with those of obese and nonobese children (Figure 1, moderate to severe OSA versus control in obese children. 2.21 ± 0.03 versus 2.02 ± 0.05, P < 0.01, in nonobese children, 2.05 ± 0.04 versus 1.99 ± 0.04, P < 0.01). In addition, log pentraxin-3 levels showed similar results in obese children as well, but findings did not achieve statistical significance in nonobese children. (Figure 2, moderate to severe OSA versus control in obese children, -0.10 ± 0.28 versus -0.43 ± 0.22, P < 0.01, in nonobese children, -0.11 ± 0.16 versus -0.26 ± 0.20, P > 0.05).
Figure 1.
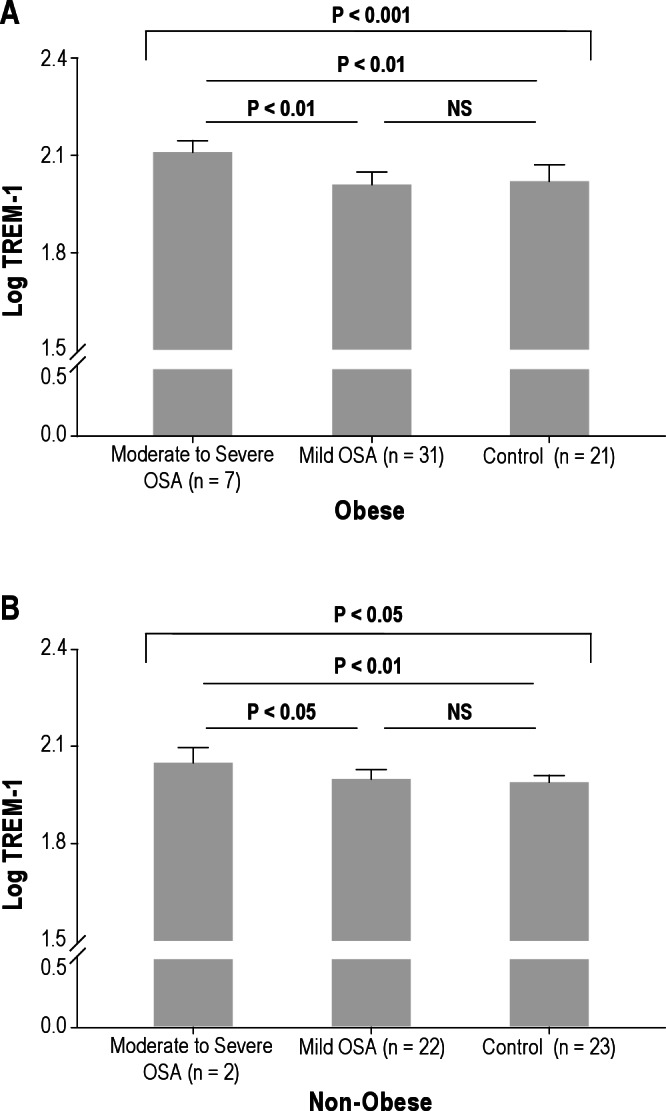
Log triggering receptor expressed on myeloid cells-1 (TREM-1) levels in children with obstructive sleep apnea and control patients among obese (A) and nonobese children (B). Obese children were defined as having a body mass index-z score > 1.65.
Figure 2.
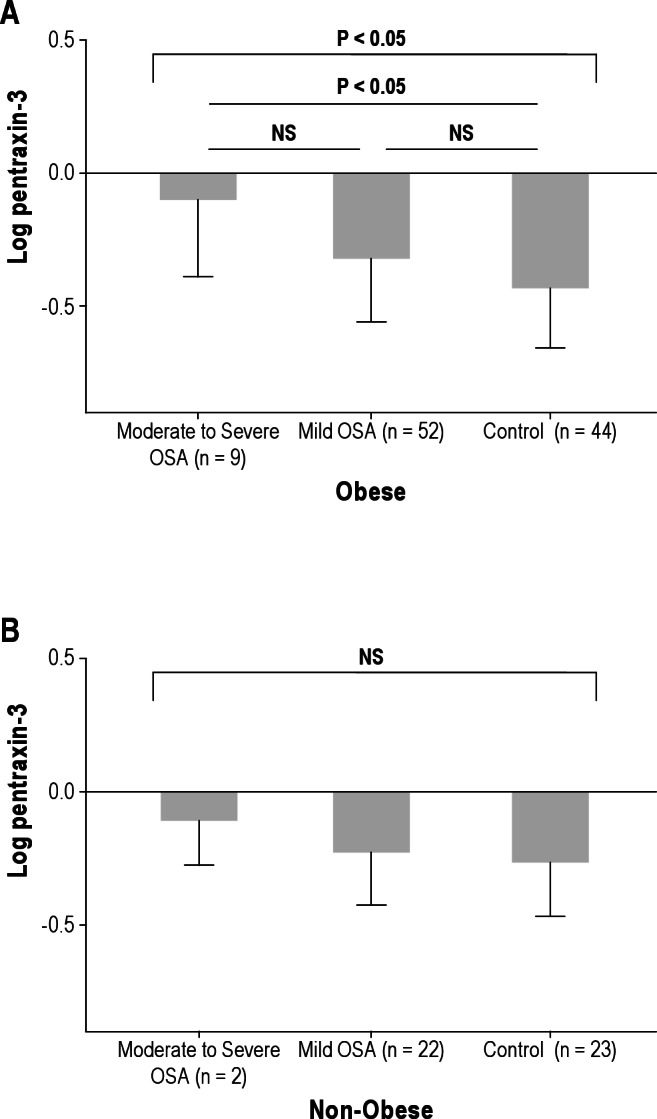
Log pentraxin-3 levels in children with obstructive sleep apnea and control patients among obese (A) and nonobese children (B). Obese children were defined as having a body mass index-z score > 1.65. NS, not significant.
Correlations between Inflammatory Markers, Circulating MPs, Lipid Profiles, Endothelial Function, and Polysomnographic Measures
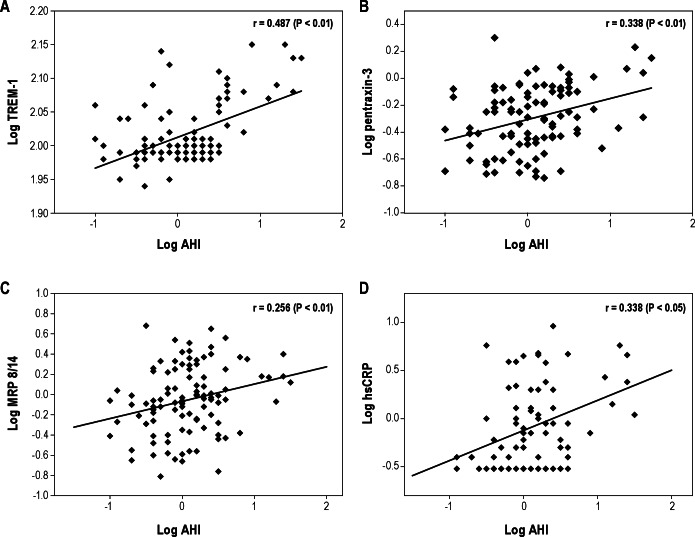
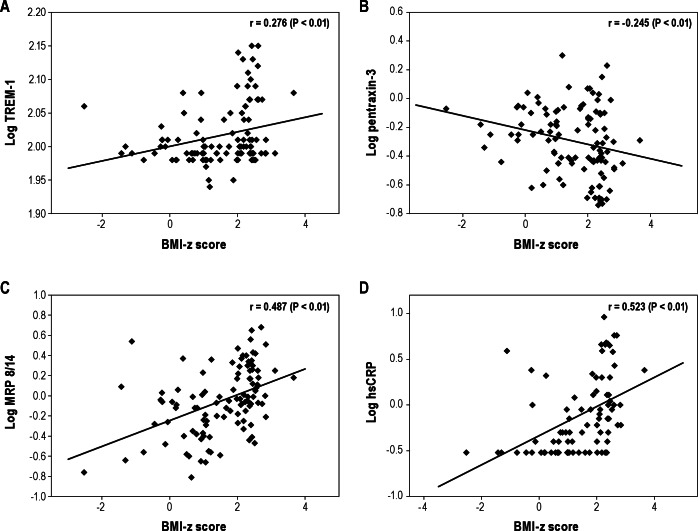
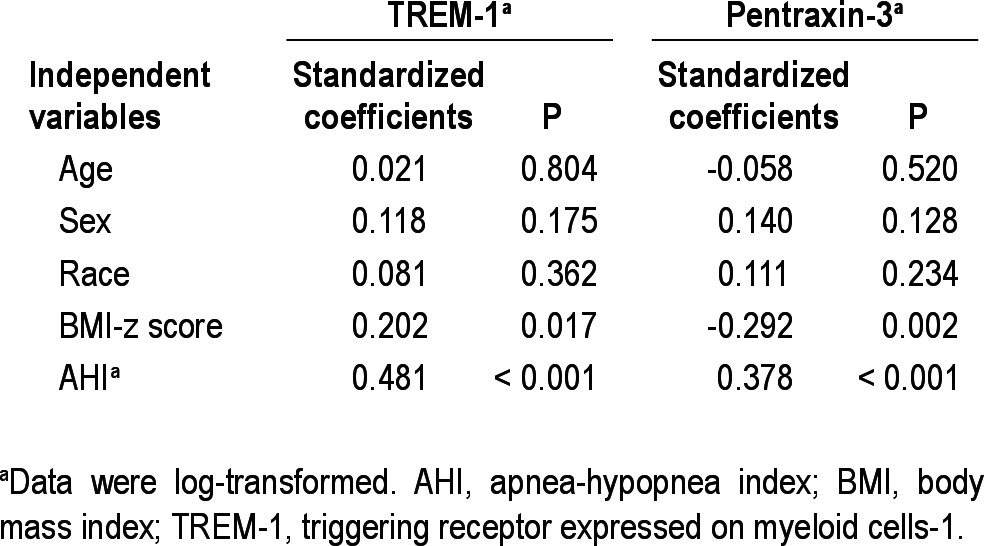
To estimate potential associations between the four inflammatory markers studied herein and polysomnographic measures, endothelial function, and various MPs, we initially performed Pearson correlation analyses (Figures 3 and 4). A significant linear correlation between log TREM-1 and log pentraxin-3 and log AHI (Figure 3A, r = 0.487, P < 0.01 for log TREM-1, Figure 3B, r = 0.338, P < 0.01 for log pentraxin-1) emerged. Log TREM-1 was positively correlated with BMI-z score (Figure 4A, r = 0.276, P < 0.01), but log pentraxin-3 was inversely associated with BMI-z score (Figure 4B, r = -0.245, P < 0.01). Furthermore, log hsCRP and log MRP 8/14 levels were significantly and positively correlated with the time to peak reperfusion in the context of postocclusive hyperemic responses (Table 3, r = 0.258, P < 0.01 and r = 0.223, P < 0.05, respectively). However, log hsCRP and log MRP 8/14 levels did not show any significant association with the various cellular MPs subsets. Interestingly, both log TREM-1 and log pentraxin-3 levels were significantly correlated with log platelet MPs (Table 3, r = 0.243, P < 0.05 and r = 0.302, P < 0.01). Furthermore, log pentraxin-3 level showed significant associations with log endothelial MPs, log endothelial MPs, and log monocyte MPs (Table 3, r = 0.230, P < 0.05 for log endothelial MPs, r = 0.269, P < 0.01 for log endothelial progenitor MPs, r = 0.242, P < 0.05 for monocyte MPs). To further explore independent predictors of log TREM-1 and log pentraxin-3 levels, we performed stepwise multiple regression analyses. In the stepwise multiple regression model, AHI was independently associated with TREM-1 levels (Table 4, standardized coefficient; 0.481, P < 0.001) and with pentraxin-3 levels (Table 4, standardized coefficient; 0.378, P < 0.001), and accounted for 29.9% and 20.8% of the variance in each biomarker respectively, after controlling for age, sex, race, and BMI-z score.
Figure 3.
Scatterplots of individual log triggering receptor expressed on myeloid cells-1. (TREM-1) (A), pentraxin-3 (B), myeloid-related protein (MRP) 8/14 (C), and high-sensitivity C-reactive protein (hsCRP) levels (D) plotted against corresponding log apnea-hypopnea index (AHI) levels. Significant linear correlations between log AHI and log TREM-1 (r = 0.487, P < 0.01), log pentraxin-3 (r = 0.338, P < 0.01), log MRP 8/14 (r = 0.256, P < 0.01), and log hsCRP (r = 0.338, P < 0.05) emerged.
Figure 4.
Scatterplots of individual log triggering receptor expressed on myeloid cells-1 (TREM-1) (A), pentraxin-3 (B), myeloid-related protein (MRP) 8/14 (C), and high-sensitivity C-reactive protein (hsCRP) (D) levels plotted against corresponding body mass index (BMI)-z scores. A significant positive linear correlation between BMI-z score and log TREM-1 (r = 0.276, P < 0.01), log MRP 8/14 (r = 0.487, P < 0.01), and log hsCRP (r = 0.523, P < 0.01) emerged and a significant negative linear correlation between BMI-z score and log pentraxin-3 (r = -0.245, P < 0.01) was apparent.
Table 3.
Correlation coefficients between TREM-1, pentraxin-3, MRP 8/14, and hsCRP levels and various variables in children
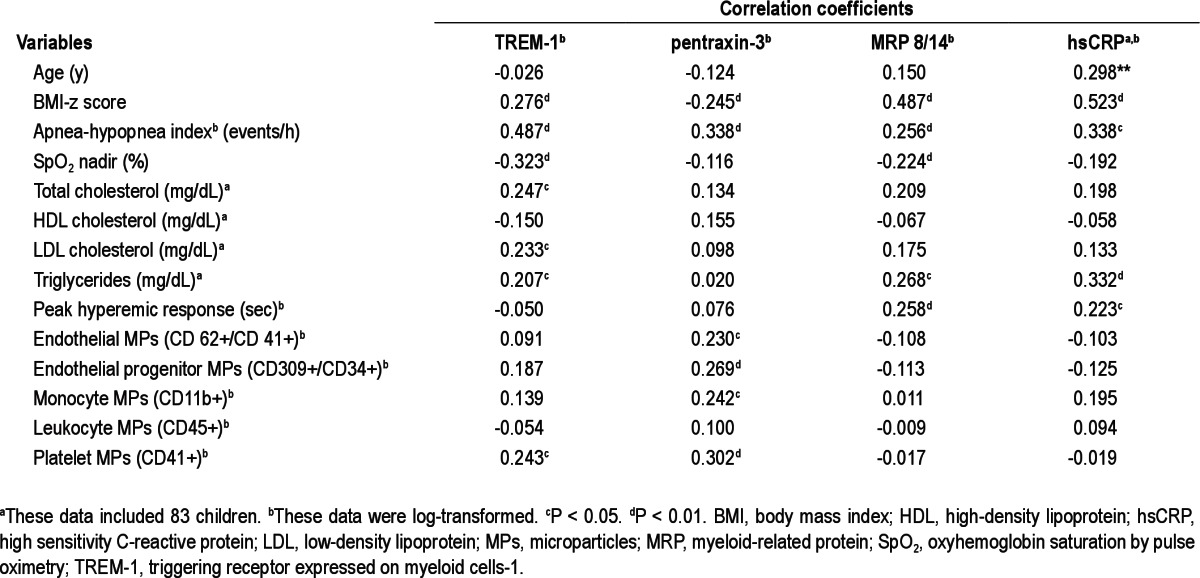
Table 4.
Multivariate regression analyses between apnea-hypopnea index and TREM-1 and pentraxin-3 levels
DISCUSSION
In the current study, we found that children with OSA have elevated plasma TREM-1 and pentraxin-3 levels. Plasma TREM-1 and pentraxin-3 levels were not only correlated with BMI-z score but were also associated with circulating MPs. Furthermore, both plasma TREM-1 and pentraxin-3 levels were independently associated with AHI in the multivariate models after controlling for potential confounders.
There is little doubt that pediatric OSA constitutes a chronic and low-grade systemic inflammatory disease.1 The inflamma-tory processes activated by the condition are very likely linked to the pathophysiology of OSA-induced end-organ morbidities, such that identification of biologically relevant inflammatory markers that possess predictive ability for adverse outcomes, e.g., CVD, neurocognitive deficits, and sleepiness, is undoubtedly an important quest. Indeed, if such efforts are successful, the panel of markers should enable allocation of a priori “similar” patients with identical AHI into risk strata permitting prior-itization of treatment or minimization of future risk. One of the major drawbacks of such an inflammatory biomarker approach, however, is that although there exists some degree of overlap among the various inflammatory markers, the relative predictive value of those markers thus far explored remains modest, thereby accounting for the search for more comprehensive sets of markers rather than reliance on a single one.23–26
TREM-1 is a 30-kDa monomeric transmembrane activating receptor and is activated by neutrophils, monocytes, and various macrophage subsets, which are the main effector cells in innate responses.6,27 Binding of TREM-1 ligand is possibly linked to activation of several transcription complexes that synergize with nuclear factor kappa-light-chain-enhancer of activated B cells to elicit transcription of proinflammatory cytokines.28 Although TREM-1 was first identified in sepsis and its release from soluble TREM-1 has been long claimed as a marker of infection, recent studies have clearly demonstrated that soluble TREM-1 levels or TREM-1 membrane expression are also enhanced in patient with noninfectious inflammatory disorders.29,30 Morales and colleagues7 recently showed that TREM like transcript-1 (TLT-1), which is a membrane protein receptor found in granules of platelets and megakaryocytes, can enhance platelet aggregation in vitro, suggesting that TLT-1 may perturb homeostastic processes by enhancing actin polymerization and result in increased platelet aggregation and adherence to the endothelium. Hermus and colleagues8 further reported that sTREM-1 levels were increased in patients with coronary artery disease, whereas Bosco et al.31 showed that hypoxia inducible factor-1, a key transcriptional factor in hypoxia-mediated responses, is implicated the transcriptional activation of TREM-1, a finding that is consistent with the presence of hypoxia response elements in the promoter region of TREM-1 gene. Therefore, plasma TREM-1 levels may not only provide a valuable marker of OSA and of the magnitude of the inflammatory response in the context of OSA, but may also be an indicator of platelet activation and increased risk for development of cardiovascular complications. Based on current findings, future exploration of TREM-1 in this contextual setting appears warranted.
Pentraxin-3 is an evolutionarily conserved, multimeric acute phase inflammatory glycoprotein that belongs to the same family as the now ubiquitously recognized hsCRP.32,33 The findings of altered pentraxin-3 levels in children with OSA were not particularly surprising considering the previously reported increases in hsCRP concentrations in both adults34,35 and children with OSA,36,37 and the reductions in such levels after OSA treatment.38,39 However, we should also point out that not all studies in adults40,41 or in children42,43 have consistently corroborated the putative association between hsCRP levels and OSA, suggesting interactions between the severity of OSA, genetic variance, and lifestyle and environmental factors.44,45 As a corollary to these observations, MRP 8/14 levels, which have been identified as an important predictor of cardiovascular disease,46,47 are increased in obese children and in children with OSA in a dose-dependent manner.22 For both of these markers, considerable variance emerges in cohorts at any level of OSA severity, further buttressing the need to incorporate multiple biomarkers in the assessment of risk for end-organ morbidity associated with OSA. Taken together, the findings pertaining to the four inflammatory proteins explored herein suggest the presence of complex and potentially divergent interactions between the resultant levels of each of these proteins in plasma and OSA disease severity, underlying presence of obesity, and the other previously mentioned factors. Accordingly, some inflammatory markers such as hsCRP may be more affected by obesity or specific sleep perturbations associated with OSA (e.g., intermittent hypoxia), whereas others may be more affected by the degree of sleep fragmentation and genetic variation, thereby requiring the use of composite biomarker approaches. In the context of pentraxin-3, because macrophages, endothelial cells, and vascular smooth muscle cells release large amounts of this protein in response to inflammatory signals such as oxidized LDL,48,49 changes in pentraxin-3 levels may provide a sensitive indicator of vascular damage. Thus, pentraxin-3 may provide more explicit information on the development and progression of atherosclerosis than other less specific markers, such as hsCRP.11 Critical exploration of the value of hsCRP is still lacking, however, and some degree of conflicting information on the reliability of hsCRP has emerged.26,36,38,50
In contrast, pentraxin-3 levels are not only increased in acute myocardial infarction but are also associated with increased mortality in such patients, therefore being proposed as a new prognostic candidate marker in ischemic heart disorders.51–55 Conversely, the assessment of pentraxin-3 as a marker of increased cardiovascular risk in the metabolic syndrome has yielded uncertain results, suggesting the need for further studies with larger numbers of patients before any definitive conclusions can be made.56,57 In the context of OSA, we are aware of only one published study by Kasai and colleagues,12 who recently showed that pentraxin-3 levels and arterial stiffness in adult patients with moderate to severe OSA were higher than in control patients, and were significantly reduced after 1 mo of continuous positive airway pressure treatment. In the current study, we found that pentraxin-3 levels are increased in children with OSA, and are not only correlated with AHI but also associated with the levels of various circulating MPs with previously demonstrated roles in atherogenesis.58,59 However, we also found that in our cohort pentraxin-3 levels did not correlate with endothelial function as determined by postocclusion hyperemic responses. These discrepancies further reinforce the concept that the phenotypic expression of pediatric OSA in general, and of any specific end-organ morbidity in particular, is the result of a complex amalgamation of a multitude of underlying contributing mechanisms, such as epigenetic alterations of DNA, genetic and environmental components, disease severity, and presence of obesity.1,60,61 Therefore, exploration of the factors regulating pentraxin-3 levels in the context of OSA and their relevance to the disease and to morbidity will be required.
Some methodologic considerations deserve comment. First, both TREM-1 and pentraxin-3 (PTX-3) can be highly expressed in different cell types.9,33,62 Specifically, pentraxin-3 is a multifunctional soluble pattern recognition receptor modulating the immunoinflammatory response and it belongs to the same pentraxin superfamily of acute-phase reactants as hsCRP. However, pentraxin-3 and hsCRP are produced by different tissues and may be involved in different pathophysiologic mechanisms. Our data also showed hsCRP was significant correlated with postocclusive hyperemic responses, but pentraxin-3 was not. Whereas hepatocytes are the main source of CRP, PTX3 is produced at sites of inflammation by a wide range of different cell types, including endothelial cells and adipocytes. Thus, it will be important to determine which cell populations are more specifically involved in the observed alterations in these inflammatory mediators in pediatric OSA. Second, our study includes a relatively modest sample size particularly among the children with moderate to severe OSA. Third, the uncertainty introduced by our findings herein on the association between post-occlusive hyperemic responses and TREM-1 and pentraxin-3 levels could reflect inherent limitations of the vascular test rather than suggest that these mediators of inflammation play no role in pediatric OSA. Fourth, the current study does not allow for establishing whether TREM-1 and pentraxin-3 are mediators of OSA-induced inflammation, or merely serve as markers of OSA-related morbidities, such as CVD and metabolic dysfunction. It is worthwhile to note that large-scale proteomic and transcriptomic approaches aiming to identify elaborate bio-marker panels would usually be generated by incorporating a finite, important number of analytes whose conglomerate receiver operator curves would clearly surpass those of a more restricted number of markers.63 Accordingly, it remains unclear whether the two specific proteins investigated in the current study would ultimately be valuable for the diagnosis of OSA or for prediction of OSA-related consequences, such as endothelial function.63 In addition, we did not explore the diurnal variation of TREM-1 and pentraxin-3 levels in children with OSA, and even though we standardized the collection times for all of our samples, this may be of future importance, particularly considering the circadian changes that other inflammatory markers display in the setting of CVD.64
In summary, children with OSA have elevated plasma TREM-1 and pentraxin-3 levels that exhibit OSA severity-related dependency, even among nonobese children. Furthermore, both plasma TREM-1 and pentraxin-3 levels emerge as being independently associated with AHI in multivariate models. These associations may in turn reflect pathogenetic roles played by TREM-1 and pentraxin-3 in promoting atherogenesis and cardiovascular risk in pediatric OSA. Additional studies will be needed to critically examine the intrinsic value of assessing the levels of these molecules in the context of evaluating children at risk for OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Dr. Kheirandish-Gozal is supported by National Institutes of Health (NIH) grant K12 HL-090003; Dr. David Gozal is supported by NIH grants HL-065270 and HL-086662; Dr. Kim is supported by the Parker B. Francis Fellowship grant.
REFERENCES
- 1.Kim J, Hakim F, Kheirandish-Gozal L, Gozal D. Inflammatory pathways in children with insufficient or disordered sleep. Respir Physiol Neurobiol. 2011;178:465–74. doi: 10.1016/j.resp.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 3.Chami HA, Resnick HE, Quan SF, Gottlieb DJ. Association of incident cardiovascular disease with progression of sleep-disordered breathing. Circulation. 2011;123:1280–6. doi: 10.1161/CIRCULATIONAHA.110.974022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gozal D, Kheirandish-Gozal L, Bhattacharjee R, Spruyt K. Neurocognitive and endothelial dysfunction in children with obstructive sleep apnea. Pediatrics. 2010;126:e1161–7. doi: 10.1542/peds.2010-0688. [DOI] [PubMed] [Google Scholar]
- 5.Tessarz AS, Cerwenka A. The TREM-1/DAP12 pathway. Immunol Lett. 2008;116:111–6. doi: 10.1016/j.imlet.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 6.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–73. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 7.Morales J, Villa K, Gattis J, et al. Soluble TLT-1 modulates platelet-endothelial cell interactions and actin polymerization. Blood Coagul Fibrinolysis. 2010;21:229–36. doi: 10.1097/MBC.0b013e3283358116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermus L, Schuitemaker JH, Tio RA, et al. Novel serum biomarkers in carotid artery stenosis: useful to identify the vulnerable plaque? Clin Biochem. 2011;44:1292–8. doi: 10.1016/j.clinbiochem.2011.08.1141. [DOI] [PubMed] [Google Scholar]
- 9.Deban L, Jaillon S, Garlanda C, Bottazzi B, Mantovani A. Pentraxins in innate immunity: lessons from PTX3. Cell Tissue Res. 2010;343:237–49. doi: 10.1007/s00441-010-1018-0. [DOI] [PubMed] [Google Scholar]
- 10.Garlanda C, Bottazzi B, Moalli F, et al. Pentraxins and atherosclerosis: the role of PTX3. Curr Pharm Des. 2011;17:38–46. doi: 10.2174/138161211795049750. [DOI] [PubMed] [Google Scholar]
- 11.Kunes P, Holubcova Z, Kolackova M, Krejsek J. Pentraxin 3(PTX 3): an endogenous modulator of the inflammatory response. Mediators Inflamm. 2012;2012:920517. doi: 10.1155/2012/920517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasai T, Inoue K, Kumagai T, et al. Plasma pentraxin3 and arterial stiffness in men with obstructive sleep apnea. Am J Hypertens. 2011;24:401–7. doi: 10.1038/ajh.2010.248. [DOI] [PubMed] [Google Scholar]
- 13.Gozal D, Kheirandish-Gozal L, Serpero LD, Sans Capdevila O, Dayyat E. Obstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomy. Circulation. 2007;116:2307–14. doi: 10.1161/CIRCULATIONAHA.107.696823. [DOI] [PubMed] [Google Scholar]
- 14.Montgomery-Downs HE, O'Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117:741–53. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 15.Standards and indications for cardiopulmonary sleep studies in children. American Thoracic Society. Am J Respir Crit Care Med. 1996;153:866–78. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]
- 16.Rechstschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Services/Brain Research Institute, University of California; 1968. [Google Scholar]
- 17.Schulz H. Phasic or transient? Comment on the terminology of the AASM manual for the scoring of sleep and associated events. J Clin Sleep Med. 2007;3:752. [PMC free article] [PubMed] [Google Scholar]
- 18.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 19.Bhattacharjee R, Kim J, Alotaibi WH, Kheirandish-Gozal L, Capdevila OS, Gozal D. Endothelial dysfunction in children without hypertension: potential contributions of obesity and obstructive sleep apnea. Chest. 2012;141:682–91. doi: 10.1378/chest.11-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wahlberg E, Olofsson P, Swendenborg J, Fagrell B. Changes in postocclusive reactive hyperaemic values as measured with laser Doppler fluxmetry after infrainguinal arterial reconstructions. Eur J Vasc Endovasc Surg. 1995;9:197–203. doi: 10.1016/s1078-5884(05)80090-5. [DOI] [PubMed] [Google Scholar]
- 21.Kim J, Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, Gozal D. Circulating microparticles in children with sleep disordered breathing. Chest. 2011;140:408–17. doi: 10.1378/chest.10-2161. [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Bhattacharjee R, Snow AB, Capdevila OS, Kheirandish-Gozal L, Gozal D. Myeloid-related protein 8/14 levels in children with obstructive sleep apnoea. Eur Respir J. 2010;35:843–50. doi: 10.1183/09031936.00075409. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Zhang SX, Gozal D. Reactive oxygen species and the brain in sleep apnea. Respir Physiol Neurobiol. 2011;174:307–16. doi: 10.1016/j.resp.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J. 2009;33:1467–84. doi: 10.1183/09031936.00086608. [DOI] [PubMed] [Google Scholar]
- 25.Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI. Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep. 2009;32:447–70. doi: 10.1093/sleep/32.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gozal D, Kheirandish-Gozal L, Bhattacharjee R, Kim J. C-reactive protein and obstructive sleep apnea syndrome in children. Front Biosci (Elite Ed) 2012;4:2410–22. doi: 10.2741/e553. [DOI] [PubMed] [Google Scholar]
- 27.Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol. 2003;3:445–53. doi: 10.1038/nri1106. [DOI] [PubMed] [Google Scholar]
- 28.Gibot S. Clinical review: role of triggering receptor expressed on myeloid cells-1 during sepsis. Crit Care. 2005;9:485–9. doi: 10.1186/cc3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murakami Y, Akahoshi T, Aoki N, Toyomoto M, Miyasaka N, Kohsaka H. Intervention of an inflammation amplifier, triggering receptor expressed on myeloid cells 1, for treatment of autoimmune arthritis. Arthritis Rheum. 2009;60:1615–23. doi: 10.1002/art.24554. [DOI] [PubMed] [Google Scholar]
- 30.Radsak MP, Taube C, Haselmayer P, et al. Soluble triggering receptor expressed on myeloid cells 1 is released in patients with stable chronic obstructive pulmonary disease. Clin Dev Immunol. 2007;2007:52040. doi: 10.1155/2007/52040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosco MC, Pierobon D, Blengio F, et al. Hypoxia modulates the gene expression profile of immunoregulatory receptors in human mature dendritic cells: identification of TREM-1 as a novel hypoxic marker in vitro and in vivo. Blood. 2011;117:2625–39. doi: 10.1182/blood-2010-06-292136. [DOI] [PubMed] [Google Scholar]
- 32.Norata GD, Garlanda C, Catapano AL. The long pentraxin PTX3: a modulator of the immunoinflammatory response in atherosclerosis and cardiovascular diseases. Trends Cardiovasc Med. 2010;20:35–40. doi: 10.1016/j.tcm.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Cieslik P, Hrycek A. Long pentraxin 3 (PTX3) in the light of its structure, mechanism of action and clinical implications. Autoimmunity. 2012;45:119–28. doi: 10.3109/08916934.2011.611549. [DOI] [PubMed] [Google Scholar]
- 34.Can M, Acikgoz S, Mungan G, et al. Serum cardiovascular risk factors in obstructive sleep apnea. Chest. 2006;129:233–7. doi: 10.1378/chest.129.2.233. [DOI] [PubMed] [Google Scholar]
- 35.Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–34. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 36.Tauman R, Ivanenko A, O'Brien LM, Gozal D. Plasma C-reactive protein levels among children with sleep-disordered breathing. Pediatrics. 2004;113:e564–9. doi: 10.1542/peds.113.6.e564. [DOI] [PubMed] [Google Scholar]
- 37.Li AM, Chan MH, Yin J, et al. C-reactive protein in children with obstructive sleep apnea and the effects of treatment. Pediatr Pulmonol. 2008;43:34–40. doi: 10.1002/ppul.20732. [DOI] [PubMed] [Google Scholar]
- 38.Kheirandish-Gozal L, Capdevila OS, Tauman R, Gozal D. Plasma C-reactive protein in nonobese children with obstructive sleep apnea before and after adenotonsillectomy. J Clin Sleep Med. 2006;2:301–4. [PMC free article] [PubMed] [Google Scholar]
- 39.Goldbart AD, Levitas A, Greenberg-Dotan S, et al. B-type natriuretic peptide and cardiovascular function in young children with obstructive sleep apnea. Chest. 2010;138:528–35. doi: 10.1378/chest.10-0150. [DOI] [PubMed] [Google Scholar]
- 40.Barcelo A, Barbe F, Llompart E, et al. Effects of obesity on C-reactive protein level and metabolic disturbances in male patients with obstructive sleep apnea. Am J Med. 2004;117:118–21. doi: 10.1016/j.amjmed.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 41.Guilleminault C, Kirisoglu C, Ohayon MM. C-reactive protein and sleep-disordered breathing. Sleep. 2004;27:1507–11. doi: 10.1093/sleep/27.8.1507. [DOI] [PubMed] [Google Scholar]
- 42.Kaditis AG, Alexopoulos EI, Kalampouka E, et al. Morning levels of C-reactive protein in children with obstructive sleep-disordered breathing. Am J Respir Crit Care Med. 2005;171:282–6. doi: 10.1164/rccm.200407-928OC. [DOI] [PubMed] [Google Scholar]
- 43.Tam CS, Wong M, McBain R, Bailey S, Waters KA. Inflammatory measures in children with obstructive sleep apnoea. J Paediatr Child Health. 2006;42:277–82. doi: 10.1111/j.1440-1754.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- 44.Rammes A, Roth J, Goebeler M, Klempt M, Hartmann M, Sorg C. Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J Biol Chem. 1997;272:9496–502. doi: 10.1074/jbc.272.14.9496. [DOI] [PubMed] [Google Scholar]
- 45.Guignard F, Mauel J, Markert M. Phosphorylation of myeloid-related proteins MRP-14 and MRP-8 during human neutrophil activation. Eur J Biochem. 1996;241:265–71. doi: 10.1111/j.1432-1033.1996.0265t.x. [DOI] [PubMed] [Google Scholar]
- 46.Croce K, Gao H, Wang Y, et al. Myeloid-related protein-8/14 is critical for the biological response to vascular injury. Circulation. 2009;120:427–36. doi: 10.1161/CIRCULATIONAHA.108.814582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schaub N, Reichlin T, Meune C, et al. Markers of plaque instability in the early diagnosis and risk stratification of acute myocardial infarction. Clin Chem. 2012;58:246–56. doi: 10.1373/clinchem.2011.172940. [DOI] [PubMed] [Google Scholar]
- 48.Deban L, Jaillon S, Garlanda C, Bottazzi B, Mantovani A. Pentraxins in innate immunity: lessons from PTX3. Cell Tissue Res. 2011;343:237–49. doi: 10.1007/s00441-010-1018-0. [DOI] [PubMed] [Google Scholar]
- 49.Deban L, Russo RC, Sironi M, et al. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat Immunol. 2011;11:328–34. doi: 10.1038/ni.1854. [DOI] [PubMed] [Google Scholar]
- 50.Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med. 2008;177:1142–9. doi: 10.1164/rccm.200711-1670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peri G, Introna M, Corradi D, et al. PTX3, a prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102:636–41. doi: 10.1161/01.cir.102.6.636. [DOI] [PubMed] [Google Scholar]
- 52.Latini R, Maggioni AP, Peri G, et al. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2004;110:2349–54. doi: 10.1161/01.CIR.0000145167.30987.2E. [DOI] [PubMed] [Google Scholar]
- 53.Inoue K, Sugiyama A, Reid PC, et al. Establishment of a high sensitivity plasma assay for human pentraxin3 as a marker for unstable angina pectoris. Arterioscler Thromb Vasc Biol. 2007;27:161–7. doi: 10.1161/01.ATV.0000252126.48375.d5. [DOI] [PubMed] [Google Scholar]
- 54.Matsui S, Ishii J, Kitagawa F, et al. Pentraxin 3 in unstable angina and non-ST-segment elevation myocardial infarction. Atherosclerosis. 2010;210:220–5. doi: 10.1016/j.atherosclerosis.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 55.Brugger-Andersen T, Ponitz V, Kontny F, et al. The long pentraxin 3 (PTX3): a novel prognostic inflammatory marker for mortality in acute chest pain. Thromb Haemost. 2009;102:555–63. doi: 10.1160/TH09-02-0137. [DOI] [PubMed] [Google Scholar]
- 56.Ogawa T, Kawano Y, Imamura T, et al. Reciprocal contribution of pentraxin 3 and C-reactive protein to obesity and metabolic syndrome. Obesity (Silver Spring) 2010;18:1871–4. doi: 10.1038/oby.2009.507. [DOI] [PubMed] [Google Scholar]
- 57.Miyaki A, Maeda S, Yoshizawa M, et al. Is pentraxin 3 involved in obesity-induced decrease in arterial distensibility? J Atheroscler Thromb. 2010;17:278–84. doi: 10.5551/jat.2741. [DOI] [PubMed] [Google Scholar]
- 58.Dursun I, Poyrazoglu HM, Gunduz Z, et al. The relationship between circulating endothelial microparticles and arterial stiffness and atherosclerosis in children with chronic kidney disease. Nephrol Dial Transplant. 2009;24:2511–8. doi: 10.1093/ndt/gfp066. [DOI] [PubMed] [Google Scholar]
- 59.Chen Y, Feng B, Li X, Ni Y, Luo Y. Plasma endothelial microparticles and their correlation with the presence of hypertension and arterial stiffness in patients with type 2 diabetes. J Clin Hypertens (Greenwich) 2012;14:455–60. doi: 10.1111/j.1751-7176.2012.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim J, Bhattacharjee R, Khalyfa A, et al. DNA methylation in inflamma-tory genes among children with obstructive sleep apnea. Am J Respir Crit Care Med. 2012;185:330–8. doi: 10.1164/rccm.201106-1026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kent BD, Ryan S, McNicholas WT. The genetics of obstructive sleep apnoea. Curr Opin Pulm Med. 2010;16:536–42. doi: 10.1097/MCP.0b013e32833ef7fe. [DOI] [PubMed] [Google Scholar]
- 62.Ford JW, McVicar DW. TREM and TREM-like receptors in inflammation and disease. Curr Opin Immunol. 2009;21:38–46. doi: 10.1016/j.coi.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gozal D. Serum, urine, and breath-related biomarkers in the diagnosis of obstructive sleep apnea in children: is it for real? Curr Opin Pulm Med. 2012;18:561–7. doi: 10.1097/MCP.0b013e328358be2d. [DOI] [PubMed] [Google Scholar]
- 64.Dominguez-Rodriguez A, Abreu-Gonzalez P, Kaski JC. Inflammatory systemic biomarkers in setting acute coronary syndromes--effects of the diurnal variation. Curr Drug Targets. 2009;10:1001–8. doi: 10.2174/138945009789577963. [DOI] [PubMed] [Google Scholar]