Abstract
Objective:
Short-sleep insomnia is associated with increased risk of diabetes. The role of altered insulin secretion and action in this association is poorly understood.
Design:
Observational study.
Setting:
Academic clinical research center.
Participants:
Nondiabetic individuals with insomnia (mean [standard deviation] age 48 [9] y, body mass index 25.6 [3.9] kg/m2) with ≤ 6 h (short sleep, n = 14) and > 6 h of sleep (n = 14) during overnight laboratory polysomnography.
Measurements and Results:
Standard oral glucose testing was used to assess glucose tolerance, beta-cell function (homeostasis model assessment [HOMA-B]; second-phase insulin secretion) and insulin resistance (HOMA-IR; insulin sensitivity index). There was no significant difference in hemoglobin A1C and fasting or 2-h blood glucose concentrations between sleep groups. Short-sleep insomnia sufferers had lower fasting and postchallenge serum insulin concentrations associated with lower estimates of fasting and glucose-stimulated insulin secretion, and increased insulin sensitivity.
Conclusions:
Individuals with short-sleep insomnia appear to have higher indices of systemic insulin sensitivity and secrete less insulin without changes in overall glucose tolerance.
Citation:
Vasisht KP; Kessler LE; Booth JN; Imperial JG; Penev PD. Differences in insulin secretion and sensitivity in short-sleep insomnia. SLEEP 2013;36(6):955-957.
Keywords: Decreased quantity and quality of sleep, oral glucose tolerance, pancreatic beta-cell function, systemic insulin resistance
INTRODUCTION
Insufficient sleep and difficulty falling or staying asleep typical of insomnia are factors that have been associated with increased risk of diabetes.1 In the Penn State cohort, individuals with insomnia who slept < 6 h during overnight laboratory polysomnography had a higher prevalence of diabetes relative to individuals with insomnia who slept > 6 h, leading Vgontzas et al.2 to propose that short-sleep insomnia may be a risk factor for such metabolic morbidity. The pathogenesis of type 2 diabetes involves deficits in insulin secretion and action, but their contribution to diabetes risk in short-sleep insomnia is poorly understood. Some reports suggest that individuals with insomnia may have increased insulin resistance,3–5 whereas the Coronary Artery Risk Development in Young Adults (CARDIA) sleep study reached opposite conclusions.6 We used an oral glucose challenge to obtain measures of glucose tolerance, insulin secretion, and insulin action in nondiabetic patients with insomnia with and without short sleep.
METHODS
Individuals with insomnia (age 30-64 y, body mass index [BMI] 20-35 kg/m2) were recruited through local advertisements. The study protocol was approved by the University of Chicago Institutional Review Board. Volunteers gave written informed consent and were paid for their participation. All participants had an Insomnia Severity Index7 > 10, Pittsburgh Sleep Quality Index8 > 5, and met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for primary insomnia. Exclusion criteria were: night work; recent travel across multiple time zones; medications that affect sleep or metabolism; any acute or unstable chronic medical condition; abnormal comprehensive metabolic panel, thyroid function tests, blood counts, or 12-lead electrocardiogram; hemoglobin A1C ≥ 6.5%; and smoking ≥ 1 pack/week. Psychiatric illness, substance abuse, and excessive alcohol consumption were excluded using the Mini-International Neuropsychiatric Interview.9
Participants had a 75-g oral glucose tolerance test at the time of screening. Plasma glucose (STAT-2300 analyzer, Yellow Spring Instruments, Yellow Springs, OH) and serum insulin (Immulite-2000, Diagnostic Products, Los Angeles, CA) were measured at 0 and 120 min to derive measures of (1) insulin secretion, including homeostatic model assessment of beta-cell function, HOMA-B (University of Oxford HOMA2-Calculator-v.2.2; http://www.dtu.ox.ac.uk/homacalculator/index.php)10 and second-phase insulin response to glucose;11 and (2) insulin action, including insulin sensitivity index (ISI)11 and homeostasis model assessment of insulin resistance, HOMA-IR (University of Oxford HOMA2-Calculator-v.2.2; http://www.dtu.ox.ac.uk/homacalculator/index.php). Individuals with diabetic-range fasting and 2-h glucose values were excluded from participation. Within 1 mo after screening, participants completed 1 night of laboratory polysomnography (Neurofax-1100EEG, Nihon-Kohden, Foothill Ranch, CA). Scheduled time-in-bed was 7.5 h. Airflow was recorded using pressure transducers and thermocouples. Thoracic and abdominal respiratory effort was monitored with piezoelectrodes. Records were scored in 30-sec epochs. Sleep latency was defined as the time between lights-off and any stage sleep. Hypopneas were defined as ≥ 10-sec reduction in airflow with oxygen desaturation ≥ 3%. Individuals with respiratory disturbance index > 14 were excluded from participation. Short-sleep insomnia was defined as polysomno-graphic sleep efficiency ≤ 80% with overnight sleep ≤ 6 h.
Familial risk for type 2 diabetes was categorized as low (no family history); increased (fewer than two first-degree or one first-degree and two second-degree relatives with diabetes); or high (at least two first-degree or one first-degree and two second-degree relatives with diabetes). Self-reported physical activity was scored as: 0 = low (walking < 90 min/week); 1 = moderate (at least 30 min of walking ≥ three times/week); and 2 = high (at least 20 min of vigorous exercise ≥ three times/ week).12 Analysis of covariance to control for between-group imbalance in familial diabetes risk and intermittent smoking (Table 1) was used to compare glucose tolerance, insulin secretion, and insulin sensitivity in patients with insomnia with and without short sleep. Results did not change (data not shown) with added control for other diabetes risk factors (age, BMI, sex, race/ethnicity, physical activity, and respiratory sleep dis turbance) that were well matched between groups (Table 1).
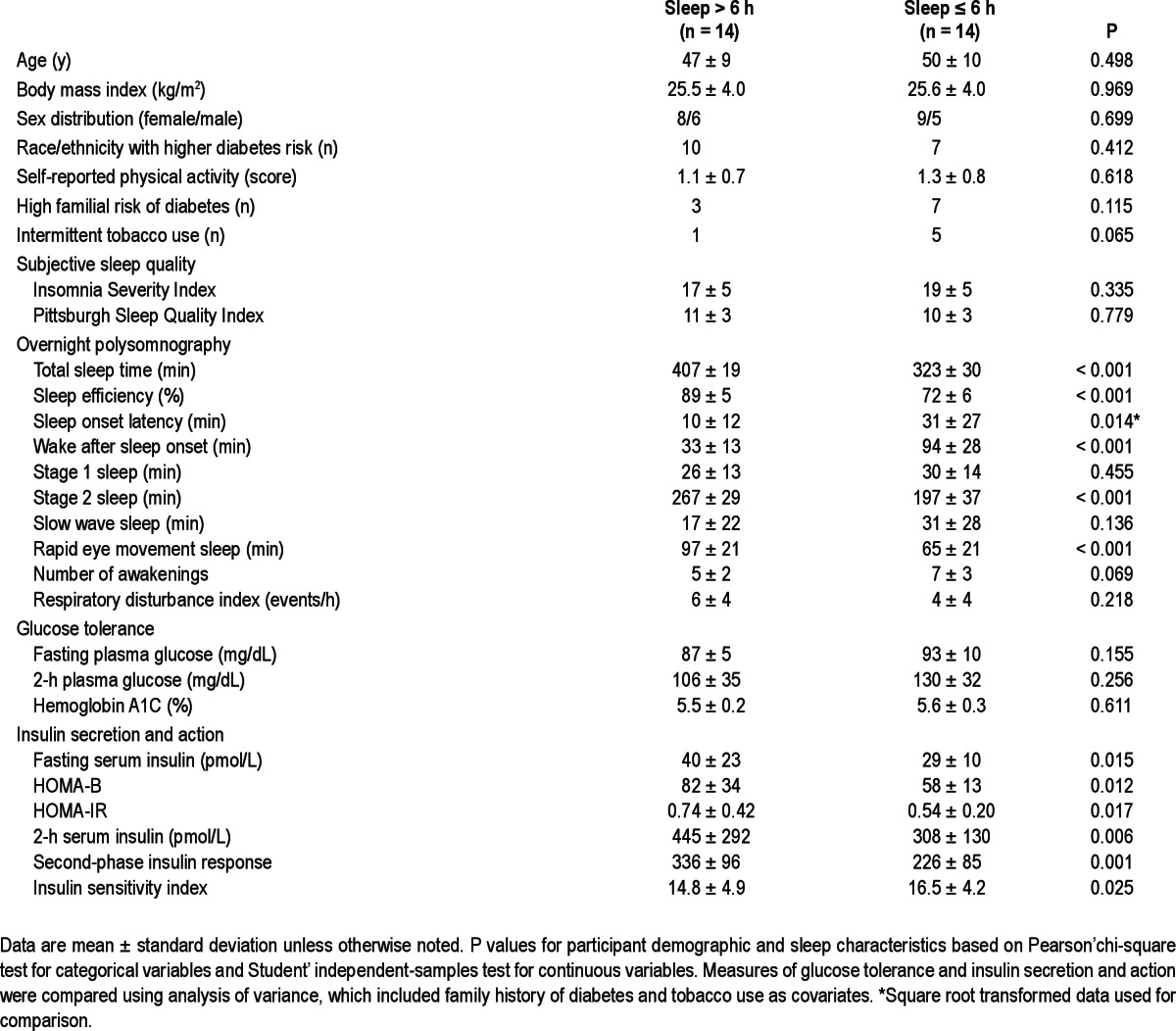
Table 1.
Characteristics of insomnia sufferers and measures of glucose regulation
RESULTS
Half of the 28 eligible participants had short-sleep insomnia by polysomnography (Table 1). They had greater difficulty falling and staying asleep, lower sleep efficiency, and less Stage 2 and rapid eye movement sleep (Table 1). Hemoglobin A1C, fasting, and 2-h blood glucose concentrations did not differ between groups (Table 1). Patients with short-sleep insomnia had lower fasting and postchallenge serum insulin concentrations, reduced indices of beta-cell function (HOMA-B; second-phase insulin secretion), and increased insulin sensitivity (HOMA-IR; ISI) (Table 1).
DISCUSSION
Short-sleep insomnia (< 6 h/night) defined by polysomnography identifies a group of patients with insomnia at increased risk of diabetes.2 In the current study, nondiabetic participants with short-sleep insomnia had reduced insulin secretion in association with higher insulin sensitivity and no significant deterioration in hemoglobin A1C and overall glycemic control (Table 1). These findings are consistent with observations in CARDIA study participants with actigraphic evidence of insomnia, whose fasting blood glucose remained unchanged in the setting of lower insulin concentrations and decreased HOMA-IR.6 However, it has been hypothesized that a similar pattern might not occur following a carbohydrate challenge.6 Our data now indicate that short-sleep insomnia is also characterized by increased postchallenge insulin sensitivity and reduced glucose-stimulated insulin secretion (Table 1).
Relying almost exclusively on fasting blood glucose and insulin measurements, previous studies have provided conflicting evidence of unchanged,13 increased,6 or decreased insulin sensitivity3–5 in insomnia sufferers. The inconsistency of these reports likely stems from their variable and often incomplete control for important variables such as race/ethnicity, physical activity, family history of diabetes, smoking, depressed mood or anxiety, and sleep disordered breathing. When we accounted for such potential contributing factors, patients with short-sleep insomnia had higher fasting and postchallenge insulin sensitivity indices (Table 1).
How do these findings relate to the association of short-sleep insomnia and diabetes risk? Primary insomnia is characterized by physiologic hyperarousal14— an energetically more costly metabolic state that may require adaptive downregulation of insulin secretion to match postabsorptive carbohydrate availability to the increased fuel needs of glucose-dependent and glucose-independent tissues.15 It is possible that when combined with preexisting defects in beta-cell function and excessive adiposity, such otherwise appropriate chronic downregulation in insulin secretion related to short-sleep insomnia could become maladaptive and translate into increased risk of diabetes. However, this speculative hypothesis could not be addressed in the current study, which was limited by its observational design, small sample size, surrogate measures of insulin secretion and sensitivity, and reliance on a single night of polysomnography to define short-sleep insomnia. Prospective interventional protocols will be needed to determine whether primary insomnia contributes to the pathogenesis of type 2 diabetes independently from other well-known metabolic risk factors.
DISCLOSURE STATEMENT
This study was partially supported by an investigator-initiated research grant from Sunovion Pharmaceuticals Inc. f/k/a Sepracor Inc. Dr. Penev is now a full-time employee of Bristol-Myers Squibb. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the volunteers for their participation, the staff of the University of Chicago Clinical Research Center, and Luis Alcantar and Harry Whitmore from the Department of Medicine for their excellent technical assistance. Preliminary data from this study were presented at the 2010 Annual Meeting of the Association of Professional Sleep Societies.
REFERENCES
- 1.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–20. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care. 2009;32:1980–5. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jennings JR, Muldoon MF, Hall M, Buysse DJ, Manuck SB. Self-reported sleep quality is associated with the metabolic syndrome. Sleep. 2007;30:219–23. doi: 10.1093/sleep/30.2.219. [DOI] [PubMed] [Google Scholar]
- 4.Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain Behav Immun. 2008;22:960–8. doi: 10.1016/j.bbi.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto N, Yamanaka G, Ishizawa K, et al. Insomnia increases insulin resistance and insulin secretion in elderly people. J Am Geriatr Soc. 2010;58:801–4. doi: 10.1111/j.1532-5415.2010.02794.x. [DOI] [PubMed] [Google Scholar]
- 6.Knutson K, Van Cauter E, Zee PC, Liu K, Lauderdale DS. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: The Coronary Artery Risk Development in Young Adults Sleep Study. Diabetes Care. 2011;34:1171–6. doi: 10.2337/dc10-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–8. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 9.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 10.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 11.Stumvoll M, Mitrakou A, Pimenta W, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. 2000;23:295–301. doi: 10.2337/diacare.23.3.295. [DOI] [PubMed] [Google Scholar]
- 12.Valdez R, Yoon PW, Liu T, Khoury MJ. Family history and prevalence of diabetes in the U.S. population: the 6-year results from the National Health and Nutrition Examination Survey (1999-2004) Diabetes Care. 2007;30:2517–22. doi: 10.2337/dc07-0720. [DOI] [PubMed] [Google Scholar]
- 13.Keckeis M, Lattova Z, Maurovich-Horvat E, et al. Impaired glucose tolerance in sleep disorders. PLoS One. 2010;5:e9444. doi: 10.1371/journal.pone.0009444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Nedeltcheva AV, Imperial JG, Penev PD. Effects of sleep restriction on glucose control and insulin secretion during diet-induced weight loss. Obesity. 2012;20:1379–86. doi: 10.1038/oby.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]


