Abstract
To quantify the downstream impact of prostate specific antigen (PSA) testing on cancer characteristics and utilization of cancer therapies among men aged 70 or older, we utilized patients diagnosed with prostate cancer in 2004–2005 in the Surveillance, Epidemiology, and End Results (SEER)-Medicare and their Medicare claims prior to their cancer diagnosis during 2000 to 2005.
Among men in the highest testing group (4–6 PSA tests), 75% were diagnosed with low- or intermediate- risk of disease but 77% received treatments within 180 days of cancer diagnosis. More than 45% of newly diagnosed patients in 2004–2005 had 4–6 PSA tests prior to their cancer diagnosis during 2000 to 2005. Men in the high testing group were 3.57 times more likely to receive cancer treatments (either surgery, radiation or hormonal therapy) when compared to men who had no prior PSA testing during the same time period. Among men aged 75+ diagnosed with low-risk cancer, men in the high testing group were 78% more likely to receive treatment than those who had no prior PSA testing.
In conclusion, given the lack of evidence of effective treatment for elderly patients diagnosed with low- and intermediate-risk prostate cancer and our inability to distinguish indolent from aggressive cancer, more frequent PSA testing among elderly population may exacerbate the risk of overdiagnosis and overtreatment.
Keywords: PSA, treatment, SEER program, Medicare
Introduction
Screening for prostate cancer by prostate specific antigen (PSA) gained widespread acceptance in the United States following a 1987 report by Stamey et al.1 More intensive PSA screening for prostate cancer has resulted in a higher prostate cancer incidence.2 Unfortunately, no therapies have been shown to extend cause-specific survival for men over aged 65 diagnosed with low- or intermediate- risk prostate cancer.3–5 Currently, the 5-year relative survival rate for patients diagnosed with localized disease is almost 100% regardless of whether patients receive therapy or not.6 While PSA testing can detect prostate cancer at an earlier stage and potentially improve survival, it is also associated with a significant risk of overdiagnosis and overtreatment.7
The U.S. Preventive Services Task Force (USPSTF) recommends against PSA screening for men over age 75 due to the concern that the risk of overdiagnosis and overtreatment may outweigh the benefits of testing.8 Despite evidence suggesting that PSA testing might be safely discontinued for most men older than 75,9 the screening rate remains high in this group.10,11 Since 2000 Medicare has paid for annual PSA testing without an upper age limit, eligibility requirements or evaluation. To quantify the downstream impact of PSA testing on utilization of treatments, we performed a nation-wide population-based study among Medicare recipients in the U.S.
Methods
Data Sources
We conducted a population-based retrospective cohort study utilizing data obtained from files linking information from the Surveillance, Epidemiology and End Results (SEER) program with Medicare claim files. The SEER regions encompass approximately 25% of the U.S. population, while the Medicare claim files cover approximately 93% of patients aged 65 years and older.12 Age-specific population projections for 2010 to 2020 were obtained from the U.S. census.13
Study Participants
Our study included 24,356 prostate cancer patients diagnosed in 2004 and 2005 in the SEER-Medicare data who were continuously enrolled both Parts A and B as their primary health insurance since 2000. Men with missing PSA values, Gleason scores or clinical stage that prevented classification by risk strata were also excluded (n=2,349). A total of 22,047 men with prostate cancer were included in the final analysis. This study was approved by the institutional review board of the University of Medicine and Dentistry of New Jersey.
Measurement of PSA Testing and Treatment Intensity
We used claims for PSA testing from Current Procedure Terminology (CPT) codes 84153 or G0103 reported in the physician/supplier and outpatient files within Medicare as measurement of PSA testing intensity.10,14 We calculated the number of claims for PSA testing from 2000 to 2005, excluding PSA testing that occurred after diagnosis of prostate cancer. More than 90% of men had only one claim for PSA testing each year. We counted multiple PSA test men received in one-year period as one single PSA test because we would like to estimate the proportion of men receiving at least one PSA testing before cancer diagnosis. The study population was divided into five groups (never, one, two, three, or four to six tests) based on the number of annual PSA tests received from 2000 to 2005.
Treatment administered following diagnosis was categorized as either active treatment or conservative management. Active therapy consisted of men receiving radical prostatectomy, radiation therapy (i.e., external beam radiation therapy and/or brachytherapy) or primary androgen deprivation therapy (PADT) within 180 days following diagnosis. Conservative management included men not receiving radical prostatectomy, radiation or PADT as recorded in the SEER-Medicare dataset.
Data Elements and Study Groupings
Patient characteristics and cancer status were measured at the time of cancer diagnosis. The health status of the study population was assessed using the Charlson co-morbidity Index,15 a summary measure based on 19 chronic diseases selected and weighted according to their association with mortality. To study the association between health status and the chance to receive PSA testing, each study participant was assigned a Charlson score based on information available from Medicare physician, inpatient, and outpatient claims during the twelve month period prior to the start of 2000. 16 Based on this information, the study population was divided into three categories: good health (Charlson score 0); average health (Charlson score 1); and poor health (Charlson score ≥ 2). Other variables known to influence PSA testing including education level, income, race, geographic location, number of physician visits, and marital status were obtained from SEER-Medicare linkages to the 2000 U.S. census data.
To assess cancer status at diagnosis, men were categorized into three risk groups using the American Joint Committee on Cancer classification system,17,18 PSA level and Gleason score: 1) low risk (stage ≤ T2a, PSA ≤ 10 ng/ml, Gleason score ≤ 6), 2) intermediate risk (stage T2b and T2c or PSA 10 to ≤20 ng/ml or Gleason score 7) or 3) high risk (stage T3 or T4 or PSA > 20 ng/ml or Gleason ≥ 8).
Data Analyses
The association between Gleason score, cancer stage, risk categories at diagnosis and the number of annual PSA tests received were evaluated by the Mantel-Haenszel statistic.19 The association between age and PSA level at diagnosis and the number of annual PSA tests received was examined by one-way analysis of variance using linear contrast. The distribution of the number of annual PSA tests received prior to diagnosis stratified by age, race, health conditions, and other characteristics present at diagnosis was evaluated by the asymptotic Kruskal-Wallis test.20
We used ordinal logistic regression models to estimate multivariate-adjusted odds ratios and 95% confidence intervals to determine the association between each patient characteristic and the number of annual PSA tests received prior to diagnosis. In addition, we used multivariable logistic regression to estimate the association between each variable, the number of PSA tests received, and the receipt of treatments. The distribution of treatments stratified by the number of PSA tests and risk groups was evaluated using the Cochran-Mantel–Haenszel statistic.
All statistical tests were two-sided and were performed by using SAS version 9.1.3 (SAS Institute, Cary, NC).
Role of the Funding Source
This study was funded by National Cancer Institute, Cancer Institute of New Jersey and Robert Wood Johnson Foundation. The funding sources had no role in the design of our analysis, its interpretation, or the decision to submit the manuscript for publication.
RESULTS
Characteristics of the patients and their pre-diagnosis PSA testing use are described in Table 1. More than 45% (10017/22047) of newly diagnosed cancer patients in 2004 and 2005 had 4–6 PSA tests during 2000 and 2005 prior to their cancer diagnosis. Men who are white, with higher education, more affluent, married and had more physician visits one year prior to cancer diagnosis are more likely to receive a higher number of PSA tests before cancer diagnosis. Increasing numbers of PSA tests prior to cancer diagnosis were associated with decreasing PSA levels at diagnosis, lower biopsy Gleason scores, lower clinical stages and lower risk disease (p<.001) (Table 2). The median PSA at diagnosis decreased from 11.9 ng/ml for patients who had never been previously tested to 7.2 ng/ml for patients who received four to six annual tests (p<.001). Among men in the highest testing group (4 to 6 PSA tests), 75% were diagnosed with low- or intermediate- risk of disease but 77% received treatments in 180 days.
Table 1.
Characteristics of men with prostate cancer by number of PSA testing received from 2000 to 2005 prior to diagnosis; SEER-Medicare database. (n=22,047)
| Number of PSA screening test from 2000 to 2005 *, n (%) | |||||
|---|---|---|---|---|---|
| Characteristics | Never (n = 1446) | 1 (n = 3692) | 2 (n = 3362) | 3 (n = 3530 ) | 4 to 6 (n = 10017) |
| Age, y | |||||
| Mean age (SD) | 77.4 (5.8) | 77.3 (5.6) | 76.8 (5.4) | 76.6 (5.1) | 76.7 (4.9) |
| Age, % | |||||
| 70–74 | 569 (39) | 1388 (38) | 1406 (42) | 1499 (42) | 3934 (39) |
| 75–79 | 413 (29) | 1146 (31) | 1007 (30) | 1093 (31) | 3422 (34) |
| 80–84 | 272 (19) | 706 (19) | 606 (18) | 637 (18) | 1941 (19) |
| 85+ | 192 (13) | 452 (12) | 343 (10) | 301 (9) | 720 (7) |
| Race, % | |||||
| White | 1109 (77) | 2915 (79) | 2708 (81) | 2956 (84) | 8562 (86) |
| Black | 241 (17) | 462 (13) | 351 (10) | 284 (8) | 614 (6) |
| Others | 96 (7) | 315 (9) | 303 (9) | 290 (8) | 841 (8) |
| Region, % | |||||
| West | 647 (45) | 1582 (43) | 1605 (48) | 1717 (49) | 4809 (48) |
| Northeast | 295 (20) | 759 (21) | 676 (20) | 707 (20) | 2231 (22) |
| North Central | 155 (11) | 533 (14) | 459 (14) | 534 (15) | 1528 (15) |
| South | 349 (24) | 818 (22) | 622 (19) | 572 (16) | 1449 (14) |
| Lived in ZIP code tabulation area in which 25% or more of adults had a college education, % | |||||
| 503 (35) | 1329 (36) | 1360 (41) | 1636 (46) | 5347 (53) | |
| Median annual income of ZIP code tabulation area, $ | 40,771 | 42,365 | 45,111 | 46,712 | 50,721 |
| Marital Status, % | |||||
| Married | 830 (57) | 2198 (59) | 2096 (62) | 2335 (66) | 6904 (69) |
| Health Status Based on Charlson Score, % | |||||
| 0 (Good) | 1251 (87) | 3089 (84) | 2706 (81) | 2806 (80) | 7964 (80) |
| 1 (Average) | 135 (9) | 412 (11) | 462 (14) | 515 (15) | 1552 (16) |
| >=2 (Poor) | 60 (4) | 191 (5) | 194 (6) | 209 (6) | 501 (5) |
| No. of physician visits one year before Cancer diagnosis** | |||||
| 1st Quartile | 972 (67) | 1495 (40) | 968 (29) | 810 (23) | 1590 (16) |
| 2nd Quartile | 190 (13) | 898 (24) | 867 (26) | 930 (26) | 2508 (25) |
| 3rd Quartile | 131 (9) | 704 (19) | 786 (23) | 938 (27) | 2938 (29) |
| 4th Quartile | 153 (11) | 595 (16) | 741 (22) | 852 (24) | 2981 (30) |
Difference across number of PSA tests received prior to diagnosis were statistically significant (p<.001) for all characteristics.
Number of physician visits one year before cancer diagnosis was grouped by quartile: 1st: 0 to 7 times; 2nd: 8 to 13; 3rd: 14 to 23; 4th: 24 and above.
Table 2.
Prognostic characteristics and treatments of men with prostate cancer by number of PSA testing received from 2000 to 2005 prior to diagnosis; SEER-Medicare database. (n=22,047)
| Cancer Characteristics | Number of PSA screening tests from 2000 to 2005* | ||||
|---|---|---|---|---|---|
| Never (n = 1446) | 1 (n = 3692) | 2 (n = 3362) | 3 (n = 3530 ) | 4 to 6 (n = 10017) | |
| Median PSA (95% Confidence Interval), ug/mL | 11.9 | 12.9 | 9.0 | 7.5 | 7.2 |
| (10.9, 13) | (12.3, 13.6) | (8.7, 9.3) | (7.2, 7.7) | (7.0, 7.3) | |
| Gleason Score, % | |||||
| Gleason 2–4 | 16 (1) | 31 (1) | 36 (1) | 33 (1) | 98 (1) |
| Gleason 5–6 | 424 (29) | 988 (27) | 1166 (35) | 1336 (38) | 4358 (44) |
| Gleason 7 | 452 (31) | 1329 (36) | 1237 (37) | 1295 (37) | 3565 (36) |
| Gleason 8–10 | 371 (26) | 1080 (30) | 774 (23) | 766 (22) | 1772 (18) |
| Unknown | 183 (13) | 264 (7) | 149 (4) | 100 (3) | 224 (2) |
| Stage, % | |||||
| T1 | 573 (40) | 1384 (38) | 1465 (44) | 1571 (45) | 4880 (49) |
| T2 | 626 (43) | 1765 (48) | 1581 (47) | 1686 (48) | 4599 (46) |
| T3/T4 | 110 (8) | 266 (7) | 165 (5) | 141 (4) | 252 (3) |
| Unknown | 137 (9) | 277 (8) | 151 (4) | 128 (4) | 286 (3) |
| Risk group, % | |||||
| Low | 279 (19) | 617 (17) | 801 (24) | 1109 (29) | 3171 (32) |
| Intermediate | 496 (34) | 1292 (35) | 1362 (41) | 1434 (41) | 4325 (43) |
| High | 671 (46) | 1786 (48) | 1199 (36) | 1087 (31) | 2521 (25) |
| Treatment in 180 days, % | |||||
| Conservative Management | 716 (50) | 954(26) | 842 (25) | 859 (24) | 2282 (23) |
| Radical Prostatectomy | 74 (5) | 238 (6) | 270 (8) | 310 (9) | 984 (10) |
| Radiation Therapy | 457 (32) | 1291 (35) | 1352 (40) | 1536 (44) | 4742 (47) |
| Hormone Therapy | 199 (14) | 1209 (33) | 898 (27) | 825 (23) | 2009 (20) |
Difference across number of PSA tests received prior to diagnosis were statistically significant (p<.001) for all characteristics.
Factors predicting the frequency of annual PSA testing prior to diagnosis are listed in Table 3. In our initial model (results not shown), age was the strongest predictor of annual testing, but surprisingly, increased Charlson score was associated with an increased frequency of annual PSA testing after adjusting for other factors. We anticipated that number of physician visits might relate to the number of PSA tests received and included this variable in our final model. In this final model, the number of physician visits in one year before cancer diagnosis was strongly associated with the chance of receiving a PSA test (Table 3). Men in poor health status was no longer related to a higher chance of receiving PSA tests after adjusting for number of physician visits when compared with men in good health status. Patients who were white, married, or had relatively higher incomes and education had a higher chance to have annual PSA tests.
Table 3.
Multivariable model of characteristics associated with receiving PSA tests prior to diagnosis and with initial treatments in 180 days, SEER-Medicare database. (n=22,047)
| Outcome | ||
|---|---|---|
| For PSA tests* | For Treatments** | |
| Characteristics | Adjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) |
| No. of PSA tests | ||
| 1 vs None | - | 2.83 (2.48, 3.24) |
| 2 vs None | - | 3.07 (2.67, 3.52) |
| 3 vs None | - | 3.21 (2.79, 3.69) |
| 4 vs None | - | 3.57 (3.14, 4.05) |
| Age | ||
| 70–74 vs 85+ | 1.73 (1.58, 1.89) | 3.52 (3.14, 3.94) |
| 75–79 vs 85+ | 1.77 (1.62, 1.94) | 2.23 (1.99, 2.49) |
| 80–84 vs 85+ | 1.54 (1.40, 1.70) | 1.38 (1.23, 1.54) |
| Race | ||
| Black vs White | 0.68 (0.63, 0.75) | 0.79 (0.71, 0.89) |
| Others vs White | 0.93 (0.85, 1.02) | 0.78 (0.70, 0.87) |
| Region | ||
| North Central vs West | 1.23 (1.14, 1.33) | 1.18 (1.07, 1.30) |
| Northeast vs West | 0.93 (0.87, 1.00) | 1.53 (1.40, 1.67) |
| South vs West | 0.85 (0.79, 0.92) | 1.13 (1.03, 1.24) |
| Lived in ZIP code tabulation area in which 25% or more of adults had a college education | ||
| Yes vs No | 1.40 (1.31, 1.49) | 1.01 (0.93, 1.09) |
| Median annual income of ZIP code tabulation area┼ | ||
| High vs Low | 1.26 (1.16, 1.36) | 1.07 (0.97, 1.19) |
| Mid vs Low | 1.13 (1.05, 1.21) | 1.06 (0.97, 1.16) |
| Marrital Status | ||
| Yes vs no | 1.27 (1.21, 1.34) | 1.62 (1.52, 1.73) |
| Charlson score | ||
| 1 (Average) vs 0 (Good) | 1.14 (1.06, 1.23) | 0.97 (0.88, 1.06) |
| >=2 (Poor) vs 0 (Good) | 0.84 (0.76, 0.95) | 0.89 (0.78, 1.03) |
| No. of physician visits one year before Cancer diagnosis ‡ | ||
| 2nd Quartile vs 1st Quartile | 2.70 (2.52, 2.89) | 1.15 (1.05, 1.26) |
| 3rd Quartile vs 1st Quartile | 3.54 (3.30, 3.79) | 1.13 (1.03, 1.25) |
| 4th Quartile vs 1st Quartile | 3.97 (3.69, 4.27) | 1.10 (1.00, 1.20) |
| Risk Group | ||
| Low vs High | - | 0.34 (0.31, 0.37) |
| Intermediate vs High | - | 0.72 (0.66, 0.78) |
Treatments include radical prostatectomy, radiation therapy and primary androgen deprivation therapy.
Modeling the risk of receiving PSA tests adjusted for all variables in the table, except Number of PSA tests and Risk Group.
Modeling the risk of receiving treatments adjusted for all variables in the table.
Median annual income of ZIP code tabulation area was categorized into High: >=$50,000; Middle: >=35,000; <50,000, Low: <35,000.
Number of physician visits one year before cancer diagnosis was grouped by quartiles: 1st: 0 to 7 times; 2nd: 7 to 13; 3rd: 13 to 23; 4th: 23 and above.
Factors predicting a higher frequency of PSA testing also predicted the likelihood of receiving cancer therapy (Table 3). Compared to men who had never been tested, the chance of receiving a cancer treatment was 2.83 (95% CI: 2.48–3.24) times in men who received only one PSA test and 3.57(95% CI: 3.14–4.05) times in men who received 4 to 6 PSA tests. This was true for men in all age categories and health conditions. Men who lived in the northeast area were more likely to receive treatments than men who lived in other regions.
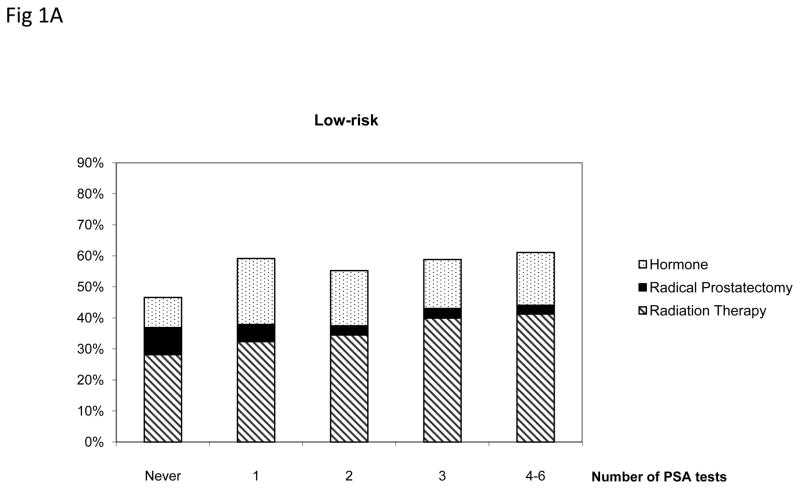
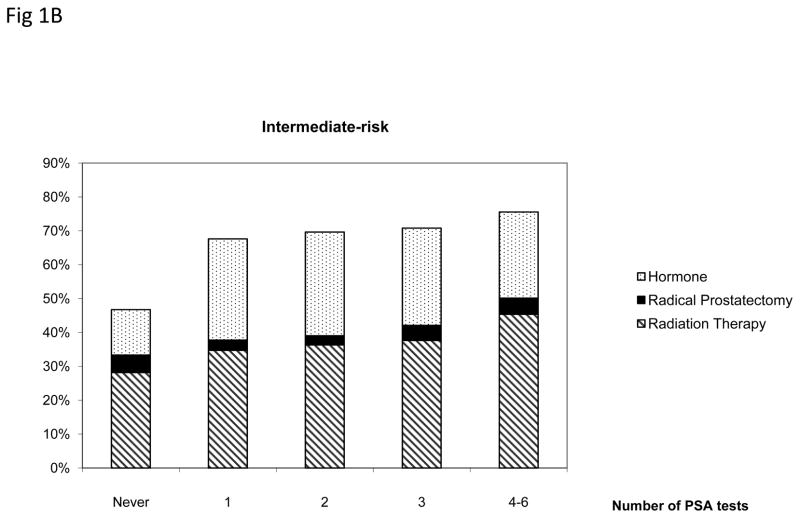
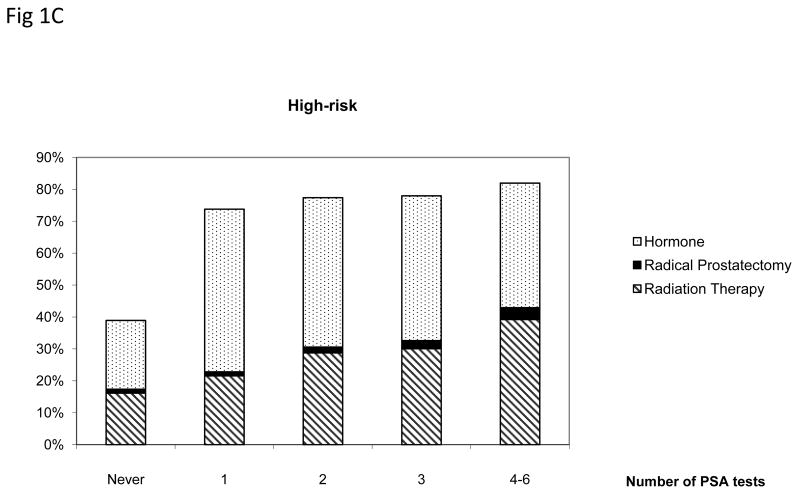
Figure 1 presents the treatment pattern among men aged 75 and older who were diagnosed with prostate cancer stratified by the number of prior annual PSA tests and cancer risk groups. Men who received more than one PSA testing prior to diagnosis were more likely to receive treatments within 180 days (p<.005). In the low-risk group, men who underwent four to six PSA tests were 1.78 times more likely to received treatments compared with men who had not had annual PSA tests (p<.005). Radical prostatectomy and radiation therapy were more common among low- and intermediate risk patients than among high-risk patients.
Figure 1.
Treatment patterns among men aged 75 and older diagnosed with low-, intermediate- and high-risk prostate cancer by the number of prostate specific antigen (PSA) tests, SEER-Medicare 2004–2005.
Only radical prostatectomy, radiation therapy or hormone therapy received within 180 days after diagnosis were included.
* Radical prostatectomy includes patients who received radical prostatectomy alone or with radiation therapy or hormone within 180 days after diagnosis. Radiation therapy includes patients who received radiation therapy alone or with hormone.
+ Difference across number of PSA tests was statistically significant for all risk groups.
DISCUSSION
Our findings suggest that men receiving more PSA tests before cancer diagnosis is associated with a higher chance in receiving cancer therapy. Among men in the highest testing group (4 to 6 PSA tests), 75% were diagnosed with low- or intermediate- risk of cancer but 77% received treatments in 180 days. Only 50% of men who had no prior PSA test received active therapy. Our previous study7 showed that aggressive local therapy was provided to most patients diagnosed with prostate cancer due to the inability to distinguish indolent from aggressive cancers. Results from this study further demonstrated that more intensive PSA testing among the elder population is likely to exacerbate the risk of overdiagnosis and overtreatment.
Screening in the U.S. is predominantly opportunistic. Opportunistic screening depends on the health care providers to recommend screening or on individuals to request screening. It involves fewer formal decisions regarding whom to screen, whether to screen and at what interval. Among men diagnosed with prostate cancer in 2004–2005, almost half have undergone annual PSA testing prior to their diagnosis and many of these men have a limited life expectancy. Our results demonstrate that PSA testing appears to correlate more with the number of physician visits rather than the risk of developing prostate cancer or the health status of the patient. The possibility that opportunistic screening could cause more harm than good has been a concern. 21 Although intensive PSA testing may diagnose patients at an earlier stage of cancer, data from ERSPC study demonstrated that cancers diagnosed from repeated screening has a minimal effect on detecting clinically significant disease, 22,23 and are not associated with reduced prostate cancer mortality.2 Despite this evidence many clinicians continue to screen Medicare eligible men annually, possibly because they over estimate the benefit and do not appreciate the potential harms of PSA screening. Many may lack the time to explain the pros and cons of PSA screening strategies while others may be concerned about medical-legal implications. 24,25
Our results support the USPSTF concern of screening men with less than a 10-year life expectancy because of the increased likelihood of harm from the consequence of screening relative to any potential benefit.26 For men aged 75 and older with low-risk prostate cancer, active surveillance may be the best treatment option when compared to the treatment-related side effects and the modest survival benefit associated with more aggressive treatment. 3–5,27 However, we found that men with low-risk cancer were treated intensively after cancer diagnosis. Men who had 4 to 6 PSA tests prior to diagnosis were much more likely to receive treatment within 180 days, particularly radiation therapy, than men who did not have PSA test. Men who had assumed they would benefit from early detection and cancer treatment before receiving a PSA test and the number of PSA tests they received prior to cancer diagnosis could be interpreted as their level of belief regarding PSA screening for prostate cancer.
Medicare started to cover annual PSA tests since 2000 despite the uncertainty surrounding the cost-effectiveness to provide PSA testing for early detection.28 In 2004, Medicare expenditures for prostate cancer ranked third highest among cancer patients after lung cancer and colorectal cancer.29 Alone with evidence suggesting the lack of benefit to screen men who are aged 75 and above,5 our data show that more frequent PSA testing and the subsequent diagnosis of additional prostate cancers often lead to more cancer therapies that may not result in reduced prostate cancer mortality. Therefore, specifying screening eligibility criteria and screening interval may yield benefits at the population level.30,31 If Medicare beneficiaries follow the USPSTF recommendation of no screening after age of 75 or screened only once in a four-year interval, many men would be spared a cancer diagnosis and treatment. Consequently, Medicare costs would decrease dramatically with a modest impact on prostate cancer mortality.
Strengths and Limitations
This is the first population based study documenting the association between PSA testing frequency and prostate cancer treatment patterns among Medicare beneficiaries. The linked dataset provided the power to study the consequence of PSA testing because of its size but limited our analysis to men diagnosed with prostate cancer at age 70 years and older. We cannot distinguish PSA screening from diagnostic PSA testing in Medicare claims where men might receive PSA test because of symptoms. However, more than 40% of men in our study diagnosed at T1c stage indicate that they were diagnosed from screening. Most of these men diagnosed at T1c stage were low- or intermediate- risk which did not need any intensive treatment necessarily, but they were mostly treated. Our analysis was unable to identify PSA testing that occurred outside of the Medicare reimbursement system. As a consequence, the prevalence of PSA testing may be even higher than recorded in our study. Furthermore, whether patients selected treatment because of their concerns about disease progression or were urged to undergo treatment by their treating physicians cannot be determined by this study.
The USPSTF recommendation against screening for men aged 75 and older is because of concerns that the risks may outweigh benefits among men with limited life expectancy. Our study supports the concerns by showing the substantial downstream effect on treatment utilization. Because prostate cancer progresses slowly and the ten year disease specific mortality is low,32 we could not evaluate the association between PSA testing and prostate cancer specific mortality. Previous studies suggest that more intensive screening and treatment is not associated with lower prostate cancer mortality among elderly men.2
Conclusion
Our study reveals the profound impact of intensive PSA testing on cancer diagnosis and treatment. Given the lack of evidence of effective treatment for men older than 65 years of age diagnosed with low- and intermediate-risk prostate cancer and our inability to distinguish indolent from aggressive cancer, intensive PSA testing is likely to exacerbate the risk of overdiagnosis and overtreatment among elderly men.
Acknowledgments
Funding: The study was supported by the following grants and awards: National Cancer Institute grant # RO1 CA 116399, Cancer Institute of New Jersey core grant NCI CA-72720-10 and Robert Wood Johnson foundation grant # 60624. The funding source had no role in the design, conduct, or analysis of this study or in the decision to submit the manuscript for publication.
Footnotes
Conflict of Interest: None to be reported.
Authorship/Disclaimer: All authors had access to the data, and a role in writing the manuscript. This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The content of the information does not reflect the position or policy of the Government or the employers, and no official endorsement should be inferred.
References
- 1.Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317(15):909–916. doi: 10.1056/NEJM198710083171501. [DOI] [PubMed] [Google Scholar]
- 2.Lu-Yao G, Albertsen PC, Stanford JL, Stukel TA, Walker-Corkery ES, Barry MJ. Natural experiment examining impact of aggressive screening and treatment on prostate cancer mortality in two fixed cohorts from Seattle area and Connecticut. BMJ. 2002;325(7367):740. doi: 10.1136/bmj.325.7367.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bill-Axelson A, Holmberg L, Ruutu M, Haggman M, Andersson SO, Bratell S, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352(19):1977–1984. doi: 10.1056/NEJMoa043739. [DOI] [PubMed] [Google Scholar]
- 4.Kawachi MH, Bahnson RR, Barry M, Busby JE, Carroll PR, Carter HB, et al. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer Early Detection. J Natl Compr Canc Netw. 2010;8(2):240–262. doi: 10.6004/jnccn.2010.0016. [DOI] [PubMed] [Google Scholar]
- 5.Bill-Axelson A, Holmberg L, Ruutu M, Garmo H, Stark JR, Busch C, et al. Radical Prostatectomy versus Watchful Waiting in Early Prostate Cancer. New England Journal of Medicine. 2011;364(18):1708–1717. doi: 10.1056/NEJMoa1011967. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer Statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 7.Shao YH, Albertsen PC, Roberts CB, Lin Y, Mehta AR, Stein MN, et al. Risk profiles and treatment patterns among men diagnosed as having prostate cancer and a prostate-specific antigen level below 4. 0 ng/ml. Arch Intern Med. 2010;170(14):1256–1261. doi: 10.1001/archinternmed.2010.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Screening for prostate cancer: U S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(3):185–191. doi: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 9.Schaeffer EM, Carter HB, Kettermann A, Loeb S, Ferrucci L, Landis P, et al. Prostate specific antigen testing among the elderly--when to stop? J Urol. 2009;181(4):1606–1614. doi: 10.1016/j.juro.2008.11.117. discussion 1613–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walter LC, Bertenthal D, Lindquist K, Konety BR. PSA screening among elderly men with limited life expectancies. JAMA. 2006;296(19):2336–2342. doi: 10.1001/jama.296.19.2336. [DOI] [PubMed] [Google Scholar]
- 11.Hudson SV, Ohman-Strickland P, Ferrante JM, Lu-Yao G, Orzano AJ, Crabtree BF. Prostate-specific antigen testing among the elderly in community-based family medicine practices. J Am Board Fam Med. 2009;22(3):257–265. doi: 10.3122/jabfm.2009.03.080136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 13.Population Projections, United States, 2004 – 2030, by state, age and sex, on CDC WONDER On-line Database, September 2005., 2005.
- 14.Freeman JL, Klabunde CN, Schussler N, Warren JL, Virnig BA, Cooper GS. Measuring breast, colorectal, and prostate cancer screening with medicare claims data. Med Care. 2002;40(8 Suppl):IV-36–42. doi: 10.1097/00005650-200208001-00005. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 17.American Joint Committee on Cancer. Manual for Staging of Cancer. 5. JB Lippincott; Philadelphia: 1997. [Google Scholar]
- 18.Hamilton AS, Gloeckler Ries L. Cancer of Prostate. In: Ries L, Young J, Keel G, Eisner M, YDLM-JH, editors. SEER Survival Monograph: Cancer Survival Among Adults: US SEER Program, 1988–2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub; Bethesda, MD: 2007. [Google Scholar]
- 19.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 20.Mehta CR, Patel NR, Tsiatis AA. Exact significance testing to establish treatment equivalence with ordered categorical data. Biometrics. 1984;40(3):819–825. [PubMed] [Google Scholar]
- 21.Recommendations on cancer screening in the European union. Advisory Committee on Cancer Prevention. Eur J Cancer. 2000;36(12):1473–1478. doi: 10.1016/s0959-8049(00)00122-2. [DOI] [PubMed] [Google Scholar]
- 22.van der Cruijsen-Koeter IW, Roobol MJ, Wildhagen MF, van der Kwast TH, Kirkels WJ, Schroder FH. Tumor characteristics and prognostic factors in two subsequent screening rounds with four-year interval within prostate cancer screening trial, ERSPC Rotterdam. Urology. 2006;68(3):615–620. doi: 10.1016/j.urology.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Roobol MJ, Grenabo A, Schroder FH, Hugosson J. Interval cancers in prostate cancer screening: comparing 2- and 4-year screening intervals in the European Randomized Study of Screening for Prostate Cancer, Gothenburg and Rotterdam. J Natl Cancer Inst. 2007;99(17):1296–1303. doi: 10.1093/jnci/djm101. [DOI] [PubMed] [Google Scholar]
- 24.Merenstein D. A piece of my mind. Winners and losers. JAMA. 2004;291(1):15–16. doi: 10.1001/jama.291.1.15. [DOI] [PubMed] [Google Scholar]
- 25.Linder SK, Hawley ST, Cooper CP, Scholl LE, Jibaja-Weiss M, Volk RJ. Primary care physicians’ reported use of pre-screening discussions for prostate cancer screening: a cross-sectional survey. BMC Fam Pract. 2009;10:19. doi: 10.1186/1471-2296-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin K, Lipsitz R, Miller T, Janakiraman S. Benefits and harms of prostate-specific antigen screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(3):192–199. doi: 10.7326/0003-4819-149-3-200808050-00009. [DOI] [PubMed] [Google Scholar]
- 27.Hayes JH, Ollendorf DA, Pearson SD, Barry MJ, Kantoff PW, Stewart ST, et al. Active surveillance compared with initial treatment for men with low-risk prostate cancer: a decision analysis. JAMA. 2010;304(21):2373–2380. doi: 10.1001/jama.2010.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barry MJ, Fleming C, Coley CM, Wasson JH, Fahs MC, Oesterling JE. Should Medicare provide reimbursement for prostate-specific antigen testing for early detection of prostate cancer? Part I: Framing the debate. Urology. 1995;46(1):2–13. doi: 10.1016/s0090-4295(99)80151-4. [DOI] [PubMed] [Google Scholar]
- 29.Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100(9):630–641. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 30.Miles A, Cockburn J, Smith RA, Wardle J. A perspective from countries using organized screening programs. Cancer. 2004;101(5):1201–1213. doi: 10.1002/cncr.20505. [DOI] [PubMed] [Google Scholar]
- 31.Ross KS, Carter HB, Pearson JD, Guess HA. Comparative efficiency of prostate-specific antigen screening strategies for prostate cancer detection. JAMA. 2000;284(11):1399–1405. doi: 10.1001/jama.284.11.1399. [DOI] [PubMed] [Google Scholar]
- 32.Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302(11):1202–1209. doi: 10.1001/jama.2009.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]