Abstract
Purpose
The 27-kDa heat shock protein (HSP27) has been implicated in wound healing in multiple tissues. We investigated the expression and localization of phosphorylated HSP27 during epithelial wound healing in the murine cornea.
Methods
Corneas of 8- to 10-week-old C57BL6 mice were wounded by epithelial debridement (n = 40). Unwounded corneas served as controls (n = 3). After 3, 7, and 14 days, phosphorylated HSP27 localization in wounded corneas was observed by confocal immunohistochemistry and double immunogold labeling transmission immunoelectron microscopy. Western blot analysis was performed to determine expression levels of phosphorylated HSP27 in scraped epithelia. Phosphorylated HSP27 localization was also separately performed with confocal immunohistochemistry 8 hours after epithelial debridement to investigate the early epithelial wound-healing process.
Results
In unwounded corneas, phosphorylated HSP27 was localized only to the superficial epithelium. In contrast, phosphorylated HSP27 was localized in the basal and superficial epithelia 3 days after corneal epithelial wounding. After 7 and 14 days, HSP27 localization was similar to that in unwounded controls. Expression levels of phosphorylated HSP27 were greater in wounded corneal epithelia on day 3 than in unwounded controls and on day 14. After 8 hours, phosphorylated HSP27 expression was prominent in the leading edge of migrating corneal epithelium.
Conclusions
Constitutive expression of phosphorylated HSP27 is limited to the superficial corneal epithelium in unwounded murine corneas. Changes in HSP27 epithelial distribution and expression levels after corneal epithelial wounding suggest that phosphorylated HSP27 plays a role in early phase of corneal epithelial wound healing.
Keywords: heat shock protein, corneal epithelium, epithelial wound healing, cornea
Heat shock proteins (HSPs) are highly conserved and classified into 2 families according to molecular size. The larger HSP family includes HSP90, HSP70, and HSP60, and the smaller family consists of HSP27.1 Human HSP27, also known as mouse HSP25, has various functions. Depending on expression and phosphorylation levels, HSP27 is an actin-capping protein in vitro and inhibits actin polymerization.2,3 HSP27 also acts as a molecular chaperone to inhibit thermal denaturation and aid in the refolding of citrate synthase in vitro.4 In vivo, HSP27 enhances pinocytosis, promotes endothelial cell migration, and provides resistance to cellular injury by stabilizing microfilaments after heat shock.5-7 In addition, HSP27 maintains endogenous antioxidants, such as glutathione, to decrease damage induced by reactive oxygen species and also acts as an antiapoptotic mediator.8-11 In murine inner retinal layers, HSP27 protected the retina from ischemic injury,12 and in human corneal epithelium, HSP27 displayed rapid phosphorylation and translocation after ultraviolet-B irradiation.13
The biological activity of HSP27 varies depending on intracellular localization, phosphorylation status, and oligomerization level.4-6,14,15 HSP27 phosphorylation is believed to play an important role in cell survival because it has been associated with the inhibition of Daxx-mediated apoptosis and Akt-mediated apoptosis.16,17 Cells that overexpress phosphorylatable HSP27 are more resistant to heat and oxidative stress than are cells that express unphosphorylatable mutants.16 In various cell types, HSP27 phosphorylation is required for cell migration, probably because of its influence on actin remodeling.18-20
In this study, we investigated the presence of phosphorylated HSP27 in murine corneal tissue and its role in corneal wound healing after full-thickness epithelial debridement.
MATERIALS AND METHODS
Animals were handled in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Eight- to 10-week-old C57BL6 mice were divided into 2 groups: unwounded controls (n = 3) and those treated with epithelial debridement (n = 10, 2 per time point).
Epithelial Debridement
Mice were anesthetized with intramuscular injection of 30 μL of 1:1 ketamine hydrochloride (100 g/mL; Hospira, Inc, Lake Forest, IL) and xylazine (20 mg/mL; X-JECT SA; Burns Veterinary Supply, Inc, Westbury, NY) and treated with topical 0.5% proparacaine eye drops (Akorn, Inc, Buffalo Grove, IL) to reduce pain. Full-thickness epithelial debridement was performed with a surgical blade after marking a 1.5-mm-diameter area in the central cornea with a 1.5-mm-diameter trephine. Two percent erythromycin ophthalmic ointment (Fougera; Fougera, Melville, NY) was applied once to the central corneal area. In the experimental group, mice were killed 0, 3, 7, and 14 days after epithelial debridement, and whole eyeballs were cryoblocked immediately. The controls were treated exactly the same as experimental subjects except for the debridement. To investigate the expression of phosphorylated HSP27 in early epithelial wound-healing process, an experimental subgroup of mice was separately killed 8 hours after epithelial debridement and their eyeballs were also cryoblocked.
Confocal Immunohistochemistry
Confocal immunohistochemistry was performed on murine corneas as described previously.21 Briefly, cryosections (8 μm) from mouse corneas were fixed in paraformaldehyde for 5 minutes. After blocking with 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS, pH 7.4), sections were incubated overnight with primary rabbit polyclonal antibodies against nonphosphorylated HSP27 (ab12351; Abcam Inc, Cambridge, MA) or primary rabbit polyclonal antibody against phosphorylated HSP27 (ab5594; hsp27 phospho S85, phosphorylated at Ser85; Abcam, Inc). After washing with PBS, sections were incubated for 30 minutes with secondary antibodies and observed using a confocal microscope (TCS 4D; Leica Microsystems, Inc, Bannockburn, IL). Primary antibody omission served as a negative control.
Transmission Immunoelectron Microscopy
Immunogold electron microscopy was performed using a rabbit polyclonal antibody against phosphorylated HSP27 (hsp27 phospho S85; Abcam, Inc) to localize phosphorylated HSP27. Corneal tissue was excised from limbus to limbus, washed 3 times in sodium phosphate buffer (pH 7.4), and fixed in 4% (vol/vol) paraformaldehyde and 1% (vol/vol) glutaraldehyde in 100 mM sodium phosphate buffer (pH 7.4) for 16 hours at 4°C. Next, tissue samples were washed 3 times for 10 minutes per wash in phosphate buffer and dehydrated for 105 minutes in a graded series of ethanol solutions [25%, 50%, 75%, 95%, and 100% (vol/vol) with 3 changes of each at 15-minute intervals]. Tissue infiltration continued in 2:1 ethanol:LR White resin and then in 1:1 and 1:2 relative ratios of each for 1 day. Pure LR White resin infiltration was completed over a 24-hour period with 3 changes, and samples were polymerized in 1 mL of pure LR White resin under vacuum at 50°C for 3 days. Cured blocks were trimmed with a razor blade and sectioned with a diamond knife on a Rie-chert Ultra Cut E ultramicrotome (Leica Microsystems, Inc). Sections were lifted onto 200-hex-mesh carbon/parlodion–coated Ni grids and dried. Thin sections on the Ni grids were incubated for 45 minutes at room temperature in blocking solution [0.8% (wt/vol) BSA, 0.1% (wt/vol) immunogold–silver stain–quality gelatin, 5% (wt/vol) normal goat serum in PBS, and 50 μL of 2% azide], washed twice in 0.8% BSA, 0.1% gelatin, and 0.025% (vol/vol) Tween 20 in PBS, and incubated overnight with antiphosphorylated HSP27 antibody at a concentration of 2.6 μg/mL in 0.8% BSA, 0.1% gelatin, and 1% normal goat serum in PBS. Subsequently, sections were washed 4 times in washing solution and incubated for 4 hours with 10-nm immunogold-labeled secondary antibody (goat antirabbit IgG; GE Healthcare, Piscataway, NJ) diluted 1:25. Grids were washed 6 times with washing solution for 5 minutes per wash and then twice with PBS, and the samples were fixed for 10 minutes in 2% (vol/vol) glutaraldehyde in PBS. Next, grids were washed 3 times in distilled water, dried, stained with 2% (wt/vol) aqueous uranyl acetate for 45 minutes, washed with distilled water, and dried again. Finally, grids were stained with lead citrate for 5 minutes, washed with distilled water, dried, and photographed with a JEOL 1200 EX transmission electron microscope (JEOL, Ltd, Tokyo, Japan). Seven randomly selected cells were observed at each of 3 time points; gold particles were localized by morphological criteria and counted. Cross-sectional areas of cell wall and cell interiors were assessed by weighing traced-out photographs and comparing them with equivalent traced standard areas.
For double immunogold labeling transmission electron microscopy, the above procedure was followed, with the exception that goat antimouse nonphosphorylated HSP27 (sc-1048; Santa Cruz Biotechnology, Inc, Santa Cruz, CA) and rabbit antimouse phosphorylated HSP27 (hsp27 phosphor S85; Abcam, Inc) were used as primary antibodies, and 25-nm immunogold-labeled donkey antigoat IgG (GE Healthcare) and 10-nm immunogold-labeled donkey antirabbit IgG (GE Healthcare) were used as secondary antibodies.
Western Blot Analysis
Corneal epithelia were isolated from 10 mice (1 eye per mouse) 0, 3, and 14 days after epithelial debridement. The isolated epithelium was lysed in 100 μL of radioimmunoprecipitation assay buffer mixed with 2 μL of protease inhibitor cocktail (Catalog No. P8340 and P7626, 1 μL each; Sigma-Aldrich, Inc, St Louis, MO) and 0.5 μL of sodium fluoride to block proteases and phosphatases and stored in refrigerator at −20°C, and the total protein concentration was estimated using the Pierce BSA Protein Assay Kit (Pierce Biotechnology, Inc, Rockford, IL). Equal amounts of protein were loaded on a sodium dodecyl sulfate–polyacrylamide gel for electrophoresis, and the electrophoresed proteins were transferred to Immobilon P membranes (Millipore, Co, Billerica, MA), blocked with 3% BSA for 60 minutes, and incubated with either rabbit anti-nonphosphorylated HSP27 antibody or rabbit antiphosphorylated HSP27 antibody. Blots were incubated with horseradish peroxidase donkey antigoat or antirabbit antibody (GE Healthcare). After washing with Tris-buffered saline–Tween-20 for 60 minutes, immunoblots were developed with an enhanced chemiluminescence reagent (PerkinElmer, Inc, Waltham, MA).
RESULTS
Constitutive Immunolocalization of Phosphorylated HSP27
Nonphosphorylated HSP27 was present in all epithelial layers of unwounded mouse corneas (Figs. 1A, B). Phosphorylated HSP27 was localized only in the superficial epithelium of unwounded corneas (Figs.1C, D). The immunogold electron microscopic photograph showed the presence of phosphorylated HSP27 within the superficial epithelium of unwounded central corneas (Fig. 1E).
FIGURE 1.
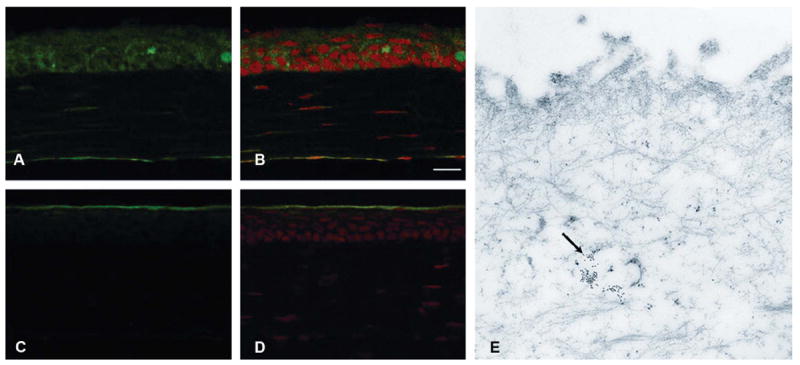
Constitutive immunolocalization of nonphosphorylated HSP27 in unwounded corneas (A) and merged image with PI staining (B). Localization of phosphorylated HSP27 in unwounded corneas (C) and merged image with propidium iodide staining (D). Localization of phosphorylated HSP27 in unwounded corneas by immunogold electron microscopy (E, indicated with black arrow). Nonphosphorylated HSP27 was present in all epithelial layers of unwounded mouse corneas (A and B), whereas phosphorylated HSP27 was localized only in the superficial epithelium of unwounded corneas (C–E).
Immunolocalization of Phosphorylated HSP27 During Corneal Wound Healing
Phosphorylated HSP27 was not detected in wounded cornea tissue on day 0 because of the absence of epithelium (Figs. 2A, B). On day 3, the epithelialization was complete within the wounded corneas. Phosphorylated HSP27 was localized to the superficial and basal epithelial layers, and the expression intensity was similar in all epithelial layers (Figs. 2C, D). After 7 days, phosphorylated HSP27 expression in the basal epithelium was less than that in the superficial epithelium (Figs. 2E, F). After 14 days, phosphorylated HSP27 was localized only to the superficial epithelium as in unwounded corneas (Figs. 2G, H).
FIGURE 2.
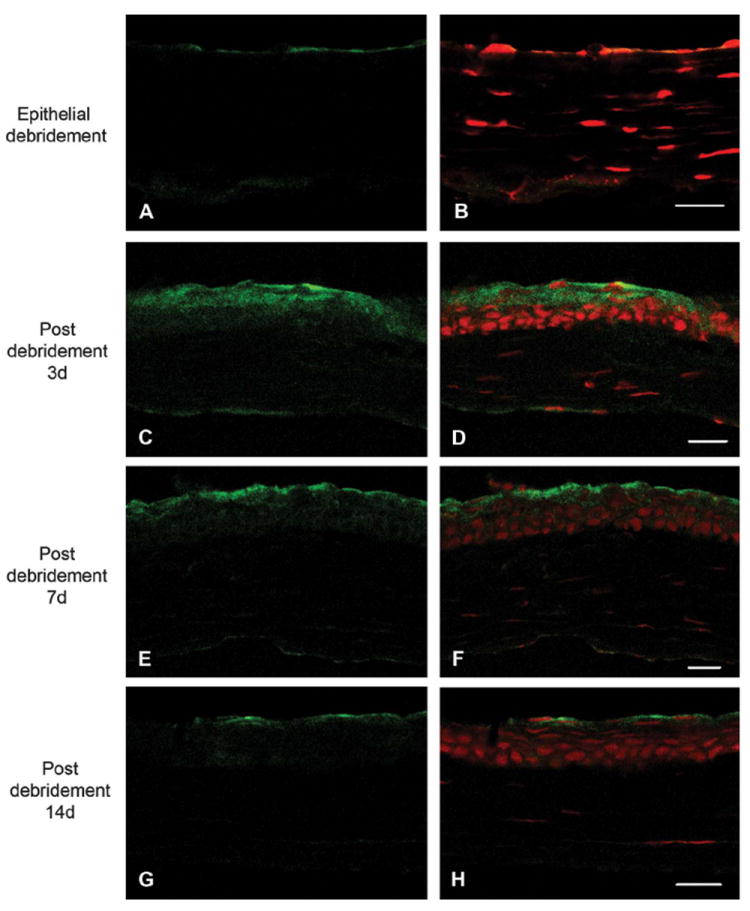
Immunolocalization of phosphorylated HSP27 in wounded central cornea at 0 days (A), 3 days (C), 7 days (E), and 14 days (G) after epithelial debridement and merged images with propidium iodide staining (B, D, F, and H), respectively. Phosphorylated HSP27 was not detected on day 0 because of the absence of epithelium (A and B). On day 3, phosphorylated HSP27 was localized to the superficial and basal epithelial layers, and the expression intensity was similar in all epithelial layers (C and D). After 7 days, phosphorylated HSP27 expression in the basal epithelium was less than that in the superficial epithelium (E and F). After 14 days, phosphorylated HSP27 was localized only to the superficial epithelium as in unwounded corneas (G and H). Scale bar = 20 μm.
At 8 hours after epithelial debridement, the epithelialization was not complete, and phosphorylated HSP27 expression was prominent at the leading edge of the migrating corneal epithelium (Figs. 3A, B). Phosphorylated HSP27 in wounded central cornea was localized to the superficial and basal epithelial layers, and the staining intensity was similar in all epithelial layers (Figs. 3C, D). However, phosphorylated HSP27 in unwounded peripheral cornea was localized only to the superficial epithelium (Figs. 3E, F).
FIGURE 3.
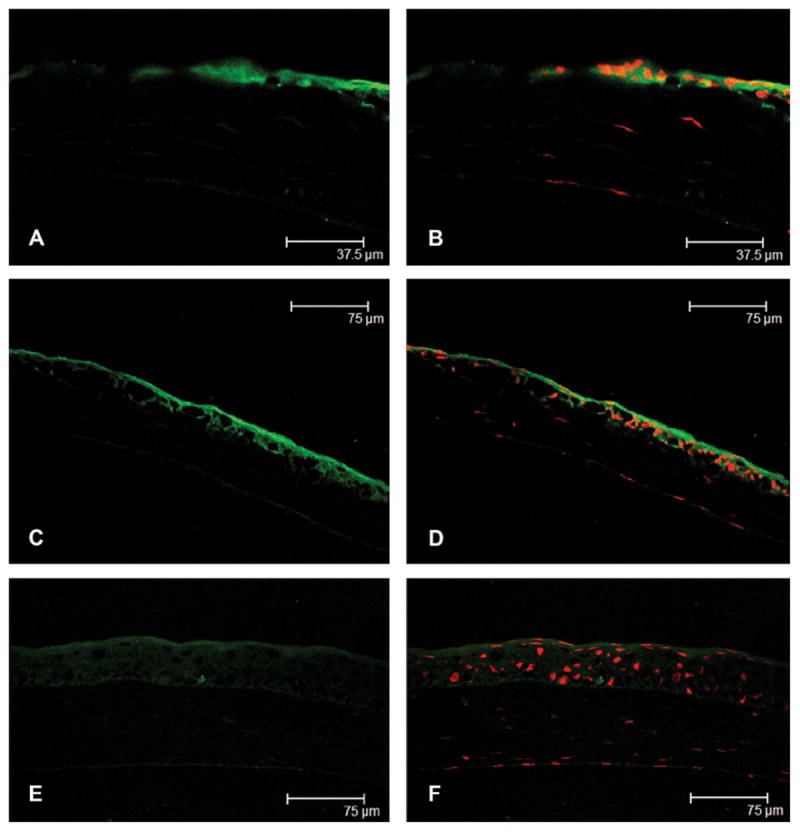
Immunolocalization of phosphorylated HSP27 from the wounded central cornea (A and C) to the unwounded peripheral cornea (E) at 8 hours after epithelial debridement and merged images with propidium iodide staining (B, D, and F), respectively. The epithelialization was not complete, and phosphorylated HSP27 expression was prominent at the leading edge of the healing central corneal epithelium (A and B). Phosphorylated HSP27 in wounded central cornea was localized to the superficial and basal epithelial layers, and the staining intensity was similar in all epithelial layers (C and D). However, phosphorylated HSP27 in unwounded peripheral corneal was localized only to the superficial epithelium (E and F).
Colocalization of Nonphosphorylated and Phosphorylated HSP27
Double immunogold labeling transmission electron microscopy revealed that the distribution of nonphosphorylated HSP27 remained unchanged in the superficial and basal epithelium over 3 time points. The distribution of phosphorylated HSP27 in the basal epithelium was sparse in unwounded corneas (Fig. 4D) and increased after 3 days (Fig. 4E) and was sparse again after 14 days (Fig. 4F), whereas that in the superficial epithelium did not differ as time went (Figs. 4A–C).
FIGURE 4.
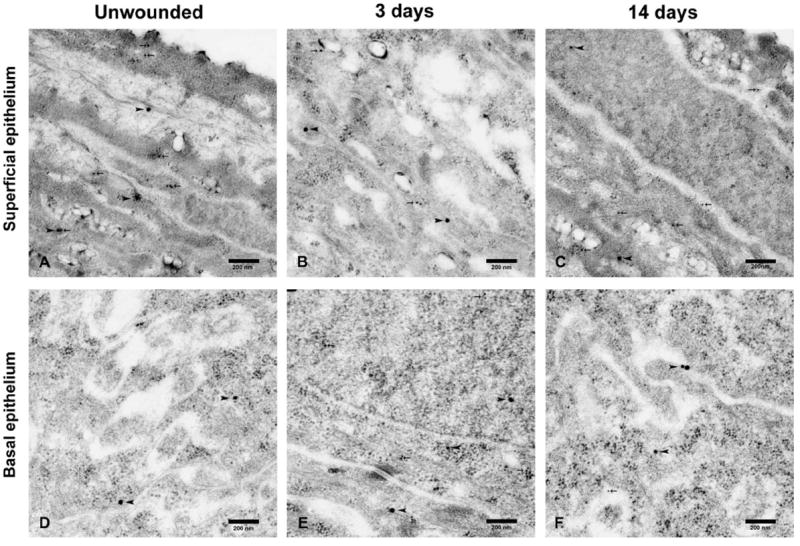
Detection of nonphosphorylated HSP27, indicated by arrow head, and phosphorylated HSP27, marked by arrow, by double immunogold labeling transmission electron microscopy in unwounded superficial (A) and basal (D) epithelial layers, superficial epithelial layers of wounded corneas after 3 days (B) and 14 days (C), and basal epithelial layers of wounded corneas after 3 days (E) and 14 days (F). The distribution of nonphosphorylated HSP27 remained unchanged in the superficial and basal epithelium over 3 time points. The distribution of phosphorylated HSP27 in the basal epithelium was sparse in unwounded corneas (D) and increased after 3 days (E) and was sparse again after 14 days (F), whereas that in the superficial epithelium did not differ as time went (A–C).
Quantification of Nonphosphorylated and Phosphorylated HSP27
Western blot analysis demonstrated that the expression of phosphorylated HSP27 was increased in corneal epithelia at 3 days compared with that in unwounded epithelia and wounded epithelia at 14 days, whereas the expression of nonphosphorylated HSP27 was consistent at each time point (Fig. 5).
FIGURE 5.
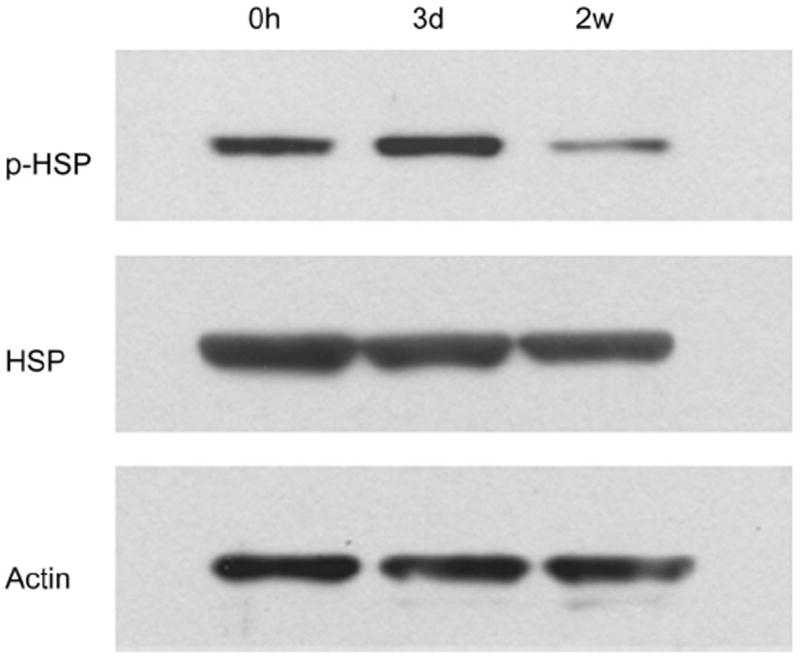
Western blot analysis with antibodies against phosphorylated HSP27 and nonphosphorylated HSP27 in unwounded corneal epithelia and epithelia at 3 days and 14 days after epithelial debridement. The expression of phosphorylated HSP27 was increased in corneal epithelia at 3 days compared with that in unwounded epithelia and wounded epithelia at 14 days, whereas the expression of non-phosphorylated HSP27 was consistent at each time point.
DISCUSSION
The heat shock 27-kDa protein 1 (HSPB1 gene) encodes the protein HSP beta-1, also called HSP27 in humans and HSP25 or HSP27 in mice. In mice, HSP25 and HSP27 are aliases for heat shock 27-kDa protein 1. The murine and human HSP27 share more than 80% primary sequence identity.22 The similarity of murine and human HSP27 has been reported in the literature.3,5,23,24 In this article, we have used HSP27 to denote the heat shock 27-kDa protein in consonance with published reports.
The biological activity of HSP27 varies with intracellular localization, phosphorylation status, and oligomerization level. Under unstressed conditions, HSP27 levels are usually low, and the protein exists as a large oligomeric unit of up to 800 kDa.20 Under stress, HSP27 phosphorylation precedes an increase in HSP27 levels and changes the quaternary structure of HSP27 from a large 600- to 800-kDa homotypic multimer to dimers and monomers.4,25 HSP27 phosphorylation occurs at several residues, Ser-15, Ser-78, Ser-82, Ser-85, and Thr-14320,26-28 and is dependent on various kinases such as mitogen-activated protein kinase–activated protein kinase 2/3, protein kinase C, cyclic guanosine monophosphate-dependent protein kinase, and protein kinase D.27,29-33 In this study, we postulated that a direct correlation exists between corneal epithelial wound healing and HSP27 phosphorylation in wild-type mouse cornea. HSP27 phosphorylation and upregulation are involved in the migration of canine tracheal myocytes, human airway epithelial cells, and bovine arterial endothelial cells.7,19,34 Contractile proteins, such as actin, myosin, and microtubule, are important in cytoarchitectural remodeling and cell migration during wound healing. Serum-starved fibroblastic cells showed a dramatic decrease in filamentous actin (F-actin) content after exiting the growth cycle, and after readdition of serum or specific growth factors, microfilaments rapidly reformed beneath the membrane and reorganized into stress fibers that extended through the cytoplasm.35,36 Actin polymerization/depolymerization occurs predominantly at the leading edge of fibroblastic cells and affects plasma membrane activities, such as ruffling, pinocytosis, extension of lamellipodia (a site of actin polymerization in fibroblastic cells), and cell motility.35-38 This dynamic process is controlled in vivo by an actin-capping protein, HSP27, which inhibits actin polymerization. HSP27 phosphorylation also alters actin dynamics. Nonphosphorylated HSP27 monomers have been reported to inhibit the polymerization of actin.2,3 Interestingly, the F-actin modulating activity of HSP27 is inhibited by HSP27 phosphorylation.3 The phosphorylation of HSP27 also affects the generation of lamellipodia microfilaments structures that aid in cellular migration.3,7,19 HPS27 phosphorylation-mediated microfilament reorganization plays a role in filament stabilization.6,18,39 However, hydrogen peroxide–inducible clone (Hic-5), a binding protein of HSP27, also binds the focal adhesion structural protein vinculin and focal adhesion kinase.40-42 Hic-5 reduces integrin-mediated cell migration by competing with paxillin in binding to sites on paxillin-binding proteins and also by inhibiting paxillin tyrosine phosphorylation.43 HSP27 phosphorylation and expression affect cellular adhesion by controlling the availability of Hic-5.44
In our experiments, we showed that phosphorylated HSP27 was localized to the superficial epithelial layer of unwounded mouse corneas. Interestingly, increased HSP27 phosphorylation was observed 3 days after injury. This finding is consistent with that of Hirano et al45 regarding the rat full-thickness skin defect model. Similar to the corneal wound, fibroblasts and myofibroblasts that are involved in skin wound healing are believed to be the source of a contractile force.45,46 Myofibroblasts are observed in actively contracting tissues, such as granulation tissue and hypertrophic scar tissue.47 HSP27 is highly phosphorylated in granulation tissue and wound edges.45
The expression pattern of phosphorylated HSP27 during corneal epithelial wound healing was noticeable. Phosphorylated HSP27 was expressed only in the superficial epithelium of unwounded corneas. In contrast, in wounded corneas after 3 days, phosphorylated HSP27 was localized to the superficial and the basal epithelial layer. After 7 days, phosphorylated HSP27 expression remained only in the superficial epithelium, and after 14 days, phosphorylated HSP27 was localized only to the superficial cell layers similar to its expression in unwounded corneas. At the early corneal epithelial wound-healing process (8 hours after epithelial debridement), phosphorylated HSP27 expression was prominent in the leading edge of migrating central corneal epithelium. During wound healing, HSP27 phosphorylation may play a role in cytoarchitectural remodeling and cell migration.
Stratified squamous epithelial cells assemble specialized protective surface barriers that are exposed to the environment and termed the cornified envelope.48 The corneal epithelium is stratified, but unlike skin, it does not have a water-impermeable layer. Like skin, various envelope precursors are expressed in human corneal epithelium.48 In this article, we showed that phosphorylated HSP27 is constitutively expressed in superficial epithelial cells of corneas that are most exposed to the environment. Given that phosphorylated HSP27 is known to possess antiapoptotic properties, its superficial localization suggests that it contributes to an innate corneal defense and corneal barrier. HSP27 is known to play a cytoprotective role through its antioxidative, antiapoptotic, and actin-stabilizing properties during cell stress. Further experimentation is required to determine the functional significance of HSP27 in ocular surface disorders and corneal epithelial wound healing. Moreover, constitutive expression of phosphorylated HSP27 could be a result of cellular stress from continuous insults from the environment.
In conclusion, constitutive expression of phosphorylated HSP27 was limited to the superficial corneal epithelium in unwounded murine corneas. Changes in HSP27 epithelial distribution and expression levels after corneal epithelial wounding proposed that phosphorylated HSP27 played a role in early phase of corneal epithelial wound healing.
Acknowledgments
Supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (NRF-2008-331-E00209); a grant (2010-464) from the Asan Institute for Life Sciences, Seoul, Korea; and National Eye Institute grant K08EY018874 (S.J.).
Footnotes
The authors state that they have no proprietary interest in the products named in this article.
References
- 1.Park JW, Moon C, Yun S, et al. Differential expression of heat shock protein mRNAs under in vivo glutathione depletion in the mouse retina. Neurosci Lett. 2007;413:260–264. doi: 10.1016/j.neulet.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 2.Miron T, Vancompernolle K, Vandekerckhove J, et al. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J Cell Biol. 1991;114:255–261. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benndorf R, Hayess K, Ryazantsev S, et al. Phosphorylation and supra-molecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J Biol Chem. 1994;269:20780–20784. [PubMed] [Google Scholar]
- 4.Rogalla T, Ehrnsperger M, Preville X, et al. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem. 1999;274:18947–18956. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- 5.Lavoie JN, Gingras-Breton G, Tanguay RM, et al. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J Biol Chem. 1993;268:3420–3429. [PubMed] [Google Scholar]
- 6.Lavoie JN, Hickey E, Weber LA, et al. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J Biol Chem. 1993;268:24210–24214. [PubMed] [Google Scholar]
- 7.Piotrowicz RS, Hickey E, Levin EG. Heat shock protein 27 kDa expression and phosphorylation regulates endothelial cell migration. FASEB J. 1998;12:1481–1490. doi: 10.1096/fasebj.12.14.1481. [DOI] [PubMed] [Google Scholar]
- 8.Arrigo AP, Firdaus WJ, Mellier G, et al. Cytotoxic effects induced by oxidative stress in cultured mammalian cells and protection provided by Hsp27 expression. Methods. 2005;35:126–138. doi: 10.1016/j.ymeth.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Mehlen P, Kretz-Remy C, Preville X, et al. Human hsp27, Drosophila hsp27 and human alphaB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFalpha-induced cell death. EMBO J. 1996;15:2695–2706. [PMC free article] [PubMed] [Google Scholar]
- 10.Garrido C, Schmitt E, Cande C, et al. HSP27 and HSP70: potentially oncogenic apoptosis inhibitors. Cell Cycle. 2003;2:579–584. [PubMed] [Google Scholar]
- 11.Jaattela M. Escaping cell death: survival proteins in cancer. Exp Cell Res. 1999;248:30–43. doi: 10.1006/excr.1999.4455. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Roth S, Laser M, et al. Retinal preconditioning and the induction of heat-shock protein 27. Invest Ophthalmol Vis Sci. 2003;44:1299–1304. doi: 10.1167/iovs.02-0235. [DOI] [PubMed] [Google Scholar]
- 13.Shi B, Han B, Schwab IR, et al. UVB irradiation-induced changes in the 27-kd heat shock protein (HSP27) in human corneal epithelial cells. Cornea. 2006;25:948–955. doi: 10.1097/01.ico.0000224643.43601.5d. [DOI] [PubMed] [Google Scholar]
- 14.Ehrnsperger M, Graber S, Gaestel M, et al. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee GJ, Roseman AM, Saibil HR, et al. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 1997;16:659–671. doi: 10.1093/emboj/16.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charette SJ, Lavoie JN, Lambert H, et al. Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol. 2000;20:7602–7612. doi: 10.1128/mcb.20.20.7602-7612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rane MJ, Pan Y, Singh S, et al. Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem. 2003;278:27828–27835. doi: 10.1074/jbc.M303417200. [DOI] [PubMed] [Google Scholar]
- 18.Guay J, Lambert H, Gingras-Breton G, et al. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci. 1997;110:357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- 19.Hedges JC, Dechert MA, Yamboliev IA, et al. A role for p38 (MAPK)/HSP27 pathway in smooth muscle cell migration. J Biol Chem. 1999;274:24211–24219. doi: 10.1074/jbc.274.34.24211. [DOI] [PubMed] [Google Scholar]
- 20.Shin KD, Lee MY, Shin DS, et al. Blocking tumor cell migration and invasion with biphenyl isoxazole derivative KRIBB3, a synthetic molecule that inhibits Hsp27 phosphorylation. J Biol Chem. 2005;280:41439–41448. doi: 10.1074/jbc.M507209200. [DOI] [PubMed] [Google Scholar]
- 21.Lu PC, Ye H, Maeda M, et al. Immunolocalization and gene expression of matrilysin during corneal wound healing. Invest Ophthalmol Vis Sci. 1999;40:20–27. [PubMed] [Google Scholar]
- 22.Gaestel M, Gross B, Benndorf R, et al. Molecular cloning, sequencing and expression in Escherichia coli of the 25-kDa growth-related protein of Ehrlich ascites tumor and its homology to mammalian stress proteins. Eur J Biochem. 1989;179:209–213. doi: 10.1111/j.1432-1033.1989.tb14542.x. [DOI] [PubMed] [Google Scholar]
- 23.Jonak C, Metze D, Traupe H, et al. The expression of the 27-kd heat shock protein in keratinization disorders: an immunohistological study. Hum Pathol. 2005;36:686–693. doi: 10.1016/j.humpath.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Leonardi R, Villari L, Caltabiano M, et al. Heat shock protein 27 expression in the epithelium of periapical lesions. J Endod. 2001;27:89–92. doi: 10.1097/00004770-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Lambert H, Charette SJ, Bernier AF, et al. HSP27 multimerization mediated by phosphorylation-sensitive intermolecular interactions at the amino terminus. J Biol Chem. 1999;274:9378–9385. doi: 10.1074/jbc.274.14.9378. [DOI] [PubMed] [Google Scholar]
- 26.Gaestel M, Schroder W, Benndorf R, et al. Identification of the phosphorylation sites of the murine small heat shock protein hsp25. J Biol Chem. 1991;266:14721–14724. [PubMed] [Google Scholar]
- 27.Landry J, Lambert H, Zhou M, et al. Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J Biol Chem. 1992;267:794–803. [PubMed] [Google Scholar]
- 28.Benjamin IJ, McMillan DR. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83:117–132. doi: 10.1161/01.res.83.2.117. [DOI] [PubMed] [Google Scholar]
- 29.Rouse J, Cohen P, Trigon S, et al. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 30.Ludwig S, Engel K, Hoffmeyer A, et al. 3pK, a novel mitogen-activated protein (MAP) kinase-activated protein kinase, is targeted by three MAP kinase pathways. Mol Cell Biol. 1996;16:6687–6697. doi: 10.1128/mcb.16.12.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maizels ET, Peters CA, Kline M, et al. Heat-shock protein-25/27 phosphorylation by the delta isoform of protein kinase C. Biochem J. 1998;332:703–712. doi: 10.1042/bj3320703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butt E, Immler D, Meyer HE, et al. Heat shock protein 27 is a substrate of cGMP-dependent protein kinase in intact human platelets: phosphorylation-induced actin polymerization caused by HSP27 mutants. J Biol Chem. 2001;276:7108–7113. doi: 10.1074/jbc.m009234200. [DOI] [PubMed] [Google Scholar]
- 33.Doppler H, Storz P, Li J, et al. A phosphorylation state-specific antibody recognizes Hsp27, a novel substrate of protein kinase D. J Biol Chem. 2005;280:15013–15019. doi: 10.1074/jbc.C400575200. [DOI] [PubMed] [Google Scholar]
- 34.White SR, Tse R, Marroquin BA. Stress-activated protein kinases mediate cell migration in human airway epithelial cells. Am J Respir Cell Mol Biol. 2005;32:301–310. doi: 10.1165/rcmb.2004-0118OC. [DOI] [PubMed] [Google Scholar]
- 35.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 36.Ridley AJ, Paterson HF, Johnston CL, et al. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 37.Theriot JA, Mitchison TJ. Actin microfilament dynamics in locomoting cells. Nature. 1991;352:126–131. doi: 10.1038/352126a0. [DOI] [PubMed] [Google Scholar]
- 38.Symons MH, Mitchison TJ. Control of actin polymerization in live and permeabilized fibroblasts. J Cell Biol. 1991;114:503–513. doi: 10.1083/jcb.114.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lavoie J, Lambert H, Hickey E, et al. Modulation of cellular thermore-sistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol. 1995;15:505–516. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jia Y, Ransom RF, Shibanuma M, et al. Identification and characterization of hic-5/ARA55 as an hsp27 binding protein. J Biol Chem. 2001;276:39911–39918. doi: 10.1074/jbc.M103510200. [DOI] [PubMed] [Google Scholar]
- 41.Fujita H, Kamiguchi K, Cho D, et al. Interaction of Hic-5, a senescence-related protein, with focal adhesion kinase. J Biol Chem. 1998;273:26516–26521. doi: 10.1074/jbc.273.41.26516. [DOI] [PubMed] [Google Scholar]
- 42.Thomas SM, Hagel M, Turner CE. Characterization of a focal adhesion protein, Hic-5, that shares extensive homology with paxillin. J Cell Sci. 1999;112:181–190. doi: 10.1242/jcs.112.2.181. [DOI] [PubMed] [Google Scholar]
- 43.Nishiya N, Tachibana K, Shibanuma M, et al. Hic-5-reduced cell spreading on fibronectin: competitive effects between paxillin and Hic-5 through interaction with focal adhesion kinase. Mol Cell Biol. 2001;21:5332–5345. doi: 10.1128/MCB.21.16.5332-5345.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirano S, Shelden EA, Gilmont RR. HSP27 regulates fibroblast adhesion, motility, and matrix contraction. Cell Stress Chaperones. 2004;9:29–37. doi: 10.1379/471.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirano S, Rees RS, Gilmont RR. MAP kinase pathways involving hsp27 regulate fibroblast-mediated wound contraction. J Surg Res. 2002;102:77–84. doi: 10.1006/jsre.2001.6315. [DOI] [PubMed] [Google Scholar]
- 46.Shi B, Isseroff RR. Arsenite pre-conditioning reduces UVB-induced apoptosis in corneal epithelial cells through the anti-apoptotic activity of 27 kDa heat shock protein (HSP27) J Cell Physiol. 2006;206:301–308. doi: 10.1002/jcp.20466. [DOI] [PubMed] [Google Scholar]
- 47.Eddy RJ, Petro JA, Tomasek JJ. Evidence for the nonmuscle nature of the “myofibroblast” of granulation tissue and hypertropic scar. An immunofluorescence study. Am J Pathol. 1988;130:252–260. [PMC free article] [PubMed] [Google Scholar]
- 48.Tong L, Corrales RM, Chen Z, et al. Expression and regulation of cornified envelope proteins in human corneal epithelium. Invest Ophthalmol Vis Sci. 2006;47:1938–1946. doi: 10.1167/iovs.05-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]


