Abstract
Background
Reporting guidelines have been available for the past 17 years since the inception of the Consolidated Standards of Reporting Trials statement in 1996. These guidelines were developed to improve the quality of reporting of studies in medical literature. Despite the widespread availability of these guidelines, the quality of reporting of medical literature remained suboptimal. In this study, we assess the current adherence practice to reporting guidelines; determine key factors associated with better adherence to these guidelines; and provide recommendations to enhance adherence to reporting guidelines for future studies.
Methods
We undertook a systematic scoping review of systematic reviews of adherence to reporting guidelines across different clinical areas and study designs. We searched four electronic databases (Cumulative Index to Nursing and Allied Health Literature, Web of Science, Embase, and Medline) from January 1996 to September 2012. Studies were included if they addressed adherence to one of the following guidelines: Consolidated Standards of Reporting Trials (CONSORT), Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), Quality of Reporting of Meta-analysis (QUOROM), Transparent Reporting of Evaluations with Nonrandomized Designs (TREND), Meta-analysis Of Observational Studies in Epidemiology (MOOSE) and Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). A protocol for this study was devised. A literature search, data extraction, and quality assessment were performed independently by two authors in duplicate. This study reporting follows the PRISMA guidelines.
Results
Our search retrieved 5159 titles, of which 50 were eligible. Overall, 86.0% of studies reported suboptimal levels of adherence to reporting guidelines. Factors associated with better adherence included journal impact factor and endorsement of guidelines, publication date, funding source, multisite studies, pharmacological interventions and larger studies.
Conclusion
Reporting guidelines in the clinical literature are important to improve the standards of reporting of clinical studies; however, adherence to these guidelines remains suboptimal. Action is therefore needed to enhance the adherence to these standards. Strategies to enhance adherence include journal editorial policies endorsing these guidelines.
Keywords: scoping, systematic, review, adherence, reporting, guidelines
Background
The medical literature is an integral component of clinical care, education, and research, as it has a serious impact on our understanding of health and disease. There are thousands of medical journals that publish articles related to clinical interventions, prognosis, diagnosis, and risks – among others – with an influence on health and life in general. For example, a quick glance at PubMed shows over 22 million citations for biomedical literature.1 It is therefore a challenge to try to assimilate data presented in the literature and make evidence-based informed decisions. Attempts to summarize these data using systematic reviews are commendable as these reviews aim to provide a summary of the state of knowledge on a specific topic and address the inconsistent findings from single studies. However, these reviews are exponential in number and may report disparate findings. Searching for systematic reviews on depression resulted in 30,038 articles,2 and in cancer resulted in 323,633.3
One way to assimilate and disseminate knowledge that can influence decision-making and provide an understanding of a certain condition is to perform a systematic review of reviews. The past few decades have given rise to a handful of such studies in several clinical areas including lifestyle interventions,4 interventions to improve mental health,5 homeopathy,6 medical education,7 spinal manipulation,8 sleep medicine,9 and cancer,10 among others. Each of these reviews of reviews is focused on a specific clinical question. There is a paucity of systematic reviews that assess the quality of reporting of clinical studies across different clinical areas, and that use different reporting guidelines. The EQUATOR (Enhancing Quality and Transparency in Health Research) network is an international initiative that supports the development and dissemination of such guidelines.11 The EQUATOR website provides guidelines for the minimum information required to report research methods and findings for various kinds of medical research.12
The evidence that is presented in the clinical literature can carry substantial weight in informing professionals and users of health care on multiple aspects of health risks, disease, health care outcomes, and delivery. However, readers of the literature are faced with conflicting results presented in various formats and styles, making interpretations and conclusions challenging even for the most informed readers. For this reason, a consensus on reporting such evidence is needed to establish the quality of such studies. It is also important to ensure that a more uniform method is used by researchers to enable the combination of results from multiple studies and reach more standardized summaries and conclusions; this can minimize heterogeneity, which often complicates meta-analyses in future studies. Furthermore, poorly reported research can cause harm to patients and lead to the use of scarce resources on ineffective treatments.13
To address the concern over the quality of reported studies and ensure transparency in reporting clinical studies, the Consolidated Standards of Reporting Trials (CONSORT)14 statement was produced as a collaborative effort to provide a checklist and flow diagram for authors to have as a guide to prepare reports on randomized controlled trials (RCTs) for publication. The CONSORT Statement was further updated in 2010 based on new evidence and an added focus on specific designs of RCTs.15 The CONSORT is a widely accepted and adopted statement that is well described in many freely accessible publications and websites. In brief, the CONSORT Statement provides a 25-item checklist describing the required criteria for inclusion when reporting RCTs. Such items include the study design, the participants, interventions, outcomes, and sample size among others. It also recommends the inclusion of a flow diagram, accounting for recruitment, randomization, allocation of interventions, and retention in the study.16 Since the introduction of the CONSORT, several extensions and modifications of the original statement have been established to improve the quality of reporting of various study types, including observational studies, systematic reviews, and meta-analysis. Despite the availability of such guidelines for reporting, the quality of reporting of clinical studies has remained suboptimal with several manuscripts in a number of clinical areas missing key items as described in the CONSORT.16–23
Evidence suggests that the use of the CONSORT criteria is associated with improved standards of reporting.24,25 However, it is not clear what the current level of adherence to reporting guidelines is, what factors are associated with improving the reporting of clinical literature, and how the results from different studies on reporting standards can be compiled to provide an overall conclusion on the current state of reporting standards.
We therefore undertook a systematic scoping review evaluating systematic reviews addressing the adherence standards to reporting guidelines published since the introduction of the CONSORT Statement in January 1996 to September 2012.
Study aims
In this study, we aimed to examine the extent of adherence to reporting guidelines in published clinical research since the introduction of the CONSORT Statement in 1996. The purpose of this systematic scoping review is to inform researchers, guideline developers, journal editors, and evidence users on the profile of reporting the existing literature and the current state of knowledge in the application of these guidelines. In particular, we will endeavor to address the following questions: (1) what is the current adherence to the reporting standards that include the CONSORT,26 Strengthening the Reporting of Observational Studies in Epidemiology (STROBE),27 Quality of Reporting of Meta-analysis – (QUOROM),28 Transparent Reporting of Evaluations with Nonrandomized Designs (TREND),29 Meta-analysis Of Observational Studies in Epidemiology (MOOSE),30 and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines?31 (2) What are the factors that are associated with adherence to reporting standards in medical literature? And (3) what guidance can we provide based on the current state of knowledge on adherence to reporting standards? More specifically the objectives of this review are to:
Report the levels of adherence to the above reporting guidelines in clinical research;
Determine the key factors associated with adherence to good reporting; and
Provide recommendations to enhance adherence to reporting guidelines for future studies.
We preselected the six guidelines above because they are among the oldest and the most popular, spanning through a wide range of study designs and clinical areas, and are therefore likely to be reported in systematic reviews, thus potentially generating a number of reviews to be included in this study.
Methods
We adopted a “systematic” scoping review approach – which is a combination of a scoping review methodology – to ensure the inclusion of broad areas of research and study designs, and a systematic review of reviews methodology.32,33
A scoping review is a relatively new type of study providing an assessment of available evidence from the literature in a broad area of research such as the compliance in the reporting of clinical studies to established guidelines. It also serves to identify gaps in the field and provide recommendations for implementation.32 The methodology of scoping reviews was first described in detail by Arksey and O’Malley32 in their pivotal paper published in 2005, which provided a foundation for carrying out a scoping review. This framework was further operationalized, and five stages were proposed to be followed when conducting a scoping review, including: (1) the identification of a research question; (2) finding the relevant studies; (3) the selection of studies to be included in the review; (4) data extraction from the included studies; and (5) assembling, summarizing, and reporting the results of the review.34
The methods of conducting a systematic review of systematic reviews follow a similar approach, but include the provision of guidelines and suggestions for clinical practice, education, and research.33 The aim of the methods and search strategy here is to ensure that the systematic review of reviews is comprehensive, thorough, and objective. We will report the results using the PRISMA (formerly QUOROM) reporting guidelines for systematic reviews.35 A protocol was specifically designed for this study outlining the study design, search strategy, and selection criteria.
Data sources and search strategy
Electronic literature databases including Cumulative Index to Nursing and Allied Health Literature (CINAHL), Web of Science, Embase, and Medline (from January 1996 [date of CONSORT] to September 2012) were searched using a comprehensive search strategy designed with the assistance of a librarian who is experienced in conducting systematic reviews. The reference lists of identified articles were also reviewed for additional studies, and a manual search of key journals like BioMed Central systematic reviews, BioMed Central Research Methodology, and the Cochrane Library was conducted to avoid missing relevant reviews. Such search strategies are well supported for this type of systematic search and retrieval of relevant studies.36,37 The databases were searched for the following key search terms: (Systematic reviews OR reviews OR quality of reporting OR completeness of reporting) AND (CONSORT OR STROBE OR QUOROM OR QUORUM OR PRISMA OR TREND OR MOOSE) OR adherence. For Web of Science, we also performed a forward citation search of the publications pertaining to reporting guidelines, whose acronyms might have other meanings, such as TREND and QUORUM. This helped us to decrease the occurrence of false positives in our search.
Initially, no language limits were set to identify the number of non-English reviews; however, a limit was then set for English language reviews only (which was necessary due to the lack of resources required to translate reviews from other languages). We also set the limits to “human” and “published complete systematic reviews.”
Inclusion criteria
Systematic reviews of clinical studies addressing the quality of reporting of the studies based on at least one of the six preselected reporting guidelines: CONSORT for RCTs; TREND for non-RCTs; STROBE for observational studies; and PRISMA (formerly QUOROM) or MOOSE for systematic reviews of RCTs or observational studies, respectively.
The systematic reviews must be complete (not abstracts only), reported in English, and investigating the quality of reporting in human studies of all age groups using one of the above guidelines.
The quality of reporting guidelines must be the primary focus of the systematic review.
Exclusion criteria
Systematic reviews were excluded if they were published as abstract only; the primary focus of the review was not on the quality of reporting; the quality of reporting was based on the standards of reporting that were different from the ones stated above, or if they were a duplicate publication of existing reviews (commentaries, letters, and editorials).
Selection of systematic reviews
Two independent reviewers examined the titles and abstracts of all citations identified in the literature search. Articles were selected for full-text review if the inclusion criteria were met and if both reviewers considered the citation potentially relevant. Disagreement at any stage of study selection was resolved by discussion and consensus between the two reviewers. If agreement could not be reached a third author was recruited to determine eligibility. Initial agreement between the two reviewers was calculated using the kappa statistic.38
Each reviewer independently:
Assessed retrieved titles and abstracts for relevance and duplication;
Screened full text articles deemed eligible for inclusion;
Decided on including or excluding articles;
Extracted relevant data using specifically designed data abstraction forms;
Appraised the quality of the included reviews. A PRISMA flow diagram of included/excluded studies is provided (Figure 1).35
Figure 1.
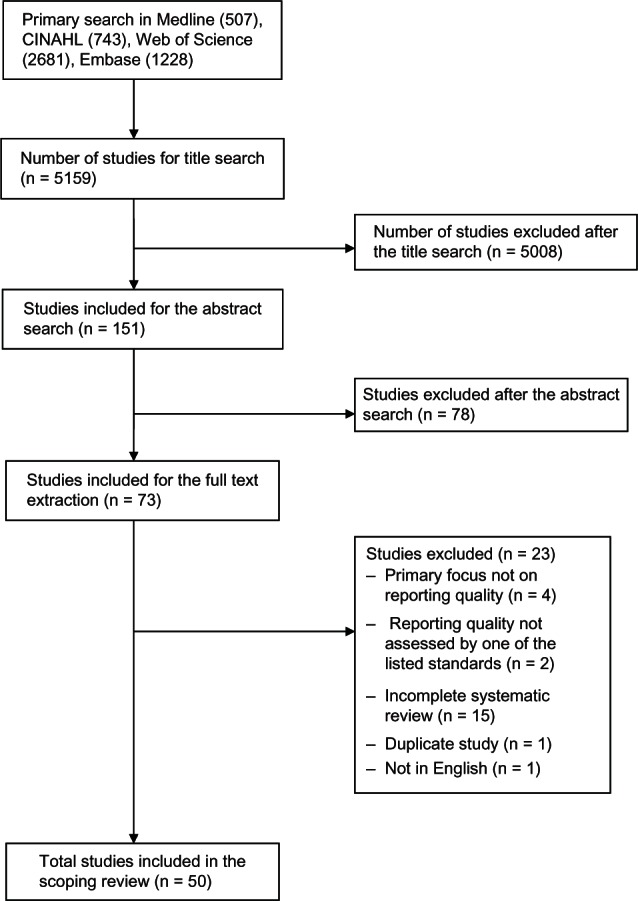
Flow diagram for study selection.
Abbreviations: CINAHL, Cumulative Index to Nursing and Allied Health Literature; n, number.
Quality assessment of systematic reviews
The quality of each systematic review that met the inclusion criteria for the study was assessed using a modified version of the assessment of multiple systematic reviews (AMSTAR, a validated tool to assess the methodological quality of systematic reviews).39–41 Certain items of AMSTAR are not relevant to this type of review and cannot be assessed (eg, item 9, “Were the methods used to combine the findings of studies appropriate?”), as pooling of data may not be feasible in all systematic reviews of methodological quality, and should relate to the study question. In addition, item 10 (“Was the likelihood of publication bias assessed?”) is irrelevant to this review, which is focused on the quality of reporting of published studies. Both of these items were omitted from the quality assessment.
We also used the modified version of the enhanced Overview Quality Assessment Questionnaire (OQAQ) to assess the quality of systematic reviews included in this study.42 In addition to these tools, we assessed the quality of the reviews based on the following criteria: the use of explicit criteria to assess individual study quality using the guidelines checklist; explicit definition of the research question using a flow diagram to explain study selection; and a formal sample size calculation for the assessment of association.
Data abstraction
A spreadsheet was created to record the following items from the selected reviews: authors, year of publication, number of primary studies included in the review, study location, study type, primary outcomes of the study, outcomes measures, and the overall results and conclusions. Two authors independently piloted the data extraction form for this review and modifications were made when necessary before reaching the final data abstraction forms used for this study. Data abstraction disagreements were resolved by discussion and consensus, and a third author extracted the data if an agreement could not be reached. Data collected from each systematic review included the study primary question, primary outcome, number of the studies included in the review, the statement investigated, quality assessment, the factors associated with adherence, the journal of publication, and whether the journal endorsed the statement in question.
Analysis
The level of agreement between raters was estimated using the kappa statistic. The adherence to reporting standards was summarized, and key determinants of adherence were identified in a narrative manner.
Results
Study selection
Our search retrieved 5159 articles from the four electronic databases searched. Following searching through the title, abstract, and full text screening, 50 articles were selected and included for data extraction and quality assessment (Figure 1). The strength of agreement between two independent raters on abstract screening was substantial (Kappa = 0.65; 95% confidence interval [CI] 0.53, 0.76; P < 0.001), and almost perfect for full text screening (Kappa = 0.94; 95% CI 0.85, 1.00; P < 0.001). Agreement was also substantial for the quality assessment using the modified OQAQ/AMSTAR checklist (Kappa = 0.63; 95% CI 0.42, 0.85; P < 0.001).
Study characteristics
Forty-one studies (82.0%) assessed RCTs using the CONSORT Statement, five (10.0%) studies used the QUOROM checklist, and two studies (4.0%) used the PRISMA tool. The final three systematic reviews (6.0%) consisted of two reviews assessing both RCTs and observational studies using the CONSORT and STROBE guidelines, and the last study used both the QUOROM and PRISMA guidelines. The systematic reviews were published in a wide variety of journals and were led by authors from many different countries (Table 1). The median and interquartile range of the number of studies included in each review were 78 and 80.5, respectively.
Table 1.
Characteristics of included studies
| First author | Year | Journal | City/country | Statement assessed | Number of studies |
|---|---|---|---|---|---|
| Al-Namankany54 | 2009 | International Journal of Pediatric Dentistry | London, UK | CONSORT | 173 |
| Areia21 | 2009 | Endoscopy | Coimbra, Portugal | CONSORT | 120 |
| Augestad44 | 2012 | Journal of the American Medical informatics Association | Tromso, Norway | CONSORT | 32 |
| Balasubramanian45 | 2006 | Annals of Surgery | Sheffield, UK | CONSORT | 69 |
| Bath55 | 1998 | Stroke | London, UK | CONSORT | 114 |
| Bereza56 | 2008 | Annals of Pharmacotherapy | Toronto, ON, Canada | QUOROM | 16 |
| Bian43 | 2006 | Journal of Chinese Integrative Medicine | Hong Kong, People’s Republic of China | CONSORT | 66 |
| Bousquet57 | 2010 | Journal of Allergy and Clinical Immunology | Montpelier, France | CONSORT | 94 |
| Capili58 | 2010 | Clinical Journal of Pain | New York, NY, USA | CONSORT | 10 |
| Cavadas59 | 2011 | International Urogynecology Journal | Porto, Portugal | CONSORT | 41 |
| Chowers60 | 2009 | Journal of Antimicrobial Chemotherapy | Kfar Saba, Israel | CONSORT | 49 |
| Cook61 | 2011 | Medical Education | Minnesota, USA | STROBE | 130 |
| de Vries62 | 2010 | Archives of Diseases in Childhood | Leeuwarden, Netherlands | CONSORT | 107 |
| Ethgen63 | 2009 | BMC Medical Research Methodology | Paris, France | CONSORT | 132 |
| Eyawo64 | 2008 | Trials | Burnaby, BC, Canada | CONSORT | 47 |
| Farrokhyar65 | 2007 | Canadian Journal of Surgery | Hamilton, ON, Canada | CONSORT | 50 |
| Froud48 | 2012 | Community Dentistry and Oral Epidemiology | London, UK | CONSORT | 23 |
| Fung49 | 2009 | Ophthalmology | San Francisco, CA, USA | CONSORT, STROBE | 36 |
| Gagnier66 | 2006 | American Journal of Medicine | Toronto, ON, Canada | CONSORT | 206 |
| Halpern67 | 2004 | International Journal of Obstetric Anesthesia | Toronto, ON, Canada | CONSORT | 99 |
| Hemels68 | 2004 | Current Medical Research and Opinion | Paris, France | QUOROM | 32 |
| Herdan69 | 2011 | Gynecological Surgery | Bamberg, Germany | CONSORT | 37 |
| Junhua70 | 2007 | The Journal of Alternative and Complementary Medicine | Tianjin, People’s Republic of China | QUOROM | 107 |
| Kiehna46 | 2011 | Journal of Neurosurgery | Charlottesville, VA, USA | CONSORT | 27 |
| Kober71 | 2006 | Journal of the National Cancer Institute | North Lyneham, Australia | CONSORT | 142 |
| Ladd25 | 2010 | Addictive Behaviors | Albuquerque, NM, USA | CONSORT | 127 |
| Li72 | 2011 | Evidence-Based Complementary and Alternative Medicine | Baltimore, MD, USA | CONSORT | 42 |
| Lu73 | 2011 | Expert Review of Anticancer therapy | Guangzhou, People’s Republic of China | CONSORT | 46 |
| Ma74 | 2011 | PLoS One | Lanzhou, People’s Republic of China | PRISMA | 369 |
| Marshman75 | 2010 | Community Dental Health | Sheffield, UK | CONSORT | 48 |
| Moberg-Mogren76 | 2006 | American Journal of Occupational Therapy | Cleveland, OH, USA | CONSORT | 14 |
| Moher47 | 2002 | BMC Pediatrics | Ottawa, ON, Canada | CONSORT | 251 |
| Montané77 | 2010 | BMC Clinical Pharmacology | Barcelona, Spain | CONSORT | 92 |
| Montgomery50 | 2011 | Trials Journal | Bristol, UK | CONSORT | 76 |
| Norton-Mabus78 | 2008 | OTJR: Occupation, Participation and Health | Toledo, OH, USA | CONSORT | 30 |
| Parsons79 | 2011 | Journal of Bone and Joint Surgery. British Volume | Coventry, UK | CONSORT, STROBE | 100 |
| Piggott80 | 2004 | Palliative Medicine | London, UK | CONSORT | 93 |
| Plint51 | 2006 | Medical Journal of Australia | Ottawa, ON, Canada | CONSORT | 8 |
| Rios81 | 2008 | Journal of Clinical Endocrinology and Metabolism | Hamilton, ON, Canada | CONSORT | 89 |
| Shea82 | 2006 | The Journal of Rheumatology | Amsterdam, Netherlands | QUOROM | 57 |
| Strech83 | 2011 | Journal of Clinical Psychiatry | Hannover, Germany | CONSORT | 105 |
| Thabane84 | 2007 | International Journal of Obesity | Hamilton, ON, Canada | CONSORT | 63 |
| Vigna-Taglianti85 | 2006 | Annals of Oncology | Torino, Italy | QUOROM | 80 |
| Walleser86 | 2011 | Journal of Clinical Epidemiology | Renens, Switzerland | CONSORT | 106 |
| Wangge52 | 2010 | PLoS One | Utrecht, Netherlands | CONSORT | 232 |
| Weir87 | 2012 | International Journal of Medical Informatics | Salt Lake City, UT, USA | PRISMA, QUOROM | 13 |
| Willis88 | 2011 | BMC Medical Research Methodology | Manchester, UK | PRISMA | 236 |
| Zhong89 | 2011 | European Journal of Integrated Medicine | Chengdu, People’s Republic of China | CONSORT | 153 |
| Zintzaras53 | 2010 | Clinical Therapeutics | Larisa, Greece | CONSORT | 18 |
| Ziogas90 | 2009 | Annals of Epidemiology | Larisa, Greece | CONSORT | 261 |
Abbreviations: CONSORT, Consolidated Standards of Reporting Trials; QUOROM, Quality of Reporting of Meta-analysis; BMC, BioMed central; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology; PLoS, Public Library of Science; PRISMA, preferred reporting items for systematic reviews and meta-analyses; OTJR, Occupational Therapy Journal of Research.
Adherence to reporting guideline
The adherence of the studies included in the systematic reviews to their respective guidelines, and the author’s conclusions, are shown in Table 2. Forty-three (86.0%) of the included studies concluded that the adherence to reporting guidelines was inadequate, poor, medium, or suboptimal, or that some improvement was needed. No combined, quantitative result was generated from the 50 systematic reviews due to differences in the measurement tools used by the individual reviews.
Table 2.
Description of the studies’ findings
| Guideline | First author | Measure of quality | Results mean (SD)/% count | Authors’ conclusion |
|---|---|---|---|---|
| CONSORT | Al-Namankany54 | Modified 34-item CONSORT checklist | Compliance varied across items and articles. Good compliance of articles to CONSORT for introduction sections (96%–98%), discussion sections (96%–98%). Poor reporting in randomization methods (5%–9%), description of sample size calculation (4%), intention-to-treat analysis (1%) | Quality of reporting of RCTs in pediatric dental journals was generally poor, with negligible improvement after the publication of CONSORT Statement |
| Areia21 | Application of CONSORT/STARD | 15.7 (2.2) | Level of adherence is medium for quality of reporting in diagnostic endoscopy | |
| Augestad44 | CONSORT adherence, Jadad | 30.75 (4), 40% of the trials had a Jadad score of ≥3 points | Level of adherence is low for quality of reporting for RCTs of disease specific clinical decision support | |
| Balasubramanian45 | Modified CONSORT score, allocation concealment as assessed by Schulz et al, Jadad score | Medians of the modified CONSORT score were 85.45 (interquartile range 81.09–86.13) and interquartile range 68.97 (62.89–73.1 1) for RCTs from medical and surgical journals, respectively; 13% clearly explained allocation concealment; 37.7% of RCTs had a Jadad score of ≥ 3 | Quality of reporting of surgical RCTs was suboptimal, and reporting in surgical journals was inferior to surgical trials in medical journals “We found that the quality of reporting of general surgical RCTs leaves considerable room for improvement” |
|
| Bath55 | In all, 33 criteria of the CONSORT Statement and 53 additional factors relevant to acute stroke or trials in general. Trial quality was also assessed with a seven-point scale | Median total report quality was 40/86 (range 15–61). Median CONSORT criterion was 19/33 (9–29) | Poor quality for acute stroke RCTs “We believe that authors should follow the CONSORT guidelines and that referees and editors should ensure this happens” |
|
| Bian43 | 63-item revised CONSORT checklist designed for Chinese Herbal Medicine clinical studies | Median score of overall reporting quality was 32% (8%) | Overall quality of reporting of CHM RCTs was poor. Need to improve reporting in clinical trials in this area. “To improve the quality of reporting of RCTs of CHM, we recommend adopting a revised CONSORT checklist that includes items specific to CHM. We also recommend that editors of CHM journals require authors to use a structured approach to presenting their trials as a condition of publication” | |
| Bousquet57 | Reporting of procedure, randomization, dropouts, strict conduct of intention-to-treat analysis, sample size calculation, which was assessed with eight items of the CONSORT Statement | Four of the 94 studies met the eight items of the CONSORT Statement criteria | RCTs in subcutaneous immunotherapy and sublingual immunotherapy had poor reporting quality. Encourage more use of CONSORT Statement | |
| Capili58 | Adherence to CONSORT/presence of harms guidelines in RCTs | I7(range: 14–21) for CONSORT; 7/22 = 77.3% for harm | Level of adherence is bad for quality of reporting, for RCTs on acupuncture, for pain reduction | |
| Cavadas59 | A 25-item 2010 revised CONSORT Statement | No combined data: only a few items were reported in less than 50% of the studies; some items were reported in more than 90% of the studies | “RCTs in [pelvic organ prolapsed] are scarce. The quality of reporting is suboptimal in many aspects and has not improved in recent years” | |
| Lu73 | Percentage of articles that reported each applicable item of the CONSORT checklist | Sample size: only one (2.2%) of the papers mentioned sample size calculation. Randomization: 12 studies (26.1 %) were deemed to have authentic randomization. Blinding: 36 papers (78.3%) provided no information about blinding of either participants or investigators. Reporting of baseline characteristics: 39 papers (84.8%) reported the details of the baseline characteristics of participants. Length of follow-up: 22 papers (47.8%). There was no information provided on the length of time for which participants were followed. Loss-to-follow-up: a total of 36 studies (78.3%) failed to report dropout rates. Statistical reporting: only one paper (2.2%) did not report what statistical methods they had used | Findings indicate that the reporting quality of RCTs needs improvement for RCTs on the treatment of cancer pain in People’s Republic of China | |
| Chowers60 | CONSORT guidelines for adverse events, adjusted to the design of the HAART trial | No combined score: harms were reported in only 24% of trials, 1/49 reported on adverse events collection method | Large variability and a lack of standard reporting of adverse events between trials; many trials did not adhere to CONSORT recommendations | |
| de Vries62 | Adequate reporting of adverse drug reactions | Mean of 3, and 18% of articles scored 6 or higher | Insufficient reporting quality in adverse event reporting in RCTs of children | |
| Ethgen63 | CLEAR NPT – a checklist to evaluate RCTs of nonpharmacological treatments | Most studies failed to report 8/12 quality indexes in the checklist. Reporting of generation of allocation sequence was adequate in 38.8% of studies, treatment allocation in 26.3%, intention-to-treat analysis in 70.0% | Inadequate reporting amongst trials involving stents. “The current reporting of results of RCTs testing stents needs to be improved to allow readers to appraise the risk of bias and the applicability of the results” | |
| Eyawo64 | Revised CONSORT checklist to assess reporting of each items on the checklist in counts (percentage) | 14/16 items ranged from 2%–7%; the other two items, sample size determination, and reporting of masking were reported in 72% and 75% of the articles | Deficiencies in the design, planning, and reporting of noninferiority and equivalence trials in ophthalmology literature | |
| Farrokhyar64 | Modified CONSORT Statement and added factor relevant to surgical trials and CABG surgery | 51.7 out of 105 (11.5) | The total reporting quality of trials in this review varied substantially between publications (35–96 out of a possible max score of 105). The results showed that there is a need for improvement in quality of reporting | |
| Froud48 | Number and percentage of studies satisfying the revised 1l-item consort checklist | Most items were reported in an adequate percentage of studies; 5/11 reported in 78%–100% of the studies | Their results suggest that cluster randomized trials in oral health are of reasonable quality with respect to the key criteria of accounting for clustering in the design and analysis | |
| Fung49 (CONSORT and STROBE) Gagnier66 | Presence or absence of CONSORT (maximum score of 37 points) statement indicators Mean CONSORT score based on 42 items and the percentage of items reported | CONSORT: median and mean values of 89% and 83%, respectively 18.92 out of 42 (5.54), and 45% of items were reported across all trials |
Overall level of reporting is acceptable and has improved since the creation of CONSORT and STROBE “We found that reports of RCTs of herbal medicine interventions reported less than half of the necessary information in their published results” Overall adherence is low |
|
| Halpern67 | Percentage of articles that reported each applicable item of the modified CONSORT checklist and count of articles complying with modified CONSORT items | In the 23 articles in Anesthesia and Analgesia, the median percentage of correct CONSORT items was 63% | Poor – total number of items that are inadequately reported is high in the current RCT literature with obstetric anesthesia | |
| Herdan69 | 22-item CONSORT checklist expressed | On average 87.4% of the CONSORT items were reported | The reporting quality has improved significantly in the period after dissemination of the CONSORT Statement; however, reporting of adverse events needs attention | |
| Kiehna46 | Quality of reporting score using CONSORT (max score of 44) and Jadad score out of 5 points | 26.4 out of 44 (range: 17–38)/67% of studies had no description or the prestudy sample size calculation, 63% did not describe whether subjects, treatment providers or assessors/analysts were blinded | The quality of reporting of RCTs in neurosurgical journals remains suboptimal | |
| Kober71 | CONSORT criteria based on a 14-item questionnaire | 75% of studies reported only six of the 13 items; only 14% reported randomization process; only 13% provided details about concealment of allocation; only 13% provided a statement on study power; only 12% used intention-to-treat analysis |
Articles of Hodgkin’s lymphoma published after 1996 do not conform to the CONSORT recommendations | |
| Ladd25 | Assessment of 36 of the items from the CONSORT Statement based on a score out of 36 | 24.43 out of 36 (3.27) | The overall level of adherence to CONSORT has improved since 1994, and continues to remain highest among studies that have been published within journals that have adopted the CONSORT guidelines | |
| Li72 | Score out of 40 based on a 40-item modified checklist based on the CONSORT Statement | 42% of the studies included explained how sample size was determined; 14% of studies described whether or not outcome assessors were blinded | The reporting quality of these trials is suboptimal and substantial improvement is required to meet the CONSORT guidelines. Almost 50% of the trials we reviewed did not satisfy more than half of the criteria in the modified CONSORT checklist, and only 23% of RCTs provided adequate details of Tai Chi intervention used in the trials | |
| Marshman75 | 56 criteria based on the CONSORT Statement | 27/56, with variation between journals (23.2 to 27.7) | Poor adherence to the CONSORT checklist in RCTs in dental health | |
| Moberg-Mogren76 | Average NMECI score (0–201 subitems scale) | 104.2(32.9) | Less than half of the articles met criteria of these subitems in selected RCTs relevant to occupational therapy | |
| Moher47 | CONSORT checklist, frequency of unclear allocation concealment, and a five-point quality assessment instrument (Jadad) | 12.7/32 of the CONSORT checklist included; 81.3% unclear allocation concealment; 1.9/5 for the Jadad assessment scale | Overall, there was no difference in the PedCAM RCTs and conventional medicine quality, with both types achieving 43% of their maximum possible outcome | |
| Montané77 | Revised CONSORT checklist, 22 items | 10.5(2.7) | Quality was good in 23 (25%) of the articles and poor in 69 (75%) of the reports for RCTs on the efficacy of analgesic drugs in postoperative pain after TOS | |
| Montgomery50 | Qualitative look | N/A | Varying level of reporting quality factorial trials of complex interventions in community settings | |
| Norton-Mabus78 | NMNECI (2l2subitems) | 119.5(25.48) | Article consistency with CONSORT Statement was less than 60%. Occupational therapy RCT had higher consistency with the instrument, scoring higher than articles in speech therapy | |
| Parsons79 (combined CONSORT and STROBE guideline) | Overall compliance calculated as the weighted mean of the compliance rates for the seven selected journals, using a previously made questionnaire | 59% (CONSORT) | Very few papers fulfilling all criteria; general lack of statistical rigor | |
| Piggott80 | Compared RCTs of three different time period cohorts, with the CONSORT (condensed, 13-item) checklist | Quality of reporting variable; 30% of trials or less used true randomization, allocation concealment, intention-to-treat analysis, and power calculations | Quality of reporting over time cohorts was variable, no consistent improvement over time. Quality of reporting remains poor for RCTs in specialized palliative care literature | |
| Plint51 | 22-item checklist from the CONSORT Statement | Standardized mean difference between CONSORT-adopting journals and nonadopters was 0.83 (95% CI, 0.46–1.19) | Journal adoption of CONSORT is associated with improved reporting of RCTs | |
| Rios81 | Overall quality score, which is a 15 point overall reporting quality score made from CONSORT checklist | 10(2.03) | Suboptimal reporting quality in an endocrine journal | |
| Strech83 | A checklist based on the CONSORT Statement | There are 72 items on the checklist; 42% were reported adequately and 25% were reported inadequately | While some trial-related information is well reported, a good part of the reporting quality of RCTs in bipolar disorder falls well below the required and practically feasible level for many aspects essential for the adequate interpretation of methodological quality and clinical relevance. Authors should be further encouraged to follow the CONSORT criteria. No consistent trend could be shown for improvement in the quality of reporting over time, or for reporting essential methodological items differently. There is a consistent trend toward better reporting in journals that endorse the URM | |
| Thabane84 | Percentage of studies satisfying each of the 44 CONSORT criteria | 26.25 (4.51 ) and 60% adherence for reporting criteria: 90% satisfied criteria for the introduction; 19% for the methods; 75% the study protocol, 70% for the results | Overall, the quality of reporting is suboptimal in RCTs of weight loss intervention. Key reporting criteria that may impact the validity and generalizability of the results were adequately reported | |
| Walleser86 | CONSORT-CRT | 34% inadequately reporting on more than half of the CONSORT-CRT criteria | The quality of reporting in CRTs needs improvement. This will hopefully improve implementation and planning | |
| Wangge52 | Extension of the CONSORT Statement for noninferiority and equivalence trial | No blinding in 34.0%, noninferiority margin in 97.8%, with only 45.7% reporting the method of determining the margin | Adherence improved slightly after CONSORT for noninferiority trials | |
| Zhong89 | Number of studies describing each of the 38 modified consort items | Of the 38 CONSORT items, only five items were described in more than 80% of the 153 included | Adherence was suboptimal for two-group parallel randomized controlled clinical trials of multiherb formulae | |
| Zintzaras53 | 17-item CONSORT checklist | 17 CONSORT checklist items were reported in 7/18 studies, and 9/17 CONSORT checklist items were reported in all 18/18 studies | Proper assessment of the credibility and generalizability of the results can be ensured by reporting quality | |
| Ziogas50 | 24-item questionnaire based on the CONSORT checklist | 75% of the studies addressed 13 out of the 24 items of the CONSORT Statement | Reporting on myeloid malignancies remains unsatisfactory and requires further improvement to properly assess the validity of clinical research | |
| PRISMA | Ma74 | Adherence to PRISMA checklist items (27 items) | Title, introduction, limitations, and conclusions were reported well in 90% or more of the studies. Most other items varied from 30%–70% of the studies | Compliance with PRISMA reporting guidelines is low for systematic reviews on TCM published in Chinese journals |
| Weir87 (combined PRISMA and QUOROM guideline) | An integrated score consisting of the number of items completed over the total numbers of items on both the PRISMA and QUOROM criteria, resulting in cored ranking from 0% to 100% (excluding the items focused on in the abstract) | Mean = 63% (range 45%–81%) on a scale of 0%–100% | Systematic reviews of empirical computerized provider order-entry research had moderate quality | |
| Willis88 | Adherence to the 27-item PRISMA checklist | Of the 236 meta-analyses included following selection: 1% reported the study protocol; 25% reported the searches used; 32% reported the results of a risk of bias assessment; and 35% reported the abstract as a structured summary | Compliance with the PRISMA statement was generally poor; none of the review completely adhered to all 27 checklist items for the published meta-analyses of diagnostic tests | |
| QUOROM | Bereza56 | 18-item QUOROM checklist, ten-item checklist OQAQ used for scientific quality | 61% ± 19% (median 60%, range 39%–94%) for the QUOROM checklist. 58% ± 28% for OQAQ | “Reporting/scientific quality was considered less than fair-to-good. Stakeholders should strive for higher scientific quality of meta-analyses” |
| Hemels68 | 18-item QUOROM checklist | On average 50.2% of the CONSORT items were reported | The overall quality of reporting in the meta-analysis of RCTs in major depressive disorder was marginally acceptable | |
| Junhua70 | 18-item QUOROM checklist, ten-item checklist OQAQ used for scientific quality | No combined score; methodological and reporting flaws in more than half of the review articles. Flaws were mainly in the literature search, characteristics of included and excluded studies, quality assessment of primary trials, and data merging | Methodology and reporting quality are poor in both systematic reviews and meta-analysis of TCM published in journals in the People’s Republic of China | |
| Shea82 | 18-item QUOROM checklist, ten-item checklist OQAQ used for scientific quality | All systematic reviews were found to have good overall quality. OQAQ mean score was 5.02 (95% CI 3.71–6.32) | Reporting quality of Cochrane musculoskeletal systematic reviews was generally good, with room for improvement | |
| Vigna-Taglianti85 | QUOROM-based checklist (score out of 50) | 29.9/50 | “Oncologists should be aware that they could be relying on poor underlying documents. Writing groups should be aware of methodological problems, and should consult the existing manuals for the preparation of guidelines” | |
| Weir87 (combined PRISMA and QUOROM guideline) | An integrated score consisting of the number of items completed over the total numbers of items on both the PRISMA and QUOROM criteria, resulting in cored ranking from 0% to 100% (excluding the items focused on in the abstract) | 63% (range 45%–81%) on a scale of 0%–100% | Systematic reviews of empirical computerized provider order-entry research were of moderate quality | |
| STROBE | Cook61 | Quality of reporting, methodological quality, and the association between methodological quality and effect size | 253 (90) | Reporting the quality of experimental research on health profession education was found to be generally suboptimal |
| Fung45 (combined CONSORT and STROBE guideline) | STROBE (maximum score of 37 points) statement indicators | STROBE mean and median: 70% and 71%, respectively | Overall level of reporting is acceptable and has improved since the creation of CONSORT and STROBE | |
| Parsons79 (combined CONSORT and STROBE guideline) | Weighted mean of the compliance rates for the seven selected journals, using a previously made questionnaire | 58% (strobe) | Very few papers fulfilling all criteria, general lack of statistical rigor |
Abbreviations: SD, standard deviation; CONSORT, Consolidated Standards of Reporting Trials; RCT, randomized controlled trial; CHM, Chinese herbal medicine; HAART, highly active antiretroviral treatment; CLEAR NPT, checklist to evaluate a report of a nonpharmacological trial; CABG, coronary artery bypass surgery; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology; NMECI, Nelson-Moberg Expanded CONSORT Consolidated Standards of Reporting Trials Instrument; PedCAM, Pediatric Complementary and Alternative Medicine Research and Education Network; TOS, thoracic outlet syndrome; N/A, not applicable; NMENCI, Nelson-Moberg, Norton Expanded Consolidated Standards of Reporting Trials Instrument; CI, confidence interval; URM, uniform requirement for manuscript; CRT, consolidated standard of reporting trial; NI, noninferiority; PRISMA, preferred reporting items for systematic reviews and meta-analyses; QUOROM, Quality of Reporting of Meta-analysis; OQAQ, Overview Quality Assessment Questionnaire; TCM, traditional Chinese medicine.
CONSORT Statement
The adherence of RCTs to the CONSORT Statement was assessed with different versions of the CONSORT checklist. These checklists ranged from eight to 63 items, except for two studies that used the 212 subitem, Nelson–Moberg–Norton Expanded CONSORT instrument and the 201 subitem Nelson–Moberg Expanded CONSORT instrument. The revisions of the CONSORT Statements were usually based on the specific field of the RCT, and the applicability of the items on the CONSORT checklist to that field. For instance, Bian et al43 used a revised 63-item CONSORT checklist designed for Chinese Herbal Medicine clinical trials. In addition to the CONSORT checklist, four studies (Augestad et al,44 Balasubramanian et al,45 Kiehna et al,46 and Moher et al)47 also used the five-point Jadad instrument to assess the quality of the individual RCTs.39
Of the 41 systematic reviews assessing RCTs reporting adherence to the CONSORT Statement, 33 (80%) of them concluded that some improvement was needed, or that the reporting quality was inadequate, poor, medium, or suboptimal (Table 3). Furthermore, the authors recommended the use of the CONSORT Statement as a guideline to improve the quality of reporting of RCTs. Eight studies did not report inadequate reporting quality of RCTs. Froud et al48 concluded that cluster randomized trials in oral health had a reasonable quality. Fung et al49 reported that the overall level of reporting was acceptable and the reporting quality has improved since the creation of CONSORT and STROBE statements. Ladd et al25 also concluded that the overall reporting quality had improved since 1994 and the articles published in journals that endorse the CONSORT Statement had the highest levels of adherence to reporting guidelines. Moher et al47 only compared the quality of pediatric complementary and alternative medicine RCTs and reported 40% of the CONSORT checklist items were included in these RCTs. Montgomery et al50 evaluated the RCTs qualitatively and found that there was a varying level of reporting quality in factorial trials of complex interventions in community settings. Plint et al51 compared RCTs from CONSORT-endorsing and nonendorsing journals, and their results suggested some improvement in the quality of reporting when the CONSORT checklist is used. Wangge et al52 suggested that adherence to reporting guidelines for noninferiority trials have improved slightly since the CONSORT Statement has been published. Lastly, Zintzaras et al53 did not comment directly on an overall quality of reporting and concluded that adhering to reporting standards can ensure proper assessment of the results.
Table 3.
Studies’ conclusions
| Type of guideline | Total number of studies | Number of studies concluding that “some improvements are needed, reporting inadequate, poor, medium, suboptimal, etc” |
|---|---|---|
| CONSORT | 41 (two combined study with both CONSORT and STROBE) | 33 (80%)21,43–46,54,55,57–60,62–67,69,71–73,75–81,83,84,86,89,90 |
| PRISMA | 3 (one combined study with both PRISMA and QUOROM) | 3 (100%)74,87,88 |
| QUOROM | 6 (one combined study with both PRISMA and QUOROM) | 3 (50%)56,70,87 |
| STROBE | 3 (two combined studies with both CONSORT and STROBE) | 2 (67%)61,79 |
| All guidelines | 50 (distinct studies) | 43 (86.0%)21,43–46,54–67,69–81,83,84,86–90 |
Abbreviations: CONSORT, Consolidated Standards of Reporting Trials; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; QUOROM, Quality of Reporting of Meta-analysis.
PRISMA, QUOROM, and STROBE statements
Three studies examined adherence to the PRISMA guidelines, and all concluded that the adherence of the assessed systematic reviews was poor or moderate. Ma et al74 and Willis et al88 used the 27-item PRISMA checklist to assess the level of adherence. Ma et al74 found that systematic reviews on traditional Chinese medicine published in Chinese journals had low adherence to the PRISMA checklist. Willis et al88 also concluded that adherence to the PRISMA checklist was generally poor for published meta-analyses of diagnostic tests. Weir et al87 used an integrated score consisting of both the PRISMA and QUOROM criteria and found that systematic reviews of empirical computerized provider order-entry research were only of moderate quality.
The assessment of studies’ adherence to the QUOROM guideline was done with the 18-item QUOROM checklist coupled with a ten-item OQAQ checklist in three studies. Bereza et al56 and Junhua et al70 reported that there was a need to improve the quality of reporting of reviews, while Shea et al82 concluded that the quality of Cochrane musculoskeletal systematic reviews was good. Hemels et al68 used only the QUOROM checklist and they concluded that the quality of meta-analyses in studies on major depressive disorder was marginally acceptable. Vigna-Taglianti et al85 used the QUOROM checklist with a specific weighting system for each of the headings and the average score was 29.9/50. No conclusions concerning adherence were made, although the authors did recommend the use of manuals to prepare guidelines for the management of breast and colon cancers. Lastly, as described in the previous paragraph, Weir et al87 used an integrated score containing both PRISMA and QUOROM criteria.
The studies by Fung et al49 and Parsons et al79 assessed the adherence of both RCTs and observational studies to their respective guidelines. Parsons et al79 found there was a general lack of statistical rigor.
Factors associated with adherence to reporting guidelines
Although we included systematic reviews assessing the adherence of research articles to four different guidelines, only systematic reviews related to the CONSORT Statement reported on the factors that were associated with adherence to the guideline (Table 4). The exception was Hemel et al,68 who concluded that the overall quality of reporting of meta-analyses using the QUOROM guidelines did not significantly change over time, and that the year of publication was not associated with change in adherence. From the CONSORT-related studies, the following are the factors that were reported to be significantly associated with an increase in adherence to the CONSORT Statement or to the quality of reporting of RCTs, as well as the number of studies reporting these factors: publication in CONSORT-endorsing journals (3); declared funding source (1); high impact factor (3); industrial funding (1); multicenter studies (1); non-Chinese reports (compared to those published in mainland China) (1); number of authors (1); reporting of allocation concealment (1); reporting in a medical journal (1); reporting method of sequence generation (1); sample size (3); trial quality (1); type of intervention (pharmacologic intervention versus nonpharmacologic intervention); and year of publication (before and after CONSORT) (9). These factors are summarized in Table 4. Having a positive outcome in RCTs (compared to a neutral or negative outcome) was the only factor reported to be significantly associated with a decrease in adherence to the CONSORT Statement (Spearman correlation = −0.192; 95% CI, −0.351 to −0.011).55 Other factors that reported but did not reach statistical significance for an association with adherence to the CONSORT Statement are also summarized in Table 4.
Table 4.
Factors associated with reporting quality of articles using the CONSORT guideline
| First author | Sample size | Factors associated with adherence ↑↓ |
|---|---|---|
| Al-Namankany54 | 173 | 1. Year of publication (↑) |
| Areia21 | 120 | 1. Publication in CONSORT-endorsing journals (↑) 2. Year of publication (↑) |
| Balasubramanian45 | 69 | 1. Number of authors (↑)* 2. Multicenter studies (↑)* 3. Declared funding source (↑)* 4. Reporting in medical journals (↑)* |
| Bath55 | 114 | 1. Trial quality (↑)* 2. Trials with positive outcome (↓)* 3. Year of publication (↑)* |
| Capili58 | 10 | 1. Journal requiring the use of CONSORT (↑) |
| Chowers60 | 49 | 1. Industry sponsored trials (industry sponsored versus nonindustry sponsored trial) (↑) 2. Year of publication (↑)* |
| de Vries62 | 107 | 1. Sponsoring (↑) |
| Ethgen63 | 132 | 1. Impact factor (↑)* 2. Publication in CONSORT-endorsing journals (↑)* |
| Farrokhyar65 | 50 | 1. Sample size (↑)* 2. Year of publication (more recent publication year [up to 2005] [2001, P = 0.822; 2002, P < 0.001; 2003, P = 0.204; 2004, P < 0.001; 2005, P < 0.001]) 3. Location of the study (UK, P = 0.900; Scandinavia, P = 0.002; Other, P = 0.003) 4. Source of funding (↓) 5. Type of primary outcome in the study-categorical (↓) |
| Herdan69 | 37 | 1. Year of publication (↑)* |
| Kiehna46 | 27 | 1. Publication in CONSORT-endorsing journals (↑)* |
| Ladd25 | 127 | 1. Year of publication (↑)* |
| Moberg-Mogren76 | 14 | 1. Year of publication (↑)* |
| Montané77 | 92 | 1. Year of publication (↑)* 2. Impact factor (↑)* 3. Studies with placebo control group (↑) |
| Montgomery50 | 76 | 1. Year of publication (↑)* |
| Plint51 | 8 | 1. Reporting method of sequence generation (↑)* 2. Allocation concealment (↑)* 3. Overall consort items (↑) |
| Rios81 | 89 | 1. Industrial funding (↑)* 2. Journal of publication (publication in JCEM) (↑)* 3. Sample size (↑)* |
| Thabane84 | 63 | 1. Sample sizes (↑)* 2. Year of publication (↑)* 3. Type of intervention (pharmacologic intervention versus nonpharmacologic intervention) (↑)* |
| Zhong89 | 153 | 1. Non-Chinese reports (compared to those published in mainland China) (↑)* 2. Publication in CONSORT-endorsing journals (↑)* |
| Ziogas90 | 261 | 1. Year of publication (↑)* 2. Impact factor (↑)* |
Notes:
Statistically significant increase/decrease, P ≤ 0.05;
positively associated with adherence;
negatively associated with adherence.
Abbreviations: CONSORT, Consolidated Standards of Reporting Trials; JCEM, The Journal of Clinical Endocrinology and Metabolism.
Quality of included studies, measured by the modified OQAQ/AMSTAR checklist
The global score of each of the studies is listed in Table 5. The mean global score of the 50 included studies was 16.6 ± 2.4. Twenty-one (42%) out of the 50 studies had a global score of 17 or more. The items with the lowest scores were question 5, “Was information on included and excluded studies provided?” and question 6, “Were the characteristics of included studies provided?” with only 16% and 32% of the studies reporting each of these items correctly, respectively.
Table 5.
Reporting quality of the 50 included systematic reviews, assessed by the modified AMSTAR/OQAQ (ten items, score out of 20)
| First author | Global score |
|---|---|
| Al-Namankany54 | 15 |
| Areia21 | 18 |
| Augestad44 | 20 |
| Balasubramanian45 | 16 |
| Bath55 | 16 |
| Bereza56 | 20 |
| Bian43 | 15 |
| Bousquet57 | 18 |
| Capili58 | 15 |
| Cavadas59 | 17 |
| Lu73 | 18 |
| Chowers60 | 12 |
| Cook61 | 18 |
| de Vries62 | 14 |
| Ethgen63 | 13 |
| Eyawo64 | 18 |
| Farrokhyar65 | 19 |
| Froud48 | 16 |
| Fung49 | 17 |
| Gagnier66 | 16 |
| Halpern67 | 14 |
| Hemels68 | 19 |
| Herdan69 | 15 |
| Junhua70 | 13 |
| Kiehna46 | 16 |
| Kober71 | 17 |
| Ladd25 | 19 |
| Li72 | 18 |
| Ma74 | 19 |
| Marshman75 | 14 |
| Moberg-Mogren76 | 16 |
| Moher47 | 14 |
| Montané77 | 15 |
| Montgomery50 | 17 |
| Norton-Mabus78 | 10 |
| Parsons79 | 17 |
| Piggott80 | 14 |
| Plint51 | 18 |
| Rios81 | 20 |
| Shea82 | 19 |
| Strech83 | 18 |
| Thabane84 | 19 |
| Vigna-Talianti85 | 15 |
| Walleser86 | 19 |
| Wangge52 | 12 |
| Weir87 | 20 |
| Willis88 | 20 |
| Zhong89 | 17 |
| Zintzaras53 | 18 |
| Ziogas90 | 15 |
Abbreviations: AMSTAR, assessment of multiple systematic reviews; OQAQ, Overview Quality Assessment Questionnaire.
Discussion
We undertook a systematic scoping review of systematic reviews to investigate the adherence to reporting guidelines that included the CONSORT, PRISMA, QUOROM, TREND, MOOSE, and STROBE statements. Our systematic review included 50 studies that fulfilled our inclusion criteria, most of which originated from North American and European countries (43/50 studies). Despite the widespread acceptance of the CONSORT Statement and its subsequent extensions, the standards of reporting of clinical studies remained suboptimal. Our study showed that 86.0% of the systematic reviews included in this study concluded that there was a suboptimal quality of reporting across multidisciplinary clinical research topics using different study designs including RCTs and observational studies. The adherence of the assessed studies to reporting standards were not specific to any field of clinical research, but rather spanned across various disciplines including diagnostic procedures, interventions, cancer trials, and alternative medicine, implying the widespread lack of adherence to reporting guidelines in the medical literature. Despite the availability of guidelines and operational definitions of how to use these guidelines to improve reporting and transparency of clinical literature (including providing checklists, flow diagrams, and explicit methods of recruitment and allocation12), the uptake of these guidelines remained low. Several shortcomings of the reporting standards of clinical literature include inadequate reporting of the methods, selective reporting of the results, or misinterpretation of the results.91 Studies have shown that the use of these guidelines was associated with better reporting of studies of acupuncture trials,92 and only minimal improvement in the adherence to reporting guidelines of studies that investigated diagnostic accuracy.93 It is possible that the lack of adherence may relate to the narrow focus of these guidelines on specific clinical areas or study designs, and therefore further guidelines need to be developed. Such new guidelines can be developed based on sets of tools and criteria, as proposed previously.94 The poor adherence to reporting guidelines seen in the clinical literature is also seen in other settings including the failure to follow the National Institute of Health guidelines for reporting sex and ethnicity in clinical trials.95 Efforts to address the gap between the standards set by the guidelines and the actual standards of the published literature are therefore needed.
The most striking observation from our study was the lack of consistency in methods of recording the adherence to the reporting guidelines, and therefore it was not possible to combine the results to provide a summary statistic. This highlights the need for a consensus statement on the reporting of methodological quality of studies addressing the adherence to CONSORT and other statements.
Despite the suboptimal adherence to reporting guidelines in most of the studies reviewed, we observed that RCTs have a better adherence to reporting standards than non-RCTs. In addition, studies published in journals endorsing the CONSORT Statement have higher adherence to reporting standards. Not surprisingly, studies published after the introduction of CONSORT showed a better reporting quality and adherence to reporting guidelines. These findings are encouraging and provide a platform to disseminate knowledge generated by this study to multiple disciplines in health research to stress the need for improvement in adherence to reporting guidelines.
The strengths of our study are that we conducted a rigorous systematic review and included studies investigating the quality of reporting across various clinical areas of research, thus adding a scoping review methodology to a systematic review. We have also extracted relevant data and attempted to provide a summary statistic; however, the diversity of the findings did not allow for the computation of results.
Our study results are limited by the lack of reviews addressing adherence standards to other guidelines (MOOSE, TREND, QUOROM), the inability to combine the overall study findings, and the unavailability of tools designed to assess the quality of systematic reviews investigating methodological quality. Furthermore, the design, conduct, analysis, and reporting of the results of the reviews including definitions of outcomes (and predictor variables) varied substantially within and between the guidelines. This is mainly due to the lack of an established framework or standard for the conduct and reporting of reviews assessing the adherence to guidelines.
The study findings are nonetheless important for educators, authors, editors, sponsors, health consumers, and research ethics boards.
Summary and recommendations
Factors that are associated with reporting standards can be grouped into four categories:
Study design: Better reporting standards were seen in studies with large sample sizes; RCT design; transparency in reporting randomization, adverse events, and secondary outcomes; and studies of drug interventions.
Timing of publication: Studies that were published more recently were associated with better quality of reporting.
Study sponsor: Studies with an industrial sponsor were also associated with a better quality of reporting.
Journal: Journals with a high impact factor and those endorsing the CONSORT Statement and its extensions tended to publish studies with better adherence to reporting standards.
Recommendations for educators
Educators are at the forefront of teaching research methodology and applications in clinical settings, and therefore they play an important role in improving the reporting standards of clinical literature. Educators need to emphasize the importance of reporting standards and incorporate the guidelines in research training. They also need to provide ongoing training through workshops at professional meetings, and highlight the factors shown to improve the quality of reporting to foster improved reporting standards of the clinical literature.
Recommendations for authors
Authors should use the reporting standards appropriate to the study design as a guide to planning and reporting studies, and provide a flow diagram and checklist that will not only improve the reporting standard and adherence to guidelines, but will also help with transparency and reproducibility of the study. The use of the guidelines will also help to minimize reporting bias. For resources on using reporting standards, see the EQUATOR Network website.12
Recommendations for editors
Studies published in journals endorsing the CONSORT and its extensions were described as having better reporting quality and increased adherence to guidelines. Therefore, editors must endorse the reporting standards as part of their journal editorial policy.
Furthermore, inclusion of the respective guideline checklist must also be part of the editorial policy. Editors need to consider assessing the adherence to reporting guidelines as a requirement for peer review, and they should revise the peer review process to incorporate these assessments.
Recommendations for sponsors
Sponsors can ensure that the quality of the study methodology and transparency are meeting these standards by requesting adherence to the respective reporting guidelines appropriate for the study design.
Recommendations for research ethics boards
Institutional Review Boards or Research Ethics Boards have a substantial responsibility to ensure ethical and sound methodological quality of clinical studies. Therefore, we recommend that Institutional Review Boards/Research Ethics Boards require that protocols be submitted for ethical approval to clearly state what reporting standards the study will be using based on the study design, and that reporting guidelines checklist are part of the application for ethics approval.
Recommendation for health consumers
In accordance with the general principles of evidence-based health care practice,96 we encourage consumers or health care users to be actively involved in their health care by discussing their care options with their providers. Understanding information presented in published studies can be an important ingredient in these discussions. We suggest that health care users consider the evaluation of the quality of the information presented in the literature by looking for a guideline statement and a checklist to ensure the study reporting followed a certain standard that is appropriate for the particular study design.
Lastly, one element that all parties need to take into consideration is the importance of conducting large studies. Large studies have been shown to have a better quality of reporting.81,84,97 Large studies are also less prone to problems of bias and have better precision.
Conclusion
Reporting guidelines help to improve the quality and transparency of clinical studies and allow for systematic reviews and meta-analyses to provide evidence worthy of changing practice, improving knowledge, and better management of health and disease. The current reporting standards and adherence to guidelines are poor and are in need of major improvement. Steps need to be taken by all involved in the conducting and reporting of clinical research in order to achieve better standards of reporting, thus minimizing bias and providing reproducible studies that can be combined to reach conclusive evidence.
Acknowledgments
Special thanks to Neera Bhatnagar for the detailed discussion on literature search strategies. This study was funded in part by funds from the CANNeCTIN program.
ZS designed and wrote the first draft of the protocol and current manuscript; LM, DK, VBD, RD, SZ, VF, BD, and MB contributed to the protocol design and writing of the manuscript; LT conceived of the idea for the study and contributed to the design and writing of the manuscript. All authors read and approved the final draft.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.US National Library of Medicine National Institutes of Health PubMed.gov [homepage on the Internet] Bethesda, MD: National Center for Biotechnology Information, US National Library of Medicine; Available from: http://www.ncbi.nlm.nih.gov/pubmedAccessed February 1, 2013 [Google Scholar]
- 2.US National Library of Medicine National Institutes of Health PubMed.gov [webpage on the Internet] Bethesda, MD: National Center for Biotechnology Information, US National Library of Medicine; 2013Available from: http://www.ncbi.nlm.nih.gov/pubmed/?term=depression+systematic+reviewAccessed February 1, 2013 [Google Scholar]
- 3.US National Library of Medicine National Institutes of Health PubMed.gov [webpage on the Internet] Bethesda, MD: National Center for Biotechnology Information, US National Library of Medicine; 2013Available from: http://www.ncbi.nlm.nih.gov/pubmed/?term=cancer+systematic+reviewAccessed February 1, 2013 [Google Scholar]
- 4.Greaves C, Sheppard K, Abraham C, et al. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health. 2011;11(1):119. doi: 10.1186/1471-2458-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tennant R, Goens C, Barlow J, Day C, Stewart-Brown S. A systematic review of reviews of interventions to promote mental health and prevent mental health problems in children and young people. Journal of Public Mental Health. 2007;6(1):25–32. [Google Scholar]
- 6.Ernst E. A systematic review of systematic reviews of homeopathy. Br J Clin Pharmacol. 2002;54(6):577–582. doi: 10.1046/j.1365-2125.2002.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloom BS. Effects of continuing medical education on improving physician clinical care and patient health: a review of systematic reviews. Int J Technol Assess Health Care. 2005 Summer;21(3):380–385. doi: 10.1017/s026646230505049x. [DOI] [PubMed] [Google Scholar]
- 8.Ernst E, Canter PH. A systematic review of systematic reviews of spinal manipulation. J R Soc Med. 2006;99(4):192–196. doi: 10.1258/jrsm.99.4.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Niet GJ, Tiemens BG, Kloos MW, Hutschemaekers GJ. Review of systematic reviews about the efficacy of non-pharmacological interventions to improve sleep quality in insomnia. Int J Evid Based Healthc. 2009;7(4):233–242. doi: 10.1111/j.1744-1609.2009.00142.x. [DOI] [PubMed] [Google Scholar]
- 10.Brouwers MC, Garcia K, Makarski J, Daraz L. The landscape of knowledge translation interventions in cancer control: What do we know and where to next? A review of systematic reviews. Implementation science: IS. 2011;6:130. doi: 10.1186/1748-5908-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richards D. The EQUATOR network and website. Evid Based Dent. 2007;8(4):117. doi: 10.1038/sj.ebd.6400533. [DOI] [PubMed] [Google Scholar]
- 12.EQUATOR Network Enhancing the QUAlity and Transparency Of health Research [homepage on the Internet] Oxford, UK; EQUATOR Network; 2011Available from: http://www.equator-network.org/home/Accessed May 13, 2012 [Google Scholar]
- 13.Simera I, Altman DG, Moher D, Schulz KF, Hoey J. Guidelines for reporting health research: the EQUATOR network’s survey of guideline authors. PLoS Med. 2008;5(6):e139. doi: 10.1371/journal.pmed.0050139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CONSORT CONSORT Transparent Reporting of Trials [homepage on the Internet] Available from: http://www.consort-statement.org/Accessed May 13, 2012 [Google Scholar]
- 15.Schulz K, Altman D, Moher D, Group The CONSORT Group CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Medicine. 2010;8(1):18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shikata S, Nakayama T, Yamagishi H. Quality of surgical randomized controlled trials for acute cholecystitis: assessment based on CONSORT and additional check items. J Hepatobiliary Pancreat Surg. 2008;15(3):297–303. doi: 10.1007/s00534-007-1268-8. [DOI] [PubMed] [Google Scholar]
- 17.Süt N, Senocak M, Uysal O, Köksalan H. Assessing the quality of randomized controlled trials from two leading cancer journals using the CONSORT statement. Hematol Oncol Stem Cell Ther. 2008;1(1):38–43. doi: 10.1016/s1658-3876(08)50059-8. [DOI] [PubMed] [Google Scholar]
- 18.Uetani K, Nakayama T, Ikai H, Yonemoto N, Moher D. Quality of reports on randomized controlled trials conducted in Japan: evaluation of adherence to the CONSORT statement. Intern Med. 2009;48(5):307–313. doi: 10.2169/internalmedicine.48.1358. [DOI] [PubMed] [Google Scholar]
- 19.Ziogas DC, Zintzaras E. Analysis of the quality of reporting of randomized controlled trials in acute and chronic myeloid leukemia, and myelodysplastic syndromes as governed by the CONSORT statement. Ann Epidemiol. 2009;19(7):494–500. doi: 10.1016/j.annepidem.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez F, Meyer N, Gourraud PA, Paul C. CONSORT adoption and quality of reporting of randomized controlled trials: a systematic analysis in two dermatology journals. Br J Dermatol. 2009;161(5):1159–1165. doi: 10.1111/j.1365-2133.2009.09382.x. [DOI] [PubMed] [Google Scholar]
- 21.Areia M, Soares M, Dinis-Ribeiro M. Quality reporting of endoscopic diagnostic studies in gastrointestinal journals: where do we stand on the use of the STARD and CONSORT statements? Endoscopy. 2010;42(2):138–147. doi: 10.1055/s-0029-1243846. [DOI] [PubMed] [Google Scholar]
- 22.Dasí F, Navarro-García MM, Jiménez-Heredia M, et al. Evaluation of the quality of publications on randomized clinical trials using the Consolidated Standards of Reporting Trials (CONSORT) statement guidelines in a Spanish tertiary hospital. J Clin Pharmacol. 2012;52(7):1106–1114. doi: 10.1177/0091270011407916. [DOI] [PubMed] [Google Scholar]
- 23.He J, Du L, Liu G, et al. Quality assessment of reporting of randomization, allocation concealment, and blinding in traditional Chinese medicine RCTs: a review of 3159 RCTs identified from 260 systematic reviews. Trials. 2011;12:122. doi: 10.1186/1745-6215-12-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith BA, Lee HJ, Lee JH, et al. Quality of reporting randomized controlled trials (RCTs) in the nursing literature: application of the consolidated standards of reporting trials (CONSORT) Nurs Outlook. 2008;56(1):31–37. doi: 10.1016/j.outlook.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Ladd BO, McCrady BS, Manuel JK, Campbell W. Improving the quality of reporting alcohol outcome studies: effects of the CONSORT statement. Addict Behav. 2010;35(7):660–666. doi: 10.1016/j.addbeh.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Hopewell S, Schulz KF, et al. CONSORT CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55. doi: 10.1016/j.ijsu.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Noah N. The STROBE initiative: STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) Epidemiol Infect. 2008;136(7):865. doi: 10.1017/S0950268808000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354(9193):1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 29.Treasure E. The TREND statement. Evid Based Dent. 2004;5(4):88–91. doi: 10.1038/sj.ebd.6400290. [DOI] [PubMed] [Google Scholar]
- 30.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 31.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. [Google Scholar]
- 33.Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011;11(1):15. doi: 10.1186/1471-2288-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brien SE, Lorenzetti DL, Lewis S, Kennedy J, Ghali WA. Overview of a formal scoping review on health system report cards. Implement Sci. 2010;5(2):2. doi: 10.1186/1748-5908-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montori VM, Wilczynski NL, Morgan D, Haynes RB. Optimal search strategies for retrieving systematic reviews from Medline: analytical survey. BMJ. 2005;330(7482):68. doi: 10.1136/bmj.38336.804167.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilczynski NL, Haynes RB. EMBASE search strategies achieved high sensitivity and specificity for retrieving methodologically sound systematic reviews. J Clin Epidemiol. 2007;60(1):29–33. doi: 10.1016/j.jclinepi.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement. 1960;20(1):37. [Google Scholar]
- 39.Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013–1020. doi: 10.1016/j.jclinepi.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Shea BJ, Bouter LM, Peterson J, et al. External validation of a measurement tool to assess systematic reviews (AMSTAR) PLoS One. 2007;2(12):e1350. doi: 10.1371/journal.pone.0001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oxman AD, Guyatt GH, Singer J, et al. Agreement among reviewers of review articles. J Clin Epidemiol. 1991;44(1):91–98. doi: 10.1016/0895-4356(91)90205-n. [DOI] [PubMed] [Google Scholar]
- 43.Bian ZX, Moher D, Dagenais S, et al. Improving the quality of randomized controlled trials in Chinese herbal medicine, part IV: applying a revised CONSORT checklist to measure reporting quality. Zhong xi yi jie he xue bao = Journal of Chinese integrative medicine. 2006;4(3):233–242. doi: 10.3736/jcim20060303. [DOI] [PubMed] [Google Scholar]
- 44.Augestad KM, Berntsen G, Lassen K, Bellika JG, Wootton R, Lindsetmo RO. Standards for reporting randomized controlled trials in medical informatics: a systematic review of CONSORT adherence in RCTs on clinical decision support. J Am Med Inform Assoc. 2012;19(1):13–21. doi: 10.1136/amiajnl-2011-000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balasubramanian SP, Wiener M, Alshameeri Z, Tiruvoipati R, Elbourne D, Reed MW. Standards of reporting of randomized controlled trials in general surgery: can we do better? Annals of surgery. 2006;244(5):663–667. doi: 10.1097/01.sla.0000217640.11224.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiehna EN, Starke RM, Pouratian N, Dumont AS. Standards for reporting randomized controlled trials in neurosurgery. J Neurosurg. 2011;114(2):280–285. doi: 10.3171/2010.8.JNS091770. [DOI] [PubMed] [Google Scholar]
- 47.Moher D, Soeken K, Sampson M, Ben-Porat L, Berman B. Assessing the quality of reports of systematic reviews in pediatric complementary and alternative medicine. BMC Pediatr. 2002;2:3. doi: 10.1186/1471-2431-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Froud R, Eldridge S, Diaz Ordaz K, Marinho VC, Donner A. Quality of cluster randomized controlled trials in oral health: a systematic review of reports published between 2005 and 2009. Community Dent Oral Epidemiol. 2012;40(Suppl 1):3–14. doi: 10.1111/j.1600-0528.2011.00660.x. [DOI] [PubMed] [Google Scholar]
- 49.Fung AE, Palanki R, Bakri SJ, Depperschmidt E, Gibson A. Applying the CONSORT and STROBE statements to evaluate the reporting quality of neovascular age-related macular degeneration studies. Ophthalmology. 2009;116(2):286–296. doi: 10.1016/j.ophtha.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 50.Montgomery AA, Astin MP, Peters TJ. Reporting of factorial trials of complex interventions in community settings: a systematic review. Trials. 2011;12:179. doi: 10.1186/1745-6215-12-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plint AC, Moher D, Morrison A, et al. Does the CONSORT checklist improve the quality of reports of randomised controlled trials? A systematic review. The Medical journal of Australia. 2006;185(5):263–267. doi: 10.5694/j.1326-5377.2006.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 52.Wangge G, Klungel OH, Roes KC, de Boer A, Hoes AW, Knol MJ. Room for improvement in conducting and reporting non-inferiority randomized controlled trials on drugs: a systematic review. PLoS One. 2010;5(10):e13550. doi: 10.1371/journal.pone.0013550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zintzaras E, Kitsios GD, Papathanasiou AA, et al. Randomized trials of dopamine agonists in restless legs syndrome: a systematic review, quality assessment, and meta-analysis. Clin Ther. 2010;32(2):221–237. doi: 10.1016/j.clinthera.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 54.Al-Namankany AA, Ashley P, Moles DR, Parekh S. Assessment of the quality of reporting of randomized clinical trials in paediatric dentistry journals. Int J Paediatr Dent. 2009;19(5):318–324. doi: 10.1111/j.1365-263X.2009.00974.x. [DOI] [PubMed] [Google Scholar]
- 55.Bath FJ, Owen VE, Bath PM. Quality of full and final publications reporting acute stroke trials: a systematic review. Stroke. 1998;29(10):2203–2210. doi: 10.1161/01.str.29.10.2203. [DOI] [PubMed] [Google Scholar]
- 56.Bereza BG, Machado M, Einarson TR. Assessing the reporting and scientific quality of meta-analyses of randomized controlled trials of treatments for anxiety disorders. Ann Pharmacother. 2008;42(10):1402–1409. doi: 10.1345/aph.1L204. [DOI] [PubMed] [Google Scholar]
- 57.Bousquet PJ, Calderon MA, Demoly P, et al. The Consolidated Standards of Reporting Trials (CONSORT) Statement applied to allergen-specific immunotherapy with inhalant allergens: a Global Allergy and Asthma European Network (GA(2)LEN) article. The Journal of Allergy and Clinical Immunology. 2011;127(1):49–56. 56, e41–e11. doi: 10.1016/j.jaci.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 58.Capili B, Anastasi JK, Geiger JN. Adverse event reporting in acupuncture clinical trials focusing on pain. Clin J Pain. 2010;26(1):43–48. doi: 10.1097/AJP.0b013e3181b2c985. [DOI] [PubMed] [Google Scholar]
- 59.Cavadas V, Branco F, Carvalho FL, Osorio L, Gomes MJ, Silva-Ramos M. The quality of reporting of randomized controlled trials in pelvic organ prolapse. Int Urogynecol J. 2011;22(9):1117–1125. doi: 10.1007/s00192-011-1426-z. [DOI] [PubMed] [Google Scholar]
- 60.Chowers MY, Gottesman BS, Leibovici L, Pielmeier U, Andreassen S, Paul M. Reporting of adverse events in randomized controlled trials of highly active antiretroviral therapy: systematic review. Antimicrob Chemother. 2009;64(2):239–250. doi: 10.1093/jac/dkp191. [DOI] [PubMed] [Google Scholar]
- 61.Cook DA, Levinson AJ, Garside S. Method and reporting quality in health professions education research: a systematic review. Med Educ. 2011;45(3):227–238. doi: 10.1111/j.1365-2923.2010.03890.x. [DOI] [PubMed] [Google Scholar]
- 62.de Vries TW, van Roon EN. Low quality of reporting adverse drug reactions in paediatric randomised controlled trials. Arch Dis Child. 2010;95(12):1023–1026. doi: 10.1136/adc.2009.175562. [DOI] [PubMed] [Google Scholar]
- 63.Ethgen M, Boutron L, Steg PG, Roy C, Ravaud P. Quality of reporting internal and external validity data from randomized controlled trials evaluating stents for percutaneous coronary intervention. BMC Med Res Methodol. 2009;9:24. doi: 10.1186/1471-2288-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eyawo O, Lee CW, Rachlis B, Mills EJ. Reporting of noninferiority and equivalence randomized trials for major prostaglandins: a systematic survey of the ophthalmology literature. Trials. 2008;9:69. doi: 10.1186/1745-6215-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farrokhyar F, Chu R, Whitlock R, Thabane L. A systematic review of the quality of publications reporting coronary artery bypass grafting trials. Can J Surg. 2007;50(4):266–277. [PMC free article] [PubMed] [Google Scholar]
- 66.Gagnier JJ, DeMelo J, Boon H, Rochon P, Bombardier C. Quality of reporting of randomized controlled trials of herbal medicine interventions. Am J Med. 2006;119(9):800, e801–e811. doi: 10.1016/j.amjmed.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 67.Halpern SH, Darani R, Douglas MJ, Wight W, Yee J. Compliance with the CONSORT checklist in obstetric anaesthesia randomised controlled trials. Int J Obstet Anesth. 2004;13(4):207–214. doi: 10.1016/j.ijoa.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 68.Hemels ME, Vicente C, Sadri H, Masson MJ, Einarson TR. Quality assessment of meta-analyses of RCTs of pharmacotherapy in major depressive disorder. Curr Med Res Opin. 2004;20(4):477–484. doi: 10.1185/030079904125003197. [DOI] [PubMed] [Google Scholar]
- 69.Herdan A, Roth R, Grass D, et al. Improvement of quality of reporting in randomised controlled trials to prevent hypotension after spinal anaesthesia for caesarean section. Gynecol Surg. 2011;8(2):121–127. doi: 10.1007/s10397-010-0648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Junhua Z, Hongcai S, Xiumei G, et al. Methodology and reporting quality of systematic review/meta–analysis of traditional Chinese medicine. J Evid Based Complementary Altern Med. 2007;13(8):797–805. doi: 10.1089/acm.2007.7195. [DOI] [PubMed] [Google Scholar]
- 71.Kober T, Trelle S, Engert A. Reporting of randomized controlled trials in Hodgkin lymphoma in biomedical journals. J Natl Cancer Inst. 2006;98(9):620–625. doi: 10.1093/jnci/djj160. [DOI] [PubMed] [Google Scholar]
- 72.Li JY, Zhang YF, Smith GS, et al. Quality of reporting of randomized clinical trials in tai chi interventions-a systematic review. Evidence-Based Complementary and Alternative Medicine. 2011;2011:383245. doi: 10.1093/ecam/nep022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu L, Zeng J, Chen Y. Quality of reporting in randomized controlled trials conducted in China on the treatment of cancer pain. Expert Rev Anticancer Ther. 2011;11(6):871–877. doi: 10.1586/era.10.236. [DOI] [PubMed] [Google Scholar]
- 74.Ma B, Guo J, Qi G, et al. Epidemiology, quality and reporting characteristics of systematic reviews of traditional Chinese medicine interventions published in Chinese journals. PLoS One. 2011;6(5):e20185. doi: 10.1371/journal.pone.0020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marshman Z, Farid F. The quality of reporting of randomised controlled trials in dental public health. Community dental health. 2010;27(4):253–256. [PubMed] [Google Scholar]
- 76.Moberg-Mogren E, Nelson DL. Evaluating the quality of reporting occupational therapy randomized controlled trials by expanding the CONSORT criteria. Am J Occup Ther. 2006;60(2):226–235. doi: 10.5014/ajot.60.2.226. [DOI] [PubMed] [Google Scholar]
- 77.Montane E, Vallano A, Vidal X, Aguilera C, Laporte JR. Reporting randomised clinical trials of analgesics after traumatic or orthopaedic surgery is inadequate: a systematic review. BMC Clin Pharmol. 2010;10(2) doi: 10.1186/1472-6904-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Norton-Mabus JC, Nelson DL. Reporting of randomized controlled trials in occupational therapy and speech therapy: evaluation using an expansion of the CONSORT statement. OTJR (Thorofare N J) 2008;28(2):64–71. [Google Scholar]
- 79.Parsons NR, Hiskens R, Price CL, Achten J, Costa ML. A systematic survey of the quality of research reporting in general orthopaedic journals. J Bone Joint Surg Br. 2011;93(9):1154–1159. doi: 10.1302/0301-620X.93B9.27193. [DOI] [PubMed] [Google Scholar]
- 80.Piggott M, McGee H, Feuer D. Has CONSORT improved the reporting of randomized controlled trials in the palliative care literature? A systematic review. Palliat Med. 2004;18(1):32–38. doi: 10.1191/0269216304pm857oa. [DOI] [PubMed] [Google Scholar]
- 81.Rios LP, Odueyungbo A, Moitri MO, Rahman MO, Thabane L. Quality of reporting of randomized controlled trials in general endocrinology literature. J Clin Endocrinol Metab. 2008;93(10):3810–3816. doi: 10.1210/jc.2008-0817. [DOI] [PubMed] [Google Scholar]
- 82.Shea B, Bouter LM, Grimshaw JM, et al. Scope for improvement in the quality of reporting of systematic reviews. From the Cochrane Musculoskeletal Group. J Rheumatol. 2006;33(1):9–15. [PubMed] [Google Scholar]
- 83.Strech D, Soltmann B, Weikert B, Bauer M, Pfennig A. Quality of reporting of randomized controlled trials of pharmacologic treatment of bipolar disorders: a systematic review. J Clin Psychiatry. 2011;72(9):1214–1221. doi: 10.4088/JCP.10r06166yel. [DOI] [PubMed] [Google Scholar]
- 84.Thabane L, Chu R, Cuddy K, Douketis J. What is the quality of reporting in weight loss intervention studies? A systematic review of randomized controlled trials. Int J Obes (Lond) 2007;31(10):1554–1559. doi: 10.1038/sj.ijo.0803640. [DOI] [PubMed] [Google Scholar]
- 85.Vigna-Taglianti F, Vineis P, Liberati A, Faggiano F. Quality of systematic reviews used in guidelines for oncology practice. Ann Oncol. 2006;17(4):691–701. doi: 10.1093/annonc/mdl003. [DOI] [PubMed] [Google Scholar]
- 86.Walleser S, Hill SR, Bero LA. Characteristics and quality of reporting of cluster randomized trials in children: reporting needs improvement. J Clin Epidemiol. 2011;64(12):1331–1340. doi: 10.1016/j.jclinepi.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 87.Weir CR, Staggers N, Laukert T. Reviewing the impact of computerized provider order entry on clinical outcomes: The quality of systematic reviews. Int J Med Inform. 2012;81(4):219–231. doi: 10.1016/j.ijmedinf.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 88.Willis BH, Quigley M. The assessment of the quality of reporting of meta-analyses in diagnostic research: a systematic review. BMC Med Res Methodol. 2011;11:163. doi: 10.1186/1471-2288-11-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhong YZ, Wei Jiang, Hongli Fan Tao. et al. Quality of reporting of two-group parallel randomized controlled clinical trials of multi-herb formulae: A survey of reports indexed in the Science Citation Index Expanded. Eur J Integr Med. 2011;3(4):E303–E310. [Google Scholar]
- 90.Ziogas DCZ E. Analysis of the quality of reporting of randomized controlled trials in acute and chronic myeloid leukemia, and myelodysplastic syndromes as governed by the CONSORT statement. Ann Epidemiol. 2009;19(7):494–500. doi: 10.1016/j.annepidem.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 91.Simera I, Moher D, Hirst A, Hoey J, Schulz KF, Altman DG. Transparent and accurate reporting increases reliability, utility, and impact of your research: reporting guidelines and the EQUATOR Network. BMC Medicine. 2010;8:24. doi: 10.1186/1741-7015-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Prady SL, Richmond SJ, Morton VM, Macpherson H. A systematic evaluation of the impact of STRICTA and CONSORT recommendations on quality of reporting for acupuncture trials. PLoS One. 2008;3(2):e1577. doi: 10.1371/journal.pone.0001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smidt N, Rutjes AW, van der Windt DA, et al. The quality of diagnostic accuracy studies since the STARD statement: has it improved? Neurology. 2006;67(5):792–797. doi: 10.1212/01.wnl.0000238386.41398.30. [DOI] [PubMed] [Google Scholar]
- 94.Moher D, Schulz KF, Simera I, Altman DG. Guidance for developers of health research reporting guidelines. PLoS medicine. 2010 Feb;7(2):e1000217. doi: 10.1371/journal.pmed.1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Geller S, Adams M, Carnes M. Adherence to federal guidelines for reporting of sex and race/ethnicity in clinical trials. J Womens Health (Larchmt) 2006;15(10):1123–1131. doi: 10.1089/jwh.2006.15.1123. [DOI] [PubMed] [Google Scholar]
- 96.Guyatt GH, Haynes RB, Jaeschke RZ, et al. Users’ Guides to the Medical Literature: XXV. Evidence-based medicine: principles for applying the Users’ Guides to patient care. Evidence-Based Medicine Working Group. JAMA. 2000;284(10):1290–1296. doi: 10.1001/jama.284.10.1290. [DOI] [PubMed] [Google Scholar]
- 97.Aleissa S, Parsons D, Grant J, Harder J, Howard J. Deep wound infection following pediatric scoliosis surgery: incidence and analysis of risk factors. Can J Surg. 2011;54(4):263–269. doi: 10.1503/cjs.008210. [DOI] [PMC free article] [PubMed] [Google Scholar]


