Summary
In comparison to total therapy 1 (TT1), the phase 3 trial total therapy 2 (TT2) evaluated the benefit of up-front administration of thalidomide (THAL); TT2 also introduced post-transplant consolidation chemotherapy. With median follow-up times of 5 and 12 years, respectively, outcome comparisons were made of 668 patient's enrolled on TT2 and 231 patients treated on TT1. Complete response (CR) rates were similar at 40% for TT1 and TT2 without THAL versus 60% on the THAL arm of TT2. CR durations were similar with either TT2 arm and both were superior to results of TT1. Event-free and overall survivals were extended from 2.6 to 5.7 years, respectively, with TT1 to 4.8 and 8.0 years with TT2. TT2's major advance vis-à-vis TT1 pertained to the subgroup without cytogenetic abnormalities (CA), supporting the role of post-transplant consolidation therapy, whereas the improved survival of the CA subgroup on the experimental versus control arm of TT2 attests to the role of THAL in this setting. Adjusting for prognostic variables in multivariate and pair-mate analyses, TT2 was superior to TT1 in terms of CR duration, event-free and overall survival. These results provide a basis for the prospective evaluation of the consolidation strategy in a randomized clinical trial design.
Keywords: multiple myeloma, prognosis, high-dose therapy
We have previously reported on total therapy 2 (TT2), a tandem transplant trial that addressed, in a randomized trial design, the role of thalidomide (THAL) in improving complete response (CR) and extending survival (Barlogie et al, 2006a). Results indicated that, while CR and event-free survival (EFS) were superior on the THAL versus no THAL arm, overall survival (OS) was not extended by the addition of THAL. An additional novel feature of the trial was the introduction of consolidation chemotherapy post-transplant in an attempt to delay or even prevent recurrence. With a median follow-up of 6 years for live patients, we now report on a final analysis of the 668 patients enrolled and address the role of THAL for subgroups with different risk features prior to TT2 and whether patients completing the intended consolidation therapy derived EFS and OS benefit compared to those who either did not qualify due to thrombocytopenia or lack of antitumour effect from chemotherapy during the induction phase. The TT2 outcome data are also examined in the context of the predecessor protocol, total therapy 1 (TT1), a tandem transplant program without consolidation chemotherapy (Barlogie et al, 1997) (Barlogie et al, 2006b,c).
Patients and methods
Treatment protocols
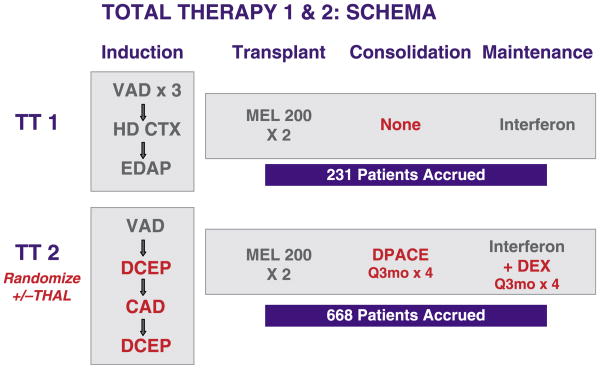
The details of TT2 have been published previously (Barlogie et al, 2006a). Briefly, TT2 consisted of four phases (Fig 1). Induction consisted of VAD (vincristine-doxorubicin-dexamethasone) (Barlogie et al, 1984), DCEP (dexamethasone plus 4 d continuous infusions of cyclophosphamide, etoposide, cis-platin) (Desikan et al, 1999), CAD (4 d continuous infusions of cyclophosphamide, doxorubicin plus dexamethasone) for peripheral blood stem cell (PBSC) collection and further DCEP. The tandem transplant phase applied melphalan 200 mg/m2 (MEL200) 3–6 months apart; those over the age of 69 years and with renal insufficiency (creatinine >176.8 μmol/l) were given a lower melphalan dose of 140 mg/m2 (MEL140); in case of less than partial response (PR) prior to the second transplant, the BEAM regimen (carmustine, etoposide, cytarabine, melphalan) was applied with the second transplant (Ramasamy et al, 2004). Consolidation consisted of DPACE (dexamethasone plus 4 d continuous infusions of cis-platin, doxorubicin, cyclophosphamide, etoposide) (Lee et al, 2003) for the last 200 patients, once no difference had been detected among the two arms to which the initial 109 patients had been randomized (DCEP every 3 months for four cycles versus DCEP alternating with CAD every 6 weeks for a total of eight cycles); those failing to recover a minimum platelet count of 50 × 109/l and others in whom DCEP was ineffective during induction (<25% further decrease in M-protein in serum and urine) were offered monthly dexamethasone. Maintenance consisted of dexamethasone pulsing every 3 months for four cycles added to interferon in the first year, and interferon was continued subsequently until relapse or undue toxicity. THAL was administered from the inception of therapy to those randomized to the experimental arm until disease relapse or undue toxicity.
Fig 1.
Treatment schemata for total therapy 1 (TT1) and total therapy (TT2). TT1 was a phase II trial whereas TT2 was a phase III trial with a control arm and an experimental arm that added thalidomide (THAL) from the initiation of therapy. In comparison to TT1, TT2 employed more intensive haematopoietic growth factor-requiring induction chemotherapy (DCEP, CAD) and introduced post-transplant consolidation chemotherapy with DPACE, administered every 3 months for four cycles. Maintenance therapy in TT2 added high-dose dexamethasone pulsing every 3 months for four cycles to interferon maintenance, which was then continued as single agent until relapse or undue toxicity. For details of regimens and abbreviations, refer to text.
TT1, the predecessor trial of TT2, also applied tandem MEL200 or MEL140 transplants but, for the second transplant when PR was not achieved after the first transplant, MEL140 plus total body irradiation (TBI, 8.5 Gy) was used instead of BEAM (see Fig 1) (Barlogie et al, 1997, 2006c). Induction chemotherapy comprised VAD for two to three cycles, cyclophosphamide for PBSC mobilization and EDAP (etoposide, dexamethasone, cytarabine, cis-platin); there was no consolidation; maintenance consisted of interferon only without dexamethasone and was applied until disease progression or toxicity.
Cytogenetic methods
Metaphase analysis was performed as previously described (Sawyer et al, 1995). Briefly, bone marrow was processed for chromosome studies by standard techniques. Cultures were set up in 10 ml of RPMI 1640 medium (Sigma, St Louis, MO, USA) supplemented with 20% fetal bovine serum (Sigma), and 1% L-glutamine/Pen-Strep solution (Gibco, Grand Island, NY, USA). Cultures were initiated with 1–3 ml of whole bone marrow. Direct harvest, 24- and 48-h unstimulated cultures were employed on most specimens. The direct harvest procedure included the combination of 1 ml 0.05% trypsin EDTA (Gibco), 9 ml of hypotonic solution (0.075 mol/l KCL) and 5 μl/ml colcemid solution (Irvine Scientific, Santa Ana, CA, USA) for 1 h at 37°C. The 24- and 48-h cultures received 50 μl colcemid solution for 1 and 2 h respectively. Twenty cells were studied in each case. The designation of ‘cytogenetic abnormalities’ (CA) required the presence of an abnormal clone, which was defined as two or more metaphases with either the same structural abnormality or the same extra chromosome, or at least three cells with the same missing chromosome.
Statistical analyses
The Kaplan–Meier method was used in estimating EFS, OS and CR duration (Kaplan & Meier, 1958), in the context of baseline laboratory parameters which included gene expression profiling (GEP)-based risk models (Shaughnessy et al, 2007) with group comparisons made using the log-rank test. EFS was defined as the time from the date of registration to death from any cause, disease progression, or relapse. Patients experiencing no event were censored at the time of last contact. OS was defined from the date of registration until death from any cause; survivors were censored at the time of last contact. CR duration was measured from the onset of CR until relapse or death from any cause. The cumulative incidence of CR was estimated using the method outlined in Gooley et al (1999); results of different trials were compared using the log-rank test. Multivariate models of prognostic factors were carried out using Cox regression (Cox, 1972). Pair-mate analysis was used to compare TT1 and TT2 patients based on standard prognostic factors found to be significant in TT2. Pair-mate analysis was also used on the subset of patients with GEP information available based on their risk score. Also examined was the influence of the level of completion of each of the scheduled treatment phases, using landmark techniques, along with the question of whether dexamethasone was inferior to chemotherapy during the consolidation phase.
Results
Clinical outcome from initiation of TT2, overall and by treatment arm and in relationship to TT1
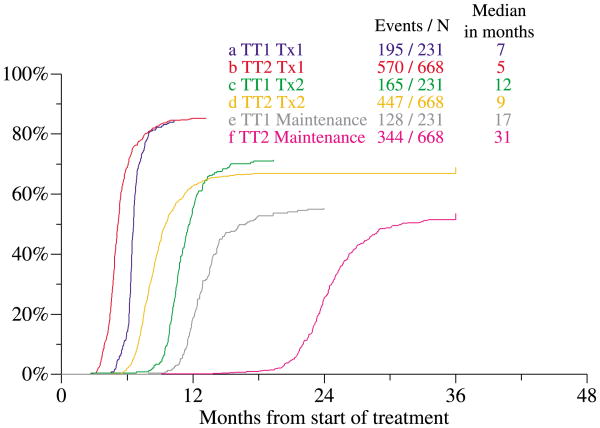
Patient characteristics were similar between the two arms of the study and, in comparison to TT1, patients on TT2 as a group were older and had higher frequencies of some adverse variables [elevations of lactate dehydrogenase (LDH) and C-reactive protein (CRP), IgA isotype] and of some favourable features (higher haemoglobin and albumin levels) (Table I). Patients had similar frequencies of CA. Figure 2 depicts the cumulative progression of patients through the protocol phases of TT1 and TT2 (both arms combined as there was no difference between them). Results showed more rapid progression through the successive protocol steps with TT2 than TT1.
Table I.
Characteristics of patients enrolled in total therapy 2 (TT 2) according to protocol arm [with or without thalidomide (THAL)] and in total therapy 1 (TT1).
| Arm | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Factor | TT1 | TT2 | P* | No THAL | THAL | P* |
| Age ≥65 years | 21/231 (9) | 136/668 (20) | <0.001 | 72/345 (21) | 64/323 (20) | 0.735 |
| Female | 88/231 (38) | 272/668 (41) | 0.483 | 135/345 (39) | 137/323 (42) | 0.388 |
| Caucasian | 206/231 (89) | 600/668 (90) | 0.782 | 308/345 (89) | 292/323 (90) | 0.630 |
| IgA isotype | 41/231 (18) | 161/668 (24) | 0.046 | 77/345 (22) | 84/323 (26) | 0.265 |
| Albumin <35 g/l | 62/231 (27) | 119/664 (18) | 0.004 | 59/343 (17) | 60/321 (19) | 0.617 |
| B2M ≥3.5 mg/l | 95/229 (41) | 243/668 (36) | 0.169 | 126/345 (37) | 117/323 (36) | 0.936 |
| Creatinine ≥176.8 μmol/l | 22/231 (10) | 62/654 (9) | 0.984 | 127/339 (37) | 136/319 (43) | 0.176 |
| CRP ≥8.0 mg/l | 70/223 (31) | 263/658 (40) | 0.022 | 98/344 (28) | 106/322 (33) | 0.215 |
| LDH >190 U/l | 49/230 (21) | 204/666 (31) | 0.007 | 37/339 (11) | 25/315 (8) | 0.194 |
| Hb <100 g/l | 78/231 (34) | 161/667 (24) | 0.004 | 80/345 (23) | 81/322 (25) | 0.553 |
| Cytogenetic abnormalities | 74/221 (33) | 197/661 (30) | 0.305 | 104/339 (31) | 93/322 (29) | 0.614 |
Values in parenthesis are in percentage.
From chi-square test.
Fig 2.
Cumulative proportions of patients completing successive treatment steps of total therapy 1 (TT1) and total therapy 2 (TT2). Abbreviations: Tx1 (1st transplant), Tx2 (2nd transplant).
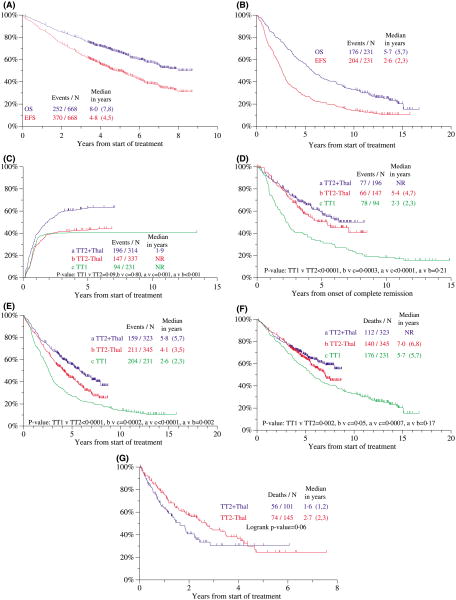
With TT2, the median follow-up of the 416 currently living patients was 5 years; and with TT1, the median follow-up of the 55 live patients was 12 years. The median durations of OS and EFS for TT2 were 8.0 and 4.8 years, respectively, compared to 5.7 and 2.6 years in the case of TT1 (Fig 3A and B). Of the 668 subjects initially enrolled in TT2, 252 have died (MM progression, 207; treatment-related toxicities, 45) and 370 have experienced an event (relapse/progression, 118; death due to MM, 207, and other causes, 45); the corresponding data for TT1 are 176 deaths (MM progression, 160; treatment-related, 16) and 204 events (relapse/progression, 28; death due to MM, 160, and other causes, 16).
Fig 3.
Kaplan–Meier plots of clinical outcomes with total therapy 2 (TT2) and total therapy 1 (TT1). (A) The median durations of overall survival (OS) and event-free survival (EFS) for all 668 patients enrolled in TT2 were 8.0 and 4.8 years respectively. (B) The median durations of OS and EFS for all 231 patients enrolled in TT1 were 5.7 and 2.6 years respectively. (C) Cumulative proportions of patients achieving complete remission (CR) counted from initiation of TT1 and TT2. Patients randomized to the thalidomide arm of TT2 (TT2 + THAL) had a significantly higher CR rate than those on the control arm of TT2 (TT2 − THAL), while there was no difference between CR rates achieved with TT1 and TT2−THAL. NR, not reached. (D) Duration of CR from onset of CR. Patients enrolled in TT2 had superior CR durations versus those treated on TT1; no difference was noted between the two arms of TT2. NR, not reached. (E) EFS of patient's enrolled in TT2 and TT1 from initiation of therapy. EFS was superior with TT2 + THAL versus TT2 − THAL, and both arms of TT2 were superior to TT1. (F) OS of patient's enrolled in TT2 and TT1 from initiation of therapy. OS was superior among patients treated with TT2 (both arms) compared to those treated with TT1, while TT2 + THAL was not superior to TT2 − THAL. Note a separation of survival curves between the two arms of TT2 emerging at 5 years. NR, not reached. (G) Postrelapse survival by treatment arm of TT2. Patients randomized initially to thalidomide (THAL) tended to fare better than those on the control arm (No THAL). (H) Treatment-related mortality with TT2 and TT1 according to age (<65 years, left; ≥65 years, right). Similar outcomes were observed in the younger age group regardless of treatment; as a group, the older age cohort experienced higher treatment-related mortality than their younger counterparts (P = 0.002). (I) EFS of patients treated with TT2 and TT1, according to the presence of cytogenetic abnormalities (CA). Overall, patients enrolled in TT2 fared better than those enrolled in TT1, regardless of CA status. Among patients without CA, both TT2 arms were individually superior to TT1, and TT2 + THAL was superior to TT2 − THAL. In the cohort with CA, patients treated with TT2 + THAL fared better than patients on TT2 − THAL, which was not superior to TT1. (J) Overall survival (OS) of patients treated with TT2 and TT1, according to the presence of CA. Overall, patients without CA fared better when enrolled in TT2 than those enrolled in TT1, while there was no difference between the two arms of TT2. Among patients with CA, those on the TT2 + THAL arm fared better than either TT1 or TT2 − THAL groups. NR, not reached.
The Kaplan–Meier plots portray outcomes for the two arms of TT2 and for TT1, with statistical differences annotated between TT1 and TT2, both combined and by study arm. The addition of THAL in TT2 raised the CR frequency to 60% from 40% noted for the control arm, which was identical to results with TT1 (P = 0.001) (Fig 3C). CR duration from onset of CR was similar for both arms of TT2 and superior to TT1 (Fig 3D). In the case of EFS, TT2 plus THAL was superior to the control arm, which, in turn, exceeded results obtained with TT1 (Fig 3E). A trend was observed for longer OS on the THAL arm versus control arm of TT2 (diverging at 5 years), both of which were superior to TT1 (Fig 3F). The postrelapse survival on TT2 tended to be longer, at a median of 2.7 years versus 1.6 years on the control versus THAL arm (P = 0.06) (Fig 3G). Treatment-related mortality (TRM) was similar in the two studies, reaching from 5% to 6% among patients younger than 65 years and approximately 10% in the older cohort (Fig 3H).
Given the dominant adverse impact of CA in both TT1 and TT2, outcomes were compared among patients with and without CA. EFS and OS were superior with TT2 (both arms) among the patients presenting without CA (Fig 3I and J). The CA subgroup benefited from the addition of THAL in TT2, whereas outcomes were similar between TT1 and the control arm of TT2 (see Fig 3I and J).
Multivariate analysis of pretreatment prognostic factor and of therapy
Table II lists, for outcomes on TT2, the independently significant prognostic factors, along with treatment arms. The presence of CA was an adverse feature for all three endpoints; elevation of LDH conferred poor OS and EFS; and increased creatinine and low albumin predicted for shorter OS. Randomization to THAL imparted longer EFS but not OS or CR duration. Relative to TT1, TT2 was superior with regard to all outcome endpoints; CA affected all three endpoints adversely, while elevated LDH levels were associated with shorter OS and EFS (Table III). These results pertained also when outcomes were compared between the individual arms of TT2 and TT1 (data not shown).
Table II.
Multivariate analysis of clinical outcomes with total therapy 2 (TT2) by protocol arm.
| Overall survival | Event-free survival | CR duration | ||||||
|---|---|---|---|---|---|---|---|---|
| Cytogenetic abnormalities (CA) | 188/634 (30%) | 1.95 (1.50, 2.53) | <0.001 | 1.31 (1.11, 1.56) | 0.002 | 83/332 (25%) | 1.95 (1.38, 2.77) | <0.001 |
| Creatinine ≥176.8 μmol/l | 60/634 (9%) | 1.57 (1.09, 2.27) | 0.017 | NS2 | NS2 | – | NS2 | NS2 |
| LDH ≥190 U/l | 197/634 (31%) | 1.47 (1.12, 1.92) | 0.005 | 1.38 (1.17, 1.64) | <0.001 | – | NS2 | NS2 |
| Albumin <35 g/l | 117/634 (18%) | 1.45 (1.07, 1.95) | 0.015 | NS2 | NS2 | – | NS2 | NS2 |
| Randomization to thalidomide | 309/634 (49%) | NS2 | NS2 | 0.84 (0.72, 0.99) | 0.032 | – | NS2 | NS2 |
Results given as Hazard Ratio (95% confidence intervals), P-value from Wald chi-square test in Cox regression.
NS2 – Multivariate results not statistically significant at 0.05 level.
Multivariate model uses stepwise selection with entry level 0.1 and variable remains if it meets the 0.05 level.
Other variables considered for OS and EFS: age, Hb, CRP, albumin and B2M; for CR duration: creatinine and B2M.
Table III.
Multivariate analysis of clinical outcome adjusting for treatment (TT2 + THAL, TT2 − THAL, TT 1).
| Overall survival | Event-free survival | CR duration | ||||||
|---|---|---|---|---|---|---|---|---|
| Cytogenetic abnormality | 262/848 (31%) | 1.84 (1.50, 2.25) | <0.001 | 1.48 (1.24, 1.78) | <0.001 | 112/433 (26%) | 1.51 (1.14, 2.01) | 0.005 |
| Creatinine ≥176.8 μmol/l | 79/848 (9%) | 1.72 (1.28, 2.31) | <0.001 | NS2 | NS2 | – | NS2 | NS2 |
| Enrolled in TT1 | 214/848 (25%) | 1.49 (1.20, 1.86) | <0.001 | 1.75 (1.46, 2.11) | <0.001 | 92/433 (21%) | 2.04 (1.53, 2.72) | <0.001 |
| LDH ≥190 U/l | 243/848 (29%) | 1.42 (1.14, 1.77) | 0.002 | 1.41 (1.17, 1.70) | <0.001 | – | NS2 | NS2 |
| Age ≥65 years | 147/848 (17%) | 1.33 (1.03, 1.71) | 0.030 | NS2 | NS2 | – | NS2 | NS2 |
| CRP ≥8 mg/l | 326/848 (38%) | 1.25 (1.02, 1.54) | 0.031 | NS2 | NS2 | – | NS2 | NS2 |
| B2M ≥3.5 mg/l | 320/848 (38%) | NS2 | NS2 | 1.44 (1.21, 1.72) | <0.001 | 153/433 (35%) | 1.41 (1.08, 1.86) | 0.013 |
Results given as Hazard Ratio (95% confidence intervals), P-value from Wald chi-square test in Cox regression.
NS2 – Multivariate results not statistically significant at 0.05 level.
Multivariate model uses stepwise selection with entry level 0.1 and variable remains if it meets the 0.05 level.
Other variables considered for OS and EFS: Hb and CRP; for CR duration: Hb, IgA and LDH.
Post-transplant survival in TT2 related to chemotherapy or dexamethasone consolidation and in relationship to TT1
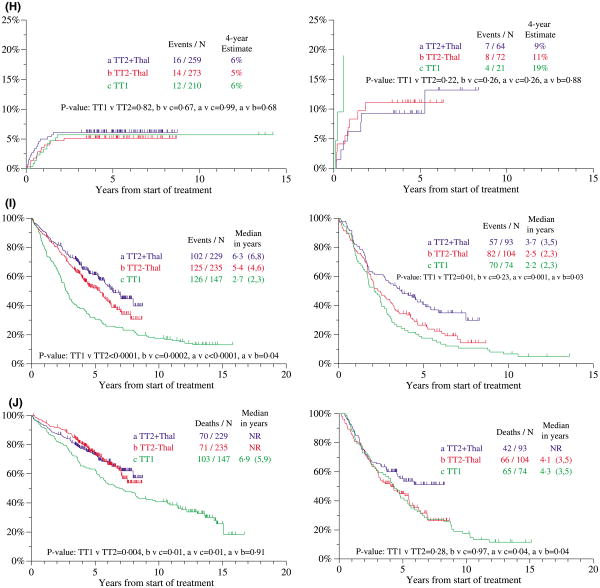
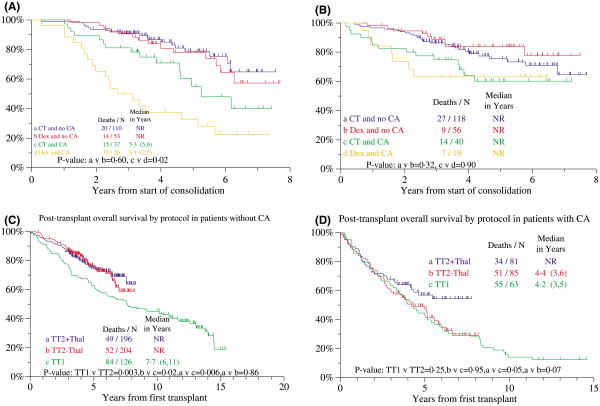
Post-transplant OS and EFS in TT2 were similar, regardless of the consolidation chemotherapy strategy employed (originally randomization between DCEP every 3 months for four cycles and DCEP alternating with CAD every 6 weeks for eight cycles; after the 109th patient enrolled, because of similar outcomes between the two consolidation arms, subsequent patients were treated with D-PACE every 3 months for four cycles) (data not shown). There was an additional provision for non-cytotoxic dexamethasone (DEX) to be given to patients either not having recovered a minimum platelet level of 100 × 109/l at 3 months from last transplant or not having responded adequately to DCEP induction; no difference in survival was noted between these two patient groups (not shown). For the control arm of TT2, consolidation chemotherapy was superior to DEX in the CA subgroup (Fig 4A), whereas such difference was not apparent for patients treated on the experimental arm of TT2 (Fig 4B).
Fig 4.
Overall survival post-transplant of patients treated with total therapy 2 (TT2) and total therapy 1 (TT1). (A) For patients treated on the control arm of TT2, overall survival from post-transplant consolidation was similar among patients without cytogenetic abnormalities (CA), whether receiving chemotherapy (CT) or dexamethasone (DEX), whereas there was a benefit in favour of CT versus DEX among patients with CA. NR, not reached. (B) For patients treated on the thalidomide arm of TT2, overall survival from post-transplant consolidation was similar among patients without CA and with CA, whether receiving CT or DEX. NR, not reached. (C) Among patients without CA, post-transplant survival was similar between the two arms of TT2, both of which were superior to TT1. NR, not reached. (D) Among patients with CA, post-transplant survival was similar between the control arm of TT2 and TT1, whereas those treated on the experimental arm of TT2 + THAL fared better than the two other cohorts. NR, not reached.
Post-transplant OS was then examined in TT2 versus TT1 in relationship to CA as the major adverse prognostic variable in both studies. Relative to TT1, patients without CA enjoyed superior OS on both TT2 arms (Fig 4C); in the presence of CA, those randomized to THAL fared better than their counterparts treated on the control arm of TT2 or with TT1 (Fig 4D). Collectively, the results presented in Fig 4 suggest a role for THAL in MM with CA, as was also noted for both EFS and OS when examined from initiation of protocol therapy (see Fig 3I and J).
Analysis of TT2 outcomes by pretreatment prognostic factors including GEP
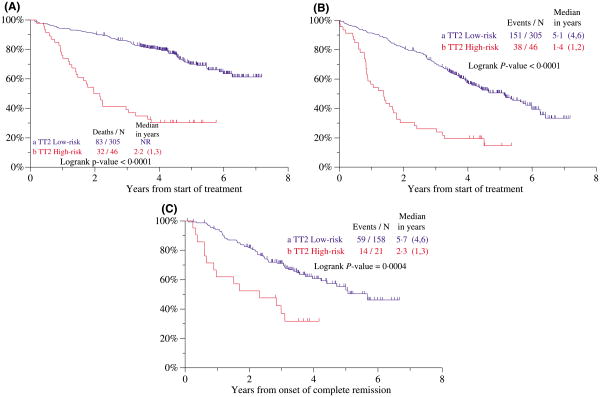
As summarized in Table IV, GEP-derived high-risk score (≥0.66) added independently to the prognostic models for OS, EFS and CR duration, displacing CA from the EFS model, whereas LDH and creatinine were retained for both OS and EFS. These findings pertained to both arms of TT2 (data not shown). Thus, the 4-year OS, EFS and CR estimates were 80%, 60% and 60%, respectively, among the 87% of patients with a low-risk score as opposed to 30%, 20% and 30%, respectively, in the 13% with high-risk score (Fig 5A–C). No significant differences were observed in OS and CR duration within GEP-defined risk groups by treatment arm whereas, for EFS, THAL benefited the low-risk subgroup (data not shown).
Table IV.
Multivariate analysis of clinical outcomes on total therapy 2 (TT2) (both arms) according to pretreatment variables inclusive of gene expression profiling (GEP)-defined risk category.
| Overall survival | Event-free survival | CR duration | ||||||
|---|---|---|---|---|---|---|---|---|
| 70 gene-defined GEP high-risk | 44/334 (13%) | 2.93 (1.84, 4.68) | <0.001 | 2.47 (1.77, 3.45) | <0.001 | 20/174 (11%) | 2.59 (1.41, 4.76) | 0.002 |
| Cytogenetic abnormalities | 108/334 (32%) | 1.93 (1.31, 2.86) | <0.001 | NS2 | NS2 | NS2 | NS2 | |
| Creatinine ≥2.0 mg/dl | 37/334 (11%) | 1.72 (1.05, 2.81) | 0.033 | 1.78 (1.25, 2.54) | 0.001 | NS2 | NS2 | |
| LDH ≥190 U/l | 114/334 (34%) | 1.59 (1.08, 2.35) | 0.019 | 1.37 (1.09, 1.74) | 0.008 | NS2 | NS2 | |
| Randomization to thalidomide | 166/334 (50%) | NS2 | NS2 | 0.75 (0.60, 0.93) | 0.008 | NS2 | NS2 |
Results given as Hazard Ratio (95% confidence intervals) P-value from Wald chi-square test in Cox regression.
NS2 – Multivariate results not statistically significant at 0.05 level.
Multivariate model uses stepwise selection with entry level 0.1 and variable remains if it meets the 0.05 level.
Other variables considered for OS and EFS: age, Hb, CRP, albumin and B2M; for CR duration: creatinine, B2M and cytogenetic abnormalities.
Fig 5.
Superior clinical outcomes among patients receiving total therapy 2 (TT2) with gene expression profiling (GEP)-defined standard-risk versus high-risk myeloma. (A) Overall survival was highly superior in the low- versus high-risk group. (B) Event-free survival was highly superior in the low- versus high-risk group. (C) Duration of complete remission (CR) was highly superior in the low- versus high-risk group.
Relapse rate in TT2 and TT1 in relationship to phases of treatment
We examined cumulative relapse frequencies after first and second transplant and maintenance phases (Fig 6). Among patients without CA, relapses occurred earlier and more frequently after both first and second transplants in TT1 compared with TT2 (Fig 6A), whereas this benefit was less pronounced in the case of CA due to earlier onset and higher rates of relapse on both trials (Fig 6B). Once in maintenance, THAL effectively reduced the relapse rate in comparison with the control arm of TT2, which, in turn, was similar to the relapse rate after TT1.
Fig 6.
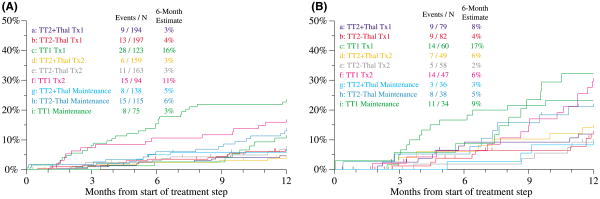
Cumulative proportions of patients relapsing in the 12 months after various treatment steps in total therapy 2 (TT2) with thalidomide (TT2 + THAL) versus TT2 without thalidomide (TT2 − THAL) versus total therapy 1 (TT1). Additional abbreviations used: Tx1, 1st transplant, Tx2, 2nd transplant. (A) Among patients without cytogenetic abnormalities, high proportions of patients relapsed soon after Tx1 and Tx2 as part of TT1, whereas such disease escape was lower in frequency and later in timing of onset in patients treated with TT2. (B) Among patients with cytogenetic abnormalities, overall relapse rates were higher and the timing of their onset earlier when compared to patients without cytogenetic abnormalities [see (A)]. Among patients enrolled in TT1, relapse rates exceeded those after TT2 for Tx1, Tx2 and maintenance; the addition of THAL in maintenance appeared to suppress relapse in comparison with TT2 − THAL.
Pair-mate analysis of clinical outcomes of patients treated with TT1, TT2 − THAL and TT2 + THAL
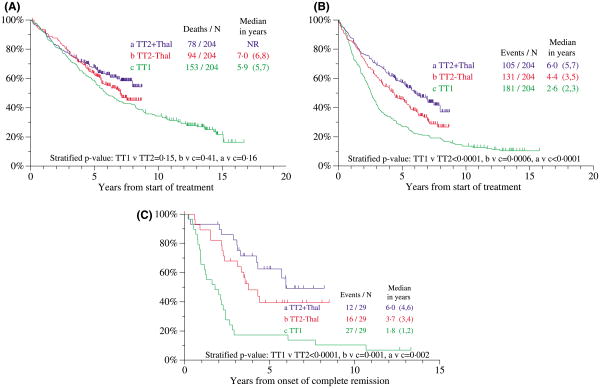
With TT1, the presence of CA and elevations of C-reactive protein (CRP ≥8.0 mg/l) and creatinine (≥176.8 μmol/l) were the three key adverse variables for OS. We identified 204 patients each in TT1 and the two arms of TT2 who were closely matched for these three variables. A trend was apparent for OS to be superior with TT2 plus THAL versus TT1, while there was no significant difference between the two arms of TT2 (Fig 7A). EFS and CR duration plots differed significantly when examined in pair-wise comparisons, so that TT2 plus THAL was superior to TT2 without THAL, which was superior to TT1 in terms of EFS (Fig 7B) and CR duration (Fig 7C).
Fig 7.
Pair-mate analysis of patients treated with total therapy 2 (TT2) and total therapy 1 (TT1). Matching was done on cytogenetic abnormalities (CA), CRP (>8 mg/l) and creatinine (≥176.8 μmol/l), found to be the leading adverse variables in TT1. (A) Overall survival tended to be superior with TT2 versus TT1. (B) Event-free survival was superior with TT2 (both arms) versus TT1. (C) Duration of complete remission (CR) was superior with TT2 (both arms) versus TT1.
Discussion
Long-term follow-up of prospective large trials with comprehensive laboratory correlates such as TT1 and TT2 is important in order to determine whether earlier observations still hold and whether and how early interventions affect long-term prognosis. In our case, all patients had at least annual visits to this institution so that the presented data have a high level of accuracy.
Within TT2, we confirmed the data of our initial publication (Barlogie et al, 2006a) that the addition of THAL increased the frequency but not the duration of CR (see Fig 3C and D) and prolonged EFS (see Fig 3E). We had attributed comparable OS durations on both study arms to a shorter postrelapse survival among patients originally randomized to THAL (Barlogie et al, 2006a), which could not be substantiated with longer follow-up (see Fig 3G). The apparent separation of survival curves on the two study arms may eventually become statistically significant (see Fig 3F). Among the subset of 351 patients with GEP data, the 13% with so-defined high-risk MM had vastly inferior OS, EFS and CR duration than their standard-risk counterparts (see Fig 5). Post-transplant consolidation chemotherapy was superior to DEX among patients presenting with CA and treated on the control arm, whereas post-transplant OS was similar for those randomized to THAL (see Fig 4A and B). These data suggest that THAL + DEX can substitute for combination chemotherapy in the setting of CA, as supported by superiority of TT2 over TT1 from initiation of therapy only in case of the experimental arm with THAL (see Fig 3I and J).
In the context of TT1, TT2 induced superior CR rates only when THAL was present (see Fig 3C). However, CR duration was superior with both arms of TT2, which also pertained to EFS and OS (see Fig 3D–F). Considering prognostic factors, the major overall benefit of TT2 was observed in the two-thirds of patients without CA (see Fig 4C); among those with CA, only patients randomized to THAL fared better with TT2 than TT1 (Fig 4D). On multivariate analysis of pretreatment variables, TT2 was an independent, significantly superior, intervention associated with superior OS, EFS and CR duration, after adjusting for critical baseline prognostic factors (see Table III). Such conclusions were also derived from a three-way pair-mate analysis for EFS and CR duration but not for OS (see Fig 7).
The essential conclusions from this long-term follow-up of two successive tandem transplant trials can be summarized as follows: (i) the introduction of post-transplant consolidation therapy in TT2 delayed the onset and decreased the magnitude of relapse especially benefiting patients presenting without CA; (ii) similar outcomes in patients with CA receiving THAL + DEX or chemotherapy without THAL as consolidation attest to the role both of THAL and chemotherapy in this high-risk setting; and (iii) as indicated by multivariate analysis, TT2 was an independent factor favouring prolonged CR duration, EFS and OS (see Table III), which was also supported by a pair-mate analysis for EFS and CR duration (see Fig 7). These observations provide an important framework for future therapeutic trials in MM.
The availability of the immunomodulatory agents, thalidomide (Singhal et al, 1999; Barlogie et al, 2001) and lenalidomide(Richardson et al, 2006a), and of the proteasome inhibitor, bortezomib, proven effective as salvage therapy for advanced and refractory MM (Richardson et al, 2003), has spawned trials with these new agents alone and in combination with DEX (Weber et al, 2003; Jagannath et al, 2005; Rajkumar et al, 2006), each other (Wang et al, 2005 Richardson et al, 2006b) and, most recently, with standard-dose melphalan in newly diagnosed patients. Remarkably high PR and near-CR/CR rates have been reported, especially with MPT (melphalan, prednisone, thalidomide) (Facon, et al 2006 Palumbo et al, 2006a), MPR (melphalan, prednisone, lenalidomide), (Palumbo et al, 2006b) VTD (bortezomib, thalidomide, DEX) (Wang et al, 2005), VMP (bortezomib, melphalan, prednisone) (Mateos et al, 2006), and even more so with V-MPT (bortezomib, melphalan, prednisone, thalidomide) (Palumbo et al, 2007). Combinations of bortezomib with liposomal doxorubicin and dexamethasone (PAD) (Oakervee et al, 2005; Orlowski et al, 2005) have also been explored successfully. Follow-up in these studies has been short, however, so that information on the durability of new agent-induced remissions and their salvage potential after relapse are not available. Eventually, prospective randomized trials are needed to determine whether the superior long-term EFS and OS reported with high-dose melphalan-based tandem transplants can also be sustained with the sole use of newer agents.
Acknowledgments
This work was supported in part by a program project grant (CA55819) from the National Institutes of Health, Bethesda, MD, USA.
References
- Barlogie B, Smith L, Alexanian R. Effective treatment of advanced multiple myeloma refractory to alkylating agents. New England Journal of Medicine. 1984;310:1353–1356. doi: 10.1056/NEJM198405243102104. [DOI] [PubMed] [Google Scholar]
- Barlogie B, Jagannath S, Vesole DH, Naucke S, Cheson B, Mattox S, Bracy D, Salmon S, Jacobson J, Crowley J, Tricot G. Superiority of tandem autologous transplantation over standard therapy for previously untreated multiple myeloma. Blood. 1997;89:789–793. [PubMed] [Google Scholar]
- Barlogie B, Desikan R, Eddlemon P, Spencer T, Zeldis J, Munshi N, Badros A, Zangari M, Anaissie E, Epstein J, Shaughnessy J, Ayers D, Spoon D, Tricot G. Extended survival in advanced and refractory multiple myeloma after single-agent thalidomide: identification of prognostic factors in a phase 2 study of 169 patients. Blood. 2001;98:492–494. doi: 10.1182/blood.v98.2.492. [DOI] [PubMed] [Google Scholar]
- Barlogie B, Tricot G, Anaissie E, Shaughnessy J, Rasmussen E, van Rhee F, Fassas A, Zangari M, Hollmig K, Pineda-Roman M, Lee C, Talamo G, Thertulien R, Kiwan E, Krishna S, Fox M, Crowley J. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. New England Journal of Medicine. 2006a;354:1021–1030. doi: 10.1056/NEJMoa053583. [DOI] [PubMed] [Google Scholar]
- Barlogie B, Tricot G, Rasmussen E, Anaissie E, van Rhee F, Zangari M, Fassas A, Hollmig K, Pineda-Roman M, Shaughnessy J, Epstein J, Crowley J. Total therapy 2 without thalidomide in comparison with total therapy 1: role of intensified induction and posttransplantation consolidation therapies. Blood. 2006b;107:2633–2638. doi: 10.1182/blood-2005-10-4084. [DOI] [PubMed] [Google Scholar]
- Barlogie B, Tricot GJ, van Rhee F, Angtuaco E, Walker R, Epstein J, Shaughnessy JD, Jagannath S, Bolejack V, Gurley J, Hoering A, Vesole D, Desikan R, Siegel D, Mehta J, Singhal S, Munshi NC, Dhodapkar M, Jenkins B, Attal M, Harousseau JL, Crowley J. Long-term outcome results of the first tandem autotransplant trial for multiple myeloma. British Journal of Haematology. 2006c;135:158–164. doi: 10.1111/j.1365-2141.2006.06271.x. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression tables and life tables. Journal of the Royal Statistical Society. 1972;34:187–202. [Google Scholar]
- Desikan R, Munshi N, Zangari M, Badros A, Anaissie E, Tricot G, Lim S, Ayers D, Spencer H, Englehart J, Barlogie B. DCEP consolidation chemotherapy (CC) after 2 cycles of melphalan-based high dose therapy (HDT) – high incidence of CR and superior outcome in comparison with matched historical controls. Blood. 1999;94:1411a. [Google Scholar]
- Facon T, Mary J, Harousseau JL, Huguet F, Berthou C, Grosbois B, Anglaret B, Azzedine A, Rodon P, Peny A. Intergroupe Francophone du Myélome. Superiority of melphalan-prednisone (MP) + thalidomide (THAL) over MP and autologous stem cell transplantation in the treatment of newly diagnosed elderly patients with multiple myeloma. Journal of Clinical Oncology. 2006;24:1. [abstract] [Google Scholar]
- Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statistics in Medicine. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Jagannath S, Durie BG, Wolf J, Camacho E, Irwin D, Lutzky J, McKinley M, Gabayan E, Mazumder A, Schenkein D, Crowley J. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. British Journal of Haematology. 2005;129:776–783. doi: 10.1111/j.1365-2141.2005.05540.x. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- Lee CK, Barlogie B, Munshi N, Zangari M, Fassas A, Jacobson J, van Rhee F, Cottler-Fox M, Muwalla F, Tricot G. DTPACE: an effective, novel combination chemotherapy with thalidomide for previously treated patients with myeloma. Journal of Clinical Oncology. 2003;21:2732–2739. doi: 10.1200/JCO.2003.01.055. [DOI] [PubMed] [Google Scholar]
- Mateos MV, Hernandez JM, Hernandez MT, Gutierrez NC, Palomera L, Fuertes M, Diaz-Mediavilla J, Lahuerta JJ, de la Rubia J, Terol MJ, Sureda A, Bargay J, Ribas P, de Arriba F, Alegre A, Oriol A, Carrera D, Garcia-Larana J, Garcia-Sanz R, Blade J, Prosper F, Mateo G, Esseltine DL, van de Velde H, San Miguel JF. Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: results of a multicenter phase 1/2 study. Blood. 2006;108:2165–2172. doi: 10.1182/blood-2006-04-019778. [DOI] [PubMed] [Google Scholar]
- Oakervee HE, Popat R, Curry N, Smith P, Morris C, Drake M, Agrawal S, Stec J, Schenkein D, Esseltine DL, Cavenagh JD. PAD combination therapy (PS-341/bortezomib, doxorubicin and dexamethasone) for previously untreated patients with multiple myeloma. British Journal of Haematology. 2005;129:755–762. doi: 10.1111/j.1365-2141.2005.05519.x. [DOI] [PubMed] [Google Scholar]
- Orlowski RZ, Voorhees PM, Garcia RA, Hall MD, Kudrik FJ, Allred T, Johri AR, Jones PE, Ivanova A, Van Deventer HW, Gabriel DA, Shea TC, Mitchell BS, Adams J, Esseltine DL, Trehu EG, Green M, Lehman MJ, Natoli S, Collins JM, Lindley CM, Dees EC. Phase 1 trial of the proteasome inhibitor bortezomib and pegylated liposomal doxorubicin in patients with advanced hematologic malignancies. Blood. 2005;105:3058–3065. doi: 10.1182/blood-2004-07-2911. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Bringhen S, Caravita T, Merla E, Capparella V, Callea V, Cangialosi C, Grasso M, Rossini F, Galli M, Cata-lano L, Zamagni E, Petrucci MT, De Stefano V, Ceccarelli M, Ambrosini MT, Avonto I, Falco P, Ciccone G, Liberati AM, Musto P, Boccadoro M. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006a;367:825–831. doi: 10.1016/S0140-6736(06)68338-4. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Falco P, Falcone A, Corradini P, Di Raimondo F, Giuliani N, Rossi G, Morabito F, Canepa L, Gozzetti A, Ambrosini MT, Zeldis J, Knight R, Foà R, Boccadoro M, Petrucci MT. Oral Revlimid® plus melphalan and prednisone (R-MP) for newly diagnosed multiple myeloma: results of a multicenter phase I/II study. Blood (ASH Annual Meeting Abstracts) 2006b;108:100. [Google Scholar]
- Palumbo A, Ambrosini MT, Benevolo G, Pregno P, Pescosta N, Callea V, Cangialosi C, Caravita T, Morabito F, Musto P, Bringhen S, Falco P, Avonto I, Cavallo F, Boccadoro M. Bortezomib, melphalan, prednisone, and thalidomide for relapsed multiple myeloma. Blood. 2007;109:2767–2772. doi: 10.1182/blood-2006-08-042275. [DOI] [PubMed] [Google Scholar]
- Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. Journal of Clinical Oncology. 2006;24:431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- Ramasamy K, Branford R, Selvaratnam R, Ho A, Duarte R, Devereux S, Pagliuca A, Mufti G. Analysis of beam (Carmustine, Etoposide, Cytosine Arabinoside, Melphalan) versus high dose melphalan (HDM) with autologous rescue in multiple myeloma (MM) Blood. 2004;104:5227. [Google Scholar]
- Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski RZ, Kuter D, Limentani SA, Lee S, Hideshima T, Esseltine DL, Kauffman M, Adams J, Schenkein DP, Anderson KC. A phase 2 study of bortezomib in relapsed, refractory myeloma. New England Journal of Medicine. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Blood E, Mitsiades CS, Jagannath S, Zeldenrust SR, Alsina M, Schlossman RL, Rajkumar SV, Desikan KR, Hideshima T, Munshi NC, Kelly-Colson K, Doss D, McKenney ML, Gorelik S, Warren D, Freeman A, Rich R, Wu A, Olesnyckyj M, Wride K, Dalton WS, Zeldis J, Knight R, Weller E, Anderson KC. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006a;108:3458–3464. doi: 10.1182/blood-2006-04-015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P, Jagannath S, Avigan D, Alsina M, Schlossman R, Mazumder A, Munshi N, Ghobrial I, Doss D, McKenney M, Farrell M, Mitsiades C, Hideshima T, Byrne C, Knight R, Birner A, Myers T, Weller E, Anderson KC. Lenalidomide plus bortezomib (Rev-Vel) in relapsed and/or refractory multiple myeloma (MM): final results of a multicenter phase 1 trial. Blood (ASH Annual Meeting Abstracts) 2006b;108:405. [Google Scholar]
- Sawyer J, Waldron J, Jagannath S, Barlogie B. Cytogenetic findings in 200 patients with multiple myeloma. Cancer Genetics and Cytogenetics. 1995;82:41–49. doi: 10.1016/0165-4608(94)00284-i. [DOI] [PubMed] [Google Scholar]
- Shaughnessy JD, Jr, Zhan F, Burington BE, Huang Y, Colla S, Hanamura I, Stewart JP, Kordsmeier B, Randolph C, Williams DR, Xiao Y, Xu H, Epstein J, Anaissie E, Krishna SG, Cottler-Fox M, Hollmig K, Mohiuddin A, Pineda-Roman M, Tricot G, van Rhee F, Sawyer J, Alsayed Y, Walker R, Zangari M, Crowley J, Barlogie B. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109:2276–2284. doi: 10.1182/blood-2006-07-038430. [DOI] [PubMed] [Google Scholar]
- Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, Munshi N, Anaissie E, Wilson C, Dhodapkar M, Zeddis J, Barlogie B. Antitumor activity of thalidomide in refractory multiple myeloma. New England Journal of Medicine. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- Wang M, Delasalle K, Giralt S, Alexanian R. Rapid control of previously untreated multiple myeloma with bortezomib-thalidomide-dexamethasone followed by early intensive therapy. Blood (ASH Annual Meeting Abstracts) 2005;106:784. [Google Scholar]
- Weber D, Rankin K, Gavino M, Delasalle K, Alexanian R. Thalidomide alone or with dexamethasone for previously untreated multiple myeloma. Journal of Clinical Oncology. 2003;21:16–19. doi: 10.1200/JCO.2003.03.139. [DOI] [PubMed] [Google Scholar]