Abstract
The uptake of arsenic by plants from contaminated soils presents a health hazard that may affect home gardeners neighboring contaminated environments. A controlled greenhouse study was conducted in parallel with a co-created citizen science program (home garden experiment) to characterize the uptake of arsenic by common homegrown vegetables near the Iron King Mine and Humboldt Smelter Superfund site in southern Arizona. The greenhouse and home garden arsenic soil concentrations varied considerably, ranging from 2.35 to 533 mg kg−1. In the greenhouse experiment four vegetables were grown in three different soil treatments and in the home garden experiment a total of 63 home garden produce samples were obtained from 19 properties neighboring the site. All vegetables accumulated arsenic in both the greenhouse and home garden experiments, ranging from 0.01 to 23.0 mg kg−1 dry weight. Bioconcentration factors were determined and show that arsenic uptake decreased in the order: Asteraceae > Brassicaceae > Amaranthaceae > Cucurbitaceae > Liliaceae > Solanaceae > Fabaceae. Certain members of the Asteraceae and Brassicaceae plant families have been previously identified as hyperaccumulator plants, and it can be inferred that members of these families have genetic and physiological capacity to accumulate, translocate, and resist high amounts of metals. Additionally, a significant linear correlation was observed between the amount of arsenic that accumulated in the edible portion of the plant and the arsenic soil concentration for the Asteraceae, Brassicaceae, Amaranthaceae, and Fabaceae families. The results suggest that home gardeners neighboring mining operations or mine tailings with elevated arsenic levels should be made aware that arsenic can accumulate considerably in certain vegetables, and in particular, it is recommended that gardeners limit consumption of vegetables from the Asteraceae and Brassicaceae plant families.
Keywords: Vegetable, Mining waste, Mine tailings, Arsenic, Home-gardens, Plant uptake
1. Introduction
Mining and industrial processes are primary sources of arsenic and heavy metal contamination in soil (Lee et al., 2005). In the United States alone there are 45 billion tons of mine waste, including waste rock and tailing material, and many of the estimated 557,650 abandoned hard rock mine sites are in arid and semiarid regions (US EPA, 2004). Mine tailings and their associated metal contaminants, such as arsenic and other heavy metals, are prone to wind dispersion and water erosion. Surface soils adjacent to, beneath, or downwind of arsenic release sources (e.g., smelters or mine tailings) often have arsenic levels at or above regulatory contaminant limits (Belluck et al., 2003). Mining operations in particular pose a potential risk to human health and the environment. Numerous studies have found an inverse relationship between arsenic levels in human urine samples and the distance of home or school environments from metal smelters and other mining operations (Csavina et al., 2012). Climate change will only exacerbate the risks posed by mining in arid and semi-arid environments like the desert Southwest, primarily due to increased temperatures and reduced precipitation (MacDonald, 2010).
Fugitive metals in receiving waterways and soils in the vicinity of mining sites can affect humans via the inadvertent consumption of metal-containing soils and dust, or through the consumption of crops grown in such soils (Murray et al., 2009; Lee et al., 2005; Cobb et al., 2000). It has been shown that arsenic in soil is the major source for arsenic uptake by crops (e.g. Huang et al., 2006). Arsenic exposure is of special concern in the US Southwest due to elevated levels that often occur naturally in drinking water sources. Thus, potential exposure via consumption of affected garden crops would add to an already elevated exposure from drinking water. Inorganic arsenic calculated as arsenite and arsenate comprise 96% of the total arsenic in vegetables (Smith et al., 2006). Intake of inorganic arsenic over a long period can lead to chronic arsenic poisoning (arsenicosis) and associated effects, including skin lesions, peripheral neuropathy, gastrointestinal symptoms, diabetes, renal system effects, cardiovascular disease and cancer, which can take years to develop depending on the level of exposure (WHO, 2010).
Due to the extent of contamination, the number of Superfund and other hazardous waste sites in the U.S. and the growing popularity of food gardening, understanding the spatial distribution of arsenic in residential soils and the uptake of arsenic in common homegrown vegetables is crucial to protect human health near these sites. In 2008, 36 million households participated in food gardening, with an average contact time of 5 h per week (National Gardening Association, 2009). The level of participation in gardening is only expected to increase, and the main reasons why Americans are food gardening are to grow better tasting and quality food, and to grow food they feel is safe (National Gardening Association, 2009). A gardener who neighbors a Superfund or hazardous waste site needs to be particularly aware of their soil quality and the potential for uptake of arsenic by the vegetables they choose to grow.
This study entitled Gardenroots, was designed to determine the concentration of arsenic in vegetable plants grown near the Iron King Mine and Humboldt Smelter Superfund (IKMHSS) site in Arizona, a site known to have elevated levels of arsenic. The objective of this study was to characterize and compare the uptake of arsenic by common homegrown vegetables grown in soils near the site. A controlled greenhouse study was conducted in parallel with a co-created citizen science program where community members, after training, collected soil, irrigation water and vegetable samples from their household garden. These samples were analyzed for arsenic content at the University of Arizona (UA). There have been several studies that have investigated the accumulation of arsenic in homegrown vegetables, but to the best of our knowledge this is the first to do so using a citizen-science program design. The residential area (Dewey–Humboldt) upon which the study was focused is adjacent to the IKMHSS site.
2. Materials and methods
2.1. Site description
The IKMHSS site is located in Dewey–Humboldt, Yavapai County, Arizona, and was listed on the U.S. Environmental Protection Agency's (US EPA) National Priorities List in 2008. The site comprises a combination of sources and releases from two separate locations: the Iron King Mine property (34°30′N, 112°15′W) and the Humboldt Smelter property (34°29′N, 112°13′W). A portion of the Town of Dewey–Humboldt is situated between the mine and the smelter (Fig. 1). The smelter operated from the late 1800s until 1969. The Iron King Mine operated from the late 1800s until the early 1960s and was a periodically active for gold, silver, copper, lead and zinc. All mining and smelting ceased by 1969.
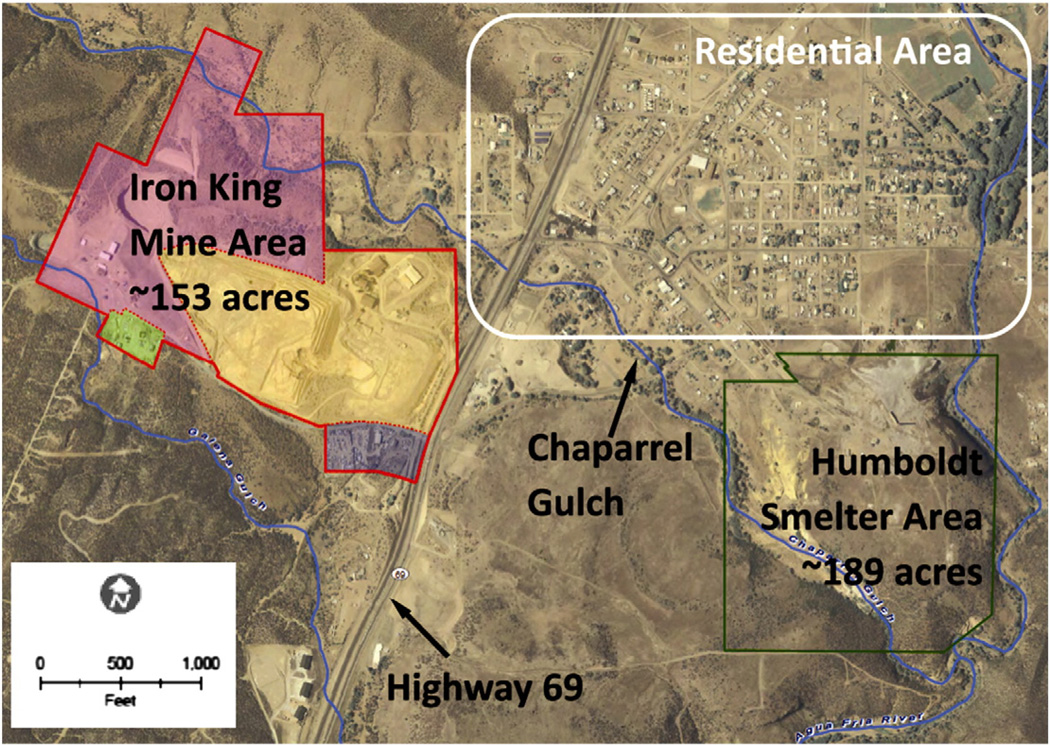
Fig. 1.
An aerial view of the Iron King Mine and Humboldt Smelter Superfund site, and the Dewey–Humboldt, Arizona residential area. Chaparral Gulch is a major waterway that runs through the Dewey–Humboldt area.
Aerial photo source: Yavapai County, GIS, 2007. Basemap source: ESRI Street Map, 2006. EA Engineering, Science, and Technology, Inc., 2010.
Large amounts of uncontrolled mine tailing waste exist on both the smelter and tailings properties. The average composite concentration of arsenic in the Iron King Mine tailings pile (0–0.61 m below ground surface) is 3,710 mg kg−1 (EA Engineering, Science, and Technology, Inc., 2010). A previous study determined that the IKMHSS mine tailings have a low pH(2.5), high EC (13.5 ms/cm), a loam texture (34.7% sand, 44.8% silt, and 20.4% clay), and TOC and TN of 1.22 g/kg and 0.0423 g/kg, respectively (Solís-Domínguez et al., 2012). The unprotected mining wastes on the two properties are point sources of pollution and are prone to eolian dispersion and water erosion. This is reflected in observations of elevated arsenic and lead concentrations in surface soil on off-site areas adjacent to the Chaparral Gulch or downwind of the mine tailings and smelter properties. The concentrations of arsenic and lead in shallow surface soil samples in these areas are higher than the concentrations of arsenic and lead in the deeper surface soil samples. The elevated lead and arsenic levels near the surface are likely due to wind dispersion or surface water transport, rather than being attributable to background conditions (EA Engineering, Science, and Technology, Inc., 2010).
2.2. Greenhouse study
The soil treatments used in the greenhouse study included surface samples (0–15 cm) that were collected at a residential site between the Iron King Mine property and the Humboldt Smelter property, and adjacent to the Chaparral Gulch in July 2010. In 2009, the US EPA sampled this residential property and identified areas with elevated levels of arsenic. Using this information, four 7-meter transects were made with transect 1 and 2 located in an area where the US EPA detected elevated levels of arsenic (120–633 mg kg−1) and transects 3 and 4 in areas where the levels of arsenic were closer to background and/or the Arizona Residential Soil Residential Level (13–25.7 mg kg−1). For each transect, a 20-liter soil sample was collected every 1.2 m. All soil samples from transects 1 and 2 were homogenized and then sieved to ≤2 mm (elevated arsenic soil). Soils from transects 3 and 4 were treated similarly (background arsenic soil). In order to replicate popular gardening practices, the two collected residential soils were mixed with 25% (w/w) MiracleGro™ Gardening Soil Mix (garden soil, Home Depot, Tucson, Arizona) and then used to create three treatments for the greenhouse study: (T1) residential soil with background levels of arsenic, mixed with 25% the garden soil; (T2) residential soil with elevated arsenic levels, mixed with 25% garden soil; and (T3) residential soil with elevated arsenic levels, mixed with 25% garden soil and 10% mine tailing waste from the Iron King Mine.
The following criteria were used to select the vegetables for the greenhouse study: 1) among the top ten most popular vegetable grown in U.S. and used by various ethnic groups (National Gardening Association); 2) used in previous research studies (Bhattacharya et al., 2010; Murray et al., 2009; Smith et al., 2009, 2008; Gaw et al., 2008; Li et al., 2006; Smith et al., 2006; Warren et al., 2003; Alam et al., 2003; Bunzl et al., 2001; Cobb et al., 2000; Kabata-Pendias and Pendias, 2001); and 3) recommended by Master Gardeners in Pima and Yavapai County, Arizona (regional knowledge). Based upon these criteria, bush bean (Fabaceae Phaseolus vulgaris), lettuce (Asteraceae Lactuca sativa), radish (Brassicaceae Raphanus sativus), and onion (Liliaceae Allium cepa) were selected and grown in the greenhouse. The experimental design was completely randomized with four replicate pots of each vegetable for each of the three treatments (N = 48). Vegetables were grown in black plastic pots with drainage (17 cm top d × 18 cm height × 13.5 cm bottom d), and filled approximately 3/4 with the treatment mixture as described by Solís-Domínguez et al., 2011. The number of seeds sown and the sowing depth varied by plant. On a per pot basis, six bush bean seeds were sown at 2.5 cm, 3 lettuce seeds were sown at 0.3 cm, 6 radish seeds were sown at 1.3 cm, and 8 onion seeds were sown at 0.64 cm. Germination occurred after approximately 3, 5, 5, and 12 d for the radish, bean, lettuce and onion, respectively. Bean pots were thinned to 1 plant per pot. Pots were watered with tap water using drip irrigation every other day (~60 mL/pot). The experiment was performed at the UA Controlled Environment Agriculture Center (Tucson, AZ) under conditions of natural light and day/night temperature of 32 °C/24 °C.
The radish, bean and lettuce plants were harvested at 66 d, and the onion was harvested at 150 d. The edible portion of the plant was carefully removed, collected in sterile bags, and transported in an ice chest to the laboratory. All vegetables were washed in a 0.1 HCl solution with nanopure water to remove all soil particles from the vegetable samples, and then oven-dried to a constant mass at 60 °C.
2.3. Home garden study
Each home garden participant collected soil samples. Participants were instructed to collect a composite soil sample from the top 15 cm of their yard and garden soils. Briefly, the participants selected six spots in a grid-like pattern in both their yard and garden areas, collected the top 15 cm of soil from each spot, then composited and mixed the soil samples thoroughly (bulk sampling) in two buckets, one designated for yard soil and the other for garden soil.
The Dewey–Humboldt community participants chose their own vegetables to grow for the study, and each household was allowed to submit up to four types of vegetables from their garden for analysis. Participants were provided verbal and written (Gardenroots Instructional Manual (www.garden-roots.org)) instructions on how to harvest and store vegetable samples. Participants were instructed to set up a washing station to: 1) remove all soil particles from vegetable using a soft bristled brush; 2) rinse the vegetable in tap water bath; 3) dip the vegetable several times in a bath of distilled water with a tablespoon of bleach; 4) air dry samples indoors for a minimum of 30 min; 5) place air-dried vegetable samples in separate ziplock plastic bags; and 6) immediately place the vegetables in a refrigerator. After all garden samples were collected and processed, each participant delivered them to the UA Yavapai Cooperative Extension office in Prescott, Arizona (25 miles from the town of Dewey–Humboldt, AZ) where they were placed in a refrigerator designated for all Gardenroots samples. All items needed for vegetable processing were provided to participants in their Gardenroots toolkit.
The vegetable samples were retrieved from the UA Yavapai Cooperative Extension office, stored on ice and transported to the main UA campus in Tucson, AZ, then the samples were washed in a 0.1 HCl solution with nanopure water, and oven-dried to a constant mass at 60 °C.
2.4. Analysis of soil and vegetable samples
Subsamples for each of the greenhouse soil treatments, the MiracleGro™ Gardening Soil, and the home garden and home yard soil samples were subjected to acid digestion following US EPA Method 3051 (US EPA SW-846, 1986). It is recognized that several digestion methods are available, and that each method has associated limitations. The EPA method was selected such that the results would be consistent with prior analyses conducted for the site by the US EPA. In addition, nitric acid was used to avoid Cl-based interferences with ICP-MS from the use of hydrochloric acid. The subsamples were treated with 2.5 mL HNO3 and microwave (CEM corporation, model number MDS 2100) digested for 1 h. The digested soil samples were analyzed using inductively coupled plasma mass spectrometry (Agilent 7500ce, Agilent Technologies, Santa Clara, CA), with a quantifiable detection limit for As of 0.11 µg L−1 or 0.0033 mg kg−1. The mean coefficient of variation for arsenic analysis was <7.6%. Quality control/assurance procedures described in US EPA Method 6020 in SW-846 Methods for Water and Waste were employed. Calibration curves include at least 5 points, and correlation coefficients were >0.995. A Continuing Calibration Blank and Continuing Calibration Verification solution were analyzed after every 20 samples. Each batch also included measurement of at least one quality control (QC) solution from a second source (i.e. standard reference, National Institute of Standards and Technology (NIST) 1643e: Trace metals in water). The range of QC responses for all the samples in this study was between 86 and 115% of the certified value. Additionally, at least one duplicate digestion sample was included per batch of 20 samples.
To determine edible tissue arsenic concentrations in vegetables from both the greenhouse study and the home garden study, samples were dried, ground with a Wiley Mill for 1 min, passed through a 30 mesh (0.595 mm) screen, and analyzed for total arsenic content. Triplicate 0.5000 g subsamples of each vegetable sample were digested using 2.5 mL HNO3, 2.5 mL H202 and 10 mL water. Baseline controls, subjected to identical processing, were employed consisting of: 1) distilled water and HNO3; and 2) standard reference tomato leaves were used as a check standard for validating accuracy and comparability within the environmental measurement community (Standard Reference Material 1573a, NIST). The metal content of digested plant tissues was determined using ICP-MS. For this study, only the edible portions of the plant were analyzed.
Once the concentration of arsenic in the soils and edible portion of the vegetable was determined, the bioconcentration factor (BCF) or transfer factor was calculated. The BCF is the ratio of the metal concentration of the edible portion of the vegetable (dry weight) to the metal concentration of the soil, BCF = Cvegetable/Csoil. All statistical analysis was performed using Microsoft Excel 2011 and JMP 9.0.
3. Results
3.1. Greenhouse study
Physiochemical parameters and arsenic concentrations for the greenhouse soil treatments are presented in Tables 1 and 2. Arsenic ranged from 27.2 to 533 mg kg−1 for the three treatments and was 3.63 mg kg−1 in the MiracleGro™ Garden Soil Mix.
Table 1.
Selected physiochemical properties of the greenhouse treatment soils.
| Soil type | pH | TOC (%) | Sand (%) | Silt (%) | Clay (%) | Conductivity | PO4 (mg/kg) | SO4 (mg/kg) |
|---|---|---|---|---|---|---|---|---|
| MiracleGro™ Soil Mix | 6.7 ± 0.04 | 26.5 ± 5.36 | —— | —— | —— | 4.26 ± 0.26 | 96.7 ± 2.28 | 205 ± 13.7 |
| T1a | 7.7 ± 0.08 | 4.62 ± 0.19 | 73.5 ± 1.28 | 15.3 ± 1.11 | 11.2 ± 0.21 | 3.43 ± 0.02 | <10 | 390 ± 11.5 |
| T2b | 7.4 ± 0.01 | 5.44 ± 0.40 | 75.2 ± 1.98 | 16.5 ± 1.41 | 8.39 ± 0.64 | 4.22 ± 0.15 | <10 | 1480 ± 78.6 |
| T3c,d | 6.6 ± 0.33 | 5.76 ± 0.48 | 75.2 ± 1.98 | 16.5 ± 1.41 | 8.39 ± 0.64 | 5.42 ± 0.65 | <10 | 2062 ± 40.5 |
T1 = greenhouse treatment 1.
T2 = greenhouse treatment 2.
T3 = greenhouse treatment 3.
T3 is T2 soils with 10% mine tailings added.
Table 2.
Arsenic in soils from greenhouse treatments and home gardens. Ranges and US back-ground values are provided for comparison.
| Soil type | Arsenic DWa (mg kg−1) |
|---|---|
| MiracleGro™ Soil Mix | 3.63 ± 0.84 |
| T1b | 27.2 ± 0.89 |
| T2c | 222 ± 3.01 |
| T3d | 533 ± 20.4 |
| Home yard soile | 46.0 (3.07–322) |
| Home garden soile | 44.1 (2.35–374) |
| Normal range of arsenic in soils | 0.1–40f |
| Range of arsenic in soils from the US | <0.1–93g |
All values are in dry mass, mg kg−1.
T1 = greenhouse treatment 1.
T2 = greenhouse treatment 2.
T3 = greenhouse treatment 3.
For home soils, average, N = 25 and (range).
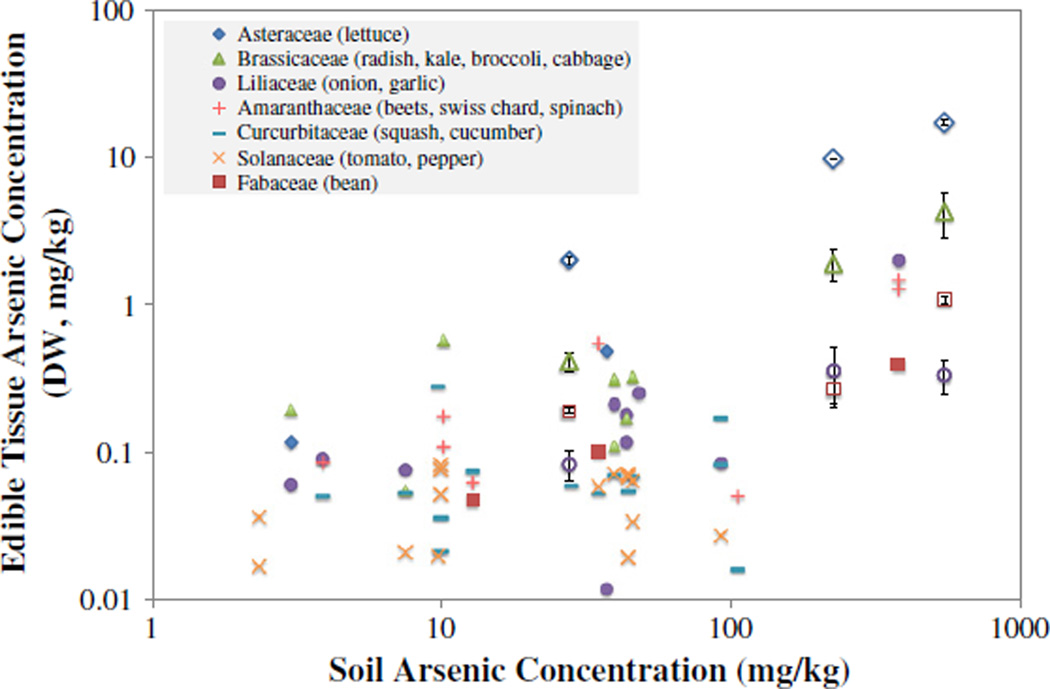
The edible tissues of the vegetables grown in the three different soil treatments had arsenic concentrations ranging over 4 orders of magnitude, from 0.0699 (onion) to 23.0 (lettuce) mg kg−1 dry weight (Fig. 2, open symbols; Supplemental Table 1). Overall, the mean arsenic accumulation in these crops for all treatments (based on edible tissue dry weight mg kg−1) decreased in order of Asteraceae (lettuce) > Brassicaceae (radish) > Fabaceae (bean) > Liliaceae (onion).
Fig. 2.
Arsenic concentration in the edible portion of common vegetables as a function of soil arsenic concentration. All points are from this study; open symbols represent vegetables grown in the greenhouse (N = 4); closed symbols represent vegetables grown in home gardens.
3.2. Home garden study
3.2.1. Arsenic in soil and water
Arsenic concentrations in irrigation water ranged from 1.40 to 2,030 µg L−1. Sixteen irrigation water samples were above the US EPA Maximum Contaminant Level of 10 µg L−1, and three of those were collected from the public potable water supply system. In total, 21 (out of 25) of the irrigation water samples were from private wells.
Community participants were also instructed to collect both yard and garden soil samples in order to determine whether, through their amending practices, they were contributing an arsenic load to their gardening soil. Arsenic concentrations in home garden and yard soil samples ranged from 2.35 to 374 mg kg−1 and 3.07 to 322 mg kg−1, respectively. The pH of these soils was near neutral. Background arsenic soil concentrations in the area are estimated to be from 18.3 to 66.3 mg kg−1 (EA Engineering, Science, and Technology, Inc., 2010) with a site-specific mean arsenic concentration value of 30.7 mg kg−1 (Arizona Department of Health Services, 2009).
As noted above, there is a large range in arsenic concentrations determined for the garden and yard soils. However, based on the households that participated in Gardenroots, there is no apparent pattern or trend in arsenic concentration as a function of proximity to the tailings pile or the smelter, which is 1 mile east of the tailings on the opposite site of Highway 69 (Fig. 1). The most elevated arsenic concentrations were observed at 2.2 miles southeast of the tailings and 1.1 miles from the smelter. It is unclear why this residential property had such elevated soil arsenic levels.
It is important to note that soil arsenic concentrations were higher for garden soil samples than for yard soil samples for 12 of the 25 community samples. It is possible that the products being used by home gardeners to amend their soils are contributing an arsenic load to their garden soils. Note that the commercially available product MiracleGro™ Garden Soil used in the greenhouse portion of this study had a relatively low average arsenic concentration of 3.6 mg kg−1.
3.2.2. Arsenic in vegetables
Sixty-three home garden produce samples were obtained from 19 properties neighboring the IKMHSS site. The vegetables were grouped by scientific family and in general, the arsenic concentration in the vegetables increases with increasing soil arsenic levels (Fig. 2). Overall, the vegetable arsenic concentrations ranged over 3 orders of magnitude, 0.01–1.96 mg kg−1 dry weight. The mean arsenic accumulation in these crops, based on edible tissue dry weight mg kg−1, decreased in order of Amaranthaceae > Liliaceae > Asteraceae > Brassicaceae > Fabaceae > Cucurbitaceae > Solanaceae (Supplemental Table 2).
3.3. Arsenic uptake by different plant families
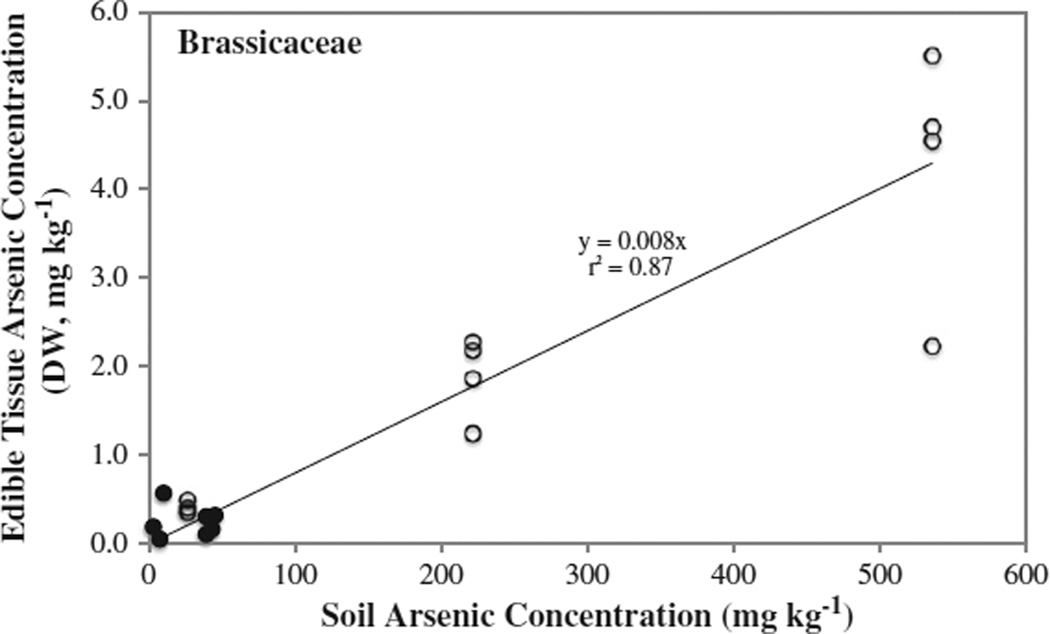
There is a direct correlation between the amount of arsenic that accumulated in the edible portion of the plant and the arsenic soil concentration for most of the vegetable families analyzed including: Asteraceae (lettuce); Brassicaceae (radish, broccoli, kale, cabbage); Amaranthaceae (beet, Swiss chard and spinach); and Fabaceae (bean) families (see Fig. 3, Table 3). In contrast, there was no correlation between arsenic uptake and soil concentration for the Solanaceae or Cucurbitaceae (Table 3). The majority of the Cucurbitaceae samples analyzed had arsenic concentrations below 0.1 mg kg−1 regardless of soil arsenic concentration, with the exception of a yellow squash sample at 0.28 mg kg−1 and green zucchini that had an arsenic concentration at 0.17 mg kg−1. Cucurbits (and solanaceas) accumulated the least amount of arsenic among the plant families examined and this may be due to the physiology of this family.
Fig. 3.
Arsenic concentration in the edible portion of the Brassicaceae as a function of soil arsenic concentration. Open symbols (○) represent vegetables grown in the greenhouse (N = 4) and closed symbols (●) represent vegetables grown in home gardens.
Table 3.
Gardenroots bioconcentration factors and correlations between the arsenic observed in vegetable families and total arsenic in the soils.
| Vegetable family | Bioconcentration factor |
Correlation of arsenic in edible tissue with arsenic in soil |
|---|---|---|
| r2 value | ||
| Asteraceae | 0.0478 | 0.791c |
| Brassicaceae | 0.0146 | 0.868c |
| Amaranthaceae | 0.00982 | 0.857b |
| Curcurbitaceae | 0.00483 | NS |
| Liliaceae | 0.00448 | 0.139a |
| Solanaceae | 0.00391 | NS |
| Fabaceae | 0.00323 | 0.557b |
NS: not significant.
Significant at 5% level.
Significant at 0.1% level.
Significant at 0.01% level.
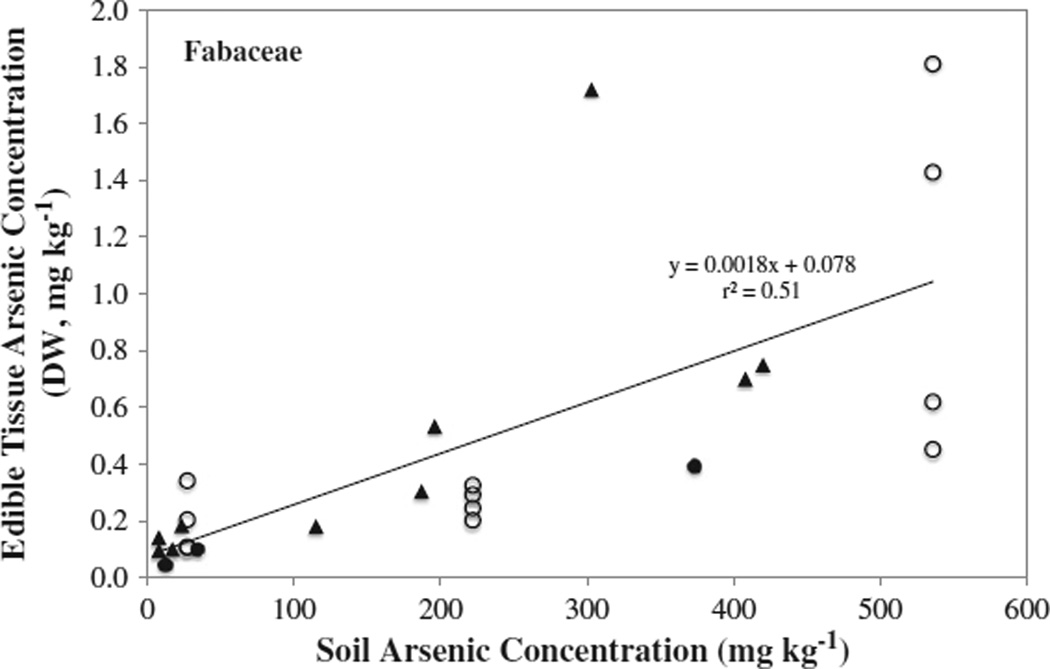
The results from the greenhouse and home gardens were combined with data reported in the literature to further examine potential correlations. Statistically significant correlations were observed at levels of: 0.01% for the Fabaceae (r2 = 0.51, N = 25, Fig. 4) and Brassicaceae (r2 = 0.42, N = 43) families, while the r2 values for the Cucurbitaceae, Solanaceae and Asteraceae families were less than or equal to 0.33 (N range = 26–33).
Fig. 4.
Arsenic concentration in the edible portion of the Fabaceae as a function of soil arsenic concentration. Values were compiled from this study and from the literature. Open symbols (○) represent vegetables grown in the greenhouse, closed symbols (●) represent vegetables grown in home gardens, and the closed triangles (▲) represent values from the literature.
3.4. Bioconcentration factors
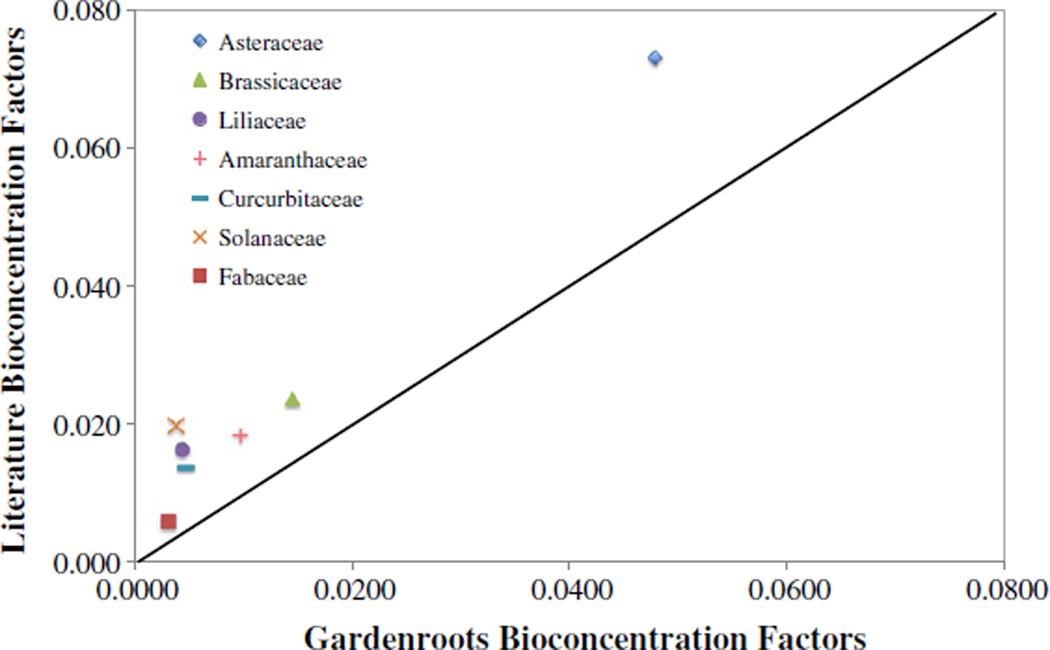
The BCF was calculated from the ratio of the metal concentration in the edible portion of the vegetable (dry weight) and the metal concentration in the soil. The average BCF for the plant families from this entire study (based on edible tissue dry weight mg kg−1) decreased in order of Asteraceae > Brassicaceae > Amaranthaceae > Cucurbitaceae > Liliaceae > Solanaceae > Fabaceae (Table 3). The Asteraceae and Brassicaceae families accumulated more arsenic than did the other families, with BCF values of 0.0478 and 0.0146, respectively. This observation is similar to the results presented in previous studies, wherein the average BCF (based on edible tissue dry weight mg kg−1) decreased in order of Asteraceae > Brassicaceae > Solanaceae > Amaranthaceae > Liliaceae > Cucurbitaceae > Fabaceae (Bhattacharya et al., 2010; Smith et al., 2008; Gaw et al., 2008; Li et al., 2006; Warren et al., 2003; Alam et al., 2003; Bunzl et al., 2001; Cobb et al., 2000). Furthermore, the Asteraceae BCF was observed to be significantly larger than those of the other families (using the Tukey–Kramer HSD test) for the literature data. This is consistent with what was observed for the Gardenroots data reported herein. It should be noted that the previous studies used for comparison were chosen because they focused on soils from or near mining waste (i.e. mine tailings, slag, and smelter) or on arsenic contaminated irrigation water and contaminated horticultural soils.
4. Discussion
4.1. Sources of arsenic in residential soils
Although this study site neighbors a Superfund site and there is evidence that the mine tailing waste has moved beyond the site and into residential areas, it's important to briefly discuss other sources of arsenic that may affect the residential and gardening soils. Disentangling the sources of arsenic in residential areas near a Superfund site can be complicated when there is also naturally occurring arsenic in the region. Arizona, and specifically Yavapai County has naturally high levels of arsenic due to: 1) granite bedrock, 2) the Colorado Plateau of northern Arizona and southern Utah, and 3) the arsenic-rich Supai Sandstone formation (Uhlman et al., 2009).
Based on existing data collected and reported in the US EPA IKMHSS site Remedial Investigation Report, the maximum soil arsenic concentration found in residential yards were northwest of the smelter area and along the Chaparral Gulch. The reports state that yards further away from the IKMHSS site are much less likely to be impacted from wind dispersion or surface water transport from sources, and vice versa (EA Engineering, Science, and Technology, Inc., 2010). We therefore anticipated the same pattern. However, there was no apparent pattern or trend in arsenic concentration as a function of proximity to the tailings pile or smelter. It should be noted that: 1) the number of samples available in the current study is not sufficient to quantitatively characterize spatial distributions; 2) wind direction in the area changes seasonally; and 3) this area has been affected by both smelting and mine tailings, which produce different sized particles that experience different magnitudes of eolian transport. Csavina et al. (2012) discuss how ultra-fine particles are often generated from smelting and slag dumps and are so small that they rapidly diffuse, coagulate and grow into the accumulation range (0.1 µm to ~1 µm particle size) in the atmosphere. Conversely, coarse particles generated by wind erosion of mine tailings are large enough to rapidly settle out of the atmosphere in minutes to hours, and have a wide distribution of metalloid concentrations.
Additionally, it was hypothesized that the garden soils would have considerably lower soil arsenic levels than the yard soils, given that the garden soils would be mixed with and thus diluted by garden amendments. Interestingly, the mean soil arsenic concentration in the yard soils (46.0 mg kg−1) and garden soils (44.1 mg kg−1) are similar, and 12 of the 25 home garden soil samples had a greater soil arsenic concentration than the yard soils. This highlights that commercially available gardening amendments may contain arsenic. Raven and Loeppert, 1997 reported measureable levels for heavy metals such as arsenic, cadmium, chromium, lead and mercury in organic fertilizers. They found that trace metal concentrations generally decreased in the following sample order: rock phosphate > sewage sludge > commercial phosphate fertilizers > organic amendments and liming materials > commercial K2O fertilizers > commercial N fertilizers. Whether or not fertilizers add significant amounts of metals to soil depends upon several factors including the existing soil metal concentration, the concentration of trace metals in the fertilizer and the fertilizer application rate (US EPA, 1999). Nonetheless, the results of this study demonstrate that the garden soil arsenic concentration did not notably decrease with the addition of amendments as hypothesized, and that in fact, 48% of the time, the arsenic concentrations in the garden soils increased. It is recommended that commercially available gardening amendments be researched further, particularly because these are widely used by gardeners.
In summary, the results of this study suggest that food gardeners surrounding mining operations test their soils prior to gardening to determine existing soil arsenic concentrations. Arsenic may be elevated due to naturally occurring sources and from the mining operation. Also, it is important for home food gardeners to understand how gardening amendments and practices may be contributing arsenic to the soils, or diluting the arsenic concentration in the garden soil mixture.
4.2. Arsenic uptake in the edible tissue of vegetables
The results presented herein show that there is a significant correlation between the levels of arsenic uptake in the edible tissue of vegetables from the Asteraceae, Brassicaceae, Amaranthaceae, and Fabaceae families and the levels of arsenic in the soil in which they were grown. Huang et al. (2006) similarly reported a significant correlation between arsenic uptake by Chinese cabbage, leaf mustard, cauliflower, and radish (all from the Brassicaceae family), as well as garlic and onion (Liliaceae family) and total arsenic in soils. They further observed an increase in the number of significant relationships (13/17 vegetables versus 8/17) when the regression was done with available arsenic (NaH2PO4-extractable As), not total arsenic. These correlations are functionally dependent and may change if examined by single vegetable species rather than by family.
When characterizing metal uptake by plants, it is important to consider the BCF, which is the ratio of metal uptake in the plant to the metal concentration in the soil. In Fig. 5, we have compared the Gardenroots BCF values for each plant family with those from selected literature studies (Bhattacharya et al., 2010; Smith et al., 2008; Gaw et al., 2008; Li et al., 2006; Warren et al., 2003; Alam et al., 2003; Bunzl et al., 2001; Cobb et al., 2000). The BCF values from the literature were generally higher than those observed in this study. This highlights that although soil arsenic concentration is an important variable, there are other factors that may influence plant uptake. One is arsenic speciation, which is known to affect plant uptake (e.g. Smith et al., 2008; Meharg and Hartley-Whitaker, 2002; Burló et al., 1999). It has been shown that plants take up arsenate, the dominant species in oxic environments, via the phosphate transport system since the phosphate ion is similar to the arsenate ion (Dixon, 1997). The second influence includes soil characteristics such as pH, organic matter, clay content, water regime and nutrient balance (i.e. phosphate) (Kabata-Pendias and Pendias, 2001). For example, arsenic in sandy soils is 5 times more available than in clay soils, and thus the toxicity threshold for sandy soils is approximately 40 mg kg−1 as compared to 200 mg kg−1 for clays (O'Neill, 1995). Additionally, it has been found that the arsenic sorption by soil increases with iron oxides (Kabata-Pendias and Pendias, 2001). Warren et al. (2003) demonstrated that adding ferrous sulfate in solution to the soils reduced lettuce, cauliflower, radish and other selected vegetables uptake of arsenic by a mean of 22%; and that the highest bioavailability was observed for soils with a high sand content. In summary, while the vegetables in this study accumulated arsenic, accumulation was lower than previously reported potentially due to one or more of the factors briefly described above.
Fig. 5.
A comparison of the average bioconcentration factor values for the current Gardenroots study and the literature values. The solid line represents a one-to-one relationship. Points above the line show plant families that have higher literature BCF values than Gardenroots values. Vice versa, points below the line would show plant families that have lower Gardenroots BCF values than literature values.
The results of this study and previous work show that some plant families, specifically the Asteraceae and Brassicaceae, accumulate more arsenic in their edible tissues than do other families. These plant families have genetic and physiological adaptations that allow them to accumulate, translocate, and resist high amounts of metals; and in general, they have an efficient root uptake, effective root-to-shoot translocation, and an enhanced tolerance to arsenic inside plant cells (Wang et al., 2009; Bondada et al., 2007; Lombi et al., 2002; Lasat et al., 1998). In general, it has been observed that leafy vegetables (lettuce, leaf mustard, water spinach) contain more arsenic in their edible parts than non-leafy vegetables (tomatoes, eggplant, beans and cowpeas) (Huang et al., 2006; Liao et al., 2005; Munoz et al., 2002; Warren et al., 2003; Cobb et al., 2000). The same pattern was observed in this study. These results indicate that plant family characteristics have a large influence on plant uptake.
Based on their growth in contaminated soils, plants can be classified into three main groups: excluders, indicators, and accumulators. Plants with BCF values ≥ 1 are often classified as hyperaccumulators (Vithanage et al., 2011). The Asteraceae and Brassicaceae families have been identified to have members classified as hyperaccumulator plants (Vithanage et al., 2011; Cheraghi et al., 2011;Maestri et al., 2010; Ghosh and Singh, 2005; Salt et al., 1998), with members of the Pteris genus (Mahmood et al., 2012) and Pityrogramma calomelanos (Francesconi et al., 2002) having been identified as arsenic hyperaccumulators. Based on this ability, these plants have been identified as potential candidates for phytoextraction, a type of phytoremediation for soil with heavy metal contamination. The first hyperaccumulators (of zinc, nickel, cadmium and selenium) characterized were members of the Brassicaceae family (Salt et al., 1998). It can be inferred that members of the Asteraceae and Brassicaceae families have genetic and physiological capacity to accumulate, translocate, and resist high amounts of metals. Thus, it is not surprising that these families had higher BCF values in this study. In summary, the family to which a plant belongs has a considerable influence on its uptake of arsenic in the edible tissue.
5. Conclusion
This study demonstrates that the soil arsenic concentration and the family to which a plant belongs influence the uptake of arsenic into the edible tissues of plants grown in mining-affected soils. All vegetables accumulated arsenic, ranging from 0.01 to 23.0 mg kg−1 dry weight. A strong correlation was observed for the Asteraceae, Brassicaceae, Amaranthaceae, and Fabaceae families between arsenic uptake and the levels of arsenic in the soil in which they were grown. Asteraceae and Brassicaceae families had larger BCF values, a pattern similar to that previously reported in the literature. Literature BCF values were generally higher than those observed in this study, highlighting that although soil arsenic concentration is an important variable, there are other soil characteristics that may influence plant uptake.
The integrated greenhouse and citizen-science field study reported here was successful in determining the concentration of arsenic in the edible portion of the vegetable plants grown near the IKMHSS site. It is recommended that home gardeners surrounding mining operations or mine tailings be made aware that their soils may have elevated soil arsenic levels and that arsenic will accumulate to different degrees in different vegetables. This study suggests that food gardeners surrounding mining operations, particularly legacy operations which may contain elevated arsenic, test their soils prior to gardening to determine arsenic soil concentrations. Particularly, it might be prudent for home gardeners who neighbor mining operations or who are in an arsenic endemic areas to limit the use of vegetables from the Asteraceae and Brassicaceae families to reduce their dietary exposure to arsenic.
Supplementary Material
HIGHLIGHTS.
-
►
We characterized As uptake by homegrown vegetables near a Superfund site in AZ.
-
►
A greenhouse study conducted in parallel with a co-created citizen science program.
-
►
Asteraceae and Brassicaceae families had the largest As bioconcentration factors.
-
►
A correlation was observed for As in vegetable vs soil for selected families.
Acknowledgments
This research was funded by the US EPA Office of Research and Development, the National Institute of Environmental Health Sciences Superfund Research Program (grant P42 ES04940), the NASA Space Grant Program, the University of Arizona TRIF Water Sustainability Program, and the Alfred P. Sloan Foundation.
The authors would like to give a special thanks to the Gardenroots' citizen science participants, the community of Dewey–Humboldt, Arizona, Mike Kopplin (the University of Arizona Superfund Research Program's Hazard Identification Core) and Atasi Ray-Maitra (the University of Arizona Water Quality Laboratory).
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.scitotenv.2012.10.095.
References
- Alam MGM, Snow ET, Tanaka A. Arsenic and heavy metal contamination of vegetables grown in Samta village, Bangladesh. Sci Total Environ. 2003;308:83–96. doi: 10.1016/S0048-9697(02)00651-4. [DOI] [PubMed] [Google Scholar]
- Arizona Department of Human Health Services. Health consultation: Iron King Mine and Humboldt Smelter, Dewey–Humboldt, Yavapai County, Arizona. [Accessed 19 June 2012];2009 Available: http://azmemory.lib.az.us/cdm/singleitem/collection/feddocs/id/1912/rec/20. [Google Scholar]
- Belluck DA, Benjamin SL, Baveye P, Sampson J, Johnson B. Widespread arsenic contamination of soils in residential areas and public spaces: an emerging regulatory or medical crisis? Int J Toxicol. 2003;22:109–128. doi: 10.1080/10915810305087. [DOI] [PubMed] [Google Scholar]
- Bhattacharya P, Samal AC, Majumdar J. Arsenic contamination in rice, wheat, pulses, and vegetables: a study in an arsenic affected area of West Bengal, India. Water Air Soil Pollut. 2010;213:3–13. [Google Scholar]
- Bondada BR, Underhill RS, Ma LQ, Davidson MR, Guyodo Y, Duran RS. Spatial distribution, localization and speciation of arsenic in the hyperaccumulating fern (Pteris vittata L.) In: Bhattacharya P, Mukherjee AB, Loeppert RH, editors. Arsenic in soil and groundwater environments: trace metals and other contaminants in environment. Amsterdam: Elsevier; 2007. pp. 299–314. [Google Scholar]
- Bunzl K, Trautmannsheimer M, Schramel P, Reifenhauser W. Availability of arsenic, copper, lead, thallium, and zinc to various vegetables grown in slag-contaminated soils. J Environ Qual. 2001;30:934–939. doi: 10.2134/jeq2001.303934x. [DOI] [PubMed] [Google Scholar]
- Burló F, Guijarro I, Carbonell-Barrachina AA, Valero D, Martínez-Sánchez F. Arsenic species: effects on and accumulation by tomato plants. J Agric Food Chem. 1999;47(3):1247–1253. doi: 10.1021/jf9806560. [DOI] [PubMed] [Google Scholar]
- Cheraghi M, Lorestani B, Yousefi N. Introduction of hyperaccumulator plants with phytoremediation potential of a lead–zinc mine in Iran. World Acad Sci Eng Technol. 2011;77 [Google Scholar]
- Cobb GP, Sands K, Waters M, Wixson BG, Dorward-King E. Accumulation of heavy metals by vegetables grown in mine wastes. Environ Toxicol Chem. 2000;19(3):600–607. [Google Scholar]
- Csavina J, Field J, Taylor MP, Gao S, Landázuri A, Betterton EA, et al. A review on the importance of metals and metalloids in atmospheric dust and aerosol from mining operations. Sci Total Environ. 2012;43:58–73. doi: 10.1016/j.scitotenv.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon HBF. The biological action of arsenic acids especially as phosphate analogues. Adv Inorg Chem. 1997;44:191–228. [Google Scholar]
- EA Engineering, Science, and Technology, Inc. Remedial investigation report. Iron King Mine and Humboldt Smelter Superfund Site Dewey–Humboldt, Yavapai County, Arizona. [accessed 19 June 2012];2010 Available: http://yosemite.epa.gov/r9/sfund/r9sfdocw.nsf/3dc283e6c5d6056f88257426007417a2/9ff58681f889089c882576fd0075ea2f!OpenDocument.
- Francesconi K, Visoottiviseth P, Sridokchan W, Goessler W. Arsenic species in an arsenic hyperaccumulating fern, Pityrogramma calomelanos: a potential phytoremediator of arsenic-contaminated soils. Sci Total Environ. 2002;284:27–35. doi: 10.1016/s0048-9697(01)00854-3. [DOI] [PubMed] [Google Scholar]
- Gaw SK, Kim ND, Northcott GL, Wilkins AL, Robinson G. Uptake of DDT, arsenic, cadmium, copper, and lead by lettuce and radish grown in contaminated horticultural soils. J Agric Food Chem. 2008;56:6584–6593. doi: 10.1021/jf073327t. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Singh SP. A review on phytoremediation of heavy metals and utilization of it's by-products. Asian J Energy Environ. 2005;6(04):214–231. [Google Scholar]
- Huang RQ, Gao SF, Wang WL, Staunton S, Wang G. Soil arsenic availability and the transfer of soil arsenic to crops in suburban areas in Fujian Province, southeast China. Sci Total Environ. 2006;368:531–541. doi: 10.1016/j.scitotenv.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Kabata-Pendias A, Pendias H. Trace elements in soils and plants. 3rd edition. Florida: CRC Press; 2001. pp. 225–233. [Google Scholar]
- Lasat MM, Baker AJM, Kochian LV. Altered Zn compartmentation in the root symplasm and stimulated Zn absorption into the leaf as mechanisms involved in Zn hyperaccumulation in Thlaspi caerulescens. Plant Physiol. 1998;118:875–888. doi: 10.1104/pp.118.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-S, Chon H-T, Kim K-W. Human risk assessment of As, Cd, Cu and Zn in the abandoned metal mine site. Environ Geochem Health. 2005;27:185–191. doi: 10.1007/s10653-005-0131-6. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang YB, Gou X, Wang G. Risk assessment of heavy metals in soils and vegetables around non-ferrous mining and smelting sites, Baiyin, China. J Environ Sci. 2006;l8:1124–1134. doi: 10.1016/s1001-0742(06)60050-8. [DOI] [PubMed] [Google Scholar]
- Liao XY, Chen T-B, Ying-Ru L. Soil As contamination and its risk assessment in areas near the industrial districts of Chenzhou City. Southern China. Environ International. 2005;31:791–798. doi: 10.1016/j.envint.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Lombi E, Zhao F, Fuhrmann M, Ma LQ, McGrath SP. Arsenic distribution and speciation in the fronds of the hyperaccumulator Pteris vittata. New Phytol. 2002;156(2):195–203. doi: 10.1046/j.1469-8137.2002.00512.x. [DOI] [PubMed] [Google Scholar]
- MacDonald GM. Water, climate change, and sustainability in the southwest. Proc Natl Acad Sci. 2010;107(50):21256–21262. doi: 10.1073/pnas.0909651107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestri E, Marmiroli M, Visioli G, Marmiroli N. Metal tolerance and hyperaccumulation: costs and trade-offs between traits and environment. Environ Exp Bot. 2010;68:1–13. [Google Scholar]
- Mahmood Q, Rashid A, Ahmad SS, Azim MR, Bilial M. Current status of toxic metals addition to environment and its consequences. In: Anju N, Ahmad I, Pereira ME, Duarte AC, Umar S, editors. The plant family Brassicaceae contribution towards phytoremediation. Dordrecht: Springer; 2012. pp. 35–71. [Google Scholar]
- Meharg AA, Hartley-Whitaker J. Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytologist. 2002;154:29–43. [Google Scholar]
- Munoz O, Diaz O, Leyton I, Nunez N, Devesa V, Suner MA, et al. Vegetables collected in the cultivated Andean area of Northern Chile: total and inorganic arsenic contents in raw vegetables. J Agric Food Chem. 2002;50:642–647. doi: 10.1021/jf011027k. [DOI] [PubMed] [Google Scholar]
- Murray H, Thompson K, Macfie SM. Site-and species-specific patterns of metals bioavailability in edible plants. Botany. 2009;87:702–711. [Google Scholar]
- National Gardening Association. The impact of home and community gardening in America. [Accessed 6 July 2010];2009 Available: http://www.gardenresearch.com/home?q=show&id=3126. [Google Scholar]
- O'Neill . Arsenic. In: Alloway BJ, editor. Heavy metals in soils. London: Blackie Academic and Professional; 1995. pp. 105–121. [Google Scholar]
- Raven KP, Loeppert RH. Trace element composition of fertilizers and soil amendments. J Environ Qual. 1997;26:551–557. [Google Scholar]
- Salt DE, Smith RD, Raskin I. Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:643–668. doi: 10.1146/annurev.arplant.49.1.643. [DOI] [PubMed] [Google Scholar]
- Smith NM, Lee R, Heitkemper DT, Cafferky KD, Haque A, Henderson AK. Inorganic arsenic in cooked rice and vegetables from Bangladeshi households. Sci Total Environ. 2006;370:294–301. doi: 10.1016/j.scitotenv.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Smith PG, Koch I, Reimer KJ. Uptake, transport and transformation of arsenate in radishes (Raphanus sativus) Sci Total Environ. 2008;390:188–197. doi: 10.1016/j.scitotenv.2007.09.037. [DOI] [PubMed] [Google Scholar]
- Smith E, Juhasz AL, Weber J. Arsenic uptake and speciation in vegetables grown under greenhouse conditions. Environ Geochem Health. 2009;31:125–132. doi: 10.1007/s10653-008-9242-1. [DOI] [PubMed] [Google Scholar]
- Solís-Domínguez FA, Valentin-Vargas A, Chorover J, Maier RM. Effect of arbuscular mycorrhizal fungi on plant biomass and the rhizosphere microbial community structure of mesquite grown in acidic lead/zinc mine tailings. Sci Total Environ. 2011;409:1009–1016. doi: 10.1016/j.scitotenv.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solís-Domínguez FA, White SA, Borrillo Hutter T, Amistadi MK, Root AA, Chorover J, et al. Response of key soil parameters during compost-assisted phytostabilization in extremely acidic tailings: effect of plant species. Environ Sci Technol. 2012;46:1019–1027. doi: 10.1021/es202846n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlman K, Rock C, Artiola JF. Arizona drinking water well contaminants. The University of Arizona Extension Bulletin AZ 1503. [Accessed 15 July 2012];2009 [Available: cals.arizona.edu/pubs/water/az1503.pdf.]. [Google Scholar]
- US EPA. Methods of analysis of hazardous solid wastes. SW-846. Washington DC Office of Solid Waste and Emergency Responses. (3rd ed.) 1986 [Google Scholar]
- USEPA (U.S. Environmental Protection Agency) Background report on fertilizer use, contaminants and regulations. [Accessed 15 July 2012];1999 Available at: www.epa.gov/oppt/pubs/fertilizer.pdf.
- USEPA (U.S. Environmental Protection Agency) Abandoned mine lands team: reference notebook. [Accessed 1 May 2011];2004 Available: http://www.epa.gov/aml/tech/refntbk.htm.
- Vithanage M, Dabrowska BB, Mukherjee AB, Bhattacharya P. Arsenic uptake by plants and possible phytoremediation applications: a brief overview. Environ Chem Lett. 2011:1–8. [Google Scholar]
- Wang B, Liu L, Gao Y, Chen J. Improved phytoremediation of oilseed rape (Brassica napus) by Trichoderma mutant constructed by restriction enzyme-mediated integration (REMI) in cadmium polluted soil. Chemosphere. 2009;74:1400–1403. doi: 10.1016/j.chemosphere.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Warren GP, Alloway BJ, Lepp NW, Singh B, Bochereau FJ, Penny C. Field trials to assess the uptake of arsenic by vegetables from contaminated soils and soil remediation with iron oxides. Sci Total Environ. 2003;311:19–33. doi: 10.1016/S0048-9697(03)00096-2. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Exposure to arsenic: a major public health concern. [Accessed 19 June 2012];2010 Available: www.who.int/ipcs/features/arsenic.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.