Abstract
Phosphatidylinositol polyphosphate lipids, such as phosphatidylinositol (3,4,5)-trisphosphate (PI(3,4,5)P3), regulate critical biological processes, many of which are aberrant in disease. These lipids often act as site-specific ligands in interactions that enforce membrane-association of protein binding partners. Herein, we describe the development of bifunctional activity probes corresponding to the headgroup of PI(3,4,5)P3 that are effective for identifying and characterizing protein binding partners from complex samples, namely cancer cell extracts. These probes contain both a photoaffinity tag for covalent labeling of target proteins as well as a secondary handle for subsequent detection or manipulation of labeled proteins. Probes bearing different secondary tags were exploited, either by direct attachment of a fluorescent dye for optical detection or by using an alkyne that can be derivatized after protein labeling via click chemistry. First, we describe the design and modular synthetic strategy used to generate multiple probes with different reporter tags of use for characterizing probe-labeled proteins. Next, we report initial labeling studies using purified protein, the PH domain of Akt, in which probes were found to label this target, as judged by on-gel detection. Furthermore, protein labeling was abrogated by controls including competition with an unlabeled PI(3,4,5)P3 headgroup analog as well as through protein denaturation, indicating specific labeling. In addition, probes featuring different linker lengths between the PI(3,4,5)P3 headgroup and photoaffinity tag led to variations in protein labeling, indicating that a shorter linker was more effective in this case. Finally, proteomic labeling studies were performed using cell extracts, labeled proteins were observed by in-gel detection and characterized using post-labeling with biotin, affinity chromatography and identification via tandem mass spectrometry. These studies yielded a total of 265 proteins, including both known and novel candidate PI(3,4,5)P3-binding proteins.
Introduction
The phosphatidylinositol polyphosphates (PIPns), also known as the phosphoinositides, represent an important family of signaling lipids that control numerous key cellular processes.1,2 These molecules contained a conserved myo-inositol headgroup that is attached via a phosphodiester linkage at the 1-position to a glycerolipid backbone. The family consists of seven naturally occurring isomers that exhibit differential patterns of phosphorylation, specifically including every combination of phosphate groups at the 3, 4, and 5 positions of the myo-inositol headgroup. A primary activity of the PIPns is to serve as site-specific ligands in protein binding events that enforce protein−membrane association.3,4 Due to the key roles of these lipids in regulating critical biological pathways, they have been linked to aberrant activities associated with diseases, including cancer and diabetes.5–8 One of the most prominent links to disease involves the role of phosphatidylinositol (3,4,5)-trisphosphate (PI(3,4,5)P3) as a ligand for Akt (protein kinase B).9–14 This binding event promotes association of Akt with the inner leaflet of the plasma membrane,15 where it is then activated through multiple phosphorylation events. Activated Akt feeds into numerous pathways that promote cell survival, and thus the enzymes that produce (phosphoinositide 3-kinase)16 and hydrolyze (PTEN)17 PI(3,4,5)P3 are among the most commonly mutated enzymes in cancer.18,19
The understanding of PIPn binding properties is hindered by complexity at multiple levels. Many proteins that bind PIPns contain one or more of a number of conserved binding module sequences, such as the PH, PX, FYVE, ENTH, ANTH, FERM, Tubby, and PROPPIN domains, members of which can be identified by sequence homology searches. New domain families have continued to be discovered in recent years and there are likely further as yet undiscovered PIPn-targeting sequences. Furthermore, complexities often arise within domain families, such as with the PH domains, where only a fraction of family members bind PIPns, and those PH-domain containing proteins that do bind PIPns exhibit broad diversity in their specificities for particular PIPn targets. Finally, other proteins that bind PIPns appear to lack any consensus binding sequence, making them more difficult to identify. As a result of these complexities, global methods for the identification and characterization of PIPn-binding proteins are of significant interest.
The primary method that has been effectively employed for global characterization of PIPn-binding proteins has involved affinity purification using solid supports decorated with different lipid motifs.20–27 Affinity chromatography using PIPn-functionalized beads was initially used to purify individual proteins,28,29 and applications have since expanded due to advancements in the technology for identifying protein binding partners. In an early example, libraries of radiolabeled proteins were screened, which led to the identification of 2 known and 1 new target of different PIPns.20 The scope of studies has expanded due to progress in mass spectrometry-based proteomic analysis, resulting in the identification of numerous PIPn-binding proteins.22,24–27,30 In a recent report, 282 PI(3,4,5)P3-binding proteins were identified using beads decorated with this headgroup that were either used directly for studies or through incorporation of the beads into liposomes to better mimic the environment of cellular membranes.25 In addition to affinity chromatography, proteome chips have also been used to probe protein−PIPn binding interactions.31 This was performed by immobilizing GST-tagged proteins from yeast, followed by microarray analysis of binding to biotin-labeled PIPn-containing liposomes. One note regarding these proteomic analyses is that the exact set of proteins that have been identified has varied among the studies. This indicates that the specific conditions that are employed for analysis affect the results, suggesting that it will be beneficial to pursue diverse approaches to elucidate the full complement of PIPn-binding proteins.
A strategy that shows great prospects for broad and efficient characterization of lipid-binding proteins is activity-based protein profiling (ABPP),32 in which protein targets are collectively characterized based on function, in this case ligand binding properties. In this approach, target proteins are collectively labeled, purified and characterized from complex biological samples such as cell extracts and live cells using small molecule probes that range from active-site directed analogs of enzyme substrates to derivatized versions of biologically active ligands. Recently, phospholipid-based activity probes have been applied to characterize both lipid-binding proteins33,34 and lipid-modifying phospholipase enzymes35 using synthetic analogs of phosphatidylcholine. A significant advantage of ABPP is that protein targets can be labeled in living cells, allowing for the probing of activities under normal physiological conditions, which is critical since proteins operate much differently in live cells than in cell extracts. In addition, the covalent labeling of protein targets in ABPP circumvents the problem of non-covalent interactions falling apart during processing, and enhances the likelihood of identifying protein targets that bind the probe with weaker affinities. Due to the benefits of ABPP, we set out to develop activity-based probes corresponding to the PIPns that are effective for proteomics towards their eventual use in live cell labeling studies.
Experimental Procedures
General Experimental
Reagents were generally purchased from Acros, Aldrich or Fisher Scientific and used as received. Dry solvents were obtained from a Pure Solv solvent delivery system purchased from Innovative Technology, Inc. Column chromatography was performed using 230–400 mesh silica gel purchased from Sorbent Technologies and C18 (17%) reverse phase column (6 mL, 2 g) purchased from Silicycle. NMR spectra were obtained using a Varian Mercury 300 spectrometer. Mass spectra were obtained with an ABI DE Pro MALDI spectrometer with high-resolution capabilities. Protein photo-cross-linking was performed using a Spectroline ENF260C UV lamp. Proteins were visualized in-gel using a Hibachi FMBio lle flatbed laser-induced fluorescence scanner (MiraiBio, Alameda, CA). Dulbecco's Phosphate-Buffered Saline (PBS) was purchased from Cellgro. Cell lines were purchased from ATCC. Heat-denatured proteins or cell extracts were generated by denaturing at 90°C for 5 minutes. PIPn headgroup−amine conjugates 4a and 4b36, Azidorhodamine 1137, and THPTA38 were each synthesized according to previously reported procedures. The GST-tagged PH domain of Akt was expressed and purified as previously reported.39 Cancer cell extracts were prepared using a previously reported procedure.37
1. Synthesis of PIPn activity-based probes
Benzophenone-Lys-OMe (6)
A reported procedure40 was used to prepare compound 6. Cbz-Lys(Boc)-OMe (5) (262 mg, 0.664 mmol) was dissolved in 5 mL methanol. And palladium(II) hydroxide (26 mg, 10% wt) was added to the solution. The reaction was stirred under hydrogen atmosphere overnight, and the reaction crude was then filtered through a pad of cellite. The filtrate was concentrated to yield Lys(Boc)-OMe, which was used in the next step without further purification. Lys(Boc)-OMe was dissolved in 10 mL N,N-dimethylformamide, to which was added 4-benzoylbenzoic acid (150 mg, 0.664 mmol), N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDCI, 153 mg, 0.797 mmol), 4-(dimethylamino)pyridine (DMAP, 97 mg, 0.791 mmol), N-methylmorpholine (NMM, 0.26 mL, 2.32 mmol). The solution was stirred at rt overnight and then concentrated under reduced pressure. The crude was purified by silica gel column chromatography with a solvent system of 50% ethyl acetate / hexanes to yield benzophenone-Lys(Boc)-OMe as a white foam (279 mg, 90% over 2 steps). The characterization of benzophenone-Lys(Boc)-OMe matches with the literature.40 Benzophenone-Lys(Boc)-OMe (0.279 g, 0.595 mmol) was then dissolved in 4 mL of trifluoroacetic acid / dichloromethane (v/v 1:1). The reaction was allowed to stir at rt for 2 h and was then concentrated under reduced pressure to yield 6 as a yellowish gum (0.272 g, 100%).
1H NMR (300MHz, CDCl3) δ 7.88 (d, J = 9Hz, 1H), 7.70-7.67 (m, 4H), 7.57-7.52 (m, 2H), 7.44-7.40 (m, 2H), 4.67-4.65 (m, 1H), 3.66 (s, 3H), 2.89-2.87 (m, 2H), 1.86-1.64 (m, 4H), 1.46-1.43 (m, 2H). 13C NMR (300MHz, CDCl3) δ 196.7, 172.6, 140.7, 136.7, 136.5, 133.4, 130.2, 130.1, 128.7, 127.5, 53.0, 52.8, 40.2, 31.3, 26.7, 22.4. MALDI-HRMS [M + Na]+ calcd for 391.1634; found 391.1632.
Benzophenone-Lys(5(6)-carboxy fluorescein)-OMe (7a)
Benzophenone-Lys-OMe (6, 175 mg, 0.475 mmol) was combined with 5(6)-carboxyfluorescein (197 mg, 0.522 mmol), N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDCI, 118 mg, 0.618 mmol), 4-(dimethylamino)pyridine (DMAP, 70 mg, 0.570 mmol), N-methylmorpholine (NMM, 0.21 mL, 1.90 mmol) in 8 mL dry N,N-dimethylformamide. The reaction was allowed to stir at rt overnight, at which point the solvent was removed under reduced pressure. The crude was dissolved in 50 mL methanol/chloroform (v/v 1:4) and extracted with 50 mL 2N hydrochloric acid aqueous solution, and the aqueous layer was washed 2 × 50 mL methanol/chloroform (v/v 1:4). The organic layers were then combined, dried with magnesium sulfate, concentrated by rotary evaporation, and then purified using column chromatography with a gradient solvent system of 5-10% methanol / chloroform to yield benzophenone-Lys(5(6)-carboxy fluorescein)-OMe (7a) as an orange solid (159 mg, 46%).
1H NMR (300MHz, CD3OD/CDCl3 (v/v 1:2)) δ 8.40-8.39 (m, 1H), 8.03-7.98 (m, 2H), 7.95-7.83 (m, 2H), 7.85-7.75 (m, 3H), 7.66-7.69 (m, 1H), 7.54-7.49 (m, 2H), 7.25-7.22 (m, 1H), 6.72-6.70 (m, 2H), 6.57-6.53 (m, 4H), 4.76-4.72 (m, 1H), 3.80 (s, 3H), 3.50-3.48 (m, 1H), 3.36-3.35 (m, 1H), 2.67 (bs, 1H), 2.06-1.94 (m, 2H), 1.74-1.46 (m, 4H). MALDI-HRMS [M + Na]+ calcd for 749.2111; found 749.2165.
Benzophenone-Lys(5(6)-carboxy fluorescein)-OH (7b)
Compound 7a (36 mg, 0.050 mmol) was suspended in 5 mL of methanol. To this solution was added 5 mL of 2M sodium hydroxide aqueous solution, and the reaction was stirred at rt for 10 min, followed by addition of 30 mL of 2N hydrochloric acid aqueous solution. An orange solid was formed immediately upon addition of the acid, which was filtered and washed with 2 × 10 mL of 2N hydrochloric acid aqueous solution to yield the crude 7b. The crude was then dissolved in acetonitrile/water and purified on a C18 reverse phase column (2g) with a gradient solvent system of 0–80% acetonitrile/water. Compound 7b eluted with ~45–60% acetonitrile/water solution. The collected fractions were concentrated by rotary evaporation to remove acetonitrile. The acetonitrile was then removed by rotary evaporation, followed by lyophilization of the water to yield benzophenone-Lys(5(6)-carboxy fluorescein)-OH (7b) as a yellow powder (19 mg, 53%).
1H NMR (300MHz, CD3CN/D2O (v/v 1:1)) δ 8.73-8.71 (m, 1H), 8.42-8.29 (m, 4H), 8.14-8.07 (m, 4H), 7.92-7.87 (m, 2H), 7.53-7.50 (m, 1H), 7.10 (s, 2H), 6.97-6.93 (m, 4H), 4.95-4.85 (m, 1H), 3.82-3.80 (m, 1H), 3.67-3.65 (m, 1H), 2.40-2.38 (m, 2H), 1.94-1.80 (m, 4H). MALDI-HRMS [M + H]+ calcd for 713.2130; found 713.2072.
Benzophenone-Lys(alkyne)-OMe (8a)
Benzophenone-Lys-OMe (6) (911 mg, 1.890 mmol) was combined with alkyne 10 (293 mg, 1.89 mmol), N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDCI, 543 mg, 2.83 mmol), 4-(dimethylamino)pyridine (DMAP, 346 mg, 2.83 mmol), and N-methylmorpholine (NMM, 2.08 mL, 18.9 mmol) in 10 mL of methanol/chloroform (v/v 1:4). The reaction was allowed to stir at rt overnight, and the solvent was then removed under reduced pressure. The crude was purified by column chromatography with a gradient solvent system of 50–100% acetone/hexanes to yield benzophenone-Lys(alkyne)-OMe (8a) as a colorless crystal (760 mg, 80%).
1H NMR (300MHz, CDCl3) δ 7.99-7.96 (m, 2H), 7.81-7.75 (m, 3H), 7.61-7.57 (m, 1H), 7.50-7.42 (m, 3H), 6.81-6.79 (m, 1H), 6.55-6.51(m, 1H), 4.74-4.67 (m, 1H), 3.95-3.93 (m, 2H), 3.75 (s, 3H), 3.27-3.15 (m, 2H), 2.42-2.41 (m, 4H), 2.17-2.14 (m, 1H), 1.90-1.86 (m, 2H), 1.53-1.42 (m, 4H). MALDI-HRMS [M + Na]+ calcd for 528.2105; found 528.2134.
Benzophenone-Lys(alkyne)-OH (8b)
Compound 8a (135 mg, 0.267 mmol) was dissolved in a mixture of 10 mL of methanol/ethanol (v/v 1:1). To this solution was added 8 mL of 2M sodium hydroxide aqueous solution. The reaction was stirred at rt for 15 min and was then extracted from 2N hydrochloric acid aqueous solution (100 mL) with 200 mL dichloromethane, and the aqueous layer was washed with 3 × 50 mL of methanol/chloroform (v/v 1:4). The combined organic layers were then dried with magnesium sulfate, filtered and concentrated to yield benzophenone-Lys(alkyne)-OH (8b) as a white solid (94 mg, 72 %).
1H NMR (300MHz, CD3OD) δ 8.01-7.99 (m, 2H), 7.84-7.76 (m, 4H), 7.65-7.63 (m, 1H), 7.55-7.53 (m, 2H), 4.59-4.57 (m, 1H), 3.95-3.93 (m, 1H), 3.65-3.61 (m, 2H), 3.21-3.19 (m, 2H), 2.57-2.45 (m, 5H), 1.99.92 (m, 2H), 1.56-1.54 (m, 4H). 13C NMR (300MHz, CDCl3) δ 196.2, 174.2, 171.9, 168.0, 140.0, 137.3, 136.8, 132.8, 129.7, 129.6, 128.3, 127.4, 78.2, 71.1, 53.1, 38.9, 30.6, 28.5, 28.2, 23.2, 21.4.MALDI-HRMS [M + Na]+ calcd for 514.1954; found 514.1974.
Benzophenone-Lys(alkyne)-hexanoate (9a)
Carboxylic acid 8b (144 mg, 0.293 mmol) was combined with 5-aminocaproic acid methyl ester hydrochloride (69 mg, 0.381 mmol), N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDCI,72 mg, 0.381 mmol), 4-(dimethylamino)pyridine (DMAP, 47 mg, 0.381 mmol), and N-methylmorpholine (NMM, 0.32 mL, 2.92 mmol) in 15 mL methanol/chloroform (v/v 1:4). The reaction was allowed to stir at rt overnight, and the solvent was then removed under reduced pressure. The crude was purified by column chromatography with a gradient solvent system of 50–100% acetone/hexanes to yield compound 9a as a colorless crystal (91 mg, 50%).
1H NMR (300MHz, CDCl3) δ 8.00-7.97 (m, 2H), 7.83-7.77 (m, 4H), 7.64-7.59 (m, 1H), 7.52-7.47 (m, 2H), 7.21-7.19 (m, 1H), 6.83-6.81(m, 1H), 4.68-4.66 (m, 1H), 3.98-3.96 (m, 2H), 3.64 (s, 3H), 3.25-3.23 (m, 4H), 2.54-2.48 (m, 4H), 2.29 (t, J = 6 Hz, 2H), 2.21-2.18 (m, 1H), 1.92-1.83 (m, 2H), 1.62-1.34 (m, 10H). 13C NMR (300MHz, CDCl3) δ 196.1, 174.1, 172.5, 172.4, 171.7, 166.8, 140.3, 136.9, 133.0, 130.1, 130.0, 128.5, 127.4, 79.7, 71.31, 53.7, 51.6, 39.4, 33.8, 31.3, 29.1, 26.3, 24.4, 22.8. MALDI-HRMS [M + Na]+ calcd for 641.2951; found 641.2953.
4-Oxo-4-(2-propyn-1-ylamino) butanoic acid (10).41
Propargylamine (1.5 mL, 21.87 mmol) was dissolved in 10 mL of N,N-dimethylformamide/acetontrile (v/v: 1/1) at 0°C. To this solution was added succinic anhydride (2.19 g, 21.87 mmol) in 10 mL of acetonitrile, and the resulting solution was stirred at rt overnight. Next, the solvent was removed under reduced pressure. The residue was then washed with hexanes and then ethyl ether to obtain crude 10 as a brownish solid. The crude product was purified by column chromatography with 50–75% ethyl acetate/hexanes to yield 10 as a pale yellowish solid (1.414 g, 42%).
1H NMR (300MHz, CD3OD) δ 3.94 (d, J = 3Hz, 1H), 2.61-2.56 (m, 2 H), 2.49-2.44 (m, 2H). 13C NMR (300MHz, CDCl3) δ 174.8, 172.7, 79.2, 70.8, 29.9, 28.7, 28.1. DART-HRMS [M + H]+ calcd for 156.0661; found 156.0670.
Benzophenone-Lys(5(6)carboxy fluorescein)-PI(3,4)P2 (1)
Acid 7b (28 mg, 0.039 mmol) was dissolved in 4 mL of N,N-dimethylformamide/tetrahydrofuran (v/v 1:2). To this stirred solution was added dicyclohexylcarbodiimide (DCC, 16 mg, 0.078 mmol) and then N-hydroxysuccinimide (9 mg, 0.078 mmol). The reaction was stirred for 8 h at rt and then filtered. The filtrate was concentrated to ~0.5 mL, to which was added 50 mL of ethyl ether, after which an orange precipitate was formed and filtered. The solid was then dissolved in 10 mL of tetrahudrofuran and filtered. The filtrate was concentrated to yield the NHS ester of 7b, which was moved to the next step without further purification. The resulting succinimidyl ester was dissolved in 1 mL of N,N-dimethylformamide, to which was added amino-tagged to a solution of PI(3,4)P2−amine 4a36 (10 mg, 0.019 mmol) in 1 mL tetraethylammonium bicarbonate (TEAB, 1 M, pH=7.5) and 0.5 mL tetrahydrofuran. The reaction was allowed to stir at rt overnight, and the solvent was then removed under reduced pressure. The solid crude product was washed with acetone (3 × 20 mL) and then dissolved in deionized water to stir with 500 mg chelex 100, sodium form at rt for 2 h. The reaction solution was then directly loaded onto a C18 reverse-phase column (2 g) and the column was eluted with deionized water (20 mL). The water elution was lyophilized to yield 1 as a yellow solid (8.0 mg, 35%).
1H NMR (300MHz, CD3OD/D2O (v/v 1:1)) δ 7.75-7.00 (m, 12 H), 6.55 (s, 2 H), 6.30-6.28 (m, 2H), 5.89-5.86 (s, 2 H), 4.30-4.28 (s, 2 H), 4.02-3.98 (m, 2H), 3.72-3.31 (m, 14H), 1.53-1.04 (m, 14H). 31P NMR (121.5 MHz, CD3OD/D2O (v/v 1:1)): 5.05, 3.64, 0.12. MALDI-HRMS [M + H]+ calcd for 1214.2696; found 1214.7771.
Benzophenone-Lys(alkyne)-PI(3,4,5)P3 (2)
Acid 9b (47 mg, 0.096 mmol) was dissolved in 5 mL of tetrahydrofuran. To this stirred solution was added N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDCI, 37 mg, 0.192 mmol) and then N-hydroxysuccinimide (22 mg, 0.192 mmol). The reaction was stirred for 8 h at rtand then concentrated. The crude was dissolved in 30 mL chloroform and washed with water (3 × 20 mL). The organic layer was concentrated and 30 mL of ethyl ether was added, after which the resulting white precipitate was filtered and redissolved in chloroform (15 mL). This solution was filtered and the filtrate was concentrated to yield the crude NHS ester of 9b as a colorless oil. The crude NHS ester was then stirred in 1 mL of N,N-dimethylformamide, to which was added 4b (10 mg, 0.0167 mmol) in 1 mL tetraethylammonium bicarbonate (TEAB, 1 M, pH=7.5). Additionally 1 mL of tetrahydrofuran was added to enhance solubility. The reaction was then allowed to stir at rt overnight. The solvent was then removed under reduced pressure, and the solid crude product was washed with acetone (3 × 20 mL). This crude was then dissolved in deionized water to stir with 500 mg of Chelex 100, sodium form at rt for 2 h. The reaction solution was then loaded directly onto a C18 reversed-phase column (2 g) and the column was eluted with deionized water (20 mL). The water elution was lyophilized to yield 2 as a white solid (11 mg, 61%).
1H NMR (300MHz, D2O) δ 7.71-7.52 (m, 7H), 7.37 (t, J = 6 Hz, 2 H), 4.24-4.18 (m, 3H), 3.88-3.63 (m, 10H), 3.03-2.97 (m, 4H), 2.79 (t, J = 6 Hz, 2H), 2.10-2.05 (t, J = 6Hz, 2H), 1.67-1.68 (m, 1 H), 1.46-1.34 (m, 10H), 1.22-1.16 (m, 9H). 31P NMR (121.5 MHz, D2O): 1.33, 1.02, 0.40, −0.89. MALDI-HRMS [M+Na]+ calcd for 1095.2178; found 1095.2285
Benzophenone-Lys(alkyne)-hexyl-PI(3,4,5)P3 (3)
Compound 9a (80 mg, 0.129 mmol) was dissolved in 2 mL of methanol. To this solution was added 2 mL of 2M sodium hydroxide aqueous solution. The reaction was stirred at rt overnight and was then added to proton resin to adjust the pH to ~4, removal of the resin by filtration. The filtrate was next concentrated to yield crude acid 9b as a colorless gum (68 mg, 87%). Acid 9b (68 mg, 0.112 mmol) was next dissolved in 6 mL of tetrahydrofuran. To this stirred solution was added N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDCI, 43 mg, 0.224 mmol) and then N-hydroxysuccinimide (26 mg, 0.224 mmol), and the reaction was stirred for 8 h and then concentrated. The crude was next dissolved in 30 mL of chloroform and washed with water (3 × 20 mL). The organic layer was concentrated and 30 mL of ethyl ether was added, after which the resulting white precipitate was filtered and redissolved in chloroform (15 mL). The solution was then filtered and the filtrate was concentrated to yield the crude NHS ester corresponding to 9b as a colorless oil. The crude NHS ester was then dissolved in 1 mL of N,N-dimethylformamide. To this solution was added 4b36 (8 mg, 0.0106 mmol) in 1 mL tetraethylammonium bicarbonate (TEAB, 1 M, pH=7.5). Additionally, 1 mL of tetrahydrofuran was added to enhance solubility. The reaction was next allowed to stir at rt overnight, at which point the solvent was removed under reduced pressure. The solid crude product was washed with acetone (3 × 20 mL) and then dissolved in deionized water to stir with 500 mg of Chelex 100, sodium form at rt for 2 h. The reaction solution was directly loaded onto a C18 reversed-phase column (2 g) and the column was eluted with deionized water (20 mL). The water elution was lyophilized to yield 3 as a white solid (11.2 mg, 93%).
1H NMR (500MHz, D2O) δ 7.86-7.80 (m, 4H), 7.76-7.74 (m, 2H), 7.67 (t, J = 3 Hz, 1H), 7.53-7.50 (m, 2H), 4.36-4.33 (m, 3H), 4.03-4.02 (m, 1H), 3.96-3.95 (m, 2H), 3.83-3.79 (m, 3H), 3.16-3.02 (m, 5H), 3.02-2.78 (m, 2H), 2.35-2.31 (m, 4H), 2.09-2.06 (m, 2H), 1.80-1.78 (m, 2H), 1.51-1.18 (m, 18H). 31P NMR (202.5 MHz, D2O): 0.70, 0.31, −0.28, − 0.90. MALDI-HRMS [M + Na]+ calcd for 1208.3024; found 1208.2186.
2. Protein and cell extract labeling studies
General procedure for protein labeling
In a transparent 96-well plate, Akt-PH (50 uL, 60 ug/mL in PBS) or cytosolic cell extracts (50 uL used in samples for probe 1, 43 uL used in samples for probe 2 and 3, 1.0 mg/mL in PBS buffer pH 7.4) were incubated with PIPn probes 1, 2 or 3 (50× stock in water) or bifunctional tag 8a (50× stock in DMSO) for 1 h at rt. The 96-well plate was then placed on ice and photolyzed for 1 h by placing it ~ 1 cm below a 365 nm Spectroline ENF260C UV lamp. Samples using probe 2 or 3 were followed by derivatization via click chemistry while samples of probe 1 were directly subjected to SDS-PAGE.
Procedure for click reaction using azido-rhodamine 11 (probe 2 and 3 only).37,42
Following the photolysis procedure, to each sample was added 50 uM rhodamine-N3 (11) (50× solution in DMSO), 1mM tris(2-carboxyethyl)phosphine (TCEP, 50× solution in water), 100 uM ligand (TBTA or THPTA (18), 17× stock in DMSO/t-butanol v/v 1:4) and 1 mM Copper sulfate pentahydrate (50× stock in water). Samples were shaken for 10 sec after each addition. After all additions, samples were incubated at rt for 1 hour. For gel analysis, each sample was quenched by adding 50 uL of SDS-PAGE sample buffer (2×). The proteins (30 uL of quenched sample per gel lane) were separated by 1D SDS-PAGE and visualized via in-gel using a fluorescence scanner.
Procedure for competition studies with Akt-PH
Akt-PH protein in PBS buffer pH 7.4 (60 ug/mL) was added into four separate wells of a 96-well microplate (50 uL each). Sample were then incubated with either 0 mM, 2 mM, 5 mM or 10 mM amino-tagged PI(3,4,5)P3 (4b) (50× stock in water) at 4 °C overnight, prior to incubation with 50 uM probe 2 (2.5 mM stock in water) for 1 h. The 96-well plate was then placed on ice and photolyzed for 1 h under 365 nm UV lamp. Next, the click reaction procedure with azido-rhodamine was 11 performed with each sample, followed by SDS-PAGE and fluorescence scanning.
3. Mass Spectrometry Proteomic Analysis
Procedure for sample preparation
To three separate tubes was added cytosolic MDA-MB-435 cancer cell extract (870 µL each, 1 mg/mL in PBS for sample I and II, or 2 mg/mL in PBS for sample III) as well as 20 uL of alkyne−PI(3,4,5)P3 probe 2 (5 mM stock solution in water for a final concentration of 100 uM in sample I and II or 1.25 mM stock solution in water for a final concentration of 25 uM in sample III). Finally, for sample I and II, a control sample was generated using the same conditions, but using bifunctional tag 8a lacking PIP headgroups; for sample III, a control sample was generated using the same condition but lacking the addition of any probe. These samples were incubated for 1 h, followed by photolysis on ice via 350 nm irradiation for 1 hour. Next, 6 uL of biotin-azide 12 (50 mM in DMSO for a final concentration of 300 uM) was added, followed by 20 uL tris-(2-carboxyethyl)phosphine (TCEP, 50 mM in water for a final concentration of 1 mM), 66 uL of TBTA ligand (1.67 mM in 1:4 DMSO/t-butanol for a final concentration of 100uM) and 20 uL of copper sulfate pentahydrate (50 mM in water a final concentration of 1 mM). These mixtures were incubated at room temperature for 1 hour. Next, to each sample was added 4 mL of methanol, 1 mL of chloroform and 3 mL of water, and the samples were vortexed and then centrifuged (4,000 rpm for 10 minutes) to pellet proteins. The protein pellet was re-dissolved in 600 uL of methanol, and 150 uL of chloroform along with 600 uL of water were added, after which the samples were vortexed and centrifuged (14,000 rpm for 3 minutes) to pellet the proteins a second time. The protein pellet was then washed with methanol (2 × 600 uL), followed by re-suspension in 500 uL of 6 M urea / 25 mM ammonium bicarbonate. Water (500 uL) was added to each sample, which was then heated to 65 °C for 5 minutes. After addition of 50 uL of TCEP (0.1 M for a final concentration of 10 mM), the sample incubation was continued at 65 °C for 15 min. After the samples were cooled to room temperature, 40 uL of iodoacetamide (0.55 M in 50 mM ammonium bicarbonate for a final concentration of 40 mM iodoacetamide) was added to each sample and the resulting solutions were incubated at 37 °C for 30 min in the dark. Next, 10% SDS was added to achieve a 2% final concentration. The samples were next heated to 65 °C for 5 min and then diluted into 0.2% SDS solution with PBS and 50 mM Tris buffer (v/v 3:3.5). The slurry was then washed with PBS (2×). To this slurry was added strepavidin agarose resin (Thermo Scientific) and the resulting samples were incubated at room temperature for 1 hour with rotation. The resin was centrifuged to pellet (4000 rpm for 3 min) and washed with 1% SDS buffer (2 × 8 mL), 6 M urea (2 × 8 ml) and then PBS buffer (2 × 10 mL). Next, to the resin was resuspended 200 uL of 2 M urea / 25 mM ammonium bicarbonate, 2 uL calcium chloride (100 mM in water for a final concentration of 1 mM) and 4 ug trypsin (sequence grade, Promega). Samples were incubated at 37°C overnight, and were then centrifuged to separate the resin from the supernatant. The supernatant was collected and acidified using 5% formic acid for LC/MS/MS analysis. The data generated from samples containing probe were compared with those from the control studies.
Mass Spectrometry and Data analysis
Digested peptide mixtures were pressure-loaded onto a biphasic (strong cation exchange/reverse phase) capillary column and analyzed by two-dimensional liquid chromatography (2D-LC) separation in combination with tandem mass spectrometry, as has previously been described.43–45 Mass spectrometry was performed using an Agilent 1200-series quaternary pump and Thermo Scientific LTQ ion trap mass spectrometer. Peptides were eluted in a 5-step MudPIT experiment (using 0%, 10%, 25%, 80%, and 100% salt bumps of 500 mM aqueous ammonium acetate) and data were collected in data-dependent acquisition mode with dynamic exclusion turned on (90 s, repeat of 1). Specifically, one full MS (MS1) scan (400–1800 m/z) was followed by 7 MS2 scans of the most abundant ions. The MS2 spectra data were extracted from the raw files using RAW Xtractor (version 1.9.1; publicly available at http://fields.scripps.edu/?q=content/download). MS2 spectra data were searched using the SEQUEST algorithm (Version 3.0) against the latest version of the mouse IPI database concatenated with the reversed database for assessment of false-discovery rates. In total the search database contained 553 protein sequence entries (549 real sequences and 4 decoy sequences). SEQUEST searches allowed for variable oxidation of methionine (+16.0), static modification of cysteine residues (+57.0 due to alkylation), no enzyme specificity and a mass tolerance set to ±1.5 Da for precursor mass and ±0.5 Da for product ion masses. The resulting MS2 spectra matches were assembled and filtered using DTASelect (version 2.0.47). The validity of peptide/spectrum matches was assessed using DTASelect and two SEQUEST-defined parameters, the cross-correlation score (XCorr), normalized difference in cross-correlation scores (DeltaCN). The search results were grouped by charge state (+1, +2, +3), tryptic status, and modification status (modified and unmodified peptides), resulting in 18 distinct subgroups. In each of these subgroups, the distribution of Xcorr and DeltaCN values for the direct and decoy database hits was obtained, then the direct and decoy subsets were separated by discriminant analysis. Outlier points in the two distributions were discarded. Full separation of the direct and decoy subsets is not generally possible so the discriminant score was set such that a false discovery rate of less than 0.73 % was determined based on the number of accepted decoy database peptides. In addition, a minimum peptide length of seven amino acids residues was imposed and protein identification required the matching of at least two peptides per protein. Such criteria resulted in the elimination of most decoy database hits. Lists of prospective PI(3,4,5)P3-binding proteins were generated by setting criteria as ≥ 5-fold (Table 1) or ≥ 2-fold (Table 2) higher spectral counts in samples containing probe 2 compared to the corresponding negative control sample. Three protein lists were generated from separate runs, and all proteins listed in Table 1 or 2 were detected in at least two of the three runs. Protein functions and domains were determined by searching two databases (www.uniprot.org/uniprot, http://pir.georgetown.edu).
Table 1.
Proteins identified from proteomic studies with greater than 5 fold enrichments of spectral counts in probe-labeled samples relative to controls. References are included for those proteins that have been identified in other global proteomic studies.
| Accession # | Abbreviation | Protein |
|---|---|---|
| P62191 | PRS4 | 26S protease regulatory subunit 4 |
| Q9BWD1 | THIC | Acetyl-CoA acetyltransferase, cytosolic |
| Q99798 | ACON | Aconitate hydratase, mitochondrial |
| P61160 | ARP2 | Actin-related protein 2 |
| P54819 | KAD2 | Adenylate kinase 2, mitochondrial |
| P00568 | KAD1 | Adenylate kinase isoenzyme 1 |
| P15121 | ALDR | Aldose reductase |
| P12814 | ACTN1 | Alpha-actinin-1 |
| O43707 | ACTN4 | Alpha-actinin-425 |
| P04083 | ANXA1 | Annexin A1 |
| P07355 | ANXA2 | Annexin A225 |
| P14868 | SYDC | Aspartyl-tRNA synthetase, cytoplasmic |
| P25705 | ATPA | ATP synthase subunit alpha, mitochondrial |
| P06576 | ATPB | ATP synthase subunit beta, mitochondrial |
| P61221 | ABCE1 | ATP-binding cassette sub-family E member 1 |
| P53396 | ACLY | ATP-citrate synthase |
| P31939 | PUR9 | Bifunctional purine biosynthesis protein PURH |
| P11586 | C1TC | C-1-tetrahydrofolate synthase, cytoplasmic |
| P16152 | CBR1 | Carbonyl reductase [NADPH] 1 OS=Homo sapiens GN=CBR1 PE=1 SV=3 |
| O75828 | CBR3 | Carbonyl reductase [NADPH] 3 |
| P68400 | CSK21 | Casein kinase II subunit alpha |
| P19784 | CSK22 | Casein kinase II subunit alpha' |
| Q00610 | CLH1 | Clathrin heavy chain 125 |
| P53621 | COPA | Coatomer subunit alpha25 |
| P21399 | ACOC | Cytoplasmic aconitate hydratase |
| Q14204 | DYHC1 | Cytoplasmic dynein 1 heavy chain 1 |
| P09417 | DHPR | Dihydropteridine reductase |
| O00429 | DNM1L | Dynamin-1-like protein |
| P30084 | ECHM | Enoyl-CoA hydratase, mitochondrial |
| Q14240 | IF4A2 | Eukaryotic initiation factor 4A-II |
| P56537 | IF6 | Eukaryotic translation initiation factor 6 |
| P55060 | XPO2 | Exportin-225 |
| P49327 | FAS | Fatty acid synthase25 |
| P21333 | FLNA | Filamin-A25 |
| P04075 | ALDOA | Fructose-bisphosphate aldolase A |
| P17931 | LEG3 | Galectin-325 |
| Q92820 | GGH | Gamma-glutamyl hydrolase |
| Q06210 | GFPT1 | Glucosamine--fructose-6-phosphate aminotransferase [isomerizing] 1 |
| P11413 | G6PD | Glucose-6-phosphate 1-dehydrogenase25 |
| P06744 | G6PI | Glucose-6-phosphate isomerase |
| P04406 | G3P | Glyceraldehyde-3-phosphate dehydrogenase25 |
| P06737 | PYGL | Glycogen phosphorylase, liver form |
| P49915 | GUAA | GMP synthase [glutamine-hydrolyzing] |
| P62826 | RAN | GTP-binding nuclear protein Ran |
| Q9NRV9 | HEBP1 | Heme-binding protein 1 |
| P12268 | IMDH2 | Inosine-5'-monophosphate dehydrogenase 2 |
| P02533 | K1C14 | Keratin, type I cytoskeletal 14 |
| P08779 | K1C16 | Keratin, type I cytoskeletal 16 |
| P35527 | K1C9 | Keratin, type I cytoskeletal 9 |
| Q9HA64 | KT3K | Ketosamine-3-kinase |
| P33176 | KINH | Kinesin-1 heavy chain25 |
| Q04760 | LGUL | Lactoylglutathione lyase |
| P42704 | LPPRC | Leucine-rich PPR motif-containing protein, mitochondrial25 |
| Q93052 | LPP | Lipoma-preferred partner |
| P40926 | MDHM | Malate dehydrogenase, mitochondrial |
| O95983 | MBD3 | Methyl-CpG-binding domain protein 3 |
| P26038 | MOES | Moesin25 |
| P35579 | MYH9 | Myosin-925 |
| P43490 | NAMPT | Nicotinamide phosphoribosyltransferase |
| P06748 | NPM | Nucleophosmin25 |
| P15531 | NDKA | Nucleoside diphosphate kinase A |
| P30041 | PRDX6 | Peroxiredoxin-6 |
| P30086 | PEBP1 | Phosphatidylethanolamine-binding protein 1 |
| P00558 | PGK1 | Phosphoglycerate kinase 1 |
| Q9Y617 | SERC | Phosphoserine aminotransferase |
| Q15365 | PCBP1 | Poly(rC)-binding protein 1 |
| P26599 | PTBP1 | Polypyrimidine tract-binding protein 1 |
| O60568 | PLOD3 | Procollagen-lysine,2-oxoglutarate 5-dioxygenase |
| P48147 | PPCE | Prolyl endopeptidase |
| Q9UL46 | PSME2 | Proteasome activator complex subunit 2 |
| P61289 | PSME3 | Proteasome activator complex subunit 3 |
| P28070 | PSB4 | Proteasome subunit beta type-4 |
| Q99497 | PARK7 | Protein DJ-1 |
| Q92597 | NDRG1 | Protein NDRG1 |
| P31150 | GDIA | Rab GDP dissociation inhibitor alpha |
| P35241 | RADI | Radixin |
| P46940 | IQGA1 | Ras GTPase-activating-like protein IQGAP125 |
| Q15019 | SEP3 | Septin-225 |
| P34897 | GLYM | Serine hydroxymethyltransferase, mitochondrial |
| P30153 | 2AAA | Serine/threonine-protein phosphatase 2A 65 kDa regulatory subunit A alpha isoform |
| P02768 | ALBU | Serum albumin |
| Q13813 | SPTA2 | Spectrin alpha chain, brain25 |
| P17987 | TCPA | T-complex protein 1 subunit alpha |
| P50991 | TCPD | T-complex protein 1 subunit delta |
| Q99832 | TCPH | T-complex protein 1 subunit eta |
| P50990 | TCPQ | T-complex protein 1 subunit theta |
| P40227 | TCPZ | T-complex protein 1 subunit zeta |
| Q8NBS9 | TXND5 | Thioredoxin domain-containing protein 5 |
| P23193 | TCEA1 | Transcription elongation factor A protein 1 |
| Q92616 | GCN1L | Translational activator GCN125 |
| P23381 | SYWC | Tryptophanyl-tRNA synthetase, cytoplasmic |
| P30085 | KCY | UMP-CMP kinase |
| Q16831 | UPP1 | Uridine phosphorylase 1 |
| P38606 | VATA | V-type proton ATPase catalytic subunit A |
| P21281 | VATB2 | V-type proton ATPase subunit B, brain isoform |
| P49748 | ACADV | Very long-chain specific acyl-CoA dehydrogenase, mitochondrial25 |
| P13010 | XRCC5 | X-ray repair cross-complementing protein 5 |
Table 2.
Proteins identified from proteomic studies with between 2-5 fold enrichments of spectral counts in probe-labeled samples relative to controls. References are included for those proteins that have been identified in other global proteomic studies.
| P61604 | CH10 | 10 kDa heat shock protein, mitochondrial |
| P31946 | 1433B | 14-3-3 protein beta/alpha |
| Q04917 | 1433F | 14-3-3 protein eta |
| P61981 | 1433G | 14-3-3 protein gamma |
| P43686 | PRS6B | 26S protease regulatory subunit 6B |
| P62195 | PRS8 | 26S protease regulatory subunit 8 |
| Q99460 | PSMD1 | 26S proteasome non-ATPase regulatory subunit 1 |
| Q6NVY1 | HIBCH | 3-hydroxyisobutyryl-CoA hydrolase, mitochondrial |
| P62841 | RS15 | 40S ribosomal protein S15 |
| P08708 | RS17 | 40S ribosomal protein S17 |
| P15880 | RS2 | 40S ribosomal protein S2 |
| P23396 | RS3 | 40S ribosomal protein S3 |
| P61247 | RS3A | 40S ribosomal protein S3a |
| P62081 | RS7 | 40S ribosomal protein S7 |
| P46781 | RS9 | 40S ribosomal protein S9 |
| Q5TFE4 | NT5D1 | 5'-nucleotidase domain-containing protein 1 |
| P17858 | K6PL | 6-phosphofructokinase, liver type25 |
| P08237 | K6PF | 6-phosphofructokinase, muscle type |
| P10809 | CH60 | 60 kDa heat shock protein, mitochondrial |
| P46778 | RL21 | 60S ribosomal protein L21 |
| P62750 | RL23A | 60S ribosomal protein L23a |
| P36578 | RL4 | 60S ribosomal protein L4 |
| P46777 | RL5 | 60S ribosomal protein L5 |
| P62424 | RL7A | 60S ribosomal protein L7a |
| P11021 | GRP78 | 78 kDa glucose-regulated protein |
| P24752 | THIL | Acetyl-CoA acetyltransferase, mitochondrial |
| O15144 | ARPC2 | Actin-related protein 2/3 complex subunit 2 |
| O15145 | ARPC3 | Actin-related protein 2/3 complex subunit 3 |
| P55263 | ADK | Adenosine kinase |
| P49588 | SYAC | Alanyl-tRNA synthetase, cytoplasmic |
| P61163 | ACTZ | Alpha-centractin |
| P08133 | ANXA6 | Annexin A6 |
| Q9BZZ5 | API5 | Apoptosis inhibitor 5 |
| Q96P48 | ARAP1 | Arf-GAP with Rho-GAP domain, ANK repeat and PH domain-containing protein 130 |
| P54136 | SYRC | Arginyl-tRNA synthetase, cytoplasmic |
| P08243 | ASNS | Asparagine synthetase [glutamine-hydrolyzing] |
| Q13057 | COASY | Bifunctional coenzyme A synthase |
| P27797 | CALR | Calreticulin |
| O43852 | CALU | Calumenin |
| O00299 | CLIC1 | Chloride intracellular channel protein 125 |
| Q9Y696 | CLIC4 | Chloride intracellular channel protein 4 |
| Q00610 | CLH1 | Clathrin heavy chain 125 |
| P53621 | COPA | Coatomer subunit alpha25 |
| Q07021 | C1QBP | Complement component 1 Q subcomponent-binding protein, mitochondrial |
| P04080 | CYTB | Cystatin-B |
| Q9UHD1 | CHRD1 | Cysteine and histidine-rich domain-containing protein 1 |
| P28838 | AMPL | Cytosol aminopeptidase |
| O00154 | BACH | Cytosolic acyl coenzyme A thioester hydrolase |
| Q96KP4 | CNDP2 | Cytosolic non-specific dipeptidase |
| P54886 | P5CS | Delta-1-pyrroline-5-carboxylate synthase |
| P15924 | DESP | Desmoplakin |
| Q16555 | DPYL2 | Dihydropyrimidinase-related protein 2 |
| P28340 | DPOD1 | DNA polymerase delta catalytic subunit |
| P49736 | MCM2 | DNA replication licensing factor MCM2 |
| P31689 | DNJA1 | DnaJ homolog subfamily A member 1 |
| Q9H223 | EHD4 | EH domain-containing protein25 |
| P13804 | ETFA | Electron transfer flavoprotein subunit alpha, mitochondrial |
| P13639 | EF2 | Elongation factor 2 |
| P49411 | EFTU | Elongation factor Tu, mitochondrial |
| P60842 | IF4A1 | Eukaryotic initiation factor 4A-I |
| P62495 | ERF1 | Eukaryotic peptide chain release factor subunit 1 |
| P60228 | EIF3E | Eukaryotic translation initiation factor 3 subunit E |
| Q15056 | IF4H | Eukaryotic translation initiation factor 4H |
| P63241 | IF5A1 | Eukaryotic translation initiation factor 5A-1 |
| P49327 | FAS | Fatty acid synthase25 |
| O75369 | FLNB | Filamin-B25 |
| P39748 | FEN1 | Flap endonuclease 1 |
| P07954 | FUMH | Fumarate hydratase, mitochondrial |
| P17931 | LEG3 | Galectin-325 |
| Q13630 | FCL | GDP-L-fucose synthase |
| P06396 | GELS | Gelsolin24 |
| O94925 | GLSK | Glutaminase kidney isoform, mitochondrial |
| Q9Y2Q3 | GSTK1 | Glutathione S-transferase kappa 1 |
| P21266 | GSTM3 | Glutathione S-transferase Mu 3 |
| P04406 | G3P | Glyceraldehyde-3-phosphate dehydrogenase25 |
| P11216 | PYGB | Glycogen phosphorylase, brain form |
| P08238 | HS90B | Heat shock protein HSP 90-beta |
| Q14103 | HNRPD | Heterogeneous nuclear ribonucleoprotein D0 |
| P52597 | HNRPF | Heterogeneous nuclear ribonucleoprotein F |
| P61978 | HNRPK | Heterogeneous nuclear ribonucleoprotein K |
| P26583 | HMGB2 | High mobility group protein B2 |
| Q16836 | HCDH | Hydroxyacyl-coenzyme A dehydrogenase, mitochondrial |
| Q9UI26 | IPO11 | Importin-11 |
| P50213 | IDH3A | Isocitrate dehydrogenase [NAD] subunit alpha, mitochondrial |
| P26440 | IVD | Isovaleryl-CoA dehydrogenase, mitochondrial |
| P14923 | PLAK | Junction plakoglobin |
| P08779 | K1C16 | Keratin, type I cytoskeletal 16 |
| P35527 | K1C9 | Keratin, type I cytoskeletal 9 |
| P04264 | K2C1 | Keratin, type II cytoskeletal 1 |
| P35908 | K22E | Keratin, type II cytoskeletal 2 epidermal |
| P33176 | KINH | Kinesin-1 heavy chain25 |
| P07195 | LDHB | L-lactate dehydrogenase B chain |
| Q32MZ4 | LRRF1 | Leucine-rich repeat flightless-interacting protein 1 |
| Q8N1G4 | LRC47 | Leucine-rich repeat-containing protein 47 |
| O60711 | LPXN | Leupaxin |
| P40925 | MDHC | Malate dehydrogenase, cytoplasmic |
| Q9UNF1 | MAGD2 | Melanoma-associated antigen D2 |
| Q15691 | MARE1 | Microtubule-associated protein RP/EB family member 125 |
| P28482 | MK01 | Mitogen-activated protein kinase 125 |
| P60660 | MYL6 | Myosin light polypeptide 625 |
| O00159 | MYO1C | Myosin-Ic |
| P51606 | RENBP | N-acylglucosamine 2-epimerase |
| Q14697 | GANAB | Neutral alpha-glucosidase AB |
| Q15233 | NONO | Non-POU domain-containing octamer-binding protein |
| Q8TAT6 | NPL4 | Nuclear protein localization protein 4 homolog |
| P12270 | TPR | Nucleoprotein TPR |
| Q8WXF1 | PSPC1 | Paraspeckle component 1 |
| P62937 | PPIA | Peptidyl-prolyl cis-trans isomerase A |
| Q13526 | PIN1 | Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
| Q13162 | PRDX4 | Peroxiredoxin-430 |
| Q9Y2H2 | SAC2 | Phosphatidylinositide phosphatase SAC2 |
| Q13492 | PICAL | Phosphatidylinositol-binding clathrin assembly protein24 |
| Q15149 | PLEC | Plectin |
| Q6P2Q9 | PRP8 | Pre-mRNA-processing-splicing factor 8 |
| Q99471 | PFD5 | Prefoldin subunit 5 |
| P02545 | LMNA | Prelamin-A/C |
| P26196 | DDX6 | Probable ATP-dependent RNA helicase DDX6 |
| P28066 | PSA5 | Proteasome subunit alpha type-5 |
| P20618 | PSB1 | Proteasome subunit beta type-1 |
| P49720 | PSB3 | Proteasome subunit beta type-3 |
| P28072 | PSB6 | Proteasome subunit beta type-6 |
| Q99436 | PSB7 | Proteasome subunit beta type-7 |
| P28062 | PSB8 | Proteasome subunit beta type-8 |
| P28065 | PSB9 | Proteasome subunit beta type-9 |
| P30101 | PDIA3 | Protein disulfide-isomerase A3 |
| P07237 | PDIA1 | Protein disulfide-isomerase |
| Q9NUQ9 | FA49B | Protein FAM49B |
| P22061 | PIMT | Protein-L-isoaspartate(D-aspartate) O-methyltransferase |
| Q9NVS9 | PNPO | Pyridoxine-5'-phosphate oxidase |
| P50395 | GDIB | Rab GDP dissociation inhibitor beta |
| P31153 | METK2 | S-adenosylmethionine synthase isoform type-2 |
| P10768 | ESTD | S-formylglutathione hydrolase |
| Q16181 | SEP3 | Septin-725 |
| Q9UHD8 | SEP10 | Septin-925 |
| Q13501 | SQSTM | Sequestosome-1 |
| P63151 | 2ABA | Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B alpha Isoform25 |
| Q15257 | PTPA | Serine/threonine-protein phosphatase 2A activator |
| P62136 | PP1A | Serine/threonine-protein phosphatase PP1-alpha catalytic subunit25 |
| P50454 | SERPH | Serpin H130 |
| Q01082 | SPTB2 | Spectrin beta chain, brain 125 |
| Q15637 | SF01 | Splicing factor 1 |
| Q13435 | SF3B2 | Splicing factor 3B subunit 2 |
| Q15393 | SF3B3 | Splicing factor 3B subunit 3 |
| P26368 | U2AF2 | Splicing factor U2AF 65 kDa subunit |
| P31948 | STIP1 | Stress-induced-phosphoprotein 1 |
| Q9UH65 | SWP70 | Switch-associated protein 7030 |
| Q99536 | VAT1 | Synaptic vesicle membrane protein VAT-1 homolog |
| P78371 | TCPB | T-complex protein 1 subunit beta |
| P48643 | TCPE | T-complex protein 1 subunit epsilon |
| P49368 | TCPG | T-complex protein 1 subunit gamma |
| O95881 | TXD12 | Thioredoxin domain-containing protein 12 |
| Q15645 | TRP13 | Thyroid receptor-interacting protein 13 |
| P20290 | BTF3 | Transcription factor BTF3 |
| Q8WXI9 | P66B | Transcriptional repressor p66-beta |
| Q9Y5L0 | TNPO3 | Transportin-3 |
| P22102 | PUR2 | Trifunctional purine biosynthetic protein adenosine-3 |
| P60174 | TPIS | Triosephosphate isomerase |
| P23381 | SYWC | Tryptophanyl-tRNA synthetase, cytoplasmic |
| P07437 | TBB5 | Tubulin beta chain |
| P04350 | TBB4 | Tubulin beta-4 chain |
| Q6IBS0 | TWF2 | Twinfilin-2 |
| O15042 | SR140 | U2-associated protein SR140 |
| Q93009 | UBP7 | Ubiquitin carboxyl-terminal hydrolase 7 |
| P61088 | UBE2N | Ubiquitin-conjugating enzyme E2 N25 |
| Q04323 | UBXN1 | UBX domain-containing protein 1 |
| P54727 | RD23B | UV excision repair protein RAD23 homolog B |
| P50552 | VASP | Vasodilator |
| Q9NQW7 | XPP1 | Xaa |
Results
Design and synthesis of PIPn headgroup activity-based probes
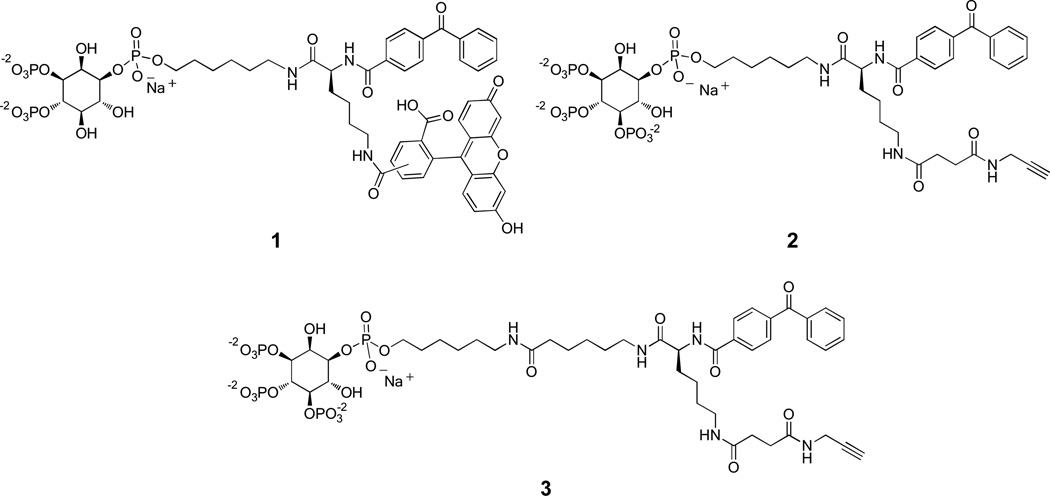
Activity-based probes generally consist of analogs of the natural substrate/ligand of interest in which two groups are introduced: 1) a reactive functional group that allows for the covalent labeling of target proteins; and 2) a secondary tag that enables selective detection and/or purification of proteins that have been successfully labeled by the probe. Activity probes corresponding to ligands that interact with proteins solely through non-covalent binding interactions typically employ photoaffinity tags46 to enforce covalent labeling of target proteins.33,34,47–49 For this purpose, we implemented the benzophenone group due to its robust nature and ease of synthesis. Based on these principles, our designs for activity probes (1−3) based on PIPn headgroups are shown in Figure 1.
Figure 1.
Design of bifunctional PIPn activity-based probes. Probes consist of the binding moiety (PIPn headgroup), linked to a Y-shaped lysine linker containing both a photoaffinity group (benzophone) and a secondary tag that consisted of either fluorescein (probe 1) or an alkyne as a bioorthogonal tag (probes 2–3).
The probes illustrated in Figure 1 contain different secondary tags including a fluorescein moiety (1) for direct in-gel detection of probe-labeled products or alkynes (2,3) as latent bioorthogonal50,51 reactive groups. The alkynes in the latter probes were employed for selective derivatization after protein labeling via click chemistry to introduce rhodamine for in-gel detection or biotin for affinity purification. This approach has proven successful for avoiding disadvantages that can result from initial attachment of bulky reporter tags.42 In order to introduce the two tags into each structure, we exploited lysine as a Y-shaped linker, with the benzophenone introduced at the α-amino group and the secondary tag appended onto the side chain. Probe 3 included a longer linker between the PIPn headgroup and the photoaffinity tag since it is known that this distance affects the magnitude of protein cross-linking. Finally, probes corresponding to both PI(3,4)P2 (1) and PI(3,4,5)P3 (2,3) were developed and studied.
In structures 1−3, the ligand motif consists of the PIPn headgroup linked via the traditional phosphodiester at the 1-position to a shortened hexyl linker attached to the reporter groups. Here, the glycerolipid backbone of the PIPns is excluded so as to present the photoaffinity tag in closer proximity to bound protein targets to promote labeling. Indeed, close proximity of the benzophenone was found to be important for successful protein labeling (see discussion below). While the short hexyl chain provides hydrophobic character to mimic the glycerolipid backbone, these simplified headgroup compounds benefit from water solubility. This circumvents the potential concern that the photoaffinity tag may be buried in the membrane core if full phospholipid analogs were used. In addition, hydrophilic probes were expected to be beneficial for avoiding nonspecific protein binding that often results from hydrophobic interactions. Finally, it is known that many proteins bind simplified soluble PIPn headgroups with high affinity outside of the membrane environment,52–56 which is particularly the case with heavily phosphorylated isomers such as PI(3,4,5)P3. For each of these reasons, we elected to utilize simplified headgroup probes bearing short hydrophobic moieties.
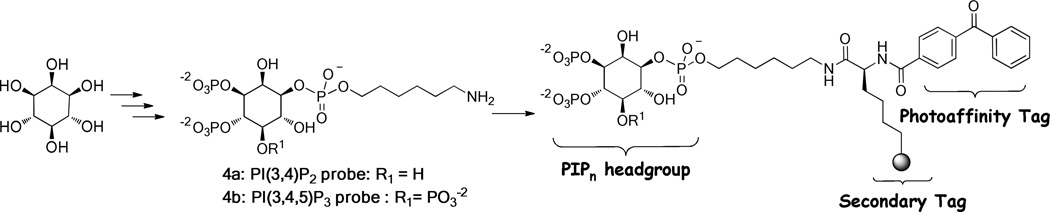
The synthesis of the described activity probes benefited from significant advances in PIPn synthetic strategies in recent years.57–59 We exploited the strategy of using amino-conjugates 60–68 of PIPn headgroups as modular intermediates that can be efficiently derivatized to produce a range of probes through amide bond formation. For this purpose, we recently described the synthesis of aminohexyl headgroup analogs of type 4 (Scheme 1) corresponding to all seven naturally occurring PIPn isomers.36 Using this strategy, probes 1–3 were each synthesized in one step from the appropriate PIPn headgroup-amino-conjugate, either 4a to produce PI(3,4)P2 probe 1 or 4b to generate PI(3,4,5)P3 probes 2 and 3.
Scheme 1.
General synthetic route to PIPn activity probes using headgroup aminoconjugates of type 4.
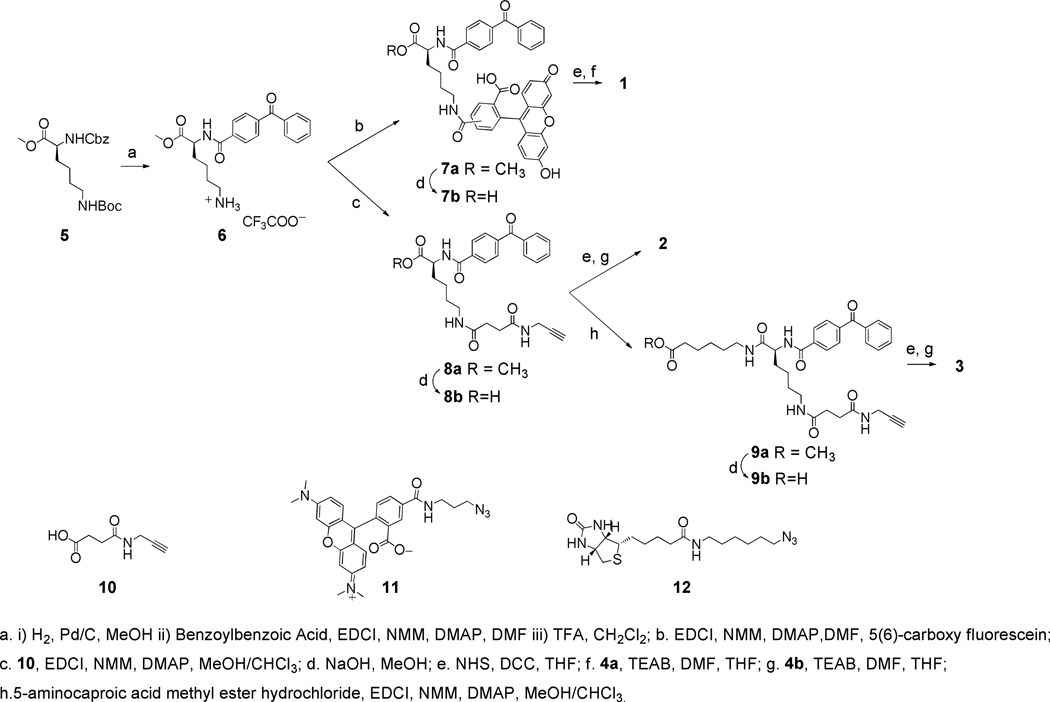
The synthetic routes employed to access the bifunctional lysine handles corresponding to each probe are depicted in Scheme 2. Each of these began with the removal of the carboxybenzyl group (Cbz) of fully protected lysine derivative 5, followed by coupling of the resulting amino group with benzoylbenzoic acid to introduce the benzophenone photoaffinity tag,69 and finally Boc deprotection to common intermediate 6. To produce PI(3,4)P2 probe 1, compound 6 was coupled to 5(6)-carboxy fluorescein to generate 7a, followed by ester hydrolysis to carboxylic acid 7b, which was then converted to the corresponding N-hydroxysuccinimidyl (NHS) ester, and finally coupled with amine 4a. Compounds 2 and 3 were both produced from carboxylic acid 8b, which was generated through coupling of amine 6 to alkynyl-carboxylic acid 10 to 8a, followed by ester hydrolysis. Compound 8b was converted to an NHS ester and coupled to amine 4b to produce PI(3,4,5)P3 probe 2. Finally, probe 3 was accessed by coupling 8b with 6-aminocaproic acid to yield 9a, followed by ester hydrolysis to 9b, and conversion to the corresponding NHS ester, which was then coupled with PI(3,4,5)P3 headgroup-amine conjugate 4b.
Scheme 2.
Synthesis of bifunctional lysine moieties bearing different secondary tags to generate PIPn activity probes 1−3.
Analysis of protein labeling by PIPn activity probes using the PH domain of Akt as a purified protein target
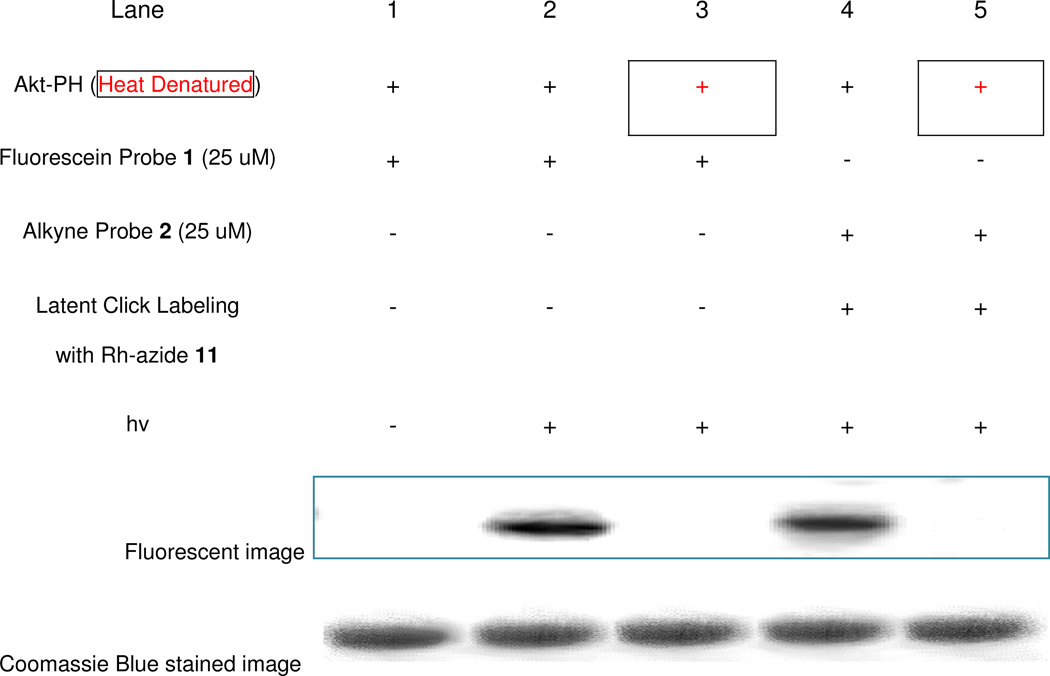
In order to obtain an initial assessment of the efficacy of the described activity probes for covalently labeling cognate protein binding partners, we performed studies employing the PH domain of Akt as a known target for both PI(3,4)P2 and PI(3,4,5)P3 (Figure 2).39,70,71 These initial studies were performed by labeling Akt-PH with probes 1 (lanes 1–3) or 2 (lanes 4–5). Labeling experiments were generally performed as follows: Akt-PH was incubated with probe 1 or 2 for 1 hour at room temperature, followed by irradiation on ice with 365 nm light for 1 hour. Experiments involving probe 1 were then directly subjected to 1D SDS-PAGE for on-gel fluorescence analysis due to the presence of fluorescein in the probe. Samples involving probe 2 utilized an intermediate step after photolysis in which click chemistry was exploited to label the alkyne of this probe through reaction with rhodamine-azide 11, followed by on-gel detection via fluorescence imaging (see section 4 for further details on post-labeling). The results of these experiments showed that Akt-PH was successfully labeled by both PI(3,4)P2−fluorescein probe 1 (Lane 2) and PI(3,4,5)P3−alkyne probe 2 (Lane 4). Please note that a color version of the fluorescence gel image in Figure 1 is also included as Figure S1 in the supplementary information.
Figure 2.
Gel images of labeling studies using purified Akt-PH (fluorescent gel image shown in grey scale). Studies indicated successful labeling of Akt-PH protein by both fluorescein-probe 1 (lane 2) and alkyne-probe 2 (lane 4, after click chemistry post-derivatization) during studies. Additionally, control studies involving no photo-cross-linking (lane 1), or heat denaturation of the protein prior to probe incubation (lanes 3 and 5) yielded no fluorescence, indicating the absence of non-specific labeling. Finally, Coomassie Blue stains indicate that the protein is still present despite the abrogation of probe labeling. Please also see color fluorescence gel scans in Figure S1 of the supplementary information.
A potential concern in any cross-linking experiment is that labeling may result from non-specific interactions of the probe with proteins that are not cognate binding partners. This is particularly the case with photoaffinity tags, which generate highly reactive intermediates upon photolysis. As such, control experiments are important for demonstrating whether labeling events are driven by specific binding interactions. One control study we performed to further scrutinize binding involved the initial heat denaturation of the protein target prior to probe incubation, which should abrogate labeling due to the loss of the protein binding domain upon destruction of its higher order structure. As seen in Figure 2, for both probe 1 (lane 3) and probe 2 (lane 5), initial heat denaturation of Akt-PH negated protein labeling, as was expected. Probe 1 was subjected to an additional control in which irradiation was not performed (lane 1), which also led to no protein labeling. These results demonstrate that the labeling of Akt-PH involves specific binding and that the photoaffinity tag is critical for on-gel detection of protein labeling.
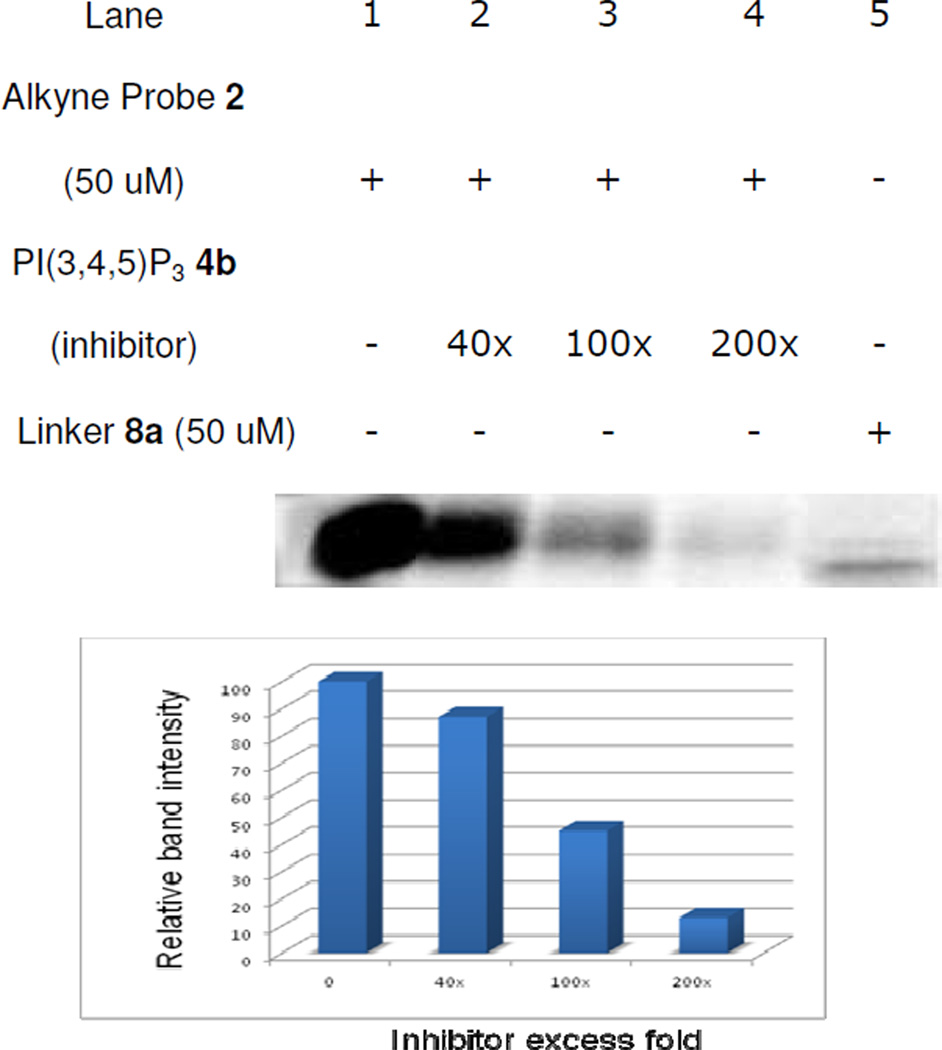
Further control studies designed to verify that the PIPn headgroup is responsible for protein labeling involved competition binding using soluble unlabeled PI(3,4,5)P3 headgroup analog 4b and probe 2. As shown in Figure 3, competition was observed with 40 times excess of inhibitor 4b and increased with larger concentrations of this competitor up to 200 times excess, which abrogated most of the labeling event (lanes 1–4). Once again, Coomassie Blue stains showed that the protein was still present in each lane (not shown). It is likely that high competitor concentrations are required since probe 2 has a competitive advantage in that it yields irreversible covalent protein labeling, while competitor 4b acts through reversible non-covalent binding. Finally, to rule out the possibility that the bifunctional lysine tag can label protein on its own, compound 8a lacking a PIPn headgroup was also tested for protein labeling (lane 5). Following incubation, cross-linking, and click chemistry with the rhodamine reporter tag, the result showed no labeling of Akt-PH, although it appears that a small impurity in the protein sample just below Akt-PH is labeled in this case. It is possible that 8a might lead to enhanced non-specific labeling due to the hydrophobicity of this compound, leading it to insert into hydrophobic pockets on random proteins. Indeed, it is plausible that probes 1 and 2 would avoid non-specific labeling events, as was observed through the control studies described herein, due to their hydrophilic nature.
Figure 3.
Competition studies involving Akt-PH labeling (fluorescent gel image shown in grey scale). In lanes 1–4, pre-incubation of Akt-PH with excess inhibitor 4b suppressed labeling by probe 2 as visualized by a reduction in fluorescence intensity of the AKt-PH band. In lane 5, the bifunctional lysine lacking the PIP headgroup did not label the Akt protein.
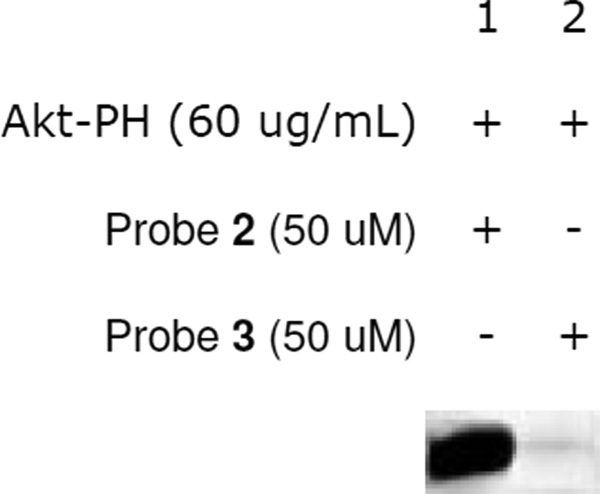
It has previously been observed that the nature of the linker between the ligand motif and a photoaffinity tag can affect the efficiency of protein labeling, in some cases leading to dramatic variations.47,48,72 In particular, differences in linker lengths can cause noticeable discrepancies in protein labeling. To explore how linker length affected the current studies, we compared probes 2 and 3, the latter containing a longer linker, in terms of the labeling of Akt-PH (Figure 4). Here, we found that probe 3 exhibited virtually no labeling of Akt-PH (lane 2) when compared to probe 2. Once again, Coomassie Blue stains showed that the protein remains present in each lane (not shown). These results can be rationalized as follows: while the longer linker provides more flexibility for the bifunctional lysine motif, the benzophenone photoaffinity group can only react with proteins in close proximity, and this flexibility may provide too much freedom such that the benzophenone does not spend enough time proximal to the bound protein. These studies again show that the linker between the ligand moiety and photoaffinity tag is critical for effective protein labeling.
Figure 4.
Akt-PH labeling studies with probes 2 and 3 indicate that the shorter linker (probe 2) results in significantly enhanced protein labeling (fluorescent gel image shown in grey scale).
Labeling of proteins in cell extracts using PIPn probes
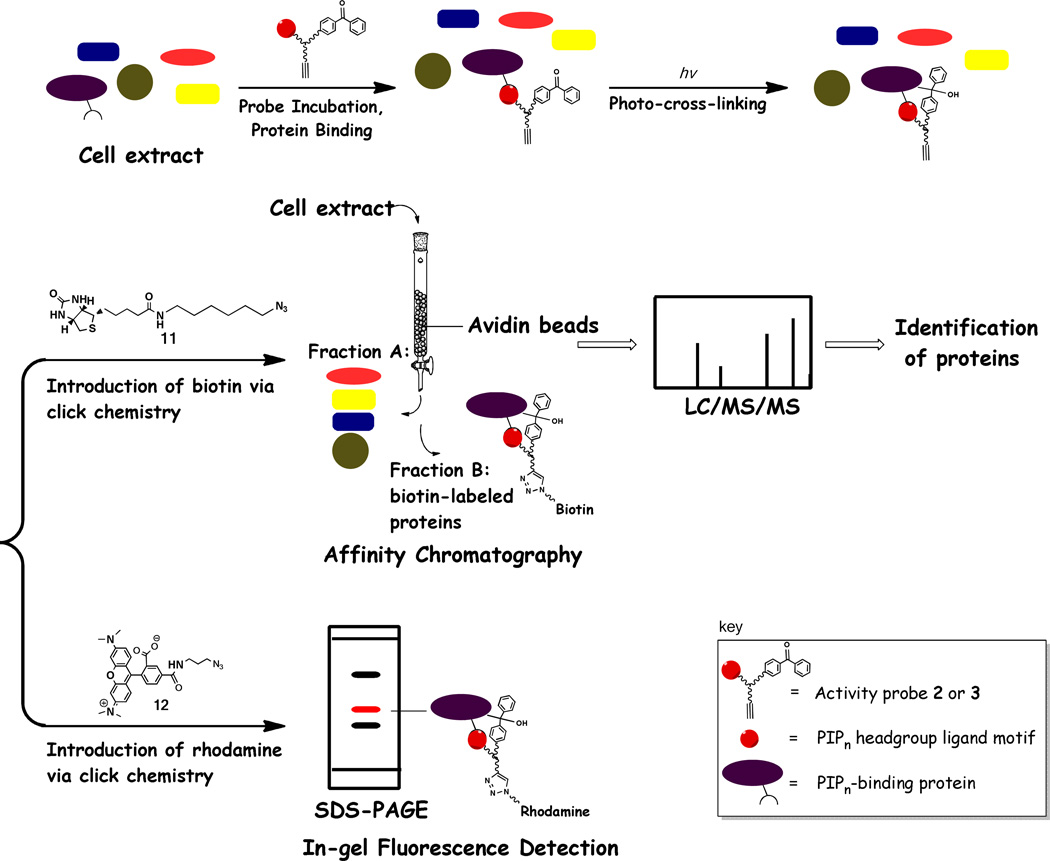
Following initial studies involving the labeling of purified Akt-PH protein, we moved on to more complex studies employing cell extracts. The strategy underlying these experiments is depicted in Figure 5, and probe 2 was generally used for studies since click chemistry allows for post-labeling with different reporter groups and this compound was more effective in protein labeling than probe 3. First, cell lysates were incubated with the probe to allow for binding of cognate protein binding partners via non-covalent interactions. Next, irradiation with 360 nm light was performed to capture these binding events through covalent cross-linking between the probe and bound proteins. The appropriate tag (rhodamine for in-gel visualization or biotin for affinity purification) was then appended to the protein-probe adducts via click chemistry. We specifically chose cancer cell extracts for studies due to the involvement of PIPn signaling in tumorigenesis.
Figure 5.
Illustration of the mechanisms of protein labeling and subsequent analysis using alkynyl-PI(3,4,5)P3 activity probes 2 and 3. Following the incubation of the probes with cell extracts, protein−probe binding events were captured through irradiation of the benzophenone tag. Next, labeled proteins were selectively derivatized via click chemistry using the secondary functional tag of the probe to introduce rhodamine (11) for in-gel fluorescence analysis or biotin (12) for protein enrichment via affinity chromatography and subsequent protein identification using liquid chromatography-tandem mass spectrometry.
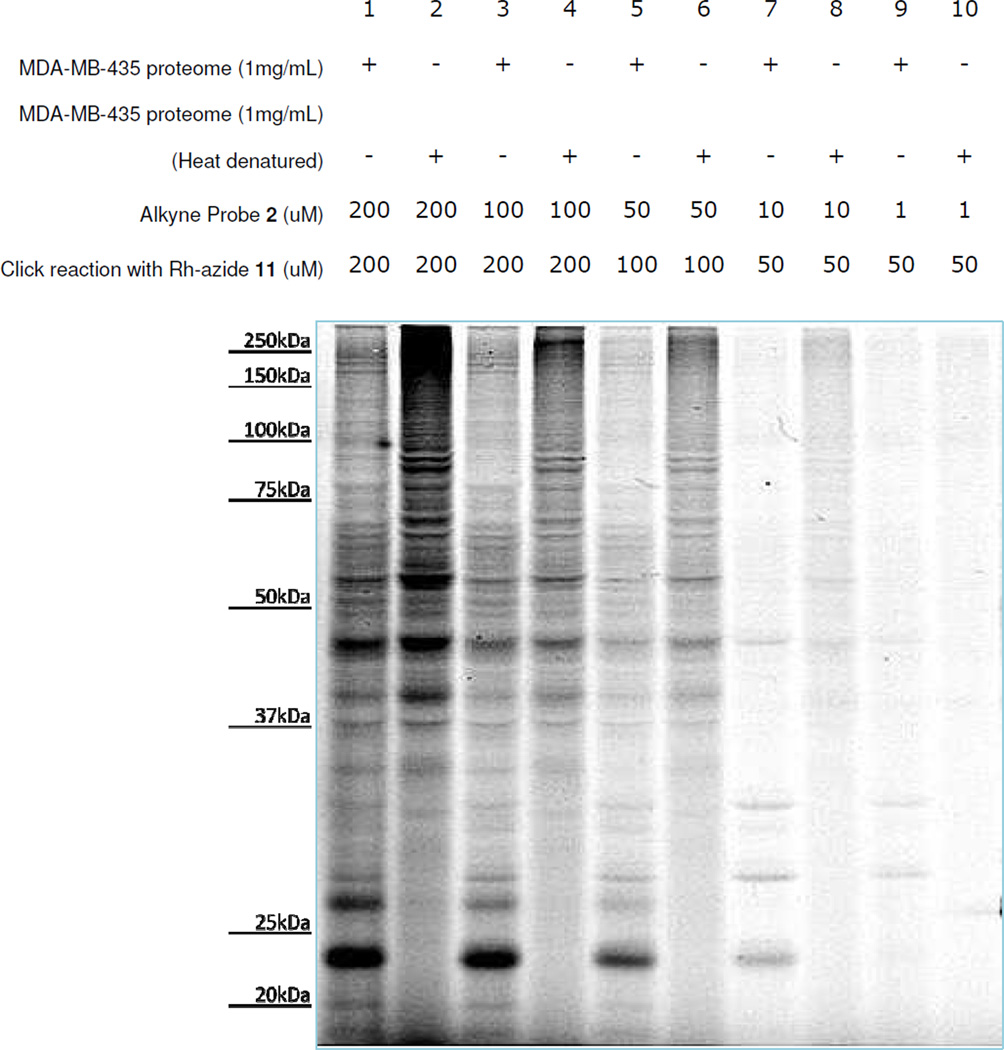
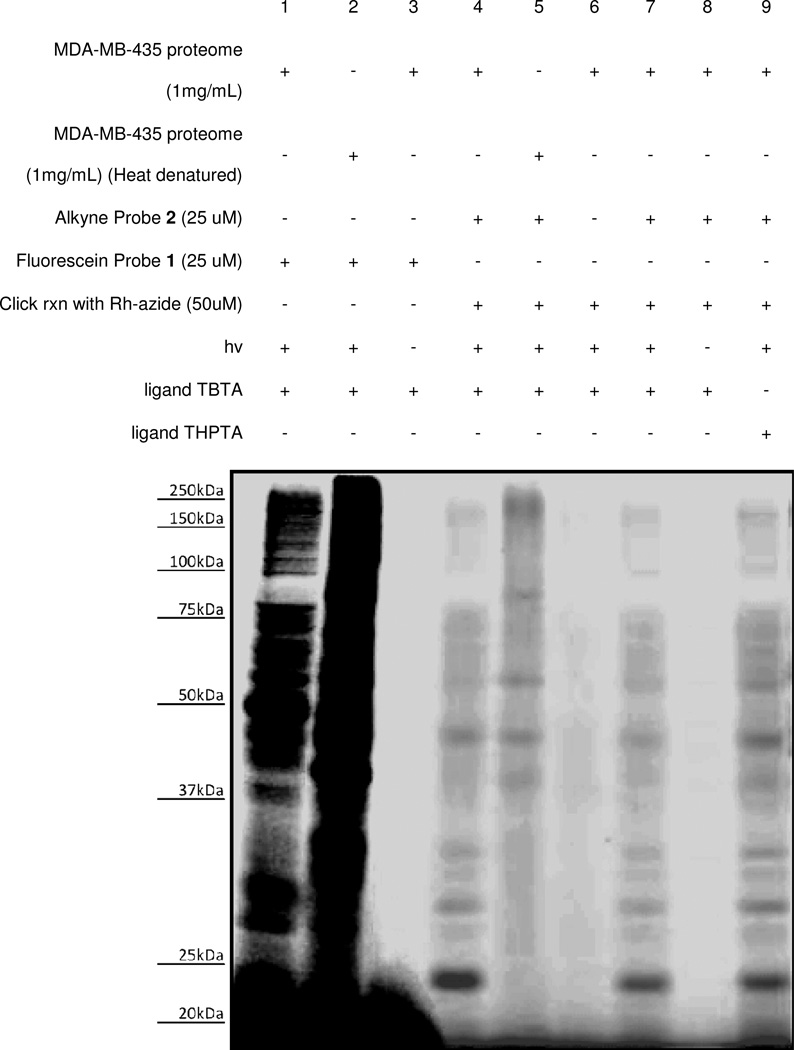
Cell extract labeling experiments began by studying a soluble proteome preparation of the human melanoma cell line MDA-MB-435 using various concentrations of probe and azido-rhodamine 11 Experiments were conducted following the protocol outlined in Figure 5 with heat-denatured proteome samples used as controls. The resulting gel showed that a number of fluorescence-labeled protein bands were present (Figure 6). In the heat-denatured controls, certain bands were found to show diminished labeling, particularly in the low mass range. It is also worthwhile to mention that some of the bands were enriched in heat-denatured samples, particularly in the higher mass range. However, it is often difficult to judge whether these enriched proteins were the same ones that were labeled in the regular sample. Images of coomassie blue stained gels showed identical bands for all lanes (now shown), indicating the presence of the same amount of proteins in each sample. From these results, probe concentrations of 25 or 50 micromolar were selected for each of the following labeling studies to optimize the balance between high-intensity labeling and low-intensity background. Please note that a color version of the fluorescence gel image in Figure 6 is also included as Figure S2 in the supplementary information.
Figure 6.
Labeling studies to ascertain effective probe concentrations (fluorescent gel image shown in grey scale). Probe concentrations were screened using MDA-MB-435 cancer cell extracts (soluble fraction) to identify concentrations that were effective for studies. Concentrations ranging from 200 nM to 1nM were used to label the same amount of the cell extracts with heat-denatured controls performed for each protein concentration. From fluorescence gel imaging results, probe concentrations of approximately 50 µM (lanes 5 and 6) were selected for other labeling studies. Please also see color fluorescence gel scans in Figure S2 of the supplementary information.
Next, both alkyne-labeled probe 2 and fluorescein-labeled probe 1 were used for labeling studies with human MDA-MB-435 soluble proteome (Figure 7). Here, studies involving fluorescein-labeled probe 1 (lane 1) clearly resulted in higher background labeling in the heat denatured control (lane 2) when compared to studies using alkyne probe 2 (lane 4 versus lane 5 (heat denatured)). It is likely that the enhancement in the labeling of heat denatured samples using probe 1 results from non-specific interactions caused by the presence of fluorescein moiety during the protein labeling event. In the heat denatured control involving probe 2 (lane 5), while a number of bands were observed to be weaker compared to the corresponding normal study (lane 4), some enhanced signals likely attributable to non-specific labeling were again observed in the heat denatured sample. Further controls including the absence of probe 2 (lane 6) and lack of irradiation (lane 8) once again demonstrated that both the probe and photocross-linking steps are necessary for protein labeling and on-gel detection. Finally, we analyzed different conditions for post-labeling via click chemistry during studies. It is known that copper-chelating ligands enhance click chemistry reactions, and two different ligand additives were analyzed herein. As with previously described experiments, lanes 1–8 utilized the ligand TBTA73 to enhance the click reaction. We compared these results to those with THPTA (compound 18, supporting information) a ligand with enhanced water solubility74 (lane 9), and found that the protein labeling results were quite similar using the different ligands. As a result, TBTA was employed in subsequent labeling studies since it is commercially available. Please note that a color version of the fluorescence gel image in Figure 6 is also included as Figure S3 in the supplementary information.
Figure 7.
Labeling of MDA-MB-435 soluble proteome using various conditions (fluorescent gel image shown in grey scale). Studies using fluorescein-probe 1 resulted in significantly stronger labeling in the heat denatured control (lane 2) compared to the normal study (lane 1), indicating that the presence of the fluorophore during labeling is problematic. Studies using probe 2 and post-labeling led to diminished labeling in heat-the denatured control (lane 5, when compared to lane 4). No probe (lane 6) and no light (lane 8) controls negated labeling, and click chemistry ligands TBTA (lane 7) and THPTA (lane 9) yielded similar results. Please also see color fluorescence gel scans in Figure S3 of the supplementary information.
Next, we analyzed multiple cancer cell proteomes (MDA-MB-435, MDA-MB-231, MCF7, and T47D) to test the general applicability of this strategy. Both the soluble and membrane fractions of each of these cell extracts were tested and compared using both alkyne probe 2 as well as fluorescein probe 1, along with heat denatured proteomes (See fluorescent gel image in Figure S4 of the Supplementary Material). Once again, fluorescein-probe 1 yielded significantly enhanced labeling in heat denatured controls, implicating post-labeling as the more effective approach for analysis. The results also demonstrated that the majority of protein labeling occurred with the soluble portion of the proteomes, which is consistent with the fact that many PIPn-binding proteins are soluble peripheral proteins that only interact with the membrane surface upon PIPnbinding. This result is further influenced by the soluble nature of the probes. Finally, it should be noted that probes 1 (PI(3,4)P2) and 2 (PI(3,4,5)P3) correspond to different PIPn headgroup isomers, and thus it would be expected that they would label a different but overlapping set of proteins.
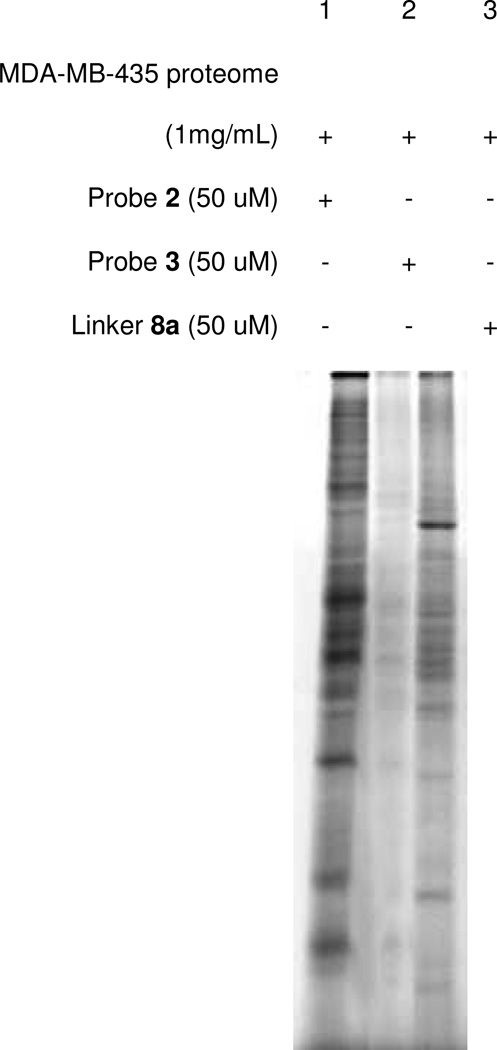
Using cell extract studies, we once again addressed the issue of the effect of linker length on protein labeling by comparing probes 2 and 3. As is seen in Figure 8, the results of these experiments mirrored those of Figure 3 in that probe 3 bearing a longer linker yielded minimal labeling compared to 2. These results further confirm the importance of linker length in probe design. Additionally, bifunctional lysine analog 8a lacking a PIPn headgroup again appeared to label different proteins than probe 2, which is likely attributable to non-specific binding resulting from the hydrophobicity of this compound.
Figure 8.
Analysis of the effect of probe linker length using MDA-MB-435 cancer cell extracts (fluorescent gel image). Labeling studies with probe 2 (shorter linker) yielded significantly enhanced labeling compared to probe 3. Control compound 8a lacking the PIPn headgroup yielded minimal protein labeling of an orthogonal subset of proteins.
Proteomic analysis for probe-based characterization of labeled proteins using MDA-MB-435 cancer cell extracts
The initial phases of this research demonstrated that PIPn activity probes are effective for labeling of protein binding targets. Following these results, we set out to apply these probes for proteomic analysis using MDA-MB-435 cell extracts to characterize protein-binding targets. Due to its effectiveness in prior protein labeling studies alkyne-PI(3,4,5)P3 probe 2 was selected for proteomics experiments, which were performed as described in Figure 5. Cell extracts were incubated with probe 2 and irradiated as described previously, followed by reaction with azido-biotin 12. Subsequently, biotinylated probe-labeled proteins were separated from unlabeled proteins through strepavidin-based affinity chromatography. Enriched proteins were then digested into peptides using trypsin, and the resulting peptide mixture was analyzed by multidimensional liquid chromatography tandem mass spectrometry (LC/LC-MS/MS) using Multidimensional Protein Identification Technology (MudPIT) technique.45 Peptide sequences were determined using the SEQUEST algorithm75 and organized and matched to the parent proteins using DTASelect.76 Spectral counting, a semi-quantitative measure of relative protein abundance, was used in this study to determine proteins enriched in the probe labeled samples.43,77,78 Additionally, control studies using lysine linker 8a lacking PIPn headgroup or lacking any probe were performed side-by-side with these analyses to determine background enrichments.
From these proteomic experiments, 265 proteins were detected with significantly increased spectral counts in probe-labeled samples compared to controls. In these studies, three probe labeled samples were run, and only those proteins that were observed in at least two of these runs and yielded at least two unique spectral counts are included. In addition, proteins that are known to be endogenously biotinylated, such as methylcrotonoyl-CoA carboxylase, were discounted. The resulting proteins are classified based on the average number of spectral counts relative to controls in Table 1 (≥5 fold above control, 97 proteins) and Table 2 (2–5 fold above controls, 168 proteins). While 2–5 fold enrichments in probe-labeled samples (proteins in Table 2) represent relatively low signals, it should be noted that a number of proteins that have previously been implicated as PI(3,4,5)P3 binders appear in Table 2, as is further discussed below. This provides evidence that 2–5 fold enrichments are sufficient for identifying putative targets. Furthermore, modest enrichments are likely attributable to the low efficiencies commonly observed in photoaffinity labeling events, low expression of certain protein targets, and/or non-specific binding of proteins during streptavidin enrichment.
Of the total of 265 proteins that were oberved, many have been identified as PI(3,4,5)P3-binding proteins in previous reports, including affinity chromatography studies. For example, 38 proteins were among the PI(3,4,5)P3 binding proteins identified by Catimel and co-workers,25 4 proteins coincide with those detected by Dixon and coworkers, 30 and 2 proteins were also identified by Pasquali and coworkers.24 Proteins that were previously observed in global proteomics screens are indicated with citations in Tables 1 and 2. In 2002, Krugmann and co-workers identified ARAP3 as a novel PI(3,4,5)P3 binding receptor,22 while, in our studies, a protein from the same family, ARAP1, was identified, which was also observed by Dixon and co-workers.30 Similary, while Dixon and co-workers reported Eukaryotic translation initiation factor 4E, we found Eukaryotic translation initiation factors 3E, 4H, 5A and 6. Furthermore, a number of proteins that were detected contain conserved binding modules that are known to target PIPns. These included proteins bearing Calponin Homology (CH) (α-actinin-1 and 4, Filamin-A and B, MARE1, Plectin, IQGA1, β-Spectrin-1), Pleckstrin Homology (PH) (ARAP1, β-Spectrin-1, and SWP70), Septin (Septins 2, 7, and 9), 4.1 Protein Ezrin Radixin Moesin (FERM) (moesin, radixin), Huntington Elongation Factor 3 PR65/A TOR (HEAT) (2AAA, GCN1L), Clathrin (Clathrin heavy chain 1) and Annexin (Annexins A1 and A2) domains. One issue with the current study that may or not be general is that a number of known PI(3,4,5)P3-binding proteins were not observed. This could result from multiple factors that are detailed in the discussion section below.
In addition to known binding properties and domains, the identified proteins also possess many common functional themes, which are again in general agreement with the conserved roles that have emerged from other PIPn-binding studies. For example, PI(3,4,5)P3 has commonly been implicated in the regulation of the actin cytoskeleton, and indeed the current studies yielded at least 12 actin-binding proteins, such as gelsolin, twinfilin-2, β-spectrin, fructose-bisphosphate aldolase A, plectin, radixin, myosins and filamins. Tables 1 and 2 also include at least 55 proteins involved in ATP-binding (21%), 7 proteins involved in GTP-binding (Dynamin-1 like protein, ERF3B, EF1A3, Septins 2, 7 and 9), 9 proteins involved in protein transport (α-coatamer, IF5A1, Exportin-2, Importin-11, RAN, Myosin-Ic, Nucleophosmin, Sequestosome-1, and Transportin-3), 3 proteins involved in cell adhesion (leupaxin, PLAK, LPP), and 3 proteins involved in signal transduction (Filamin-B, Leupaxin, 2ABA). Finally, at least 86 of the labeled proteins participate in protein binding (32%), and many common enzymatic activities were present, including at least 29 hydrolases, 19 transferases, 13 oxidoreductases, 14 kinases, 9 isomerases, and 5 lyases.
Another point of interest involves the dependence of protein labeling results on the particular system that is being analyzed. In this case, the fact that studies were performed using a cancer cell line may lead to certain processes, such as actin skeleton regulation, being particularly represented due to disease-related upregulation. In future studies, we will seek to exploit the ability to use ABPP to identify proteomic variations by comparing profiles of normal cells with those associated with a particular conditions, which has previously been performed for cancer,37,79–81 malaria82,83, and obesity84 cell lines. Finally, proteins detected during the current studies that were not previously known to bind PI(3,4,5)P3 offer putative new targets for this ligand. As is always the case with global proteomic approaches, further work is necessary to confirm specific binding interactions, but these techniques provide a highly efficient avenue for uncovering prospective targets. Furthermore, certain putative targets exhibit important ramifications for future studies. For example, in the current studies, the labeling of PARK7, associated with Parkinson’s disease, opens up interesting possibilities and could be scrutinized further.
Discussion
In this study, we designed, synthesized and applied bifunctional activity-based probes corresponding to PIPns to label and identify protein targets of these critical ligands. Probes consisting of PIPn headgroup analogs decorated with both a photoaffinity tag for cross-linking to bound proteins and a secondary tag for subsequent analysis were found to be effective for labeling protein targets. In particular, the use of an alkyne secondary tag followed by click chemistry provided a versatile approach for both on-gel fluorescence detection and biotin-based affinity purification that also minimized non-specific protein labeling. The latter problem is likely caused by the bulky reporter groups that are present within the probe structure when click chemistry is not used for post-labeling. Initial studies involving the purified PH domain of Akt yielded effective labeling of this protein that was abrogated using competition binding, heat denaturation, no probe and no light controls, indicating specific labeling resulting from a discrete binding interaction. The study of probes containing different linker lengths between the PI(3,4,5)P3 headgroup and bifunctional tag indicated that a shorter linker (probe 2) resulted in greatly enhanced protein labeling in this particular case. While this does not necessarily mean that the linker length in this probe is optimal, it does clearly show that the spacing of the photoaffinity group is critical for subsequent protein labeling. In fact, the ideal spacer length would likely vary depending upon the specific 3-dimensional structure of the protein target and the proximity of residues. Nevertheless, there should be a range of distances in which cross-linking is feasible before the distance and flexibility of the photoaffinity tag becomes too great for significant protein labeling.
Following optimization of labeling studies and controls using cancer cell extracts, probe 2 was exploited for proteomic analysis for collective identification of protein targets from these samples, which resulted in the identification 265 proteins. The inclusion of previously known PI(3,4,5)P3-binding targets, proteins bearing conserved PIPn-binding domains, and many proteins involved in processes associated with PIPns provides evidence that this approach is effective for detection of specific protein targets. As a result, the other proteins that were observed represent solid candidate target effectors for further study. It should also be noted that the described probe strategy may also pull down proteins that bind the soluble inositol phosphates (InsPs).85,86 In this case, probe 2 could also be considered an analog of inositol-(1,3,4,5)-tetrakisphosphate, in which the phosphate at the 1-position has been modified for linkage via a phosphodiester, a linkage strategy has previously been pursued to develop probes corresponding to the InsPs.60–68 Ultimately, the binding properties of InsPs and PIPns are often difficult to differentiate due to their similar structures, and indeed members from each family have been observed to compete for the same protein binding targets.87–90
One issue with the current study is that a number of known PI(3,4,5)P3-binding proteins were not observed. This could be due to a number of factors including the nature of the probe structures employed for studies, challenges associated with the cross-linking of sufficient quantities of protein for detection, and the use of cell extracts for experiments. One aspect that will affect the proteins that are observed pertains to the structure of the probe that is employed for protein labeling. Previous work has led to the categorization of PIPn-binding domains based on binding mode, as some proteins bind predominately to the phosphorylated myo-inositol headgroup (S-type proteins), others interact with the interfacial membrane region (I-type proteins), and finally a third binding mode involves substantial penetration of the protein into the hydrophobic membrane core (H-type proteins).4 Since probe 2 corresponds to a soluble headgroup analog, it would be expected that this compound would be more effective at labeling S-type proteins than H-type proteins. This effect can be seen in the data since proteins bearing domains that primarily target headgroups, such as the S-type ANTH and PH modules, were found, while those requiring membrane penetration, such as the H-type ENTH and PX domains, were not.
As discussed previously, soluble activity probes were expected to be beneficial for protein labeling studies to present the photoaffinity tag proximal to bound proteins and avoid non-specific hydrophobic interactions. While this approach may limit the labeling of proteins that require the membrane environment for binding, it will be challenging to devise a single probe structure that will be effective for labeling the majority of PIPn-binding proteins due to their diverse binding modes. Instead, multiple probes with different structural features will likely be necessary. The need for different structural probes has already been observed in affinity chromatography studies, where isolated beads and those associated with liposomes were found to pull out different and only mildly overlapping sets of proteins.25 In future studies, we will pursue different probe structures to understand the effect this has on proteins that are labeled. Since PIPn-binding proteins exhibit broad variation in binding mode in terms of headgroup/membrane binding, localization, multivalency, and requirements for other ligands, diverse probe strategies will likely be necessary to fully elucidate the complex scope of binding targets.
Successful photolabeling of proteins relies on a number of factors including the amount of protein that is effectively labeled, the efficiency of cross-linking and the placement of the photoaffinity tag in close proximity to residues on the bound protein. Low abundance proteins are challenging to detect, which is compounded by the low cross-linking efficiencies commonly observed with photoaffinity groups. Furthermore, proteins should only be labeled when they are in the form in which ligand binding is activated, and lipid-binding proteins are tightly regulated in both a spatial and temporal manner. In addition, the ideal placement of the photoaffinity tag will likely vary for each protein target depending upon the 3-dimensional arrangement of nearby residues in the binding domain. This represents a more general rationale as to why it is unlikely that a single probe structure would be equally effective for labeling all target proteins. While this approach may not pull out every target, it nevertheless provides an efficient means for identifying a set of putative protein targets from highly complex samples. Finally, studies performed using cell extracts may not be ideal since they do not take place in the native environment inside the cell. A primary benefit of ABPP is that this strategy is amenable to analysis using live cells and organisms. Now that these initial PIPn activity probes have proven effective for labeling and identifying target proteins, we are currently working on modifying probes and conditions to undertake studies in living samples.
Supplementary Material
Footnotes
Supplemental Information
Supplemental Information includes procedures, characterization data, and color gel images. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Best MD, Zhang HL, Prestwich GD. Inositol polyphosphates, diphosphoinositol polyphosphates and phosphatidylinositol polyphosphate lipids: Structure, synthesis, and development of probes for studying biological activity. Nat. Prod. Rep. 2010;27:1403–1430. doi: 10.1039/b923844c. [DOI] [PubMed] [Google Scholar]
- 2.Conway SJ, Miller GJ. Biology-enabling inositol phosphates, phosphatidylinositol phosphates and derivatives. Nat. Prod. Rep. 2007;24:687–707. doi: 10.1039/b407701f. [DOI] [PubMed] [Google Scholar]
- 3.Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat. Rev. Mol. Cell Biol. 2008;9:99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 4.Cho W, Stahelin RV. Membrane-protein interactions in cell signaling and membrane trafficking. Annu. Rev. Biophys. Biomol. Struct. 2005;34:119–151. doi: 10.1146/annurev.biophys.33.110502.133337. [DOI] [PubMed] [Google Scholar]
- 5.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 6.Pendaries C, Tronchere H, Plantavid M, Payrastre B. Phosphoinositide signaling disorders in human diseases. FEBS Lett. 2003;546:25–31. doi: 10.1016/s0014-5793(03)00437-x. [DOI] [PubMed] [Google Scholar]
- 7.Vicinanza M, D'Angelo G, Di Campli A, De Matteis MA. Phosphoinositides as regulators of membrane trafficking in health and disease. Cell. Mol. Life Sci. 2008;65:2833–2841. doi: 10.1007/s00018-008-8353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wymann MP, Schneiter R. Lipid signalling in disease. Nat. Rev. Mol. Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 9.Assinder SJ, Dong QH, Kovacevic Z, Richardson DR. The TGF-beta, PI3K/Akt and PTEN pathways: established and proposed biochemical integration in prostate cancer. Biochem. J. 2009;417:411–421. doi: 10.1042/BJ20081610. [DOI] [PubMed] [Google Scholar]
- 10.Duronio V. The life of a cell: apoptosis regulation by the PI3K/PKB pathway. Biochem. J. 2008;415:333–344. doi: 10.1042/BJ20081056. [DOI] [PubMed] [Google Scholar]
- 11.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 12.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–414. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stokoe D, Stephens LR, Copeland T, Gaffney PRJ, Reese CB, Painter GF, Holmes AB, McCormick F, Hawkins PT. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 16.Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: Implications for development, immunity, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- 17.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 18.Samuels Y, Wang ZH, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell DM, Riggins GJ, Willson JKV, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554–554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 19.Chow LML, Baker SJ. PTEN function in normal and neoplastic growth. Cancer Lett. 2006;241:184–196. doi: 10.1016/j.canlet.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 20.Rao VR, Corradetti MN, Chen JA, Peng JR, Yuan JY, Prestwich GD, Brugge JS. Expression cloning of protein targets for 3-phosphorylated phosphoinositides. J. Biol. Chem. 1999;274:37893–37900. doi: 10.1074/jbc.274.53.37893. [DOI] [PubMed] [Google Scholar]
- 21.Painter GF, Thuring JW, Lim ZY, Holmes AB, Hawkins PT, Stephens LR. Synthesis and biological evaluation of a PtdIns(3,4,5)P-3 affinity matrix. Chem. Commun. 2001:645–646. [Google Scholar]
- 22.Krugmann S, Anderson KE, Ridley SH, Risso N, McGregor A, Coadwell J, Davidson K, Eguinoa A, Ellson CD, Lipp P, Manifava M, Ktistakis N, Painter G, Thuring JW, Cooper MA, Lim ZY, Holmes AB, Dove SK, Michell RH, Grewal A, Nazarian A, Erdjument-Bromage H, Tempst P, Stephens LR, Hawkins PT. Identification of ARAP3, a novel PI3K effector regulating both Arf and Rho GTPases, by selective capture on phosphoinositide affinity matrices. Mol. Cell. 2002;9:95–108. doi: 10.1016/s1097-2765(02)00434-3. [DOI] [PubMed] [Google Scholar]
- 23.Lim ZY, Thuring JW, Holmes AB, Manifava M, Ktistakis NT. Synthesis and biological evaluation of a PtdIns(4,5)P-2 and a phosphatidic acid affinity matrix. J. Chem. Soc. Perkin Trans. 2002;1:1067–1075. [Google Scholar]
- 24.Pasquali C, Bertschy-Meier D, Chabert C, Curchod ML, Arod C, Booth R, Mechtler K, Vilbois F, Xenarios I, Ferguson CG, Prestwich GD, Camps M, Rommel C. A chemical proteomics approach to phosphatidylinositol 3-kinase signaling in macrophages. Mol. Cell. Proteomics. 2007;6:1829–1841. doi: 10.1074/mcp.T600066-MCP200. [DOI] [PubMed] [Google Scholar]
- 25.Catimel B, Yin MX, Schieber C, Condron M, Patsiouras H, Catimel J, Robinson D, Wong LSM, Nice EC, Holmes AB, Burgess AW. PI(3,4,5)P3 Interactome. J. Proteome Res. 2009;8:3712–3726. doi: 10.1021/pr900320a. [DOI] [PubMed] [Google Scholar]
- 26.Catimel B, Schleber C, Condron M, Patsiouras H, Connolly L, Catimel J, Nice EC, Burgess AW, Holmes AB. The PI(3,5)P2 and PI(4,5)P2 Interactomes. J. Proteome Res. 2008;7:5295–5313. doi: 10.1021/pr800540h. [DOI] [PubMed] [Google Scholar]
- 27.Conway SJ, Gardiner J, Grove SJA, Johns MK, Lim Z-Y, Painter GF, Robinson DEJE, Schieber C, Thuring JW, Wong LS-M, Yin M-X, Burgess AW, Catimel B, Hawkins PT, Ktistakis NT, Stephens L, Holmes AB. Synthesis and biological investigation of phosphatidylinositol phosphate affinity probes. Org. Biomol. Chem. 2010;8:66–76. doi: 10.1039/b913399b. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka K, ImajohOhmi S, Sawada T, Shirai R, Hashimoto Y, Iwasaki S, Kaibuchi K, Kanaho Y, Shirai T, Terada Y, Kimura K, Nagata S, Fukui Y. A target of phosphatidylinositol 3,4,5-trisphosphate with a zinc finger motif similar to that of the ADP-ribosylation-factor GTPase-activating protein and two pleckstrin homology domains. Eur. J. Biochem. 1997;245:512–519. doi: 10.1111/j.1432-1033.1997.00512.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang DS, Ching TT, St Pyrek J, Chen CS. Biotinylated phosphatidylinositol 3,4,5-trisphosphate as affinity ligand. Anal. Biochem. 2000;280:301–307. doi: 10.1006/abio.2000.4525. [DOI] [PubMed] [Google Scholar]
- 30.Dixon MJ, Gray A, Boisvert FM, Agacan M, Morrice NA, Gourlay R, Leslie NR, Downes CP, Batty IH. A screen for novel phosphoinositide 3-kinase effector proteins. Mol. Cell. Proteomics. 2011;10 doi: 10.1074/mcp.M110.003178. 10.1074/mcp.M1110.003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu H, Bilgin M, Bangham R, Hall D, Casamayor A, Bertone P, Lan N, Jansen R, Bidlingmaier S, Houfek T, Mitchell T, Miller P, Dean RA, Gerstein M, Snyder M. Global analysis of protein activities using proteome chips. Science. 2001;293:2101–2105. doi: 10.1126/science.1062191. [DOI] [PubMed] [Google Scholar]
- 32.Cravatt BF, Wright AT, Kozarich JW. Activity-based protein profiling: From enzyme chemistry. Annu. Rev. Biochem. 2008;77:383–414. doi: 10.1146/annurev.biochem.75.101304.124125. [DOI] [PubMed] [Google Scholar]
- 33.Gubbens J, Ruijter E, de Fays LEV, Damen JMA, de Kruijff B, Slijper M, Rijkers DTS, Liskamp RMJ, de Kroon A. Photocrosslinking and click chemistry enable the specific detection of proteins interacting with phospholipids at the membrane interface. Chem. Biol. 2009;16:3–14. doi: 10.1016/j.chembiol.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Gubbens J, de Kroon A. Proteome-wide detection of phospholipidprotein interactions in mitochondria by photocrosslinking and click chemistry. Mol. Biosyst. 2010;6:1751–1759. doi: 10.1039/c003064n. [DOI] [PubMed] [Google Scholar]
- 35.Tully SE, Cravatt BF. Activity-based probes that target functional subclasses of phospholipases in proteomes. J. Am. Chem. Soc. 2010;132:3264–3265. doi: 10.1021/ja1000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong D, Bostic HE, Smith MD, Best MD. Synthesis of modular headgroup conjugates corresponding to all seven phosphatidylinositol polyphosphate isomers for convenient probe generation. Eur. JOrg. Chem. 2009:4170–4179. [Google Scholar]
- 37.Speers AE, Cravatt BF. Profiling enzyme activities in vivo using click chemistry methods. Chem. Biol. 2004;11:535–546. doi: 10.1016/j.chembiol.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Hong V, Presolski SI, Ma C, Finn MG. Analysis and Optimization of Copper-Catalyzed Azide-Alkyne Cycloaddition for Bioconjugation. Angew. Chem. Int. Edit. 2009;48:9879–9883. doi: 10.1002/anie.200905087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manna D, Albanese A, Park WS, Cho W. Mechanistic basis of differential cellular responses of phosphatidylinositol 3,4-bisphosphate- and phosphatidylinositol 3,4,5-trisphosphate-binding pleckstrin homology domains. J. Biol. Chem. 2007;282:32093–32105. doi: 10.1074/jbc.M703517200. [DOI] [PubMed] [Google Scholar]
- 40.Smith MD, Gong DH, Sudhahar CG, Reno JC, Stahelin RV, Best MD. Synthesis and convenient functionalization of azide-labeled diacylglycerol analogues for modular access to biologically active lipid probes. Bioconjugate Chem. 2008;19:1855–1863. doi: 10.1021/bc8001002. [DOI] [PubMed] [Google Scholar]
- 41.Guichard G, Fournel S, Trouche N, Wieckowski S. Preparation of new multimeric molecules, particularly CD40 ligands, for use in the preparation of drugs. 2008 [Google Scholar]
- 42.Speers AE, Adam GC, Cravatt BF. Activity-based protein profiling in vivo using a coppeRI)-catalyzed azide-alkyne 3+2 cycloaddition. J. Am. Chem. Soc. 2003;125:4686–4687. doi: 10.1021/ja034490h. [DOI] [PubMed] [Google Scholar]
- 43.Jessani N, Niessen S, Wei BQQ, Nicolau M, Humphrey M, Ji YR, Han WS, Noh DY, Yates JR, Jeffrey SS, Cravatt BF. A streamlined platform for high-content functional proteomics of primary human specimens. Nat. Methods. 2005;2:691–697. doi: 10.1038/nmeth778. [DOI] [PubMed] [Google Scholar]
- 44.Peng JM, Elias JE, Thoreen CC, Licklider LJ, Gygi SP. Evaluation of multidimensional chromatography coupled with tandem mass spectrometry (LC/LCMS/ MS) for large-scale protein analysis: The yeast proteome. J. Proteome Res. 2003;2:43–50. doi: 10.1021/pr025556v. [DOI] [PubMed] [Google Scholar]
- 45.Washburn MP, Wolters D, Yates JR. Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat. Biotechnol. 2001;19:242–247. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- 46.Dorman G, Prestwich GD. Using photolabile ligands in drug discovery and development. Trends Biotechnol. 2000;18:64–77. doi: 10.1016/s0167-7799(99)01402-x. [DOI] [PubMed] [Google Scholar]
- 47.Ballell L, Alink KJ, Slijper M, Versluis C, Liskamp RMJ, Pieters RJ. A new chemical probe for proteomics of carbohydrate-binding proteins. ChemBioChem. 2005;6:291–295. doi: 10.1002/cbic.200400209. [DOI] [PubMed] [Google Scholar]
- 48.Ballell L, van Scherpenzeel M, Buchalova K, Liskamp RMJ, Pieters RJ. A new chemical probe for the detection of the cancer-linked galectin-3. Org. Biomol. Chem. 2006;4:4387–4394. doi: 10.1039/b611050a. [DOI] [PubMed] [Google Scholar]
- 49.van Scherpenzeel M, van der Pot M, Arnusch CJ, Liskamp RMJ, Pieters RJ. Detection of galectin-3 by novel peptidic photoprobes. Bioorg. Med. Chem. Lett. 2007;17:376–378. doi: 10.1016/j.bmcl.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 50.Sletten EM, Bertozzi CR. Bioorthogonal chemistry: Fishing for selectivity in a sea of functionality. Angew. Chem., Int. Edit. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Best MD. Click chemistry and bioorthogonal reactions: unprecedented selectivity in the labeling of biological molecules. Biochemistry. 2009;48:6571–6584. doi: 10.1021/bi9007726. [DOI] [PubMed] [Google Scholar]
- 52.Ferguson KM, Lemmon MA, Schlessinger J, Sigler PB. Structure of the high-affinity complex of inositol trisphosphate with a phospholipase-C pleckstrin homology domain. Cell. 1995;83:1037–1046. doi: 10.1016/0092-8674(95)90219-8. [DOI] [PubMed] [Google Scholar]
- 53.Garcia P, Gupta R, Shah S, Morris AJ, Rudge SA, Scarlata S, Petrova V, McLaughlin S, Rebecchi MJ. The pleckstrin homology domain of phospholipase C-delta(1) binds with high affinity to phosphatidylinositol 4,5- bisphosphate in bilayer membranes. Biochemistry. 1995;34:16228–16234. doi: 10.1021/bi00049a039. [DOI] [PubMed] [Google Scholar]
- 54.Hirose K, Kadowaki S, Tanabe M, Takeshima H, Iino M. Spatiotemporal dynamics of inositol 1,4,5-trisphosphate that underlies complex Ca2+ mobilization patterns. Science. 1999;284:1527–1530. doi: 10.1126/science.284.5419.1527. [DOI] [PubMed] [Google Scholar]
- 55.Kavran JM, Klein DE, Lee A, Falasca M, Isakoff SJ, Skolnik EY, Lemmon MA. Specificity and promiscuity in phosphoinositide binding by Pleckstrin homology domains. J. Biol. Chem. 1998;273:30497–30508. doi: 10.1074/jbc.273.46.30497. [DOI] [PubMed] [Google Scholar]
- 56.Lemmon MA, Ferguson KM, Obrien R, Sigler PB, Schlessinger J. Specific and high-affinity binding of inositol phosphates to an isolated pleckstrin homology domain. Proc. Natl. Acad. Sci. U. S. A. 1995;92:10472–10476. doi: 10.1073/pnas.92.23.10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prestwich GD. Touching all the bases: Synthesis of inositol polyphosphate and phosphoinositide affinity probes from glucose. Acc. Chem. Res. 1996;29:503–513. [Google Scholar]
- 58.Gu QM, Prestwich GD. Synthesis of phosphotriester analogues of the phosphoinositides PtdIns(4,5)P-2 and PtdIns(3,4,5)P-3. J. Org. Chem. 1996;61:8642–8647. [Google Scholar]
- 59.Painter GF, Grove SJA, Gilbert IH, Holmes AB, Raithby PR, Hill ML, Hawkins PT, Stephens L. General synthesis of 3-phosphorylated myoinositol phospholipids and derivatives. J. Chem. Soc., Perkin Trans. 1999;1:923–935. [Google Scholar]
- 60.Henne V, Mayr GW, Grabowski B, Koppitz B, Soling HD. Smisynthetic derivatives of inositol 1,4,5-trisphosphate substituted at the 1-phosphate group - Effects on calium release from permeabilized guinea-pig parotid acinar cells and comparison with binding to alolase-A. Eur. J. Biochem. 1988;174:95–101. doi: 10.1111/j.1432-1033.1988.tb14067.x. [DOI] [PubMed] [Google Scholar]
- 61.Dorman G, Chen J, Prestwich GD. Synthesis of D-myo-P-1-(Oaminopropyl)- inositol-1,4,5-trisphosphate affinity probes from alpha d-glucose. Tetrahedron Lett. 1995;36:8719–8722. [Google Scholar]
- 62.Inoue T, Kikuchi K, Hirose K, Iino M, Nagano T. Synthesis and evaluation of 1-position-modified inositol 1,4,5-trisphosphate analogs. Bioorg. Med. Chem. Lett. 1999;9:1697–1702. doi: 10.1016/s0960-894x(99)00256-5. [DOI] [PubMed] [Google Scholar]
- 63.Marecek JF, Estevez VA, Prestwich GD. New tetherable derivatives of myo-inositol 2,4,5-trisphosphates and 1,3,4-trisphosphates. Carbohyd. Res. 1992;234:65–73. doi: 10.1016/0008-6215(92)85039-3. [DOI] [PubMed] [Google Scholar]
- 64.Nakanishi W, Kikuchi K, Inoue T, Hirose K, Iino M, Nagano T. Hydrophobic modifications at 1-phosphate of inositol 1,4,5-trisphosphate analogues enhance receptor binding. Bioorg. Med. Chem. Lett. 2002;12:911–913. doi: 10.1016/s0960-894x(02)00044-6. [DOI] [PubMed] [Google Scholar]
- 65.Olszewski JD, Dorman G, Elliott JT, Hong Y, Ahern DG, Prestwich GD. Tethered benzophenone reagents for the synthesis of photoactivatable ligands. Bioconjugate Chem. 1995;6:395–400. doi: 10.1021/bc00034a009. [DOI] [PubMed] [Google Scholar]
- 66.Prestwich GD, Marecek JF, Mourey RJ, Theibert AB, Ferris CD, Danoff SK, Snyder SH. Tethered IP3 - synthesis and biochemical applications of the 1- O-(3-aminopropyl) ester of inositol 1,4,5-trisphosphate. J. Am. Chem. Soc. 1991;113:1822–1825. [Google Scholar]
- 67.Schafer R, Nehlssahabandu M, Grabowsky B, Dehlingerkremer M, Schulz I, Mayr GW. Synthesis and application of photoaffinity analogs of inositol 1,4,5- trisphosphate selectively substituted at the 1-phosphate group. Biochem. J. 1990;272:817–825. doi: 10.1042/bj2720817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tegge W, Ballou CE. Syntheses of D-myo-Inositol 1,4,5-trisphosphate affinity ligands. Carbohyd. Res. 1992;230:63–77. doi: 10.1016/s0008-6215(00)90513-5. [DOI] [PubMed] [Google Scholar]
- 69.Smith MD, Sudhahar CG, Gong D, Stahelin RV, Best MD. Modular synthesis of biologically active phosphatidic acid probes using click chemistry. Mol. Biosyst. 2009:962–972. doi: 10.1039/b901420a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frech M, Andjelkovic M, Ingley E, Reddy KK, Falck JR, Hemmings BA. High affinity binding of inositol phosphates and phosphoinositides to the Pleckstrin homology domain of RAC protein kinase B and their influence on kinase activity. J. Biol. Chem. 1997;272:8474–8481. doi: 10.1074/jbc.272.13.8474. [DOI] [PubMed] [Google Scholar]
- 71.James SR, Downes CP, Gigg R, Grove SJA, Holmes AB, Alessi DR. Specific binding of the Akt-1 protein kinase to phosphatidylinositol 3,4,5- trisphosphate without subsequent activation. Biochem. J. 1996;315:709–713. doi: 10.1042/bj3150709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baker JG, Middleton R, Adams L, May LT, Briddon SJ, Kellam B, Hill SJ. Influence of fluorophore and linker composition on the pharmacology of fluorescent adenosine A(1) receptor ligands. Brit. J. Pharmacol. 2010;159:772–786. doi: 10.1111/j.1476-5381.2009.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. Bioconjugation by coppeRI)-catalyzed azide-alkyne [3+2] cycloaddition. J. Am. Chem. Soc. 2003;125:3192–3193. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]
- 74.Chan TR, Hilgraf R, Sharpless KB, Fokin VV. Polytriazoles as coppeRI)-stabilizing ligands in catalysis. Org. Lett. 2004;6:2853–2855. doi: 10.1021/ol0493094. [DOI] [PubMed] [Google Scholar]
- 75.Yates, Eng JK, McCormack AL, Schieltz D. Method to correlate tandem mass-spectra of modified peptides to amino-acid sequences in the protein database. Anal. Chem. 1995;67:1426–1436. doi: 10.1021/ac00104a020. [DOI] [PubMed] [Google Scholar]
- 76.Tabb DL, McDonald WH, Yates JR. DTASelect and contrast: Tools for assembling and comparing protein identifications from shotgun proteomics. J. Proteome Res. 2002;1:21–26. doi: 10.1021/pr015504q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zybailov B, Coleman MK, Florens L, Washburn MP. Correlation of relative abundance ratios derived from peptide ion chromatograms and spectrum counting for quantitative proteomic analysis using stable isotope labeling. Anal. Chem. 2005;77:6218–6224. doi: 10.1021/ac050846r. [DOI] [PubMed] [Google Scholar]
- 78.Usaite R, Wohlschlegel J, Venable JD, Park SK, Nielsen J, Olsson L, Yates JR. Characterization of global yeast quantitative proteome data generated from the wild-type and glucose repression Saccharomyces cerevisiae strains: The comparison of two quantitative methods. J. Proteome Res. 2008;7:266–275. doi: 10.1021/pr700580m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chiang KP, Niessen S, Saghatelian A, Cravatt BF. An enzyme that regulates ether lipid signaling pathways in cancer annotated by multidimensional profiling. Chem. Biol. 2006;13:1041–1050. doi: 10.1016/j.chembiol.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 80.Jessani N, Liu YS, Humphrey M, Cravatt BF. Enzyme activity profiles of the secreted and membrane proteome that depict cancer cell invasiveness. Proc. Natl. Acad. SciU.SA. 2002;99:10335–10340. doi: 10.1073/pnas.162187599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paulick MG, Bogyo M. Application of activity-based probes to the study of enzymes involved in cancer progression. Curr. Opin. Gen. Dev. 2008;18:97–106. doi: 10.1016/j.gde.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Greenbaum DC, Baruch A, Grainger M, Bozdech Z, Medzihradszky KF, Engel J, DeRisi J, Holder AA, Bogyo M. A role for the protease falcipain 1 in host cell invasion by the human malaria parasite. Science. 2002;298:2002–2006. doi: 10.1126/science.1077426. [DOI] [PubMed] [Google Scholar]
- 83.Arastu-Kapur S, Ponder EL, Fonovic UP, Yeoh S, Yuan F, Fonovic M, Grainger M, Phillips CI, Powers JC, Bogyo M. Identification of proteases that regulate erythrocyte rupture by the malaria parasite Plasmodium falciparum. Nat. Chem. Biol. 2008;4:203–213. doi: 10.1038/nchembio.70. [DOI] [PubMed] [Google Scholar]
- 84.Barglow KT, Cravatt BF. Discovering disease-associated enzymes by proteome reactivity profiling. Chem. Biol. 2004;11:1523–1531. doi: 10.1016/j.chembiol.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 85.Irvine RF. Inositide evolution - towards turtle domination? J. Physiol. 2005;566:295–300. doi: 10.1113/jphysiol.2005.087387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Irvine RF, Schell MJ. Back in the water: The return of the inositol phosphates. Nat. Rev. Mol. Cell Biol. 2001;2:327–338. doi: 10.1038/35073015. [DOI] [PubMed] [Google Scholar]
- 87.Razzini G, Berrie CP, Vignati S, Broggini M, Mascetta G, Brancaccio A, Falasca M. Novel functional PI 3-kinase antagonists inhibit cell growth and tumorigenicity in human cancer cell lines. FASEB. J. 2000;14:1179–1187. doi: 10.1096/fasebj.14.9.1179. [DOI] [PubMed] [Google Scholar]
- 88.Piccolo E, Vignati S, Maffucci T, Innominato PF, Riley AM, Potter BVL, Pandolfi PP, Broggini M, Iacobelli S, Innocenti P, Falasca M. Inositol pentakisphosphate promotes apoptosis through the PI3-K/Akt pathway. Oncogene. 2004;23:1754–1765. doi: 10.1038/sj.onc.1207296. [DOI] [PubMed] [Google Scholar]
- 89.Riley AM, Guedat P, Schlewer G, Spiess B, Potter BVL. A conformationally restricted cyclic phosphate analogue of inositol trisphosphate: Synthesis and physicochemical properties. J. Org. Chem. 1998;63:295–305. [Google Scholar]
- 90.Maffucci T, Piccolo E, Cumashi A, Iezzi M, Riley AM, Saiardi A, Godage HY, Rossi C, Broggini M, Iacobelli S, Potter BVL, Innocenti P, Falasca M. Inhibition of the phosphatidylinositol 3-kinase/Akt pathway by inositol pentakisphosphate results in antiangiogenic and antitumor effects. Cancer Res. 2005;65:8339–8349. doi: 10.1158/0008-5472.CAN-05-0121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.