Light not only is indispensable as an energy source for life on earth but also serves as an essential environmental cue conveying the information of daily and seasonal time to organisms across different kingdoms. Although the molecular mechanisms underlying light responses are actively explored in various light-sensitive organisms, these studies are either hindered by the complexity of the systems or an incomplete familiarity with the light signaling components involved in the scheme. Therefore, study of a simple and well-characterized model system is desirable to expand our knowledge of basic properties underlying the regulation of biological light responses. This review will briefly introduce the basic light sensing machinery in Neurospora crassa, a filamentous fungus, and then focus on the most recent advances in employing Neurospora as a model to study light signaling cascades, photoadaptation, and circadian clock-modulated effects in eukaryotic cells. Also, we will summarize the functions of a number of putative photoreceptors in Neurospora, and discuss the implications of the study of Neurospora to the field of fungal photobiology and some challenges for future studies.
Photobiology and the basic light sensing machinery in Neurospora
Light responses in the fungal kingdom, a family comprising an estimated 1.5 million species (Casadevall et al., 2008), have been extensively studied for several decades in the long-established model system Neurospora crassa. Light affects a variety of physiological processes in Neurospora, including entrainment and resetting of the circadian clock, biosynthesis of the photo-protective pigments, induction of asexual conidiospores, development of sexual structures, and the direction of ascospore dispersal (Bahn et al., 2007; Corrochano, 2007; Dunlap and Loros, 2005; Herrera-Estrella and Horwitz, 2007; Idnurm and Heitman, 2005a). Underlying these biological phenomena is the regulation of many Neurospora genes by light. Many studies have focused on understanding light-induced gene regulation of individual or specific groups of genes (Arpaia et al., 1995a; Bell-Pedersen et al., 1996; Corrochano et al., 1995; Kaldenhoff and Russo, 1993; Lauter et al., 1992). Recently, of the 5,600 detected genes on a whole-genome microarray, approximately 5.6% or 314, responded to a light stimulus by a relatively rapid increase in transcript amount (Chen et al., 2009). This ratio is higher but close to a prior estimate (3%) from spotted cDNA microarrays covering 1343 genes (Lewis et al., 2002). Based on the timing of induction, most of these light-responsive genes can be classified as either early or late light responders, with average peaks of mRNA expression at approximately 15 to 30 and 60 to 90 minutes, respectively. Reflecting the temporal regulation of various cellular events, genes related to distinct functional categories are enriched in different stages of light responses.
To perceive light, organisms must be equipped with proteins capable of reacting to light, mostly through a light-dependent, reversible covalent binding to a chromophore through which the energy of light is absorbed and transferred. The proteins competent in responding to light with a reversible conformational change (i.e. photocycle) are referred to as photoreceptors. After decades of studies, all known light responses in Neurospora are restricted to near UV/blue-light, suggesting the presence of a blue-light photoreceptor(s) (Chen and Loros, 2009; Linden et al., 1997; Sargent and Briggs, 1967). Extensive genetic screenings and molecular characterization have lead to the identification of only two blind mutants, wc-1 and wc-2 (Ballario et al., 1996; Collett et al., 2002; Lee et al., 2003; Linden et al., 1997; Linden and Macino, 1997). WC-1 is both a flavin adenine dinucleotide (FAD) binding photoreceptor and a GATA zinc finger transcription factor (Ballario et al., 1996; Froehlich et al., 2002; He et al., 2002). Through their PAS (PER-ARNT-SIM) domains (Ballario et al., 1998; Cheng et al., 2002; Cheng et al., 2003; Crosthwaite et al., 1997), WC-1 and WC-2, another GATA zinc finger transcription factor, form the White Collar Complex (WCC) to transfer light signals to their target genes. WC-1 senses light through a flavin chromophore binding LOV domain (light, oxygen or voltage) (Froehlich et al., 2002; He et al., 2002). LOV domains are a PAS domain variant initially found in the phototropins, a family of blue-light sensitive plant photoreceptors first identified in Arabidopsis (Christie et al., 1999), and also found in the Neurospora VVD protein discussed below. Upon illumination, the chromophore becomes covalently bound to a cysteine in the LOV domain and undergoes a photochemical cycle, or photocycle, reflecting structural changes in the protein and these changes can be monitored by absorbance or fluorescence spectroscopy (Briggs, 2007). After light activation, WC-1 polymerizes and the WCC transiently binds to the promoter of early light-responsive genes at defined elements within 15 minutes or less (Chen et al., 2009; Cheng et al., 2003; Froehlich et al., 2002; He and Liu, 2005; Olmedo et al., 2010a) to activate transcription, presumably through chromatin remodeling events. WCC-dependent histone modifications have been associated with the light activation of al-3 (Grimaldi et al., 2006) and frq (Belden et al., 2007) genes. Notably, over-expression of WC-1 in the dark is not sufficient to activate gene expression (Lewis et al., 2002). Through a transient light-induced phosphorylation, hyperphosphorylated WC-1 is degraded and removed from the light-responsive elements (He and Liu, 2005; Schafmeier et al., 2005; Talora et al., 1999). Although WC-1 and WC-2 control most light responses in Neurospora (Chen et al., 2009; Collett et al., 2002; Lee et al., 2003), some transient, low amplitude light responses have been found in the absence of wc-1 (Chen et al., 2009). In addition to its ability to initiate light responses, the WCC has function in the dark as a central circadian clock component in Neurospora, responsible for activating expression of the frequency (frq) gene. Studies exploring this function have been reviewed in detail elsewhere (Dunlap et al., 2007; Heintzen and Liu, 2007; Liu and Bell-Pedersen, 2006).
Light signaling cascades in Neurospora: light-responsive transcription factors pave the way
To differentially activate light responses in a temporal fashion, the WCC might initiate a hierarchical activation of regulatory factors to induce waves of light responses with distinct timing, presumably through a sequential activation of transcription factors. As predicted, by following light responses in a number of light-inducible transcription factor knockout strains, a GATA family transcription factor called sub-1 (submerged protoperithecia-1 whose knockout strain is defective in protoperithecial formation (Colot et al., 2006)), was identified as both light-responsive and essential for regulating most of the late light responsive genes (Chen et al., 2009). Consistent with sub-1’s induction as an early light responsive gene, the WCC can directly bind to the early light-responsive elements (ELREs) on the sub-1 promoter within 15 minutes after lights on. Interestingly, several genes affected in the Δsub-1 strain remain transcriptionally responsive to light stimuli but with a lower amplitude, indicating that SUB-1 is not the sole regulator for activating these genes, and may modulate the action of other regulator(s) accordingly. In contrast to the well-established binding kinetics between the WCC and the ELREs, the molecular connection between SUB-1 and late light-responsive elements (LLREs) remains to be determined (Chen et al., 2009). Several transcription factors in addition to WC-1 and SUB-1 are identified to be light-responsive, but the respective knockout strains do not show changes in gene induction within the two hours of light stimulation given to vegetative hyphae growing in liquid culture, regardless of their various defects in light-regulated development (Chen and Loros, 2009; Chen et al., 2009). This suggests that their roles in propagating light signals might become evident at specific, yet to be identified, developmental stages or metabolic conditions.
Notably, despite differences in the timing of induction and the signaling components involved, the microarray analysis also indicates that the late light responses still share many regulatory features with the early light responses (e.g. complete dependence upon functional WCC to respond to light; the induction amplitude modulated by both FRQ and VVD, see discussion below).
Photoadaptation in Neurospora: a vivid solution
VVD has been the most intensely studied photoreceptor in Neurospora after the WCC, but whose molecular actions have been less understood. Mutants in vvd were identified due to the unique bright orange appearance of the mycelia when grown in constant light (Heintzen et al., 2001; Schwerdtfeger and Linden, 2001; Shrode et al., 2001), presumably due to the persistent activation of carotenoid biosynthesis (Chen et al., 2009; Olmedo et al., 2010b). Studies have shown that VVD acts as a universal repressor to WCC activity for most light responses (Chen et al., 2009; Heintzen et al., 2001; Schwerdtfeger and Linden, 2001; Schwerdtfeger and Linden, 2003; Shrode et al., 2001). In vvd mutants, once gene transcription is turned on by light-activated WCC, the level of transcription will remain up-regulated for many hours in constant light, referred to as “photoadaptation defects”. In contrast, in a wild-type strain, elevated levels of light-induced gene transcription is transient, usually returning to pre-induction baseline within two to four hours (Arpaia et al., 1999; Chen et al., 2009; Lauter and Yanofsky, 1993; Navarro-Sampedro et al., 2008; Schwerdtfeger and Linden, 2001; Schwerdtfeger and Linden, 2003). Some genes display features of a slower or imperfect adaptation and may stay elevated after 4 hours of constant light, like frq or vvd while others, although repressed, do not return to dark baseline levels within this time frame, and others, such as al-3, sub-1 and wc-1, that display a fast return to dark, baseline levels. Interestingly, in the presence of vvd, once photoadaptation repression takes place, light responses can be transcriptionally re-activated by a new light stimulus if the culture was returned to the dark (Arpaia et al., 1999; Schwerdtfeger and Linden, 2001) or by an increase in light intensity if the culture has remained in the light (Arpaia et al., 1999; Chen et al., 2010; Schwerdtfeger and Linden, 2003).
Molecularly, VVD is a small, 21 kDa flavin-binding blue-light photoreceptor consisting a LOV domain and an N-terminal cap (Zoltowski et al., 2007). Upon light activation, the formation of a protein-flavin covalent bond within the LOV domain induces a reversible conformational change with a very slow photocycle (Zoltowski et al., 2009). Point mutations in either cysteine 108 that forms a cysteinyl-flavin adduct in response to blue light, or cysteine 71, at the hinge predicted to be essential for allowing the N-terminal cap to swing in response to light, result in a loss-of-function VVD (Schwerdtfeger and Linden, 2003; Zoltowski et al., 2007). In solution, VVD forms a rapidly exchanging dimer in light, raising the possibility that the VVD LOV domain might interact in vivo with PAS domains from other proteins like those in WC-1 or WC-2 (Lamb et al., 2009; Zoltowski and Crane, 2008). In addition to repressing the WCC activity in constant light, VVD also regulates various WCC-mediated circadian clock properties in darkness, including gating of light input to the clock, maintenance of a daytime oscillator promoting dusk resetting, and temperature compensation of the clock phase (Elvin et al., 2005; Heintzen et al., 2001; Hunt et al., 2007). Until recently, the subcellular localization of VVD and the WCC appeared to conflict with the strong genetic data supporting a robust connection between the two proteins. Fractionation studies found VVD to localize exclusively in the cytosol while the majority of the WCC resides in the nucleus (Denault et al., 2001; Hong et al., 2008; Schafmeier et al., 2008; Schwerdtfeger and Linden, 2003). Small proteins might leak out of the nucleus during the process of nuclei purification and recently two groups have independently demonstrated nuclear, as well as cytoplasmic, localization of GFP-VVD as well as biochemical interaction between VVD and the WCC (Chen et al., 2010; Hunt et al., 2010). Using live cell imaging, a fully-functional version of GFP-conjugated VVD (48 kDa) accumulates in the nucleus upon light induction, and this accumulation is shown to be independent of light activation. Additionally, VVD can directly interact with the WCC and the level of interaction between VVD and the WCC is correlated with the levels of WCC repression in constant light. Raising the amount of VVD protein in the cell before exposure to light results in effective repression of the light responses within 15 minutes, indicating that VVD alone is sufficient to alter the dynamics of photoadaptation, thus suggesting a molecular explanation of the photoadaptation process. Similar work is underway in the Brunner lab (Malzahn et al., 2010).
Light responses and the clock in Neurospora: frequency determines the amplitude
In natural environments with light-dark cycles, the circadian clock is essential for organisms to anticipate and be prepared for upcoming changes in the environment (Dodd et al., 2005; Michael et al., 2003; Woelfle et al., 2004). In Neurospora as in many other light-sensitive organisms, light resets the phase and entrains the period of the biological clock (Crosthwaite et al., 1995), while the clock also regulates the amplitude of light inputs (Chen et al., 2009; Heintzen et al., 2001; Merrow et al., 2001) and photoperiodism (Tan et al., 2004). Molecularly, the WCC not only is the primary photoreceptor complex in the Neurospora cell but also is the positive factor at the core of the circadian oscillator (Crosthwaite et al., 1997; Dunlap and Loros, 2006; Liu and Bell-Pedersen, 2006). A well-described transcription-translation-based auto-regulatory negative feedback loop (primarily involving both WC proteins, FRQ and an associated helicase are integral to the Neurospora clock system. On a daily basis and in the dark the WCC complex is responsible for the rhythmic activation of frq transcription, providing the “positive element” to the feedback loop. FRQ protein, as the “negative element” in the negative-feedback loop of the clock, blocks its own transcription by mediating repression of WCC transcriptional activity by physically binding to the WCC (Dunlap, 1998; Froehlich et al., 2003; Heintzen and Liu, 2007; Schafmeier et al., 2005). There are numerous points of regulation and delay built into the basic feedback loop, including at the highly complex frequency transcription unit, in the surprisingly large number of phosphorylations events on the FRQ and WC-1 proteins by several kinases resulting in a temporally regulated cascade of phospo-isoforms and finally, the creation of additional interlocked feedback loops when FRQ positively regulates WC-1 protein synthesis (Lee et al., 2000) and increases the abundance of wc-2 mRNA (Cheng et al., 2001). Depending on time-of-day, the activation of the WCC by light is affected by the amount of FRQ in the system (Heintzen et al., 2001; Merrow et al., 2001), which oscillates dramatically in a circadian manner. By following light responses in both frq+ and the long-period frq7 strains, microarray analysis confirms and expands this clock-modulated effects to both early and late light responses (Chen et al., 2009). In the absence of the clock (i.e. in a frq-null strain), the expression of WC-1 is low (Lee et al., 2000). Some light responses may be severely affected (Merrow et al., 2001; Tan et al., 2004), while some genes remain robustly light-induced (Arpaia et al., 1993; Arpaia et al., 1995b), presumably due to gene-specific requirements for the amount of the WC-1 in the system (Lee et al., 2003).
In addition to affecting the amplitude of light responses, the circadian clock is also involved in sensing seasonal changes of day length for proper responses or development (i.e. photoperiodism) as demonstrated in plants, insects, and animals (Hazlerigg and Wagner, 2006; Imaizumi, 2010; Nelson et al., 2009). In Neurospora, variations in photoperiod have been shown to affect various developmental processes, including asexual conidia production, sexual protoperithecia formation, and the accumulation of carotenoid pigments. As expected, a functional clock is required for these effects (Tan et al., 2004). However, the detailed molecular mechanisms remain elusive. Given the complicated and intertwined relationship between light responses and the clock, Neurospora may be an attractive simple system for studies in the field.
Other light signaling components in Neurospora
In addition to forward genetic screens, the Neurospora Genome Project (Galagan et al., 2003) identified three additional putative photoreceptors: two phytochrome orthologs (phy-1 and phy-2) and one cryptochrome orthologue (cry) based on a bioinformatics approach. Although phytochromes in Arabidopsis thaliana are bona fide red-light/far red-light photoreceptors and mediate various developmental processes (Franklin and Quail, 2010), the biological function of phytochromes in Neurospora remains to be determined due to lack of either phenotypic or molecular abnormality in the knockout strains (Chen et al., 2009; Froehlich et al., 2005). However, Neurospora PHY-2 is capable of binding either biliverdin or phycocyanobilin with a photocycle in vitro (Froehlich et al., 2005), suggesting full photoreceptor capability involved in a yet to be discovered function. Cryptochromes on the other hand are either blue-light photoreceptors or circadian clock components in plants and animals (Guo et al., 1998; Lin and Todo, 2005; Ma et al., 2001; Somers et al., 1998; Yang et al., 2000). Genetic and biochemical analysis (Froehlich et al., 2010) indicate that Neurospora CRY is a DASH-type cryptochrome but displays no photolyase activity in vivo. The purified CRY protein is capable of binding chromophores (i.e. flavin-adenine dinucleotide and methenlytetrahydrofolate) and single/double stranded ribonucleotides. Both the transcript and the protein of cry are strongly induced by light in a wc-1 dependent manner, and the transcript is circadianly regulated. However, besides a small delay of clock phase under conditions of circadian entrainment, genome-wide microarray analysis and assays for light-regulated phenotypes failed to identify any significant difference between the wild-type and the Δcry strain (Froehlich et al., 2010). Another recent study using different methodology and strains reports small but statistically validated changes in expression of a few genes (Olmedo et al., 2010b), see below. Given regulatory similarities of the circadian clock machinery among eukaryotic model systems, it is somewhat surprising that the cryptochrome in Neurospora is neither an essential clock component, nor an indispensable photoreceptor involved in clock resetting.
Based on sequence homology with archaeal opsins, nop-1 (New eukaryotic opsin-1) was first identified as a putative green-light photoreceptor (Bieszke et al., 1999a). Biochemical analysis indicates that NOP-1 binds to retinal and is capable of undergoing a slow photocycle (Bieszke et al., 1999b). Although extensive examination of Δnop-1 strains failed to detect any defects in light-regulated processes, several conidiation-specific genes appear to be affected in the Δnop-1 strain at certain developmental stages during asexual development (Bieszke et al., 2007). However, under vegetative growth conditions, microarray analysis indicates that both early and late light responses are not affected in the absence of nop-1 (Chen et al., 2009).
Interestingly, although most light-responses appear to be unaffected in the absence of phy-2, cry and nop-1 (Chen et al., 2009; Froehlich et al., 2010; Froehlich et al., 2005), a recent study by Olmedo et al (Olmedo et al., 2010b) provides evidence that under conditions of high-intensity white light, these photoreceptors may modify light responses in a gene-specific manner, possibly through modification of WCC activity. For example, the light induction of con-10 is slightly enhanced in the absence of phy-2, while the induction of the con-6, al-1, and vvd genes in the same strain are not affected. In addition to alternations seen in the Δphy-2 strain, similar effects were also observed in Δcry-1 and Δnop-1 strains. Although the influence of these photoreceptors appears subtle, target-specific, developmental age and stage-specific, and evident only under specific light conditions, these results suggest that the light signaling cascades in Neurospora are complicated and communication among various photoreceptors may be essential for organisms living in their native habitats within a range of light conditions.
In contrast to the well-defined blue-light phenotypes in Neurospora crassa, red-light-regulated phenotypes are more evident in Aspergillus nidulans. Through a phytochrome (fphA) and its direct downstream target veA, red-light promotes conidiation and represses the formation of fruiting bodies in Aspergillus (Blumenstein et al., 2005; Purschwitz et al., 2008). A mutation in the veA gene causes impaired red-light signaling, excessive asexual conidiation, and repressed sexual development (Mooney and Yager, 1990). Although neither red-light-regulated responses nor red-light-mediated phenotypes have been documented in Neurospora crassa (Chen et al., 2009; Froehlich et al., 2005), a veA orthologue, Neurospora ve-1, is sufficient to rescue the phenotypes of a veA null mutant in Aspergillus (Bayram et al., 2008). Except for some light-independent developmental defects (i.e. shortened aerial hyphae and increased asexual conidiation) (Bayram et al., 2008), the Neurospora Δve-1 strain was recently found to have a three-fold lower accumulation of carotenoids in contrast to a wild-type strain after exposing to white light for 24 hours (Olmedo et al., 2010b), supporting ve-1’s potential role in the Neurospora light signaling response. It seems likely that additional roles for Neurospora PHY-1 and PHY-2 will emerge as additional stages of the life cycle are examined more closely. Nevertheless, it is somewhat surprising to find that Neurospora and Aspergillus, while both belonging to the Ascomycota, contain such a similar set of light-responding receptor proteins yet appear to use these photoreceptors in very different ways to control physiological responses to light, for example the differential response to red light to direct developmental responses. This may in part be a reflection of the quite large evolutionary distance between the two genera, very roughly estimated to be in the neighborhood of 300 million years, about the same distance between divergence of birds and mammals (Berbee and Taylor, 2010), and the quite different developmental outputs, preferred substrates and overall lifestyles of these fungi.
In addition to photoreceptors, transcription factors, and a number of signaling components, several enzymes, including PKC (protein kinase C) (Arpaia et al., 1999; Franchi et al., 2005; Loros, 2005), NGF-1 (a yeast homologue of gcn-5 histone acetyl-transferase) (Grimaldi et al., 2006), and NDK-1 (nucleoside diphosphate kinase-1) (Ogura et al., 2001), have been implicated in affecting light responses in Neurospora. Alternations in PKC activity lead to changes in the level of the WC-1 protein. PKC appears to interact with WC-1 in a light-dependent manner when it is ectopically over-expressed (Franchi et al., 2005). In contrast, a ngf-1 mutant was found to be defective in both the acetylation of histone H3 K14 and light responses (Grimaldi et al., 2006), suggesting a connection between chromatin remodeling events and transcriptional activation by light. In the ndk-1 mutants, several light-regulated developmental processes are severely affected (Lee et al., 2006; Ogura et al., 2001). However, unlike other light signaling components in Neurospora, the knockout or knockdown strains of pkc, ngf-1 and ndk-1 are either non-viable or show severe growth defects (Loros and Dunlap labs unpublished data). Clearly, it may be difficult to separate the specific activities of these kinases in regulating light responses from general pleiotropic effects arising from their roles in other cellular functions. Similarly, the enzymes involved in the detoxification of ROS (reactive oxygen species), such as CAT-1 (catalase-1) and SOD-1 (superoxide dismutase-1), are reported to affect light-regulated phenotypes in Neurospora (Iigusa et al., 2005; Wang et al., 2007; Yoshida and Hasunuma, 2004; Yoshida et al., 2008). Given a wide-range of biological processes in which ROS might participate, the crosstalk between ROS and the WCC activity is likely to be indirect.
Comparing Neurospora’s light sensing machinery to other fungi
The successful molecular analysis of the photobiology machinery in Neurospora crassa has fostered several subsequent studies in other fungi, demonstrating the presence of conserved light sensing components and regulatory pathways, found across phyla from the Ascomycota, Basidiomycota, and Zygomycota. Many of these efforts have been reviewed in detail elsewhere (Corrochano, 2007; Dunlap and Loros, 2006; Herrera-Estrella and Horwitz, 2007; Idnurm and Heitman, 2005a; Purschwitz et al., 2006). Our discussion here focuses on two recent studies in Phycomyces and Trichoderma, serving as examples highlighting Neurospora’s utility and future implications for the field of fungal photobiology.
The Zygomycetes sit at the base of the fungal evolutionary tree and are the causative agents of several serious mycoses. Phycomyces, as an organism, while still very poorly understood, is yet the best understood of all the Zygomycetes. Max Delbrück and co-workers began working on the Zygomycete fungus Phycomyces blakesleeanus as a model organism for signaling by examining phototropism (Varju et al., 1961), the ability to grow or bend towards light, leaving a legacy of mutants aberrant in their response to light called the mad genes (Bergman et al., 1973). Although these phototropism mutants (madA through madJ) were isolated 40 years ago, the mutants with the most severe phenotypes (i.e. madA and madB), remained unidentified until recently. By cloning and analysis of genes with homologous sequences to the Neurospora white-collar proteins, madA and madB were found to be wc-1 and wc-2 orthologs (Idnurm et al., 2006; Sanz et al., 2009), allowing researchers to begin getting a handle on these not well understood but incredibly important organisms. As with the WC-1 and the WC-2 in Neurospora, MADA and MADB form a transcription factor complex able to drive light responses at the level of gene expression (Sanz et al., 2009).
Trichoderma reesei is an ascomycete fungus with an im portant industrial application to produce cellulolytic and hemicellulolytic enzymes to degrade biomass (Martinez et al., 2008). Light not only affects the sporulation and growth of Trichoderma, but also regulates its capability to express cellobiohydrolase 1 (cbh1, a cellulase gene) (Schmoll et al., 2005). The blr1 and blr2 genes, orthologs of Neurospora wc-1 and wc-2, are essential to activate most light responses in Trichoderma (Casas-Flores et al., 2004; Rosales-Saavedra et al., 2006). A vvd homologue has been recently identified in Trichoderma called ENVOY (env1) and research shows that the env1 transcript is strongly induced by light (Schmoll et al., 2005) with kinetics similar to the vvd transcript. Moreover, the env1 mutants also display photoadaptation defects in constant light, similar to the Δvvd strain, allowing mutant strains to grow with continuous exposure to light (Castellanos et al., 2010). It seems reasonable to predict that the ENVOY might repress the activity of BLR1 and BLR2 through a direct physical interaction as VVD and the WCC do in Neurospora (Chen et al., 2010; Hunt et al., 2010).
Future challenges for studying fungal photobiology
Recent studies with Aspergillus and Neurospora have brought to light some challenges in studying fungal photobiology. In addition to red-light, Aspergillus nidulans was recently shown to be sensitive to blue-light under certain light conditions, mostly through its WC-1 and WC-2 orthologs, LreA and LreB (Purschwitz et al., 2008). By following light-regulated formation of asexual and sexual structures, blue-light was found to enhance the effects of red-light to promote conidiation while partially restoring the amount of cleistothecia repressed by red-light. Furthermore, by performing co-immunoprecipitation assays, Purschwitz et al are able to show a direct interaction between the red light-sensing phytochrome photoreceptor FphA (Blumenstein et al., 2005; Idnurm and Heitman, 2005b) and LreA/LreB, suggesting that red-light and blue-light photoreceptors might act as a complex to differentially regulate various light responses. In Neurospora, although red-light-regulated phenotypes remain to be discovered, several genes whose light induction depends on the WCC are affected in the absence of phy-2 under high-light conditions (Olmedo et al., 2010b), suggesting the existence of some coordinated actions between blue-light and red-light photoreceptors in Neurospora. In addition to communication between photoreceptors, there may be other variables, either developmental or environmental, that determine how fungi perceive and respond to light stimuli, many of them currently unexamined. For instance, light-responsive genes involved in the development of sexual structures might be repressed in the process of asexual sporulation and vice versa. The environmental variables include light intensity, the composition of the light spectrum, the duration of light exposure (i.e. repression from photoadaptation), developmental stage, growth conditions, and the time-of-day effects (i.e. influences from the circadian clock). As demonstrated in Neurospora, the role of these variables could become more profound when the research focus is gradually shifted from the understanding of the primary photoreceptors to the secondary players in the system.
Summary and conclusion
Light signaling components and light responses in Neurospora crassa are summarized in Table 1 and Figure 1. Examination of photoperception and light responses in Neurospora has been instructive in elucidating some of the basic properties underlying light responsiveness in eukaryotic cells, including light signaling cascades, photoadaptation, and circadian clock-modulated effects. Furthermore, light responses have been shown to affect various aspects of fungal physiology, including growth, development, metabolism, and virulence (Chen et al., 2009; Idnurm and Crosson, 2009; Idnurm and Heitman, 2005a; Tisch and Schmoll, 2010). Many light-sensitive fungi are known animal and/or plant pathogens or have industrial applications. In addition to understanding the basic biology of the fungi, light application might represent a direct and powerful way to manipulate fungal activities. We expect that the future studies on the mechanisms of light responsiveness in Neurospora crassa, one of the best characterized members of the fungal kingdom, will continue to be illuminating and of great interest.
Table I.
Light signaling components in Neurospora crassa
| NCU #1 | Gene symbol |
Structural features |
Light- induced? |
Light responses/phenotypes in the knockout strain | References |
|---|---|---|---|---|---|
| Components affecting all light responses | |||||
| NCU02356.4 | wc-1 | photoreceptor & transcription factor |
Yes | Blind to all known light-regulated responses; k/o shows repressed carotenegenesis |
Ballario et al., 1996; Froehlich et al., 2002; He et al., 2002; Cheng et al., 2003; Lee et al., 2003; He and Liu , 2005; Liu and Bell-Pedersen, 2006; Chen et al., 2009 |
| NCU00902.4 | wc-2 | transcription factor | Yes | Blind to all known light-regulated responses; k/o shows repressed carotenogenesis |
Ballario et al., 1996; Froehlich et al., 2002; He et al., 2002; Collett et al., 2002; Chen et al., 2009 |
| NCU03967.4 | vvd | photoreceptor | Yes | Regulates photoadaptation upon prolonged light exposure; k/o shows enhanced carotenogenesis |
Heintzen et al., 2001; Shrode et al., 2001; Schwerdtfeger and Linden, 2001; Schwerdtfeger and Linden, 2003; Chen et al., 2009; Olmedo et al., 2010b |
| NCU02265.4 | frq | transcriptional regulator |
Yes | Regulates light input in a circadian manner; influences expression of WC-1; k/o shows repressed conidiation |
Heintzen et al., 2001; Lee et al., 2000; Denault et al., 2001; Heintzen and Liu., 2007; Chen et al., 2009 |
| Components affecting a subset of light responses | |||||
| NCU01154.4 | sub-1 | transcription factor | Yes | Some early and most late light responses are severely impaired ; k/o shows submerged protoperithecia in the sexual cycle |
Colot et al., 2006; Chen et al., 2009 |
| NCU00582.4 | cry | photoreceptor | Yes | Affects amplitude of light responses for a few genes; k/o has a slight phase delay under conditions of circadian entrainment. |
Chen et al., 2009; Olmedo et al., 2010b; Froehlich et al., 2010 |
| NCU05790.4 | phy-2 | photoreceptor | No | Affects amplitude of light responses for a few genes | Froehlich et al., 2005; Chen et al., 2009; Olmedo et al., 2010b |
| NCU10055.4 | nop-1 | photoreceptor | No | Affects amplitude of light responses for a few genes | Bieszke et al., 2007; Chen et al., 2009; Olmedo et al., 2010b |
| NCU01731.4 | ve-1 | undetermined | No | k/o shows shortened aerial hyphae, increased conidiation and reduced carotenogenesis |
Bayram et al., 2008; Olmedo et al., 2010b |
| Components with undetermined affects on light responses | |||||
| NCU02713.4 | csp-1 | transcription factor | Yes | k/o defective in conidiospore maturation | Chen et al., 2009; Lambreghts et al., 2009 |
| NCU04179.4 | sah-1 | transcription factor | Yes | k/o shows shortened aerial hyphae | Colot et al., 2006; Chen et al., 2009 |
| NCU06407.4 | vad-3 | transcription factor | Yes | k/o slows basal and aerial hyphal extension | Colot et al., 2006; Chen et al., 2009 |
| NCU03643.4 | none | transcription factor | Yes | k/o shows no phenotype | Chen et al., 2009 |
| NCU04834.4 | phy-1 | putative photoreceptor |
No | k/o shows no phenotype | Froehlich et al., 2005; Chen et al., 2009; Olmedo et al., 2010b |
NCU numbers are from Neurospora annotation
(http://www.broad.mit.edu/annotation/genome/neurospora/Home.html).
Smith et al., 2010 in press added in proof
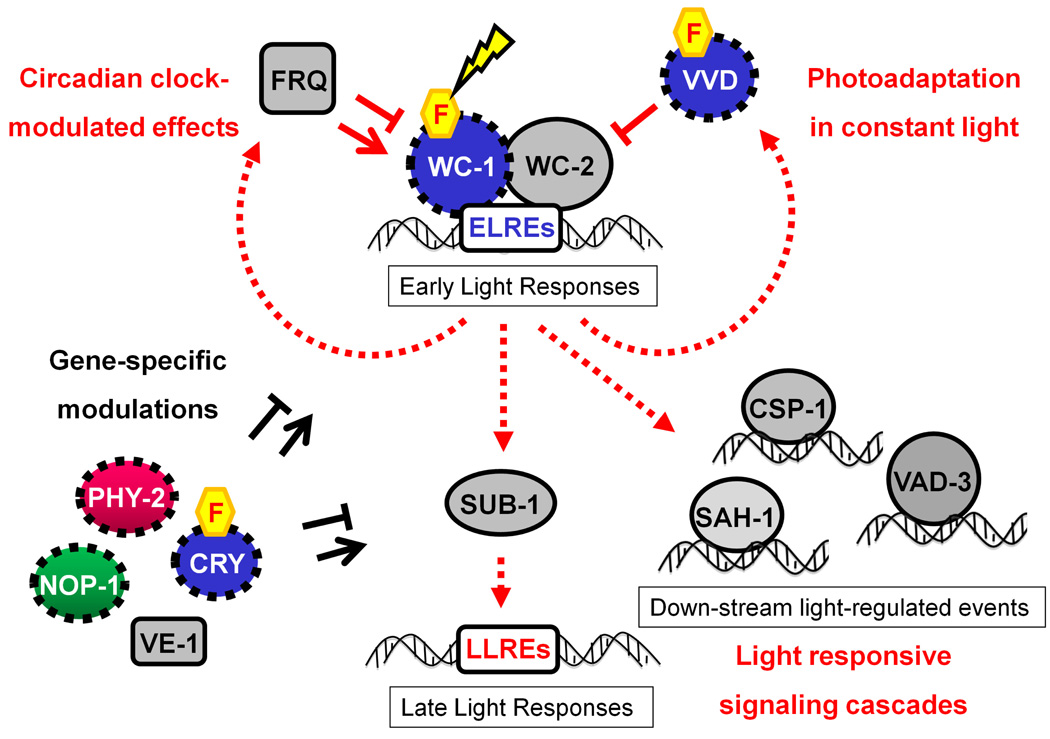
Figure 1. Light responses in Neurospora crassa.
In the presence of light, the White-collar complex (WCC), made up of WC-1 and WC-2, transiently binds to the early light-responsive elements (ELREs) in the promoters of early light-responsive genes to activate the first wave of light induced transcription (i.e. early light responses), including the expression of frq, encoding the clock protein FRQ, the photoreceptor VVD, the transcription factor SUB-1, and others. Depending on the specific time-of-day at which light is imposed, FRQ modulates the WCC activity in a circadian manner to determine the amplitude of the light responses. In response to light, VVD protein is highly expressed and accumulates in the nucleus to repress transcriptional activity through a physical interaction with the WCC. Meanwhile, the induction of SUB-1, essential for most late light responses, results in a second wave of light-induced gene expression, in many cases regulated through identified late light response elements (LLRE’s) on their promoters. Several secondary photoreceptors and other proteins might affect light responses in a gene-specific or developmental stage-specific manner, including CRY, PHY-2, NOP-1, and VE-1. Finally, the roles of light-inducible transcription factors, such as CSP-1, SAH-1 and VAD-3, are yet to be determined. Components capable of sensing light directly via a chromophore are shown by the wavelength color they absorbed and marked with dashed lines. Flavin moieties are shown by yellow hexagons.
Acknowledgements
This work was supported by grants from the National Institutes of Health to J.J.L. (RO1 GM08336), to J.C.D (PO1GM68087), and by the core grant to the Norris Cotton Cancer Center at Dartmouth. We thank Judith Hertog for critical reading of the manuscript and the Fungal Genetics Stock Center, University of Missouri, Kansas City, for their invaluable service to the fungal community.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arpaia G, et al. Light and development regulate the expression of the albino-3 gene in Neurospora crassa. Dev Biol. 1995a;170:626–635. doi: 10.1006/dbio.1995.1242. [DOI] [PubMed] [Google Scholar]
- Arpaia G, et al. Involvement of protein kinase C in the response of Neurospora crassa to blue light. Mol Gen Genet. 1999;262:314–322. doi: 10.1007/s004380051089. [DOI] [PubMed] [Google Scholar]
- Arpaia G, et al. The interplay of light and the circadian clock. Independent dual regulation of clock-controlled gene ccg-2(eas) Plant Physiol. 1993;102:1299–1305. doi: 10.1104/pp.102.4.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia G, et al. Light induction of the clock-controlled gene ccg-1 is not transduced through the circadian clock in Neurospora crassa. Mol Gen Genet. 1995b;247:157–163. doi: 10.1007/BF00705645. [DOI] [PubMed] [Google Scholar]
- Bahn YS, et al. Sensing the environment: lessons from fungi. Nat Rev Microbiol. 2007;5:57–69. doi: 10.1038/nrmicro1578. [DOI] [PubMed] [Google Scholar]
- Ballario P, et al. Roles in dimerization and blue light photoresponse of the PAS and LOV domains of Neurospora crassa white collar proteins. Mol Microbiol. 1998;29:719–729. doi: 10.1046/j.1365-2958.1998.00955.x. [DOI] [PubMed] [Google Scholar]
- Ballario P, et al. White collar-1, a central regulator of blue light responses in Neurospora, is a zinc finger protein. EMBO J. 1996;15:1650–1657. [PMC free article] [PubMed] [Google Scholar]
- Bayram O, et al. Neurospora crassa ve-1 affects asexual conidiation. Fungal Genet Biol. 2008;45:127–138. doi: 10.1016/j.fgb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Belden WJ, et al. Execution of the circadian negative feedback loop in Neurospora requires the ATP-dependent chromatin-remodeling enzyme CLOCKSWITCH. Mol Cell. 2007;25:587–600. doi: 10.1016/j.molcel.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Bell-Pedersen D, et al. Distinct cis-acting elements mediate clock, light, and developmental regulation of the Neurospora crassa eas (ccg-2) gene. Mol Cell Biol. 1996;16:513–521. doi: 10.1128/mcb.16.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbee ML, Taylor JW. Dating the molecular clock in fungi – how close are we? Fungal Biology Reviews. 2010 (in press). [Google Scholar]
- Bergman K, et al. Mutants of Phycomyces with abnormal phototropism. Mol Gen Genet. 1973;123:1–16. doi: 10.1007/BF00282984. [DOI] [PubMed] [Google Scholar]
- Bieszke JA, et al. The nop-1 gene of Neurospora crassa encodes a seven transmembrane helix retinal-binding protein homologous to archaeal rhodopsins. Proc Natl Acad Sci U S A. 1999a;96:8034–8039. doi: 10.1073/pnas.96.14.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieszke JA, et al. The fungal opsin gene nop-1 is negatively-regulated by a component of the blue light sensing pathway and influences conidiation-specific gene expression in Neurospora crassa. Curr Genet. 2007;52:149–157. doi: 10.1007/s00294-007-0148-8. [DOI] [PubMed] [Google Scholar]
- Bieszke JA, et al. A eukaryotic protein, NOP-1, binds retinal to form an archaeal rhodopsin-like photochemically reactive pigment. Biochemistry. 1999b;38:14138–14145. doi: 10.1021/bi9916170. [DOI] [PubMed] [Google Scholar]
- Blumenstein A, et al. The Aspergillus nidulans phytochrome FphA represses sexual development in red light. Curr Biol. 2005;15:1833–1838. doi: 10.1016/j.cub.2005.08.061. [DOI] [PubMed] [Google Scholar]
- Briggs WR. The LOV domain: a chromophore module servicing multiple photoreceptors. J Biomed Sci. 2007;14:499–504. doi: 10.1007/s11373-007-9162-6. [DOI] [PubMed] [Google Scholar]
- Casadevall A, et al. The Fungal Kingdom: Diverse and Essential Roles in Earth's Ecosystem. American Academy of Microbiology. 2008 [PubMed] [Google Scholar]
- Casas-Flores S, et al. BLR-1 and BLR-2, key regulatory elements of photoconidiation and mycelial growth in Trichoderma atroviride. Microbiology. 2004;150:3561–3569. doi: 10.1099/mic.0.27346-0. [DOI] [PubMed] [Google Scholar]
- Castellanos F, et al. Crucial factors of the light perception machinery and their impact on growth and cellulase gene transcription in Trichoderma reesei. Fungal Genetics and Biology. 2010 doi: 10.1016/j.fgb.2010.02.001. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- Chen CH, et al. Direct physical interaction between VVD and WCC regulates photoadaptation in Neurospora crassa. 2010 doi: 10.1073/pnas.1011190107. (manuscript submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Loros JJ. Neurospora sees the light: Light signaling components in a model system. Commun Integr Biol. 2009;2:448–451. doi: 10.4161/cib.2.5.8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, et al. Genome-wide analysis of light-inducible responses reveals hierarchical light signalling in Neurospora. EMBO J. 2009;28:1029–1042. doi: 10.1038/emboj.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, et al. PAS domain-mediated WC-1/WC-2 interaction is essential for maintaining the steady-state level of WC-1 and the function of both proteins in circadian clock and light responses of Neurospora. Mol Cell Biol. 2002;22:517–524. doi: 10.1128/MCB.22.2.517-524.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, et al. Interlocked feedback loops contribute to the robustness of the Neurospora circadian clock. Proc Natl Acad Sci U S A. 2001;98:7408–7413. doi: 10.1073/pnas.121170298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, et al. WHITE COLLAR-1, a multifunctional Neurospora protein involved in the circadian feedback loops, light sensing, and transcription repression of wc-2. J Biol Chem. 2003;278:3801–3808. doi: 10.1074/jbc.M209592200. [DOI] [PubMed] [Google Scholar]
- Christie JM, et al. LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): binding sites for the chromophore flavin mononucleotide. Proc Natl Acad Sci U S A. 1999;96:8779–8783. doi: 10.1073/pnas.96.15.8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett MA, et al. Light and clock expression of the Neurospora clock gene frequency is differentially driven by but dependent on WHITE COLLAR-2. Genetics. 2002;160:149–158. doi: 10.1093/genetics/160.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colot HV, et al. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc Natl Acad Sci U S A. 2006;103:10352–10357. doi: 10.1073/pnas.0601456103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrochano LM. Fungal photoreceptors: sensory molecules for fungal development and behaviour. Photochem Photobiol Sci. 2007;6:725–736. doi: 10.1039/b702155k. [DOI] [PubMed] [Google Scholar]
- Corrochano LM, et al. Light and developmental regulation of the gene con-10 of Neurospora crassa. Dev Biol. 1995;167:190–200. doi: 10.1006/dbio.1995.1016. [DOI] [PubMed] [Google Scholar]
- Crosthwaite SK, et al. Neurospora wc-1 and wc-2: transcription, photoresponses, and the origins of circadian rhythmicity. Science. 1997;276:763–769. doi: 10.1126/science.276.5313.763. [DOI] [PubMed] [Google Scholar]
- Crosthwaite SK, et al. Light-induced resetting of a circadian clock is mediated by a rapid increase in frequency transcript. Cell. 1995;81:1003–1012. doi: 10.1016/s0092-8674(05)80005-4. [DOI] [PubMed] [Google Scholar]
- Denault DL, et al. WC-2 mediates WC-1-FRQ interaction within the PAS protein-linked circadian feedback loop of Neurospora. EMBO J. 2001;20:109–117. doi: 10.1093/emboj/20.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AN, et al. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science. 2005;309:630–633. doi: 10.1126/science.1115581. [DOI] [PubMed] [Google Scholar]
- Dunlap JC. Common threads in eukaryotic circadian systems. Curr Opin Genet Dev. 1998;8:400–406. doi: 10.1016/s0959-437x(98)80109-3. [DOI] [PubMed] [Google Scholar]
- Dunlap JC, Loros JJ. In: Neurospora Photoreceptors. Briggs WR, Spudich J, editors. Berlin: Wiley-VCH; 2005. [Google Scholar]
- Dunlap JC, Loros JJ. How fungi keep time: circadian system in Neurospora and other fungi. Curr Opin Microbiol. 2006;9:579–587. doi: 10.1016/j.mib.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Dunlap JC, et al. A circadian clock in Neurospora: how genes and proteins cooperate to produce a sustained, entrainable, and compensated biological oscillator with a period of about a day. Cold Spring Harb Symp Quant Biol. 2007;72:57–68. doi: 10.1101/sqb.2007.72.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvin M, et al. The PAS/LOV protein VIVID supports a rapidly dampened daytime oscillator that facilitates entrainment of the Neurospora circadian clock. Genes Dev. 2005;19:2593–2605. doi: 10.1101/gad.349305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, et al. Protein kinase C modulates light responses in Neurospora by regulating the blue light photoreceptor WC-1. Mol Microbiol. 2005;56:334–345. doi: 10.1111/j.1365-2958.2005.04545.x. [DOI] [PubMed] [Google Scholar]
- Franklin KA, Quail PH. Phytochrome functions in Arabidopsis development. J Exp Bot. 2010;61:11–24. doi: 10.1093/jxb/erp304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich AC, et al. Genetic and molecular characterization of a cryptochrome from the filamentous fungus Neurospora crassa. Eukaryot Cell. 2010;5:738–750. doi: 10.1128/EC.00380-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich AC, et al. White Collar-1, a circadian blue light photoreceptor, binding to the frequency promoter. Science. 2002;297:815–819. doi: 10.1126/science.1073681. [DOI] [PubMed] [Google Scholar]
- Froehlich AC, et al. Rhythmic binding of a WHITE COLLAR-containing complex to the frequency promoter is inhibited by FREQUENCY. Proc Natl Acad Sci U S A. 2003;100:5914–5919. doi: 10.1073/pnas.1030057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich AC, et al. Genetic and molecular analysis of phytochromes from the filamentous fungus Neurospora crassa. Eukaryot Cell. 2005;4:2140–2152. doi: 10.1128/EC.4.12.2140-2152.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galagan JE, et al. The genome sequence of the filamentous fungus Neurospora crassa. Nature. 2003;422:859–868. doi: 10.1038/nature01554. [DOI] [PubMed] [Google Scholar]
- Grimaldi B, et al. The Neurospora crassa White Collar-1 dependent blue light response requires acetylation of histone H3 lysine 14 by NGF-1. Mol Biol Cell. 2006;17:4576–4583. doi: 10.1091/mbc.E06-03-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, et al. Regulation of flowering time by Arabidopsis photoreceptors. Science. 1998;279:1360–1363. doi: 10.1126/science.279.5355.1360. [DOI] [PubMed] [Google Scholar]
- Hazlerigg DG, Wagner GC. Seasonal photoperiodism in vertebrates: from coincidence to amplitude. Trends Endocrinol Metab. 2006;17:83–91. doi: 10.1016/j.tem.2006.02.004. [DOI] [PubMed] [Google Scholar]
- He Q, et al. White collar-1, a DNA binding transcription factor and a light sensor. Science. 2002;297:840–843. doi: 10.1126/science.1072795. [DOI] [PubMed] [Google Scholar]
- He Q, Liu Y. Molecular mechanism of light responses in Neurospora: from light-induced transcription to photoadaptation. Genes Dev. 2005;19:2888–2899. doi: 10.1101/gad.1369605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzen C, Liu Y. The Neurospora crassa circadian clock. Adv Genet. 2007;58:25–66. doi: 10.1016/S0065-2660(06)58002-2. [DOI] [PubMed] [Google Scholar]
- Heintzen C, et al. The PAS protein VIVID defines a clock-associated feedback loop that represses light input, modulates gating, and regulates clock resetting. Cell. 2001;104:453–464. doi: 10.1016/s0092-8674(01)00232-x. [DOI] [PubMed] [Google Scholar]
- Herrera-Estrella A, Horwitz BA. Looking through the eyes of fungi: molecular genetics of photoreception. Mol Microbiol. 2007;64:5–15. doi: 10.1111/j.1365-2958.2007.05632.x. [DOI] [PubMed] [Google Scholar]
- Hong CI, et al. Closing the circadian negative feedback loop: FRQ-dependent clearance of WC-1 from the nucleus. Genes Dev. 2008;22:3196–3204. doi: 10.1101/gad.1706908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SM, et al. The PAS/LOV protein VIVID controls temperature compensation of circadian clock phase and development in Neurospora crassa. Genes Dev. 2007;21:1964–1974. doi: 10.1101/gad.437107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SM, et al. The role of VIVID in light and temperature responses of the Neurospora circadian system. Fungal Genetics Reports. 2010;57:13. [Google Scholar]
- Idnurm A, Crosson S. The photobiology of microbial pathogenesis. PLoS Pathog. 2009;5:e1000470. doi: 10.1371/journal.ppat.1000470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idnurm A, Heitman J. Light controls growth and development via a conserved pathway in the fungal kingdom. PLoS Biol. 2005a;3:e95. doi: 10.1371/journal.pbio.0030095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idnurm A, Heitman J. Photosensing fungi: phytochrome in the spotlight. Curr Biol. 2005b;15:R829–R832. doi: 10.1016/j.cub.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Idnurm A, et al. The Phycomyces madA gene encodes a blue-light photoreceptor for phototropism and other light responses. Proc Natl Acad Sci U S A. 2006;103:4546–4551. doi: 10.1073/pnas.0600633103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iigusa H, et al. Oxygen and hydrogen peroxide enhance light-induced carotenoid synthesis in Neurospora crassa. FEBS Lett. 2005;579:4012–4016. doi: 10.1016/j.febslet.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Imaizumi T. Arabidopsis circadian clock and photoperiodism: time to think about location. Curr Opin Plant Biol. 2010;13:83–89. doi: 10.1016/j.pbi.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldenhoff R, Russo VE. Promoter analysis of the bli-7/eas gene. Curr Genet. 1993;24:394–399. doi: 10.1007/BF00351847. [DOI] [PubMed] [Google Scholar]
- Lamb JS, et al. Illuminating solution responses of a LOV domain protein with photocoupled small-angle X-ray scattering. J Mol Biol. 2009;393:909–919. doi: 10.1016/j.jmb.2009.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambreghts R, et al. A high-density single nucleotide polymorphism map for Neurospora crassa. Genetics. 2009;181:767–781. doi: 10.1534/genetics.108.089292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauter FR, et al. Developmental and light regulation of eas, the structural gene for the rodlet protein of Neurospora. Genes Dev. 1992;6:2373–2381. doi: 10.1101/gad.6.12a.2373. [DOI] [PubMed] [Google Scholar]
- Lauter FR, Yanofsky C. Day/night and circadian rhythm control of con gene expression in Neurospora. Proc Natl Acad Sci U S A. 1993;90:8249–8253. doi: 10.1073/pnas.90.17.8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, et al. Photomorphogenetic characteristics are severely affected in nucleoside diphosphate kinase-1 (ndk-1)-disrupted mutants in Neurospora crassa. Mol Genet Genomics. 2006;275:9–17. doi: 10.1007/s00438-005-0044-1. [DOI] [PubMed] [Google Scholar]
- Lee K, et al. Roles for WHITE COLLAR-1 in circadian and general photoperception in Neurospora crassa. Genetics. 2003;163:103–114. doi: 10.1093/genetics/163.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, et al. Interconnected feedback loops in the Neurospora circadian system. Science. 2000;289:107–110. doi: 10.1126/science.289.5476.107. [DOI] [PubMed] [Google Scholar]
- Lewis ZA, et al. Overexpression of White Collar-1 (WC-1) activates circadian clock-associated genes, but is not sufficient to induce most light-regulated gene expression in Neurospora crassa. Mol Microbiol. 2002;45:917–931. doi: 10.1046/j.1365-2958.2002.03074.x. [DOI] [PubMed] [Google Scholar]
- Lin C, Todo T. The cryptochromes. Genome Biol. 2005;6:220. doi: 10.1186/gb-2005-6-5-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden H, et al. Blue light regulation in Neurospora crassa. Fungal Genet Biol. 1997;22:141–150. doi: 10.1006/fgbi.1997.1013. [DOI] [PubMed] [Google Scholar]
- Linden H, Macino G. White collar 2, a partner in blue-light signal transduction, controlling expression of light-regulated genes in Neurospora crassa. EMBO J. 1997;16:98–109. doi: 10.1093/emboj/16.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Bell-Pedersen D. Circadian rhythms in Neurospora crassa and other filamentous fungi. Eukaryot Cell. 2006;5:1184–1193. doi: 10.1128/EC.00133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loros J. A kinase for light and time. Mol Microbiol. 2005;56:299–302. doi: 10.1111/j.1365-2958.2005.04546.x. [DOI] [PubMed] [Google Scholar]
- Ma L, et al. Light control of Arabidopsis development entails coordinated regulation of genome expression and cellular pathways. Plant Cell. 2001;13:2589–2607. doi: 10.1105/tpc.010229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malzahn E, et al. Molecular mechanism of photo-Adaptation in Neurospora crassa. Fungal Genetics Reports. 2010;57(suppl):12. [Google Scholar]
- Martinez D, et al. Genome sequencing and analysis of the biomass-degrading fungus Trichoderma reesei (syn. Hypocrea jecorina) Nat Biotechnol. 2008;26:553–560. doi: 10.1038/nbt1403. [DOI] [PubMed] [Google Scholar]
- Merrow M, et al. Circadian regulation of the light input pathway in Neurospora crassa. EMBO J. 2001;20:307–315. doi: 10.1093/emboj/20.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael TP, et al. Enhanced fitness conferred by naturally occurring variation in the circadian clock. Science. 2003;302:1049–1053. doi: 10.1126/science.1082971. [DOI] [PubMed] [Google Scholar]
- Mooney JL, Yager LN. Light is required for conidiation in Aspergillus nidulans. Genes Dev. 1990;4:1473–1482. doi: 10.1101/gad.4.9.1473. [DOI] [PubMed] [Google Scholar]
- Navarro-Sampedro L, et al. A genetic selection for Neurospora crassa mutants altered in their light regulation of transcription. Genetics. 2008;178:171–183. doi: 10.1534/genetics.107.079582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, et al. Photoperiodism: The Biological Calendar. Oxford University Press; 2009. [Google Scholar]
- Ogura Y, et al. A point mutation in nucleoside diphosphate kinase results in a deficient light response for perithecial polarity in Neurospora crassa. J Biol Chem. 2001;276:21228–21234. doi: 10.1074/jbc.M011381200. [DOI] [PubMed] [Google Scholar]
- Olmedo M, et al. Regulation by blue light of the fluffy gene encodi n g a major regulator of conidiation in Neurospora crassa. Genetics. 2010a;184:651–658. doi: 10.1534/genetics.109.109975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmedo M, et al. A complex photoreceptor system mediates the regulation by light of the conidiation genes con-10 and con-6 in Neurospora crassa. Fungal Genet Biol. 2010b;47:352–363. doi: 10.1016/j.fgb.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Purschwitz J, et al. Seeing the rainbow: light sensing in fungi. Curr Opin Microbiol. 2006;9:566–571. doi: 10.1016/j.mib.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Purschwitz J, et al. Functional and Physical Interaction of Blue- and Red-Light Sensors in Aspergillus nidulans. Curr Biol. 2008;18:255–259. doi: 10.1016/j.cub.2008.01.061. [DOI] [PubMed] [Google Scholar]
- Rosales-Saavedra T, et al. Novel light-regulated genes in Trichoderma atroviride: a dissection by cDNA microarrays. Microbiology. 2006;152:3305–3317. doi: 10.1099/mic.0.29000-0. [DOI] [PubMed] [Google Scholar]
- Sanz C, et al. Phycomyces MADB interacts with MADA to form the primary photoreceptor complex for fungal phototropism. Proc Natl Acad Sci U S A. 2009;106:7095–7100. doi: 10.1073/pnas.0900879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent ML, Briggs WR. The Effects of Light on a Circadian Rhythm of Conidiation in Neurospora. Plant Physiol. 1967;42:1504–1510. doi: 10.1104/pp.42.11.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafmeier T, et al. Circadian activity and abundance rhythms of the Neurospora clock transcription factor WCC associated with rapid nucleo-cytoplasmic shuttling. Genes Dev. 2008;22:3397–3402. doi: 10.1101/gad.507408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafmeier T, et al. Transcriptional feedback of Neurospora circadian clock gene by phosphorylation-dependent inactivation of its transcription factor. Cell. 2005;122:235–246. doi: 10.1016/j.cell.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Schmoll M, et al. Envoy, a PAS/LOV domain protein of Hypocrea jecorina (Anamorph Trichoderma reesei), modulates cellulase gene transcription in response to light. Eukaryot Cell. 2005;4:1998–2007. doi: 10.1128/EC.4.12.1998-2007.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerdtfeger C, Linden H. Blue light adaptation and desensitization of light signal transduction in Neurospora crassa. Mol Microbiol. 2001;39:1080–1087. doi: 10.1046/j.1365-2958.2001.02306.x. [DOI] [PubMed] [Google Scholar]
- Schwerdtfeger C, Linden H. VIVID is a flavoprotein and serves as a fungal blue light photoreceptor for photoadaptation. EMBO J. 2003;22:4846–4855. doi: 10.1093/emboj/cdg451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrode LB, et al. vvd is required for light adaptation of conidiation-specific genes of Neurospora crassa, but not circadian conidiation. Fungal Genet Biol. 2001;32:169–181. doi: 10.1006/fgbi.2001.1264. [DOI] [PubMed] [Google Scholar]
- Smith KM, et al. Transcription factors in light and circadian clock signaling networks revealed by genome-wide mapping of direct targets for Neurospora WHITE COLLAR COMPLEX. Eukaryot Cell. 2010 doi: 10.1128/EC.00154-10. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers DE, et al. Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science. 1998;282:1488–1490. doi: 10.1126/science.282.5393.1488. [DOI] [PubMed] [Google Scholar]
- Talora C, et al. Role of a white collar-1-white collar-2 complex in blue-light signal transduction. EMBO J. 1999;18:4961–4968. doi: 10.1093/emboj/18.18.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, et al. Photoperiodism in Neurospora crassa. J Biol Rhythms. 2004;19:135–143. doi: 10.1177/0748730404263015. [DOI] [PubMed] [Google Scholar]
- Tisch D, Schmoll M. Light regulation of metabolic pathways in fungi. Appl Microbiol Biotechnol. 2010;85:1259–1277. doi: 10.1007/s00253-009-2320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varju D, et al. Interplay between the reactions to light and to gravity in Phycomyces. J Gen Physiol. 1961;45:47–58. doi: 10.1085/jgp.45.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, et al. Loss of Catalase-1 (Cat-1) results in decreased conidial viability enhanced by exposure to light in Neurospora crassa. Mol Genet Genomics. 2007;277:13–22. doi: 10.1007/s00438-006-0170-4. [DOI] [PubMed] [Google Scholar]
- Woelfle MA, et al. The adaptive value of circadian clocks: an experimental assessment in cyanobacteria. Curr Biol. 2004;14:1481–1486. doi: 10.1016/j.cub.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Yang HQ, et al. The C termini of Arabidopsis cryptochromes mediate a constitutive light response. Cell. 2000;103:815–827. doi: 10.1016/s0092-8674(00)00184-7. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Hasunuma K. Reactive oxygen species affect photomorphogenesis in Neurospora crassa. J Biol Chem. 2004;279:6986–6993. doi: 10.1074/jbc.M310060200. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, et al. Conidiation rhythm and light entrainment in superoxide dismutase mutant in Neurospora crassa. Mol Genet Genomics. 2008;279:193–202. doi: 10.1007/s00438-007-0308-z. [DOI] [PubMed] [Google Scholar]
- Zoltowski BD, Crane BR. Light activation of the LOV protein vivid generates a rapidly exchanging dimer. Biochemistry. 2008;47:7012–7019. doi: 10.1021/bi8007017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoltowski BD, et al. Conformational switching in the fungal light sensor Vivid. Science. 2007;316:1054–1057. doi: 10.1126/science.1137128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoltowski BD, et al. Mechanism-based tuning of a LOV domain photoreceptor. Nat Chem Biol. 2009;5:827–834. doi: 10.1038/nchembio.210. [DOI] [PMC free article] [PubMed] [Google Scholar]