Abstract
The process of modality selection and how it works is a critical determinant of peritoneal dialysis (PD) utilization. This very complex process has not been well analyzed. Here, we break it down into 6 steps and point out how problems at each step can significantly reduce the proportion of endstage renal disease patients initiating PD. It is important that any program wising it to grow its use of PD understand the steps and the points at which problems may be arising. Examples are presented.
Key words: Chronic kidney disease, modality selection
The percentage of end-stage renal disease (ESRD) patients treated with peritoneal dialysis (PD) has declined in many developed countries since the mid-1990s (1,2). This decline has occurred despite the apparent cost advantages of PD and despite initiatives to grow the modality (3,4). Marked differences in PD utilization are evident both between and within countries, and it is widely believed that non-medical issues contribute to those disparities (5,6). Centers or individual physicians may claim that they give their patients a free choice between the PD and hemodialysis (HD) modalities and yet achieve markedly different degrees of PD utilization. These factors suggest a need for greater attention to be given to the actual process of modality selection as conducted at the level of individual centers. We have attempted to dissect the process, breaking it down into key steps, using standard definitions that centers can use to compare their results with regard to modality selection (7,8).
We propose that there are 6 key steps that have to be taken if a center is to successfully initiate a patient on PD (Figure 1). The purpose of this approach is to ensure that each patient is given the opportunity to advance as far as possible through the process. The underlying philosophy is that the nephrology team has to determine if a patient is suitable to do PD, but that the final modality choice should rest with the patient. We realize that this approach does not always hold and that, for a variety of reasons, the modality choice is sometimes made by the team rather than by the patient. To ensure that the process is followed in all cases, we recommend a systematic approach in which a multidisciplinary team meeting discusses each individual case as the patient moves through the successive steps. To facilitate this approach, we have used a web-based computer software program—Dialysis Measurement, Analysis and Reporting (DMAR: Oliver Medical Management, Toronto, ON, Canada)—into which data are entered on a common platform by trained staff using standardized definitions.
Figure 1.
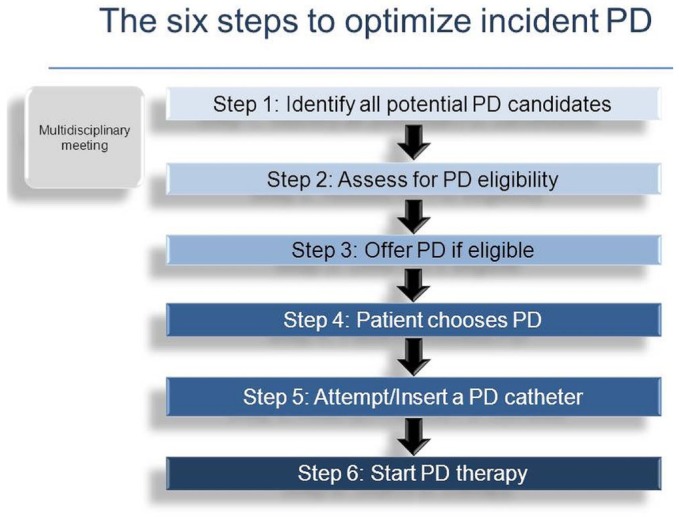
— Pathway to peritoneal dialysis (PD) for the patient.
STEP 1: IDENTIFICATION OF ALL POTENTIAL CANDIDATES FOR PD
The first step may seem self-evident, but it is vital that centers identify all possible candidates for PD so that valid measurements of PD use can be calculated and so that meaningful comparisons with other centers can be made.
Candidates include, of course, all new ESRD patients, but also those with failing grafts and those transferring from elsewhere into the center concerned. New ESRD patients include those with advanced chronic kidney disease (CKD) identified in the pre-dialysis clinic, but also those presenting late and needing to start dialysis urgently. The latter group will typically not have received modality education before dialysis initiation.
A problematic group is the patients who initially present with apparently acute or acute on chronic kidney injury. Many of these patients will recover sufficient renal function to stop dialysis, but others will not. Traditionally, registries do not identify these patients as ESRD until 90 days of dialysis have been completed. Clinicians also tend to think this way and so may not consider offering modality selection until many weeks or months have passed.
Patients starting dialysis urgently are considered less likely ever to do PD, which may become a self-fulfilling prophecy if they continue to be thought of as “non-ESRD” for weeks or months on end and are not encouraged to enter a modality education process (9). We would argue that those patients should be considered candidates for modality selection at a much earlier stage. Otherwise, a vital opportunity may be lost because of permanent decisions made by the patients during the “non-ESRD” period. With those situations in mind, we propose that a patient be defined as a candidate for PD after 30 days of dialysis dependence or once outpatient dialysis has occurred. They should then be initiated into the modality selection process. We realize that some of these patients will recover independent kidney function, but we would argue that it is better to provide modality choice for too many rather than to too few patients. The use of these definitions will ensure that all possible candidates are evaluated for PD.
Patients with failed grafts also deserve special attention. Transplant programs may be late in referring these patients to CKD clinics, and there may be less time to prepare them for dialysis or to put them through an elective modality selection process. These patients may then end up using HD by default.
To summarize, we define the PD candidate population as comprising
all patients initiated on dialysis with a diagnosis of ESRD (this is typically based on the opinion of the nephrology team and on knowledge of the patient’s prior renal function).
all patients receiving outpatient dialysis.
all patients with more than 30 consecutive days of dialysis dependence, even if they have a presumed diagnosis of acute kidney injury.
all patients with a failed graft requiring dialysis.
all ESRD patients transferred from other centers.
The 30-day definition is important here, because it ensures relatively early consideration of modality education and selection for patients who start dialysis urgently. Some of these patients will recover renal function, temporarily or permanently; but an objective definition promotes an early focus on patients who typically have a low rate of PD use. The multidisciplinary team meeting should play a central role in defining this PD candidate population.
STEP 2: ASSESSMENT FOR PD ELIGIBILITY
Once a patient is defined as a potential PD candidate, the medical team has to assess that patient’s eligibility to do PD. Ideally, this assessment will have been made in the CKD clinic, long before initiation of dialysis. However, early assessment will not have occurred in the large proportion of patients who start dialysis urgently (9-11). In that group, the assessment will typically have to start after dialysis initiation. A systematic approach to assessment for PD eligibility in a multidisciplinary team meeting with discussion of all possible PD candidates is, in our opinion, very helpful and highly recommended.
It should be noted that this assessment is not an issue of the patient choosing or not choosing to do PD, but rather of whether the team has considered the patient to be eligible for, or capable of, doing PD. This step requires an assessment of any contraindications or barriers to the patient doing PD and of the supports potentially available to the patient.
A contraindication is a factor that absolutely disqualifies the patient from doing PD regardless of physician or patient choice (7,8). Examples include massive obesity, large abdominal hernias that cannot be repaired, previous extensive abdominal surgery, and a place of residence that does not permit the patient to do PD and that cannot easily be changed (Table 1). Barriers to PD are factors that make the modality a challenge, but that do not, on their own, contraindicate it (7,8). Examples are psychiatric disease, poor hygiene, general frailty, and impaired vision (Table 2). Barriers, by definition, can be overcome if sufficient supports are available to the patient. In other words, barriers are really obstacles to self-care rather than to PD per se.
TABLE 1.
Most Frequent Contraindications to Peritoneal Dialysis (PD)
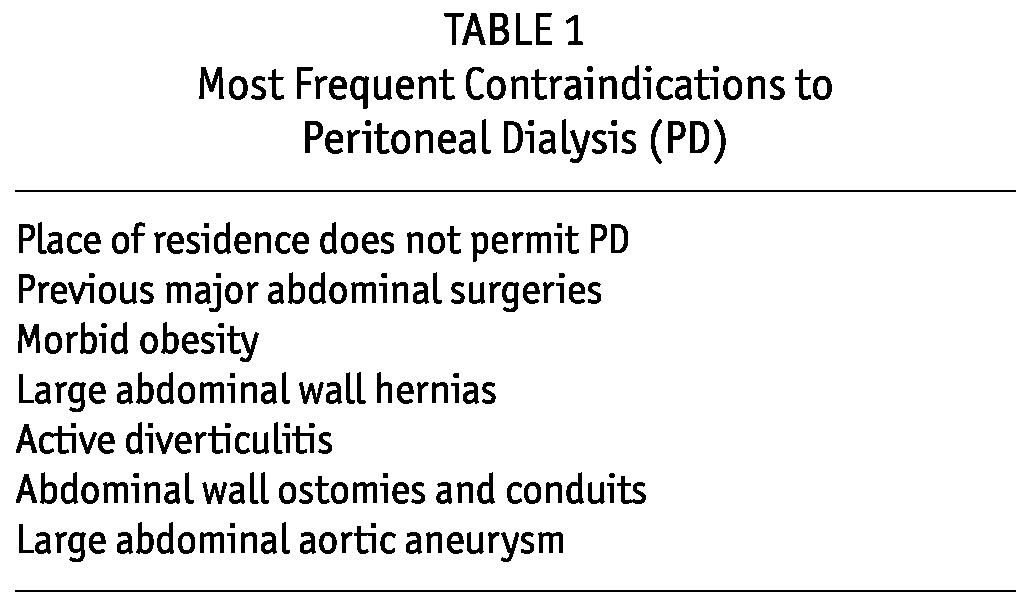
TABLE 2.
Most Frequent Barriers to Peritoneal Dialysis
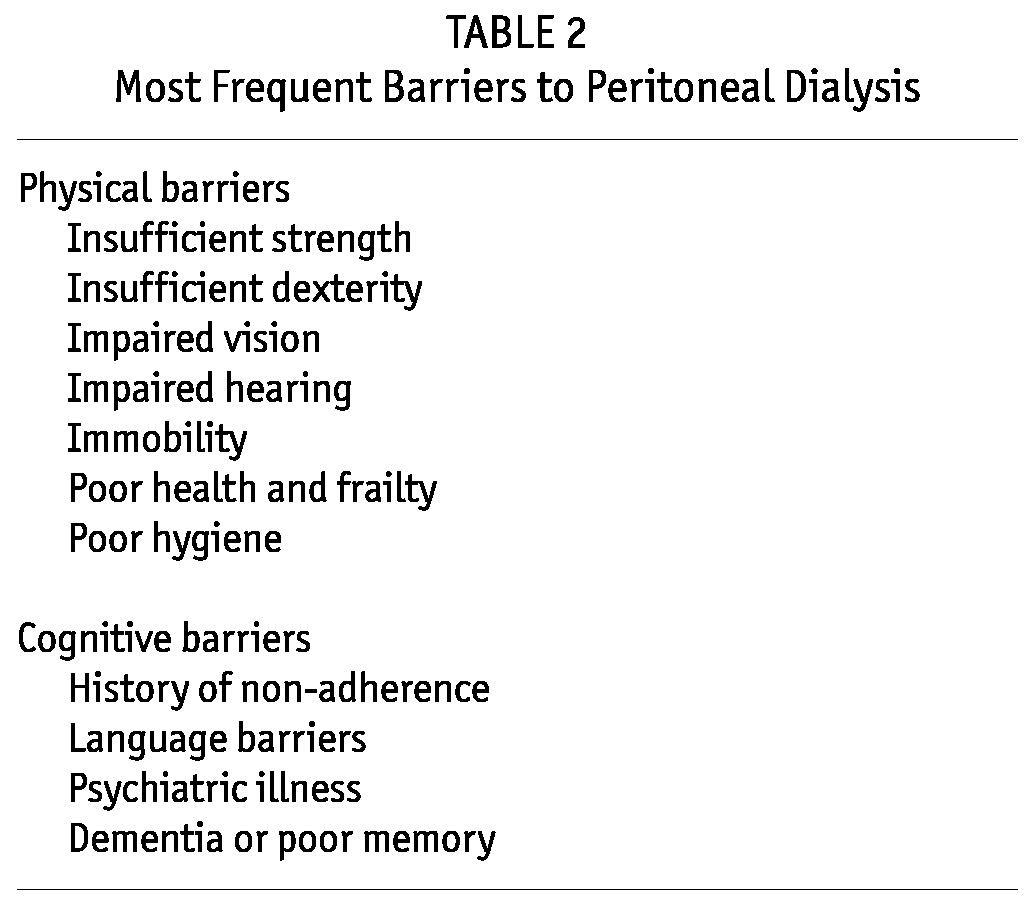
A patient can be deemed ineligible for PD by the team based on just one contraindication or on one or more barriers that, in the judgment of the team, simply cannot be overcome even if the patient is willing to try. Ability to overcome barriers is of course influenced by the degree of help that is available to the patient, whether that help is in the form of support from family or friends, from paid helpers or visiting nurses, or from other health care professionals (7,8). By definition, contraindications are not affected by support.
One method to distinguish barriers from contraindications is to ask the question “If PD were to be performed for the patient by a nurse, could this patient receive PD?” If the answer is no, then the condition is a contraindication; if the answer is yes, then the condition is usually a barrier. The assessment may be very brief if the patient has a blatant contraindication or if the patient is relatively healthy and obviously eligible. In contrast, the assessment of eligibility may be quite complex and drawn out, requiring major input from a social worker or PD nurse.
All candidates for PD as defined in step 1 should be assessed for PD eligibility either before or after dialysis initiation. The only justifiable exceptions are those who are expecting pre-emptive transplantation; those who transfer elsewhere for care; or those who die, become palliative, or recover renal function before such an assessment can be made. In practice, many candidate patients are never assessed for PD eligibility, typically because they “slip through the cracks” or because no defined process is in place. Lack of assessment is particularly likely to happen in patients who start HD urgently because of acute or acute on chronic kidney disease and who do not recover renal function. Such patients may initially be too ill to be assessed. The issue may then be forgotten, or the patient may be perceived to be “doing well” on HD. Again, the multidisciplinary team meeting is helpful in ensuring that the full modality selection process occurs in these situations.
Of patients assessed, the proportion that are declared eligible for PD will vary widely (7,8,12-14). Key factors will include the age and overall comorbidity burden of the population being accepted for dialysis. However, notable differences will typically occur between programs in the same country and even between patients attending individual nephrologists within the same program. Some of the explanations for the differences will be related to varying perceptions and biases on the part of the nephrology team. Others will reflect the services available to patients in their homes. For example, variations are often seen in the degree of obesity or in the number and nature of previous abdominal surgeries that physicians consider contraindications to PD. Differences in eligibility may also be related to the degree of home care support available to given patients. For example, for frail elderly patients who reside in an area in which a visiting homecare worker or nurse can set up the PD cycler each day, the rate of PD eligibility may run higher than it would in an area in which such support is not available. The element of experience and confidence may also be such that, compared with newer PD programs, more established PD programs might be more “aggressive” about offering PD to “marginal” patients.
When centers are compared, the estimated proportion of patients eligible to do PD is highly variable, typically running between 40% and 80%—thus being a major determinant of a program’s ultimate PD utilization percentage (7,8,12-14). The whole topic of perceived eligibility and ineligibility to do PD is an area that needs extensive research, and we think that application of the process we are suggesting would be helpful in highlighting inappropriate differences between PD programs.
STEP 3: OFFER OF MODALITY CHOICE TO THE PD-ELIGIBLE PATIENT
In step 3, each eligible patient should be offered the opportunity to do PD. Ideally, this “choice” is offered as part of a modality education process that might include group lectures, one-to-one sessions, peer education, and exposure to written material, videos, and web sites. Again, ideally, the choice and associated modality education should have been presented before dialysis initiation, but as with determination of PD eligibility, the offer may not have occurred—whether because of omission, procrastination by the patient or the medical team, or acute dialysis initiation. If the patient is already on HD, a choice might not be offered because of lack of a defined process, because the patient was too ill initially and the issue was then forgotten, or because the patient is perceived to be “doing well” on HD.
As was the case with the first two steps, ensuring that modality choice has been offered to every eligible patient must be facilitated by a clearly defined process involving a multidisciplinary team meeting and recordkeeping for every patient. It would be helpful if information about the decision made is also shared with the patient to avoid any confusion.
STEP 4: PATIENT CHOICE
Once eligible patients are offered a modality choice, most studies suggest that approximately half will select PD (15-18), and the question of how to define the modality chosen arises.
The simplest method is to record the patient’s stated decision after modality education has been completed. The objection to that approach is that some patients change their mind between the time of the initial decision and the time at which they actually have to start dialysis. A revised choice may reflect a change in functional status during the interval or a realization by the patient or the family that home dialysis may be more challenging or less desirable than was initially thought. In other cases, the patient chooses PD, but unexpectedly requires HD before arrangements for PD catheter placement can be made. A more demanding definition of “choosing PD” is the patient undergoing an attempt at PD catheter insertion, indicating that the patient is committed to PD as therapy. Although this definition has the advantage of being very clear, it does not take account the difficulties that some programs encounter in arranging for PD catheter insertion in a timely fashion. We have therefore chosen to use the patient’s modality decision, recorded after a modality education process, as the definition of patient choice having occurred.
Some patients in the CKD clinic may persistently refuse to attend modality education classes or to make (or even consider) a modality choice. Some may wish to defer that decision to the nephrology team. Others may be in a state of denial and may feel too overwhelmed to make any decision. In these situations, it might be best for the team to make the decision for the patient, perhaps in consultation with family members. This approach might sometimes provoke the patient into making a more active decision. Similarly, some patients already on HD consistently ask to defer the issue of modality selection, perhaps because they feel overwhelmed, because they believe that they will recover kidney function, or because they do not wish to switch away from HD. If they persist in this approach, they are realistically best defined as choosing not to do PD.
If fewer than a third of patients considered eligible for PD eventually choose the modality, it suggests that insufficient opportunity and encouragement to do PD is being provided. Conversely, if more than two thirds choose PD, it suggests that patients are being aggressively pushed to choose the modality, which may be similarly undesirable. There is a theoretical concern that mandating patients to receive PD may lead to higher rates of technique failure, although that concern is currently unproven. As mentioned earlier, a 50% choice for PD among eligible candidates is typical of a center providing a balanced approach.
STEP 5: PD CATHETER PLACEMENT
The next step in the path to PD is insertion of the peritoneal catheter. A significant proportion of patients who choose to do PD after being offered an informed choice never undergo catheter insertion and eventually start on HD (19). These HD starts may occur because of long wait lists to see the surgeon who places PD catheters, a procedure that is not always considered high priority in centers in which access to the operating room is limited. More often, the problem is that renal function deteriorates more rapidly than expected, and dialysis must be initiated relatively urgently. In most renal programs, urgent start on PD is not routinely practiced because of lack of an individual to place catheters at short notice and because of concerns about the feasibility of training a patient to do PD shortly after urgent catheter placement (10).
Programs that have inadequate surgical support may depend on nephrologists to place catheters. Reliance on nephrologists may result in a lack of access to PD for patients with previous abdominal surgeries or obesity, because many nephrologists will not wish to place catheters in such patients outside an operating room setting. In some centers, nephrologists are less selective and do almost all catheter placements (20). Recently, more centers have been using interventional radiologists to place catheters (21).
STEP 6: SUCCESSFUL INITIATION OF PD
Successful initiation of PD is best defined as the point at which a patient has completed training and is actually carrying out or receiving PD in the patient’s own place of residence.
A significant proportion of patients who undergo a PD catheter placement attempt do not succeed in becoming successful home PD patients. Here, the major possibilities are either that catheter insertion is unsuccessful or that the catheter itself does not function adequately to allow home PD. Catheter nonfunction rates of 10% - 15% are not unusual, though good centers will report rates of less than 5% (22,23). About half the cases of inadequate function will respond to corrective measures, but the others will be lost permanently to PD, either because the problem persists or because the patient refuses the required interventions. A second possibility is that the training itself fails because the patient or family member is found to be unable or unwilling to learn to carry out the required procedures in a safe manner. Less often, patients change their mind or experience an unexpected change in health status between catheter insertion and PD training. In general, more than 85% of patients who have an attempted PD catheter placement should eventually do home PD successfully. Anything less reflects a problem with either catheter placement or the original assessment of patient eligibility.
PROCESS SUMMARY
Ideally, the 6 steps we have described should occur in order, leading to elective initiation of the preferred modality of dialysis. However, in patients starting dialysis urgently, the sequence of steps may change. Identification as a candidate, assessment for eligibility, and patient choice might follow rather than precede initiation of dialysis. The initial modality will typically be HD because only rarely do programs use PD in urgent dialysis starts (10). The “urgent start” group is a large proportion of all ESRD cases—at least 20% - 40% and more than 60% if the definition is expanded to include all inpatient starts (24). In this group, PD utilization is often very low, and therefore the process is most important and, potentially, most fruitful in programs aiming to increase overall PD use.
In practice, and for the purposes of the present analysis, we consider this group of patients to have successfully initiated PD if they do so any time within 6 months of HD initiation. In other words, actual PD starts and switches to PD within the first 6 months are both included. The 6-month cut-off is arbitrary because some patients will still switch after 6 months on HD; however, to allow for comparisons a “line” has to be drawn somewhere.
Notwithstanding the foregoing complexities, the 6 steps to PD are represented as one pathway in Figure 1.
The approach described by the 6 steps depends on a standardized process and associated definitions. The multidisciplinary team meeting plays a critical role in ensuring that no patient is missed. Central to the success of this approach has been our use of the DMAR system. All data elements are reviewed by experts to ensure accuracy and consistency in coding. Metrics reflecting the movement of patients through each step in the process of care are reported back to renal replacement therapy programs, benchmarked against their peers. The programs can then identify their strengths and weakness and can target resources to key steps that require attention (7,8). The “apples to apples” comparisons thus facilitated give programs confidence that their local practice is being compared in a valid manner.
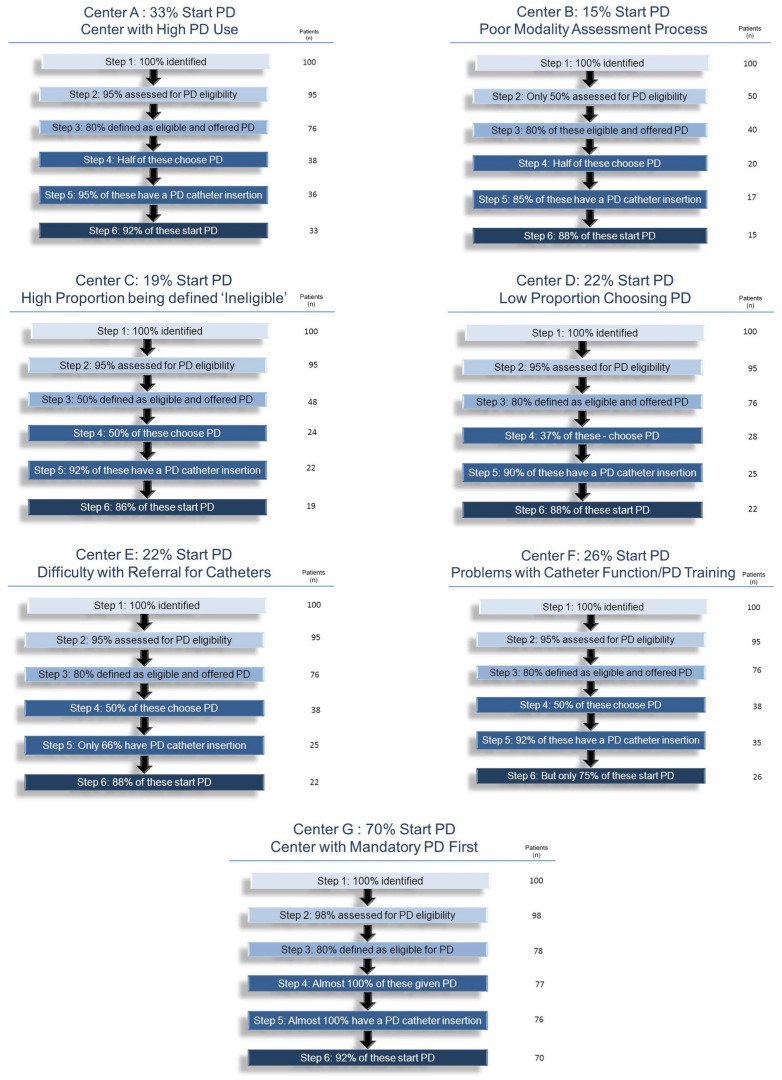
Examples: To demonstrate the utility of the process, we created some hypothetical centers that are based on actual observations from our network of renal replacement therapy programs.
Center A has a high rate of PD utilization. Figure 2(A) shows that 80% of patients are being declared eligible for PD, 50% of eligible patients are choosing PD, and in turn, 90% of them end up at home on PD, yielding an incident PD rate of 33%. Comparing data from center B, it would be helpful to understand why that center has a notably lower incident PD rate despite an apparent common commitment to maximize the use of PD.
Figure 2.
— (A-G) Comparison of pathways to peritoneal dialysis (PD) in 7 theoretical centers. For each center, the column to the right shows how 100 patients in the particular program might progress through the 6 steps.
As can be seen from Figure 2(B), both centers are equally successful in defining their ESRD populations, and both are equally likely to declare patients eligible to do PD. The centers show very little difference in the proportions of patients offered PD who choose it and of those who had catheters inserted and who successfully completed training. The difference can be found in the proportions of patients assessed for PD eligibility; only 50% were assessed in center B compared with 95% in center A. The effect is large, with only 15% of incident ESRD patients initiating PD in center B. Center B lacks a comprehensive and systematic modality assessment process, which is making a huge difference.
Another common scenario is seen in comparing hypothetical center C with center A. As can be seen from Figure 2(C), both centers are successful in defining their ESRD populations and in assessing almost all patients for PD eligibility. The centers also show very little difference in terms both of the proportion of patients offered PD who actually choose it and of the proportion choosing PD who were successfully initiated on that modality. However, the proportions of patients deemed eligible for PD are notably different, being 80% at center A, but only 50% at center C. The result, as can be seen from Figure 2, is that center A successfully initiated PD in 33% of incident ESRD patients, but that center C initiated only 19%—a relative difference of about 40%. Center C is being more selective in offering PD. Further investigation might suggest that physicians at center C have been unduly conservative with respect to conditions such as obesity, previous surgery, and hernias during the consideration of contraindications to PD, or with respect to the degree to which barriers are being considered impossible to overcome.
In a third example, center A is compared with center D [Figure 2(D)]. Center D also has a lower PD start rate than center A despite a similar percentage of its patients being declared eligible for PD and being offered the choice to do it, and despite a similarly high success rate in converting PD choice to eventual PD utilization. Here, the difference is found in the percentage of patients choosing PD: only 36% are making that choice at center D, compared with 50% at center A. As a result, 22% started PD at center D compared with 33% at center A, a relative difference of 25%. Center D might have an issue with its modality education process, possibly presenting home PD in a less favorable light. Perhaps cyclers are not being made available, or perhaps there is an issue with the degree of support that is being offered in the home after PD initiation.
In the fourth example, shown in Figure 2(E), the rate of PD utilization is lower in center E than in center A despite similar rates of PD eligibility and choice. Here, the difference is in the rate at which patients choosing PD eventually come to have a catheter inserted. The catheter insertion rate is 65% in center E and 95% in center A. The end result is 22% rather than 33% of incident ESRD patients starting PD. The problem is probably access to a surgeon or physician who will place catheters in a timely manner. This problem is common in centers that depend on a surgeon. Often, long delays result in the patient acutely starting HD and then abandoning the idea of doing PD.
In the fifth example, shown in Figure 2(F), center F has a lower rate of PD utilization than center A despite similar rates of PD eligibility and choice. Here, the difference is in the rate of conversion of PD catheter placement to successful PD use at home, which is 75% instead of 90%, with the result that incident PD use is 26% rather than 33%. This observation raises the possibility of a technical problem with catheter placement or function. Alternatively, the problem may be with the approach to teaching PD to patients. Perhaps center F is quicker to give up on patient training and to declare individuals unsafe to carry out PD at home. That situation might, in turn, reflect less availability of home support in some centers compared with others.
Finally, Figure 2(G) shows a hypothetical center G with a mandatory “PD First” policy, which therefore does not give free choice to patients. Here, 70% of incident ESRD patients start with PD; only those who are truly unable are excluded.
Note that differences in the earlier steps—for example, the rate of modality assessment or of defined PD eligibility—tend to have the greatest detrimental effect on incident PD use.
Using the DMAR approach, centers can assess how they are performing at each of the 6 steps in the process of care. They can then target resources to the specific steps that need attention to optimize incident PD use.
OTHER ASPECTS
To apply the described methodology in a useful and valid manner, it is necessary to track a substantial number of patients through the modality selection process. The ideal number is about 100. In large centers, reaching that goal should not take much longer than 1 - 2 years. Data collected over longer periods of time may increase patient numbers, but carries the risk that older data no longer reflects current practice, which is always changing.
It should also be noted that programs might vary in the percentage of patients choosing to start PD. Such variations are typically a result of differences in the characteristics of the candidate patients, rather than differences in the criteria for eligibility, in the presentation of choice, or in the ability to initiate willing patients successfully. Some programs deal with populations that are older, or more urban, or more rural, or more affluent, or that include higher proportions of particular ethnic groups. Some centers have a larger proportion of urgent starts or of unresolved acute kidney injury patients that might influence eligibility for, or choice of, PD. As mentioned earlier, centers with high pre-emptive transplant rates may have proportionately fewer healthy younger patients and thus more patients who are perhaps less willing and able to do PD. In centers having more patients with failed grafts, proportionately more patients might be willing to take on home dialysis. Accordingly, comparisons have to be interpreted against the background of these baseline factors, which are therefore recorded as part of the process.
PERCENTAGE PD PREVALENCE
The process set out here deals only with incident ESRD patients and the proportion of them who do PD. The rate of PD utilization in a program is, of course, a measure of prevalence and so will be influenced not only by the percentage of patients who successfully start PD, but also by the program’s ability to retain those patients on the modality. Retention, however, is another complex measure; it will be discussed in a future paper.
CONCLUSIONS
We present an approach to the “black box” that is the process of selecting a renal replacement therapy modality for patients with ESRD. We apply this approach using a customized software program and regular interdisciplinary team meetings at our respective centers. As a result, we have been able to dissect out the key steps involved in modality selection. Moreover, the use of the same approach by multiple centers has made it possible to compare results and draw valid conclusions concerning why higher rates of PD use in incident ESRD patients are seen in some centers compared with others.
Adherence by the nephrology team to the process ensures that each patient is adequately assessed for modality and, if deemed eligible, given the opportunity to do PD. We believe that every patient has the right to be at least considered for PD. A similar process can be used to ensure that every patient is also assessed for kidney transplantation or even home HD in programs in which that option is available.
DISCLOSURES
MJO and RRQ are co-inventors of the Dialysis Measurement Analysis and Reporting (DMAR) system.
References
- 1. van Biesen W, Veys N, Lameire N, Vanholder R. Why less success of the peritoneal dialysis programmes in Europe? Nephrol Dial Transplant 2008; 23:1478–81 [DOI] [PubMed] [Google Scholar]
- 2. Blake PG, Finkelstein FO. Why is the proportion of patients doing peritoneal dialysis declining in North America? Perit Dial Int 2001; 21:107–14 [PubMed] [Google Scholar]
- 3. Just PM, Riella MC, Tschosik EA, Noe LL, Bhattacharyya SK, de Charro F. Economic evaluations of dialysis treatment modalities. Health Policy 2008; 86:163–80 [DOI] [PubMed] [Google Scholar]
- 4. Oreopoulos DG, Coleman S, Doyle E. Reversing the decreasing peritoneal dialysis trend in Ontario. Perit Dial Int 2008; 28:201–3 [PubMed] [Google Scholar]
- 5. Nissenson AR, Prichard SS, Cheng IK, Gokal R, Kubota M, Maiorca R, et al. Non-medical factors that impact on ESRD modality selection. Kidney Int 1993; 40:S120–7 [PubMed] [Google Scholar]
- 6. Just PM, de Charro F, Tchosik EA, Noe LL, Bhattacharraya SK, Riella MC. Reimbursement and economic factors influencing dialysis modality choice around the world. Nephrol Dial Transplant 2008; 23:2365–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oliver MJ, Quinn RR, Richardson EP, Kiss AJ, Lamping DL, Manns BJ. Home care assistance and the utilization of peritoneal dialysis. Kidney Int 2007; 71:673–8 [DOI] [PubMed] [Google Scholar]
- 8. Oliver MJ, Garg AX, Blake PG, Johnson JF, Verrelli M, Zacharias JM, et al. Impact of contraindications, barriers to self-care, and support on incident peritoneal dialysis utilization. Nephrol Dial Transplant 2010; 25:2737–44 [DOI] [PubMed] [Google Scholar]
- 9. Lameire N, Wauters JP, Teruel JL, Van Biesen W, Vanholder R. An update on the referral pattern of patients with end-stage renal disease. Kidney Int Suppl 2002; (80):27–34 [DOI] [PubMed] [Google Scholar]
- 10. Povlsen JV, Ivarsen P. How to start the late referred ESRD patient urgently on chronic APD. Nephrol Dial Transplant 2006; 21(Suppl 2):ii56–9 [DOI] [PubMed] [Google Scholar]
- 11. Smart NA, Titus TT. Outcomes of early versus late nephrology referral in chronic kidney disease: a systematic review. Am J Med 2011; 124:1073–80 [DOI] [PubMed] [Google Scholar]
- 12. Mendelssohn DC, Mullaney RL, Jung B, Blake PG, Mehta RL. What do American nephrologists think about dialysis modality selection? Am J Kidney Dis 2001; 37:22–9 [DOI] [PubMed] [Google Scholar]
- 13. Goovaerts T, Jadoull M, Goffin E. Influence of a predialysis education program (PDEP) on the mode of renal replacement therapy. Nephrol Dial Transplant 2005; 20:1842–7 [DOI] [PubMed] [Google Scholar]
- 14. Morton RL, Snelling P, Webster AC, Rose J, Masterson R, Johnson DW, et al. Dialysis modality preferences of patients with CKD and family caregivers: a discrete-choice study. Am J Kidney Dis 2012; 60:102–11 [DOI] [PubMed] [Google Scholar]
- 15. Prichard SS. Treatment modality selection in 150 consecutive patients starting ESRD therapy. Perit Dial Int 1996; 16:69–72 [PubMed] [Google Scholar]
- 16. Golper T. Patient education: can it maximize the success of therapy? Nephrol Dial Transplant 2001; 16(Suppl 7):20–4 [DOI] [PubMed] [Google Scholar]
- 17. Manns BJ, Taub K, Vanderstraeten C, Jones H, Mills C, Visser M, et al. The impact of education on chronic kidney disease patients’ plans to initiate dialysis with self-care dialysis: a randomized trial. Kidney Int 2005; 68:1777–83 [DOI] [PubMed] [Google Scholar]
- 18. Lacson E, Jr, Wang W, DeVries C, Leste K, Hakim RM, Lazarus M, et al. Effects of a nationwide predialysis program on modality choice, vascular access and patient outcomes. Am J Kidney Dis 2011; 58:235–42 [DOI] [PubMed] [Google Scholar]
- 19. Liebman SE, Bushinsky DA, Dolan JG, Veazie P. Differences between dialysis modality selection and initiation. Am J Kidney Dis 2012; 59:550–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li PK, Chow KM. Importance of peritoneal dialysis catheter insertion by nephrologists: practice makes perfect. Nephrol Dial Transplant 2009; 24:3274–6 [DOI] [PubMed] [Google Scholar]
- 21. Brunier G, Hillier JA, Drayton S, Pugash RA, Tobe SW. A change to radiological PD catheter insertion: three-month outcomes. Perit Dial Int 2010; 30:528–33 [DOI] [PubMed] [Google Scholar]
- 22. Crabtree JH. Selected best demonstrated practices in peritoneal dialysis access. Kidney Int Suppl 2006; (103):S27–37 [DOI] [PubMed] [Google Scholar]
- 23. Crabtree JH. Who should place peritoneal dialysis catheters? Perit Dial Int 2010; 30:142–50 [DOI] [PubMed] [Google Scholar]
- 24. Quinn RR, Hux JE, Oliver MJ, Austin PC, Tonelli M, Laupacis A. Selection bias explains differential mortality between dialysis modalities. J Am Soc Nephrol 2011; 22:1534–42 [DOI] [PMC free article] [PubMed] [Google Scholar]