Abstract
♦ Background: The effects of novel biocompatible peritoneal dialysis (PD) solutions on human peritoneal membrane pathology have yet to be determined. Quantitative evaluation of human peritoneal biopsy specimens may reveal the effects of the new solutions on peritoneal membrane pathology.
♦ Methods: Peritoneal specimens from 24 PD patients being treated with either acidic solution containing high-glucose degradation products [GDPs (n = 12)] or neutral solution with low GDPs (n = 12) were investigated at the end of PD. As controls, pre-PD peritoneal specimens, obtained from 13 patients at PD catheter insertion, were also investigated. The extent of peritoneal fibrosis, vascular sclerosis, and advanced glycation end-product (AGE) accumulation were evaluated by quantitative or semi-quantitative methods. The average densities of CD31-positive vessels and podoplanin-positive lymphatic vessels were also determined.
♦ Results: Peritoneal membrane fibrosis, vascular sclerosis, and AGE accumulation were significantly suppressed in the neutral group compared with the acidic group. The neutral group also showed lower peritoneal equilibration test scores and preserved ultrafiltration volume. The density of blood capillaries, but not of lymphatic capillaries, was significantly increased in the neutral group compared with the acidic and pre-PD groups.
♦ Conclusions: Neutral solutions with low GDPs are associated with less peritoneal membrane fibrosis and vascular sclerosis through suppression of AGE accumulation. However, contrary to expectation, blood capillary density was increased in the neutral group. The altered contents of the new PD solutions modified peritoneal membrane morphology and function in patients undergoing PD.
Key words: AGEs, angiogenesis, biocompatible solution, GDPs, peritoneal fibrosis, vascular sclerosis
Peritoneal dialysis (PD) solution should not contain bioincompatible elements such as high glucose, low pH, and glucose degradation products (GDPs) (1,2). Those bioincompatible elements promote peritoneal fibrosis, hyalinizing vasculopathy, deposition of advanced glycation end-products (AGEs), and angiogenesis (3,4), which in turn lead to membrane hyperpermeability and reduced ultrafiltration capacity because of the involvement of several factors, including transforming growth factor-β, vascular endothelial growth factor, aquaporin-1, and endothelial nitric oxide synthase (5-8).
Recently, biocompatible PD solutions have been developed to prevent PD-related peritoneal damage and potentially to prolong the duration of PD treatment. These biocompatible PD solutions include neutral-pH solutions buffered with lactate or bicarbonate, low-GDP solutions that do not require heat sterilization, and solutions with icodextrin and amino acids instead of glucose as osmolar substances; all have been used in combination with conventional PD solutions (9). Lactate-buffered neutral PD solutions with low GDPs achieved using a two-chambered bag system were introduced for clinical use in Japan more than a decade ago. These biocompatible solutions are expected to reduce morphologic and functional peritoneal deterioration and the incidence rate of encapsulating peritoneal sclerosis, a devastating complication of PD.
The effectiveness of the new solutions for the prevention of peritoneal fibrosis and angiogenesis has been demonstrated experimentally (10-13). Several clinical studies have evaluated their effects (14,15), and the results of those studies indicated their usefulness in maintaining peritoneal integrity. However, the histologic effects of the new solutions on human peritoneal membrane pathology have not been evaluated.
We hypothesized that use of neutral low-GDP solutions may improve peritoneal membrane deterioration by suppression of peritoneal AGE accumulation. To confirm that hypothesis, we set out in the present study to elucidate the histologic effects of low-GDP solutions by evaluating peritoneal biopsy samples obtained from patients treated solely with either conventional acidic solutions with high GDPs or neutral low-GDP solutions, both of which are buffered with lactate. Possible relationships between the use of the new PD solutions and peritoneal membrane pathology are discussed in terms of their clinical relevance.
METHODS
PATIENT COHORT
Between 1980 and 2010, we treated 379 patients with PD and performed 205 peritoneal biopsies at our center. The present study enrolled 29 patients at catheter insertion (pre-PD) and 176 at catheter removal (post-PD). To eliminate any influence of peritonitis on peritoneal histology, patients with a history of peritonitis during PD therapy were excluded from the study. Because neutral solutions have been used clinically only since 2002 at our center, a limited number of patients (n = 12) had been treated solely using such solutions (Dianeal-N: Baxter, Tokyo, Japan; Perisate N: JMS, Tokyo, Japan). Patients for the acidic group were selected from among 43 patients treated solely with acidic solution (Dianeal: Baxter; Perisate: JMS), who were matched with the patients of the neutral group based on age, sex, PD duration, and number in the group (n = 12). All patients underwent peritoneal biopsy at the time of PD catheter removal or when they withdrew from PD therapy. All clinical information was collected from medical records. Approval for the study was obtained from the Tokyo Women’s Medical University ethics committee.
Clinical data included age, sex, primary cause of renal failure, PD duration, use of high-glucose solution (≥2.27 g/dL), residual renal function (represented by residual urine volume in milliliters per day), ultrafiltration volume [UFV (milliliters per day)], AGE accumulation score, peritoneal equilibration test (PET) category at 6 - 12 months after PD initiation or before PD withdrawal, and administration of angiotensin converting-enzyme inhibitor or angiotensin II receptor blocker, statins, and vitamin D (Table 1). Patients who were prescribed high-glucose solution once or more daily to maintain fluid balance were defined as users of high-glucose solution. Ultrafiltration failure was defined as a daily UFV below 400 mL.
TABLE 1.
Clinical Characteristics of Patients on Acidic and Neutral Dialysate at the End of Peritoneal Dialysis (PD)
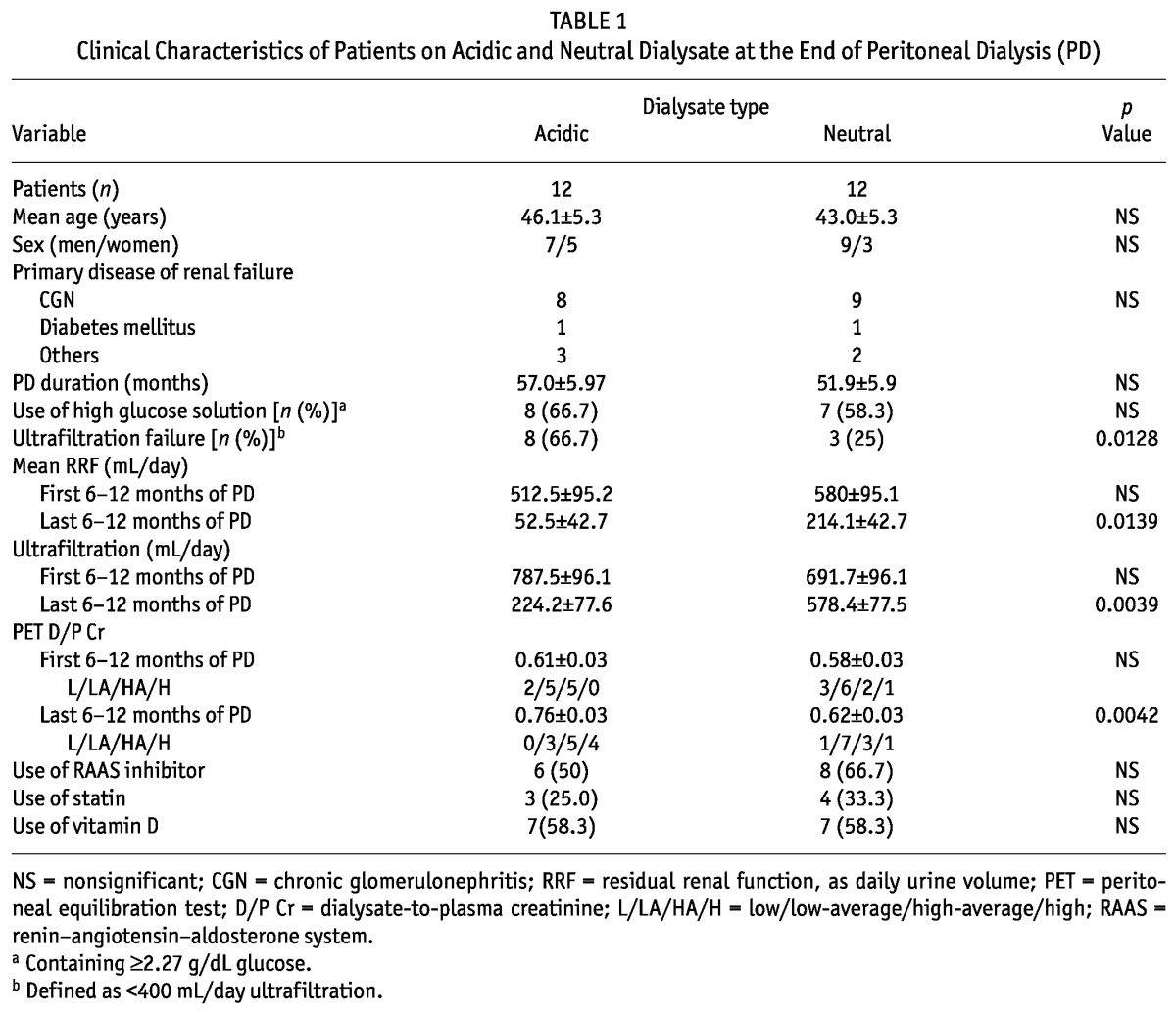
The reasons for PD withdrawal in the study cohort were PD-related problems such as ultrafiltration failure (acidic group: n = 8; neutral group: n = 3), desire to transfer to hemodialysis (acidic group: n = 2; neutral group: n = 2), renal transplantation (acidic group: n = 2; neutral group: n = 5), and other (neutral group: n = 2).
As a control, pre-PD samples were selected randomly from 13 of 29 patients who underwent peritoneal biopsy at the time of PD catheter insertion. Among the 13 patients who provided pre-PD samples (9 men, 4 women), mean age was 48.2 ± 4.2 years, mean serum creatinine was 8.01 ± 0.63 mg/dL, estimated glomerular filtration rate was 6.96 ± 0.60 mL/min/1.73 m2, and primary causes of renal failure were chronic glomerulonephritis (n = 8), diabetes mellitus (n = 1), and other (n = 4).
HISTOLOGIC EVALUATION FOR PERITONEAL FIBROSIS AND VASCULAR SCLEROSIS
Peritoneal biopsy specimens were cut by scalpel to approximately 1 cm×≤5 mm and processed using routine pathology procedures. Two pathology experts, blinded to the clinical backgrounds of the patients, jointly reviewed the samples to arrive at a consensus evaluation. The extent of peritoneal fibrosis and of vascular sclerosis was evaluated as previously reported (16). Briefly, for the evaluation of peritoneal fibrosis, we calculated the average thickness (μm) at 5 random points within the submesothelial compact zone (SMC) between the basal border of the surface mesothelial cells and the upper border of peritoneal adipose tissue. For the evaluation of vascular sclerosis, the average ratio of lumen-to-vessel diameter was calculated at 5 randomly selected postcapillary venules (PCVs) with external diameters of 25 - 50 μm.
IMMUNOHISTOCHEMISTRY
The primary antibodies used were a mouse monoclonal antibody against the AGE carboxymethyllysine (TransGenic, Kobe, Japan); a mouse monoclonal antibody against human CD31 (Dako Cytomation, Glostrup, Denmark), a marker of vascular endothelial cells; and a mouse monoclonal antibody against human podoplanin (AngioBio, Del Mar, CA, USA), a marker of lymphatic endothelial cells. The Envision+ System (Dako Cytomation) was used as the secondary antibody, and the antibody reaction was visualized using diaminobenzidine tetrahydrochloride (0.02%). Cell nuclei were counter-stained with hematoxylin. To examine colocalization of CD31 and podoplanin in lymphatic endothelial cells, we performed double staining for CD31 and podoplanin using Alexa Fluor 488- or 568-labelled goat anti-mouse immunoglobulin G (Invitrogen, Carlsbad, CA, USA) as the secondary antibody and DAPI (Sigma-Aldrich, St. Louis, MO, USA) to fluorescently stain cell nuclei.
EVALUATION OF AGE ACCUMULATION
Accumulation of AGE in the interstitium and vasculature was evaluated by a semi-quantitative grading method: grade 0, no AGE accumulation; grade 1, mild accumulation; grade 2, moderate accumulation; and grade 3, severe accumulation (17). The total grade score for AGE accumulation in the interstitium and vasculature were analyzed in association with PD duration.
EVALUATION OF VESSEL DENSITY
The total of CD31-positive vessels and podoplanin-positive lymphatic vessels located in the peritoneal interstitium were assessed under 200× magnification using a Leica DMD108 digital microscope (Leica Microsystems, Wetzlar, Germany). The type of CD31-positive vessel (capillary or PCV) was determined based on the external diameter (in micrometers) along the short axis: vessels less than 15 μm in diameter were defined as capillaries; those 15 - 100 μm in diameter were defined as PCVs. The density of each vessel type was calculated as the average number of vessels per area (square millimeter), assessed in 5 randomly selected fields of the specimen.
STATISTICAL ANALYSIS
Data were compared by analysis of variance and are presented as mean ± standard error of the mean. Correlation coefficients were assessed for significance using the F-test. The statistical analysis was performed using the JMP software application (version 8: SAS Institute, Cary, NC, USA). In all analyses, p < 0.05 was considered to indicate statistical significance.
RESULTS
CLINICAL FINDINGS
Age and PD duration did not differ significantly between the acidic- and neutral-solution groups (Table 1). High-glucose PD solution was used equally in both groups. The incidence of ultrafiltration failure was significantly higher in the acidic group. At the start of PD, neither residual renal function nor UFV differed between the groups. However, at the end of PD, residual renal function and UFV were both significantly higher in the neutral group than in the acidic group. At the start of PD, PET results were not significantly different; however, by the end of PD, the dialysate-to-plasma ratio of creatinine had become significantly higher in the acidic group. The groups showed no significant difference in the use of angiotensin converting-enzyme inhibitors or angiotensin II receptor blockers, statins, or vitamin D.
PERITONEAL HISTOLOGY
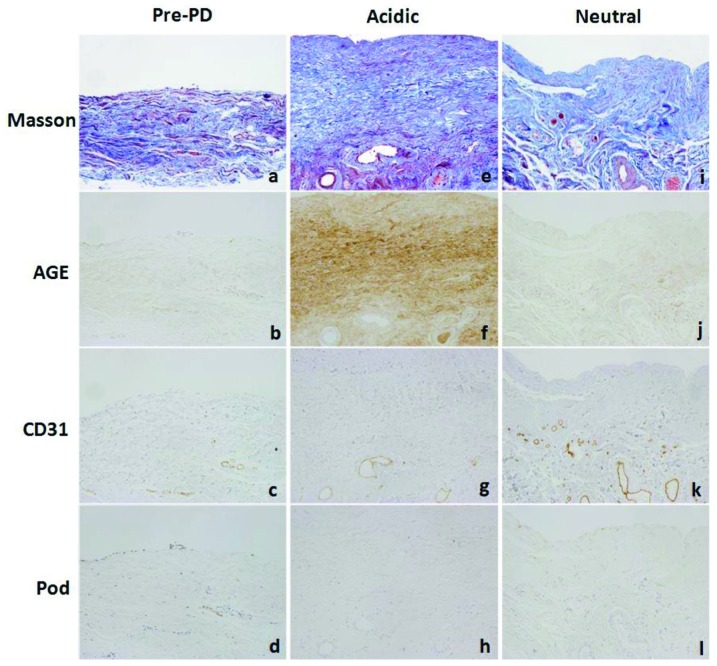
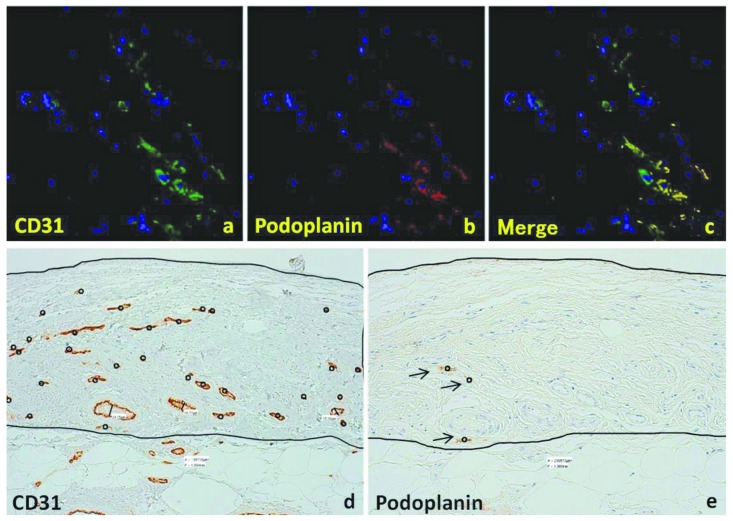
Figure 1 shows peritoneal histology findings and results of immunohistochemistry for AGE, CD31, and podoplanin in representative cases from the pre-PD and in the acidic- and neutral-solution groups. Peritoneal specimens from the acidic group showed significant fibrosis in the SMC, accompanied by hyalinizing degeneration of collagen fibers. The walls of PCVs and capillaries are thickened and hyalinized in the acidic group [Figure 1(e)]; such alterations tended to be reduced in the neutral group [Figure 1(i)]. In the acidic group compared with the neutral group, AGE accumulation was noted in the interstitium, and the vascular walls showed more intense staining [Figure 1(f) and (j) respectively]. Slight AGE accumulation was observed in the pre-PD peritoneal samples [Figure 1(b)]. Staining for CD31 revealed increased vascularity in the neutral group [Figure 1(k)] compared with the pre-PD [Figure (c)] and acidic groups [Figure 1(g)]; however, podoplanin staining showed scant distribution of lymphatic vessels, without an increase in density in any group [Figure 1(d,h,l)]. Figure 2(a-c) shows the colocalization of CD31 and podoplanin in lymphatic endothelial cells. Figure 2(d) shows an example of vessel density analysis, counting the number of capillaries and PCVs detected by CD31 staining divided by the area of the SMC. The density of podoplanin-positive lymphatic capillaries was evaluated in the same manner [Figure 2(e)].
Figure 1.
— Peritoneal histology and immunohistochemistry for advanced glycosylation end-products (AGEs), CD31, and podoplanin (Pod) in (a-d) the pre-peritoneal dialysis (PD) group and (e-h) the acidic and (i-l) neutral dialysate groups. 200× original magnification. Peritoneal samples from the acidic dialysate group show significant fibrosis in the submesothelial compact zone, with accompanying hyalinizing degeneration of collagen fibers. In the acidic group, AGEs also accumulated more intensely. Staining for CD31 revealed increased vascularity in the neutral group; however, staining for podoplanin revealed no increase of podoplanin-positive lymphatic vessels, and no differences between the groups. Masson = Masson trichrome stain.
Figure 2.
— Double immunostaining with for CD31 and podoplanin for vessel density analysis. (a) CD31-positive vessels (green). (b) Podoplanin-positive vessels (red). (c) Merge of CD31 and podoplanin staining. (d) Vessel density analysis for a representative CD31-positive sample (n = 30, black circles). The area of the submesothelial compact zone is 0.199 mm2, yielding a vessel density of 150.7/mm2). (e) Vessel density analysis for a representative podoplanin-positive lymphatic vessel (n = 3, black arrows). The area of the submesothelial compact zone is 0.209 mm2, yielding a vessel density of 14.4/mm2).
QUANTITATIVE COMPARISON OF PERITONEAL MEMBRANE PATHOLOGY
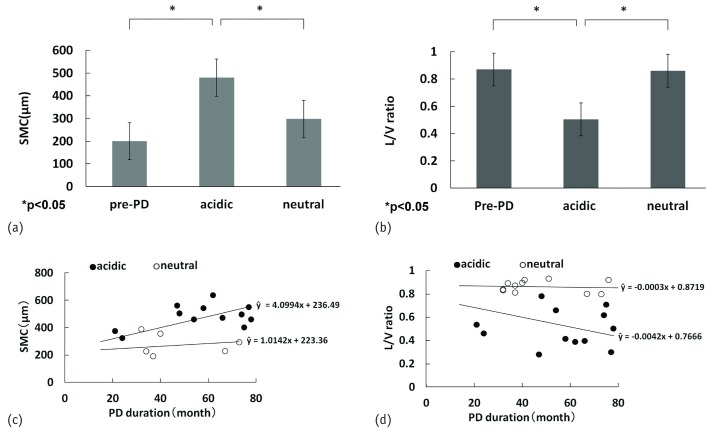
The average SMC thickness was significantly greater in the acidic group (482.5 ± 24.3 μm) than in the neutral (281.4 ± 34.4 μm) and pre-PD (201.0 ± 36.1 μm) groups, but no significant difference was observed between the pre-PD and neutral groups [Figure 3(a)]. The average ratio of lumen-to-vessel diameter in PCVs was significantly smaller in the acidic group (0.50 ± 0.03) than in the pre-PD (0.87 ± 0.02) and neutral (0.86 ± 0.03) groups, but no significant difference was observed between the pre-PD and neutral groups [Figure 3(b)].
Figure 3.
— Quantitative comparison of peritoneal fibrosis and vascular sclerosis and their correlation with peritoneal dialysis (PD) duration. (a) Thickness of the submesothelial compact zone (SMC). (b) Ratio of lumen-to-vessel (L/V) diameter. (c) Correlation between SMC and PD duration. (d) Correlation between L/V ratio and PD duration. The SMC was thinner and the L/V ratio was greater, significantly so, in the group on neutral dialysate compared with the group on acidic dialysate. The SMC thickness and L/V ratio were significantly correlated with PD duration in the group on acidic dialysate; no significant correlation was found in the group on neutral dialysate.
In the acidic group, a significant correlation was observed between SMC thickness and PD duration [r = 0.821, p < 0.0001; Figure 3(c)], and a weak correlation was observed between the ratio of lumen-to-vessel diameter and PD duration [r = -0.592, p = 0.00200; Figure 3(d)]; however, no significant correlation was observed in the neutral group [Figure 3(c,d)].
GRADE OF AGE ACCUMULATION
The grade of AGE accumulation in the interstitium and vascular wall was significantly higher in the acidic group than in the neutral and pre-PD groups. The total grade of AGE accumulation was also significantly higher in the acidic group than in the neutral and pre-PD groups (Figure 4).
Figure 4.
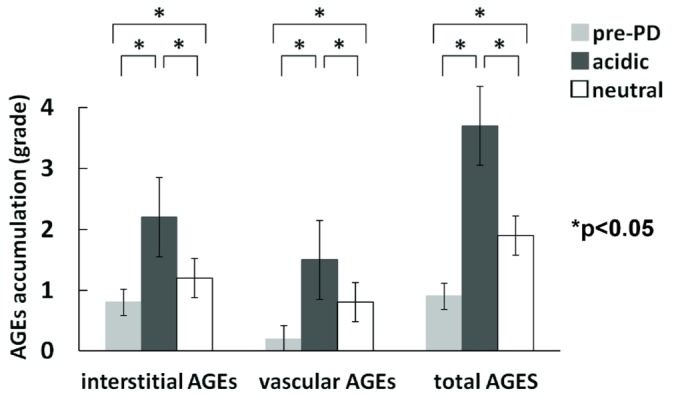
— Comparison of peritoneal accumulation of advanced glycosylation end-products (AGEs)—interstitial, vascular, and total. The grades of AGE accumulation in the interstitium and vascular wall were significantly higher in the group on acidic dialysate than in the group on neutral dialysate. The total grade of AGE accumulation was also significant higher in the group on acidic dialysate.
PERITONEAL VESSEL DENSITY
The capillary density detected by staining for CD31 was significantly higher in the neutral group (90.4 ± 3.3/mm2) than in the acidic (30.6 ± 3.5/mm2) and pre-PD groups [51.3 ± 3.3/mm2, Figure 5(a)]. However, the podoplanin-positive lymphatic capillary density showed no significant difference between groups (neutral: 12.3 ± 0.9/mm2; acidic: 11.1 ± 1.1/mm2; pre-PD: 10.6 ± 0.8/mm2). The density of CD31-positive capillaries was significantly and positively correlated with PD duration in the neutral group (r = 0.7168, p = 0.0006) and negatively correlated with PD duration in the acidic group [r = -0.603, p = 0.00080; Figure 5(b)].
Figure 5.
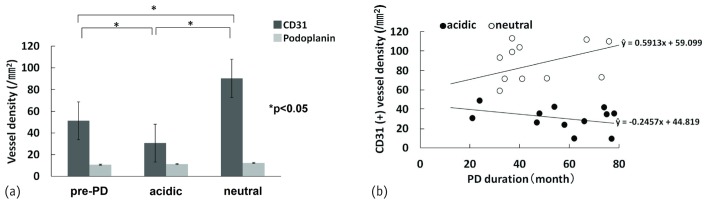
— Quantitative comparison of capillary and lymphatic vessel density and correlation between capillary density and peritoneal dialysis (PD) duration. (a) The capillary density determined by CD31 staining was significantly higher in the group on neutral dialysate than in the group on acidic dialysate or not yet on PD. However, lymphatic vessel density did not differ significantly between the groups. (b) The density of CD31-positive (CD31+) capillaries was positively correlated with PD duration in the group on neutral dialysate, but it was negatively correlated with PD duration in the group on acidic dialysate.
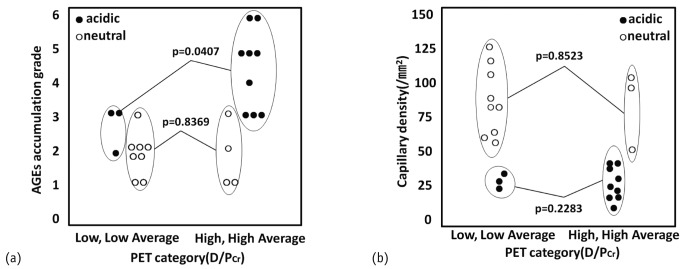
PET CATEGORY
In the acidic group, the total grade of AGE accumulation was significantly higher in the high-average and high (HA/H) PET categories than in the low and low-average (L/LA) categories [p = 0.0407, [Figure 6(a)]; however, capillary density in the acidic group did not differ between the L/LA and HA/H categories [Figure 6(b)]. In the neutral group, no association between PET category and grade of AGE accumulation or capillary density was observed [Figure 6(a,b)]. These results indicate that the severity of AGE accumulation, but not capillary density, is associated with peritoneal hyperpermeability as determined by a PET.
Figure 6.
— Accumulation of advanced glycosylation end-products (AGEs) and capillary density compared between patients grouped by low and low-average (L/LA) and high-average and high (HA/H) transport categories as determined by dialysate-to-plasma ratio of creatinine (D/P Cr) in a peritoneal equilibration test (PET). (a) In the group on acidic dialysate, the total grade of AGE accumulation was significantly higher in the HA/H patients than in the L/LA patients; however, (b) the capillary density did not differ between the L/LA and HA/H patients. No correlation was observed between PET category and AGE accumulation or capillary density in the group on neutral dialysate.
DISCUSSION
Recently developed PD solutions that eliminate various bioincompatible factors such as low pH, lactate, glucose, and GDPs have been expected to reduce PD-related peritoneal deterioration. Although the precise effect of each factor is not fully understood, several experimental (12,13) and clinical (14,15,18) trials have identified factors that play a causative role in the pathogenesis of PD-induced peritoneal damage and determined how the new solutions help to preserve peritoneal integrity.
In a chronic exposure model in rats, Mortier et al. (12) demonstrated that it is mainly GDPs and the associated AGE formation that induces the morphology changes of peritoneal fibrosis and angiogenesis and the functional alterations of vascular endothelial growth factor production and ultrafiltration loss. Krediet et al. reported that high concentrations of lactate (19) and glucose (20) have greater effects on peritoneal angiogenesis, and high GDPs have a stronger effect on peritoneal fibrosis, probably through AGE accumulation.
In a clinical trial, Davies et al. (18) demonstrated that high glucose exposure (also with high GDPs) was associated with increased peritoneal permeability and ultrafiltration loss after 5 years of PD treatment. With regard to the role of GDPs, Rippe et al. (14) performed a clinical trial to compare markers of peritoneal transport and mesothelial integrity between conventional high-GDP solution and low-GDP solution for 2 years. Although peritoneal transport and ultrafiltration were not significantly different, markers of peritoneal integrity—cancer antigen 125 and procollagen I C-terminal peptide—were significantly higher in patients treated with low-GDP solution, suggesting that, compared with conventional solution, the new solution may cause less mesothelial and interstitial damage (14). Furthermore, in the Euro-Balance Study, Williams et al. (15) demonstrated that a neutral lactate-buffered low-GDP solution increased effluent markers of peritoneal integrity (cancer antigen 125 and procollagen I C-terminal peptide) and decreased circulating AGE levels after a 12-week clinical trial, suggesting the promising effects of neutral and low-GDP solution as reflected in improved local peritoneal homeostasis and reduced AGE formation (15). In that context, the present study is the first to demonstrate the histologic effects of the new biocompatible solutions in human peritoneal specimens. The new biocompatible solutions currently available in Japan are neutral lactate-buffered low-GDP solutions (Dianeal-N; Perisate N; Midperic: Terumo, Tokyo, Japan).
The extent of peritoneal fibrosis and vascular wall hyalinosis were associated with AGE accumulation (17), and those alterations were suppressed with use of the new solutions containing low levels of GDPs. On the other hand, in the present study, peritoneal vascular density reflecting peritoneal angiogenesis was markedly increased with use of the new solutions, with blood and not lymphatic capillaries being affected. That result was confirmed by CD31 positivity and podoplanin negativity demonstrated by immunostaining of the endothelial cells lining the vessels.
Peritoneal angiogenesis has been thought to be induced by PD solution (21). However, vascularity findings in human peritoneum after PD treatment have been controversial since 2000. Some studies reported increased peritoneal vascular density in patients with peritoneal sclerosis (22) and ultrafiltration failure (23-25); others found no significant increase in total vessel density in the SMC in patients receiving PD (26). Numata et al. (27) reported that increased peritoneal transport is associated with relative vascular area, but not with relative vessel number.
In the present study, the cause of increased vascular density in the neutral group compared with the acidic group remains uncertain. Krediet et al. (13) proposed that high concentrations of glucose and lactate may be the principal factors promoting angiogenesis. Considering that hypothesis, we speculate that the new biocompatible solutions may eventually reduce the toxic effects of GDPs and may an promote angiogenic responses with high glucose and lactate levels, which are still present in the new solutions.
Lymphangiogenesis has been reported to occur in various diseases of kidney (28) and peritoneum in an experimental PD model (29), and lymphatic vessels are speculated to be involved in peritoneal transport and PD-related peritoneal disorders. However, the vessels that increased in density in the neutral group were not lymphatic vessels but blood vessels, and the role of lymphatic vessels in the pathogenesis of PD-related peritoneal deterioration was not demonstrated in the present study.
Although increased vascular density was observed in the neutral group, it was not correlated with peritoneal hyperpermeability to small molecules (creatinine and glucose) as determined by a PET. In contrast, the grade of AGE accumulation was significantly correlated with peritoneal hyperpermeability, indicating that GDPs may play a more important role in peritoneal hyperpermeability through AGE accumulation. Factors other than vascular density—such as impaired endothelial cell function or vascular integrity induced by GDPs—might have an important role in regulating peritoneal permeability.
CONCLUSIONS
Histologic evaluation of peritoneal biopsy specimens revealed that neutral solutions with low GDPs can prevent peritoneal fibrosis and vascular sclerosis by suppressing AGE accumulation. We also found an increased density of blood vessels, but not lymphatic vessels, in peritoneum of patients using neutral low-GDP solution, which was not associated with peritoneal hyperpermeability as determined by a PET. Thus, the altered contents of the new PD solutions might modify peritoneal morphology and function in patients undergoing PD.
DISCLOSURES
The authors have no financial conflicts of interest to declare.
Acknowledgments
We thank Akihito Sannomiya, MD PhD, a surgeon in the Department of Surgical Nephrology, for specimens of peritoneum. The statistical analysis in this study was supported by Satoru Shimizu, PhD, of the Medical Research Institute of Tokyo Women’s Medical University.
References
- 1. Jörres A, Bender TO, Finn A, Witowski J, Fröhlich S, Gahl GM, et al. Biocompatibility and buffers: effect of bicarbonate-buffered peritoneal dialysis fluids on peritoneal cell function. Kidney Int 1998; 54:2184–93 [DOI] [PubMed] [Google Scholar]
- 2. Jörres A, Witowski J. Lessons from basic research for PD treatment. Perit Dial Int 2005; 25(Suppl 3):S35–8 [PubMed] [Google Scholar]
- 3. Topley N, Kaur D, Petersen MM, Jörres A, Williams JD, Faict D, et al. In vitro effects of bicarbonate and bicarbonate-lactate buffered peritoneal dialysis solutions on mesothelial and neutrophil function. J Am Soc Nephrol 1996; 7:218–24 [DOI] [PubMed] [Google Scholar]
- 4. Combet S, Miyata T, Moulin P, Pouthier D, Goffin E, Devuyst O. Vascular proliferation and enhanced expression of endothelial nitric oxide synthase in human peritoneum exposed to long-term peritoneal dialysis. J Am Soc Nephrol 2000; 11:717–28 [DOI] [PubMed] [Google Scholar]
- 5. Pecoits-Filho R, Araújo MR, Lindholm B, Stenvinkel P, Abensur H, Romão JE, Jr, et al. Plasma and dialysate IL-6 and VEGF concentrations are associated with high peritoneal solute transport rate. Nephrol Dial Transplant 2002; 17:1480–6 [DOI] [PubMed] [Google Scholar]
- 6. Rougier JP, Guia S, Hagège J, Nguyen G, Ronco PM. PAI-1 secretion and matrix deposition in human peritoneal mesothelial cell cultures: transcriptional regulation by TGF-β1. Kidney Int 1998; 54:87–98 [DOI] [PubMed] [Google Scholar]
- 7. Aroeira LS, Aguilera A, Selgas R, Ramírez-Huesca M, Pérez-Lozano ML, Cirugeda A, et al. Mesenchymal conversion of mesothelial cells as a mechanism responsible for high solute transport rate in peritoneal dialysis: role of vascular endothelial growth factor. Am J Kidney Dis 2005; 46:938–48 [DOI] [PubMed] [Google Scholar]
- 8. Devuyst O, Combet S, Cnops Y, Stoenoiu MS. Regulation of NO synthase isoforms in the peritoneum: implications for ultrafiltration failure in peritoneal dialysis. Nephrol Dial Transplant 2001; 16:675–8 [DOI] [PubMed] [Google Scholar]
- 9. Garcia-Lopez E, Lindholm B, Tranæus A. Biocompatibility of new peritoneal dialysis solutions: clinical experience. Perit Dial Int 2000; 20(Suppl 5):S48–56 [PubMed] [Google Scholar]
- 10. Nakamoto H, Imai H, Ishida Y, Yamanouchi Y, Inoue T, Okada H, et al. New animal models for encapsulating peritoneal sclerosis—role of acidic solution. Perit Dial Int 2001; 21(Suppl 3):349–53 [PubMed] [Google Scholar]
- 11. López-Novoa JM, Bernabeu C. The physiological role of endoglin in the cardiovascular system. Am J Physiol Heart Circ Physiol 2010; 299:H959–74 [DOI] [PubMed] [Google Scholar]
- 12. Mortier S, Faict D, Schalkwijk CG, Lameire NH, De Vriese AS. Long-term exposure to new peritoneal dialysis solutions: effects on the peritoneal membrane. Kidney Int 2004; 66:1257–65 [DOI] [PubMed] [Google Scholar]
- 13. Krediet RT, Zweers MM, van Westrhenen R, Zegwaard A, Struijk DG. Effects of reducing the lactate and glucose content of PD solutions on the peritoneum. Is the future GLAD? NDT Plus 2008; 1(Suppl 4):iv56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rippe B, Simonsen O, Heimbürger O, Christensson A, Haraldsson B, Stelin G, et al. Long-term clinical effects of a peritoneal dialysis fluid with less glucose degradation products. Kidney Int 2001; 59:348–57 [DOI] [PubMed] [Google Scholar]
- 15. Williams JD, Topley N, Craig KJ, Mackenzie RK, Pischetsrieder M, Lage C, et al. The Euro-Balance Trial: the effect of a new biocompatible peritoneal dialysis fluid (Balance) on the peritoneal membrane. Kidney Int 2004; 66:408–18 [DOI] [PubMed] [Google Scholar]
- 16. Honda K, Hamada C, Nakayama M, Miyazaki M, Sherif AM, Harada T, et al. Impact of uremia, diabetes, and peritoneal dialysis itself on the pathogenesis of peritoneal sclerosis: a quantitative study of peritoneal membrane morphology. Clin J Am Soc Nephrol 2008; 3:720–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Honda K, Nitta K, Horita S, Yumura W, Nihei H, Nagai R, et al. Accumulation of advanced glycation end products in the peritoneal vasculature of continuous ambulatory peritoneal dialysis patients with low ultra-filtration. Nephrol Dial Transplant 1999; 14:1541–9 [DOI] [PubMed] [Google Scholar]
- 18. Davies SJ, Phillips L, Naish PF, Russell GI. Peritoneal glucose exposure and changes in membrane solute transport with time on peritoneal dialysis. J Am Soc Nephrol 2001; 12:1046–51 [DOI] [PubMed] [Google Scholar]
- 19. van Westrhenen R, Zweers MM, Kunne C, de Waart DR, van der Wal AC, Krediet RT. A pyruvate-buffered dialysis fluid induces less peritoneal angiogenesis and fibrosis than a conventional solution. Perit Dial Int 2008; 28:487–96 [PubMed] [Google Scholar]
- 20. van Westrhenen R, Vlijm A, Hiralall JK, Krediet RT. Experimental study on long-term exposure to a biocompatible, hypertonic, pyruvate-buffered dialysis solution. Perit Dial Int 2008; 28(Suppl 5):S43–7 [PubMed] [Google Scholar]
- 21. Rippe B. Peritoneal angiogenesis in response to dialysis fluid. Contrib Nephrol 2009; 163:60–6 [DOI] [PubMed] [Google Scholar]
- 22. Mateijsen MA, van der Wal AC, Hendriks PM, Zweers MM, Mulder J, Struijk DG, et al. Vascular and interstitial changes in the peritoneum of CAPD patients with peritoneal sclerosis. Perit Dial Int 1999; 19:517–25 [PubMed] [Google Scholar]
- 23. Simonsen O, Sterner G, Carlsson O, Wieslander A, Rippe B. Improvement of peritoneal ultrafiltration with peritoneal dialysis solution buffered with bicarbonate/lactate mixture. Perit Dial Int 2006; 26:353–9 [PubMed] [Google Scholar]
- 24. Jones S, Holmes CJ, Krediet RT, Mackenzie R, Faict D, Tranæus A, et al. Bicarbonate/lactate-based peritoneal dialysis solution increases cancer antigen 125 and decreases hyaluronic acid levels. Kidney Int 2001; 59:1529–38 [DOI] [PubMed] [Google Scholar]
- 25. Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, et al. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 2002; 13:470–9 [DOI] [PubMed] [Google Scholar]
- 26. Sherif AM, Nakayama M, Maruyama Y, Yoshida H, Yamamoto H, Yokoyama K, et al. Quantitative assessment of the peritoneal vessel density and vasculopathy in CAPD patients. Nephrol Dial Transplant 2006; 21:1675–81 [DOI] [PubMed] [Google Scholar]
- 27. Numata M, Nakayama M, Nimura S, Kawakami M, Lindholm B, Kawaguchi Y, et al. Association between an increased surface area of peritoneal microvessels and a high peritoneal solute transport rate. Perit Dial Int 2003; 23:116–22 [PubMed] [Google Scholar]
- 28. Sakamoto I, Ito Y, Mizuno M, Suzuki Y, Sawai A, Tanaka A, et al. Lymphatic vessels develop during tubulointerstitial fibrosis. Kidney Int 2009; 75:828–38 [DOI] [PubMed] [Google Scholar]
- 29. Fabbrini P, Schilte MN, Zareie M, ter Wee PM, Keuning ED, Beelen RH, et al. Celecoxib treatment reduces peritoneal fibrosis and angiogenesis and prevents ultrafiltration failure in experimental peritoneal dialysis. Nephrol Dial Transplant 2009; 24:3669–76 [DOI] [PubMed] [Google Scholar]