Abstract
♦ Introduction: Automated assisted peritoneal dialysis (AAPD) has been shown to be successful as renal replacement therapy for elderly and physically incapable end-stage renal disease (ESRD) patients. In early 2003, a pioneer AAPD program was initiated at GAMEN Renal Clinic in Rio de Janeiro, Brazil.
♦ Objective: We evaluated the results of an AAPD program offered as an option to elderly ESRD patients with physical or cognitive debilities or as last resort to patients with vascular access failure or hemodynamic instability during hemodialysis.
♦ Methods: A cohort of 30 consecutive patients started AAPD from January 2003 to March 2008 and was followed to July 2009. Demographics, clinical and laboratory parameters, causes of death, and patient and technique survival were analyzed.
♦ Results: Median age of the patients was 72 years (range: 47 - 93 years), with 60% being older than 65. The Davies score was greater than 2 in 73% of patients, and the Karnofsky index was less than 70 in 40%. The overall peritonitis rate was 1 episode in 37 patient-months. The total duration of AAPD ranged from 3 to 72 months. Patient survival was 80% at 12 months, 60% at 24 months, and 23.3% at 48 months. The most common cause of death was cardiovascular problems (70%).
♦ Conclusions: In this clinical observational study, AAPD fulfilled its expected role, offering an opportune, reliable, and effective homecare alternative for ESRD patients with no other renal replacement therapy options.
Key words: Assisted automated peritoneal dialysis, elderly patients, Brazil
The increase in life expectancy observed in most countries of the world brings as a consequence the aging of the global population. This phenomenon creates an enormous challenge by raising issues such as independence, health care promotion, disease prevention, and maintenance or improvement of quality of life (QOL) for elderly people (1-3).
Regardless of the patient’s age, some chronic diseases—such as end-stage renal disease—are associated with an increased prevalence of frailty, which reduces the functional capacity of the patient, increasing that person’s dependency (4).
The major challenges for Brazil in the 21st century will be caring for a population of more than 32 million elderly people, mostly of a low socio-economic and education level, with a high incidence of chronic and disabling diseases (5,6). In the census of the Brazilian Society of Nephrology posted in March 2008, 36.3% of all dialysis patients were more than 60 years of age (7), and in the census of 2009, that number rose to 40% (8).
For elderly patients, peritoneal dialysis (PD) offers advantages of particular interest, such avoidance of the need to travel to the dialysis center three times each week (9), less hemodynamic instability (9), lower risk of central venous catheter-associated bacteremia (10), and better control of blood pressure (10,11). Beyond the cognitive, social, physical, and psychological limitations (12,13) of such patients, many associated comorbidities may also be present, complicating implementation of the PD technique, because many elderly patients live by themselves and have no family support (14). Thus, assistance for their treatment becomes crucial (15). Since the publication of the pioneering and successful French experience in 1977 (16), when home care nurses treated elderly people (more than 75 years of age) with assisted continuous ambulatory peritoneal dialysis (ACAPD), it has been shown that this alternative can provide successful renal replacement therapy (RRT) for a great number of physically incapable patients and also for those who are elderly and frail.
Several European countries already use assisted automated peritoneal dialysis (AAPD), including Denmark, France, the Netherlands, Norway, Sweden, the United Kingdom, and Belgium (17). In France, one study that included 1613 incident PD patients over 75 years of age (15) showed that 82% were treated with assisted PD (ACAPD or AAPD). In Denmark, Povlsen and Ivarsen (18) reported their experience using automated peritoneal dialysis (APD) and trained nurses with 64 patients on AAPD, with overall 1- and 2-year survivals of 58% and 48% respectively.
In Brazil, dialysis patients have universal health coverage: 90% by the public health system (19), and the remaining 10% by private or corporate health insurance. For hemodialysis (HD), reimbursement is paid as “fee for service”; for PD, all PD supplies are paid directly by the public health system to either Baxter International or Fresenius Medical Care (the PD supplies providers in Brazil, both located in São Paulo), and physicians are reimbursed only for the training period and for the monthly visits thereafter (20).
In early 2003, a pilot AAPD program was initiated at the GAMEN Renal Clinic in Rio de Janeiro to give home assistance to elderly or disabled patients who either chose PD as their first option or who no longer met the hemodynamic conditions or had a proper vascular access to be able to continue on HD, therefore requiring PD as last resort. The aim of the present paper is to describe the results of that AAPD program, offered as a first option or a last resort to elderly or physically incapable end-stage renal disease patients.
METHODS
This cohort study was performed at a single center (GAMEN Renal Clinic, Rio de Janeiro, Brazil) during the period January 2003 - July 2009. The clinic maintains a program of chronic PD (continuous ambulatory PD and APD) with 95 patients, representing approximately 20% of its total number of RRT patients. The GAMEN Renal Clinic has a health care team consisting of dialysis nurses, nephrologists, nurse assistants, and a social worker. Because the home assistance costs of AAPD are not covered by the Brazilian public health care reimbursement system for dialysis, the extra therapy costs have been funded entirely by the GAMEN Renal Clinic itself. Those costs comprise the number of hours that the nurse assistant dedicates to starting APD at the patient’s home, including the time and cost of transportation to and from the home.
All patients were treated using APD with Dianeal PD solutions and a cycler (HomeChoice: Baxter International). The AAPD protocol consists of 6 - 8 cycles per night, on 7 consecutive days, always having at least one 4.25% Dianeal bag in the daily prescription. Some patients take a dialysis-free day, usually on a Sunday. The connection to the dialysis machine is always performed by a nurse assistant, and during the first 15 days of therapy at home, the nurse assistant also disconnects the patient at the end of therapy in the morning. However, thereafter, to cut costs, a family member or the patient is encouraged to perform the disconnection, given that it is an extremely simple and fast procedure to execute. This routine procedure has been described by Verger et al. (21).
When we started the AAPD program, the clinic hired a new nurse assistant who was trained by our head PD nurse to be responsible for home support. The total training process (theoretical and practical) lasted for 20 hours.
For the present study, we included all consecutive incident patients starting AAPD from January 2003 to July 2009, who had a physical dependency or who were living by themselves (or both), and who lacked the ability to perform their own treatment, as well as patients who had been undergoing HD with vascular access failure or hemodynamic instability.
Once a month, medical and laboratory evaluations were performed at the clinic. A peritoneal equilibration test was performed and Kt/V, creatinine clearance, and residual renal function were measured at baseline and whenever necessary afterward (22).
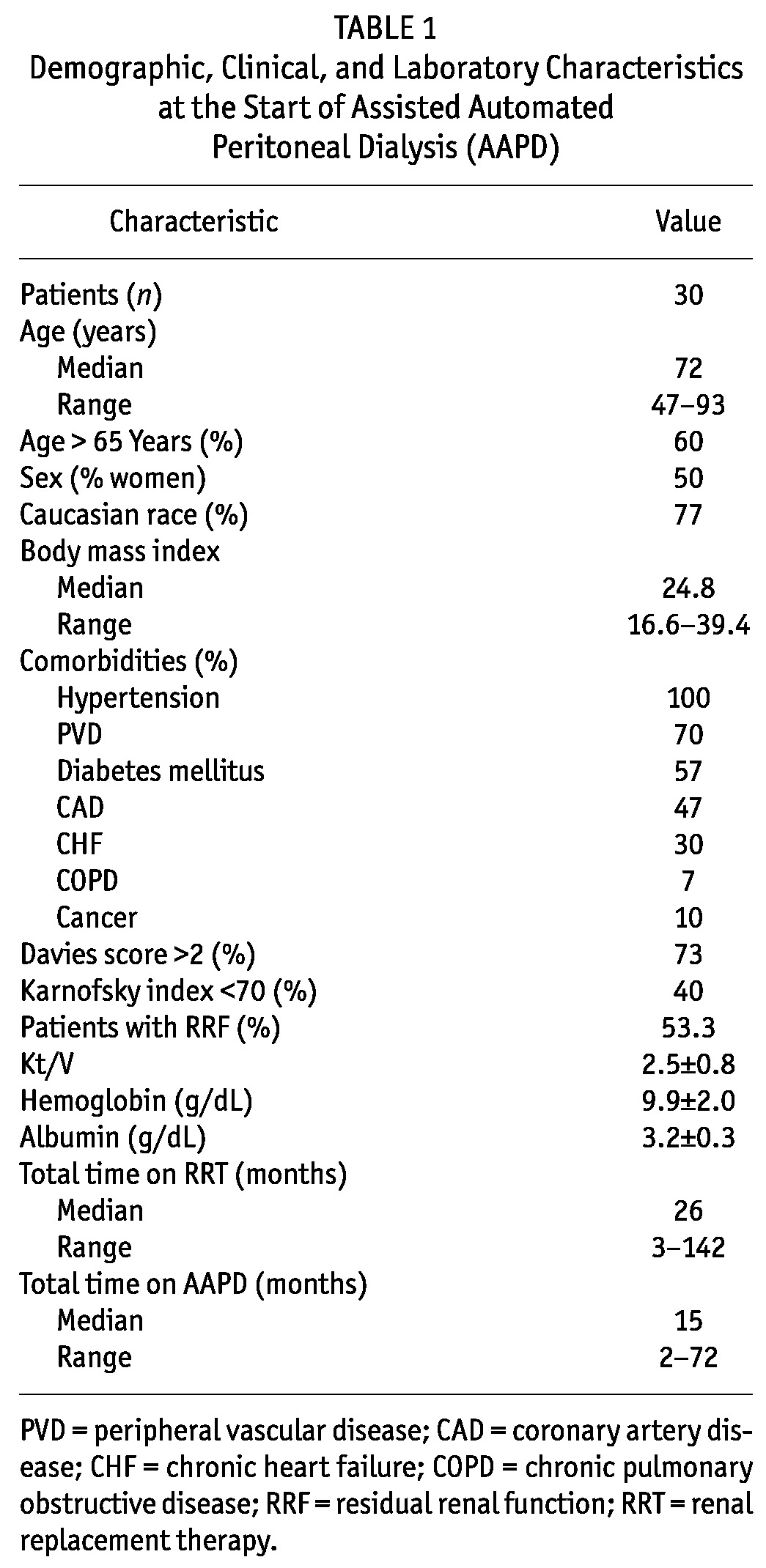
We collected data on age, sex, race, cause of chronic kidney disease (CKD), indication for AAPD, body mass index (23), and comorbidities from the medical records. The Davies comorbidity score was used to assess the severity of comorbid conditions (24). Physical performance was assessed using the Karnofsky index. Creatinine, urea, potassium, hemoglobin, and albumin were evaluated using routine methods. Overall time on dialysis, time on AAPD, and peritonitis rates were also registered.
The study was conducted in accordance with the Declaration of Helsinki, and all participants provided written informed consent before enrollment.
STATISTICAL ANALYSIS
The demographic, clinical, and laboratory data were evaluated, and a descriptive analysis was generated. Normally distributed variables are expressed as mean ± standard deviation (unless otherwise noted), and non-normally distributed variables are expressed as medians and ranges or percentages. Survival was analyzed using the Kaplan-Meier method. The principal outcome was mortality or technique failure. The definition of mortality was death of the patient during AAPD therapy or, at most, 3 months after a switch from AAPD to HD. Technique failure was defined as drop-out from AAPD because of peritoneal membrane failure or transfer to HD because of peritonitis. It is important to highlight that technique survival was defined as the patient remaining in the AAPD program during the observation period or dying during therapy for any reason other than peritonitis or peritoneal membrane failure. Patients were censored when lost to follow-up, switched to HD, or transplanted, or at study end. Statistical significance was set at the level of p < 0.05. All statistical analyses were performed using the SPSS software application (version 15: SPSS, Chicago, IL, USA).
RESULTS
A cohort of 30 consecutive patients started AAPD from January 2003 to March 2008, and they were followed till July 2009. Of those 30 patients, 9 (30%) started with AAPD as their first dialysis therapy (PD-first group), and the remaining 21 (70%) were transferred from HD to AAPD (HD-first group) either because of hemodynamic instability (8 patients) or vascular access failure (13 patients).
Table 1 describes the demographic, clinical, and biochemical characteristics of the study patients. In this cohort, 18 (60%) were more than 65 years of age. Among these older PD patients, 9 (30%) were more than 80 years of age. All 9 PD-first patients had received prior nephrology follow-up; none of the HD-first patients had received prior nephrology care.
TABLE 1.
Demographic, Clinical, and Laboratory Characteristics at the Start of Assisted Automated Peritoneal Dialysis (AAPD)
The peritoneal equilibration test revealed that 33% of patients were categorized as low-average, 48% as high-average, and 19% as high transporters.
During the observation period, more than half the patients (53%) never experienced a peritonitis episode, and the overall peritonitis rate was 1 episode in 37 patient-months. In the cultures of PD fluid that were carried out, 78% were positive (6% fungi, 37% gram-positive, and 57% gram-negative micro-organisms), and 22% were negative.
Overall, the median time on RRT was 26 months, ranging from 3 months to 142 months (mean: 38.9 ± 32.1 months), and the median time on AAPD was 15 months, ranging from 3 months to 72 months (mean: 19.9 ± 15.9 months).
During the study period, 51% of the patients were hospitalized. Among those patients, cardiovascular disease (30%) was the most important cause of hospitalization, followed by non-therapy-related sepsis (28%), therapy-related sepsis (11%), stroke (8%), and other conditions (23%).
The most common cause of death was cardiovascular disease (69.6%), followed by non-therapy-related sepsis (21.7%), and therapy-related sepsis (8.7%, Figure 1). Patient survival on AAPD at 12, 24, and 48 months was 60%, 23.3%, and 3%. Overall patient survival on dialysis at 12, 24, and 48 months was 80%, 60%, and 23.3% respectively [Figure 2(A,B)].
Figure 1.
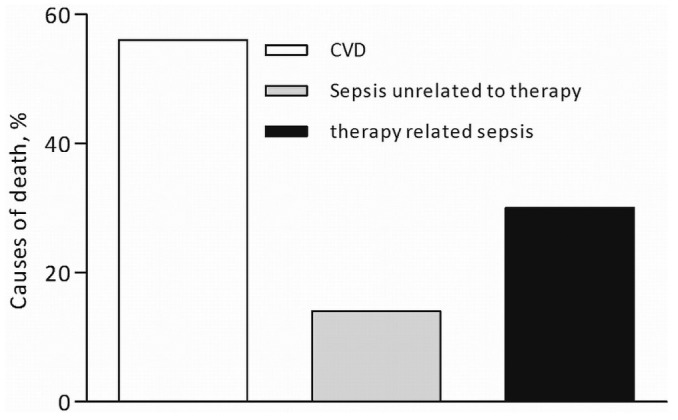
— Causes of death during the study.
Figure 2.
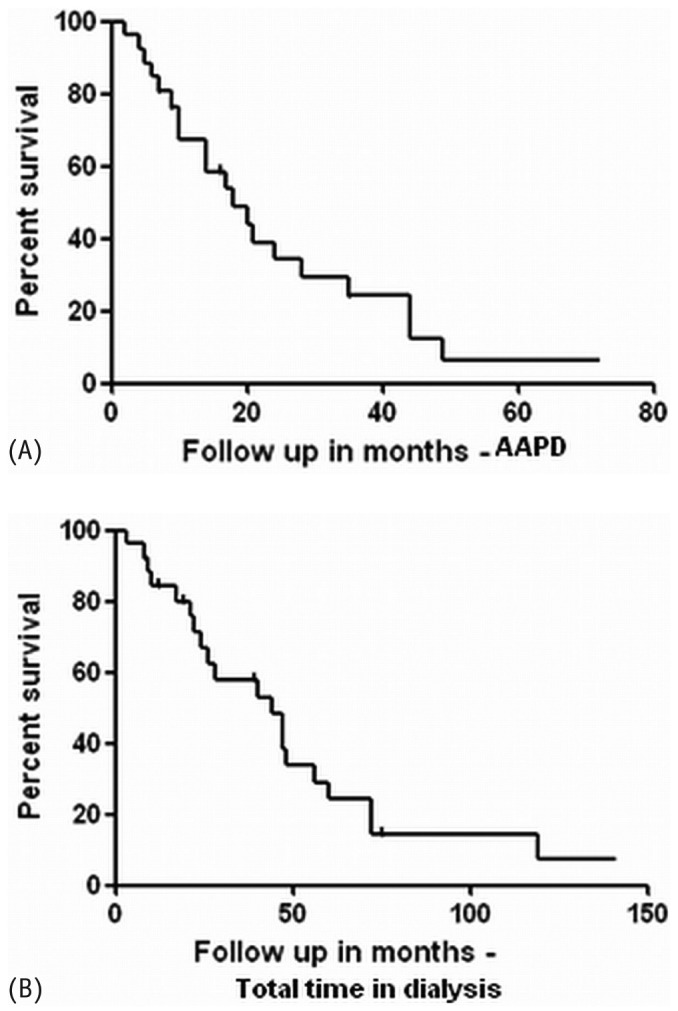
— Kaplan-Meier curves for survival in patients during (A) time on assisted automated peritoneal dialysis and (B) total time on dialysis.
In Brazil, the monthly reimbursement paid by the public service for HD is US$1113.71 (based on US$85.67 per dialysis session and 13 sessions per month), and for continuous ambulatory PD, it is US$900.28. For APD, the reimbursement is higher: US$1177.29 per month. For both continuous ambulatory PD and APD, the reimbursement medical fee for the initial training period and the subsequent monthly visits is US$84.86 per patient per month. The extra cost for our AAPD program is basically the nurse assistant’s salary (US$560.00 per month), plus the public transportation cost (approximately US$150 per month). Each nurse assistant is responsible for 4 patients. The quoted costs include the number of hours that the nurse assistant dedicates to starting APD at the patient’s home and the time and costs of transportation to and from the home. These extra AAPD costs have been absorbed entirely by the GAMEN Renal Clinic. No cost-effectiveness study has compared AAPD with HD for these patients.
DISCUSSION
Previous reports about assisted PD have typically come from wealthy countries with substantial resources dedicated to health care. The present study does not provide major additional information; however, this pilot experience shows that, even in a country in which assisted PD is not covered by health care insurance, the therapy is viable, with reasonably good outcomes. It is also important to highlight the fact that the availability of AAPD allows nephrologists to convert elderly patients from HD to PD, especially those who cannot tolerate HD. For elderly patients, PD and HD are complementary palliative therapies.
Palliative care is an approach that improves QOL for patients and their families in all disease stages, including those undergoing treatment for curable illnesses and those living with chronic disease (25). In the case of AAPD, care is provided by a team of nephrologists, nurses, assistant nurses, and social workers who work together, providing an extra and essential layer of support.
Our study with 30 incident AAPD patients is rather small, limiting the possibility of subgroup analyses and resulting in quite large confidence intervals. However, one study from France (26), not much larger and with a shorter follow-up time, has been reported. Data from South America have not been reported so far. The decision to report this AAPD experience is based solely on the fact that new approaches to therapy need pure clinical reports if they are to be developed into sound and successful options. The PD technique would not have achieved the position it has today as an important RRT option if it were not for small, purely clinical, studies carried out during the last few decades (27-29). The information provided here may shed some light on, or contribute to the establishment of, AAPD for a well-defined group of CKD patients as a reliable option and also as palliative care. This experience of assisted PD in a developing country as a reliable and effective home care approach may have important positive implications for health care policy in the developing world as more resources are allocated for home and palliative care.
For elderly patients with CKD, PD is regarded as a proper and ideal method when assistance is needed for its utilization (30-32). Among the factors that hamper the choice of PD for elderly patients, those related to the inherent difficulties of advanced age, such as difficulties in learning or performing the procedure, must be considered (14). Furthermore, Jagose and co-workers reported that about 61.2% of patients over 80 years of age need assistance to perform PD exchanges, to care for the exit site, and to make proper use of prescribed drugs (33). In our study, 60% of the patients were elderly, with 30% being more than 80 years of age, and all patients needed assistance to perform their own dialysis, even patients who were not elderly. It has been demonstrated that it is easier for elderly patients to accept this procedure and that they have greater treatment adherence and motivation (34).
In respect to underlying diseases in our study cohort, diabetic nephropathy was the most frequent; the most frequent comorbidity was hypertension. Those data accord with the findings reported for all PD patients in the BRAZPD study (35). Compared with findings in the BRAZPD study (35), the Karnofsky index and Davies score were worse in our patient cohort—probably because the study population was older than that in BRAZPD, in which the mean age was 54 ± 19 years, and 37% of the patients were more than 65 years of age.
Lobbedez et al. (26) studied 36 assisted PD (AAPD and ACAPD) patients and observed a relatively high peritonitis rate, with 50% presenting at least 1 episode annually. In contrast, Povlsen and Ivarsen (18), performing AAPD, reported a peritonitis rate higher than that observed in the present study. The patients in our study achieved peritonitis rates within PD guideline targets (36,37). Although we did not investigate gastrointestinal diseases in our patients, such conditions might explain the high prevalence of gram-negative peritonitis found in cultures of PD effluent (36).
Hospitalization during PD treatment is often only a result of technique-related infectious complications (38), and so our unusual observation of no hospitalization for peritonitis in the PD group cannot be explained, given the small number of patients evaluated in the present study. Verger et al. (21) reported that, compared with family-assisted AAPD patients, those assisted a private nurse had a higher risk of developing peritonitis, and recent findings (39) showed that low rates of peritonitis in patients on assisted PD could be explained by the assistance being given by a family member. Nevertheless, in our study, the PD group had no hospitalizations for peritonitis even though most were being attended by a trained nurse assistant.
In evaluating patients who underwent AAPD, Povlsen and Ivarsen (18) showed crude patient survival rates of 58% and 48% at 12 and 24 months respectively. The study by Lobbedez et al., in which ACAPD was used more often than AAPD, had a patient survival of 90% at 6 months and 83% at 12 months, with technique survival of 85% at 6 months and 58% at 12 months (26). Oliver et al. (12), who followed 28 patients on assisted PD for 28 months, reported a death rate of 0.12 per patient-year of follow-up, and technique survival of 81% at 12 months. In the present study, overall patient survival on dialysis was 82% at 12 months and 58% at 24 months; technique survival was 96.6% at 12 months and 95% at 24 months.
Besides the retrospective design and the small number of patients in the present study, the lack of a formal QOL assessment for these patients is another limitation. The subjective impression of the AAPD team during the period that these patients were treated was that QOL improved for most of them and for their relatives. However, given that QOL is a meaningful outcome for patients on dialysis (39), a prospective observational study, including a QOL evaluation, may help to address this very important matter.
CONCLUSIONS
This observational Brazilian study using AAPD as either a first RRT option for elderly patients with physical or cognitive debilities, lack of familial support, and financial conditions too poor to afford caregivers or as the only or last option for HD patients with vascular access failure or hemodynamic instability indicates that AAPD is a reliable and effective home care choice for end-stage renal disease patients with no other RRT options.
DISCLOSURES
The authors have no financial conflicts of interest to declare.
Acknowledgments
The authors thank Professor Friedo W. Dekker for reviewing the manuscript.
References
- 1. von Faber M, Bootsma-van der Wiel A, van Exel E, Gussekloo J, Lagaay AM, van Dongen E, et al. Successful aging in the oldest old: who can be characterized as successfully aged? Arch Intern Med 2001; 161:2694–700 [DOI] [PubMed] [Google Scholar]
- 2. Albert SM, Im A, Raveis VH. Public health and the second 50 years of life. Am J Public Health 2002; 92:1214–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization (WHO). Active Aging: A Policy Framework (Portuguese). Brasília, Brazil: WHO; 2005: 7–12 [Google Scholar]
- 4. Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol 2007; 18:2960–7 [DOI] [PubMed] [Google Scholar]
- 5. Ramos LR. Determinant factors for healthy aging among senior citizens in a large city: the Epidoso Project in Sao Paulo (Portuguese). Cad Saude Publica 2003; 19:793–8 [DOI] [PubMed] [Google Scholar]
- 6. Vladeck BC. Economic and policy implications of improving longevity. J Am Geriatr Soc 2005; 53(Suppl 9):S304–7 [DOI] [PubMed] [Google Scholar]
- 7. Sesso R, Lopes AA, Thomé FS, Bevilacqua JL, Romão Junior JE, Lugon J. Brazilian Dialysis Census Report (Portuguese). J Bras Nefrol 2008; 30:233–8 [Google Scholar]
- 8. Sesso RCC, Lopes AA, Thomé FS, Lugon J, Burdmann EA. Brazilian Dialysis Census (Portuguese). J Bras Nefrol 2010; 32:380–4 [PubMed] [Google Scholar]
- 9. Brown EA, Johansson L, Farrington K, Gallagher H, Sensky T, Gordon F, et al. Broadening Options for Long-term Dialysis in the Elderly (BOLDE): differences in quality of life on peritoneal dialysis compared to haemodialysis for older patients. Nephrol Dial Transplant 2010; 25:3755–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blake PG. Peritoneal dialysis: a “kinder, gentler” treatment for the elderly? Perit Dial Int 2008; 28:435–6 [PubMed] [Google Scholar]
- 11. Dimkovic N, Aggarwal V, Khan S, Chu M, Bargman J, Oreopoulos DG. Assisted peritoneal dialysis: what is it and who does it involve? Adv Perit Dial 2009; 25:165–70 [PubMed] [Google Scholar]
- 12. Oliver MJ, Quinn RR, Richardson EP, Kiss AJ, Lamping DL, Manns BJ. Home care assistance and the utilization of peritoneal dialysis. Kidney Int 2007; 71:673–8 [DOI] [PubMed] [Google Scholar]
- 13. Brown EA. Peritoneal dialysis for older people: overcoming the barriers. Kidney Int Suppl 2008; (108):S68–71 [DOI] [PubMed] [Google Scholar]
- 14. Jager KJ, Korevaar JC, Dekker FW, Krediet RT, Boeschoten EW. on behalf of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) Study Group. The effect of contraindications and patient preference on dialysis modality selection in ESRD patients in the Netherlands. Am J Kidney Dis 2004; 43:891–9 [DOI] [PubMed] [Google Scholar]
- 15. Castrale C, Evans D, Verger C, Fabre E, Aguilera D, Ryckelynck JP, et al. Peritoneal dialysis in elderly patients: report from the French Peritoneal Dialysis Registry (RDPLF). Nephrol Dial Transplant 2010; 25:255–62 [DOI] [PubMed] [Google Scholar]
- 16. Issad B, Benevent D, Allouache M, Durand PY, Aguilera D, Milongo R, et al. 213 Elderly uremic patients over 75 years of age treated with long-term peritoneal dialysis: a French multicenter study. Perit Dial Int 1996; 16(Suppl 1):S414–18 [PubMed] [Google Scholar]
- 17. Brown EA, Dratwa M, Povlsen JV. Assisted peritoneal dialysis—an evolving dialysis modality. Nephrol Dial Transplant 2007; 22:3091–2 [DOI] [PubMed] [Google Scholar]
- 18. Povlsen JV, Ivarsen P. Assisted automated peritoneal dialysis (AAPD) for the functionally dependent and elderly patient. Perit Dial Int 2005; 25(Suppl 3):S60–3 [PubMed] [Google Scholar]
- 19. Pecoits-Filho R, Campos C, Cerdas-Calderon M, Fortes P, Jarpa C, Just P, et al. Policies and health care financing issues for dialysis in Latin America: extracts from the roundtable discussion on the economics of dialysis and chronic kidney disease. Perit Dial Int 2009; 29(Supp 2):S222–6 [PubMed] [Google Scholar]
- 20. Andrade MV, Junoy JP, Andrade EI, Acurcio Fde A, Sesso R, Queiroz OV, et al. Allocation of initial modality for renal replacement therapy in Brazil. Clin J Am Soc Nephrol 2010; 5:637–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Verger C, Duman M, Durand PY, Veniez G, Fabre E, Ryckelynck JP. Influence of autonomy and type of home assistance on the prevention of peritonitis in assisted automated peritoneal dialysis patients. An analysis of data from the French Language Peritoneal Dialysis Registry. Nephrol Dial Transplant 2007; 22:1218–23 [DOI] [PubMed] [Google Scholar]
- 22. U.S. National Kidney Foundation (NKF). NKF KDOQI guidelines (Web page). New York, NY: NKF; 2006. [Available online at: http://www.kidney.org/professionals/kdoqi/guideline_upHD_PD_VA/pd_guide3.htm; accessed 6 March 2012] [Google Scholar]
- 23. Diet, nutrition and the prevention of chronic diseases. World Health Organ Tech Rep Ser 2003; 916:i-viii,1-149 [PubMed] [Google Scholar]
- 24. Davies SJ, Russell L, Bryan J, Phillips L, Russell GI. Comorbidity, urea kinetics, and appetite in continuous ambulatory peritoneal dialysis patients: their interrelationship and prediction of survival. Am J Kidney Dis 1995; 26:353–61 [DOI] [PubMed] [Google Scholar]
- 25. World Health Organization (WHO). Cancer. WHO definition of palliative care (Web page). Geneva, Switzerland: WHO; n.d. [Available at: http://www.who.int/cancer/palliative/definition/en; accessed 16 March 2012] [Google Scholar]
- 26. Lobbedez T, Moldovan R, Lecame M, Hurault de Ligny B, El Haggan W, Ryckelynck JP. Assisted peritoneal dialysis. Experience in a French renal department. Perit Dial Int 2006; 26:671–6 [PubMed] [Google Scholar]
- 27. Boen ST. History of peritoneal dialysis. In: Nolph KD, ed. Peritoneal Dialysis. Dordrecht, Netherlands: Kluwer Academic Publishers; 1989: 1–12 [Google Scholar]
- 28. Moncrief JW, Popovich RP, Nolph KD. The history and current status of continuous ambulatory peritoneal dialysis. Am J Kidney Dis 1990; 16:579–84 [DOI] [PubMed] [Google Scholar]
- 29. Oreopoulos DG, Khanna R, McCready W, Katirtzoglou A, Vas S. Continuous ambulatory peritoneal dialysis in Canada. Dial Transplant 1980; 9:224–6 [Google Scholar]
- 30. Kadambi P, Troidle L, Gorban-Brennan N, Kliger AS, Finkelstein FO. APD in the elderly. Semin Dial 2002; 15:430–3 [DOI] [PubMed] [Google Scholar]
- 31. Dimkovic N, Oreopoulos DG. Assisted peritoneal dialysis as a method of choice for elderly with end-stage renal disease. Int Urol Nephrol 2008; 40:1143–50 [DOI] [PubMed] [Google Scholar]
- 32. Brown EA. How to address barriers to peritoneal dialysis in the elderly. Perit Dial Int 2011; 31(Suppl 2):S83–5 [DOI] [PubMed] [Google Scholar]
- 33. Jagose JT, Afthentopoulos IE, Shetty A, Oreopoulos DG. Successful use of continuous ambulatory peritoneal dialysis in octogenarians. Adv Perit Dial 1996; 12:126–31 [PubMed] [Google Scholar]
- 34. Dimkovic NB, Prakash S, Roscoe J, Brissenden J, Tam P, Bargman J, et al. Chronic peritoneal dialysis in octogenarians. Nephrol Dial Transplant 2001; 16:2034–40 [DOI] [PubMed] [Google Scholar]
- 35. Fernandes N, Bastos MG, Cassi HV, Machado NL, Ribeiro JA, Martins G, et al. The Brazilian Peritoneal Dialysis Multicenter Study (BRAZPD): characterization of the cohort. Kidney Int Suppl 2008; (108):S145–51 [DOI] [PubMed] [Google Scholar]
- 36. Nessim SJ, Bargman JM, Austin PC, Story K, Jassal SV. Impact of age on peritonitis risk in peritoneal dialysis patients: an era effect. Clin J Am Soc Nephrol 2009; 4:135–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chow KM, Szeto CC, Leung CB, Kwan BC, Law MC, Li PK. A risk analysis of continuous ambulatory peritoneal dialysis-related peritonitis. Perit Dial Int 2005; 25:374–9 [PubMed] [Google Scholar]
- 38. Genestier S, Meyer N, Chantrel F, Alenabi F, Brignon P, Maaz M, et al. Prognostic survival factors in elderly renal failure patients treated with peritoneal dialysis: a nine-year retrospective study. Perit Dial Int 2010; 30:218–26 [DOI] [PubMed] [Google Scholar]
- 39. Xu R, Zhuo M, Yang Z, Dong J. Experience with assisted peritoneal dialysis in China. Perit Dial Int 2012; 32:94–101 [DOI] [PMC free article] [PubMed] [Google Scholar]


