Abstract
♦ Background: Whether peritoneal protein leakage predicts risk for peritonitis in patients on peritoneal dialysis (PD) is unknown. In this observational cohort study, we aimed to determine that association and, further, to explore if it might be explained by systemic inflammation.
♦ Methods: We prospectively followed 305 incident PD patients to first-episode peritonitis, censoring, or the end of the study. Demographics, comorbidity score, biochemistry, and peritoneal protein clearance (PrC) were collected at baseline. The predictors of first-episode peritonitis were analyzed prospectively.
♦ Results: During follow-up, 14 868 patient months and 251 episodes of peritonitis were observed. The baseline PrC was 73.2 mL/day (range: 53.2 - 102 mL/day). Patients with a high PrC were prone to be older and malnourished. They also had a higher comorbidity score and higher C-reactive protein values. In 132 first episodes of peritonitis, baseline PrC was shown to be a significant independent predictor after adjustment for age, sex, body mass index, diabetes, residual renal function, hemoglobin, and peritoneal transport rate. Systemic inflammatory markers such as serum albumin, C-reactive protein, and interleukin-6 could not explain the association of PrC and high risk for peritonitis.
♦ Conclusions: Baseline peritoneal protein leakage was able to independently predict risk for peritonitis, which is not explained by systemic inflammation. The underlying mechanisms should be explored in future.
Key words: Peritonitis, protein leakage, inflammation
Peritoneal protein leakage, estimated by peritoneal albumin excretion or peritoneal protein clearance (PrC), has been shown in patients on peritoneal dialysis (PD) to be associated with the prevalence of peripheral arterial disease, cardiovascular events, and higher mortality (1-4). Peritoneal protein leakage actually depends on peritoneal large-pore transport, which is, for the most part, correlated with systemic or local inflammatory status and endothelial dysfunction (4-6). That association might explain the predictive role of PrC in the foregoing outcome events.
However, evidence of an association of peritoneal protein leakage and peritonitis risk are few. To our knowledge, inflammation and altered cytokine balance in the uremic state are accompanied by impaired immune function (7), which is suspected to be associated with a high burden of infection. It is therefore necessary to explore whether peritoneal protein leakage, a surrogate marker of inflammation and endothelial dysfunction (3,8-11), is correlated with peritonitis risk. Recently, Pérez-Fontán et al. reported that peritoneal protein excretion during baseline peritoneal equilibration tests independently predicted first-episode peritonitis in 269 patients (12). The association remained even after adjustment for serum albumin and C-reactive protein.
We were therefore interested to verify that association between peritoneal protein leakage and peritonitis risk in a larger cohort of PD patients. To further determine if the association is independent of systemic inflammation, we also measured serum albumin (13), C-reactive protein, and interleukin-6. As shown in a previous study, interleukin-6 is a stronger predictor than C-reactive protein of total and cardiovascular mortality in hemodialysis patients (14). If inflammation estimated by all those inflammatory markers cannot explain the correlation of protein leakage and peritonitis risk, more research will need to be performed to explore the potential mechanisms for this phenomenon.
METHODS
SUBJECTS
Our cohort study enrolled a total of 305 incident patients who started PD in our unit from July 2002 to February 2007 and who lived longer than 6 months. All patients could visit a physician at least once every 3 months and consented to send dialysate effluent to our (or the nearest) hospital for culturing when peritonitis occurred. Demographics and comorbidities were recorded within the week preceding PD catheter implantation, including age, sex, height, weight, body mass index (BMI), and score on the Charlson comorbidity index (CCI). The baseline values for biochemistry, residual renal function, inflammatory markers, and peritoneal transport characteristics (including small-solute transport rate and protein losses) were those evaluated during the first 3 or 6 months. All patients were prescribed lactate-buffered glucose dialysate, delivered using a twin-bag connection system (Baxter Healthcare, Guangzhou, China) by the continuous ambulatory PD modality. The study was approved by the Medical Ethical Committee of Peking University. Written informed consent was obtained from each patient.
DEFINITION OF OUTCOME EVENTS
Peritonitis and calculation of the peritonitis rate was defined as recommended by the International Society for Peritoneal Dialysis guidelines (15). Peritonitis was also treated using the recommended standard antibiotic protocols. Initial antimicrobial therapy for peritonitis consisted, in general, of intraperitoneal administration of a third-generation cephalosporin plus cefazolin (15). Appropriate antibiotic therapy was continued for 14 - 21 days depending on the actual organism.
We examined first-episode peritonitis-free survival as the main outcome. We also examined peritonitis-associated death and switch to hemodialysis as treatment failure. Death related to peritonitis was defined as death of a patient with active peritonitis or within 2 weeks of a peritonitis episode (15).
BIOCHEMISTRY, INFLAMMATORY MARKERS, PERITONEAL TRANSPORT TYPE, AND PROTEIN LEAKAGE
Biochemical parameters including hemoglobin (Hb), serum albumin, blood urea nitrogen, and serum creatinine were examined using an automatic Hitachi chemistry analyzer. Serum high-sensitivity C-reactive protein (CRP) and interleukin-6 (IL-6) were measured as markers of inflammation. Immunonephelometric analysis was used to measure CRP, and IL-6 was measured using an ELISA method. The ELISA kits were obtained from RB Corporation (Rapidbio, West Hills, CA, USA).
One day before a regular clinic visit, 24-hour dialysate effluent and urine were collected. Residual renal function (RRF) was calculated by measuring the urea and creatinine clearances in urine. The 24-hour dialysate-to-plasma ratio for creatinine (D/Pcr) was calculated to determine small-solute transport characteristics (16,17). Peritoneal total protein losses were measured in 24-hour effluent using the biuret reaction analytical method. Protein clearance (PrC) was calculated using the equation
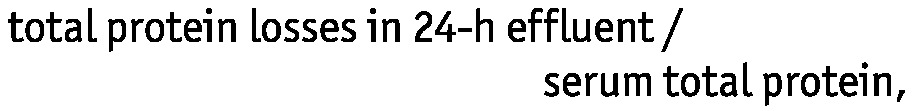 |
expressed per milliliter of plasma per day (18). The duration between the PrC measurements and the occurrence of peritonitis was at least 1 month.
STATISTICAL ANALYSES
Continuous data that are normally distributed are termed “parametric data,” and those that are not normally distributed, “nonparametric data.” Parametric data are presented as mean ± standard deviation. Nonparametric data are presented as median values with the interval from the 25th to the 75th percentile. Categorical variables are expressed as a percentage or ratio. Patient data were compared using the chi-square test for categorical variables and the Mann-Whitney U-test for continuous variables. Partial correlation analysis was used to analyze the relationships between serum albumin, peritoneal transport characteristics, and markers of inflammation, with adjustments for age, sex, and body area. Multivariate Cox proportional hazards models were built to analyze the predictive role of PrC in the risk for first-episode peritonitis. Analyses were censored at death, transplantation, and transfer to hemodialysis. To examine the impact of PrC on peritonitis risk, variables such as age, sex, BMI, diabetes status, RRF, Hb, and D/Pcr were included in base model. Models 1, 2, and 3 then incorporated serum albumin, CRP, and IL-6 respectively to examine whether the association could be explained by markers of inflammation. All of the reported p values are two-tailed, and statistical significance was set at 0.05. Statistical analyses were performed using the SPSS software package (version 13.0: SPSS, Chicago, IL, USA).
RESULTS
SUBJECT DEMOGRAPHICS
The 305 incident PD patients (129 men, 176 women) had a mean age of 59.4 ± 14.2 years, a mean BMI of 23.4 ± 3.8 kg/m2, and a diabetes prevalence of 40.3%. The median CCI score was 5 (range: 3 - 8) in our population.
PRC AND DEMOGRAPHIC AND CLINICAL PARAMETERS
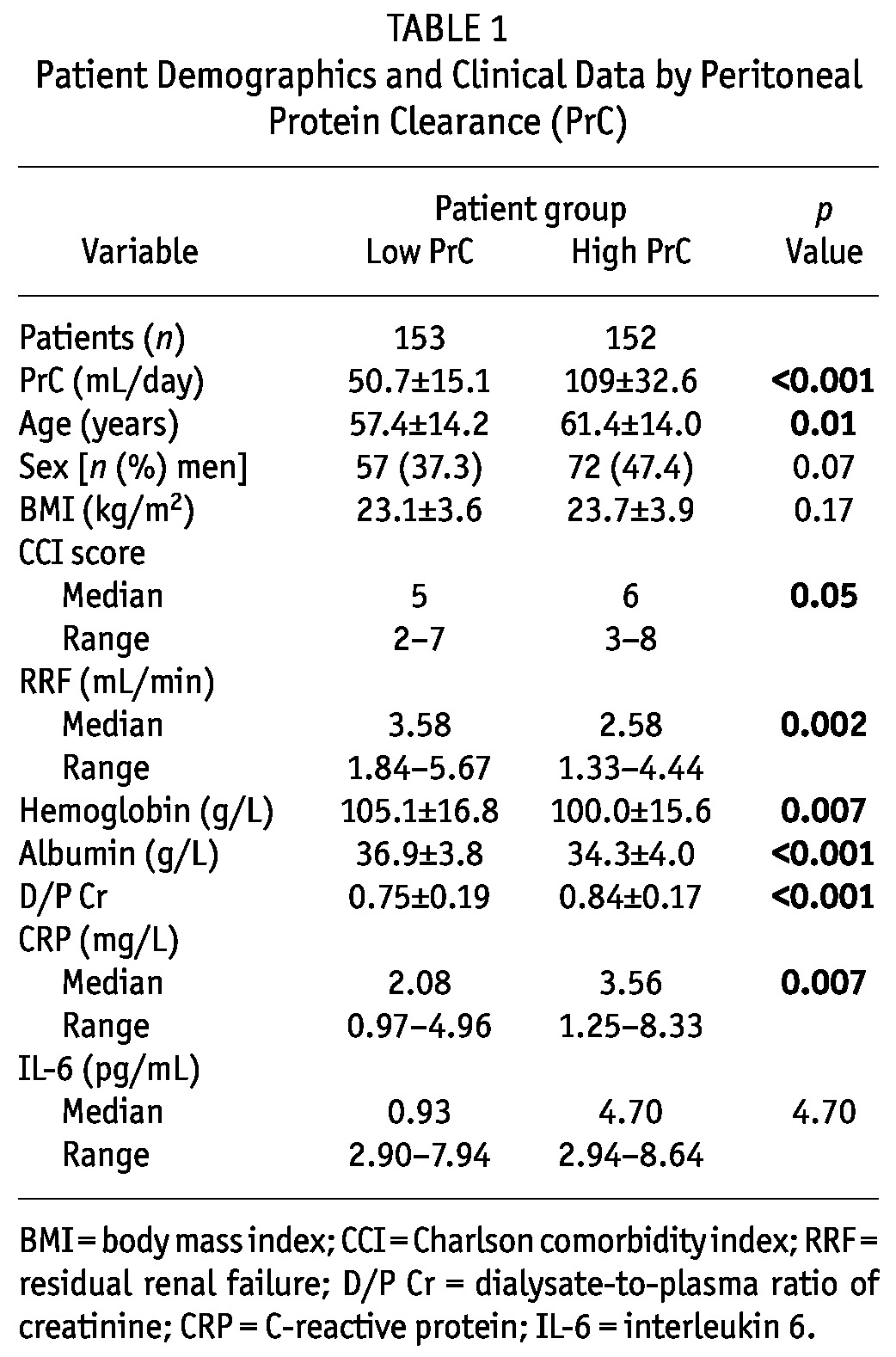
Table 1 shows the baseline clinical characteristics of the patients dichotomized by median PrC. Participants with a high PrC were more likely to be older and sicker, with a higher CCI score (p < 0.05). They also had less RRF and lower values for Hb and serum albumin (p < 0.001 - 0.01). Peritoneal transport rates and serum CRP were significantly higher in patients in the high PrC group (p < 0.001 - 0.01). The gender balance, BMI, and serum IL-6 were not significantly different between the groups (p > 0.05).
TABLE 1.
Patient Demographics and Clinical Data by Peritoneal Protein Clearance (PrC)
CORRELATION OF SERUM ALBUMIN, PERITONEAL TRANSPORT CHARACTERISTICS, AND INFLAMMATION
We examined the partial correlations of serum albumin, D/Pcr, PrC, CRP, and IL-6. Serum albumin was significantly associated with CRP and IL-6, with correlation coefficients of -0.19 and -0.22 respectively (p < 0.01). The PrC correlated with serum CRP (correlation coefficient: 0.16; p = 0.01), but not with IL-6 (p > 0.05). The D/Pcr was not significantly associated with serum CRP or IL-6 (p > 0.05).
PRC AND OUTCOME EVENTS
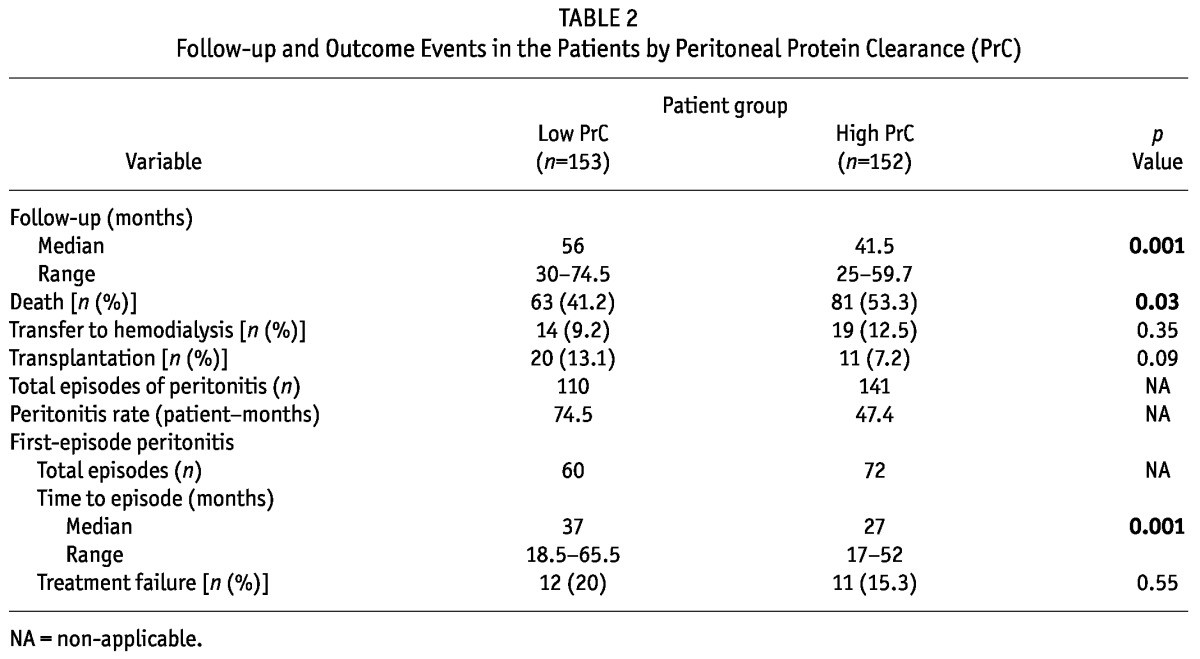
During the observation period, 144 patients died, 33 patients transferred to hemodialysis, and 31 patients underwent kidney transplantation. The patients with high PrC had a shorter dialysis duration because of a higher death rate (p < 0.05). The peritonitis rate in the high PrC group was numerically higher than it was in the low PrC group. A total of 132 first episodes of peritonitis in 305 patients were observed during the study period, with 26 episodes (19.7%) being attributable to enteric organisms; 53 (40.2%), to non-enteric organisms; and 4 (3%), to fungi. In 4 episodes (3%), the infection was polymicrobial; 31 episodes (23.5%) were culture-negative; and 14 episodes (10.6%) had no culture results. The time to first-episode peritonitis was also shorter in the high PrC group (p = 0.001). This first-episode peritonitis resulted in 6 (4.5%) permanent transfers to hemodialysis and 17 (12.9%) deaths. The remaining 109 patients (82.6%) remained on PD. The treatment failure percentage was not significantly different between the low and high PrC groups (p = 0.55, Table 2).
TABLE 2.
Follow-up and Outcome Events in the Patients by Peritoneal Protein Clearance (PrC)
ASSOCIATIONS BETWEEN PRC, INFLAMMATION, AND PERITONITIS RISK
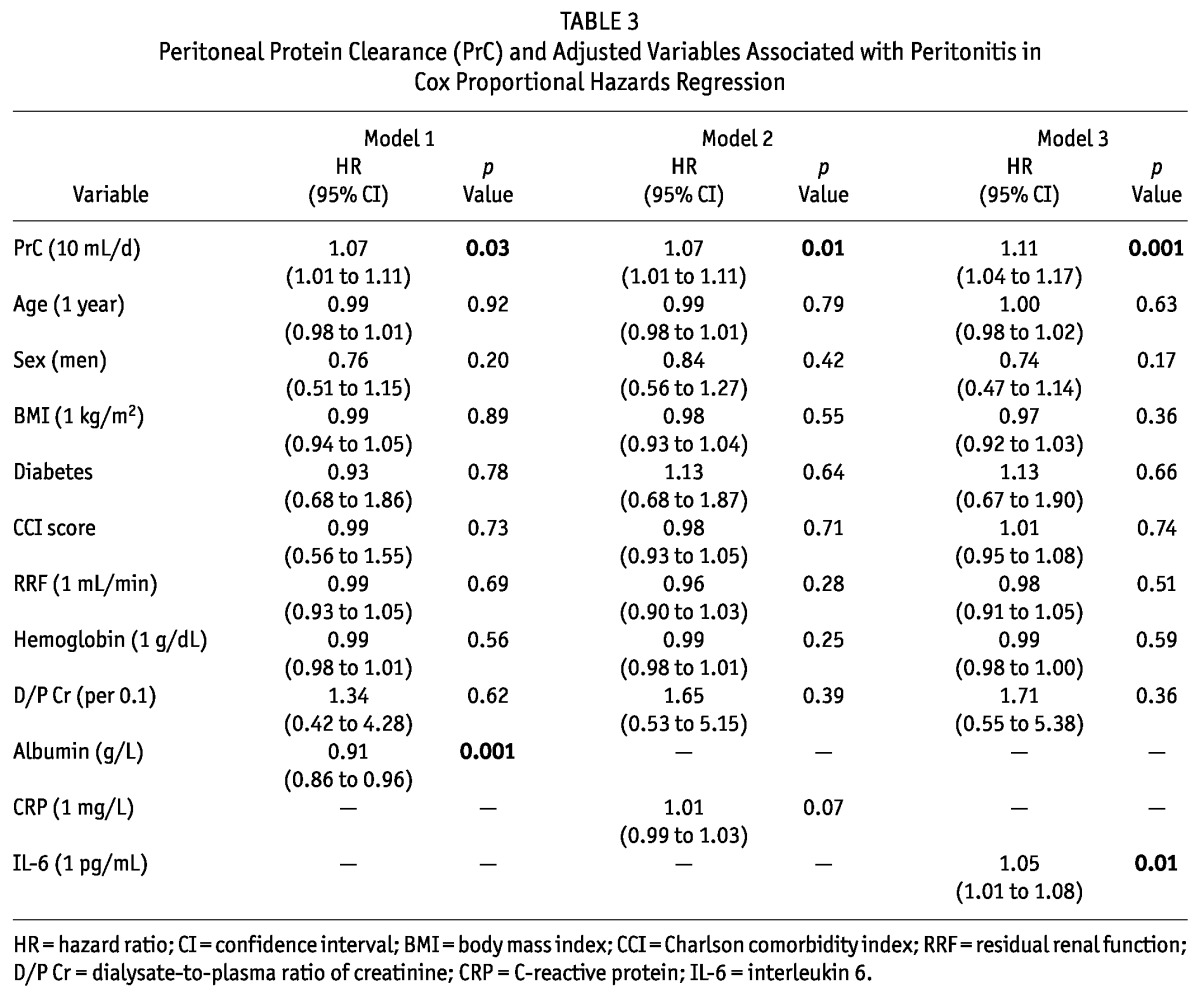
The predictive role of PrC for first-episode peritonitis was examined in multivariate Cox regression models. In the base model, baseline PrC was significantly associated with risk for peritonitis after adjustment for age, sex, BMI, diabetes, RRF, Hb, and D/Pcr [hazard ratio (HR): 1.08 (1.03 - 1.14); p = 0.003]. Models 1, 2, and 3 then incorporated serum albumin, CRP, and IL-6 respectively. The PrC still predicted peritonitis risk (Table 3) even adjusted for serum albumin [HR: 1.07 (1.01 - 1.11); p = 0.03], for CRP [HR: 1.07 (1.01 - 1.11); p = 0.01], and for IL-6 [HR: 1.11 (1.04 - 1.17); p = 0.001].
TABLE 3.
Peritoneal Protein Clearance (PrC) and Adjusted Variables Associated with Peritonitis in Cox Proportional Hazards Regression
DISCUSSION
Dialysis-related peritonitis contributes to treatment failure, hospitalization, and the death rate in PD populations (19-21). Nowadays, peritonitis rates are typically less than 1 episode in 30 patient-months in most series (22-25), which is unacceptably high. Exploring new risk factors for peritonitis is extremely critical for clinicians. As shown in our data, high PrC values can predict an increased risk for first-episode peritonitis. This association remained even adjustment for systemic inflammation estimated by serum albumin, CRP, and IL-6. Those findings support and extend previous observations in recent years (1-3,6,12,26).
The PrC is viewed as a surrogate marker of large-pore transport, which is potentially a result of inflammation (4,18,27,28). In comparison, a fast peritoneal transport rate proportional to an increased small-pore area might be less influenced by capillary vasodilatation as a result of inflammation (28). Indeed, the relationship between large-pore transport (measured as JvL or PrC) and CRP, rather than small-pore transport and CRP has been suggested (4,5). The present data further support that suggestion. The predictive role of PrC in peritonitis is therefore suspected to be linked to systemic inflammation. Interestingly, that suspicion was not verified in the Pérez-Fontán study, which indicated that the association between baseline peritoneal protein excretion during a peritoneal equilibration test and peritonitis is independent of inflammatory status estimated by albumin and CRP (12). Our findings further support the report by those authors.
Whether local inflammation in the peritoneal cavity partly contributes to the increased risk for peritonitis with high PrC needs to be explored. As reported, the inflammatory status of the adipose tissue local to the peritoneal cavity can cause high production of vascular endothelial growth factor (VEGF) and leptin (29). The VEGF and leptin lead to vasodilatation and intercellular fenestra, a critical mechanism for the transport of large molecules throughout the peritoneal capillary endothelium (30). Most recently, Balafa et al. indicated that high peritoneal protein losses are associated with high levels of cancer antigen 125 and the presence of a large mesothelial cell mass (31). The latter finding suggests peritoneal production of VEGF factor (32) or IL-6 (33), independent of comorbidity or systematic inflammation. Whether local inflammation is associated with malfunctioning of phagocytosis by peritoneal macrophages, thus contributing to the occurrence of peritonitis (34,35) requires exploration in the future.
On the other hand, some types of protein that can leak into the peritoneal cavity affect white cell function (36). Unfortunately, proteomic analysis of PD effluent was not performed in our study, which hampers any attempt to explore whether the types of the leaked proteins may be more significant than the total amount of protein in the association with risk of peritoneal infection.
Our study has several strengths. It was performed in a large PD cohort with relatively long-term follow-up. The patients were thoroughly examined for peritoneal protein leakage and inflammatory markers at baseline, and for culture results once peritonitis occurred, which gave us a unique opportunity to explore potential associations. The demographic characteristics, distribution of causative organisms, and associations of PrC and clinic parameters demonstrated here are typical of those previously reported, suggesting the generalizability of our findings to other PD cohorts elsewhere.
At the same time, we realize the limitations of the study. First, the mortality rate is high because of the relatively long follow-up. Some patients may die before peritonitis occurs. In that case, relationships between any factor and first-episode peritonitis should be interpreted cautiously. Also, even though most recognized confounders were considered, we cannot preclude an unknown factor confounding the observed associations by being associated with both exposure and outcome. For example, some potential risk factors, such as the effect of initial training or occurrence of exit-site and tunnel function were not evaluated. Moreover, the cause-and-effect relationship between peritoneal protein leakage and peritonitis risk needs to be verified in a future study. The present study design could not explore potential mechanisms for the associations of peritoneal protein leakage, systemic inflammation, and peritonitis risk. Obviously, simultaneous measurements of systemic and local markers of inflammation, and proteomic analysis of PD effluent are needed in future to explore such mechanisms.
CONCLUSIONS
Our study revealed that baseline values of peritoneal protein leakage are significantly independent predictors of peritonitis even with adjustment for systemic inflammation. The potential mechanisms of this phenomenon remain to be explored. Our findings also encourage us to search for new biomarkers local to the peritoneal cavity in the prediction of peritonitis risk.
DISCLOSURES
The authors have no financial conflicts of interest to declare.
Acknowledgments
The authors express their appreciation to the patients and nursing staff of the peritoneal dialysis center of First Hospital, Peking University, for their participation in the study reported here. The study was supported in part by a Capital Characteristic Clinic Research Grant from the Beijing Science and Technology Committee (Z111107058811110); a New Century Excellent Talents from the Education Department, China; a Ketosteril Research Award from Fresenius Kabi Deutschland GmbH; and a Baxter Clinical Research Award from Baxter Corporation, China.
References
- 1. Perl J, Huckvale K, Chellar M, John B, Davies SJ. Peritoneal protein clearance and not peritoneal membrane transport status predicts survival in a contemporary cohort of peritoneal dialysis patients. Clin J Am Soc Nephrol 2009; 4:1201–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Szeto CC, Chow KM, Lam CW, Cheung R, Kwan BC, Chung KY, et al. Peritoneal albumin excretion is a strong predictor of cardiovascular events in peritoneal dialysis patients: a prospective cohort study. Perit Dial Int 2005; 25:445–52 [PubMed] [Google Scholar]
- 3. Sánchez-Villanueva R, Bajo A, Del Peso G, Fernandez-Reyes MJ, González E, Romero S, et al. Higher daily peritoneal protein clearance when initiating peritoneal dialysis is independently associated with peripheral arterial disease (PAD): a possible new marker of systemic endothelial dysfunction? Nephrol Dial Transplant 2009; 24:1009–14 [DOI] [PubMed] [Google Scholar]
- 4. Van Biesen W, Van der Tol A, Veys N, Dequidt C, Vijt D, Lameire N, et al. The personal dialysis capacity test is superior to the peritoneal equilibration test to discriminate inflammation as the cause of fast transport status in peritoneal dialysis patients. Clin J Am Soc Nephrol 2006; 1:269–74 [DOI] [PubMed] [Google Scholar]
- 5. Wang T, Heimbürger O, Cheng HH, Bergström J, Lindholm B. Does a high peritoneal transport rate reflect a state of chronic inflammation? Perit Dial Int 1999; 19:17–22 [PubMed] [Google Scholar]
- 6. Dong J, Xu Y, Li Y, Yang Z. Does association with volume status and inflammation account for the increased death risk from high peritoneal protein clearance in peritoneal dialysis? Blood Purif 2010; 30:127–34 [DOI] [PubMed] [Google Scholar]
- 7. Behling-Kelly E, Czuprynski CJ. Endothelial cells as active participants in veterinary infections and inflammatory disorders. Anim Health Res Rev 2007; 8:47–58 [DOI] [PubMed] [Google Scholar]
- 8. Ochodnicky P, Henning RH, van Dokkum RP, de Zeeuw D. Microalbuminuria and endothelial dysfunction: emerging targets for primary prevention of end-organ damage. J Cardiovasc Pharmacol 2006; 47(Suppl 2):S151–62 [DOI] [PubMed] [Google Scholar]
- 9. Jensen JS, Borch-Johnsen K, Jensen G, Feldt-Rasmussen B. Microalbuminuria reflects a generalized transvascular albumin leakiness in clinically healthy subjects. Clin Sci (Lond) 1995; 88:629–33 [DOI] [PubMed] [Google Scholar]
- 10. Nannipieri M, Rizzo L, Rapuano A, Pilo A, Penno G, Navalesi R. Increased transcapillary escape rate of albumin in microalbuminuric type II diabetic patients. Diabetes Care 1995; 18:1–9 [DOI] [PubMed] [Google Scholar]
- 11. Pedrinelli R, Penno G, Dell’Omo G, Bandinelli S, Giorgi D, Di Bello V, et al. Microalbuminuria and transcapillary albumin leakage in essential hypertension. Hypertension 1999; 34:491–5 [DOI] [PubMed] [Google Scholar]
- 12. Pérez-Fontán M, Rodríguez-Carmona A, Barreda D, López-Muñiz A, Blanco-Castro N, García-Falcón T. Peritoneal protein transport during the baseline peritoneal equilibration test is an accurate predictor of the outcome of peritoneal dialysis patients. Nephron Clin Pract 2010; 116:c104–13 [DOI] [PubMed] [Google Scholar]
- 13. Friedman AN, Fadem SZ. Reassessment of albumin as a nutritional marker in kidney disease. J Am Soc Nephrol 2010; 21:223–30 [DOI] [PubMed] [Google Scholar]
- 14. Panichi V, Maggiore U, Taccola D, Migliori M, Rizza GM, Consani C, et al. Interleukin-6 is a stronger predictor of total and cardiovascular mortality than C-reactive protein in haemodialysis patients. Nephrol Dial Transplant 2004; 19:1154–60 [DOI] [PubMed] [Google Scholar]
- 15. Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, Holmes C, et al. Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int 2005; 25:107–31 [PubMed] [Google Scholar]
- 16. Rocco MV, Jordan JR, Burkart JM. Determination of peritoneal transport characteristics with 24-hour dialysate collections: dialysis adequacy and transport test. J Am Soc Nephrol 1994; 5:1333–8 [DOI] [PubMed] [Google Scholar]
- 17. Rocco MV, Jordan JR, Burkart JM. 24-Hour dialysate collection for determination of peritoneal membrane transport characteristics: longitudinal follow-up data for the dialysis adequacy and transport test (DATT). Perit Dial Int 1996; 16:590–3 [PubMed] [Google Scholar]
- 18. Haraldsson B. Assessing the peritoneal dialysis capacities of individual patients. Kidney Int 1995; 47:1187–98 [DOI] [PubMed] [Google Scholar]
- 19. Pérez Fontan M, Rodríguez-Carmona A, García-Naveiro R, Rosales M, Villaverde P, Valdés F. Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Perit Dial Int 2005; 25:274–84 [PubMed] [Google Scholar]
- 20. Blake PG. Complicated peritonitis—the biggest cause of technique failure. Perit Dial Int 2008; 28:327–8 [PubMed] [Google Scholar]
- 21. Yang CY, Chen TW, Lin YP, Lin CC, Ng YY, Yang WC, et al. Determinants of catheter loss following continuous ambulatory peritoneal dialysis peritonitis. Perit Dial Int 2008; 28:361–70 [PubMed] [Google Scholar]
- 22. Chow KM, Szeto CC, Leung CB, Kwan BC, Law MC, Li PK. A risk analysis of continuous ambulatory peritoneal dialysis-related peritonitis. Perit Dial Int 2005; 25:374–9 [PubMed] [Google Scholar]
- 23. Nasso L. Our peritonitis continuous quality improvement project: where there is a will there is a way. CANNT J 2006; 16:20–3 [PubMed] [Google Scholar]
- 24. Chow KM, Szeto CC, Law MC, Fun Fung JS, Kam-Tao Li P. Influence of peritoneal dialysis training nurses’ experience on peritonitis rates. Clin J Am Soc Nephrol 2007; 2:647–52 [DOI] [PubMed] [Google Scholar]
- 25. Kotsanas D, Polkinghorne KR, Korman TM, Atkins RC, Brown F. Risk factors for peritoneal dialysis-related peritonitis: can we reduce the incidence and improve patient selection? Nephrology (Carlton) 2007; 12:239–45 [DOI] [PubMed] [Google Scholar]
- 26. Heaf JG, Sarac S, Afzal S. A high peritoneal large pore fluid flux causes hypoalbuminaemia and is a risk factor for death in peritoneal dialysis patients. Nephrol Dial Transplant 2005; 20:2194–201 [DOI] [PubMed] [Google Scholar]
- 27. Gokal R. Taking peritoneal dialysis beyond the year 2000. Perit Dial Int 1999; 19(Suppl 3):S35–42 [PubMed] [Google Scholar]
- 28. Dulaney JT, Hatch FE., Jr Peritoneal dialysis and loss of proteins: a review. Kidney Int 1984; 26:253–62 [DOI] [PubMed] [Google Scholar]
- 29. Chen HC, Smith SJ, Tow B, Elias PM, Farese RV., Jr Leptin modulates the effects of acyl CoA:diacylglycerol acyltransferase deficiency on murine fur and sebaceous glands. J Clin Invest 2002; 109:175–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest 2007; 117:2362–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Balafa O, Halbesma N, Struijk DG, Dekker FW, Krediet RT. Peritoneal albumin and protein losses do not predict outcome in peritoneal dialysis patients. Clin J Am Soc Nephrol 2011; 6:561–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Esch S, Zweers MM, Jansen MA, de Waart DR, van Manen JG, Krediet RT. Determinants of peritoneal solute transport rates in newly started non-diabetic peritoneal dialysis patients. Perit Dial Int 2004; 24:554–61 [PubMed] [Google Scholar]
- 33. Oh KH, Jung JY, Yoon MO, Song A, Lee H, Ro H, et al. Intraperitoneal interleukin-6 system is a potent determinant of the baseline peritoneal solute transport in incident peritoneal dialysis patients. Nephrol Dial Transplant 2010; 25:1639–46 [DOI] [PubMed] [Google Scholar]
- 34. Betjes MG, Tuk CW, Visser CE, Zemel D, Krediet RT, Arisz L, et al. Analysis of the peritoneal cellular immune system during CAPD shortly before a clinical peritonitis. Nephrol Dial Transplant 1994; 9:684–92 [DOI] [PubMed] [Google Scholar]
- 35. Lewis S, Holmes C. Host defense mechanisms in the peritoneal cavity of continuous ambulatory peritoneal dialysis patients. 1. Perit Dial Int 1991; 11:14–21 [PubMed] [Google Scholar]
- 36. Sritippayawan S, Chiangjong W, Semangoen T, Aiyasanon N, Jaetanawanitch P, Sinchaikul S, et al. Proteomic analysis of peritoneal dialysate fluid in patients with different types of peritoneal membranes. J Proteome Res 2007; 6:4356–62 [DOI] [PubMed] [Google Scholar]


