Abstract
♦ Background: The efficacy of the phosphate binder lanthanum carbonate has been demonstrated for hemodialysis patients, but no studies have focused on patients undergoing continuous ambulatory peritoneal dialysis (CAPD). We evaluated whether lanthanum carbonate could control phosphate levels in patients on CAPD.
♦ Methods: In this 48-week open-label prospective study, 28 patients on CAPD with a phosphate level of 6 mg/dL or greater were given lanthanum carbonate titrated from 750 mg to 2250 mg daily to achieve a target serum phosphate level of less than 6 mg/dL. The primary efficacy endpoint was reduction of serum phosphate to less than 6 mg/dL. Serum levels of calcium and parathyroid hormone were also evaluated, as were the Ca×P product and adverse effects.
♦ Results: From week 4 to the end of the study at week 48, we observed a significant reduction of serum phosphate to 5.25 ± 0.97 mg/dL from 6.88 ± 1.06 mg/dL at study start (p < 0.01). At the end of the study, 78.6% of participants had achieved the target of less than 6 mg/dL. Because no change of serum calcium occurred, the Ca×P product declined significantly during the study. Intact parathyroid hormone declined gradually over the study period, but the change had not reached significance at the end of the study (p = 0.11). The mean final dose of lanthanum carbonate was 946 mg daily. The only adverse effect reported was mild nausea in 1 patient.
♦ Conclusions: Lanthanum carbonate is an effective phosphate binder that can control serum phosphate and Ca×P product in CAPD patients with hyperphosphatemia. Lanthanum carbonate was well tolerated in our population.
Key words: Lanthanum carbonate, continuous ambulatory peritoneal dialysis, CAPD, hyperphosphatemia, Ca×P product, parathyroid hormone
Hyperphosphatemia is a silent killer in the dialysis population, and phosphate control is critical. Although serum phosphate is not initially high, phosphate retention eventually contributes to the development of cardiovascular disease in dialysis patients (1-3). Active management of hyperphosphatemia is therefore essential in patients on dialysis. Restriction of dietary phosphate intake and administration of phosphate binders are the standard methods used in attempting to prevent hyperphosphatemia in dialysis patients.
Dietary restriction can reduce serum concentrations of phosphate, fibroblast growth factor 23, and parathyroid hormone (PTH), although the normal range will not be reached (4). As a result, oral phosphate binders are frequently required. In dialysis patients, phosphate excretion is essentially absent, and so oral phosphate binders are given to limit phosphate absorption (5). Secondary hyperparathyroidism may also contribute to hyperphosphatemia by enhancing the release of calcium phosphate from bone (6).
At present, the major medications for lowering serum phosphate are calcium-containing phosphate binders and non-calcium-containing phosphate binders such as sevelamer hydrochloride and lanthanum carbonate. Calcium carbonate, particularly if combined with inappropriate vitamin D therapy, can give rise to hypercalcemia and potentially to metastatic calcification (7). Sevelamer hydrochloride is a nonabsorbable agent that contains neither calcium nor aluminum, but that lowers bicarbonate levels, potentially promoting metabolic acidosis (8). Another non-calcium-containing phosphate binder is lanthanum carbonate, which has been shown in multiple studies using various dosing regimens to be effective for reducing phosphate levels in dialysis patients (9).
Despite the many reports about the efficacy of lanthanum carbonate in hemodialysis patients, few studies have been conducted in patients undergoing continuous ambulatory peritoneal dialysis (CAPD) (9-19). Accordingly, the aim of the present study was to evaluate the efficacy and safety of lanthanum carbonate in CAPD patients with hyperphosphatemia.
METHODS
SUBJECTS
Men and women more than 20 years of age who had been on CAPD for at least 12 weeks were eligible to enter the study if their serum phosphate was 6.0 mg/dL or greater. Patients were considered suitable for inclusion if they were receiving CAPD for end-stage renal disease and had a serum phosphate level consistently at or over 6.0 mg/dL, confirmed in two consecutive measurements during an 8-week observation period. All subjects used a dialysate with 2.5% calcium and performed 4 exchanges of a 2-L bag over 24 hours.
Exclusion criteria included serum phosphate less than 6.0 mg/dL or more than 10 mg/dL, corrected serum calcium less than 8.0 mg/dL or more than 11.0 mg/dL, and intact PTH exceeding 800 pg/mL after the 8-week observation period at the start of the study, as well as other clinically significant laboratory abnormalities and significant gastrointestinal disorders such as colonic diverticulosis or malignancy.
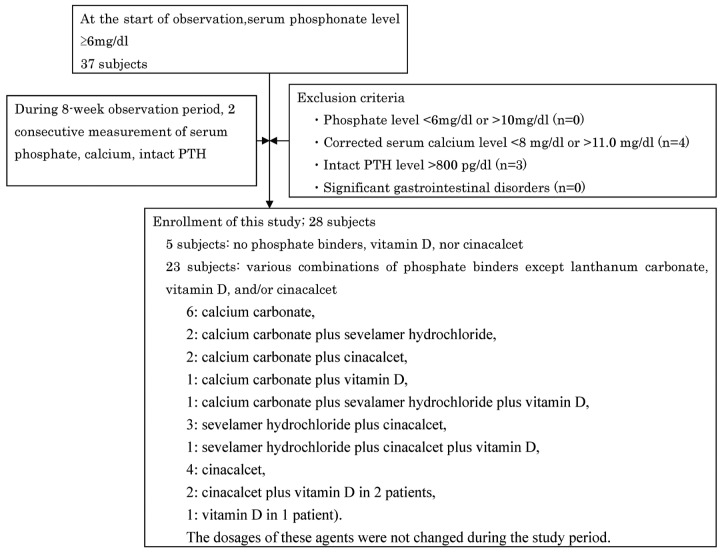
Although 35 candidates had a serum phosphate level of 6 mg/dL or more at the start of observation, the study enrolled just 28 subjects at the end of observation. Exclusion criteria eliminated 4 patients with a corrected serum calcium of less than 8.0 mg/dL or more than 11.0 mg/dL and 3 patients with an intact PTH level exceeding 800 pg/mL during the observation period (Figure 1).
Figure 1.
— Study enrollment.
Of the 28 study patients, 23 were being treated with various combinations of phosphate binders, vitamin D, and cinacalcet (calcium carbonate in 6 patients, calcium carbonate plus sevelamer hydrochloride in 2 patients, calcium carbonate plus cinacalcet in 2 patients, calcium carbonate plus vitamin D in 1 patient, calcium carbonate plus sevelamer hydrochloride plus vitamin D in 1 patient, sevelamer hydrochloride plus cinacalcet in 3 patients, sevelamer hydrochloride plus cinacalcet plus vitamin D in 1 patient, cinacalcet in 4 patients, cinacalcet plus vitamin D in 2 patients, and vitamin D in 1 patient). The dosages of those agents were not changed during the study period.
The study protocol was reviewed and approved by the ethics committee at each study site, and informed consent was obtained from each subject.
STUDY DESIGN
This non-comparative open-label dose-escalation study set out to assess the efficacy and safety of lanthanum carbonate for reducing serum phosphate in CAPD patients with hyperphosphatemia.
After the 8-week observation period and before dose titration with lanthanum carbonate began, confirmation was obtained that the patients who met the inclusion criteria still had a serum phosphate level of 6.0 mg/dL or greater. Phosphate binders (calcium carbonate or sevelamer hydrochloride), vitamin D, and cinacalcet could be continued without any change of dose—but could not be initiated—during the study.
At 4, 8, 12, 24, and 48 weeks, venous blood samples were obtained from all patients after an overnight fast. Serum phosphate, calcium, albumin, and other biochemical parameters were determined using an automated multi-analyzer. Intact PTH was measured using a commercially available electrochemiluminescence detection system. The dialysate-to-plasma ratio of creatinine (D/P Cr) was calculated to assess peritoneal membrane function.
Every 8 weeks, the dose of lanthanum carbonate was titrated from an initial daily dose of 750 mg to a maximum of 2250 mg according to the patient’s serum phosphate. Patients took their daily dose of chewable tablets in 3 equal portions after meals. Drug compliance was confirmed by interview with the patients every 4 weeks.
ANALYSIS OF EFFICACY AND SAFETY
The primary endpoint for the study was reduction in serum phosphate to between 3.5 mg/dL and 6.0 mg/dL at the end of the treatment period. Secondary endpoints included corrected serum calcium, Ca×P product, and intact PTH. Achievement of the primary endpoint in more than 50% of subjects was considered to indicate the efficacy of lanthanum carbonate.
A safety analysis summarized the incidence of adverse events and drug-related laboratory abnormalities.
STATISTICAL ANALYSIS
Results are reported as mean ± standard deviation, with p < 0.05 indicating significance. A D’Agostino-Pearson K2 omnibus test was used to confirm normal distribution of the data. After normal distribution was confirmed, changes in the variables throughout the study were analyzed using a repeated-measures analysis of variance. A Bonferroni test assessed differences between values at the start and throughout the study. If normal distribution was not confirmed, a Friedman test was performed. Correlations between serum phosphate and age, body mass index, D/P Cr, serum phosphate, serum calcium, and intact PTH at week 0 were determined by linear regression analysis.
RESULTS
DEMOGRAPHIC PROFILE
In the 28 CAPD patients (16 men, 12 women; mean age: 57.4 ± 9.0 years), underlying renal diseases were chronic glomerulonephritis in 22 patients, diabetic nephropathy in 4, and nephrosclerosis in 2. All 28 patients completed the 48 weeks of treatment. One subject suffered from mild nausea for about 7 days, but the nausea subsided as treatment with lanthanum carbonate continued.
DOSE OF LANTHANUM CARBONATE
In most patients, lanthanum carbonate was maintained at the initial dose of 750 mg daily. The final daily dose was 750 mg in 22 patients (78.6%), 1000 mg in 1 patient (3.6%), 1500 mg in 3 patients (10.7%), and 2250 mg in 2 patients (7.1%). The mean dose of lanthanum carbonate was 946 mg daily.
SERUM PHOSPHATE
From the start of lanthanum carbonate therapy, mean serum phosphate declined significantly and progressively over the study period, with mean serum phosphate being 6.88 ± 1.06 mg/dL, 6.14 ± 1.30 mg/dL, 5.96 ± 1.47 mg/dL, 5.63 ± 1.23 mg/dL, 5.33 ± 1.15 mg/dL, and 5.25 ± 0.97 mg/dL at 0, 4, 8, 12, 24, and 48 weeks respectively (Figure 2, p < 0.01). The mean reduction in serum phosphate from start to end of the study was 1.63 mg/dL. The percentage of patients with a serum phosphate level less than 6 mg/dL was 35.7%, 53.6%, 60.7%, 75.0%, and 78.6% at 4, 8, 12, 24, and 48 weeks respectively (Figure 3).
Figure 2.
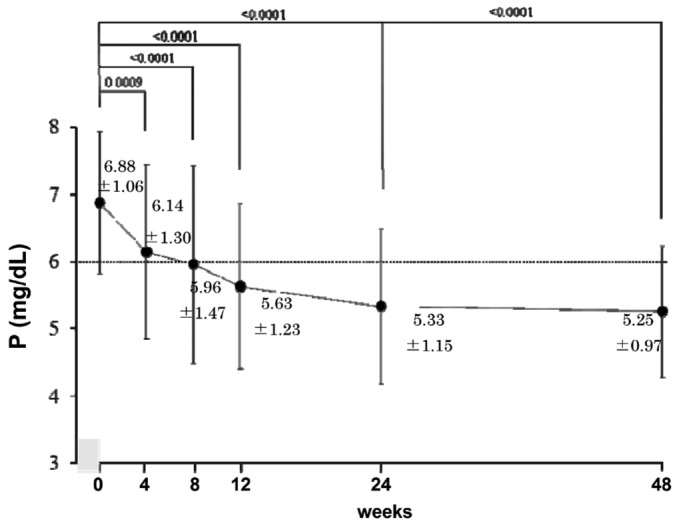
— Serum phosphate level throughout the study (mean ± standard deviation). The mean phosphate level declined significantly and progressively during the study.
Figure 3.
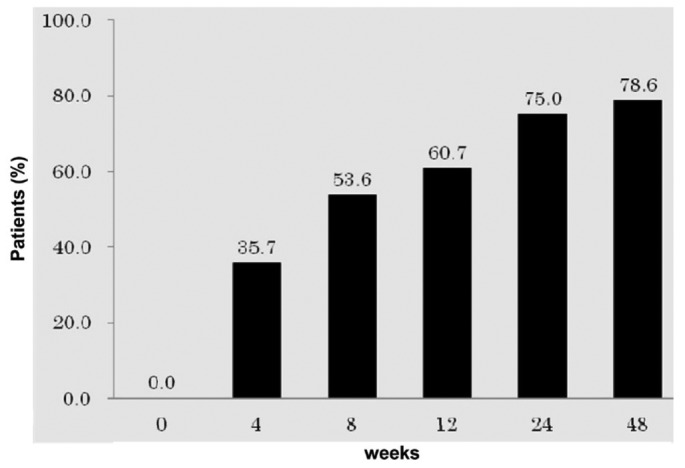
— The percentage of patients with serum phosphate less than 6 mg/dL increased throughout the study period.
SERUM CALCIUM AND INTACT PTH
The mean corrected serum calcium did not change during the study period (Figure 4). Changes of the Ca×P product were consistent with those of serum phosphate (60 ± 9 mg2/dL2 at 0 weeks to 46 ± 8 mg2/dL2 at 48 weeks, p < 0.01, Figure 5). Serum intact PTH declined gradually over the study period, but the change had not reached significance at study end (p = 0.11, Figure 6).
Figure 4.
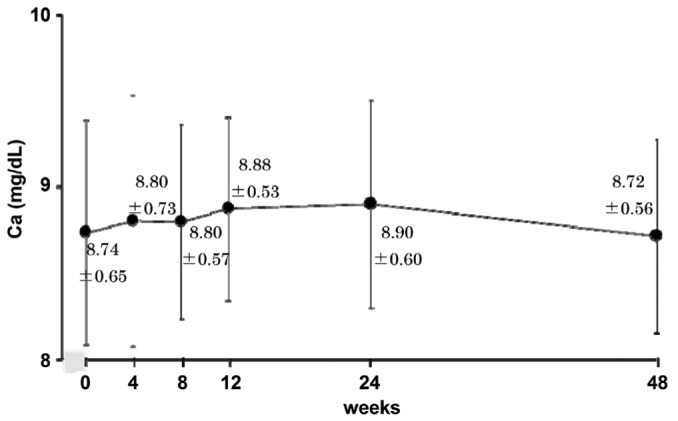
— Serum calcium (mean ± standard deviation) was unchanged throughout the study.
Figure 5.
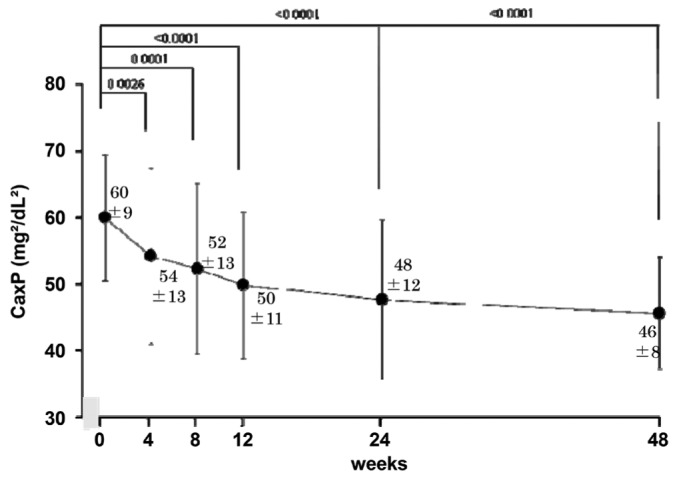
— Ca×P product (mean ± standard deviation) declined significantly and progressively throughout the study.
Figure 6.
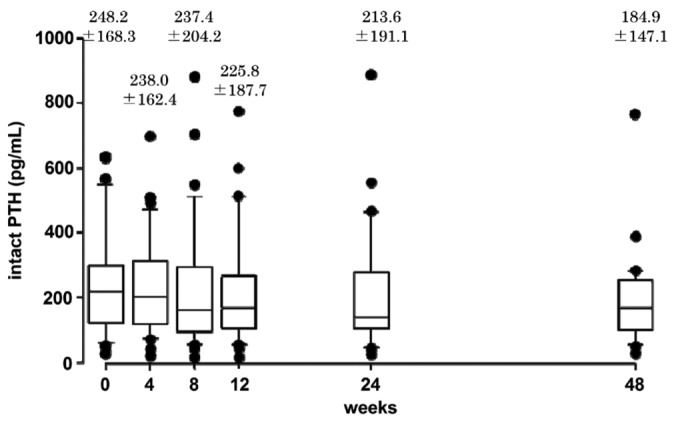
— Serum intact parathyroid hormone [PTH (mean ± standard deviation)] declined gradually throughout the study, but had not reached significance at the end of the study.
RELATIONSHIP BETWEEN SERUM PHOSPHATE AND OTHER CLINICAL PARAMETERS
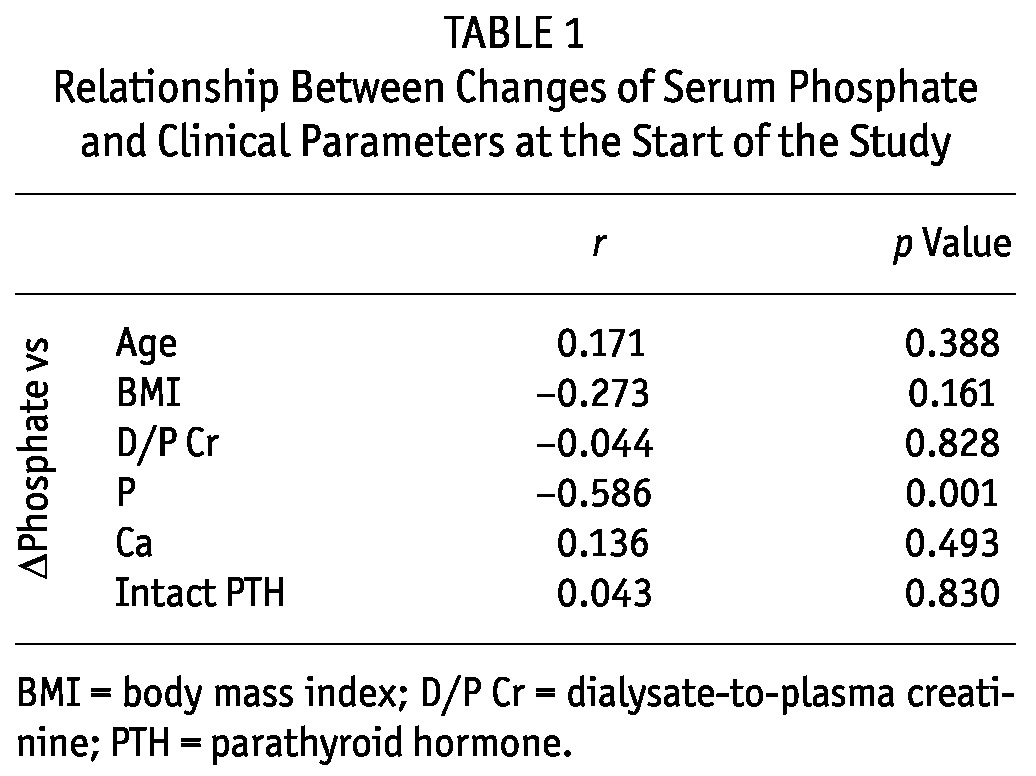
The change in serum phosphate correlated inversely with serum phosphate at 0 weeks, and lanthanum carbonate was more effective in CAPD patients with a higher baseline serum phosphate. We observed no relationship between change in serum phosphate and age, body mass index, D/P Cr, corrected serum calcium, or intact PTH (Table 1).
TABLE 1.
Relationship Between Changes of Serum Phosphate and Clinical Parameters at the Start of the Study
SAFETY
Treatment with lanthanum carbonate was generally well tolerated. During the study period, 1 patient complained of nausea for 7 days at 4 weeks after treatment start. The symptom was transient and subsided as the patient continued to take lanthanum carbonate.
No clinically or statistically significant changes of hematologic or biochemistry test results were observed.
DISCUSSION
The present study set out to evaluate the efficacy of lanthanum carbonate for controlling serum phosphate in CAPD patients with hyperphosphatemia. Of 28 subjects, 5 received lanthanum carbonate monotherapy for hyperphosphatemia; the other 23 patients had phosphate levels of 6 mg/dL or greater despite receiving standard treatment for hyperphosphatemia. Most of the subjects therefore had hyperphosphatemia resistant to standard treatment.
The primary endpoint of our study was reduction in serum phosphate to less than 6 mg/dL. At the end of the study, 78.6% of the subjects had achieved that target. Lanthanum carbonate reduced serum phosphate without increasing corrected serum calcium. As a result, the Ca×P product remained within the normal range. It is noteworthy that 78.6% of patients were able to be maintained on the initial lanthanum carbonate dose of 750 mg daily, with only 3.6%, 14.8%, and 11.1% of patients needing up-titration to 1000 mg, 1500 mg, and 2250 mg daily respectively. The optimum dose of lanthanum carbonate for controlling hyperphosphatemia in CAPD patients therefore seems to be lower than that for patients on hemodialysis (10-15,17), because the median dose required to improve hyperphosphatemia in hemodialysis patients is 1250 mg daily (17,20). Two possible explanations for that difference can be suggested: First, of 28 patients in the present study, 14 (50.0%) were taking calcium carbonate, and 8 (28.6%) were using sevelamer hydrochloride. Therefore, 78.6% of the patients were already taking other phosphate binders that were continued during the study. Second, in CAPD patients, a slow but continuous transfer of phosphate occurs between plasma and dialysate, with net diffusion into dialysate. In contrast, patients on hemodialysis show a trough level of serum phosphate during dialysis that rebounds to the pre-dialysis level within 2 - 3 hours after completion of the session. Accordingly, net removal of phosphate is likely to be higher with CAPD than with hemodialysis (21), which may partly account for the decline of serum phosphate with a lower dose of lanthanum carbonate in the present study and in other reports (22).
Our study population showed a low incidence of adverse events. As with other phosphate binders, the most common adverse events caused by lanthanum carbonate are gastrointestinal symptoms such as nausea and vomiting that lead to withdrawal from treatment. Use of lanthanum carbonate was well tolerated by our CAPD patients, and no subject dropped out because of adverse effects. The relatively low dose of lanthanum carbonate (mean: 946 mg daily) that was sufficient to control serum phosphate levels may have contributed to the low rate of side effects.
Two principal treatment modalities—restriction of dietary phosphate intake and administration of phosphate binders—are used in attempting to prevent or reverse hyperphosphatemia in patients undergoing dialysis. Many patients require oral phosphate binders to control their serum phosphate. The major drugs available to lower serum phosphate are classified as calcium-containing or non-calcium-containing. There is no evidence that the use of one type of agent rather than another results in a significant clinical benefit with respect to mortality and morbidity. However, relying on a calcium-containing phosphate binder (calcium carbonate) increases the risk of a positive calcium balance, particularly when combined with vitamin D therapy (23,24). Sevelamer hydrochloride is a nonabsorbable cationic polymer that binds phosphate through ion exchange; it contains neither calcium nor aluminum. The incidence of adverse events with sevelamer hydrochloride is higher compared with that with calcium carbonate (25). Lanthanum carbonate is another non-aluminum- and non-calcium-containing phosphate binder that is available for the management of hyperphosphatemia in patients with end-stage renal disease. After oral administration, it dissociates in the upper gastrointestinal tract to release lanthanum ions that bind with phosphate and effectively decrease gastrointestinal phosphate absorption through the formation, with dietary phosphate, of highly insoluble complexes that are subsequently excreted (15,16,26,27). The present study shows that lanthanum carbonate is an effective phosphate binder that can control serum phosphate levels in patients on CAPD, with treatment achieving a significantly lower serum phosphate and Ca×P product almost without side effects.
Various animal and human studies have revealed that lanthanum is minimally absorbed and not metabolized, accumulating at low concentrations in bone and liver (16,26,27). Lanthanum carbonate tablets can be visualized clearly on plain radiographs, with a density between that of bone and metal. Patients taking lanthanum carbonate will therefore have the drug in the gastrointestinal tract at the time of radiologic examinations, and they should be instructed to chew their tablets well (28).
The present study has several limitations, including a small sample size, no blinding, and no comparison with a placebo control. In particular, the lack of a control arm is a major limitation. Patients may have improved their adherence to medication and diet during the study, which might account for much of the reduction in serum phosphate. A control arm could have compensated for such potential confounders. Given the lack of a control arm, the efficacy of lanthanum carbonate was assessed on the basis of the percentage of subjects achieving the primary endpoint. A placebo-controlled study of lanthanum carbonate that included hemodialysis patients (n = 15) and CAPD patients (n = 21) was previously performed by Al-Baaj et al. (19), and they achieved results similar to ours. Their patients had a phosphate level of 4.03 - 5.58 mg/dL at a lanthanum dose of 375 - 2250 mg daily; the patients were then randomized to continue maintenance therapy with lanthanum carbonate (n = 17) or to receive placebo (n = 19). Acceptable phosphate levels were maintained in 64.7% of the lanthanum carbonate-treated patients compared with 21.4% of those in the placebo group. The mean daily dose of lanthanum carbonate calculated from the data in that study was 1213 mg.
We also could not assess the difference between lanthanum carbonate as add-on therapy compared with monotherapy because of the small number of patients receiving monotherapy. Moreover, lanthanum carbonate was generally well tolerated during this study, but adverse effects could emerge during long-term treatment.
CONCLUSIONS
Lanthanum carbonate is an effective phosphate binder that lowers serum phosphate in CAPD patients without increasing serum calcium. Lanthanum carbonate was well tolerated in our population of CAPD patients. In future, the long-term efficacy and safety of lanthanum carbonate for CAPD patients should be investigated.
DISCLOSURES
The authors have no financial conflicts of interest to declare.
References
- 1. Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol 2009; 20:397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fukagawa M, Kazama JJ. With or without the kidney: the role of FGF23 in CKD. Nephrol Dial Transplant 2005; 20:1295–8 [DOI] [PubMed] [Google Scholar]
- 3. Slatopolsky E. The intact nephron hypothesis: the concept and its implications for phosphate management in CKD-related mineral and bone disorder. Kidney Int Suppl 2011; (121):S3–8 [DOI] [PubMed] [Google Scholar]
- 4. Friedman EA. Consequences and management of hyperphosphatemia in patients with renal insufficiency. Kidney Int Suppl 2005; (95):1–7 [DOI] [PubMed] [Google Scholar]
- 5. Delmez JA, Slatopolsky E. Hyperphosphatemia: its consequences and treatment in patients with chronic kidney disease. Am J Kidney Dis 1992; 19:303–17 [DOI] [PubMed] [Google Scholar]
- 6. Felsenfeld AJ, Rodriguez M. Phosphorous, regulation of plasma calcium, and secondary hyperparathyroidism: a hypothesis to integrate a historical and modern perspective. J Am Soc Nephrol 1999; 10:878–90 [DOI] [PubMed] [Google Scholar]
- 7. Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 2005; 68:1815–24 [DOI] [PubMed] [Google Scholar]
- 8. Gallieni M, Cozzolino M, Brancaccio D. Transient decrease of serum bicarbonate levels with sevelamer hydrochloride as the phosphate binder. Kidney Int 2000; 57:1776–7 [DOI] [PubMed] [Google Scholar]
- 9. Behets GJ, Verberckmoes SC, D’Haese PC, De Broe ME. Lanthanum carbonate: a new phosphate binder. Curr Opin Nephrol Hypertens 2004; 13:403–9 [DOI] [PubMed] [Google Scholar]
- 10. Joy MS, Finn WF. on behalf of the LAM-302 Study Group. Randomized, double blind, placebo-controlled, dose-titration, phase III study assessing the efficacy and tolerability of lanthanum carbonate: a new phosphate binder for the treatment of hyperphosphatemia. Am J Kidney Dis 2003; 42:96–107 [DOI] [PubMed] [Google Scholar]
- 11. Hutchison AJ, Speake M, Al-Baaj F. Reducing high phosphate levels in patients with chronic renal failure undergoing dialysis: a 4-week, dose-finding, open-label study with lanthanum carbonate. Nephrol Dial Transplant 2004; 19:1902–6 [DOI] [PubMed] [Google Scholar]
- 12. Finn WF, Joy MS, Hladik G. on behalf of the Lanthanum Study Group. Efficacy and safety of lanthanum carbonate for reduction of serum phosphorous in patients with chronic renal failure receiving hemodialysis. Clin Nephrol 2004; 62:193–201 [DOI] [PubMed] [Google Scholar]
- 13. Hutchison AJ, Barnett ME, Krause R, Kwan JT, Siami GA. on behalf of the SPD405-309 Lanthanum Study Group. Long-term efficacy and safety profile of lanthanum carbonate: results for up to 6 years of treatment. Nephron Clin Pract 2008; 110:c15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chiang SS, Chen JB, Yang WC. Lanthanum carbonate (Fosrenol): efficacy and tolerability in the treatment of hyperphosphatemic patients with end-stage renal disease. Clin Nephrol 2005; 63:461–70 [DOI] [PubMed] [Google Scholar]
- 15. Finn WF. on behalf of the SPD 405-307 Lanthanum Study Group. Lanthanum carbonate versus standard therapy for the treatment of hyperphosphatemia: safety and efficacy in chronic maintenance hemodialysis patients. Clin Nephrol 2006; 65:191–202 [DOI] [PubMed] [Google Scholar]
- 16. Persy VP, Behets GJ, Bervoets AR, De Broe ME, D’Haese PC. Lanthanum: a safe phosphate binder. Semin Dial 2006; 19:195–9 [DOI] [PubMed] [Google Scholar]
- 17. Shigematsu T. on behalf of the Lanthanum Carbonate Research Group. Lanthanum carbonate effectively controls serum phosphate without affecting serum calcium levels in patients undergoing hemodialysis. Ther Apher Dial 2008; 12:55–61 [DOI] [PubMed] [Google Scholar]
- 18. Kawanishi H, Ishida M, Ishizaki M, Takuma Y, Tamura H, Kobayashi S, et al. Lanthanum carbonate treatment of patients with hyperphosphatemia undergoing CAPD. Perit Dial Int 2008; 28:673–5 [PubMed] [Google Scholar]
- 19. Al-Baaj F, Speake M, Hutchison AJ. Control of serum phosphate by oral lanthanum carbonate in patients undergoing haemodialysis and continuous ambulatory peritoneal dialysis in a short-term, placebo-controlled study. Nephrol Dial Transplant 2005; 20:775–82 [DOI] [PubMed] [Google Scholar]
- 20. D’Haese PC, Spasovski GB, Sikole A, Hutchison A, Freemont TJ, Sulkova S, et al. A multicenter study on the effects of lanthanum carbonate (Fosrenol) and calcium carbonate on renal bone disease in dialysis patients. Kidney Int Suppl 2003; (85):S73–8 [DOI] [PubMed] [Google Scholar]
- 21. Coburn JW. Mineral metabolism and renal bone disease: effects of CAPD versus hemodialysis. Kidney Int Suppl 1993; 40:S92–100 [PubMed] [Google Scholar]
- 22. Joffe P, Pódenphant J, Heaf JG. Bone histology in CAPD patients: a comparison with hemodialysis and conservatively treated chronic uremics. Adv Perit Dial 1989; 5:171–6 [PubMed] [Google Scholar]
- 23. Tonelli M, Pannu N, Manns B. Oral phosphate binders in patients with kidney failure. N Engl J Med 2010; 362:1312–24 [DOI] [PubMed] [Google Scholar]
- 24. Sprague SM. A comparative review of the efficacy and safety of established phosphate binders: calcium, sevelamer, and lanthanum carbonate. Curr Med Res Opin 2007; 23:3167–75 [DOI] [PubMed] [Google Scholar]
- 25. Suki WN, Zabaneh R, Cangiano JL, Reed J, Fischer D, Garrett L, et al. Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int 2007; 72:1130–7 [DOI] [PubMed] [Google Scholar]
- 26. Swainston Harrison T, Scott LJ. Lanthanum carbonate. Drugs 2004; 64:985–96 [DOI] [PubMed] [Google Scholar]
- 27. Pennick M, Dennis K, Damment SJ. Absolute bioavailability and disposition of lanthanum in healthy human subjects administered lanthanum carbonate. J Clin Pharmacol 2006; 46:738–46 [DOI] [PubMed] [Google Scholar]
- 28. Chuang CL, Chiou SY, Li SY, Jian DY, Chen JY. The case: a peritoneal dialysis patient with an unusual abdominal film. Treatment with lanthanum carbonate. Kidney Int 2007; 72:1291–2 [DOI] [PubMed] [Google Scholar]