Abstract
Leaf type is an important trait that closely associates with crop yield. WRKY transcription factors exert diverse regulatory effects in plants, but their roles in the determination of leaf type have not been reported so far. In this work, we isolated a WRKY transcription factor gene TaWRKY71-1 from a wheat introgression line SR3, which has larger leaves, superior growth capacity and higher yield than its parent common wheat JN177. TaWRKY71-1 specifically expressed in leaves, and produced more mRNA in SR3 than in JN177. TaWRKY71-1 localized in the nucleus and had no transcriptional activation activity. TaWRKY71-1 overexpression in Arabidopsis resulted in hyponastic rosette leaves, and the hyponastic strength was closely correlative with the transcription level of the transgene. The spongy mesophyll cells at abaxial side of leaves were drastically compacted by TaWRKY71-1 overexpression. In TaWRKY71-1 overexpression Arabidopsis, the expression of IAMT1 that encodes a methyltransferase converting free indole-3-acetic acid (IAA) to methyl-IAA ester (MeIAA) to alter auxin homeostatic level was induced, and the induction level was dependent on the abundance of TaWRKY71-1 transcripts. Besides, several TCP genes that had found to be restricted by IAMT1 had lower expression levels as well. Our results suggest that TaWRKY71-1 causes hyponastic leaves through altering auxin homeostatic level by promoting the conversion of IAA to MeIAA.
Introduction
Plant type closely associates with the crop yield, and is therefore a crucial trait in crop breeding. Appropriate leaf shape is an important aspect of ideal plant type, because the three-dimensional structure of the plant leaf affects light capture, carbon fixation, and gas exchange for photosynthesis [1]. In rice, the rolled leaf is regarded as a critical element for the ideal phenotype [2], [3]. Moderate leaf rolling improves photosynthetic efficiency, accelerates dry-matter accumulation, increases yield, reduces the solar radiation on leaves, and decreases leaf transpiration under drought stress [4]. Therefore, to unravel the mechanism controlling leaf curling is beneficial for breeding crops with the desired architecture and stress tolerance.
Leaf curling is orchestrated by the complicated machineries with hormones and non-hormone factors involved. Auxin has been widely reported to play an essential role in the determination of the leaf morphology [5], [6]. Auxin signaling mutant auxin-resistant 1 (axr1) results in epinastic leaves [7], while phototrophic hypocotyl 4/auxin response factor 7 (nph4/arf7) produces hyponastic (up-curled) leaf phenotypes [8]. Auxin synthesis defect mutants sporocyteless-Dominant I (spl-D) and petal loss-Dominant (ptl-D) display a hyponastic leaves phenotype [9], but its overproduction mutants such as rooty [10], sur2 [11], [12], yucca [13], and iaaM overexpression lines [14] all have epinastic leaves phenotype. Apart from the synthesis and degradation, auxin homeostasis between free auxin and its conjugates that are modified by monosaccharide, disaccharide or methyl has proved to regulate leaf curling. Overexpression of the Arabidopsis UGT84B1, the homologue of maize IAGlu that catalyzes the formation of IAA glucose ester, results in rounded, wrinkled, and curly leaves [15]. The dominant mutant iamt1-D of IAMT1 (At5g55250), whose product appears to encode an IAA methyltransferase that specifically converts free IAA to methyl-IAA ester (MeIAA) in vitro [16], [17], causes severe hyponastic leaves [18]. In iamt1-D, the expression of some known leaf-curling associated genes such as several TCPs and HASTY is reduced. However, how IAMT1-mediated homeostasis between IAA and MeIAA is modulated is still not known.
WRKY transcription factors, named by their WRKY domain, are one of the large families of transcriptional regulators in plants. The WRKY domain is about 60 residues in length, containing the WRKYGQK residues and a zinc-finger structure at the C-terminus. The zinc-finger structure is either Cx4–5Cx22–23HxH or Cx7Cx23HxC. The WRKY transcription factors are divided into three groups based on the number of WRKY domains and their zinc fingers structure. Group I proteins contain two WRKY domains and C2H2 structures, group II proteins contain one WRKY domain and C2H2 structures, and group III proteins contain one WRKY domain and C2HC structures. Group II proteins were further divided into IIa, IIb, IIc, IId and IIe based on the primary amino acid sequence. WRKY proteins act as repressors or activators to play important roles in diverse processes such as plant development and response to environmental stimuli, and their functions in leaves include senescence and resistance to Xanthomonas oryzae (bacterial rice leaf blight) (see review [19]). The issue that whether or not WRKY transcription factors regulate plant type has not been reported so far.
To identify WRKY transcription factor that may function in leaf type (curling), we screened the microarry-based transcriptomic data between the wheat (Triticum aestivum) introgression cultivar SR3 and its parent common wheat JN177. SR3 has larger leaves, more vigorous growth capacity, higher yield as well as superior salt tolerance than JN177 [20]. We isolated a wheat WRKY transcription factor, TaWRKY71-1, specifically expressed in leaves with higher transcription level in SR3 than in JN177. Its overexpression in Arabidopsis resulted in hyponastic leaf phenotype, promoted the expression of IAMT1 and several TCP genes, and tightened spongy tissue cells. These findings indicate that TaWRKY71-1 resulted in hyponastic leaves by shifting auxin homeostasis from IAA to MeIAA.
Materials and Methods
Plant Materials
The wheat introgression line SR3 that was selected from asymmetric somatic hybrids between a bread wheat cultivar JN177 and Thinopyrum ponticum was used in this study [20]. Seeds of SR3 were germinated in distilled water at 25°C and 60% humidity under a photoperiod of 16 h/8 h for seven days, and then uniform seedlings were transferred into half-strength Hoagland’s liquid medium under the same condition for another seven days. Leaves and roots of fourteen-day-old seedlings with similar height were collected separately and stored in liquid nitrogen for RNA extraction and further analysis.
Isolation and Sequence Analysis of TaWRKY71-1 from Wheat
The sequence of the selected probe in the microarry was subjected to BLASTn against wheat EST database at NCBI, and all matched EST sequences were assembled into a contig sequence using the CAP3 package. According to the contig sequence (designated as TaWRKY71-1 for it was an allelic gene of TaWRKY71 isolated from wheat cultivar Chinese Spring), the gene-specific primer pairs covering the open reading frame (ORF) were designed (forward primer: 5′-CAGCGTCCATTCCATTACAGTAG-3′; reverse primer: 5′-CGTCTTCGTCGTTGGTCAGG-3′). Then the ORF and the genomic sequence of TaWRKY71-1 were obtained from cDNA and genomic DNA of SR3, respectively, by PCR.
Isolation of TaWRKY71-1 Promoter and Prediction of cis-elements
The promoter sequence of TaWRKY71-1 was predicted by BLAST the Chinese Spring draft genome assembly (http://www.cerealsdb.uk.net/CerealsDB/Documents/DOC_search_reads.php) with the genomic sequence of TaWRKY71-1 as query. According to the predicted promoter sequence, the specific primers (the forward primer 5′-CGTTTTTGAAAGAGTGAGTCGAGC-3′ and the reverse primer 5′-CGGCTGGTTGTTGTAGTGGTTATTGC-3′) were designed, and TaWRKY71-1 promoter sequence was amplified from genomic DNA. cis-elements in the promoter sequence were analyzed using Plant-CARE available online (http://bioinformatics.psb.ugent. be/webtools/plantcare/html/).
Subcellular Localization of TaWRKY71-1
TaWRKY71-1 ORF without termination codon was amplified by PCR with primers containing BglII and HindIII sites (underlined) at their 5′ ends, 5′-AGATCTCAGCGTCCATTCCATTACAGTAG-3′ and 5′-AAGCTTATTTATGTCCCTGGTCGGCGAT-3′. After being verified by sequencing, the fragment was introduced into pBI221 vector, creating an in-frame fusion TaWRKY71-1::GFP gene under the control of CaMV 35S promoter. The fusion construct (pBI221-TaWRKY71-1::GFP) and control vector (pBI221-GFP) were transformed into Arabidopsis protoplasts [21] and onion epidermis cells [22], respectively. After incubation at 21–23°C for 16 h, GFP was detected by a confocal fluorescence microscopy (Zeiss LSM700).
Transcriptional Activation Analysis
For transcriptional activation analysis, the full-length of TaWRKY71-1 cDNA was amplified using the following primers, which possess EcoRI and BglII sites (underlined), 5′-GAATTCATGGATCCATGGGTCAGCA-3′ and 5′-AGATCTCGTCTTCGTCGTTGGTCAGG-3′ to build the pGBKT7-TaWRKY71-1 vector (Clontech) containing the GAL4 DNA binding domain. The pGBKT7-TaWRKY71-1 and pGBKT7 (negative control) were transformed into yeast strain AH109. The transformed yeast cells were selected on synthetic defined (SD) plates without Trp, and analyzed on SD plates without Trp, His and Ade.
Generation of TaWRKY71-1 Overexpression Arabidopsis Lines
The ORF of TaWRKY71-1 was cloned into the XbaI and SmaI sites of pSTART vector to create the pSTART-TaWRKY71-1 construct driven by CaMV35S promoter using the forward primer 5′-TCTAGACAGCGTCCATTCCATTACAGTAG-3′ and the reverse primer 5′-CCCGGGCGTCTTCGTCGTTGGTCAGG-3′. The pSTART-TaWRKY71-1 construct in A. tumefaciens EHA105 was transformed into Arabidopsis (Columbia-0) using floral dipping method [23], and the transformed Arabidopsis was grown in soil under a 16 h/8 h photoperoid, 60∼80% relative humidity, 21–23°C regime. Seeds of transformed Arabidopsis were screened in 1/2 MS agar plate containing kanamycin and cephalosporin in dark at 4°C for 72 h followed by under a 16 h/8 h photoperoid, 60∼80% relative humidity, 21∼23°C regime for two weeks. Homozygous T3 progeny bred from a T2 population which segregated in the correct ratio 3∶1 were selected and confirmed by semi-quantitative RT-PCR (sqRT-PCR) with total cDNAs as template, which was used for further analysis.
RNA Extraction and Gene Expression Assay
Total RNA of wheat and Arabidopsis seedling samples was extracted using TRIZol reagent (Takara), and the first strand of cDNA was prepared using RNAiso plus kit (Invitrogen) according to the manual. sqRT-PCR for gene expression assay was performed in a 20µL reaction volume with the thermal cycling procedure that denaturation at 95°C for 5 min, followed by 28/38 cycles of 94°C/30 s, 55°C/30 s, 72°C/45 s, with a final extension of 72°C/5 min. Real time quantitative PCR (qRT-PCR) for Arabidopsis marker gene expression assay was performed in a 10µL reaction volume with the thermal cycling procedure that denaturation at 95°C for 2 min, followed by 40 cycles of 94°C/10 s, 60°C/10 s, 72°C/20 s, and then melting curve. A constitutively expressed wheat TaACTIN (AB181991) and Arabidopsis TUBLIN (AT5G62690) were used as internal reference for sqRT-PCR, and Arabidopsis ACTIN2 (AT3G18780) for qRT-PCR. sqRT-PCR primers were: TaWRKY71-1, forward 5′-CAGTTCACCGACATGGTCAC-3′, reverse 5′-GTCGCCACGAGTATGGTCTT-3′; wheat TaACTIN, forward 5′-GTTCCAATCTATGAGGGATACACGC-3′, reverse 5′-GAACCTCCACTGAGAACAACATTACC-3′; Arabidopsis TUBLIN, forward 5′-CTCAAGAGGTTCTCAGCAGTA-3′, reverse 5′-TCACCTTCTTCATCCGCAGTT-3′. Primers used for qRT-PCR analysis of Arabidopsis genes were: TCP3, forward 5′-TAGCTTCAACGCAACAGAGC-3′, reverse 5′-GGTTCTGTGTATTGCCTCGTG-3′; TCP4, forward 5′-CACGACGGTCTCACTCACAA-3′, reverse 5′-AATCTAAGTCAAGCTTCAATGTGC-3′; TCP10, forward 5′-CCACGGAGAAGAAGCTACTCA-3′, reverse 5′-TCATCATGAATTTGAACCTCCA-3′; TCP17, forward 5′-TGGCTCTTAGAAGTTGCCAAA-3′, reverse 5′-TGAAAACCAGGTGGGAATTG-3′; TCP24, forward 5′-GCTCATGACAAGAATCTGAAGAAA-3′, reverse 5′-TGTTGCAGTGATAAACTTTTGAATTT-3′; ARF8, forward 5′-GACATGAAGCTGTCAACATCTGG-3′, reverse 5′-TAGGTTGCTTACTCGGTATCC-3′; IAMT1, forward 5′-CAACAACGGCATACAAACGG-3′, reverse 5′-TCTCTGCTGCTACCAAACCC-3′; HASTY, forward 5′-AGAGGGCAGAGCAAAGGTTC-3′, reverse 5′-TTCAAGAGGAAACCCACCATAG-3′; ACTIN2, forward 5′-CCGGTATTGTGCTGGATTCT-3′, reverse 5′-TTACAATTTCCCGCTCTGCT-3′. Three biological replicates and three technical replicates for each sample were performed.
Leaf Paraffin Section Analysis
For paraffin section analysis, the mature rosette sixth leaves were collected and fixed in Carnoy’s solution (60∶30:10, ethanol:chloroform:glacial acetic acid, v/v/v) for 12 h. Samples were washed with 60% (v/v) ethanol, immersed in 30% (v/v) hydrofluoric acid for approximately 7 to 10 d, and then dehydrated with a graded ethanol series and embedded in paraffin. Sections (approximately 8–10 mm thick) were cut with a microtome (Leica), stained with 1% (w/v) safranin O (Amresco) and 1% (w/v) fast green FCF (Merck), examined with a microscope and photographed.
Results
TaWRKY71-1 Encoded a Group IIa WRKY Transcription Factor
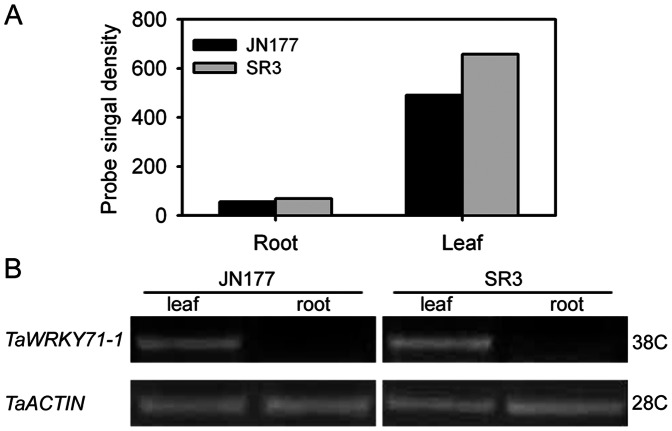
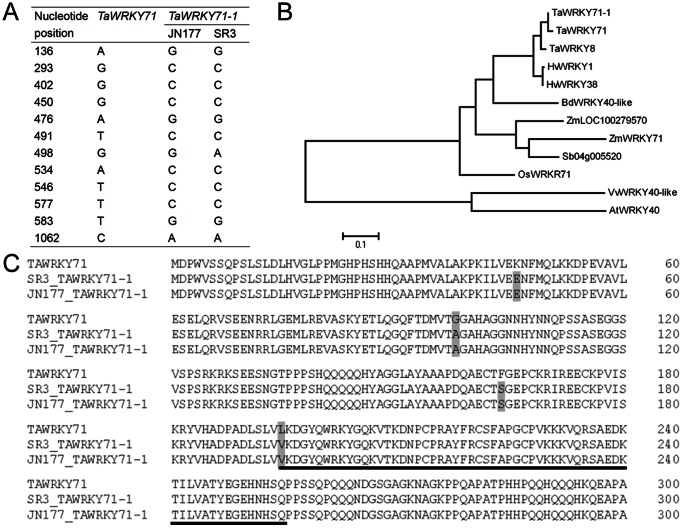
To identify wheat WRKY transcription factors that may account for leaf type, we screened the probes referring to WRKY transcription factor from the microarray data of SR3 and JN177 seedlings at three-leaf stage, and found a probe that predominantly transcribed in leaves and had a higher expression level in SR3 than in JN177 (Fig. 1A). The gene referring to the probe was isolated from SR3, and it consisted of a CDS with 1068 nucleotides in length that was separated by a 107-nucleotide intron. This gene had high identity to TaWRKY71 (Genbank accession ID: 100049023) of wheat cultivar Chinese Spring with only twelve single nucleotide polymorphisms (SNPs) (Fig. 2A), indicating that it was an allelic gene of TaWRKY71, so this gene was termed as TaWRYK71-1. TaWRKY71-1 encoded a protein of 355 amino acid residues with a predicted molecular weight of 38.79 kDa and a theoretical pI of 7.72. Among the characterized proteins, TaWRKY71-1 showed the most homology with TaWRKY71 (Fig. 2B). TaWRKY71-1 possessed a single WRKY domain (Fig. 2C), and based on the primary amino acid sequence of this domain, it has been assigned to group IIa of WRKY transcription factors.
Figure 1. TaWRKY71-1 are specifically transcribed in wheat leaves with a higher expression level in SR3.
A: The hybridization signals of the probe referring to TaWRKY71-1 in the microarray using wheat seedlings harvested at three-leaf stage. Data present as mean ± standard deviation. B: The sqRT-PCR using two-week old wheat seedlings. A wheat actin gene was used as the internal reference. JN177, a common wheat cultivar; SR3, a wheat introgression cultivar selected from somatic hybrids between JN177 and its close relative grass Thinopyrum ponticum via asymmetric somatic hybridization. 28C, 28 cycles; 38C, 38 cycles.
Figure 2. TaWRKY71-1 encodes a group IIa of WRKY transcription factor.
A: the statistics of single nucleotide polymorphisms of TaWRKY71-1 from SR3 and JN177 and its homologous gene TaWRKY71 from wheat cultivar Chinese Spring. B: The phylogenetic analysis of TaWRKY71-1 and its aligned WRKY transcription factors from NCBI at the amino acid level. C: Sequence alignment of TaWRKY71-1 from SR3 and JN177and its homologous gene TaWRKY71 from Chinese Spring. Underlined peptide sequence is a WRKY domain. Ta, Triticum aestivum; Hv, Hordeum vulgare; Bd, Brachypodium distachyon; Zm, Zea mays; Sb, Sorghum bicolor; Os, Oryza sativa; Vv, Vitis vinifera; At, Arabidopsis thaliana.
Twelve allelic variations (SNPs) in the CDS region between TaWRYK71-1 and TaWRYK71 (Fig. 2A) brings about four substitutions of amino acid residues between their deduced peptide sequences (one substitution occurred in WRKY domain) (Fig. 2C). SR3 genome had taken place whole-genome-scale genetic (DNA sequence) and epigenetic (DNA methylation) variations during asymmetric somatic hybridization ([24], unpublished data). To determine whether these SNPs came from the natural mutation between Chinese Spring and JN177 or from the variation during asymmetric somatic hybridization, we isolated the homologous sequence of TaWRKY71-1 from JN177. Among the twelve SNPs, eleven SNPs came from the natural mutation (present between TaWRKY71 and JN177 TaWRKY71-1, but not between SR3 and JN177), and the other one came from asymmetric somatic hybridization (present between SR3 and JN177, but not between TaWRKY71 and JN177 TaWRKY71-1) (Fig. 2A). The SNP induced by asymmetric somatic hybridization was synonymous nucleotide substitution, which did not result in the change of amino acid residue (Fig. 2C).
TaWRKY71-1 Expression and its Promoter Analysis
To confirm the microarray result, TaWRKY71-1 expression profile was assayed with sqRT-PCR. TaWRKY71-1 transcribed in the leaves of two-week-old seedlings in both SR3 and JN177, but its transcript was not detected in roots (Fig. 1B). Moreover, there had higher expression level in SR3 than in JN177 (Fig. 1B). Our unpublished data indicated that some genes with differential transcriptional levels between SR3 and JN177 partially owe to the genetic and epigenetic variations in their promoter regions. To know whether or not these variations also account for the differential expression of TaWRKY71-1 between two cultivars, the 739bp fragment present upstream of TaWRKY71-1 coding sequence was isolated from SR3 and JN177 genomic DNA. The promoter sequences between two cultivars were absolutely identical, and their methylation frequencies were also comparable (data not shown). This suggests that the differential expression of TaWRKY71-1 between SR3 and JN177 is modulated by its upstream regulatory factors rather than the promoter sequence itself.
There had a number of putative cis-acting elements involved in light response (Box I, G-box, GATA-motif, Sp1, TCCC-motif), gibberellin response (GARE-motif), meristem specific activation (CCGTCC-box), and lignin synthesis (AC-I) (Table 1), which closely relate to plant development and growth. Along with its higher expression in SR3, it could be implied that TaWRKY71-1 is correlated with vigorous growth and large leaf of SR3. Besides, the promoter also possessed the elements that are associated with the response to hormones such as ABA (ABRE) and jasmonic acid (CGTCA-motif, TGACG-motif) as well as to fungal elicitor and pathogen (Box-W1, W box). These hormones have proved to participate in plant development and response to environmental stimuli, suggesting TaWRKY71-1 might be involved in these processes. Consistent with this suggestion, TaWRKY71-1 was found to be induced by several abiotic stresses (data not shown).
Table 1. The predicted cis-elements in TaWRKY71-1 promoter sequence.
| Element | Position (Strand)a | Core sequence | Function |
| A-box | 171 (+), 344 (+) | CCGTCC | cis-acting regulatory element |
| ABRE | 411 (+) | CCTACGTGGC | cis-acting element involved in the abscisic acid responsiveness |
| AC-I | 176 (+), 177 (+) | CCCACCTACC | lignin synthesis associated |
| Box I | 4 (−) | TTTCAAA | light responsive element |
| Box-W1 | 106 (+) | TTGACC | fungal elicitor responsive element |
| CCAAT-box | 186 (−) | CAACGG | MYBHv1 binding site |
| CCGTCC-box | 171 (+), 344 (+) | CCGTCC | cis-acting regulatory element related to meristem specific activation |
| CGTCA-motif | 271 (+) | CGTCA | cis-acting regulatory element involved in the MeJA-responsiveness |
| G-box | 364 (+), 561 (+) | CACGAC | cis-acting regulatory element involved in light responsiveness |
| GARE-motif | 243 (−) | TCTGTTG | gibberellin-responsive element |
| GATA-motif | 98 (−) | AAGGATAAGG | part of a light responsive element |
| Sp1 | 178 (+), 339 (+), 182 (+), 481 (+) | CC(G/A)CCC | light responsive element |
| TCCC-motif | 629 (+) | TCTCCCT | part of a light responsive element |
| TGACG-motif | 271 (−) | TGACG | cis-acting regulatory element involved in the MeJA-responsiveness |
| W box | 106 (+) | TTGACC | pathogen- or elicitor-responsive |
Position, the nucleotide number upstream the start codon ATG; Strand,+and - present the element exists in sense strand and antisense strand, respectively.
TaWRKY71-1 Localized in the Nucleus
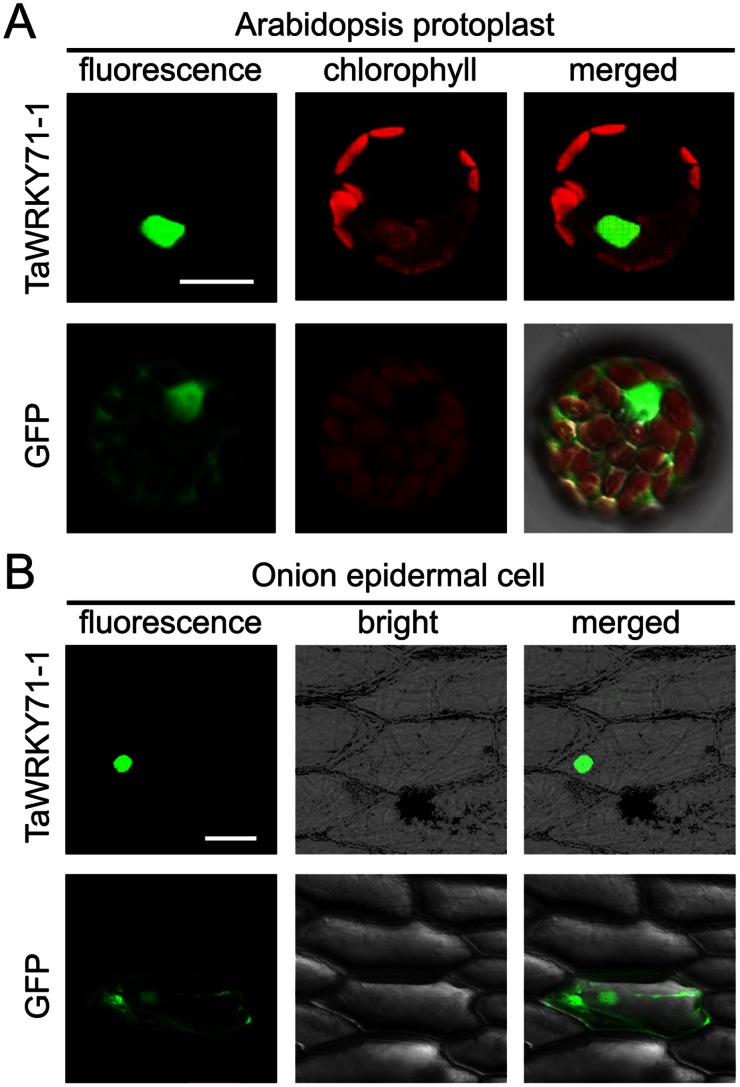
TaWRKY71-1 protein was predicted to localize in the nucleus using WoLF PSORT (http://wolfpsort.org/). To confirm this at the cellular level, the transient expression vector pBI221 expressing in-frame TaWRKY71-1:GFP fusion protein (pBI221-TaWRKY71-1-GFP) and the negative control (pBI221-GFP) were transformed into Arabidopsis protoplasts and onion epidermal cells. In both systems, TaWRKY71-1-GFP fusion proteins were restricted to the cell nucleus while the control GFP protein was observed throughout the entire cell (Fig. 3). This result shows that TaWRKY71-1 is a nuclear protein, further indicating that TaWRKY71-1 is a transcription factor.
Figure 3. TaWRKY71-1 localizes in the nucleus.
Transiently expressed TaWRKY71-1 in-frame fused with GFP in Arabidopsis mesophyll protoplasts (A) and onion epidermis cells (B). Bar in A 10µm and in B 100µm.
TaWRKY71-1 had no Transcriptional Activation Activity in Yeast
To determine the transcriptional activation activity of TaWRKY71-1 protein, a fusion protein containing a GAL4 DNA binding domain (GAL4-BD) and the whole ORF of TaWRKY71-1 was constructed (pGBKT7-TaWRKY71-1). The fusion plasmid pGBKT7-TaWRKY71-1 and pGBKT7 (negative control) were respectively transformed into yeast strain AH109 containing the upstream activating sequence (UAS), which could be specifically bound by GAL4 binding domain. All transformants containing pGBKT7-TaWRKY71-1 or pGBKT7 grew well on selective medium without tryptophan (SD-Trp), whereas they did not grow on selective medium without tryptophan, histdine and adenine (SD-Trp-His-Ade) (Fig. 4). These results indicated that TaWRKY71-1 indeed could not transactivate the expression of both reporter genes HIS3 and ADE2 as the negative control in yeast, suggested that TaWRKY71-1 may not be a transcriptional activator.
Figure 4. TaWRKY71-1 has no transactivation activity.
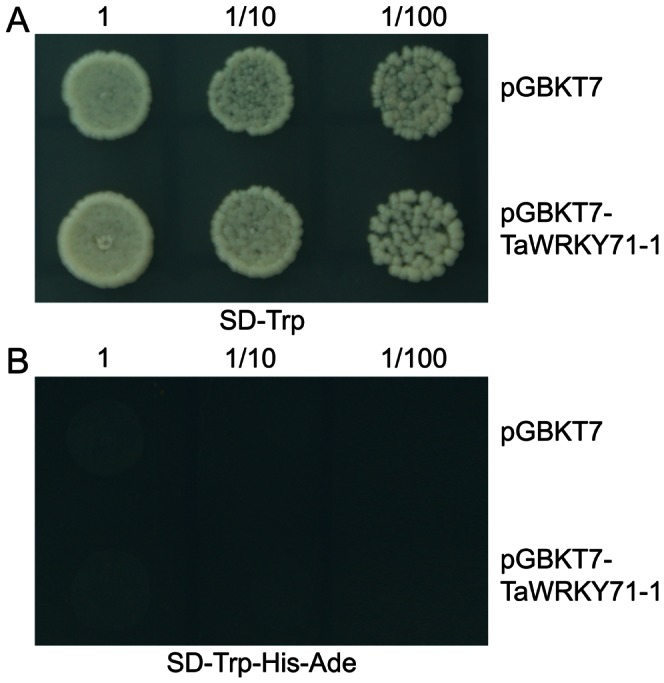
The full length ORF of TaWRKY71-1 was constructed into pGBKT7, and transformed yeasts were selected on both SD-Trp and SD-Trp-His-Ade media, respectively.
TaWRKY71-1 Overexpression in Arabidopsis Raised Hyponastic Leaves
TaWRKY71-1 was transformed into Arabidopsis for checking its role in leaf development, and more than ten independent transgenic overexpression (OE) lines were obtained. These OE lines and empty vector control line (VC) were comparable with respect to the growth (plant size and height) and reproduction (flowing time, seed size and yield) phenotypes (data not shown). The rosette leaves of OE plants appeared indistinguishable from those of the VC plants when they first emerged, but their mature leaves obviously curled upward with different extents (Fig. 5A, data not shown). According to the curvature, the hyponastic leaves of these transgenic lines were classified into slight, moderate and drastic grades, which were illustrated by three OE lines OE1, OE2 and OE3, respectively (Fig. 5A). sqRT-PCR showed that the transcriptional level of TaWRKY71-1 was the lowest in the lines (OE1) with the slight grade of hyponastic leaves, but the highest in the lines (OE3) with the drastic grade of hyponastic leaves (Fig. 5B; data not shown), which well coincided with the strength of leaf hyponasty. These findings demonstrate that TaWRKY71-1 plays an important and direct role in modulating leaf hyponasty.
Figure 5. TaWRKY71-1 overexpression results in hyponastic leaves in Arabidopsis.
A: Phenotypic comparison of four-week old Arabidopsis seedlings. B: sqRT-PCR of transgenic TaWRKY71-1 in Arabidopsis, and an Arabidopsis tublin gene was used as the internal reference. Col-0, the ecotype Columbia-0; VC, Vector control; OE1–3, three independent transgenic Arabidopsis lines overexpressing TaWRKY71-1.
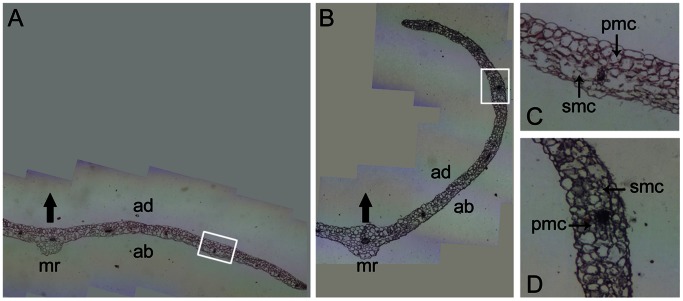
To elucidate how TaWRKY71-1 functions leaf hyponasty, the transverse sections of middle part of the mature rosette leaves were observed (Fig. 6). The cell sizes and densities of upper epidermis between the OE and VC lines both had no significant difference, as was found in lower epidermis as well. The palisade mesophyll cells at adaxial side had comparable sizes and densities between OE and VC leaves. In comparison with those of the VC leaves, the spongy mesophyll tissue cells at abaxial side of the OE leaves had similar size, but they tightened compactly with larger distribution density.
Figure 6. TaWRKY71-1 overexpression tightens spongy mesophyll tissue by the paraffin section assay.
A, B: Transverse sections of middle part of the 6th mature rosette leaves of VC (A) and OE3 (B) lines. C, D: The enlarged view of white pane-labeled section in panels A and B, respectively. VC, Vector control; OE3, an independent transgenic Arabidopsis lines overexpressing TaWRKY71-1. Arrows in A and B show the adaxial direction. mr, main rib; ad, adaxial side; ab, abaxial side; pmc, palisade mesophyll cells; smc, spongy mesophyll cells.
TaWRKY71-1 Altered the Expression of some Leaf Hyponasty Associated Genes
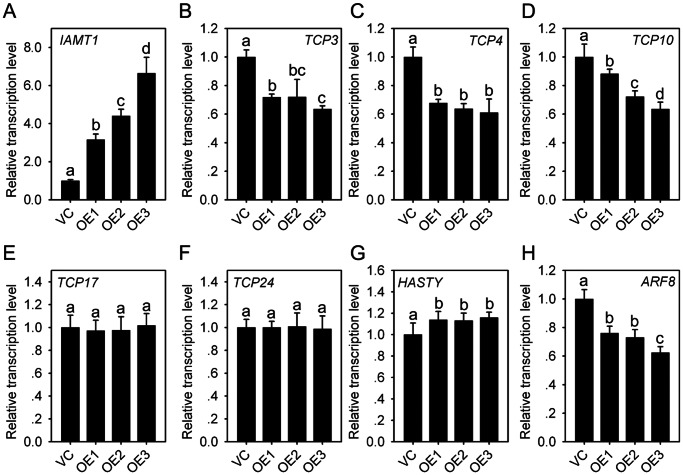
To primarily elucidate the molecular mechanism of TaWRKY71-1 in leaf hyponasty, the expression profiles of some genes which are involved in regulating leaf hyponasty development were examined. IAMT1 was upregulated in the OE lines, and its induction level is the lowest in OE1 and the highest in OE3 (Fig. 7A). Among four TCP genes that proved to participate leaf hyponasty [25] and to be restricted by IAMT1 [18], TCP3, TCP4 and TCP10 were downregulated in the OE lines (Fig. 7B–D), whereas TCP24 was not influenced by TaWRKY71-1 (Fig. 7F); TCP17 that is not disturbed by IAMT1 showed constant expression level between VC and OE lines as well (Fig. 7E). Opposite to IAMT1 dominant mutant, TaWRKY71-1 overexpression enhanced but did not decrease the expression of HASTY. Other leaf hyponasty development associated genes, AGAMOUS, AXR, BDL, CLF, DLF, KANADI, PEAPOD, PTL, SHY, AS1, AS2, KAN2, all had similar expression level between the VC and OE lines by sqRT-PCR (data not shown). Besides, ARF8, an auxin response factor that is induced by endogenous auxin [26], was also downregulated (Fig. 7G). Moreover, alike IAMT1, the change in expression strength of TCP10, ARF8 as well as TCP3 and TCP4 among three OE lines was closely correlated with the transcription levels of TaWRKY71-1, reasonably revealing the direct regulation of IAA-MeIAA homeostasis by TaWRKY71-1 during leaf hyponasty development.
Figure 7. TaWRKY71-1 alters the expression of IAMT1 -mediated leaf curvature genes.
VC, Vector control; OE1–3, Three independent transgenic Arabidopsis lines overexpressing TaWRKY71-1. The leaves of four-week old Arabidopsis seedlings were harvested for real-time PCR. Arabidopsis actin gene ACTIN2 was used as the internal reference. The graphs represent one of the same results from three independent experiments. Data present as mean ± standard deviation. Columns without the same lowercase letter are significantly different using the lowest significant difference test of one-way ANOVA (P<0.05).
Discussion
Here, we isolated a wheat TaWRKY71 allelic gene TaWRKY71-1 with four amino acid residue substitutions from a wheat introgression cultivar SR3. TaWRKY71-1 contains a typical WRKY domain and localizes in the nucleus (Fig. 2C; Fig. 3), indicating that it is a WRKY transcription factor. However, TaWRKY71-1 has no transcriptional activation activity in yeast (Fig. 4). Along with the finding that OsWRKY71, a homologous protein of TaWRKY71-1, did not show transactivation activity in yeast and rice cells [27], [28], it could be suggested that TaWRKY71-1 may serve as a transcriptional suppressor.
Ectopic overexpression of TaWRKY71-1 in Arabidopsis results in hyponastic leaves (Fig. 5). MeIAA is a crucial component in the auxin pool, which appears to determine the homeostatic level of auxin that is suggested to play important roles in the regulation of leaf hyponasty [15]. IAMT1 functions in the alteration of auxin homeostasis from IAA to MeIAA [16], [17], and its iamt1-D dominant mutant (overexpression) results in a hyponastic leaf phenotype [18]. Importantly, in comparison with the wild-type, the heterozygous iamt1-D displays partially hyponastic leaves, but the homozygous iamt1-D has dramatically hyponastic leaves, showing the effect of IAMT1 on leaf hyponasty depends on its mRNA abundance. Consist with this, transgenic Arabidopsis plants with higher TaWRKY71-1 transcription level possess more obviously hyponastic leaves (Fig. 5), and their IAMT1 induction extent is more significant as well (Fig. 7). Moreover, increased expression of IAMT1 leads to down-regulation of leaf developmental genes TCP3, TCP4, TCP10, and TCP24; the higher the IAMT1 expression level, the lower the TCP mRNA steady state level [18]. These TCP genes are highly similar to the snapdragon curvature regulation gene CINCINNATA, and have been shown to participate in regulating leaf curvature development [25], so it appears that IAMT1 causes the hyponastic leaf phenotypes through decreasing the transcription of TCPs. Following the finding in iamt1-D, the expression levels of TCP3, TCP4, and TCP10 are down-regulated in TaWRKY71-1 overexpression Arabidopsis, and the downregulation strength is dependent on the mRNA abundance of TaWRKY71-1 (Fig. 7). These data provide a strong evidence for that the role of TaWRKY71-1 in leaf hyponasty is achieved via altering auxin homeostatic level by promoting IAMT1-catalyzed the shift of free IAA to MeIA.
The regulatory mechanism of the homeostatic level between IAA and MeIAA has not been well documented, in which the issue how IAMT1 is modulated is still now unclear. Our data provides a feasible way to understand this issue. IAMT1 is induced by transgenic TaWRKY71-1 in Arabidopsis (Fig. 7), while TaWRKY71-1, alike its homologue in rice, has no transcriptional activation activity in yeast (Fig. 4). This suggests that there should have at least one gene in the TaWRKY71-1–IAMT1 regulatory cascade, and this gene could be inhibited by TaWRKY71-1 and serve as a suppressor of IAMT1. The transcription level of IAMT1 is strongly positively correlated to that of transgenic TaWRKY71-1, indicating that the regulatory effect of TaWRKY71-1 on IAMT1 is substantially accomplished at transcriptional-translational (direct linear correlation between the abundance of transcripts and proteins) but not post-translational (not due to protein modification-mediated activity modulation) level. The component(s) in the putative TaWRKY71-1–IAMT1 regulatory cascade are, at least very likely, transcription factor(s), which transduce the TaWRKY71-1′s order in a single-threaded manner. The combination with screening TaWRKY71 target genes and the transcription factors directly regulating IAMT1 will help understand the regulatory mechanism of IAMT1-mediated leaf hyponasty.
Leaf curvature has proved to owe to the asymmetric cell elongation (cell size) and proliferation (cell number) between adaxial and abaxial sides of leaves. However, how IAMT1-mediated alteration of auxin homeostasis from free IAA to MeIAA causes hyponastic leaves is not discussed. TaWRKY71-1 does not affect cell sizes and densities of both upper, lower epidermises and palisade mesophyll cells, but tightens spongy mesophyll tissue (Fig. 6). It was found that overexpression of E2F transcription factor in tobacco causes epinastic leaves, which is due to an accelerated proliferation of adaxial palisade mesophyll cells in the outer region and by repressed cell division on the abaxial spongy mesophyll side [29]. The mutant of auxin-associated gene STENOFOLIA, encoding a WUSCHEL-like homeobox transcriptional regulator, in Medicago truncatula leads to the diminishment of the difference in shape between palisade mesophyll cells (adaxial side) and spongy mesophyll cells (abaxial side) [30]. Together with these findings, our results suggest that auxin homeostatic level could substantially modulate leaf curvature development by changing the structure of spongy mesophyll tissue, showing the complicated anatomical basis of leaf hyponasty.
Unlike IAA–sugar and IAA–amino acid conjugates, MeIAA is essentially nonpolar, so it has a more efficient uptake than free IAA through diffusing through membranes rather than requiring an active transport system. This offers IAA esters the auxin activities similar to free IAA and in some cases even more potent than free IAA. Auxin response factors are crucial components in auxin signaling pathway and are often induced by increased auxin level [31]. However, ARF8, which has been found to be induced by auxin and influences cell expansion and proliferation during petal growth [32], is restricted by TaWRKY71-1 in a transcription level dependent behavior (Fig. 7). One putative explanation is that apart from their similarities, MeIAA and free IAA may perform regulatory functions in different ways as well. Moreover, TaWRKY71-1 induced the expression of TCP24 and HASTY (Fig. 7), two IAMT1-restricted leaf curvature genes [18]. This implies that TaWRKY71-1 may control the expression of these genes independent of IAA-MeIAA homeostasis. Besides, a novel transcriptional repressor TIE1 was recently proposed to serve as an inhibitory modulator of TCP activities, and its overexpression leads to hyponastic leaves [33]. We found that the promoter of TIE1 possesses W-box that is specifically bound by WRKY transcription factors, suggesting TaWRKY71-1 possibly also modulate TCPs via regulating TIE1. These speculations sound interesting and worthy of being elucidated.
In summary, we isolated a wheat WRKY transcription factor gene TaWRKY71-1 that cause hyponastic leaves in Arabidopsis through modulating IAA and MeIAA levels. Our results provide a molecular clue to know how IAMT1-mediated auxin homeostasis is regulated. Moderate hyponasty of leaves is an important aspect of ideal plant type for crop yield improvement. Thus, TaWRKY71-1 could be used for improving plant type in crop molecular breeding.
Funding Statement
This work was supported by National Transgenic Project (2011ZX08002-002), National Basic Research 973 Program of China (2012CB114204; 2009CB118300), Major Program of Natural Science Foundation of China (31030053), Natural Science Foundation of China (31171175), Excellent Young Scientist Award Foundation of Shandong Province (BS2009SW023), College Innovation Foundation of Jinan City (200906021). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Govaerts YM, Jacquemoud S, Verstraete MM, Ustin SL (1996) Three-dimensional radiation transfer modeling in a dicotyledon leaf. Appl Opt 35: 6585–6598. [DOI] [PubMed] [Google Scholar]
- 2. Yuan L (1997) Hybrid rice breeding for super high yield. Hybrid Rice 12: 1–6. [Google Scholar]
- 3. Chen Z, Pan XB, Hu J (2001) Relationship between rolling leaf and ideal plant type of rice (Oryza sativa L.) Jiangsu Agricultural Res. 22: 81–89. [Google Scholar]
- 4. Lang YZ, Zhang ZJ, Gu XY, Yang JC, Zhu QS (2004) A physiological and ecological effect of crimpy leaf character in rice (Oryza sativa L.) II. Photosynthetic character, dry mass production and yield forming. Acta Agron Sin 30: 883–887. [Google Scholar]
- 5. Cheng Y, Dai X, Zhao Y (2007) Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis . Plant Cell 19: 2430–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li X, Qin G, Chen Z, Gu H, Qu LJ (2008) A gain-of-function mutation of transcriptional factor PTL results in curly leaves, dwarfism and male sterility by affecting auxin homeostasis. Plant Mol Biol 66: 315–327. [DOI] [PubMed] [Google Scholar]
- 7. Lincoln C, Britton JH, Estelle M (1990) Growth and development of the axr1 mutants of Arabidopsis . Plant Cell 2: 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harper RM, Stowe-Evans EL, Luesse DR, Muto H, Tatematsu K, et al. (2000) The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell 12: 757–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li LC, Qin GJ, Tsuge T, Hou XH, Ding MY, et al. (2008) SPOROCYTELESS modulates YUCCA expression to regulate the development of lateral organs in Arabidopsis . New Phytol 179: 751–764. [DOI] [PubMed] [Google Scholar]
- 10. King JJ, Stimart DP, Fisher RH, Bleecker AB (1995) A Mutation Altering Auxin Homeostasis and Plant Morphology in Arabidopsis . Plant Cell 7: 2023–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Delarue M, Prinsen E, Onckelen HV, Caboche M, Bellini C (1998) Sur2 mutations of Arabidopsis thaliana define a new locus involved in the control of auxin homeostasis. Plant J 14: 603–611. [DOI] [PubMed] [Google Scholar]
- 12. Barlier I, Kowalczyk M, Marchant A, Ljung K, Bhalerao R, et al. (2000) The SUR2 gene of Arabidopsis thaliana encodes the cytochrome P450 CYP83B1, a modulator of auxin homeostasis. Proc Natl Acad Sci USA 97: 14819–14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, et al. (2001) A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291: 306–309. [DOI] [PubMed] [Google Scholar]
- 14. Romano CP, Robson PR, Smith H, Estelle M, Klee H (1995) Transgene-mediated auxin overproduction in Arabidopsis: hypocotyl elongation phenotype and interactions with the hy6–1 hypocotyl elongation and axr1 auxin-resistant mutants. Plant Mol Biol 27: 1071–1083. [DOI] [PubMed] [Google Scholar]
- 15. Jackson RG, Kowalczyk M, Li Y, Higgins G, Ross J, et al. (2002) Over-expression of an Arabidopsis gene encoding a glucosyltransferase of indole-3-acetic acid: phenotypic characterisation of transgenic lines. Plant J 32: 573–583. [DOI] [PubMed] [Google Scholar]
- 16. D’Auria JC, Chen F, Pichersky E (2003) The SABATH family of methyltransferases in Arabidopsis thaliana and other plant species. Recent Adv Phytochem 37: 95–125. [Google Scholar]
- 17. Zubieta C, Ross JR, Koscheski P, Yang Y, Pichersky E, et al. (2003) Structural basis for substrate recognition in the salicylic acid carboxyl methyltransferase family. Plant Cell 15: 1704–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qin G, Gu H, Zhao Y, Ma Z, Shi G, et al. (2005) An indole-3-acetic acid carboxyl methyltransferase regulates Arabidopsis leaf development. Plant Cell 17: 2693–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rushton PJ, Somssich IE, Ringler P, Shen QJ (2010) WRKY transcription factors. Trends Plant Sci 15: 247–258. [DOI] [PubMed] [Google Scholar]
- 20. Xia G, Xiang F, Zhou A, Wang H, Chen H (2003) Asymmetric somatic hybridization between wheat (Triticum aestivum L.) and Agropyron elongatum (Host) Nevishi. Theor Appl Genet 107: 299–305. [DOI] [PubMed] [Google Scholar]
- 21. Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572. [DOI] [PubMed] [Google Scholar]
- 22. Lee SS, Cho HS, Yoon GM, Ahn JW, Kim HH, et al. (2003) Interaction of NtCDPK1 calcium-dependent protein kinase with NtRpn3 regulatory subunit of the 26S proteasome in Nicotiana tabacum . Plant J 33: 825–840. [DOI] [PubMed] [Google Scholar]
- 23. Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . Plant J 16: 735–743. [DOI] [PubMed] [Google Scholar]
- 24. Liu S, Gao X, Xia G (2008) Characterizing HMW-GS alleles of decaploid Agropyron elongatum in relation to evolution and wheat breeding. Theor Appl Genet 116: 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nath U, Crawford BC, Carpenter R, Coen E (2003) Genetic control of surface curvature. Science 299: 1404–1407. [DOI] [PubMed] [Google Scholar]
- 26. Ulmasov T, Hagen G, Guilfoyle TJ (1999) Activation and repression of transcription by auxin-response factors. Proc Natl Acad Sci USA 96: 5844–5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chujo T, Kato T, Yamada K, Takai R, Akimoto-Tomiyama C, et al. (2008) Characterization of an elicitor-induced rice WRKY gene, OsWRKY71 . Biosci Biotechnol Biochem 72: 240–245. [DOI] [PubMed] [Google Scholar]
- 28. Liu X, Bai X, Wang X, Chu C (2007) OsWRKY71, a rice transcription factor, is involved in rice defense response. J Plant Physiol 164: 969–979. [DOI] [PubMed] [Google Scholar]
- 29. Kosugi S, Ohashi Y (2003) Constitutive E2F expression in tobacco plants exhibits altered cell cycle control and morphological change in a cell type-specific manner. Plant Physiol 132: 2012–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tadege M, Lin H, Bedair M, Berbel A, Wen J, et al. (2011) STENOFOLIA regulates blade outgrowth and leaf vascular patterning in Medicago truncatula and Nicotiana sylvestris . Plant Cell 23: 2125–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kiehl TR, Shen D, Khattak SF, Jian Li Z, Sharfstein ST (2011) Observations of cell size dynamics under osmotic stress. Cytometry A 79: 560–569. [DOI] [PubMed] [Google Scholar]
- 32. Varaud E, Brioudes F, Szecsi J, Leroux J, Brown S, et al. (2011) AUXIN RESPONSE FACTOR8 regulates Arabidopsis petal growth by interacting with the bHLH transcription factor BIGPETALp. Plant Cell 23: 973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao Q, Guo D, Wei B, Zhang F, Pang C, et al. (2013) The TIE1 Transcriptional Repressor Links TCP Transcription Factors with TOPLESS/TOPLESS-RELATED Corepressors and Modulates Leaf Development in Arabidopsis. Plant Cell: DOI: tpc.113.109223. [DOI] [PMC free article] [PubMed]