Abstract
Background
Understanding the mechanisms by which natural populations cope with environmental stress is paramount to predict their persistence in the face of escalating anthropogenic impacts. Reef-building corals are increasingly exposed to local and global stressors that alter nutritional status causing reduced fitness and mortality, however, these responses can vary considerably across species and populations.
Methodology/Principal Findings
We compare the expression of 22 coral host genes in individuals from an inshore and an offshore reef location using quantitative Reverse Transcription-PCR (qRT-PCR) over the course of 26 days following translocation into a shaded, filtered seawater environment. Declines in lipid content and PSII activity of the algal endosymbionts (Symbiodinium ITS-1 type C2) over the course of the experiment indicated that heterotrophic uptake and photosynthesis were limited, creating nutritional deprivation conditions. Regulation of coral host genes involved in metabolism, CO2 transport and oxidative stress could be detected already after five days, whereas PSII activity took twice as long to respond. Opposing expression trajectories of Tgl, which releases fatty acids from the triacylglycerol storage, and Dgat1, which catalyses the formation of triglycerides, indicate that the decline in lipid content can be attributed, at least in part, by mobilisation of triacylglycerol stores. Corals from the inshore location had initially higher lipid content and showed consistently elevated expression levels of two genes involved in metabolism (aldehyde dehydrogenase) and calcification (carbonic anhydrase).
Conclusions/Significance
Coral host gene expression adjusts rapidly upon change in nutritional conditions, and therefore can serve as an early signature of imminent coral stress. Consistent gene expression differences between populations indicate that corals acclimatize and/or adapt to local environments. Our results set the stage for analysis of these processes in natural coral populations, to better understand the responses of coral communities to global climate change and to develop more efficient management strategies.
Introduction
Biotopes across the globe are increasingly affected by anthropogenic activities and changing environmental conditions [1]. On coral reefs, coral cover has declined rapidly over the past few decades (e.g., [2], [3], [4]) and up to 33% of reef-building coral species now face an elevated risk of extinction [5]. The sources of stress that underpin coral declines operate at local (e.g. eutrophication and pollution) as well as at global (e.g. elevated ocean temperature and acidification from atmospheric CO2) scales. The degree to which reef corals will persist into the future depends on the severity of chronic stress and the frequency of acute stress (e.g., [6], [7]–[11]). The point at which physiological tolerances are exceeded and stress occurs (e.g. bleaching) varies among coral populations and species (e.g., [9], [12], [13]). Understanding the physiological and genetic basis of this variability is a central theme in coral biology that has broad relevance to evolutionary ecology and climate change adaptation research [9], [11].
Corals are able to optimise their fitness under local environmental regimes through physiological and genetic adaptation (e.g., [14]). Physiological adaptation describes the process of tuning of the organism's physiological performance to a varying environment within its lifetime and is often interchangeably referred to as phenotypic plasticity or acclimatisation [14]–[17]. Acclimatisation can occur over relatively short time scales (typically hours to days) and, while the rate and magnitude of change is assumed to be limited by genetic make-up, it is an important physiological mechanism and can be adaptive [9], [14], [18], [19]. Stress responses and acclimatisation in corals are often inferred from changes in endosymbiotic dinoflagellate (Symbiodinium) densities and photosynthesis-related physiological responses. Variation in PSII performance has been documented among symbiont types [20], [21], along light and depth gradients [22]–[24], across latitudes [25], [26], historical temperature regime [27] and recent thermal history [28]–[30]. It has, however, been shown that host-specific responses to stress may occur before changes in symbiont densities or photo-physiology are evident [31]–[33]. Even though many host-specific traits are likely to play an important role in coping with chronic and acute environmental change [9], [34] our understanding of host-specific acclimatisation mechanisms remains less understood [14].
The nutritional needs of corals are met from auto and heterotrophic sources, which relative importance may vary among environmental regimes, microbial symbiont communities and species [35], [36]. The effects of auto and heterotrophy on coral physiology are complexly interlinked [37], [38]. For example, variation in light regimes and symbiont communities influence the energetic status of corals through changes in photosynthesis (e.g., [39], [40]), which can be enhanced with feeding to boost coral growth rates [38]. Energy budgets affect corals' relative allocation into skeletal and tissue growth [41], reproduction [42] and stress tolerance [43]–[45]. Environmental conditions that cause coral nutritional stress through changes in auto and heterotrophic capacity are expected to increase in the future [46], [47]. Therefore, an enhanced understanding of the physiological processes that underpin changes in coral nutritional status will therefore allow new insights into the current and future health of corals.
Gene expression is now used more commonly to understand the stress response and evolutionary ecology of corals [14], [48]. Global gene expression can elucidate how whole organisms respond to environmental stimuli and suggests molecular targets of adaptation that can be further studied with better precision using qRT-PCR [49], [50]. While post-translational changes may occur, a large proportion of differentially expressed genes (73–86%) affect downstream protein levels, and gene expression, therefore, has the potential to reveal metabolic responses to the environment [51], [52]. Analyses of changes in gene expression during metamorphosis, establishment, maintenance and stress of photosymbiosis (including bleaching) have previously been reported in corals (e.g., [53], [54], [55]). Less attention has been devoted to understanding how gene expression is affected by changes in the environment that do not cause bleaching but may affect coral physiology and condition (a.k.a. capacity adaptation [14]) but see [56]–[60]. Lastly, we are only beginning to explore how coral gene expression plasticity is modulated by genetic factors (e.g., [57], [61], [62]).
In this study, we investigate how gene expression, lipid content of the holobiont and symbiont PSII activity in corals from two source populations responded following translocation into a controlled laboratory environment with reduced potential for auto and heterotrophy for one month. Previously, we used microarray analysis to demonstrate widespread differential expression of metabolic genes and fluorescent proteins between two time points consistent with nutritional limitation [57]. Here, we extend this study to more accurately quantify expression of 25 genes implicated in metabolism, CO2 transport, oxidative stress, immunity and symbiosis, as well as measure photosynthesis and lipid content, over a finer timescale.
Results
Changes in gene expression depending on coral origin and over time
The normalized relative expression of 22 target genes (Table 1) was compared between two populations from inshore (Orpheus Island, OI) and offshore (Davies Reef, DR) environments. Gene expression varied up to eight fold across the experiment and samples clustered into three groups (columns in Figure 1). The first cluster contained the field samples from both locations and the second and third clusters contained the subsequent laboratory samples separated by location. The genes (rows in Figure 1) partitioned into two main clusters based predominantly on their expression in the field. The first cluster contained 14 genes that were up-regulated after translocation into the laboratory, including two genes with roles in immunity (C3 and NFKB) and three stress genes (Fer, Prx6 and Catalase). This cluster also contained one distinctive sub-cluster of three genes associated with calcification and metabolism (i.e., Carbonic Anhydrase [CA], Galaxin and Aldehyde dehydrogenase [Aldh3]), demonstrating a tendency towards differential expression between corals from different populations (higher in Davies Reef corals, lower in Orpheus Island corals). The second cluster contained seven genes that were down-regulated in the laboratory with little differentiation between locations except C-type lectin (Ctl) which appeared to be up-regulated in corals from Orpheus Island in the laboratory but down-regulated in corals from Davies Reef (Figure 1). The second cluster also contained a gene with a putative role in light perception (Rdh1) and four metabolic genes (Ndh1b, Dgat1, COX15 and FadD). Triacylglycerol lipase (Tgl) did not belong to any of the two clusters and displayed the greatest differential expression of all genes examined, being uniformly up-regulated in the laboratory.
Table 1. Genes included in the RT-qPCR assay, their abbreviations and putative function.
| Protein name | Abbreviation | Putative functional role |
| Complement-component 3 | C3 | Essential for activating the complement system in the immune response [87], [120] |
| Nuclear factor Kappa-B, Subunit 2 | Nf-kb2 | Activated in response to environmental stress, pathogens and chemicals [87], [120] |
| Hemolytic lectin | Cel3 | Cell surface recognition and metamorphosis [88] |
| C type lectin | Ctl | Cell surface recognition, immunity and symbiosis [88], [89] |
| Urokinase Plasminogen Activation Receptor | uPar | Serine protease involved in the plasminogen activation system [59], [95] |
| Superoxide dismutase | MnSod | Involved in the antioxidant defense system [105] |
| Catalase | Cat | Involved in the antioxidant defense system [105] |
| Soma ferritin | Sof | Involved in ferroxidase activity and cellular homeostasis by minimising Fe2+ availability for ROS production [100] |
| H+ transporting ATPase | H-ATPase | Involved in the ATP synthesis and photosynthesis [57], [65] |
| Na+/K+ exchanging ATPase | Na,K-ATPase | Actives transport of ions leading to a low sodium and a high potassium concentrations in cells [9] |
| Cytochrome c oxidase subunit XV | Cox15 | Essential components of the mitochondrial respiratory chain (e.g., [121], [122]) |
| Aldehyde dehydrogenase 3 | Aldh3 | Involved in the oxidation of aldehydes generated by the metabolism of a broad range of compounds, including alcohols, amino-acids, vitamins, steroids or lipids (e.g., [121], [122] |
| NADH dehydrogenase I chain B | Ndh1b | |
| Peroxiredoxin 6 | Prx6 | Involved in the antioxidant defense system [91] |
| Triacylglycerol lipase | Tgl | Role in releasing fatty acids from the triacylglycerol storage form [79] |
| Retinol dehydrogenase 1 | Rdh1 | Involved in the retinoid metabolism, vision and heat stress response [82], [83] |
| Diacylglycerol O-acyltransferase | Dgat1 | Catalyzes the formation of triglycerides from diacylglycerol, using AcylCoA [79] |
| Acyl-CoA oxidase | Aco | Involved in the peroxisomal beta-oxidation of fatty acids [79] |
| Carbonic anhydrase | CA | Involved in CO2 transport associated with photosynthesis, respiration and calcification (e.g., [65]) |
| Major yolk protein | Myp | Constitutes source of nutrients during gametogenesis [123], [124] |
| Long-chain fatty-acid-CoA ligase | FadD | Catalyzes the bioactivation of fatty acids, leading to the formation of acylCoA thioesters [79] |
| Actin1 | Actin1 | Involved in cell motility and cell structure |
| Collagen | Coll | Involved in structural support in animal tissues; dominant in mesoglea, possibly associated with symbiosis [87] |
| Galaxin | Gal | Constituent in the organic cellular matrix with a role in biomineralisation [54], [125]. |
| Fibrinogen | Fib | Role in symbiosis [86] |
| 60S Ribosomal protein L9-like isoform | RIBOL9 | Internal control gene [105] |
| Unknown transcript | Ctg1913 | Internal control gene [105] |
Putative functional role is based on http://www.ncbi.nlm.nih.gov/protein, http://expasy.org/, cited sources and references therein.
Figure 1. Heat map of gene expression, averaged across all samples representing each combination of factors (population and time-point).
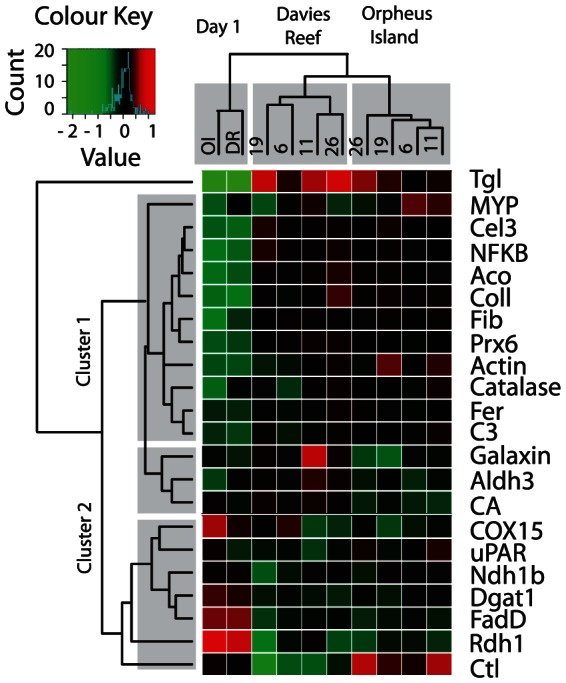
The trees correspond to hierarchical clustering of conditions (columns) and genes (rows). The conditions fall into three clusters: one uniting all initial samples taken in the field (“Day 1”, “OI” – Orpheus Island, “DR” – Davies reef), and two clusters containing subsequent common garden samples, one cluster per population. The numbers above the columns (6, 11, 19, 26) indicate days of sampling while in the common garden. The genes fall into two main clusters: the ones increasing in expression in the common garden compared to expression in the field (cluster 1), and the ones decreasing in expression (cluster 2). The color scale corresponds to log2-transformed deviance from the global mean for each gene; the maximal range of expression variation was 8-fold (Tgl).
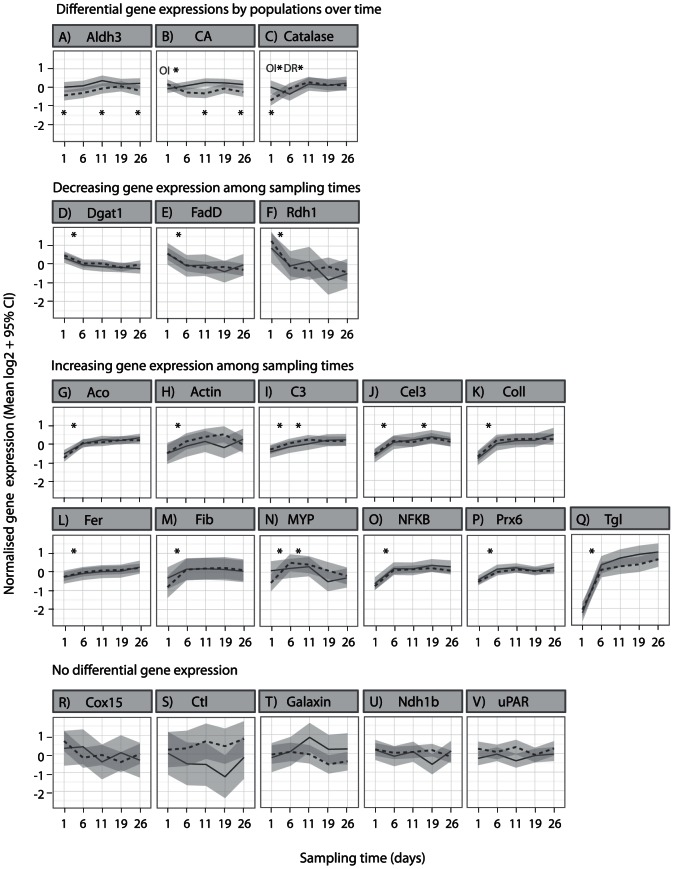
Population-specific changes in gene expression over time were significant in three genes (Adj. P LRT<0.013, Fig. 2 A–C). Population-specific expression was time-dependent in CA and Catalase (Cat) (Adj. P MCMC<0.018) but not in Aldh3. The significance of model terms and pairwise comparisons for all genes can be found in File S1. Expression of CA and Aldh3 was greater in Davies Reef corals at several sampling times whereas Cat was expressed at a higher level in field sampled Davies Reef corals but became similar between populations in the laboratory (Adj. P MCMC<0.022). This occurred after an initial increase in expression of Orpheus Island corals and a later decrease in Davies Reef corals (Figure 2 A–C, Adj. P MCMC<0.018).
Figure 2. Temporal changes in gene expression of corals from two locations translocated to a common laboratory environment.
Lines represent means of normalized log2 transformed expression values among replicate colonies (solid line = Davies Reef, dashed line = Orpheus Island). The grey-shaded areas represent 95% confidence intervals of means. Plots are organised by the significance of model factors (FDR adj. P LRT<0.022). Model-derived significant differences over time-points are indicated with stars above means (and are population specific in A–C, FDR adj. P MCMC<0.024 or 0.018). Significant differences between populations at specific time-points are indicated by stars below means in A–C (FDR adj. P MCMC<0.022).
Gene expression changed over time in 15 genes (Adj. P LRT<0.013). In three of these genes (Diacylglycerol O-acyltransferase [Dgat1], Long-chain fatty-acid-CoA ligase [FadD] and Retinol dehydrogenase [Rdh1]) expression declined and a significant drop in gene expression was identified between the field and the first laboratory sample (Figure 2 D–F). Gene expression increased either sharply after five days in the laboratory or gradually across the sampling times in the remaining 12 genes (Figure 2 G–Q). Variation in the expression of five genes was not significantly explained by our model terms (Figure 2 R–V).
Changes in energetic content and photophysiology
Total standardized lipid changed among sampling times and source populations (P LRT = 0.001; Model fit in File S1). Total standardized lipid was significantly higher in Orpheus Island corals in the field and declined at approximately the same rate among sampling times. In contrast, the lipid content of Davies Reef corals was initially lower than in corals from Orpheus Island and did not decline significantly until the last sampling time (Figure 3).
Figure 3. Variation in standardised lipid content of corals among sampling times and source populations.
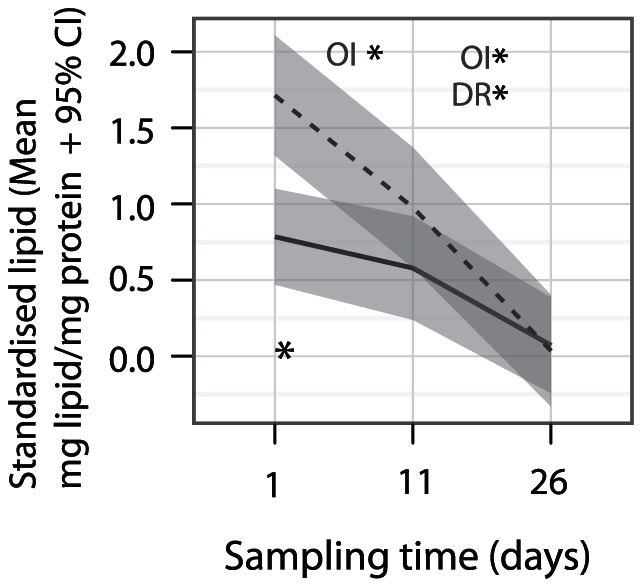
Lines represent mean standardized lipid content (mg lipid per g coral/mg protein per g coral) among replicate colonies (solid line = Davies Reef, dashed line = Orpheus Island). Grey-shaded areas represent the 95% confidence intervals of means. Population specific model-derived significant differences between consecutive sampling times are indicated above the plots (FDR adj. P MCMC<0.018) and differences between populations below the plots (FDR adj. P MCMC<0.024).
We fitted rapid light curves to a hyperbolic tangent function to derive information about the light dependency of PSII activity of symbionts in response to the translocation into the shaded laboratory environment. Our analyses showed that relative ETRmax and Ek varied significantly over time (p<3.7×10−8) but not between corals sourced from different populations (Model fits in File S1). Fv/Fm consistently remained above 0.6 indicating healthy PSII function and was similar between populations, although a decline, particularly evident in Orpheus Island corals, occurred after 19 days in the laboratory (Figure 4C). The transition to lowered relative ETRmax and Ek was evident at 11 to 18 days in the shaded laboratory setting (Figure 4A–B).
Figure 4. Temporal changes in photo-physiology of Symbiodinium C2 in corals translocated to a common laboratory environment.
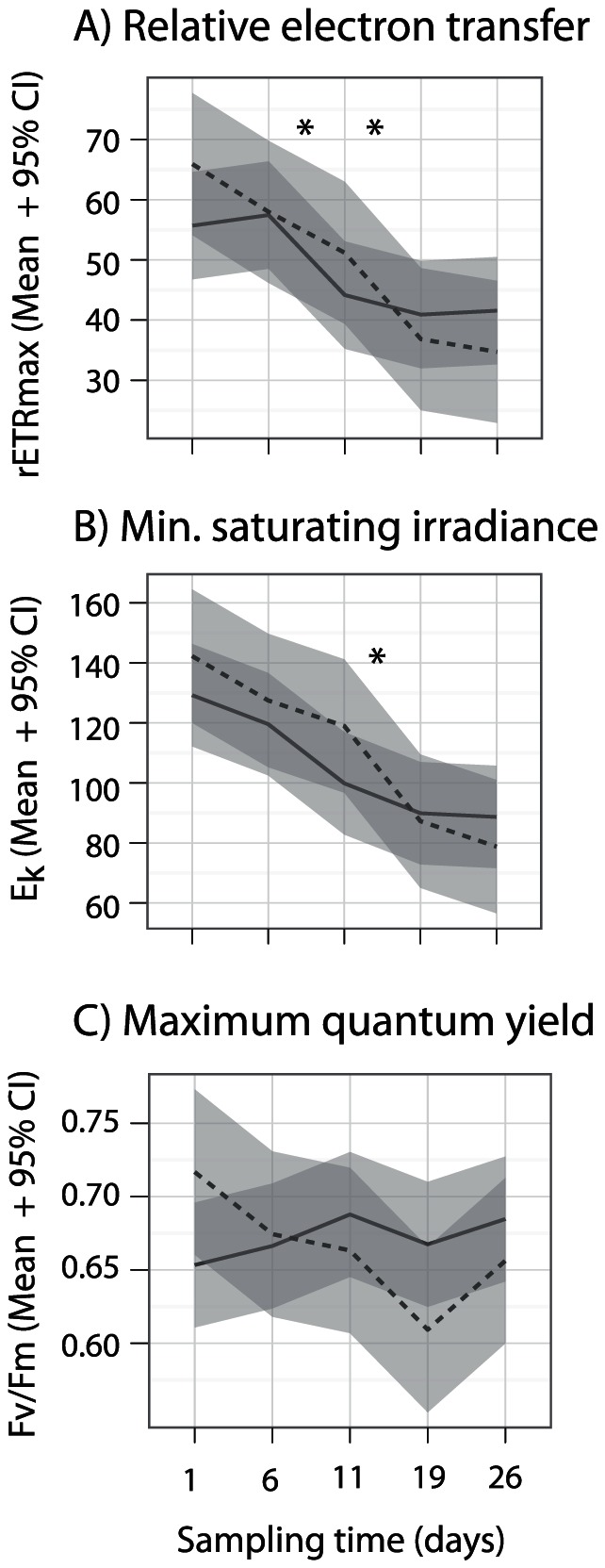
Panels represent A) mean relative electron transfer rate, B) mean minimum saturating irradiance and C) mean maximum quantum yield among replicate colonies (solid line = Davies Reef, dashed line = Orpheus Island). Grey-shaded areas represent the 95% confidence intervals of the means. Model-derived significant differences between consecutive sampling times are indicated above the plots (FDR adj. p<0.024).
Discussion
The corals examined here displayed both immediate and longer-term responses to changing environmental conditions associated with translocation from two field locations into a shaded laboratory condition with limited potential for heterotropic feeding (i.e., supplied with 1 µm filtered water). These environmentally driven changes in gene expression corresponded well with changes in physiology and many were statistically significant after five days in the laboratory. Changes in lipid content and PSII activity of symbionts were more variable and our results, therefore, add gene expression to host specific responses that can be detected earlier in the acclimatisation process compared to, for example, changes in PSII function of symbionts [31], [32].
Populations of coral may differ in their phenotype, however, the degree to which this difference is shaped by genetic or physiological adaptation is still not well understood (e.g., [14], [57], [63]). Here, three out of 22 surveyed genes showed statistically significant population-specific response to the common garden conditions. This may indicate genetic divergence, or physiological acclimatisation to the conditions at each locality. Persistent differences between populations throughout the common garden period observed for Carbonic Anhydrase (CA) and Aldehyde Dehydrogenase 3 (Aldh3) are particularly tempting to interpret as a consequence of genetic divergence between populations. In contrast, the difference in Catalase was only detectable at the initial time-point and therefore most likely reflected short-term acclimatisation to local conditions. Cytosolic α - Carbonic Anhydrase (CA) catalyses the reversible hydration of CO2 to HCO3 − (e.g., [64]) and modulates CO2 levels in response to respiration, photosynthesis and calcification (e.g., [65], [66]). The expression of CA has previously been associated with photosynthetic activity and incident light levels (e.g., [67], [68]) but did not differ between field sampled populations here, despite higher average light levels at the offshore location [69]. CA also did not respond negatively to lower incident light levels in the laboratory as has been shown previously [67]. Aldh3 acts in the oxidation of aldehydes and was down-regulated in response to starvation in the liver of rats [70] and in response to low temperature acclimation in blue-fin tuna [71]. The higher expression in Davies Reef corals that also showed the slowest rate of decline in standardised total lipid may therefore suggest that the laboratory was less nutritionally challenging for this coral population compared to corals from Orpheus Island.
The energetic status of corals can be defined by the content of total or specific protein, lipid and carbohydrates and is a good indicator of coral fitness including survival following environmental stress [44], [46], [72]–[74]. Patterns of total standardised lipid content revealed here support the hypothesis that corals found the common garden conditions to be nutritionally challenging and coped by the utilisation of stored lipid reserves. We only detected three genes that consistently declined over the sampling times and two of these (FadD, Dgat1) have roles lipid metabolism [75], a process previously suggested to be under positive selection in two Acropora species [76]. Triacylglycerols and wax esters are the two primary classes of lipids used for storage in corals, which are specifically mobilized during bleaching [77], [78]. Dgat1 catalyses the formation of triacylglycerols [79] and its down-regulation suggests that A. millepora decrease or cease storing their lipid during times of low food availability. In contrast, Tgl, which has the inverse role and releases fatty acids from the triacylglycerol storage form [79] increased in expression across the sampling times (Figure 2). Although declines in standardised lipid content were very variable between individual colonies and a consistent decline was only detectable in both populations after 25 days in the laboratory, the changes in expression of genes related to lipid metabolism were evident after just five days in the common garden environment (Figure 3). Because of this robust response, environmental regulation of lipid metabolism genes represent a particularly attractive avenue for further investigation of the physiological mechanisms that underpin coral energetic condition and the potential for acclimatisation in this trait.
Our results indicated no location-specific difference in photo-physiology prior to or following translocation despite large differences in the average ambient light environments. This suggests that autotrophic capacity was similar between corals from the two environments. A gradual reduction in rETRmax and Ek occurred in corals from both locations and supports acclimatisation to the shaded common laboratory environment. Shade acclimatisation is known to occur in corals exposed to low-light environments over comparable time scales [80], [81]. The expression of CA did not reflect patterns of PSII acclimation but was relatively constant throughout the laboratory sampling duration. In contrast, Retinol dehydrogenase (Rdh1) was down-regulated in corals from both locations in response to the laboratory environment. This enzyme is involved in vision in higher organisms and can respond to light environments by retinoic acid receptor activation or can act in transcriptional regulation [82], [83]. Our results suggest that Rdhl responds to changes in ambient light environments in a similar fashion to PSII acclimation.
Collagen (Col) and a Hemolytic Cel-III type lectin (Cel3) showed increased expression after translocation into the laboratory. Col is a major component of connective tissue in animals and the mesoglea in corals [84], [85]. A role of Col in Cnidarian symbiosis has been proposed based on expression in symbiotic vs aposymbiotic states [86] and symbiont containing cells vs those without [87]. The expression of Ctl, a C-type lectin was not significantly explained by our model terms, however, this gene is widely hypothesised to play a role in the onset and maintenance of symbiosis possibly through immune regulation [86], [88], [89]. It was also strongly down-regulated following acute heat stress [90] likely concomitant with changes in photosynthesis known to occur under these conditions [30]. Its expression dipped on the fourth sampling time concomitant with a decline in the maximum quantum yield (Figure 2S and 3C). Our results therefore support a role of these genes in the photo-symbiotic adaptation response of corals here (e.g., [27]), however, further research is needed to better understand the role and regulation of these genes in corals. Simultaneous investigations of host gene expression, Symbiodinium densities, pigment concentrations and symbiont specific gene expression will benefit future attempts to better understand how the physiological adaptation of photosymbionts affect coral gene expression, health and fitness.
Bay et al. [57] reported a widespread down-regulation of many metabolic genes between the field and the ten day sampling time in the laboratory, possibly indicating that metabolic arrest was occurring. Metabolic arrest is a general physiological trade-off that occurs during stress because synthesis of stress repair proteins is prioritised [91]. Here, we uncovered an up-regulation of two genes associated with immunity (NFKB and C3) and three genes associated with oxidative stress (Fer, Prx6, Cat), supporting the hypothesis that the laboratory treatment did impose some stress on the corals despite the absence of obvious disease or bleaching. The up-regulation of oxidate stress genes could also be a response to Reactive Oxygen Species (ROS) production as a result of increased Tgl activity [92]. Mn-SOD and uPAR did not respond to our experimental treatment. Urokinase Plasminogen Activation Receptor (uPAR) is involved in cellular signalling, adhesion, wound healing and tissue regeneration [93], [94] and expression correlates with temperature and salinity in wild corals [59]. uPAR shares a precursor with Pdcyst-rich, a gene with a putative role in calcification that was down-regulated during experimental thermal stress in Pocillopora damicornis, possibly because of a trade-off mechanism between growth and stress response [95].
Our results demonstrate the utility of gene expression data to detect rapid changes in physiological status as well as to understand long-term acclimatisation and/or adaptation in natural coral populations. As the co-regulation patterns and functional roles of coral genes are increasingly understood, transcriptomic data, combined with physiological and energetic analyses, will play a growing role in revealing how populations and species of corals may vary in their response to local and global stressors [49], [50], [96]. This approach will facilitate the use of coral energetic status to assess the health of coral populations and to forecast their response to stress including bleaching.
Materials and Methods
Sampling design
We compared the acclimatisation response of the reef building coral, Acropora millepora, from two source populations: Pioneer Bay, Orpheus Island (OI: 16 km off the coast and inshore, 18°36′35″S, 146°29′16″E) and Davies Reef lagoon (DR: 78 km off the coast and offshore, 18°50′11″S, 147°38′00″E) on the Great Barrier Reef, Australia. All necessary permits were obtained for the described field studies (Great Barrier Reef Marine Park Authority Permit number G06/15571.1). At offshore locations shallow corals routinely experience light levels >800 µmol photons/m2/s and SPM<1.5 mg/L compared with 300 µmol photons/m2/s and SPM<1.5 mg/L at inshore locations [69]. The laboratory environment was maintained at ambient field temperature (∼27.5°C), shaded (max. 100 µmol photons/m2/s) and flow-through seawater was filtered to 1 µm to remove all SPM. Three replicate branches from seven replicate colonies per population were sampled in the field and four times in the laboratory over the following month. Colonies ranged from 15 to 22 cm in diameter (mean diameter = 18.5 cm), which corresponds to ca. 225 branches. Sampled branches were removed from the central section of colonies and constituted ∼7% of colony biomass. All colonies were the same colour morph (pink) and hosted the same type of photosynthetic endosymbionts (Symbiodinium ITS-1 C2 sensu [97]). Colonies were separated by >10 meters to minimise the potential for collection of clones. The two populations we sampled were likely genetically differentiated as demonstrated with allozymes and microsatellites for nearby, and similarly spaced sampling locations [98], [99]. Corals were sampled at midday (±15 min.) for gene expression analysis and after dark for photophysiology and energetic analyses.
Gene expression analysis
We used the GeXP analysis system (Beckman Coulter) to develop and implement a multiplex reverse transcription (RT) qPCR assay to analyse the expression of 29 genes [100]–[103]. Target genes were selected to represent a range of biological functions including metabolism, tissue and skeletal growth, immunity, symbiosis and oxidative stress (Table 1). We designed specific primers for 29 genes (26 target and 3 control genes) and optimized the assay following principles outlined in [100], [101], [104]. Amplicons were restricted to sizes of 100 to 260 base pairs (bp) and were spaced by at least five bp. We used the KanR gene as a spike-in reference gene with an amplification product of 325 bp length (File S2). Of the 29 genes included in the assay, 28 amplified consistently in all samples but VgnP did not, and was therefore eliminated from all further analyses.
The mRNA was extracted with poly-T magnetic beads using manufacturer's recommendations (Invitrogen) [105]. The concentration and purity of mRNA in each sample was evaluated using a Nanodrop spectrophotometer. Reverse transcription (RT) of mRNA to cDNA was achieved in 10 µL reaction volumes containing 10 ng sample mRNA, 2.5 µL of KanR RNA (5 ng/µL), 2 µL of RT Buffer 5X (GenomeLabTM GeXP Start Kit) and 2 µL of RT Reverse Primer Plex (Specific primer concentrations in File S2). Negative controls (no template and no reverse transcriptase) were included to test for mRNA and DNA contamination and never produced amplification products. Reverse transcription was conducted in a thermocycler (96-well Applied Biosystems 2720) at 48°C for 1 min, 37°C for 5 min, 42°C for 60 min with a final denaturation at 95°C for 5 min. To quantify technical variation associated with RT, PCR amplification and electrophoresis were performed three times on each mRNA extraction.
Polymerase Chain Reaction (PCR) was performed on cDNA in 10 µL reaction volumes containing 2 µL of PCR Buffer, 2 µL of MgCl2 (25 mM, ABgene), 1 µL of Forward Primer Plex (containing all forward primers at 200 nM) and 0.35 µL of Thermo-Start DNA Polymerase (ABgene). The PCR thermal cycling program included a 10 min denaturation step at 95°C, followed by 35 cycles of 30 s at 94°C, 30 s at 55°C and 1 min at 68.5°C. 1 µL of 1∶20 diluted PCR product was analysed on automated capillary sequencer (CEQ™ 8800 Genetic Analysis System, Beckman-Coulter) with 0.2 µL of ET-400 DNA size standard (Beckman-Coulter). Electrophoregrams were visualized in the GenomeLab™ Genetic Analysis System software (Beckman-Coulter), filtered then imported in GenomeLabTM eXpress Profiler software where peak binning was performed to associate fragment sizes with genes and to quantify the area under the peak used to evaluate the expression level of the associated gene [104].
We first pre-normalised the data by expressing each gene as a fraction of the internal spike-in control (i.e. Kanamycin). GeNorm was then used to determine the most stable genes for use in normalisation [106]. We identified H+ and Na+/K+ ATPases and MnSOD as the three most stable genes, but not the a priori control genes (CTG_1319 and Ribol9). We then applied a normalisation factor (NF) based on the geometric mean of the expression values of these three most stable genes. The normalised gene expression ratios of remaining target genes were further analysed and the a priori control genes, as well as the actual normalisation genes were excluded leaving 22 genes for further analysis.
PAM analysis
Rapid light curve analysis is a tool to assess photochemical efficiency through PSII electron flow using pulse-amplitude fluorometry (PAM) [107] and is useful for rapidly assessing photo-physiological changes [80], [108]. Three terminal branches from the centre of each coral colony were collected at dusk and a rapid light curve conducted within 1 hour of sunset in a shallow seawater bath using an Imaging-PAM fluorometer (Walz) yielding 2D images of photo-physiological parameters of interest. A single area of interest (3 mm in diameter) was selected approximately 1 cm from the tip of each branch as previous studies have shown that Symbiodinium may be absent or in low abundance in apical polyps [109]. Each rapid light curve comprised nine incremental 10 sec irradiance steps and photochemical efficiency of PSII was assessed at each irradiance level using the saturating pulse methodology [110]. Maximum (Fv/Fm) and effective quantum yields (ΔF/Fm′) were calculated as described in Bay et al. [57] and used to derive the relative electron transport rate (rETR) at each irradiance step. The descriptive parameters (rETRmax, a.u. [maximum relative electron transport rate], and Ek, µmol photons m−2 s−1 [minimum saturating irradiance]) were modeled based on a hyperbolic tangent formula devised by Platt et al. [111]. The instrumental settings used were described in Bay et al. [57].
Energetic content
Two branches from each colony were snap frozen in liquid nitrogen following photo-physiological analysis. Each branch was crushed into a fine powder, divided into two aliquots (20% for protein and 80% for lipid analyses), freeze-dried overnight in pre-weighed containers, then re-weighed to determine the sample dry weight. Total lipid was extracted using a Dichloromethane/Methanol extraction method, and lipid content was determined gravimetrically [112]. In brief, 2∶1 Dichloromethane/Methanol was twice added to freeze-dried samples, agitated and left overnight at 4°C then filtered using Whatman GF/C filters. To purify the extracts 0.88% KCl was added and the upper aqueous phase was removed after separation for 24 hours at 4°C. An additional two wash steps were conducted in a similar fashion with 1∶1 Methanol: Distilled Water after which extracts were dried overnight at 60°C in pre-weighed foil trays and weighed. Proteins were extracted with 0.5 M NaOH at 90°C for one hour, centrifuged for 1500 g for 10 min before supernatant was removed. Total protein content in the supernatant was quantified using a Petersen - Lowry Protein Assay (Bio-Rad DC II) using BSA as a standard. Absorbance was measured at 750 nm in a spectrophotometer (BioTek Powerwave). Three technical replicates were analysed per sample (Coefficient of Variation (CV)<10%). Lipid and protein contents were standardized to dry sample weight, after which lipid was expressed as lipid/protein and averaged across the two replicate branches per colony.
Statistical analysis
The data were analyzed using linear mixed model approach, with population, time-point, and their interaction as fixed factors, and a specific coral colony as a scalar random factor. This approach allows a more accurate estimation of among-group variance, and is less sensitive to violations of parametric assumptions and missing data compared to repeated measures ANOVA [113], [114]. The analysis was implemented using lme4 package in R [115]. The significance of each of the fixed factors was assessed by means of likelihood ratio tests between the corresponding nested models. The Benjamini – Yekutieli correction, appropriate for dependent tests [116], [117], was used to control type 1 error rate of gene expression (FDR corrected α for 22 LogLikelihood ratio tests (LRT) = 0.0132) and photo-physiology (FDR corrected for three tests αLRT = 0.027). The optimality of the selected model was also confirmed by a large weight of the Akaike information criterion. Gene expression was analyzed on a gene-by-gene basis, using log2-transformed deviances from the global mean for the particular gene as the response variable [33]. The standardized lipid, and photophysiology data were directly used as response variables.
Depending on which fixed effects were significant, we evaluated the pairwise differences between consecutive time-points from the results of Markov Chain Monte Carlo (MCMC) sampling after implementing the optimal linear mixed model in MCMCglmm [118]. In MCMCglmm, the default prior was used, corresponding to the maximum-likelihood estimates of the parameters. We compared consecutive time-points and conducted four test when only the time factor was significant (FDR corrected αMCMC = 0.024) and eight tests when population specific responses were detected (FDR corrected α MCMC = 0.018). We also compared populations at individual time-points when the time by population interaction was significant (i.e., five tests with FDR corrected α MCMC = 0.022).
Hierarchical clustering of gene expression profiles and heatmap visualisation was done using function heatmap.2 of the package gplots; plotting of gene expression and physiological time series was done using ggplot function of the ggplot2 package [33]. All the analyses were performed in R statistical software environment [119].
Supporting Information
Likelihood ratio tests, Akaike weights and pairwise differences from gene expression and physiological analyses.
(PDF)
Genes included in the gene expression assay, abbreviations, accession numbers, concentration of primer in Reverse Primer Plex (nM), length of the transcript (bp) and Oligo sequences.
(PDF)
Acknowledgments
We are grateful for field assistance by David Abrego, Eneour Puill-Stephan and Madeleine van Oppen. James Scott (University of Texas at Austin) provided statistical advise. This work was undertaken using the aquarium and laboratory facilities at the Australian Institute of Marine Science.
Funding Statement
Funding was provided by the Marine and Tropical Sciences Research Facility, the ARC Centre of Excellence for Coral Reef Studies and the Australian Institute of Marine Science. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annual Review of Ecology and Systematics 37: 637–369. [Google Scholar]
- 2. Bruno JF, Selig ER (2007) Regional Decline of Coral Cover in the Indo-Pacific: Timing, Extent, and Subregional Comparisons. PLoS ONE 2: e711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gardner TA, Cote IM, Gill GA, Grant A, Watkinson AR (2003) Long-Term Region-Wide Declines in Caribbean Corals. Science 301: 958–960. [DOI] [PubMed] [Google Scholar]
- 4. De'ath G, Fabricius KE, Sweatman H, Puotinen M (2012) The 27–year decline of coral cover on the Great Barrier Reef and its causes. Proceedings of the National Academy of Science http://www.pnas.org/cgi/doi/10.1073/pnas.1208909109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carpenter KE, Abrar M, Aeby G, Aronson RB, Banks S, et al. (2008) One-Third of Reef-Building Corals Face Elevated Extinction Risk from Climate Change and Local Impacts. Science 321 [DOI] [PubMed] [Google Scholar]
- 6. Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, et al. (2007) Coral reefs under rapid climate change and ocean acidification. Science 318: 1737–1742. [DOI] [PubMed] [Google Scholar]
- 7. Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, et al. (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301: 929–933. [DOI] [PubMed] [Google Scholar]
- 8. West JM, Salm RV (2003) Resistance and resilience to coral bleaching: Implications for coral reef conservation and management. Conservation Biology 17: 956–967. [Google Scholar]
- 9. Hofmann GE, Todgham AE (2010) Living in the Now: Physiological Mechanisms to Tolerate a Rapidly Changing Environment. Annual Review of Physiology 72: 127–145. [DOI] [PubMed] [Google Scholar]
- 10. Jackson JBC (2008) Ecological extinction and evolution in the brave new ocean. Proceedings of the National Academy of Science 105: 11458–11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Knowlton N (2001) The future of coral reefs. Proceedings of the National Academy of Science 98: 5419–5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hansen MM, Olivieri I, Waller DM, Nielsen NN (2012) Monitoring adaptive genetic responses to environmental change. Molecular Ecology 21: 1311–1329. [DOI] [PubMed] [Google Scholar]
- 13. van Oppen MJH, Gates RD (2006) Conservation genetics and the resilience of reef-building corals. Molecular Ecology 15: 3863–3883. [DOI] [PubMed] [Google Scholar]
- 14. Brown BE, Cossins AR (2011) The Potential for Temperature Acclimatisation of Reef Corals in the Face of Climate Change In: Dubinsky Z, Stambler N, editors. Coral Reefs: An Ecosystem in Transition. Heidelberg: Springer 421–434. [Google Scholar]
- 15. Coles SL, Brown BE (2003) Coral bleaching - Capacity for acclimatization and adaptation. Advances in Marine Biology 46: 183–223. [DOI] [PubMed] [Google Scholar]
- 16. Pigliucci M (1993) Plasticity versus genetics? Trends in Ecology & Evolution 8: 379–379. [DOI] [PubMed] [Google Scholar]
- 17. Pigliucci M (2005) Evolution of phenotypic plasticity: where are we going now? Trends in Ecology & Evolution 20: 481–486. [DOI] [PubMed] [Google Scholar]
- 18. Edmunds PJ, Gates RD (2008) Acclimatization in tropical reef corals. Marine Ecology-Progress Series 361: 307–310. [Google Scholar]
- 19.Huey R, Berrigan D (1996) Testing evolutionary hypotheses of acclimation. In: Johnston I, Bennett A, editors. Animals and Temperature Phenotypic and evolutionary adaptation. Cambridge: Cambridge University Press. pp. 205–238.
- 20. Berkelmans R, van Oppen MJH (2006) The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proceedings of the Royal Society B-Biological Sciences 273: 2305–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rowan R, Knowlton N, Baker A, Jara J (1997) Landscape ecology of algal symbionts creates variation in episodes of coral breaching. Nature 388: 265–269. [DOI] [PubMed] [Google Scholar]
- 22. Toller WW, Rowan R, Knowlton N (2001) Zooxanthellae of the Montastraea annularis species complex: Patterns of distribution of four taxa of Symbiodinium on different reefs and across depths. Biological Bulletin 201: 348–359. [DOI] [PubMed] [Google Scholar]
- 23. Hennige SJ, Suggett DJ, Warner ME, McDougall KE, Smith DJ (2009) Photobiology of Symbiodinium revisited: bio-physical and bio-optical signatures. Coral Reefs 28: 179–195. [Google Scholar]
- 24. Cooper TF, Ulstrup KE, Dandan SS, Heyward AJ, Kuhl M, et al. (2011) Niche specialization of reef-building corals in the mesophotic zone: metabolic trade-offs between divergent Symbiodinium types. Proceedings of the Royal Society B-Biological Sciences 278: 1840–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ulstrup KE, Berkelmans R, Ralph PJ, van Oppen MJH (2006) Variation in bleaching sensitivity of two coral species across a latitudinal gradient on the Great Barrier Reef: the role of zooxanthellae. Marine Ecology-Progress Series 314: 135–148. [Google Scholar]
- 26. Cooper TF, Ulstrup KE (2011) Mesoscale variation in the photophysiology of the reef building coral Pocillopora damicornis along an environmental gradient. Estuarine Coastal and Shelf Science 83: 186–196. [Google Scholar]
- 27. Howells EJ, Beltran VH, Larsen NW, Bay LK, Willis BL, et al. (2012) Coral thermal tolerance shaped by local adaptation of photosymbionts. Nature Climate Change 2: 116–120. [Google Scholar]
- 28. Middlebrook R, Hoegh-Guldberg O, Leggat W (2008) The effect of thermal history on the susceptibility of reef-building corals to thermal stress. Journal of Experimental Biology 211: 1050–1056. [DOI] [PubMed] [Google Scholar]
- 29. Putnam H, Edmunds PJ, Fan T-Y (2010) Effect of a fluctuating thermal regime on adult and larval reef corals. Invertebrate Biology 129: 199–209. [Google Scholar]
- 30. Oliver TA, Palumbi SR (2011) Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs 30: 429–440. [Google Scholar]
- 31. Ainsworth TD, Hoegh-Guldberg O, Heron SF, Skirving WJ, Leggat W (2008) Early cellular changes are indicators of pre-bleaching thermal stress in the coral host. Journal of Experimental Marine Biology and Ecology 364: 63–71. [Google Scholar]
- 32. Dunn SR, Thomason JC, Le Tissier MDA, Bythell JC (2004) Heat stress induces different forms of cell death in sea anemones and their endosymbiotic algae depending on temperature and duration. Cell Death and Differentiation 11: 1213–1222. [DOI] [PubMed] [Google Scholar]
- 33. Kenkel CD, Aglyamova G, Alamaru A, Bhagooli R, Capper R, et al. (2011) Development of Gene Expression Markers of Acute Heat-Light Stress in Reef-Building Corals of the Genus Porites . PLoS ONE 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baird AH, Bhagooli R, Ralph PJ, Takahashi S (2008) Coral bleaching: the role of the host. Trends in Ecology & Evolution 24: 16–20. [DOI] [PubMed] [Google Scholar]
- 35. Houlbreque F, Ferrier-Pages C (2009) Heterotrophy in Tropical Scleractinian Corals. Biological Reviews 84: 1–17. [DOI] [PubMed] [Google Scholar]
- 36.Ferrier-Pagès C, Hoogenboom M, Houlbrèque F (2011) The role of plankton in coral trophodynamics. In: Dubinsky Z, Stambler N, editors. Coral Reefs: An Ecosystem in Transition. Berlin: Sprinver Verlag. pp. 215–229.
- 37. Anthony KRN, Fabricius KE (2000) Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. Journal of Experimental Marine Biology and Ecology 252: 221–253. [DOI] [PubMed] [Google Scholar]
- 38. Houlbreque F, Tambutte E, Ferrier-Pages C (2003) Effect of zooplankton availability on the rates of photosynthesis, and tissue and skeletal growth in the scleractinian coral Stylophora pistillata . Journal of Experimental Marine Biology and Ecology 296: 145–166. [Google Scholar]
- 39. Hoogenboom M, Beraud E, Ferrier-Pages C (2010) Relationship between symbiont density and photosynthetic carbon acquisition in the temperate coral Cladocora caespitosa . Coral Reefs 29: 21–29. [Google Scholar]
- 40. Cantin NE, van Oppen MJH, Willis BL, Mieog JC, Negri AP (2009) Juvenile corals can acquire more carbon from high-performance algal symbionts. Coral Reefs 28: 405–414. [Google Scholar]
- 41. Hoogenboom M, Rodolfo-Metalpa R, Ferrier-Pages C (2010) Co-variation between autotrophy and heterotrophy in the Mediterranean coral Cladocora caespitosa . Journal of Experimental Biology 213: 2399–2409. [DOI] [PubMed] [Google Scholar]
- 42. Leuzinger S, Willis BL, Anthony KRN (2012) Energy allocation in a reef coral under varying resource availability. Marine Biology 159: 177–186. [Google Scholar]
- 43. Grottoli AG, Rodrigues LJ, Palardy JE (2006) Heterotrophic plasticity and resilience in bleached corals. Nature 440: 1186–1189. [DOI] [PubMed] [Google Scholar]
- 44. Rodrigues LJ, Grottoli AG (2007) Energy reserves and metabolism as indicators of coral recovery from bleaching. Limnology and Oceanography 52: 1874–1882. [Google Scholar]
- 45. Connolly SR, Lopez-Yglesias MA, Anthony KRN (2012) Food availability promotes rapid recovery from thermal stress in a scleractinian coral. Coral Reefs 31: 951–960. [Google Scholar]
- 46. Anthony KRN, Hoogenboom MO, Maynard JA, Grottoli AG, Middlebrook R (2009) Energetics approach to predicting mortality risk from environmental stress: a case study of coral bleaching. Functional Ecology 23: 539–550. [Google Scholar]
- 47. Donner SD, Skirving WJ, Little CM, Oppenheimer M, Hoegh-Guldberg O (2005) Global assessment of coral bleaching and required rates of adaptation under climate change. Global Change Biology 11: 2251–2265. [DOI] [PubMed] [Google Scholar]
- 48. Foret S, Kassahn KS, Grasso LC, Hayward DC, Iguchi A, et al. (2007) Genomic and microarray approaches to coral reef conservation biology. Coral Reefs 26: 475–486. [Google Scholar]
- 49. Dalziel AC, Rogers SM, Schulte PM (2009) Linking genotypes to phenotypes and fitness: how mechanistic biology can inform molecular ecology. Molecular Ecology 18: 4997–5017. [DOI] [PubMed] [Google Scholar]
- 50. Ranz JM, Machado CA (2006) Uncovering evolutionary patterns of gene expression using microarrays. Trends in Ecology & Evolution 21: 29–37. [DOI] [PubMed] [Google Scholar]
- 51. Newman JRS, Ghaemmaghami S, Ihmels J, Breslow DK, Noble M, et al. (2006) Single-cell proteomic analysis of S. cerevisiae reveals the architecture of biological noise. Nature 441: 840–846. [DOI] [PubMed] [Google Scholar]
- 52. Lu P, Vogel C, Wang R, Yao X, Marcotte EM (2007) Absolute protein expression profiling estimates the relative contributions of transcriptional and translational regulation. Nature Biotechnology 25: 117–124. [DOI] [PubMed] [Google Scholar]
- 53. Desalvo MK, Voolstra C, Sunagawa S, Schwarz JA, Stillman JH, et al. (2008) Differential gene expression during thermal stress and bleaching in the Caribbean coral Montastraea faveolata . Molecular Ecology 17: 3952–3971. [DOI] [PubMed] [Google Scholar]
- 54. Grasso LC, Negri AP, Foret S, Saint R, Hayward DC, et al. (2011) The biology of coral metamorphosis: Molecular responses of larvae to inducers of settlement and metamorphosis. Developmental Biology 353: 411–419. [DOI] [PubMed] [Google Scholar]
- 55. Voolstra C, Schwarz JA, Schnetzer J, Sunagawa S, Desalvo MK, et al. (2009) The host transcriptome remains unaltered during the establishment of coral-algal symbioses. Molecular Ecology 18: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 56. Bay LK, Nielsen HB, Jarmer H, Seneca F, van Oppen MJH (2009) Transcriptomic variation in a coral reveals pathways of clonal organisation. Marine Genomics 2: 119–125. [DOI] [PubMed] [Google Scholar]
- 57. Bay LK, Ulstrup KE, Nielsen HB, Jarmer H, Goffard N, et al. (2009) Microarray analysis reveals transcriptional plasticity in the reef building coral Acropora millepora. . Molecular Ecology 18: 3062–3075. [DOI] [PubMed] [Google Scholar]
- 58. Edge SE, Morgan MB, Gleason DF, Snell TW (2005) Development of a coral cDNA array to examine gene expression profiles in Montastraea faveolata exposed to environmental stress. Marine Pollution Bulletin 51: 507–523. [DOI] [PubMed] [Google Scholar]
- 59. Edge SE, Morgan MB, Snell TW (2008) Temporal analysis of gene expression in a field population of the Scleractinian coral Montastraea faveolata . Journal of Experimental Marine Biology and Ecology 355: 114–124. [Google Scholar]
- 60. Morgan MB, Edge SE, Snell TW (2005) Profiling differential gene expression of corals along a transect of waters adjacent to the Bermuda municipal dump. Marine Pollution Bulletin 51: 524–533. [DOI] [PubMed] [Google Scholar]
- 61. Meyer E, Davies S, Wang S, Willis BL, Abrego D, et al. (2009) Genetic variation in responses to a settlement cue and elevated temperature in the reef-building coral Acropora millepora . Marine Ecology-Progress Series 392: 81–92. [Google Scholar]
- 62. Meyer E, Aglyamova GV, Matz MV (2011) Profiling gene expression responses of coral larvae (Acropora millepora) to elevated temperature and settlement inducers using a novel RNA-Seq procedure. Molecular Ecology 20: 3599–3616. [DOI] [PubMed] [Google Scholar]
- 63. Barshis DJ, Stillman JH, Gates RD, Toonen RJ, Smith LW, et al. (2010) Protein expression and genetic structure of the coral Porites lobata in an environmentally extreme Samoan back reef: does host genotype limit phenotypic plasticity? Molecular Ecology 19: 1705–1720. [DOI] [PubMed] [Google Scholar]
- 64.Allemand D, Tambutte E, Zoccola D, Tambutte S (2011) Coral Calcification, Cells to Reefs. In: Dubinsky Z, Stambler N, editors. Coral Reefs: An Ecosystem in Transition. Heidelberg: Springer. pp. 119–150.
- 65. Furla P, Allemand D, Orsenigo M-N (2000) Involvement of H+ - ATPase and carbonic anhydrase in inorganic carbon uptake for endosymbiont photosynthesis. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology 278: R870–R881. [DOI] [PubMed] [Google Scholar]
- 66. Weis VM (1993) Effect of dissolved inorganic carbon concentration on the photosynthesis of the symbiotic sea anemone Aiptasia pulchella Carlgren: role of carbonic anhydrase. Journal of Experimental Marine Biology and Ecology 174: 209–225. [Google Scholar]
- 67. Weis VM, Smith GJ, Muscatine L (1989) A “CO2-supply” mechanism in zooxanthellate cnidarians: role of carbonic anhydrase. Marine Biology 100: 195–202. [Google Scholar]
- 68. Weis VM (1991) The induction of carbonic anhydrase in the symbiotic sea anemone Aiptasia pulchella . Biological Bulletin 180: 496–504. [DOI] [PubMed] [Google Scholar]
- 69. Cooper TF, Uthicke S, Humphrey C, Fabricius KE (2007) Gradients in water column nutrients, sediment parameters, irradiance and coral reef development in the Whitsunday Region, central Great Barrier Reef. Estuarine Coastal and Shelf Science 74: 458–470. [Google Scholar]
- 70. Maly IP, Sasse D (1988) Nutritional and gonadal effects on the intra-acinar profiles of low-Km and high-Km aldehyde dehydrogenase activity in rat liver. Histochemistry 88: 387–393. [DOI] [PubMed] [Google Scholar]
- 71. Castilho PC, Buckley BA, Somero G, Block BA (2009) Heterologous hybridization to a complementary DNA microarray reveals the effect of thermal acclimation in the endothermic bluefin tuna (Thunnus orientalis). Molecular Ecology 18: 2092–2102. [DOI] [PubMed] [Google Scholar]
- 72. Fitt WK, Gates RD, Hoegh-Guldberg O, Bythell JC, Jatkar A, et al. (2009) Response of two species of Indo-Pacific corals, Porites cylindrica and Stylophora pistillata, to short-term thermal stress: The host does matter in determining the tolerance of corals to bleaching. Journal of Experimental Marine Biology and Ecology 373: 102–110. [Google Scholar]
- 73. Grottoli AG, Rodrigues LJ, Palardy JE (2006) Heterotropic plasticity and resilience in bleached corals. Nature 440: 1186–1189. [DOI] [PubMed] [Google Scholar]
- 74. Middlebrook R, Anthony KRN, Hoegh-Guldberg O, Dove S (2010) Heating rate and symbiont productivity are key factors determining thermal stress in the reef-building coral Acropora formosa . Journal of Experimental Biology 213: 1026–1034. [DOI] [PubMed] [Google Scholar]
- 75. Yuyama I, Watanabe T, Takei Y (2011) Profiling Differential Gene Expression of Symbiotic and Aposymbiotic Corals Using a High Coverage Gene Expression Profiling (HiCEP) Analysis. Marine Biotechnology 13: 32–40. [DOI] [PubMed] [Google Scholar]
- 76. Voolstra CR, Sunagawa S, Matz MV, Bayer T, Aranda M, et al. (2011) Rapid Evolution of Coral Proteins Responsible for Interaction with the Environment. PLoS ONE 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Grottoli AG, Rodrigues LJ, Juarez C (2004) Lipids and stable carbon isotopes in two species of Hawaiian corals, Porites compressa and Montipora verrucosa, following a bleaching event. Marine Biology 145: 621–631. [Google Scholar]
- 78. Yamashiro H, Oku H, Onaga K (2005) Effect of bleaching on lipid content and composition of Okinawan corals. Fisheries Science 71: 448–453. [Google Scholar]
- 79. Tocher DR (2003) Metabolism and Functions of Lipids and Fatty Acids in Teleost Fish. Reviews in Fisheries Science 11: 107–184. [Google Scholar]
- 80. Ulstrup KE, Ralph PJ, Larkum AWD, Kuhl M (2006) Intra-colonial variability in light acclimation of zooxanthellae in coral tissues of Pocillopora damicornis . Marine Biology 149: 1325–1335. [Google Scholar]
- 81. Anthony KRN, Hoegh-Guldberg O (2003) Kinetics of photoacclimation in corals. Oecologia 134: 23–31. [DOI] [PubMed] [Google Scholar]
- 82. Kortschak RD, Samuel G, Saint R, Miller DJ (2003) EST analysis of the Cnidarian Acropora millepora reveals extensive gene loss and rapid sequence divergence in the model invertebrates. Current Biology 13: 2190–2195. [DOI] [PubMed] [Google Scholar]
- 83. Desalvo MK, Sunagawa S, Voolstra CR, Medina M (2010) Transcriptomic responses to heat stress and bleaching in the elkhorn coral Acropora palmata . Marine Ecology Progress Series 402: 97–113. [Google Scholar]
- 84. Tambutté S, Holcomb M, Ferrier-Pagès C, Reynaud S, Tambutté E, et al. (2011) Coral biomineralization: From the gene to he environment. Journal of Experimental Marine Biology and Ecology 408: 58–78. [Google Scholar]
- 85. Young SD (1973) Collagen and other mesoglea protein from the coral Lobophyllia corymbosa (anthozoa, scleractinia). International Journal of Biochemistry 4: 339–344. [Google Scholar]
- 86. Schwarz JA, Brokstein PB, Voolstra C, Terry AY, Miller DJ, et al. (2008) Coral life history and symbiosis: Functional genomic resources for two reef building Caribbean corals, Acropora palmata and Montastraea faveolata . BMC Genomics 9: 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ganot P, Moya A, Magnone V, Allemand D, Furla P, et al. (2011) Adaptations to Endosymbiosis in a Cnidarian-Dinoflagellate Association: Differential Gene Expression and Specific Gene Duplications. PLoS Genetics 7: e1002187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Grasso LC, Maindonald J, Rudd S, Hayward DC, Saint R, et al. (2008) Microarray analysis identifies candidate genes for key roles in coral development. BMC Genomics 9: 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kvennefors CE, Leggat W, Hoegh-Guldberg O, Degnan BM, Barnes AC (2008) An ancient and variable mannose-binding lectin from the coral Acropora millepora binds both pathogens and symbionts Developmental and Comparative. Immunology 32: 1582–1592. [DOI] [PubMed] [Google Scholar]
- 90. Barshis DJ, Ladner JT, Oliver TA, Seneca FO, Traylor-Knowles N, et al. (2013) Genomic basis for coral resilience to climate change. Proceedings of the National Academy of Science 110: 1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tomanek L (2011) Environmental Proteomics: Changes in the Proteome of Marine Organisms in Response to Environmental Stress, Pollutants, Infection, Symbiosis, and Development. Annual Review of Marine Science 3: 373–399. [DOI] [PubMed] [Google Scholar]
- 92. Schrader M, Fahimi HD (2006) Peroxisomes and oxidative stress. Biochimica et Biophysica Acta 1763: 1755–1766. [DOI] [PubMed] [Google Scholar]
- 93. Blasi F, Carmeliet P (2002) uPAR: A versatile signalling orchestrator. Nature Reviews: Molecular Cell Biology 3: 932–943. [DOI] [PubMed] [Google Scholar]
- 94. Smith HW, Marshall CJ (2010) Regulation of cell signalling by uPAR. Nature Reviews: Molecular Cell Biology 11: 23–36. [DOI] [PubMed] [Google Scholar]
- 95. Vidal-Dupiol J, Adjeroud M, Roger E, Foure L, Duval D, et al. (2009) Coral bleaching under thermal stress: putative involvement of host/symbiont recognition mechanisms. BMC Physiology 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Cossins A, Fraser J, Hughes M, Gracey A (2006) Post-genomic approaches to understanding the mechanisms of environmentally induced phenotypic plasticity. Journal of Experimental Biology 209: 2328–2336. [DOI] [PubMed] [Google Scholar]
- 97. van Oppen MJH, Palstra FP, Piquet AMT, Miller DJ (2001) Patterns of coral-dinoflagellate associations in Acropora: significance of local availability and physiology of Symbiodinium strains and host-symbiont selectivity. Proceedings of the Royal Society of London Series B-Biological Sciences 268: 1759–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Smith-Keune C, van Oppen M (2006) Genetic structure of a reef-building coral from thermally distinct environments on the Great Barrier Reef. Coral Reefs 25: 493–502. [Google Scholar]
- 99. van Oppen MJH, Peplow LM, Kininmonth S, Berkelmans R (2011) Historical and contemporary factors shape the population genetic structure of the broadcast spawning coral, Acropora millepora, on the Great Barrier Reef. Molecular Ecology 20: 4899–4914. [DOI] [PubMed] [Google Scholar]
- 100. Souter P, Bay LK, Andreakis N, Csaszar N, Seneca FO, et al. (2011) A multilocus, temperature stress-related gene expression profile assay in Acropora millepora, a dominant reef-building coral. Molecular Ecology Resources 11: 328–334. [DOI] [PubMed] [Google Scholar]
- 101. Drew JE, Mayer C-D, Farquharson AJ, Young P, Barrera LN (2011) Custom Design of a GeXP Multiplexed Assay Used to Assess Expression Profiles of Inflammatory Gene Targets in Normal Colon, Polyp, and Tumor Tissue. Journal of Molecular Diagnostics 13: 233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Webster MS, Pantile R, Botte E, Abdo D, Andreakis N, et al. (2013) A complex life cycle in a warming planet: gene expression in thermally stressed sponges. Molecular Ecology doi: 10.1111/mec.12213. [DOI] [PubMed] [Google Scholar]
- 103. Rocker MM, Willis BL, Bay LK (2012) Thermal stress-related gene expression in corals with different Symbiodinium types. Cairns Australia [Google Scholar]
- 104. Rai AJ, Kamath RM, Gerald W, Fleisher M (2009) Analytical validation of the GeXP analyzer and design of a workflow for cancer biomarker discovery using multiplexed gene-expression profiling. Analytical and Bioanalytical Chemistry 393: 1505–1511. [DOI] [PubMed] [Google Scholar]
- 105. Csaszar NBM, Seneca FO, van Oppen MJH (2009) Variation in antioxidant gene expression in the scleractinian coral Acropora millepora under laboratory thermal stress. Marine Ecology-Progress Series 392: 93–102. [Google Scholar]
- 106. Vandesompele J, de Preter K, Pattyn F, Poppe B, van Roy N, et al. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ralph PJ, Gademann R (2005) Rapid light curves: A powerful tool to assess photosynthetic activity. Aquatic Botany 82: 222–237. [Google Scholar]
- 108. Geel C, Versluis W, Snel JFH (1997) Estimation of oxygen evolution by marine phytoplankton from measurements of the efficiency of photosystem II electron flow. Photosynthesis Research 51: 61–70. [Google Scholar]
- 109. Hill R, Schreiber U, Gademann R, Larkum AWD, Kuhl M, et al. (2004) Spatial heterogeneity of photosynthesis and the effect of temperature induced bleaching conditions in the three species of corals. Marine Biology 144: 633–640. [Google Scholar]
- 110.Schreiber U (2004) Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: An overview. In: Papageorgiou G, Govindjee, editors. Chlorophyll a Fluorescence: A signature of Photosynthesis. New York: Springer. pp. 279–319.
- 111. Platt T, Gallegos CL, Harrison WG (1980) Photoinhibition of photosynthesis in natural assemblages of marine-phytoplankton. Journal of Marine Research 38: 687–701. [Google Scholar]
- 112. Harland AD, Fixter LM, Davies PS, Anderson RA (1992) Effect of light on the total lipid-content and storage lipids of the symbiotic sea-anemone Anemonia viridis . Marine Biology 112: 253–258. [Google Scholar]
- 113. Cnaan A, Laird NM, Slasor P (1997) Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data.. Statistics in Medicine 16: 2349–2380. [DOI] [PubMed] [Google Scholar]
- 114. Krueger C, Tian L (2004) A Comparison of the General Linear Mixed Model and Repeated Measures ANOVA Using a Dataset with Multiple Missing Data Points. Biological Research for Nursing 6: 151–157. [DOI] [PubMed] [Google Scholar]
- 115. Bates D (2005) Fitting linear mixed models in R. R News 5: 4. [Google Scholar]
- 116. Benjamini Y, Yekutieli D (2001) The control of false discovery rate under dependency. Annals of Statistics 29: 1165–1188. [Google Scholar]
- 117. Narum SR (2006) Beyond Bonferroni: Less conservative analyses for conservation genetics. Conservation Genetics 7: 783–787. [Google Scholar]
- 118. Hadfield JD (2010) MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. Journal of Statistical Software 33: 1–22. [PMC free article] [PubMed] [Google Scholar]
- 119.R Development Core Team (2012) R: A language and environment for statistical computing. Vienna, Austria: R foundation for statistical computing.
- 120. Miller DJ, Hemmrich G, Ball EE, Hayward DC, Khalturin K, et al. (2007) The innate immune repertoire in Cnidaria -ancestral complexity and stochastic gene loss. Genome Biology 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Carr HS, Winge DR (2003) Assembly of Cytochrome c Oxidase within the Mitochondrion. Accounts of Chemical Research 36: 309–316. [DOI] [PubMed] [Google Scholar]
- 122. Barrientos A, Barrosa MH, Valnot I, Rotig A, Rustin P, et al. (2002) Cytochrome oxidase in health and disease. Gene 286: 53–63. [DOI] [PubMed] [Google Scholar]
- 123. Unuma T, Yamamoto T, Akiyama T, Shiraishi M, Ohta H (2003) Quantitative changes in yolk protein and other components in the ovary and testis of the sea urchin Pseudocentrotus depressus . Journal of Experimental Biology 206: 365–372. [DOI] [PubMed] [Google Scholar]
- 124. Imagawa S, Nakano Y, Watanabe T (2004) Molecular analysis of a major soluble eggprotein in the scleractinian coral Favites chinensis . Comparative Biochemistry and Physiology 137: 11–19. [DOI] [PubMed] [Google Scholar]
- 125. Reyes-Bermudez A, Lin Z, Hayward DC, Miller DJ, Ball EE (2009) Differential expression of three galaxin related genes during settlement and metamorphosis in the scleractinian coral Acropora millepora . BMC Evolutionary Biology 9: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Likelihood ratio tests, Akaike weights and pairwise differences from gene expression and physiological analyses.
(PDF)
Genes included in the gene expression assay, abbreviations, accession numbers, concentration of primer in Reverse Primer Plex (nM), length of the transcript (bp) and Oligo sequences.
(PDF)