Abstract
During the last two decades, large-scale high biomass algal blooms of the dinoflagellate Prorocentrum donghaiense Lu have occurred frequently in the East China Sea (ECS). The role of increasing nutrient concentrations in driving those blooms is well-established, but the source population that initiates them is poorly understood. We hypothesized that the front of Taiwan Warm Current (TWC) may serve as a ‘seed bank’ that initiates P. donghaiense blooms in the ECS, as the physiochemical conditions in the TWC are suitable for the growth of P. donghaiense. In order to test this hypothesis, two surveys at different spatio-temporal scales were conducted in 2010 and 2011. We found a strong correlation in space and time between the abundance of P. donghaiense and the TWC. The spatial extent of the P. donghaiense bloom coincided with the TWC front in both 2010 and 2011. During the early development of the blooms, P. donghaiense concentration was highest at the TWC front, and then the bloom mass shifted inshore over the course of our 2011 survey. The TWC also moved inshore, albeit after the appearance of P. donghaiense. Overall, these results support our hypothesis that P. donghaiense blooms develop from the population at the TWC front in the ECS, suggesting the role of the ocean current front as a seed bank to dinoflagellate blooms.
Introduction
Large-scale high biomass algal blooms of the dinoflagellate Prorocentrum donghaiense Lu have occurred frequently in the East China Sea (ECS) over the last two decades. These blooms are massive, sometimes extending over thousands of square kilometers, and can persist for nearly one month [1], [2]. The blooms are considered to be driven by increasing nutrient inputs from the Changjiang River (the largest river in China) and upwelling [3]–[5].
One aspect of the P. donghaiense bloom that is poorly understood is its initiation. A hypothesis is that a “pelagic seed bank” of low number of P. donghaiense cells offshore may act as an inoculum. The hypothesis, proposed by Smayda (2002), postulates that algal cells that have accumulated at ocean current fronts may act as bloom inoculums in upwelling zones [6]. The proposed mechanism is in contrast to the initiation mechanism that has been described for cyst-forming dinoflagellate species (e.g. Alexandrium fundyense) that are inoculated through the germination of their benthic cysts [7], [8]. No such cyst stage has been described for P. donghaiense [4]. Therefore its initiation is more likely to rely on mechanisms like the “pelagic seed bank” that deliver vegetative cells to nutrient rich waters like upwelling zones.
There is an upwelling belt in the ECS, about 40 km in width between the 20 m and 50 m isobaths and parallel to the Zhejiang coast line [9]–[11]. The upwelling is mainly induced by the continental slope and the Taiwan Warm Current (TWC) [12], [13]. As a branch of the Kuroshio Current (KC), the TWC is characterized by a high temperature and salinity, making it suitable for the survival and growth of P. donghaiense cells even in winter time [14], [15].
In this study, we tested the “pelagic seed bank” hypothesis for the initiation of P. donghaiense blooms in the ECS via introduction from the TWC front. Two surveys at different spatio-temporal scales were conducted in 2010 and 2011 (Fig. 1) with the objective of delimiting the TWC and the extent and abundance of P. donghaiense cells. The bloom pattern and development were examined in the surveys.
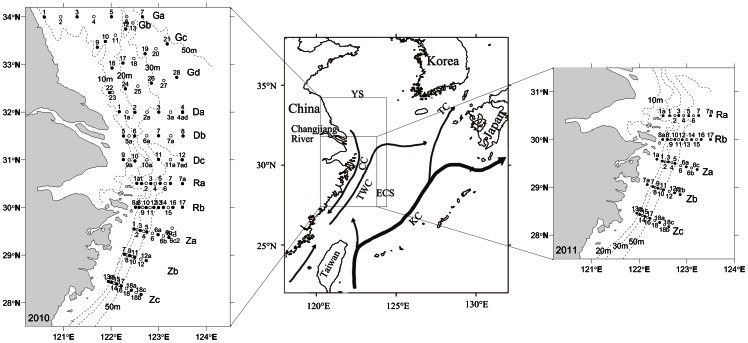
Figure 1. Sampling sites and Circulation pattern in the East China Sea (ECS) (modified from [4], [16]).
Left panel and right panel show the station locations in 2010 and 2011, respectively. Closed circle indicates comprehensive stations and open circle indicates hydrological stations, with labels above or below the station symbols, respectively. Transect labels are marked on the right of transects. Dot line indicates the isobath line. Middle panel, YS: Yellow Sea; KC: Kuroshio Current; TWC: Taiwan Warm Current; CC: Coastal Current (seasonal current with northward in summer and southward in winter); TC: Tsushima Current.
Results
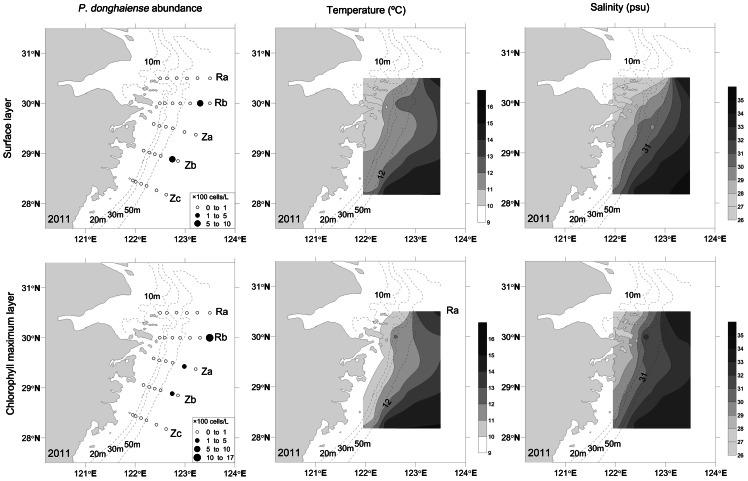
P. donghaiense cells were present above our detection limit of 2 cells/L at 38 of 41 stations during the 2010 comprehensive survey. The number of dinoflagellate species recorded varied from 1 to 9 (including P. donghaiense; mean±SD = 3.61±1.95). The relative abundance of P. donghaiense versus total dinoflagellates varied from 28.60% to 100%, with an average of 96.80±9.12%. Few diatom species were recorded. In 2011, P. donghaiense cells were only present at stations Rb16, Rb17, Za6a and Zb12a in the first cruise but at all stations in the last cruise (Fig. 2).
Figure 2. Distribution of P. donghaiense (left panels), temperature (middle panels) and salinity (right panels) in surface and chorophyll maximum layers in the ECS in the first cruise in 2011.
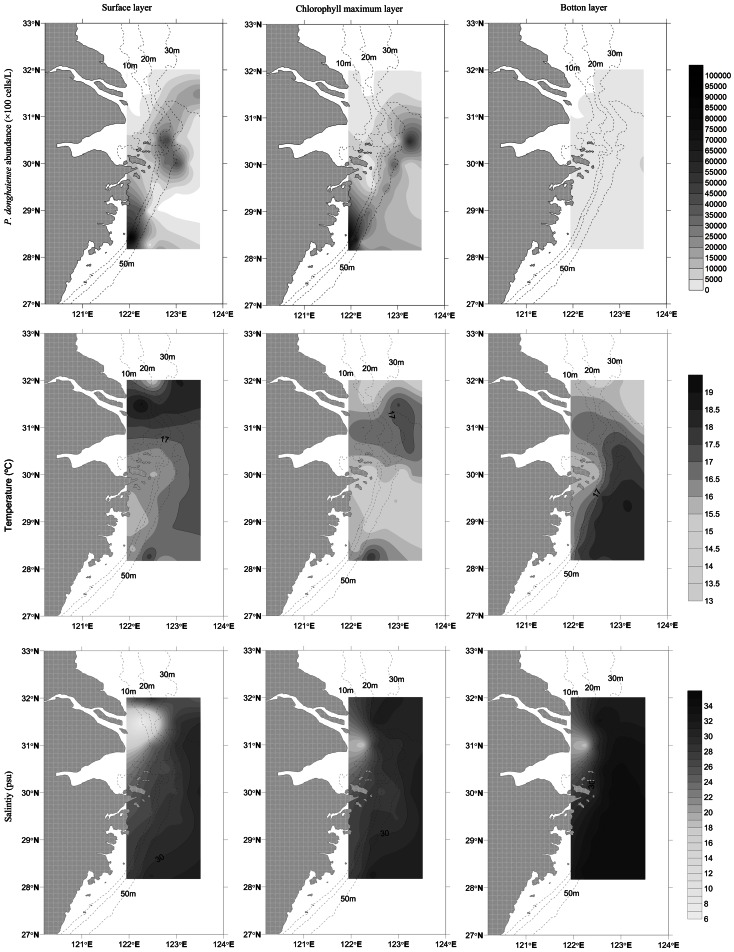
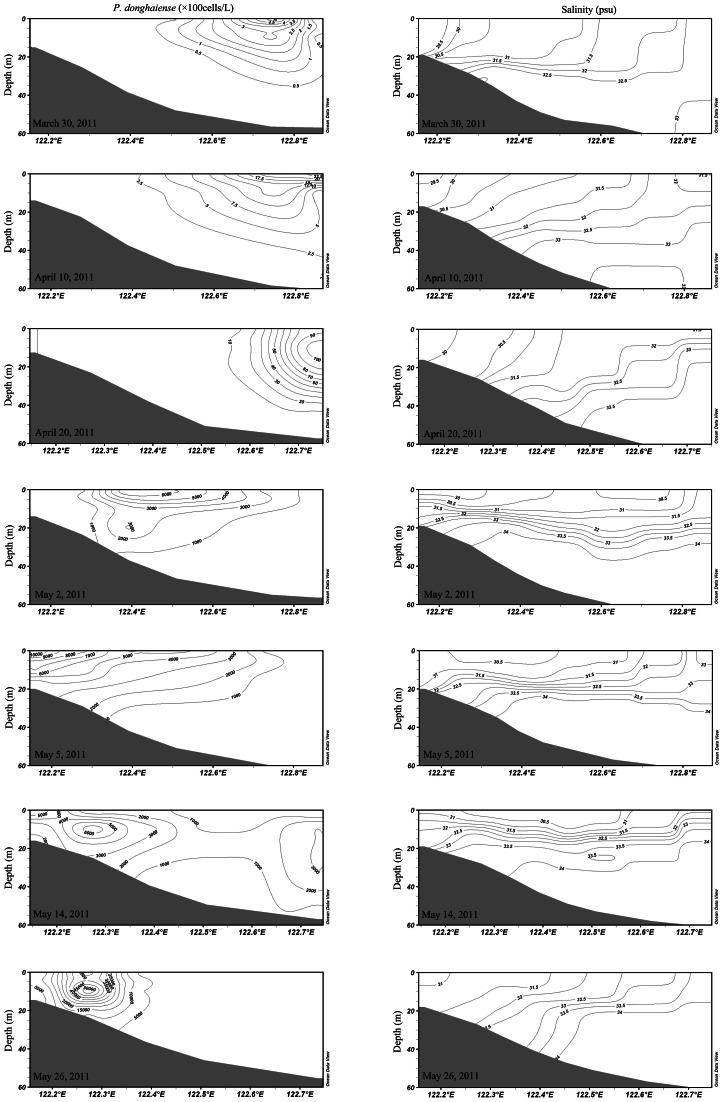
P. donghaiense cell concentrations were highest along the upwelling zone near the coast (Figs. 3 and 4). At the surface, P. donghaiense concentrations were high at the Zhoushan Fishary Ground and in the area at 122°E, 28.5°N. In the chlorophyll maximum layer, a high abundance belt was observed around the 50 m isobath. In bottom layer, the abundance was low. The salinity distribution showed clearly that there was a fresh water plume in the Changjiang River estuary (Figs. 3 and 4). The pattern of P. donghaiense abundance was closely related with that of salinity, with rapid development of the P. donghaiense bloom during stratified conditions (Figs. 4 and 5). Moreover, the highest concentrations of P. donghaiense were located at the 10 m depth, near the TWC front on all transects (Figs. 4 and 5).
Figure 3. Distribution of P. donghaiense (top panels), temperature (middle panels) and salinity (bottom panels) in three different layers in the ECS in 2010.
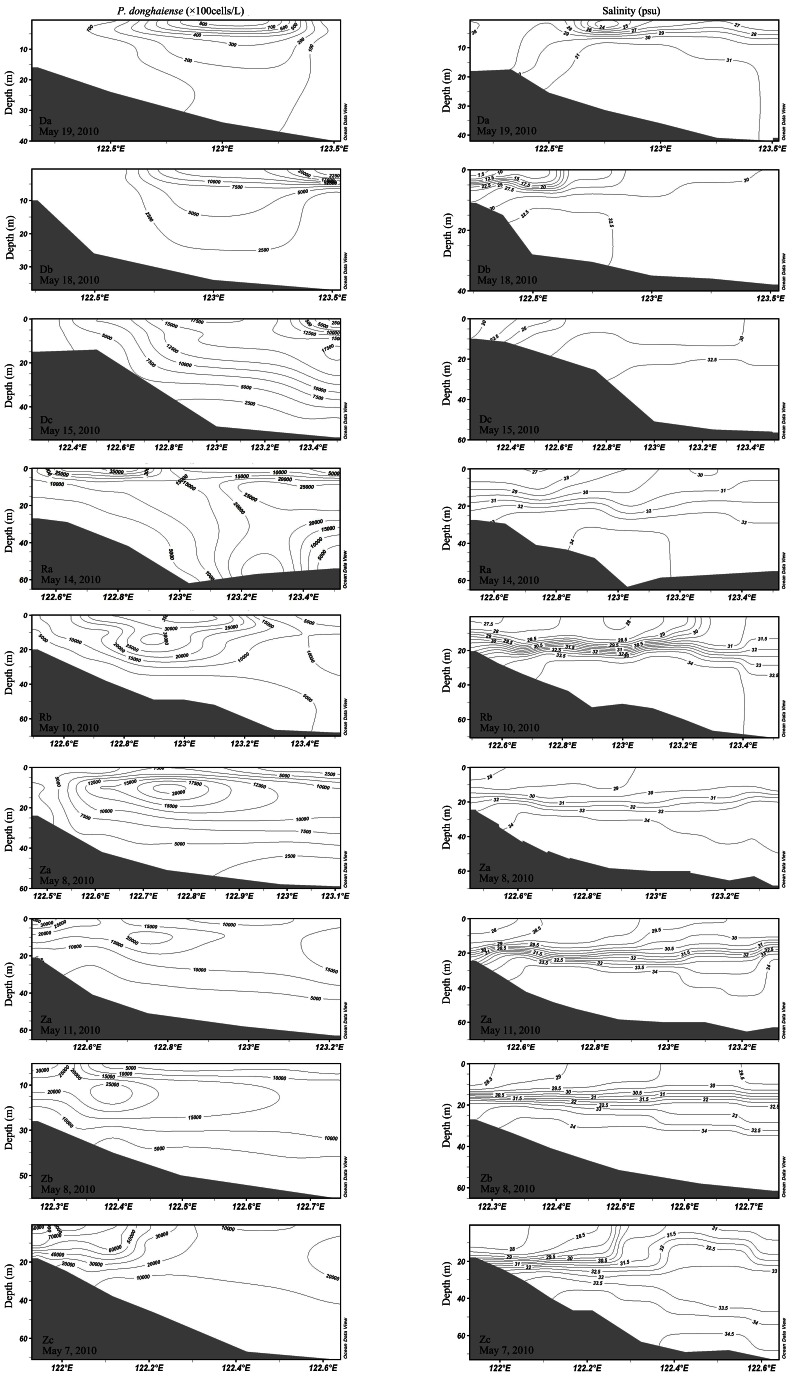
Figure 4. Vertical profiles of P. donghaiense abundance (left panels) and salinity (right panels) of nine transects (2 replicates for the transect Za) investigated in 2010.
Figure 5. Vertical profiles of P. donghaiense abundance (left panels) and salinity (right panels) on the transect Zb in seven cruises in 2011.
The highest concentrations of P. donghaiense formed early at the TWC front, and then spread inshore over the course of 2 months in 2011, coinciding with the incursion inshore of the TWC indicated by high salinity (Fig. 5). On the transect Zb, the cell abundance core was located at 122.8°E on March 30, 2011, displaying a low abundance of about 450 cells/L. It moved inshore to 122.1°E on May 26, 2011, exhibiting a high abundance of about 7×106 cells/L. P. donghaiense bloom expanded rapidly in stratified water mass with relatively stable environment (Fig. 5). Interestingly, the core of maximum abundance of P. donghaiense bloom moved inshore from May 8–11, 2010 on the transect Za, while the water mass stratification did not change too much (Fig. 4).
Discussion
Like most dinoflagellate blooms, large-scale P. donghaiense blooms in the ECS are affected by multiple factors including eutrophication from human activities [5]. In this study, we highlighted the role of the TWC front as a “pelagic seed bank” to inshore P. donghaiense blooms.
The TWC front as a “pelagic seed bank”
The strong spatio-temporal match between P. donghaiense concentrations and the TWC is consistent to the hypothesis that the TWC front may serve as a seed bank to P. donghaiense blooms in the ECS (Figs. 2, 3, 4, 5). Algal cells aggregate along frontal zones because of the physical aggregation and barrier effect [6], [17], [18]. These aggregations are often evident as extraordinarily long bloom patches. Examples include a 300 km long patch of Lingulodinium polyedrum that has been observed in the California Current [19] and a 2000 km patch of Akashiwo sanguineum that has been observed within the Peru Current [19], [20]. Here, a similar belt pattern of P. donghaiense was recorded in two years, at the frontal zone between the Coastal Current (CC) and TWC (Figs. 2, 3, 4). The highest cell concentrations within these patches occurred at depths of approximately 10 m, consistent with the boundary of the TWC front in both survey years. These observations supported our hypothesis that the TWC front acts as a “pelagic seed bank” from which the P. donghaiense population could develop and expand to adjacent areas closer to the coast (Figs. 4 and 5).
We did not investigate whether P. donghaiense existed within the TWC here, but it was clear that P. donghaiense was present at the TWC front in early spring (Fig. 2). The high P. donghaiense concentration moved inshore with the TWC front after bloom initiation (Fig. 5). We suggested that the movement of the bloom inshore was caused by three factors: changes in the nearshore current flows, the swimming behavior of P. donghaiense and nutrient distribution.
With regard to changes in the nearshore current flows, the TWC front may play a role in driving P. donghaiense blooms inshore, similar to the transportation of Cochlodinium polykrikoides blooms by the Tsushima Current (TC) in the southern coast of Korea [18]. P. donghaiense bloom could develop inshore even in stable stratified water mass, for example, the movement of the bloom on the transect Za in 2010 (Fig. 4). This species has been recorded in the frontal zone of the TWC throughout the year during larger-scale surveys (unpublished data and [5]). P. donghaiense has also been recorded at higher latitudes (along the coasts of South Korea and Japan) where the TWC is entrained into the KC (Fig. 1 and [21]). However, P. donghaiense has not been recorded within the Yellow Sea, most likely because water mass in this region is dominated by fresh water flows from the Changjiang River and cold Yellow Sea coastal current [5].
The promotion of bloom development by water stratification is also partly attributable to the swimming behavior and chain formation of this species [1]. P. donghaiense has the characteristics cited as critical for pelagic seed bank species by Smayda (2002) when he proposed their existence, i.e., tolerates the turbulence and velocities in frontal systems, develop frontal zone populations and their successful advective transfer from these sites leads to blooms elsewhere [8].
Nutrients
In the TWC, low nutrient concentrations likely limited P. donghaiense growth even though the temperature and salinity conditions were suitable for survival of this species [22]. In contrast, temperature was 8–12°C in the coastal zone during the winter, too low for P. donghaiense survival, but nutrient concentrations were relatively high due to enrichment from the Changjiang River and mixing via the CC [8], [23] and upwelling along the coastal zone [4], [9]. Therefore, P. donghaiense blooms would be triggered by increasing temperature within the coastal zone, especially since this species is more competitive in water with low phosphorus and excess nitrogen as a result of diatom blooms in early spring [3], [24].
Movement of the TWC also impacted the distribution of nutrient concentrations within the ECS, especially via the interaction of the TWC with the movement of fresh water along the coast. Nutrient concentrations decreased along transects from the coastal zone to offshore areas [25]. This nutrient gradient likely led to the stimulation of P. donghaiense blooms once they reached inshore areas. Nutrient concentrations are also enriched at depth along the TWC front because of the aggregation effect [8], which helps P. donghaiense easily inhabit this water column.
Temperature and salinity
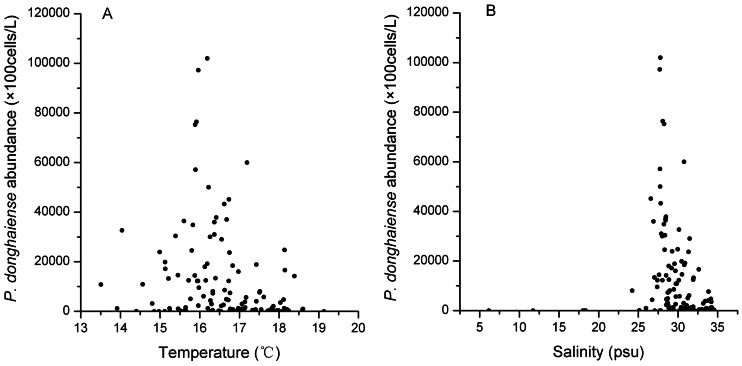
Temperature and salinity are both critical for the survival and distribution of P. donghaiense [15]. In winter, the low temperature of coastal water (<12°C) is unfavorable for growth or even survival by P. donghaiense [26]. In spring, a temperature and salinity window opens for P. donghaiense blooms through the movement of the TWC close to shore. Previous studies have reported that the optimal temperature range for P. donghaiense growth is between 20 and 27°C in cultures [15], and between 18.5 and 21.3°C in situ [27]. However in this study, the highest P. donghaiense concentration occurred at lower temperature, around 16°C (Fig. 6). This likely reflects the reality that many phytoplankton species live in suboptimal conditions in nature because of the trade-offs among many different environmental conditions [28]. In the ECS, nutrient concentrations were quite suitable for growth but the temperature was low within the coastal zone, while the temperature was optimal but the nutrient concentrations were low offshore.
Figure 6. Relationships between P. donghaiense abundance and temperature (A) and salinity (B).
P. donghaiense is likely to benefit from the combination of high temperature, high salinity and high nutrient water conditions within the interaction zone between the TWC and coastal fresh water. Once growth is initiated, P. donghaiense blooms may then expand to adjacent areas. This type of interaction may also disperse P. donghaiense into coastal areas without immediately triggering a bloom as was observed in the ECS during the 2005 season when springtime warming was delayed [29], [30]. In all cases – the 2005 season and the 2010 and 2011 seasons – the tight coupling of P. donghaiense with the TWC front in space and time suggests a pelagic seed bank strategy for the introduction of these cells into the nutrient rich coastal zone. The importance of such offshore source populations should be investigated further as they may play a significant role in the development of other dinoflagellate blooms.
Materials and Methods
We declare that all necessary permits are obtained for the described field studies. No specific permissions are required for these locations. The location is not privately-owned or protected in any way. No endangered or protected species are involved in the field studies.
Study area
Survey data was collected over the course of 10 research cruises. The first of these was conducted from May 7–24, 2010. The remaining nine cruises were conducted in 2011, from March 29 to April 2, April 9–10, April 19–20, April 28, May 2, May 4–7, May 13–15, May 22 and May 25–27, each along the selected transects of five transects (Ra, Rb, Za, Zb and Zc) between 28°N and 34°N (see Fig. 1). Most transects crossed the 20 m and 60 m isobaths in the coastal area of the ECS and the Yellow Sea (YS).
Within the study area, there is a seasonal switch whereby the Coastal Current (CC) flows southwestward in winter and northeastward in summer. Due to the winter-time direction of flow, the CC mixes with the fresh water from the Changjiang River during winter, transferring the nutrients from the estuary to the coastal region of Zhejiang Province [5]. In contrast, the flow of the TWC does not change seasonally (Fig. 1). The balance between these currents determines the movement of the TWC front [11]. Near 31.5°N, in the area offshore to the Changjiang River estuary, the fresh water of Changjiang River as well as the TWC turns from northeastward to eastward in late spring (Fig. 1) [5], [31].
Sample collection
There were two types of stations: (1) comprehensive station in which both the hydrological and biological parameters were investigated; and (2) hydrological station in which only the hydrological parameter was investigated. At each comprehensive station, a CTD multiparameter sonde (SBE 19plus, Sea-Bird Electronics, Inc. USA) was used to profile from the sea surface to the bottom of the water column to determine the depth of chlorophyll maximum layer before sampling. Water samples were collected using 30 L Niskin bottles in surface layer, chlorophyll maximum layer and bottom layer. Extra sampling depths were added at selected stations. Water sample (500 ml) was transferred into 550 ml Polyethylene terephthalate (PET) bottles and was then fixed with 3–5% acidic Lugol's solution. At each station, environmental parameters such as temperature, salinity, density, turbulence, dissolved oxygen (DO), chlorophyll-a (Chla), irradiance and pH were recorded at every station at 0.5 m depth interval using the CTD.
Field samples were concentrated to 50 ml after sedimentation for more than 24 h in laboratory. And then, 1 ml subsample was transferred to 1 ml counting chamber (Sedgewick Rafter Counting Cell, PYSER-SGI LIMITED, UK) for observation using a light microscope (LEICA DMI 4000B, Germany) at 100× and 400× magnification. This step was repeated if the plankton abundance is low. Cell concentrations (cells/L) were estimated as 100×Cn/V, where Cn was the number of cells counted and V (ml) was the total volume of subsamples.
Data analysis
The distributions of P. donghaiense and salinity were plotted with the Surfer software package (Version 8.0, 2002, Golden Software, Inc. USA) from biological observations taken at the comprehensive stations and hydrological observations from all stations.
Special emphasis was given to the transect Zb in our analysis because it was occupied during seven cruises and our hydrological data from these cruises showed substantial movement of the TWC front. Water salinity was used as a proxy for the TWC front because its variability was strongly correlated with water density in all of our surveys. Contour maps of P. donghaiense cells abundance and salinity on each transect were drawn using the Ocean Data View (ODV, 2007, http://odv.awi.de/).
Acknowledgments
We thank Dr. Zhenyi Chao and Dr. Qi Chen for their contributions in the collection of data. We also greatly acknowledge valuable comments from Dr. Michael Brosnahan and two anonymous referees.
Funding Statement
This study was supported by CEOHAB-II National 973 Program (2010CB428702; 2010CB428704), National Science Foundation (41176141), Grant from the scientific research fund of the Second Institute of Oceanography, SOA (SZ1040), Foundation of Key Laboratory of Integrated Marine Monitoring and Applied Technologies for Harmful Algal Blooms, SOA (MATHAB20100310) and Zhejiang Provincial Natural Science Foundation (Y5110185). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lu D, Goebel J, Qi Y, Zou J, Han X, et al. (2005) Morphological and genetic study of Prorocentrum donghaiense Lu from the East China Sea, and comparison with some related Prorocentrum species. Harmful Algae 4: 493–505. [Google Scholar]
- 2. Li J, Glibert PM, Zhou M (2010) Temporal and spatial variability in nitrogen uptake kinetics during harmful dinoflagellate blooms in the East China Sea. Harmful Algae 9: 531–539. [Google Scholar]
- 3. Wong GTF, Gong GC, Liu KK, Pai SC (1998) ‘Excess nitrate’ in the East China Sea. Estuarine, Coastal and Shelf Science 46: 411–418. [Google Scholar]
- 4. Zhou M, Yan T, Zou J (2003) Preliminary analysis of the characteristics of red tide areas in Changjiang River estuary and its adjacent sea. Chinese Journal of Applied Ecology 14: 1031–1038. [PubMed] [Google Scholar]
- 5. Zhou MJ, Shen ZL, Yu RC (2008) Responses of a coastal phytoplankton community to increased nutrient input from the Changjiang (Yangtze) River. Continental Shelf Research 28: 1483–1489. [Google Scholar]
- 6. Smayda TJ (2002) Turbulence, watermass stratification and harmful algal blooms: an alternative view and frontal zones as “pelagic seed banks”. Harmful Algae 1: 95–112. [Google Scholar]
- 7. Kremp A (2001) Effects of cyst resuspension on germination and seeding of two bloom-forming dinoflagellates in the Baltic Sea. Marine Ecolgoy Progress Series 216: 57–66. [Google Scholar]
- 8. Joycea LB, Pitcher GC, Randt AD, Monteiro PMS (2005) Dinoflagellate cysts from surface sediments of Saldanha Bay, South Africa: an indication of the potential risk of harmful algal blooms. Harmful Algae 4: 309–318. [Google Scholar]
- 9. Luo Y (1998) Numerical modelling of upwelling in coastal areas of the East China Sea. Transactions of Oceanology and Limnology 3: 1–6. [Google Scholar]
- 10. Luo Y, Yu G (1998) Numerical studies of wind-and TWC-driver upwelling in coastal areas of the East China Sea. Journal of Ocean University of Qingdao 28: 536–542. [Google Scholar]
- 11. Qiao F, Yang Y, Lv X, Xia C, Chen X, et al. (2006) Coastal upwelling in the East China Sea in winter. Journal of Geophysical Research 111: 1–11.20411040 [Google Scholar]
- 12. Cao X (1986) Prelimary study on the seasonal process of the coastal upwelling of Zhejiang in the East China Sea. Journal of Fish China 10: 51–69. [Google Scholar]
- 13. Jing Z, Qi Y, Hua Z (2007) Numerical study on upwelling and its seasonal variation along Fujian and Zhejiang coast. Journal of Hehai University (Natural Sciences) 35: 464–470. [Google Scholar]
- 14. Chen CTA, Ruo R, Pai SC, Liu CT, Wong GTF (1995) Exchange of water masses between the East China Sea and the Kuroshio off northeastern Taiwan. Continental Shelf Research 15: 19–39. [Google Scholar]
- 15. Xu N, Duan S, Li A, Zhang C, Cai Z, et al. (2010) Effects of temperature, salinity and irradiance on the growth of the harmful dinoflagellate Prorocentrum donghaiense Lu. Harmful Algae 9: 13–17. [Google Scholar]
- 16. Naimiea CE, Blain CA, Lynch DR (2001) Seasonal mean circulation in the Yellow Sea – a model-generated climatology. Continental Shelf Research 21: 667–695. [Google Scholar]
- 17. Park GH, Lee K, Koo CM, Lee HW (2005) A sulfur hexafluoride-based Lagrangian study on initiation and accumulation of the red tide Cochlodinium polykrikoides in southern coastal waters of Korea. Limnology and Oceanography 50: 578–586. [Google Scholar]
- 18. Lee DK (2008) Cochlodinium polykrikoides blooms and eco-physical conditions in the South Sea of Korea. Harmful Algae 7: 318–323. [Google Scholar]
- 19.Lasker R, Zweifel JR (1978) Growth and survival of first-feeding northern anchovy larvae (Engraulis mordax) in patches containing different proportions of large and small prey. In: Steele JH (Ed.), Spatial Pattern in Plankton Communities. Plenum Press, New York. 329–354.
- 20. Packard TT, Dugdate RC, Goering JJ, Barber RT (1978) Nitrate reductase activity in the sub-surface waters of the Peru Current. Journal of Marine Research 36: 59–76. [Google Scholar]
- 21. Wang H, Lu D, Huang H, Xia P, Dai X, et al. (2011) Comparison of morphological structure and ITS sequence between two Prorocent rum strains from the East China Sea and Masan Bay of Korea. Journal of Marine Science 29: 42–48. [Google Scholar]
- 22. Jiao N, Yang Y, Koshikawa H, Watanabe M (2002) Influence of hydrographic conditions on picoplankton distribution in the East China Sea. Aquatic Microbial Ecology 30: 37–48. [Google Scholar]
- 23. Xia W, Wang J, Tan L, Wang Q (2011) Variation of bacteria biomass and its possible controlling factors in the East China Sea. Oceanic and Coastal Sea Research 10: 135–141. [Google Scholar]
- 24. Zhu M, Xu Z, Li R, Wang Z, Shi X (2009) Interspecies competition for nutrients between Prorocentrum donghaiense Lu and Skeletonema costatum (Grev.) Cleve in mesocosm experiments. Acta Oceanologica Sinica 28: 72–82. [Google Scholar]
- 25. Xia W, Wang J, Tan L, Wang Q (2011) Variation of bacteria biomass and its possible controlling factors in the East China Sea. Oceanic and Coastal Sea Research 10: 135–141. [Google Scholar]
- 26.Chen D, Sun X, Pu Y (1992) Marine atlas of Bohai Sea, Yellow Sea, East China Sea. China Ocean Press, Beijing. 13–33.
- 27. Li Y, Lu S, Jiang T, Xiao Y, You S (2011) Environmental factors and seasonal dynamics of Prorocentrum populations in Nanji Islands National Nature Reserve, East China Sea. Harmful Algae 10: 426–432. [Google Scholar]
- 28.GEOHAB (2008) Global Ecology and Oceanography of Harmful Algal Blooms, GEOHAB Core Research Project: HABs in Stratified Systems. In: Gentien P, Reguera B, Yamazaki H, Fernand L, Berdalet E, Raine R (Eds.) IOC and SCOR, Paris, France, and Newark, Delaware, USA. 59 p.
- 29.Lu D, Göbel J, Gao Y, Qi Y, Zou J, et al.. (2008) Succession pattern of HAB species before large-scale blooms of dinoflagellates in the East China Sea in spring 2004/2005. In: Moestrup (ed.) Proceedings of the 12th. International Conference on harmful Alage. International Society for the Study of Harmful Alage and Intergovermental Oceanographic Commission of UNESCO, 2008 Copenhagen, 132–134.
- 30. Zhu D, Lu D, Wang Y, Su J (2008) The low temperature characteristics in Zhejiang coastal region in the early spring of 2005 and its influence on harmful algae bloom. Acta Oceanologica Sinica 31: 31–39. [Google Scholar]
- 31. Lu D, Göbel J (2001) Study on taxonomy and distribution of Ornithocercus in the East China Sea. Donghai Marine Science 19: 11–18. [Google Scholar]